Alternative and Sustainable Technologies for Freshwater Generation: From Fog Harvesting to Novel Membrane-Based Systems
Abstract
1. Introduction
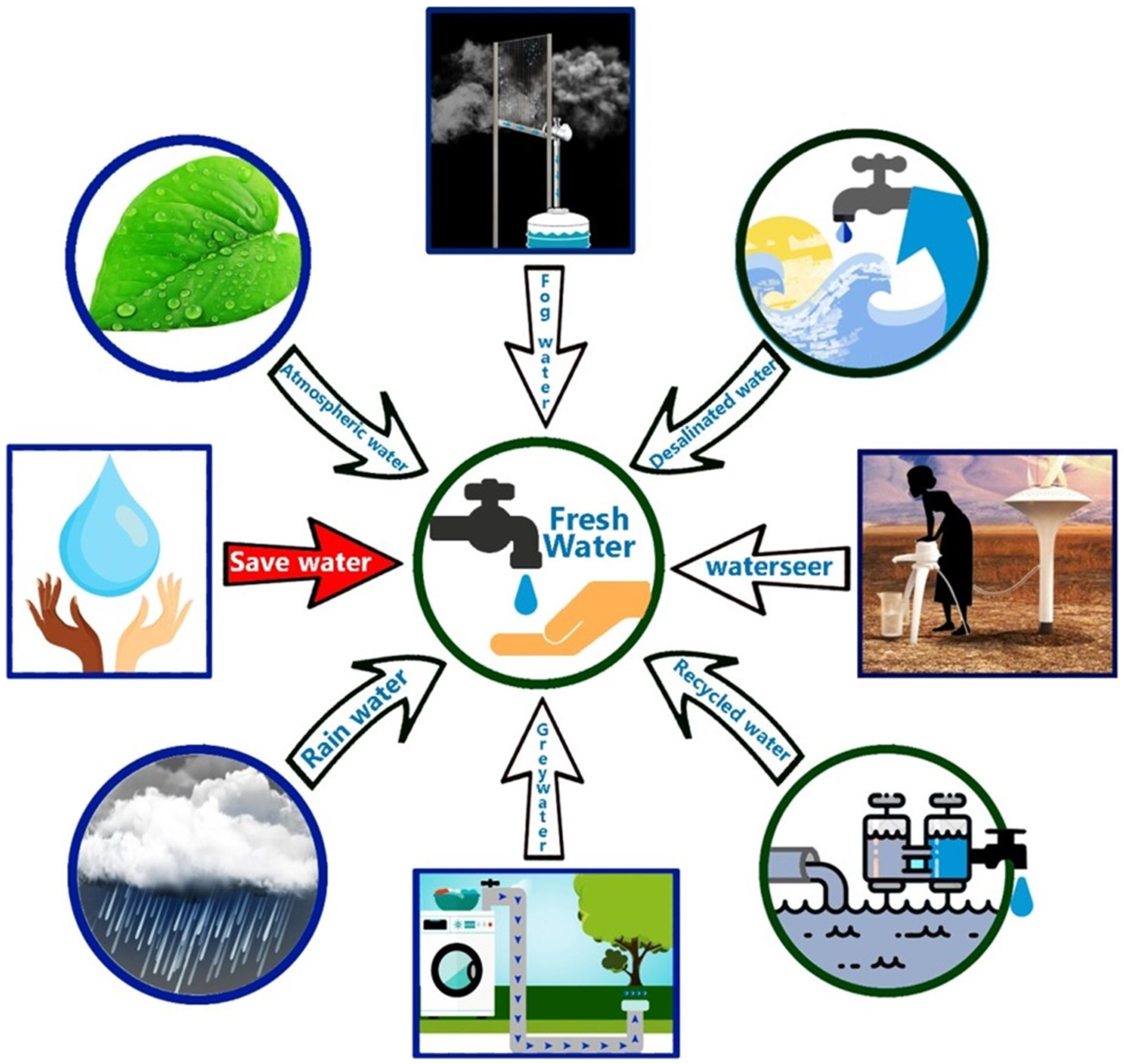
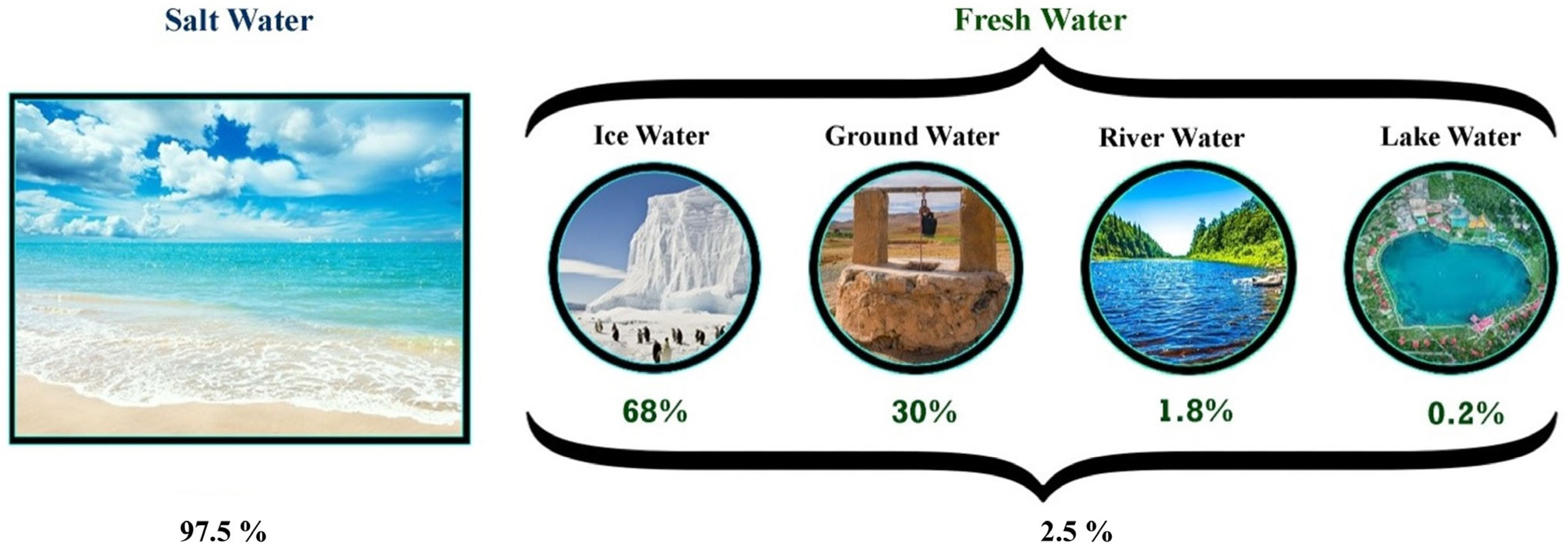
2. Alternative Water Resources
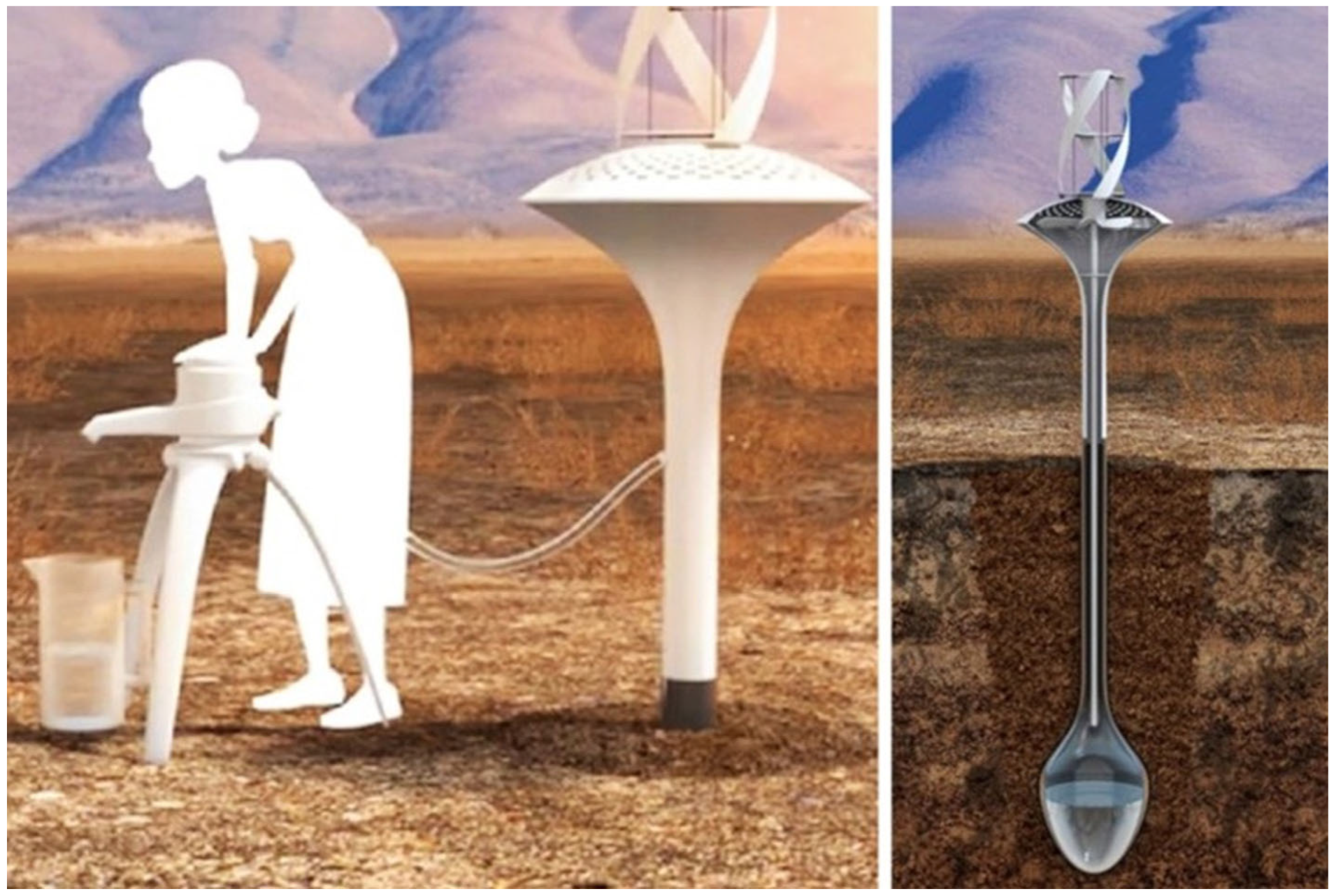
2.1. WaterSeer
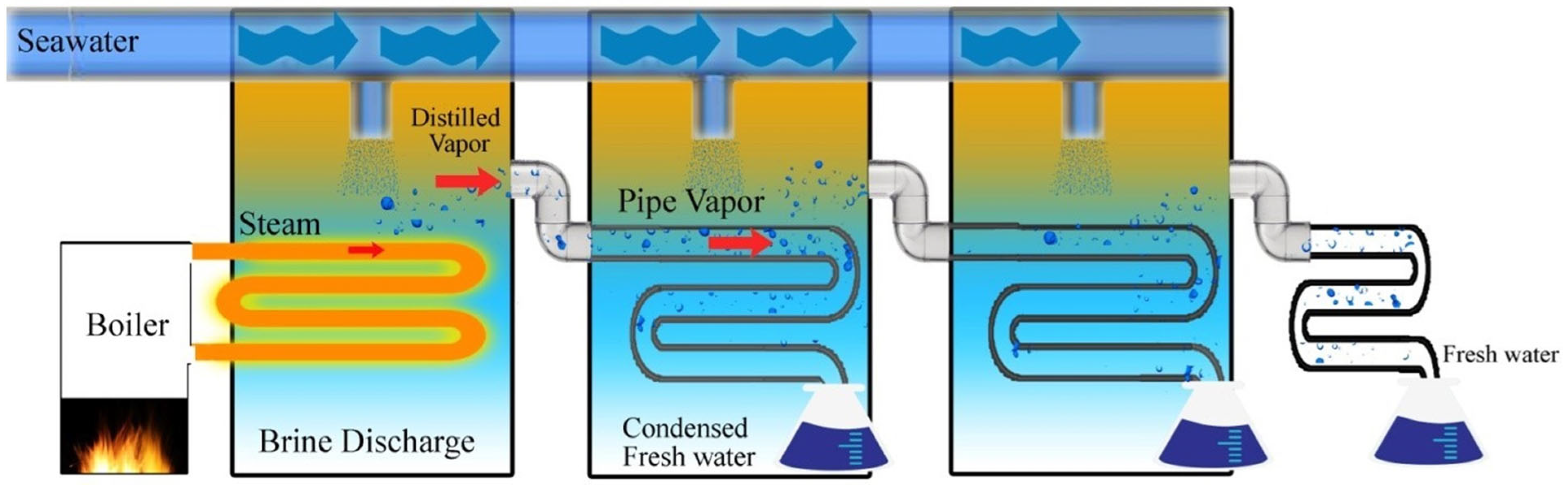
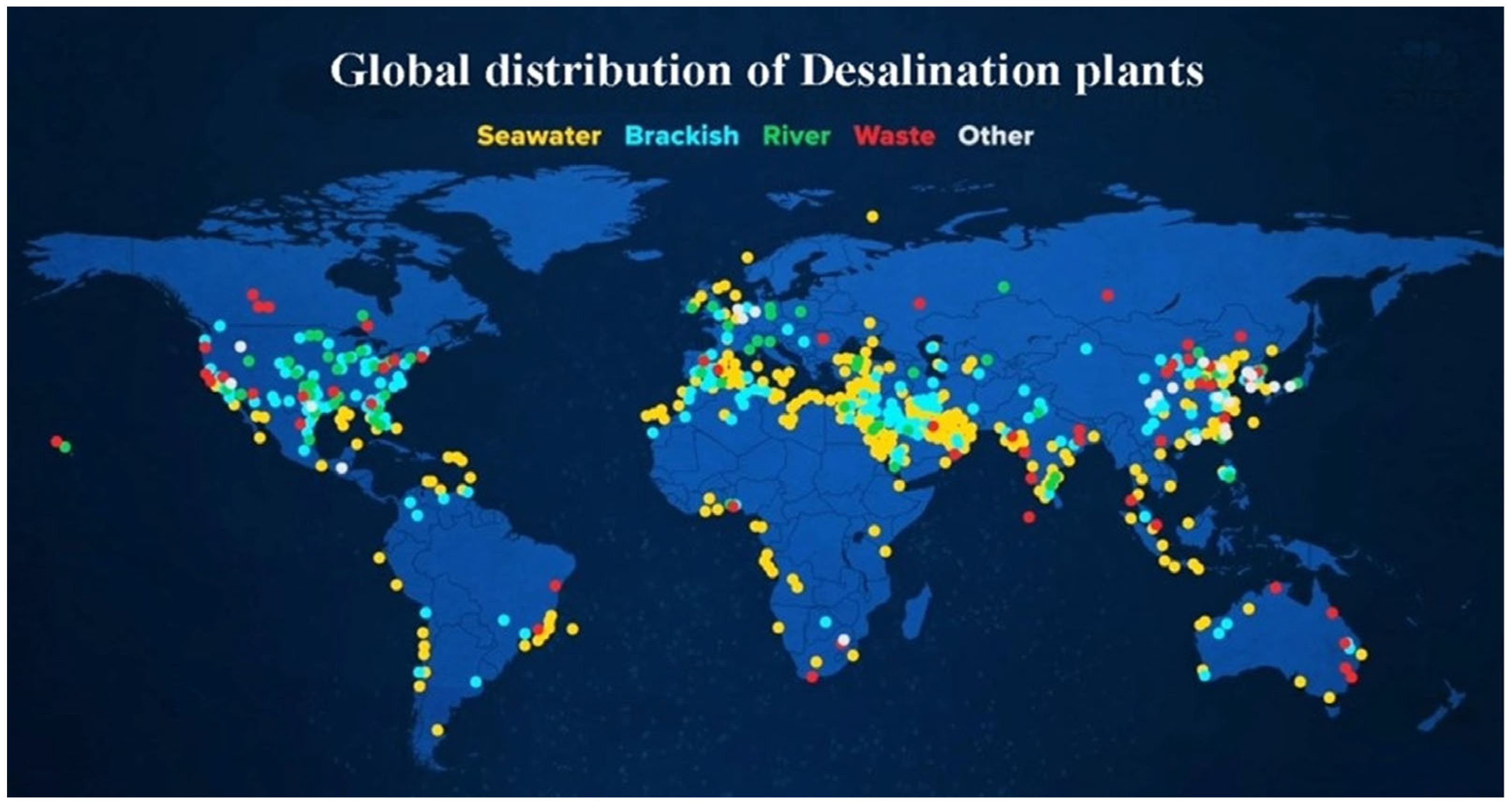
2.2. Desalination
2.3. Desolenator
2.4. Fog Harvesting
2.5. Dew Collection
2.5.1. Dew Point
2.5.2. Dew Collector
2.5.3. Water Extraction from Atmospheric Air
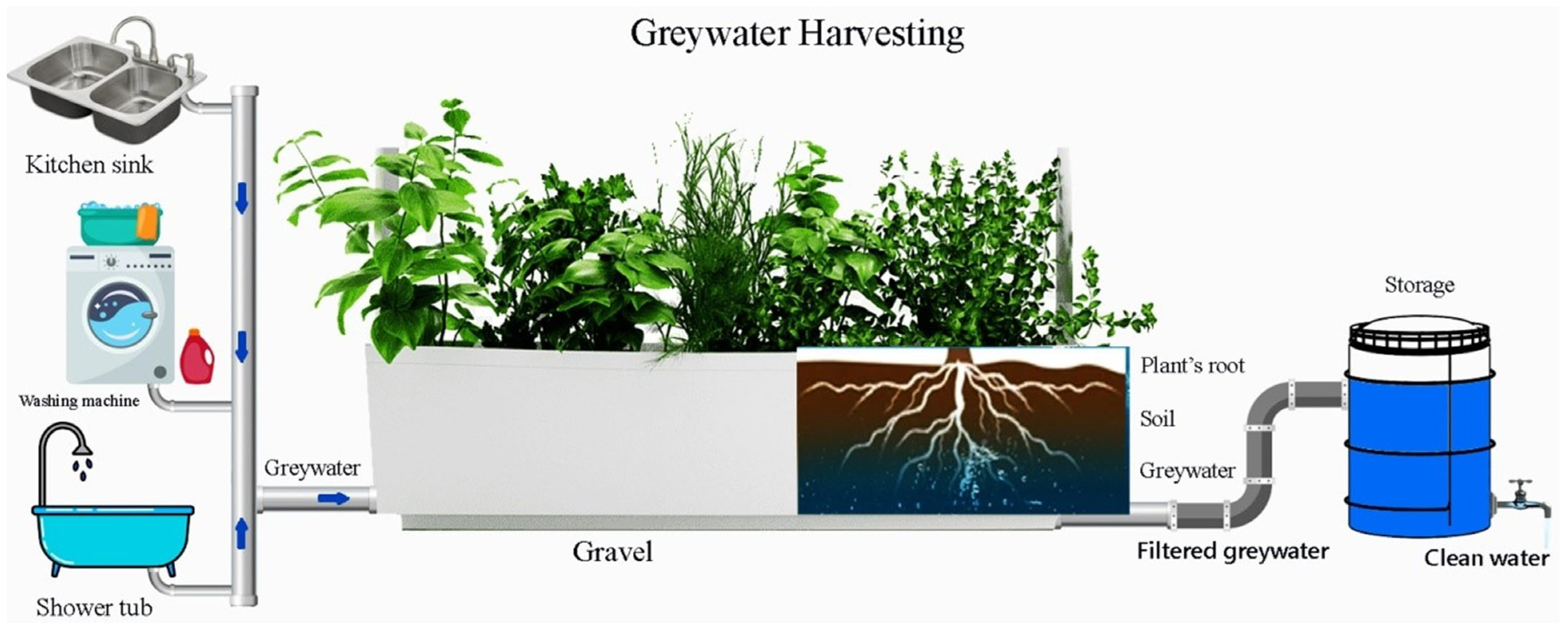
2.6. Greywater
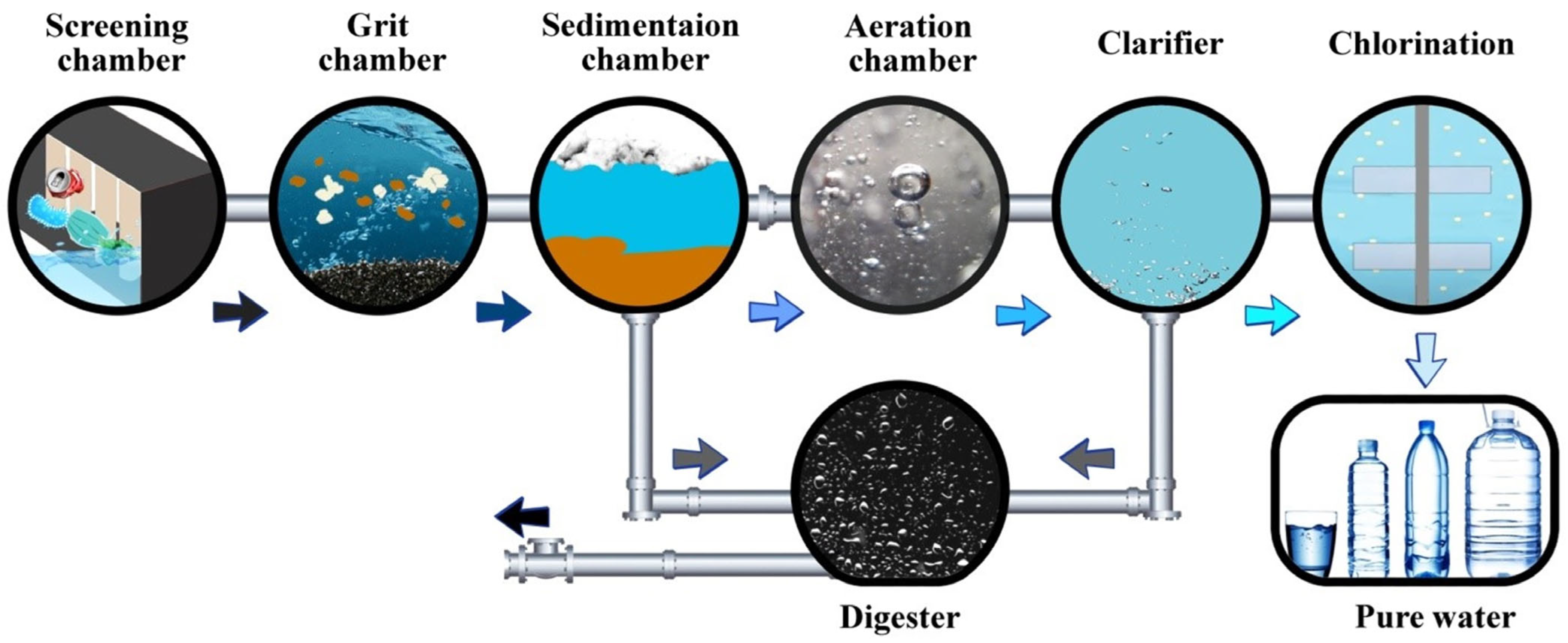
2.7. Wastewater Treatment
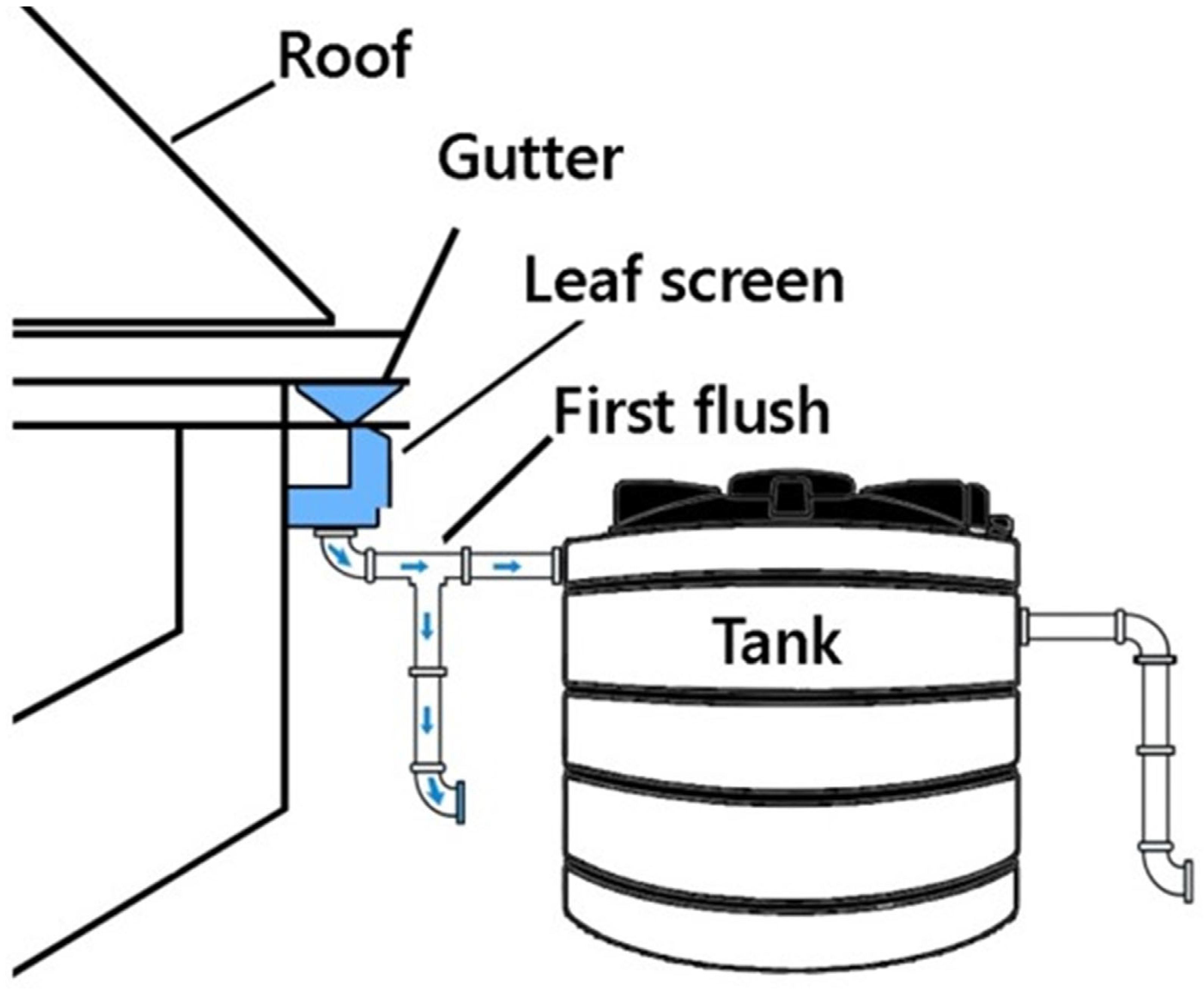
2.8. Rain Harvesting
2.9. Humidification–Dehumidification (HDH) Desalination
3. Produced Water Treatment
4. Water Quality Concerns
5. Water Scarcity and Its Effects
5.1. Climate Change
5.2. Pollution
5.3. Agriculture
5.4. Population Growth
5.5. Disappearing Wetlands
5.6. Damaged Ecosystems
6. Technological Advancement
7. Discussion
8. Summary and Future Direction
- The analysis shows that while large-scale desalination plants are technologically mature and capable of delivering high recovery ratios (40–60%), their high energy demand, brine disposal issues, and infrastructure requirements limit their sustainability and accessibility in many regions. In contrast, textile-based fog harvesting emerges as a particularly promising alternative due to its energy efficiency, low cost, and adaptability to local manufacturing and deployment.
- While the technologies vary in maturity and scalability, each provides valuable potential under appropriate environmental, social, and economic conditions. For example, large-scale RO plants are suited to coastal cities with robust infrastructure, while fog harvesters and dew condensers offer passive solutions for remote or arid regions with limited resources. Likewise, household-level systems (WaterSeer and Desolenator) are promising for decentralized, off-grid communities.
- Rather than serving as a prescriptive implementation manual, this article aims to support decision-making by offering a comparative lens through which alternative water technologies can be evaluated based on local needs and constraints. As climate stress intensifies and freshwater demands grow, further research should focus on hybrid solutions, integration with renewable energy systems, and context-specific deployment strategies to improve the sustainability and accessibility of clean water supplies globally.
- Many studies report water yield, energy consumption, or efficiency using different units and environmental conditions, making comparisons difficult across technologies. Technologies such as fog and dew harvesting or passive solar desalination are often evaluated only in lab or pilot-scale settings, with insufficient long-term field studies.
- Combining fog harvesting, atmospheric water generation, and renewable-powered desalination remains largely theoretical; there is a lack of integrated designs tailored to local conditions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Multi-Effect Distillation (MED) | Multi-Stage Flash (MSF) |
| Reverse Osmosis (RO) | Carbon nanotubes (CNTs) |
| Recovery Ratio (RR) | nanofiltration (NF) |
| zero liquid discharge (ZLD) | polyethylene (PE) |
| Climate-KIC is a Knowledge and Innovation Community (KIC) | Cobalt intercalated metal–organic framework/Polyaniline (Co-MOF/PANI) |
| fog collector element (FCE) | standard fog collector (SFC) |
| large fog collector (LFC) | captured efficiency (ɳcapt) |
| aerodynamics collection efficiency (ɳAC) | drainage efficiency (ɳdrain) |
| Total efficiency (ɳtot) | filtered by (φ) |
| incident to (χ) | Stokes number (Stk) |
| World Health Organization (WHO) | U.S. Geological Survey (USGS) |
| San Antonio Creek integrated model (SACIM) | San Antonio Creek Valley watershed (SACVW) |
| total dissolved solids, ppm (TDS) | microalgal-bacterial granular sludge (MBGS) |
References
- Choi, P.J.; Lee, J.; Jang, A. Interconnection between renewable energy technologies and water treatment processes. Water Res. 2024, 261, 122037. [Google Scholar] [CrossRef]
- Azeem, M.; Guérin, A.; Dumais, T.; Caminos, L.; Goldstein, R.E.; Pesci, A.; Rivera, J.d.D.; Torres, M.J.; Wiener, J.; Luis Campos, J. Optimal Design of Multi-Layer Fog Collectors. ACS Appl. Mater. Interfaces 2020, 12, 7736–7743. [Google Scholar] [CrossRef]
- Azeem, M.; Noman, M.T.; Wiener, J.; Petru, M.; Louda, P. Structural design of efficient fog collectors: A review. Environ. Technol. Innov. 2020, 20, 101169. [Google Scholar] [CrossRef]
- Geere, J.-A.L.; Hunter, P.R.; Jagals, P. Domestic water carrying and its implications for health: A review and mixed methods pilot study in Limpopo Province, South Africa. Environ. Health 2010, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Portman, M.E.; Vdov, O.; Schuetze, M.; Gilboa, Y.; Friedler, E. Public perceptions and perspectives on alternative sources of water for reuse generated at the household level. Water Reuse 2022, 12, 157–174. [Google Scholar] [CrossRef]
- Sloggy, M.R.; Suter, J.F.; Rad, M.R.; Manning, D.T.; Goemans, C. Changing climate, changing minds? The effects of natural disasters on public perceptions of climate change. Clim. Change 2021, 168, 25. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, Y. Advancements in sustainable desalination with ocean thermal energy: A review. Desalination 2024, 586, 117770. [Google Scholar] [CrossRef]
- Mishra, R.K.; Dubey, S.C. Fresh water availability and its global challenge. Br. J. Multidiscip. Adv. Stud. 2023, 4, 1–78. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, H.; Kong, H. Key pathways for efficient solar thermal desalination. Energy Convers. Manag. 2024, 299, 117806. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Kool, D.; Agra, E.; Drabkin, A.; Duncan, A.; Fendinat, P.; Leduc, S.; Lupovitch, G.; Nambwandja, A.; Ndilenga, N.; Thị, T.N. The overlooked non-rainfall water input sibling of fog and dew: Daily water vapor adsorption on a! Nara hummock in the Namib Sand Sea. J. Hydrol. 2021, 598, 126420. [Google Scholar] [CrossRef]
- Khalil, M.M.; Kara-Ali, A.; Assad, M. Potential of harvesting water from fog and dew water over semi-arid and arid regions in Syria. Water Supply 2022, 22, 874–882. [Google Scholar] [CrossRef]
- Tian, C.; Du, K.; Wang, L.; Zhang, X.; Li, F.; Jiao, W.; Beysens, D.; Kaseke, K.F.; Medici, M.-G. Stable isotope variations of dew under three different climates. Sci. Data 2022, 9, 50. [Google Scholar] [CrossRef]
- Raveesh, G.; Goyal, R.; Tyagi, S. Advances in atmospheric water generation technologies. Energy Convers. Manag. 2021, 239, 114226. [Google Scholar] [CrossRef]
- Hristov, J.; Barreiro-Hurle, J.; Salputra, G.; Blanco, M.; Witzke, P. Reuse of treated water in European agriculture: Potential to address water scarcity under climate change. Agric. Water Manag. 2021, 251, 106872. [Google Scholar] [CrossRef]
- Jeliazkov, A.; Martínez-Fernández, V.; Altanov, V.Y.; Beisel, J.-N.; Buijse, A.D.; Consuegra, S.; Felin, S.; de Leaniz, C.G.; Graf, W.; He, F. A global systematic map of knowledge of inland commercial navigation effects on freshwater ecosystems. J. Environ. Manag. 2024, 370, 122474. [Google Scholar] [CrossRef] [PubMed]
- Sarvar-Ardeh, S.; Rashidi, S.; Rafee, R.; Li, G. Recent advances in the applications of solar-driven co-generation systems for heat, freshwater and power. Renew. Energy 2024, 225, 120256. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, P.; Xu, J.; Wang, R.; Li, T. Progress and perspectives of sorption-based atmospheric water harvesting for sustainable water generation: Materials, devices, and systems. Sci. Bull. 2023, 69, 671–687. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumar, A. A comprehensive review of fresh water production from atmospheric air–techniques, challenges and opportunities. Environ. Dev. Sustain. 2024, 26, 1–36. [Google Scholar] [CrossRef]
- Salehi, A.A.; Ghannadi-Maragheh, M.; Torab-Mostaedi, M.; Torkaman, R.; Asadollahzadeh, M. A review on the water-energy nexus for drinking water production from humid air. Renew. Sustain. Energy Rev. 2020, 120, 109627. [Google Scholar] [CrossRef]
- Peeters, R.; Vanderschaeghe, H.; Rongé, J.; Martens, J.A. Energy performance and climate dependency of technologies for fresh water production from atmospheric water vapour. Environ. Sci. Water Res. Technol. 2020, 6, 2016–2034. [Google Scholar] [CrossRef]
- Beysens, D.; Milimouk, I.; Nikolayev, V.; Muselli, M.; Marcillat, J. Using radiative cooling to condense atmospheric vapor: A study to improve water yield. J. Hydrol. 2003, 276, 1–11. [Google Scholar] [CrossRef]
- Ouazaa, S.; Burguete, J.; Zapata, N. Solid-set sprinklers irrigation of field boundaries: Experiments and modeling. Irrig. Sci. 2016, 34, 85–103. [Google Scholar] [CrossRef][Green Version]
- Prins, F.X.; Etale, A.; Ablo, A.D.; Thatcher, A. Water scarcity and alternative water sources in South Africa: Can information provision shift perceptions? Urban Water J. 2023, 20, 1438–1449. [Google Scholar] [CrossRef]
- Azimibavil, S.; Dehkordi, A.J. Dynamic simulation of a Multi-Effect Distillation (MED) process. Desalination 2016, 392, 91–101. [Google Scholar] [CrossRef]
- Aly, S.; Manzoor, H.; Simson, S.; Abotaleb, A.; Lawler, J.; Mabrouk, A.N. Pilot testing of a novel Multi Effect Distillation (MED) technology for seawater desalination. Desalination 2021, 519, 115221. [Google Scholar] [CrossRef]
- Toth, A.J. Modelling and optimisation of multi-stage flash distillation and reverse osmosis for desalination of saline process wastewater sources. Membranes 2020, 10, 265. [Google Scholar] [CrossRef]
- Cohen, Y.; Glater, J. A tribute to Sidney Loeb-The pioneer of reverse osmosis desalination research. Desalination Water Treat. 2010, 15, 222–227. [Google Scholar] [CrossRef]
- Islam, M.; Sultana, A.; Saadat, A.; Shammi, M.; Uddin, M. Desalination technologies for developing countries: A review. J. Sci. Res. 2018, 10, 77–97. [Google Scholar] [CrossRef]
- Kumar, M.; Adham, S.S.; Pearce, W.R. Investigation of seawater reverse osmosis fouling and its relationship to pretreatment type. Environ. Sci. Technol. 2006, 40, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; He, B.; Zhang, T.; Yang, B.; Yu, W.; Hu, L.; Jiang, G. 3D-printed flexible thermoplastic polyurethane membrane for ultrafast oil/water separation. Chem. Eng. J. 2025, 503, 158500. [Google Scholar] [CrossRef]
- Meng, S.; Yao, C.; Liu, G.; Chen, H.; Hu, T.; Zhang, Z.; Yang, J.; Yang, W. A 3d-printed bionic membrane with autonomously passive unidirectional liquid transfer capability for water condensation, collection, and purification. ACS Appl. Mater. Interfaces 2024, 16, 62892–62901. [Google Scholar] [CrossRef] [PubMed]
- Nyadroh, C.; Keshavarz, T.; Kyazze, G. The effect of immobilisation of electroactive bacteria as 3D-printed bioelectrodes on water desalination in microbial desalination cells. Desalination Water Treat. 2025, 322, 101235. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M.; Azeem, M. Single phase anatase encapsulated-glass fiber-reinforced composites for battery management. J. Mater. Sci. 2025, 60, 12766–12781. [Google Scholar] [CrossRef]
- Liu, X.; Ma, S.; He, P.; Wang, M.; Duan, X.; Jia, D.; Colombo, P.; Zhou, Y. 3D printing of green and environment-friendly rGO@ ZnO/GP for removal of methylene blue from wastewater. J. Phys. Chem. Solids 2023, 174, 111158. [Google Scholar] [CrossRef]
- Navarro-López, D.E.; Sánchez-Huerta, T.M.; Flores-Jimenez, M.S.; Tiwari, N.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Garcia-Gonzalez, A.; Fuentes-Aguilar, R.Q.; Sanchez-Ante, G.; Corona-Romero, K. Nanocomposites based on doped ZnO nanoparticles for antibacterial applications. Colloids Surf. Physicochem. Eng. Asp. 2022, 652, 129871. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Kontoleon, K.J.; Ashraf, M.A.; Petru, M. Polymer-Based Composites for Sorption, Energy Storage and Sensing Applications. Sep. Purif. Rev. 2024, 53, 1–14. [Google Scholar] [CrossRef]
- Amor, N.; Adach, K.; Noman, M.T.; Hazuka, F.; Fijalkowski, M. Advances in heat exchangers for heat and mass transfer: A review. J. Text. Inst. 2024, 115, 2678–2692. [Google Scholar] [CrossRef]
- Setiawan, T.; Alvin Setiadi, G.; Mukti, R.R. Deployable and retrievable 3D-printed zeolite–polymer composites for wastewater treatment: A review. Ind. Eng. Chem. Res. 2023, 62, 18159–18177. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Khalil, A.; Ahmed, F.E.; Hilal, N. The emerging role of 3D printing in water desalination. Sci. Total Environ. 2021, 790, 148238. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.-m. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Pinto, F.S.; Marques, R.C. New era/new solutions: The role of alternative tariff structures in water supply projects. Water Res. 2017, 126, 216–231. [Google Scholar] [CrossRef]
- Saleh, L.; Mezher, T. Techno-economic analysis of sustainability and externality costs of water desalination production. Renew. Sustain. Energy Rev. 2021, 150, 111465. [Google Scholar] [CrossRef]
- Moharram, N.A.; Bayoumi, S.; Hanafy, A.A.; El-Maghlany, W.M. Techno-economic analysis of a combined concentrated solar power and water desalination plant. Energy Conver. Manag. 2021, 228, 113629. [Google Scholar] [CrossRef]
- Honarparvar, S.; Zhang, X.; Chen, T.; Alborzi, A.; Afroz, K.; Reible, D. Frontiers of membrane desalination processes for brackish water treatment: A review. Membranes 2021, 11, 246. [Google Scholar] [CrossRef]
- Patel, S.K.; Qin, M.; Walker, W.S.; Elimelech, M. Energy efficiency of electro-driven brackish water desalination: Electrodialysis significantly outperforms membrane capacitive deionization. Environ. Sci. Technol. 2020, 54, 3663–3677. [Google Scholar] [CrossRef]
- Honarparvar, S.; Saravi, S.H.; Reible, D.; Chen, C.-C. Comprehensive thermodynamic modeling of saline water with electrolyte NRTL model: A study of aqueous Sr2+-Na+-Cl−-SO42− quaternary system. Fluid Phase Equilibria 2018, 470, 221–231. [Google Scholar] [CrossRef]
- Panagopoulos, A. Energetic, economic and environmental assessment of zero liquid discharge (ZLD) brackish water and seawater desalination systems. Energy Convers. Manag. 2021, 235, 113957. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, H.-U.-R.; Nasim, H.A.; Ayub, A.; Ashfaq, M.; Rauf, A.; Shah, S.S.A.; Ahmad, M.M.; Nawaz, H.; Hussain, E. Synthesis and characterization of water stable polymeric metallo organic composite (PMOC) for the removal of arsenic and lead from brackish water. Toxin Rev. 2022, 41, 577–587. [Google Scholar] [CrossRef]
- Wang, X.; Noman, M.T.; Petrů, M.; Kang, G. Enhancing dynamic viscoelastic performance of flax fiber/fly ash hollow cenosphere-reinforced composites coated by in situ fabrication of ultrasonically synthesized nanotitanium dioxide. J. Appl. Polym. Sci. 2024, 141, e55843. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Bi-functional renewable biopolymer wrapped CNFs/Ag doped spinel cobalt oxide as a sensitive platform for highly toxic nitroaromatic compound detection and degradation. Chemosphere 2022, 291, 132998. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.; Yu, W.-C.; Balram, D.; Noman, M.T.; Amor, N. Silver doped dodecahedral metal-organic framework anchored RGO nanosheets for nanomolar quantification of priority toxic pollutant in aquatic environment. J. Alloys Comp. 2022, 922, 166180. [Google Scholar] [CrossRef]
- Kumar, L.; Soomro, J.; Khoharo, H.; Assad, M.E.H. A comprehensive review of solar thermal desalination technologies for freshwater production. AIMS Energy 2023, 11, 293–318. [Google Scholar] [CrossRef]
- Zheng, Y.; Cáceres González, R.A.; Hatzell, M.C.; Hatzell, K.B. Challenges and roadmap for solar-thermal desalination. ACS EST Eng. 2023, 3, 1055–1082. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Guo, Z. Harps with directed arrangement spines enables efficient fog harvesting. Chem. Eng. J. 2025, 521, 166738. [Google Scholar] [CrossRef]
- Wan, Y.; Yin, Y.; Yu, H.; Wang, Y.; Xue, J. Bioinspired functional surface with curved topography and dual heterogeneous wettability for enhanced fog collection. J. Water Process Eng. 2025, 77, 108546. [Google Scholar] [CrossRef]
- Hu, F.; Tian, G.; Zhang, H.; Guo, Z. Biomimetic multi-functional 3D fog collector: Independent of fog flow direction. Colloids Surf. Physicochem. Eng. Asp. 2024, 703, 135191. [Google Scholar] [CrossRef]
- Lei, J.; Guo, Z. A fog-collecting surface mimicking the Namib beetle: Its water collection efficiency and influencing factors. Nanoscale 2020, 12, 6921–6936. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Anderson, M.J.; Tulkoff, J.B.; Kennedy, B.S.; Boreyko, J.B. Fog harvesting with harps. ACS Appl. Mater. Interfaces 2018, 10, 11979–11986. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Wang, Y.; Wang, P. Efficient and Anisotropic Fog Harvesting on a Hybrid and Directional Surface. Adv. Mater. Interfaces 2017, 4, 1600801. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Liu, J.; Liu, X.; Jin, Z. A twice electrochemical-etching method to fabricate superhydrophobic-superhydrophilic patterns for biomimetic fog harvest. Sci. Rep. 2017, 7, 8816. [Google Scholar] [CrossRef]
- Liu, H.; Xie, W.-Y.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Constructing hierarchically hydrophilic/superhydrophobic ZIF-8 pattern on soy protein towards a biomimetic efficient water harvesting material. Chem. Eng. J. 2019, 369, 1040–1048. [Google Scholar] [CrossRef]
- Schemenauer, R.S.; Cereceda, P. A proposed standard fog collector for use in high-elevation regions. J. Appl. Meteorol. Climatol. 1994, 33, 1313–1322. [Google Scholar] [CrossRef]
- Holmes, R.; de Dios Rivera, J.; de la Jara, E. Large fog collectors: New strategies for collection efficiency and structural response to wind pressure. Atmos. Res. 2015, 151, 236–249. [Google Scholar] [CrossRef]
- de Dios Rivera, J. Aerodynamic collection efficiency of fog water collectors. Atmos. Res. 2011, 102, 335–342. [Google Scholar] [CrossRef]
- Park, K.-C.; Chhatre, S.S.; Srinivasan, S.; Cohen, R.E.; McKinley, G.H. Optimal design of permeable fiber network structures for fog harvesting. Langmuir 2013, 29, 13269–13277. [Google Scholar] [CrossRef] [PubMed]
- Regalado, C.M.; Ritter, A. The design of an optimal fog water collector: A theoretical analysis. Atmos. Res. 2016, 178, 45–54. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Torregrosa, A.; Weiss-Penzias, P.S.; Zhang, B.J.; Sorensen, D.; Cohen, R.E.; McKinley, G.H.; Kleingartner, J.; Oliphant, A.; Bowman, M. Fog water collection effectiveness: Mesh intercomparisons. Aerosol Air Qual. Res. 2018, 18, 270–283. [Google Scholar] [CrossRef]
- Beysens, D. The formation of dew. Atmos. Res. 1995, 39, 215–237. [Google Scholar] [CrossRef]
- Clus, O.; Ouazzani, J.; Muselli, M.; Nikolayev, V.; Sharan, G.; Beysens, D. Comparison of various radiation-cooled dew condensers using computational fluid dynamics. Desalination 2009, 249, 707–712. [Google Scholar] [CrossRef]
- Jacobs, A.; Heusinkveld, B.; Berkowicz, S. Passive dew collection in a grassland area, The Netherlands. Atmos. Res. 2008, 87, 377–385. [Google Scholar] [CrossRef]
- Sharan, G.; Clus, O.; Singh, S.; Muselli, M.; Beysens, D. A very large dew and rain ridge collector in the Kutch area (Gujarat, India). J. Hydrol. 2011, 405, 171–181. [Google Scholar] [CrossRef]
- Beysens, D.; Clus, O.; Mileta, M.; Milimouk, I.; Muselli, M.; Nikolayev, V. Collecting dew as a water source on small islands: The dew equipment for water project in Biševo (Croatia). Energy 2007, 32, 1032–1037. [Google Scholar] [CrossRef]
- Tomaszkiewicz, M.; Abou Najm, M.; Beysens, D.; Alameddine, I.; El-Fadel, M. Dew as a sustainable non-conventional water resource: A critical review. Environ. Rev. 2015, 23, 425–442. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Hsu, M.; Bhate, N.; Yang, W.; Deng, T. Spatial control in the heterogeneous nucleation of water. Appl. Phys. Lett. 2009, 95, 094101. [Google Scholar] [CrossRef]
- Nioras, D.; Ellinas, K.; Constantoudis, V.; Gogolides, E. How different are fog collection and dew water harvesting on surfaces with different wetting behaviors? ACS Appl. Mater. Interfaces 2021, 13, 48322–48332. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Z. Global water vapor variability and trend from the latest 36 year (1979 to 2014) data of ECMWF and NCEP reanalyses, radiosonde, GPS, and microwave satellite. J. Geophys. Res. Atmos. 2016, 121, 11442–11462. [Google Scholar] [CrossRef]
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; Lopez-Maldonado, E.A.; Ihara, I. Methods to prepare biosorbents and magnetic sorbents for water treatment: A review. Environ. Chem. Lett. 2023, 21, 2337–2398. [Google Scholar] [CrossRef]
- Entezari, A.; Esan, O.C.; Yan, X.; Wang, R.; An, L. Sorption-based atmospheric water harvesting: Materials, components, systems, and applications. Adv. Mater. 2023, 35, 2210957. [Google Scholar] [CrossRef]
- Siddiqui, M.; Azam, M.A.; Khan, M.M.; Iqbal, S.; Khan, M.U.; Raffat, Y. Current trends on extraction of water from air: An alternative solution to water supply. Int. J. Environ. Sci. Technol. 2023, 20, 1053–1080. [Google Scholar] [CrossRef]
- Shin, I.C.; Lee, U.; Park, S.; Lee, J. Toward Net-Zero Energy Cooling: Dynamic Radiative Coolers with Near-Zero Energy Input. Adv. Funct. Mater. 2025, 35, e08007. [Google Scholar] [CrossRef]
- Gude, V.G. Exergy evaluation of desalination processes. ChemEngineering 2018, 2, 28. [Google Scholar] [CrossRef]
- Azeem, M.; Amor, N.; Petru, M.; Wiener, J.; Noman, M.T. A novel approach for assessing fog collection efficiency using hydrophobic surfaces. Chaos Solitons Fract. 2025, 198, 116625. [Google Scholar] [CrossRef]
- Babatunde, O.; Munda, J.; Hamam, Y. Hybridized off-grid fuel cell/wind/solar PV/battery for energy generation in a small household: A multi-criteria perspective. Int. J. Hydrogen Energy 2022, 47, 6437–6452. [Google Scholar] [CrossRef]
- Dawoud, M.A.; Sallam, G.R.; Abdelrahman, M.A.; Emam, M. The performance and feasibility of solar-powered desalination for brackish groundwater in Egypt. Sustainability 2024, 16, 1630. [Google Scholar] [CrossRef]
- Azeem, M.; Noman, M.T.; Petru, M.; Shahid, M.; Khan, M.Q.; Wiener, J. Surface wettability of vertical harps for fog collection. Surf. Interfaces 2022, 30, 101842. [Google Scholar] [CrossRef]
- Moser, M.; Trieb, F.; Fichter, T.; Kern, J. Renewable desalination: A methodology for cost comparison. Desalination Water Treat. 2013, 51, 1171–1189. [Google Scholar] [CrossRef]
- Dorzhiev, S.S.; Bazarova, E.G.; Pimenov, S.V.; Dorzhiev, S.S. Application of renewable energy sources for water extraction from atmospheric air. Energy Rep. 2021, 7, 343–357. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Tan, S.C. Device design and optimization of sorption-based atmospheric water harvesters. Device 2023, 1, 100099. [Google Scholar] [CrossRef]
- Elewa, M.M. Emerging and conventional water desalination technologies powered by renewable energy and energy storage systems toward zero liquid discharge. Separations 2024, 11, 291. [Google Scholar] [CrossRef]
- Lord, J.; Thomas, A.; Treat, N.; Forkin, M.; Bain, R.; Dulac, P.; Behroozi, C.H.; Mamutov, T.; Fongheiser, J.; Kobilansky, N. Global potential for harvesting drinking water from air using solar energy. Nature 2021, 598, 611–617. [Google Scholar] [CrossRef]
- Almassad, H.A.; Abaza, R.I.; Siwwan, L.; Al-Maythalony, B.; Cordova, K.E. Environmentally adaptive MOF-based device enables continuous self-optimizing atmospheric water harvesting. Nat. Commun. 2022, 13, 4873. [Google Scholar] [CrossRef]
- Luo, F.; Liang, X.; Chen, W.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. High-efficient and scalable solar-driven MOF-based water collection unit: From module design to concrete implementation. Chem. Eng. J. 2023, 465, 142891. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Cui, Z.; Du, S.; Wang, R. Continuous atmospheric water production coupled with humidity regulation enabled by a MOF-based humidity pump. Nano Energy 2024, 125, 109596. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and applications of ZnO nanostructures (ZONSs): A review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 99–141. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; de Oliveira Vaz, L.; Peres, M.; Merlo, S.S. Microbiological risk from non-potable reuse of greywater treated by anaerobic filters associated to vertical constructed wetlands. J. Water Process Eng. 2021, 39, 101751. [Google Scholar] [CrossRef]
- Wurochekke, A.; Mohamed, R.; Al-Gheethi, A.; Atiku, H.; Amir, H.; Matias-Peralta, H. Household greywater treatment methods using natural materials and their hybrid system. J. Water Health 2016, 14, 914–928. [Google Scholar] [CrossRef]
- Murray, A.; Drechsel, P. Why do some wastewater treatment facilities work when the majority fail? Case study from the sanitation sector in Ghana. Waterline 2011, 3, 135–149. [Google Scholar] [CrossRef]
- Hasan, H.A.; Muhammad, M.H. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Bilgin, M.; Yurtsever, M.; Karadagli, F. Microplastic removal by aerated grit chambers versus settling tanks of a municipal wastewater treatment plant. J. Water Process Eng. 2020, 38, 101604. [Google Scholar] [CrossRef]
- Walsh, C.J.; Booth, D.B.; Burns, M.J.; Fletcher, T.D.; Hale, R.L.; Hoang, L.N.; Livingston, G.; Rippy, M.A.; Roy, A.H.; Scoggins, M. Principles for urban stormwater management to protect stream ecosystems. Freshw. Sci. 2016, 35, 398–411. [Google Scholar] [CrossRef]
- Mao, J.; Xia, B.; Zhou, Y.; Bi, F.; Zhang, X.; Zhang, W.; Xia, S. Effect of roof materials and weather patterns on the quality of harvested rainwater in Shanghai, China. J. Clean. Prod. 2021, 279, 123419. [Google Scholar] [CrossRef]
- Naghdi, S.; Bozorg-Haddad, O.; Khorsandi, M.; Chu, X. Multi-objective optimization for allocation of surface water and groundwater resources. Sci. Total Environ. 2021, 776, 146026. [Google Scholar] [CrossRef]
- Afshar, A.; Khosravi, M.; Ostadrahimi, L.; Afshar, A. Reliability-based multi-objective optimum design of nonlinear conjunctive use problem; cyclic storage system approach. J. Hydrol. 2020, 588, 125109. [Google Scholar] [CrossRef]
- Afshar, A.; Khosravi, M.; Molajou, A. Assessing adaptability of cyclic and non-cyclic approach to conjunctive use of groundwater and surface water for sustainable management plans under climate change. Water Resour. Manag. 2021, 35, 3463–3479. [Google Scholar] [CrossRef]
- Woolfenden, L.R.; Engott, J.A.; Larsen, J.; Cromwell, G. Simulation of Groundwater and Surface-Water Resources of the San Antonio Creek Valley Watershed, Santa Barbara County, California; US Geological Survey: Reston, VA, USA, 2022; pp. 1–76. ISSN 2328-0328. [Google Scholar]
- Ashour, A.M.; Jaffar, H.M.; Kadhim, S.A.; Rashid, F.L.; Bouabidi, A.; Rajhi, W.; Al-Shammrei, S.; Alshammari, M. Experimental assessment of humidification and dehumidification performance in an evaporative-thermoelectric desalination system. Desalination 2025, 616, 119347. [Google Scholar] [CrossRef]
- Sonkar, M.; Kanathala, Y.; Naik, B.K. Recent Developments in Solar-Driven Adsorption and Humidification-Dehumidification based Hybrid Desalination System: A state-of-the-art review. Sol. Compass 2025, 15, 100125. [Google Scholar] [CrossRef]
- Luberti, M.; Capocelli, M. Enhanced humidification–dehumidification (HDH) systems for sustainable water desalination. Energies 2023, 16, 6352. [Google Scholar] [CrossRef]
- Abdullah, A.; Aljaghtham, M.; Kandeal, A.; Sharshir, S.W. Techno-economic investigation of an entirely solar-powered hybrid HDH/RO desalination plant for moderate water demand under various operating conditions. Results Eng. 2024, 24, 103200. [Google Scholar] [CrossRef]
- Mudhafar, M.A. Evaluation of a novel high-Performance HP-HDH desalination system integrated with PV module and electrolyzer for fresh water and hydrogen productions. Renew. Energy 2025, 255, 123738. [Google Scholar] [CrossRef]
- Ziauddin, M.; Alnaimat, F.; Mathew, B. A novel humidification and dehumidification desalination system powered by direct solar energy. Case Stud. Therm. Eng. 2025, 72, 106236. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, W. Performance analysis of a heat pump coupled humidification-dehumidification desalination system with a large temperature difference using an ejector. Appl. Therm. Eng. 2025, 271, 126302. [Google Scholar] [CrossRef]
- Ghorbani, G.; Safarzadeh, H.; Khanmohammadi, S. Conceptual design and multi-optimization of small-scale solar water production based on integration of Humidification-Dehumidification and solar still desalination. Desalination Water Treat. 2025, 323, 101317. [Google Scholar] [CrossRef]
- Orfi, J.; Sherif, R.; AlFaleh, M. Conventional and Emerging Desalination Technologies: Review and Comparative Study from a Sustainability Perspective. Water 2025, 17, 279. [Google Scholar] [CrossRef]
- Gohil, P.P.; Desai, H.; Kumar, A.; Kumar, R. Current status and advancement in thermal and membrane-based hybrid seawater desalination technologies. Water 2023, 15, 2274. [Google Scholar] [CrossRef]
- Alkaisi, A.; Mossad, R.; Sharifian-Barforoush, A. A review of the water desalination systems integrated with renewable energy. Energy Procedia 2017, 110, 268–274. [Google Scholar] [CrossRef]
- Wijewardane, S.; Ghaffour, N. Inventions, innovations, and new technologies: Solar Desalination. Sol. Compass 2023, 5, 100037. [Google Scholar] [CrossRef]
- Selvam, A.; Jain, G.; Chaudhuri, R.G.; Mandal, M.K.; Chakrabarti, S. Avant-Garde Solar–Thermal Nanostructures: Nascent Strategy into Effective Photothermal Desalination. Sol. RRL 2022, 6, 2200321. [Google Scholar] [CrossRef]
- Winchester, B.; Huang, G.; Beath, H.; Sandwell, P.; Cen, J.; Nelson, J.; Markides, C.N. Lifetime optimisation of integrated thermally and electrically driven solar desalination plants. npj Clean Water 2024, 7, 65. [Google Scholar] [CrossRef]
- Rostami, M.H.; Najafi, G.; Minaei, S.; Khoshtaghaza, M.H. Design, optimization and performance evaluation of water-energy nexus of an atmospheric water harvesting system (AWHS) based on subsurface cooling and solar power. Case Stud. Therm. Eng. 2025, 72, 106378. [Google Scholar] [CrossRef]
- Forrest, N.; Stein, Z.; Wiek, A. Transferability and scalability of sustainable urban water solutions–A case study from the Colorado River Basin. Resour. Conserv. Recycl. 2020, 157, 104790. [Google Scholar] [CrossRef]
- Azeem, M.; Noman, M.T.; Khan, M.Z.; Ali, A.; Wiener, J.; Petru, M.; Kejzlar, P.; Masin, I. Efficient fog collector with superhydrophobic coated surface. J. Text. Inst. 2024, 115, 1048–1058. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, R.; Zhou, C.; Tian, Y.; Wang, L. Asymmetric fibers for efficient fog harvesting. Chem. Eng. J. 2021, 415, 128944. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Z.; Shi, Y.; Zhao, X.; Fan, S.; Chen, Z. Fundamental limits of the dew-harvesting technology. Nanoscale Microscale Thermophys. Eng. 2020, 24, 43–52. [Google Scholar] [CrossRef]
- Xiang, T.; Xie, S.; Chen, G.; Zhang, C.; Guo, Z. Recent advances in atmospheric water harvesting technology and its development. Mater. Horiz. 2025, 12, 1084–1105. [Google Scholar] [CrossRef]
- Thavalengal, M.S.; Jamil, M.A.; Mehroz, M.; Xu, B.B.; Yaqoob, H.; Sultan, M.; Imtiaz, N.; Shahzad, M.W. Progress and prospects of air water harvesting system for remote areas: A comprehensive review. Energies 2023, 16, 2686. [Google Scholar] [CrossRef]
- Alghoul, M.; Poovanaesvaran, P.; Sopian, K.; Sulaiman, M. Review of brackish water reverse osmosis (BWRO) system designs. Renew. Sustain. Energy Rev. 2009, 13, 2661–2667. [Google Scholar] [CrossRef]
- Dilaver, M.; Çelebi, M.D.; Ağtaş, M.; Koyuncu, I. Brackish water RO concentrate treatment and water recovery using cost lowering integrated technology. J. Environ. Chem. Eng. 2022, 10, 108463. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, X.; Gao, S.; Wang, X.; Zhang, R. Progress in research and application of nanofiltration (nf) technology for brackish water treatment. Membranes 2021, 11, 662. [Google Scholar] [CrossRef]
- Leong, J.Y.C.; Balan, P.; Chong, M.N.; Poh, P.E. Life-cycle assessment and life-cycle cost analysis of decentralised rainwater harvesting, greywater recycling and hybrid rainwater-greywater systems. J. Clean. Prod. 2019, 229, 1211–1224. [Google Scholar] [CrossRef]
- Oh, K.S.; Leong, J.Y.C.; Poh, P.E.; Chong, M.N.; Von Lau, E. A review of greywater recycling related issues: Challenges and future prospects in Malaysia. J. Clean. Prod. 2018, 171, 17–29. [Google Scholar] [CrossRef]
- Prakash, O.; Tripathy, P.; Zade, A.; Sharma, A.; Juneja, C.; Hiwrale, I.; Shukla, V.; Kannan, K.; Pal, S. Multifaceted dimensions of greywater recycling in advancing sustainable development goals: A comprehensive review. Environ. Dev. Sustain. 2024, 27, 1–30. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Malik, M.H.; Farooq, A.; Noman, M.T.; Khan, N. Development of Maghemite Nanoparticles to Effectively Adsorb Methylene Blue Dye from Water. Water Air Soil Pollut. 2024, 235, 244. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Wiener, J.; Farooq, A.; Saskova, J.; Noman, M.T. Development of maghemite glass fibre nanocomposite for adsorptive removal of methylene blue. Fibers Polym. 2018, 19, 1735–1746. [Google Scholar] [CrossRef]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-light triggered photocatalytic performance of ZnO.SiO2 nanocomposite for water pollutant compound methyl orange dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef]
- de Sá Silva, A.C.R.; Bimbato, A.M.; Balestieri, J.A.P.; Vilanova, M.R.N. Exploring environmental, economic and social aspects of rainwater harvesting systems: A review. Sustain. Cities Soc. 2022, 76, 103475. [Google Scholar] [CrossRef]
- Raimondi, A.; Quinn, R.; Abhijith, G.R.; Becciu, G.; Ostfeld, A. Rainwater harvesting and treatment: State of the art and perspectives. Water 2023, 15, 1518. [Google Scholar] [CrossRef]
- Chen, G.; Wu, P.; Wang, J.; Zhang, P.; Jia, Z. Ridge–furrow rainfall harvesting system helps to improve stability, benefits and precipitation utilization efficiency of maize production in Loess Plateau region of China. Agric. Water Manag. 2022, 261, 107360. [Google Scholar] [CrossRef]
- Essa, F.A.; Othman, M.M.; Abdullah, A.; Abou Al-Sood, M.M.; Omara, Z. Critical issues on advancements and challenges in HDH desalination units. Results Eng. 2024, 22, 102180. [Google Scholar] [CrossRef]
- Nabil, I.; Abdalla, A.M.; Mansour, T.M.; Shehata, A.I.; Dawood, M.M.K. Salinity impacts on humidification dehumidification (HDH) desalination systems. Environ. Sci. Pollut. Res. 2024, 31, 1907–1925. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Valipour, M.S.; Rashidi, S. Performance enhancement techniques in humidification–dehumidification desalination systems: A detailed review. J. Therm. Anal. Calorim. 2025, 150, 8413–8442. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.; Boels, L.; Lammertink, R.; De Vos, W. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.; Jafari, I.; Parniankhoy, B. Oil and gas produced water management: A review of treatment technologies, challenges, and opportunities. Chem. Eng. Commun. 2017, 204, 990–1005. [Google Scholar] [CrossRef]
- Karim, M.R. Assessment of rainwater harvesting for drinking water supply in Bangladesh. Water Sci. Technol. Water Supply 2010, 10, 243–249. [Google Scholar] [CrossRef]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Dolan, F.; Lamontagne, J.; Link, R.; Hejazi, M.; Reed, P.; Edmonds, J. Evaluating the economic impact of water scarcity in a changing world. Nat. Commun. 2021, 12, 1915. [Google Scholar] [CrossRef]
- Abdelkader, M.; Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A. Combined use of modal analysis and machine learning for materials classification. Materials 2021, 14, 4270. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M. Investigating horn power and impact of sonication on TiO2@ cotton composites with machine learning and computer vision. Measurement 2025, 252, 117424. [Google Scholar] [CrossRef]
- Amor, N.; Noman, M.T.; Petru, M.; Sebastian, N.; Balram, D. Machining performance of TiO2 embedded-glass fiber reinforced composites with snake optimizer. Measurement 2024, 227, 114253. [Google Scholar] [CrossRef]
- Amor, N.; Noman, M.T.; Petru, M.; Sebastian, N.; Balram, D. Design and optimization of machinability of ZnO embedded-glass fiber reinforced polymer composites with a modified white shark optimizer. Exp. Syst. Applic. 2024, 237, 121474. [Google Scholar] [CrossRef]
- Priya, P.; Nguyen, T.C.; Saxena, A.; Aluru, N.R. Machine learning assisted screening of two-dimensional materials for water desalination. ACS Nano 2022, 16, 1929–1939. [Google Scholar] [CrossRef]
- Ahmad, U.; Abdala, A.; Ng, K.C.; Akhtar, F.H. Machine learning-driven design of membranes for saline and produced water treatment across scales. Environ. Sci. Water Res. Technol. 2025, 11, 2080–2099. [Google Scholar] [CrossRef]
- Bonny, T.; Kashkash, M.; Ahmed, F. An efficient deep reinforcement machine learning-based control reverse osmosis system for water desalination. Desalination 2022, 522, 115443. [Google Scholar] [CrossRef]
- Ashraf, W.M.; Jamil, M.A.; Uddin, G.M.; Shboul, B.; Ishfaq, K.; Ng, K.C.; Dixon, M.; Xu, B.B.; Shahzad, M.W. Machine learning assisted improved desalination pilot system design and experimentation for the circular economy. J. Water Process Eng. 2024, 63, 105535. [Google Scholar] [CrossRef]
- Reza, A.F.; Singh, R.; Verma, R.K.; Singh, A.; Ahn, Y.-H.; Ray, S.S. An integral and multidimensional review on multi-layer perceptron as an emerging tool in the field of water treatment and desalination processes. Desalination 2024, 586, 117849. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Farghali, M.; Bakr, S.S.; Eltaweil, A.S.; Rashwan, A.K.; Abd El-Monaem, E.M. Machine learning for membrane design in energy production, gas separation, and water treatment: A review. Environ. Chem. Lett. 2024, 22, 505–560. [Google Scholar] [CrossRef]





| TDS Range (mg/L) | Dominant Ionic Constituents | Classification Notes |
|---|---|---|
| ~1800 | NaHCO3–SO42− (approx. 1/3 of total anions) | Low-salinity brackish water; moderate treatment required |
| ~2500 | CaSO4–Na+, Mg2+ (each ~25% of total cations) | Scaling likely; pretreatment recommended |
| ~8400 | NaCl | High salinity; similar treatment as seawater RO |
| ~1400 | Mixed ions with high silica content | Challenging fouling potential; advanced pre-filtration needed |
| Basis | Categories | Description | References |
|---|---|---|---|
| Moisture Extraction |
| Methods based on extracting water via condensation, sorbent materials, or a combination of both. | [79,80,81] |
| Power Consumption |
| Passive systems use natural cooling; active systems rely on mechanical/electrical input. | [82,83,84] |
| Power Supply Source |
| Defines how the system is powered: central grid, fuel-powered, battery, or renewable energy like solar or wind. | [85,86,87,88] |
| Technology | Water Yield (Approx.) | Energy Source | Scale | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Desalination (RO, MSF, MED) | 40–60% recovery ratio | Electricity/fossil/solar | Large-scale | High yield, established infrastructure | High energy demand, brine disposal issues, expensive infrastructure | [117,118,119] |
| Desolenator | ~15 L/day (small unit) | Solar | Household | Off-grid, sustainable, durable | Small-scale only, relatively slow output, high upfront cost | [120,121,122] |
| WaterSeer | ~11–50 L/day (ideal conditions) | Wind/Geothermal | Individual | Passive, renewable, low maintenance | Limited validated performance, conceptual stage, not field-proven | [123,124] |
| Fog Harvesting | Up to 103 mL/min/m2 | Passive | Community | Eco-friendly, high efficiency (harp design) | Climate dependent, limited to fog-prone areas, moderate yields | [125,126] |
| Dew Collection | 0.6 L/m2/night | Radiative cooling | Household | Low-cost, suitable for arid areas | Low productivity, requires large cooling surfaces, dependent on night conditions | [127,128,129] |
| Brackish Water RO | Moderate to high (varies) | Electric/solar | Regional | Lower energy demand than seawater RO | Brine disposal issues, moderate energy demand, risk of membrane fouling/scaling | [130,131,132] |
| Greywater Recycling | 50% of household use | Gravity/solar pumps | Household | Reduces freshwater demand, eco-friendly | Requires secondary treatment for safety, public acceptance issues | [133,134,135] |
| Wastewater Treatment | Up to 90% reclamation | Various | Municipal | Large-scale reuse, supports agriculture | Public acceptance concerns, risk of residual contaminants | [136,137,138] |
| Rain Harvesting | Variable (site-dependent) | Gravity | Household/community | Enhances groundwater, simple design | Rainfall-dependent, limited storage capacity | [139,140,141] |
| HDH Desalination | 1–5 L/h/m2 (solar-driven) | Solar thermal, waste heat | Small-medium | Low-cost, scalable, ideal for off-grid use | Still pilot-scale, moderate yields, require large surface areas | [142,143,144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeem, M.; Noman, M.T.; Amor, N.; Petru, M. Alternative and Sustainable Technologies for Freshwater Generation: From Fog Harvesting to Novel Membrane-Based Systems. Textiles 2025, 5, 43. https://doi.org/10.3390/textiles5040043
Azeem M, Noman MT, Amor N, Petru M. Alternative and Sustainable Technologies for Freshwater Generation: From Fog Harvesting to Novel Membrane-Based Systems. Textiles. 2025; 5(4):43. https://doi.org/10.3390/textiles5040043
Chicago/Turabian StyleAzeem, Musaddaq, Muhammad Tayyab Noman, Nesrine Amor, and Michal Petru. 2025. "Alternative and Sustainable Technologies for Freshwater Generation: From Fog Harvesting to Novel Membrane-Based Systems" Textiles 5, no. 4: 43. https://doi.org/10.3390/textiles5040043
APA StyleAzeem, M., Noman, M. T., Amor, N., & Petru, M. (2025). Alternative and Sustainable Technologies for Freshwater Generation: From Fog Harvesting to Novel Membrane-Based Systems. Textiles, 5(4), 43. https://doi.org/10.3390/textiles5040043








