Recent Progress on Green-Derived Tin Oxide (SnO2) for the Degradation of Textile Dyes: A Review
Abstract
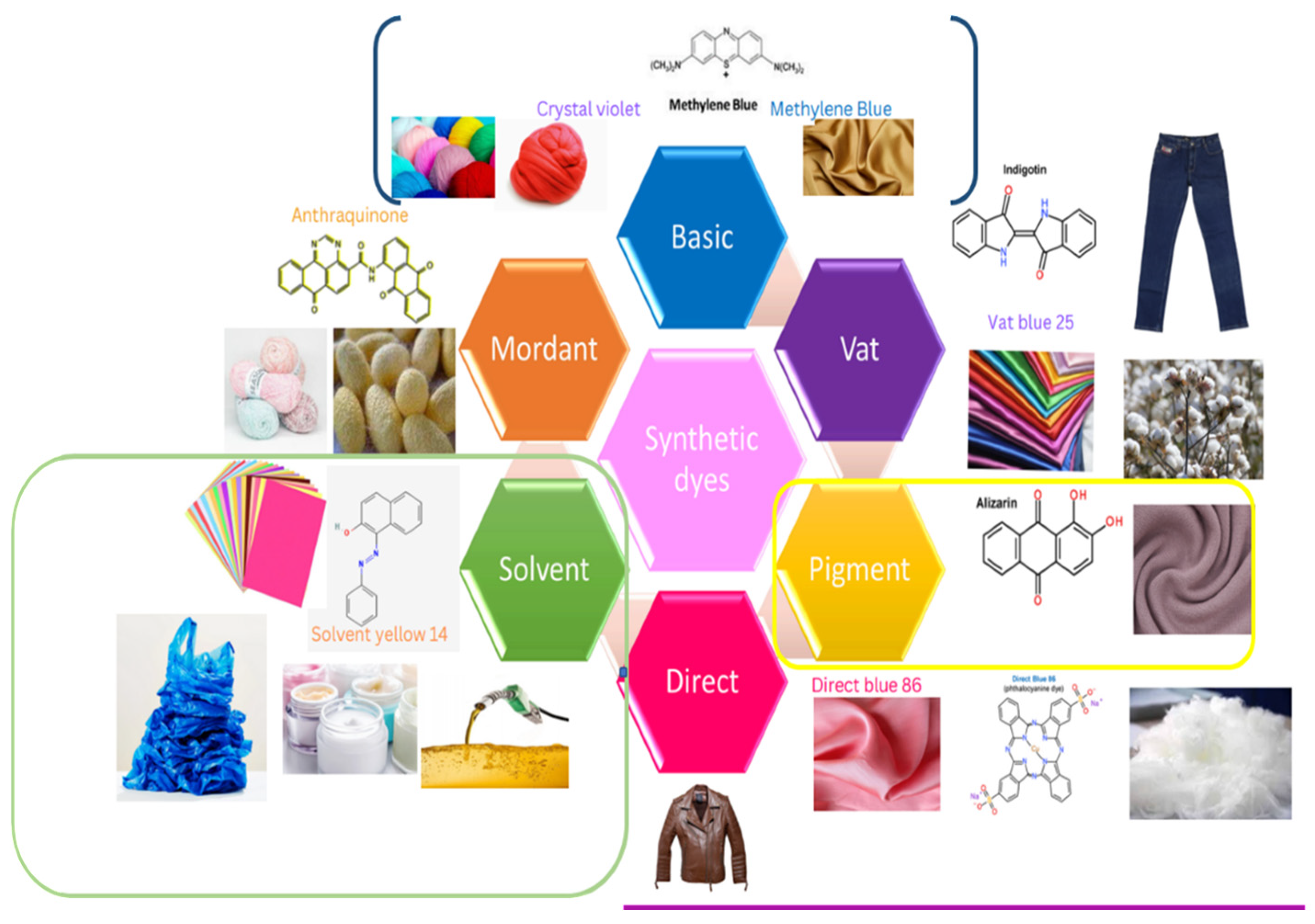
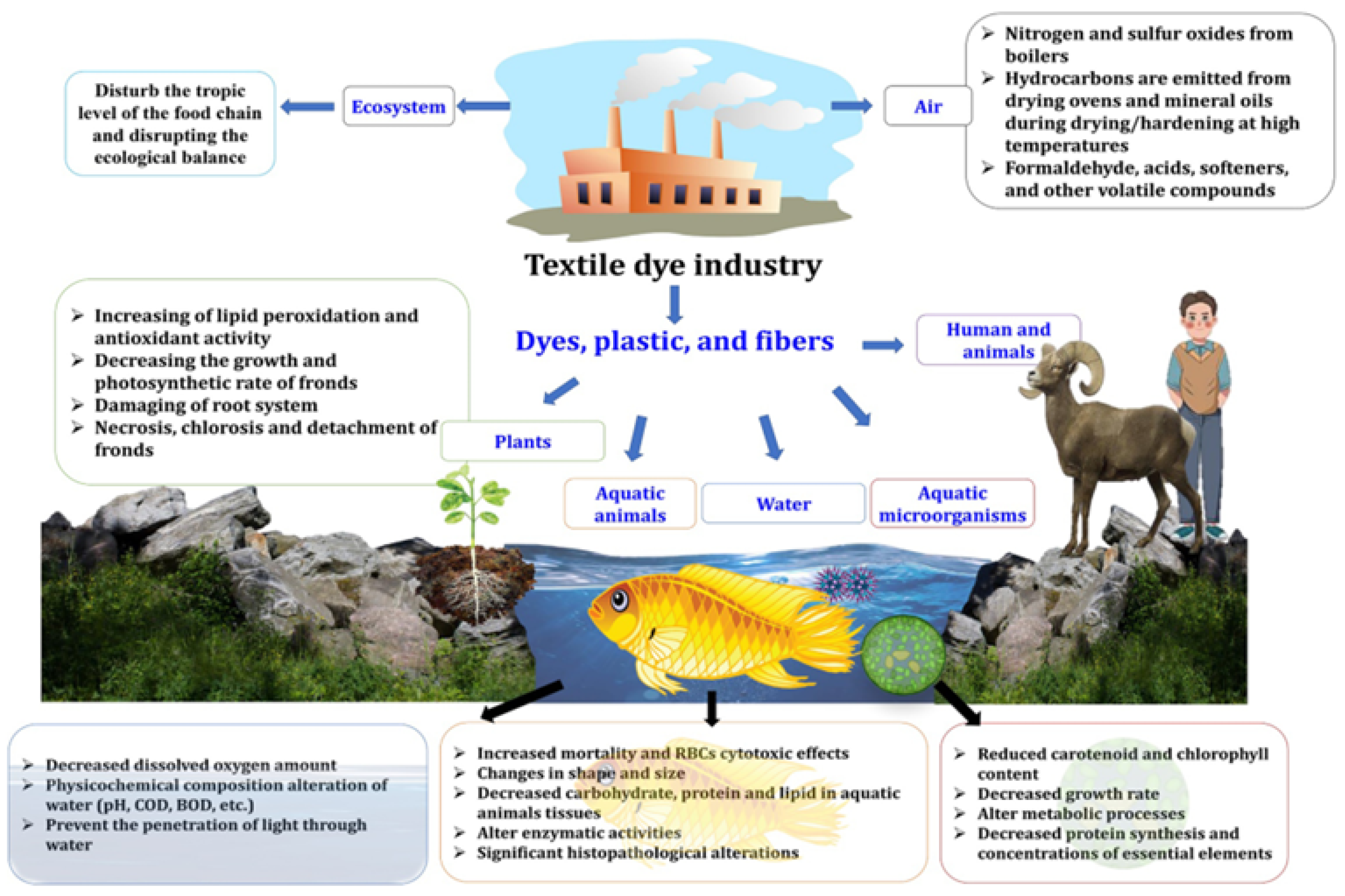
1. Introduction
2. SnO2 Nanoparticles
3. Synthesis of SnO2 Nanoparticles
| Remediation Methods | Functions | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Lithography | Techniques that employed concentrated electron or light beam to synthesize or fabricate structures or patterns at the nanoscale. | Commercial fabrication on nano science devices Capacity to create a cluster of the required shape and size from a single nanoparticle. | Require high equipment maintenance Costly equipment Fluids produce bubbles during synthesis | [37,46,47,48] |
| Ball milling | It uses a mechanical process to break larger particles into smaller particle sizes | Produce ultra-fine nanoparticles Provides higher yield Greater densification Homogeneity | Requires high energy Irregular morphologies Potential of contamination. | [49] |
| Sputtering | Process of depositing nanoparticles on a surface using a metal-based material after ions have clashed with the surface. | Strong adherence Homogeneity Capacity to deposit complex compounds | Low deposition rates High maintenance Risk of film stress and contamination Limited scalability for large-area coatings | [37,49,50] |
| Electrospinning | It is an electrostatic method that fabricates fiber materials by pulling a polymer solution under a high electric field, resulting in nanofiber production. | Creates fibers with a high surface-to-volume ratio Adjustable porosity Uniform structure | High maintenance Low biocompatibility Long biodegradation | [51,52] |
| Precipitation | Used for producing nanoparticles by combining chemical components in a solution, resulting in the formation of solid precipitates. | High purity Large surface area materials Simple | Use of toxic reagents and chemicals High sludge production Difficulty in separation | [49,53] |
| Hydrothermal | A process that creates nanoparticles by carrying out reactions at high temperatures and pressures in an autoclave reactor in a sealed vessel | Controlled particle size and structure Surface functionality Optical properties of the resultant nanomaterials | Uses high temperature Lengthy response periods High energy consumption | [40,54] |
| Microwave | Uses microwave irradiation to heat reaction mixtures that form nanoparticles. |
|
| [55] |
| Chemical vapor deposition | Used to decompose gaseous reactants using light, heat, and plasma to form solid nanoparticles | No need for capping/reducing agents High purity nanoparticles | Use of higher temperature and pressure | [56] |
| Spray pyrolysis | Entails atomizing metal solutions while maintaining a high temperature and gas flow rate in order to break down the metal and create nanoparticles | Regulated crystallinity, composition, chemical uniformity Adaptability for large-area films | Difficulty in real-time observation and modelling of nanoparticle formation High-temperature requirements Relatively slow deposition rates | [38] |
| Biological | A process that uses microbes (bacteria, fungi, plants, and algae) to eliminate pollutants | Simple Eco-friendly Cost effective | Slow process Microbes require extra attention. Non-selective Low degradation efficiency | [57,58] |
4. Green Synthesis of SnO2 Nanoparticles
5. Green-Derived SnO2 as Photocatalysts for Dye Degradation
6. Limitations of Green SnO2 and Their Modification for Dye Degradation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hamdi, A.M.; Rinner, U.; Sillanpää, M. Tin dioxide as a photocatalyst for water treatment: A review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M. A green and novel approach for the synthesis of SnO2 nanoparticles and its exploitation as a catalyst in the degradation of methylene blue under solar radiation. Mater. Lett. 2015, 145, 74–78. [Google Scholar] [CrossRef]
- Elango, G.; Kumaran, S.M.; Kumar, S.S.; Muthuraja, S.; Roopan, S.M. Green synthesis of SnO2 nanoparticles and its photocatalytic activity of phenolsulfonphthalein dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 176–180. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Shah, A.; Khan, A.R.; Rehman, M.U.; Ahmad, P.; Khan, B.; Ali, G. Green synthesis and characterization of tin dioxide nanoparticles for photocatalytic and antimicrobial studies. Mater. Res. Express 2020, 7, 025012. [Google Scholar] [CrossRef]
- Ligaray, M.; Kim, N.; Park, S.; Park, J.-S.; Park, J.; Kim, Y.; Cho, K.H. Energy projection of the seawater battery desalination system using the reverse osmosis system analysis model. Chem. Eng. J. 2020, 395, 125082. [Google Scholar] [CrossRef]
- Paździor, K.; Klepacz-Smółka, A.; Ledakowicz, S.; Sójka-Ledakowicz, J.; Mrozińska, Z.; Żyłła, R. Integration of nanofiltration and biological degradation of textile wastewater containing azo dye. Chemosphere 2009, 75, 250–255. [Google Scholar] [CrossRef]
- Pradinaud, C.; Northey, S.; Amor, B.; Bare, J.; Benini, L.; Berger, M.; Boulay, A.-M.; Junqua, G.; Lathuillière, M.J.; Margni, M.; et al. Defining freshwater as a natural resource: A framework linking water use to the area of protection natural resources. Int. J. Life Cycle Assess. 2019, 24, 960–974. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ismail, A.A.; Osama, I.; Ibrahim, I.; Kandil, A.-H.T. Photocatalytic decomposition of dyes using ZnO doped SnO2 nanoparticles prepared by sol-vothermal method. Arab. J. Chem. 2014, 7, 71–77. [Google Scholar] [CrossRef]
- Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the dye containing wastewater. Ecotoxilogical and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Devi, T.B.; Ahmaruzzaman, M. l-lysine monohydrate mediated facile and environment friendly syn-thesis of SnO2 nanoparticles and their prospective applications as a catalyst for the reduction and photodegradation of aro-matic compounds. J. Environ. Chem. Eng. 2016, 4, 2976–2989. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, O.S.; Jang, J. Facile synthesis of SnO2 nanofibers decorated with N-doped ZnO nanonodules for visible light photocatalysts using single-nozzle co-electrospinning. J. Mater. Chem. 2012, 22, 14565. [Google Scholar] [CrossRef]
- Jeyaraj, S.; Saral, A.M. Synthesis of SnO2 Nanoparticles using Brassica juncea (L.) Czern Seed Extract: Characteriza-tion and Biological Applications. Chem. Sel. 2025, 10, e202404490. [Google Scholar] [CrossRef]
- Suresh, K.C.; Surendhiran, S.; Manoj Kumar, P.; Kumar, E.R.; Khadar, Y.A.S.; Balamurugan, A. Green synthesis of SnO2 nanoparticles using Delonix elata leaf extract: Evaluation of its structural, optical, morphological and photocatalytic properties. SN Appl. Sci. 2020, 2, 1735. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, H.; Kukkar, D.; Mukamia, V.K.; Kumar, S.; Rawat, M. Green synthesis of SnO2 NPs for solar light induced photocalytic applications. Mater. Res. Express 2019, 6, 115007. [Google Scholar] [CrossRef]
- Tasisa, Y.E.; Sarma, T.; Sahu, T.K.; Krishnara, R. Phytosynthesis and characterization of tin-oxide nanoparticles (SnO2-NPs) from Croton macrostachyus leaf extract and its application under visible light photocatalytic activities. Sci. Rep. 2024, 14, 10780. [Google Scholar] [CrossRef]
- Najjar, M.; Hosseini, H.A.; Masoudi, A.; Hashemzadeh, A.; Darroudi, M. Preparation of tin oxide (IV) nanoparticles by a green chemistry method and investigation of its role in the removal of organic dyes in water purification. Res. Chem. Intermed. 2020, 46, 2155–2168. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Buniyamin, I.; Khusaimi, Z.; Rusop, M. Utilization of tin oxide nanoparticles synthesized through plant-mediated methods and their application in photocatalysis: A brief review. Int. J. Chem. 2023, 24, 116–123. [Google Scholar]
- González, E.Q.; Medina, E.L.; Robles, R.V.Q.; Gálvez, H.E.G.; Lopez, Y.A.B.; Viveros, E.V.; Molina, F.A.; Nestor, A.R.V.; Morales, P.A.L. A Study of the Optical and Structural Properties of SnO2 Nanoparticles Synthesized with Tilia cordata Applied in Methylene Blue Degradation. Symmetry 2022, 14, 2231. [Google Scholar] [CrossRef]
- Ahmad, W.; Pandey, A.; Ahmed, S.; Nur-e-Alam, M. Sambucus Canadensis leaf extract mediated synthesis and analysis of antibacterial and photocatalytic degradation property of stannous oxide nanoparticles. Nanotechnol. Environ. Eng. 2023, 8, 707–716. [Google Scholar] [CrossRef]
- Gomathi, E.; Jayapriya, M.; Arulmozhi, M. Environmental benign synthesis of tin oxide (SnO2) nanoparticles using Actinidia deliciosa (Kiwi) peel extract with enhanced catalytic properties. Inorg. Chem. Commun. 2021, 130, 108670. [Google Scholar] [CrossRef]
- Haritha, E.; Roopan, S.M.; Madhavi, G.; Elango, G.; Al-Dhabi, N.A.; Arasu, M.V. Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies. J. Photochem. Photobiol. B Biol. 2016, 162, 441–447. [Google Scholar] [CrossRef]
- Honarmand, M.; Golmohammadi, M.; Naeimi, A. Biosynthesis of tin oxide (SnO2) nanoparticles using jujube fruit for photocatalytic degradation of organic dyes. Adv. Powder Technol. 2019, 30, 1551–1557. [Google Scholar] [CrossRef]
- Kumar, M.; Mehta, A.; Mishra, A.; Singh, J.; Rawat, M.; Basu, S. Biosynthesis of tin oxide nanoparticles using Psidium Guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett. 2018, 215, 121–124. [Google Scholar] [CrossRef]
- Luque, P.A.; Chinchillas-Chinchillas, M.J.; Nava, O.; Lugo-Medina, E.; Martínez-Rosas, M.E.; Carrillo-Castillo, A.; Vilchis-Nestor, A.R.; Madrigal-Muñoz, L.E.; Garrafa-Gálvez, H.E. Green synthesis of tin dioxide nanoparticles using Camellia sinensis and its application in photocatalytic degradation of textile dyes. Optik 2021, 229, 166259. [Google Scholar] [CrossRef]
- Narasaiah, B.P.; Banoth, P.; Sohan, A.; Mandal, B.K.; Bustamante Dominguez, A.G.; De Los Santos Valladares, L.; Kollu, P. Green Biosynthesis of Tin Oxide Nanomaterials Mediated by Agro-Waste Cotton Boll Peel Extracts for the Remediation of Environmental Pollutant Dyes. ACS Omega 2022, 7, 15423–15438. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.; Manikandan, E.; Rajendran, V.; Maaza, M. Physical & enhanced photocatalytic properties of green synthesized SnO2 nanoparticles via Aspalathus linearis. J. Alloys Compd. 2016, 681, 561–570. [Google Scholar] [CrossRef]
- Jafeesh, J.; Aswathy Aromal, S.; Krishnan, R.R. Bio synthesis and characterization of tin oxide for photocatalytic, anti-microbial and anti-oxidant applications. Mater. Chem. Phys. 2023, 307, 128027. [Google Scholar] [CrossRef]
- Kaur, M.; Prasher, D.; Sharma, A.; Ghosh, D.; Sharma, R. Natural sunlight driven photocatalytic dye degradation by biogenically synthesized tin oxide (SnO2) nanostructures using Tinospora crispa stem extract and its anticancer and antibacterial applications. Environ. Sci. Pollut. Res. 2023, 30, 38869–38885. [Google Scholar] [CrossRef]
- Manimaran, M.; Muthuvel, A.; Said, N.M. Microwave-assisted green synthesis of SnO2 nanoparticles and their photocatalytic degradation and antibacterial activities. Nanotechnol. Environ. Eng. 2023, 8, 413–423. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Ameen, F.; Alsamhary, K.; Dawoud, T.; Al-Khattaf, F.; AlNadhari, S. Tin oxide nanoparticles (SnO2-NPs) synthesis using Galaxaura elongata and its anti-microbial and cytotoxicity study: A greenery approach. Appl. Nanosci. 2023, 13, 519–527. [Google Scholar] [CrossRef]
- Borah, D.; Saikia, P.; Rout, J.; Gogoi, D.; Ghosh, N.N.; Bhattacharjee, C.R. Sustainable green synthesis of SnO2 quantum dots: A stable, phase-pure and highly efficient photocatalyst for degradation of toxic dyes. Mater. Today Sustain. 2024, 26, 100770. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthika, D.; Rosaiah, P.; Devanesan, S.; Mythili, R.; Dhananjaya, M.; Joo, S.W. Fabrication of SnO2/NGO hybrid nanocomposite as an effective photocatalyst for binary dye degradation under sunlight illumination. Process Saf. Environ. Prot. 2024, 188, 398–405. [Google Scholar] [CrossRef]
- Snigdha; Gautam, A.; Gautam, N.; Singh, K.B.; Upadhyay, D.D.; Pandey, G. Facile green synthesis of SnO2:Zn@g-C3N4 heterojunction nanocomposite using Murraya paniculata leaves extract for effective photocatalytic degradation of organic dye malachite green by sunlight illumination. J. Mol. Struct. 2024, 1308, 138013. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Hintsho-Mbita, N.C. Green Derived Zinc Oxide (ZnO) for the Degradation of Dyes from Wastewater and Their Antimicrobial Activity: A Review. Catalysts 2022, 12, 833. [Google Scholar] [CrossRef]
- Heyradi, S. Biosynthesis of the Ag Doped SnO2 Nanorods Supported Bentonite Clay with Enhanced Photocatalytic Activity Against Crystal Violet Dye. J. Inorg. Organomet. Polym. Mater. 2025, 35, 2162–2173. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T.; Gebretnsae, H.G. Green and Cost-Effective Synthesis of Tin Oxide Nanoparticles: A Review on the Synthesis Methodologies, Mechanism of Formation, and Their Potential Applications. Nanoscale Res. Lett. 2021, 16, 97. [Google Scholar] [CrossRef]
- Nipa, S.T.; Akter, R.; Raihan, A.; Rasul, S.b.; Som, U.; Ahmed, S.; Alam, J.; Khan, M.R.; Enzo, S.; Rahman, W. State-of-the-art biosynthesis of tin oxide nanoparticles by chemical precipitation method towards photocatalytic application. Environ. Sci. Pollut. Res. 2022, 29, 10871–10893. [Google Scholar] [CrossRef]
- Ren, P.; Qi, L.; You, K.; Shi, Q. Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved For-maldehyde Gas Sensing. Nanomaterials 2022, 12, 228. [Google Scholar] [CrossRef]
- Buniyamin, I.; Asli, N.A.; Akhir, R.M.; Jafar, S.M.; Eswar, K.A.; Mahmood, M.K.A.; Idorus, M.Y.; Shamsudin, M.S.; Rahman, A.F.M.M.; Mahmood, M.R.; et al. Biofabricated SnO2 Nanoparticles Derived from Leaves Extract of Morinda citrifolia and Pandanus amaryllifolius for Photocatalytic Degradation. J. Clust. Sci. 2025, 36, 3. [Google Scholar] [CrossRef]
- Kanwal, A.; ur Rehman, M.; Sattar, R.; Thebo, K.H.; Kazi, M. Highly efficient tin oxide and polyaniline-tin-oxide nanostructured materials for photocatalytic degradation of organic dyes. J. Mol. Struct. 2024, 1312, 138454. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K. Tyrosine mediated synthesis of SnO2 nanoparticles and their photocatalytic activity towards Violet 4 BSN dye. J. Mol. Liq. 2016, 221, 415–421. [Google Scholar] [CrossRef]
- Rathinabala, R.; Thamizselvi, R.; Padmanabhan, D.; Alshahateet, S.F.; Fatimah, I.; Kassegn Sibhatu, A.; Kassegn Weldegebrieal, G.; Izwan Abd Razak, S.; Sagadevan, S. Sun light-assisted enhanced photocatalytic activity and cytotoxicity of green synthesized SnO2 nanoparticles. Inorg. Chem. Commun. 2022, 143, 109783. [Google Scholar] [CrossRef]
- Sundara Selvam, P.S.; Ganesan, D.; Rajangam, V.; Raji, A.; Kandan, V. Green Synthesis of SnO2 Nanoparticles for Catalytic Degradation of Rhodamine B. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 661–676. [Google Scholar] [CrossRef]
- Zalevsky, Z.; Livshit, P.; Gur, E. New Approaches to Image Processing Based Failure Analysis of Nano-Scale ULSI Devices; William Andrew: Norwich, NY, USA, 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Pimpin, A.; Srituravanich, W. Reviews on micro- and nanolithography techniques and their applications. Eng. J. 2012, 16, 37–56. [Google Scholar] [CrossRef]
- Sharma, E.; Rathi, R.; Misharwal, J.; Sinhmar, B.; Kumari, S.; Dalal, J.; Kumar, A. Evolution in Lithography Techniques: Microlithography to Nanolithography. Nanomaterials 2022, 12, 2754. [Google Scholar] [CrossRef]
- Abdullah, H.I.; Al-Amiery, A.A.; Al-Baghdadi, S.B. The using of nanomaterials as catalysts for photodegradations. J. Phys. Conf. Ser. 2021, 185, 012052. [Google Scholar] [CrossRef]
- Kour, D.; Kaur, T.; Kumari, S.; Bassi, A.; Khan, S.S.; Gusain, M.; Shoket, H.; Sindhu, M.; Sharma, B.; Singh, S. Advances and innovations in green nanotechnology for agro-environmental sustainability. Nanotechnol. Environ. Eng. 2025, 10, 49. [Google Scholar] [CrossRef]
- Nalbandian, M.J. Development and Optimization of Chemically-Active Electro Spun Nanofibers For treatment of Impaired Water Sources. Ph.D. Thesis, University of California Riverside, Riverside, CA, USA, 2014. [Google Scholar]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Robkhob, P.; Tang, I.M.; Thongmee, S. Magnetic properties of the dilute magnetic semiconductor Zn1-xCoxO nanoparticles. J. Supercond. Nov. Magn. 2019, 32, 3637–3645. [Google Scholar] [CrossRef]
- De Silva, G. Polarized Light and Optical Activity. Bachelor’s Thesis, University of Colombo, Colombo, Sri Lanka, 2020. [Google Scholar] [CrossRef]
- Sabzi, M.; Mousavi Anijdan, S.H.; Shamsodin, M.; Farzam, M.; Hojjati-Najafabadi, A.; Feng, P.; Park, N.; Lee, U. A Review on Sustainable Manufacturing of Ceramic-Based Thin Films by Chemical Vapor Deposition (CVD): Reactions Kinetics and the Deposition Mechanisms. Coatings 2023, 13, 188. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mathobela, S.; Hintsho-Mbita, N.C. Biosynthesized Bimetallic (ZnOSnO2) Nanoparticles for Photocatalytic Degradation of Organic Dyes and Pharmaceutical Pollutants. Catalysts 2022, 12, 334. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; Arenas-Alatorre, J.A. Green Synthesis of Nanoparticles: A Biological Approach. In Advances in Green Chemistry—Prevention-Assurance-Sustainability; Kinjal, J.-S., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Minh, D.T.C.; Do Dat, T.; Quan, T.T.; Nam, N.T.H.; Thi Thanh Huong, Q.; Dat, N.M.; Hieu, N.H. Phyto-synthesis of tin oxide nanoparticles using Diospyros mollis leaves extract doped graphitic carbon nitride for photocatalytic methylene blue degradation and hydrogen peroxide production. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133454. [Google Scholar] [CrossRef]
- Kaur, M.; Prasher, D.; Dhiman, V.; Murugesan, P.; Ghosh, D.; Sharma, R. Green energy induced photocatalytic decomposition of methylene blue and crystal violet dyes by strontium doped tin oxide nanoparticles and its antibacterial activity. Phys. B Condens. Matter 2023, 661, 414924. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based pho-tocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Abduh, N.; Al-Adoyni, A.-B. Green Synthesis of ZnO/SnO hybrid nanocomposites for degradation of cationic and an-ionic dyes under sunlight radiation. Materials 2023, 16, 7398. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Kamenická, B.; Švec, P.; Weidlich, T. Separation of Anionic Chlorinated Dyes from Polluted Aqueous Streams Using Ionic Liquids and Their Subsequent Recycling. Int. J. Mol. Sci. 2023, 24, 12235. [Google Scholar] [CrossRef] [PubMed]
- Kamenická, B.; Weidlich, T. A Comparison of Different Reagents Applicable for Destroying Halogenated Anionic Textile Dye Mordant Blue 9 in Polluted Aqueous Streams. Catalysts 2023, 13, 460. [Google Scholar] [CrossRef]
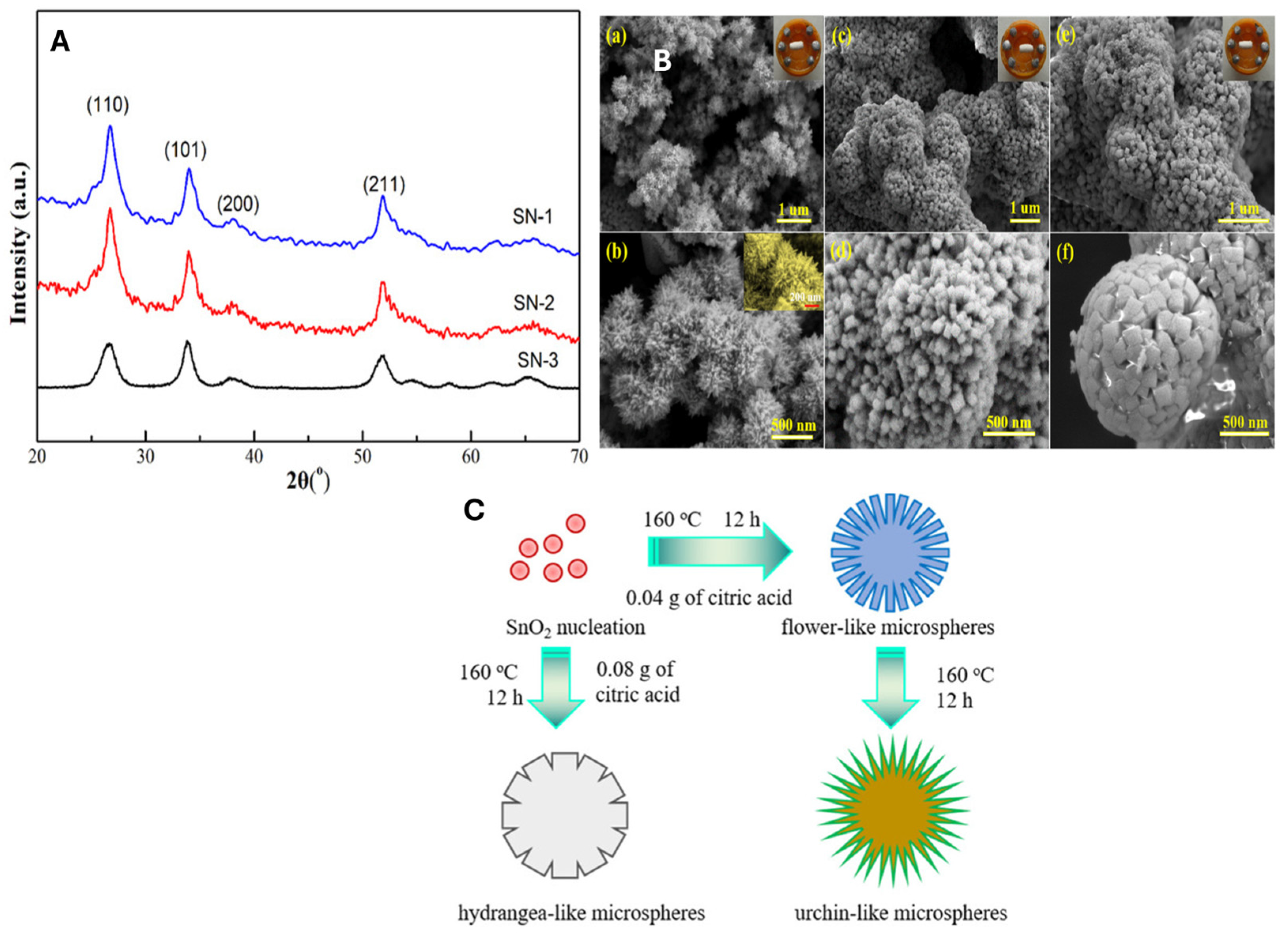
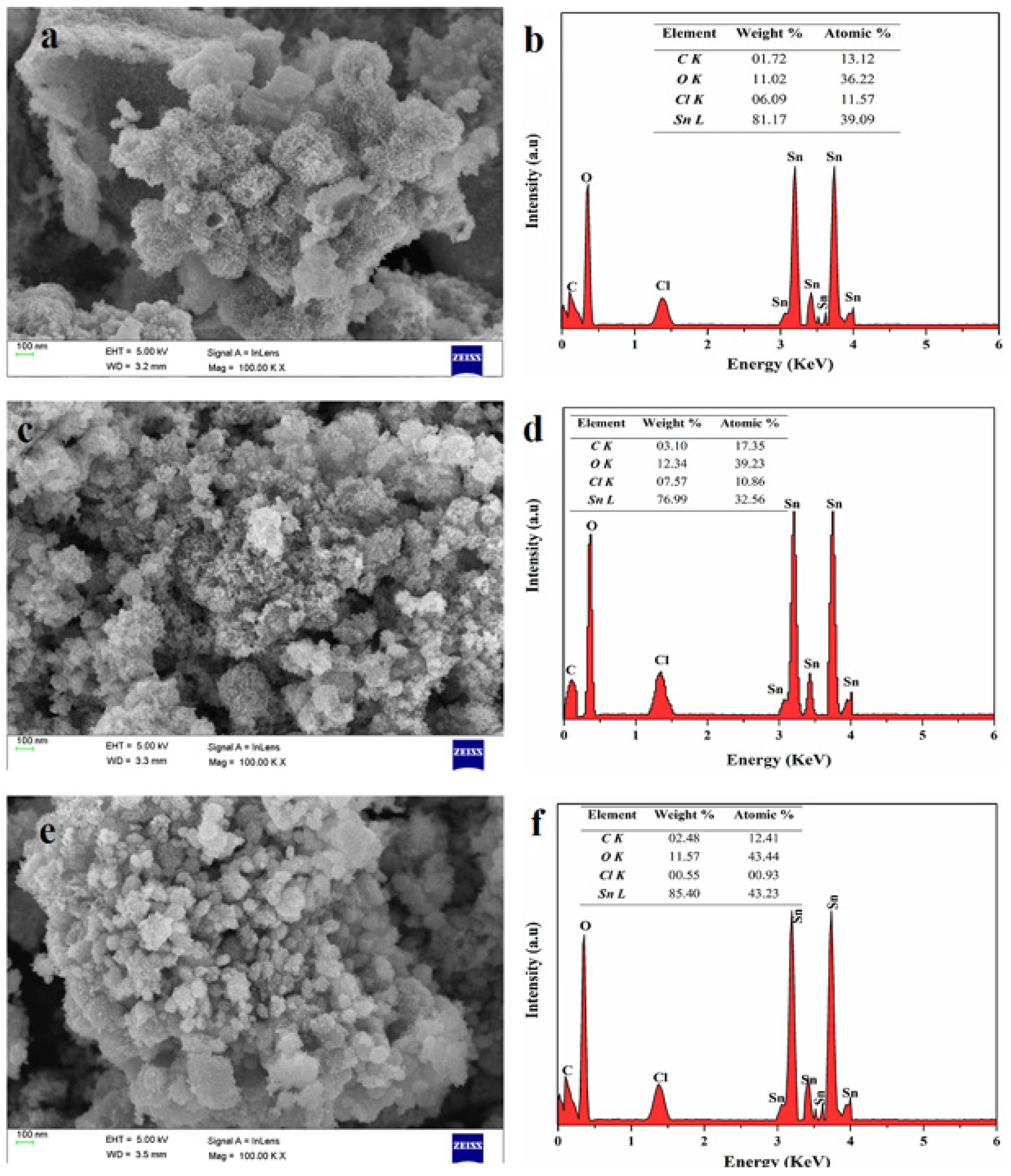
| Reductant | Product Calcination Temperature and Duration | Extract Dosage | Extract Temperature and Duration | Morphology and Phase Identification | Particle Size (nm) | Refs. |
|---|---|---|---|---|---|---|
| Sambucus Canadensis leaf extract | 350 °C for 30 min | 20 g in 250 mL | 60 °C for 12 h | Plant like | - | [21] |
| Actinidia deliosa (Kiwi) peel extract | 300 °C for 3 h | 1:10 ratio | 60 °C for 2 h | Sphere | 5–10 | [22] |
| Catunaregam spinosa (barks roots) | 450 °C for 2 h | 300 g in 500 mL | 60 °C for 2 h | Sphere | 47 ± 2 | [23] |
| Jujube fruit extract | 500 °C for 1 h | 200 g in 200 mL | 80 °C for 30 min | Sphere | 18 | [24] |
| Psidium Guajava leaf extract | 400 °C for 4 h | - | 60 °C for 4 h | Sphere Tetragonal rutile | 8 | [25] |
| Camelia sinesis | 400 °C for 1 h | 0.5 g in 50 mL 1 g in 50 mL 2 g in 50 mL | 60 °C for 1 h | Quazi-spherical Hexagonal rutile | - | [26] |
| Cotton bool waste Peel extract | 200 °C for 3 h 500 °C for 3 h 700 °C for 3 h | 3 g in 100 mL | 80 °C for 30 min | Sphere Tetragonal | 4 8 13 | [27] |
| Aspalathus linearis | 200 °C for 3 h 700 °C for 3 h 900 °C for 3 h | - | - | Quasi-spherical | 1–5 | [28] |
| Persia Americana | 300 °C for 3 h | - | - | Sphere Tetragonal rutile | 4 | [5] |
| Daphne mucronata (D. mucronata) leaf extract | Oven dried 100 °C for 6 h | 20 g in 500 mL | 100 °C for 1 h | Different shapes | 64 | [6] |
| Garcinia Cambogia | 500 °C for 2 h | 10 g in 100 mL | 100 °C for 5 min. | Sphere Tetragonal | 14 11 10 | [29] |
| Tnospora crispa (TCSE) stem extract | 550 °C for 4 h | 4 g in 150 mL | 100 °C | Rods and spheres | - | [30] |
| Solanum nigrum leaf extract | 600 °C for 6 h | 10 g in 250 mL | 85 °C in 20 min | Sphere | 5 & 18 | [31] |
| Galaxaura elongata (red algae). | 500 °C for 3 h | 2 g in 200 mL | 70 °C for 48 h | Sphere Tetragonal | 35 | [32] |
| Croton macrostachyus leaf extract | 500 °C for 3 h | 10 g in 200 mL | 60 °C for 4 h | Sphere | 32 | [17] |
| marine alga, Ulva sp. | 400 °C for 3 h | 2 g in 100 mL | 70 °C for 20 min | Sphere and cuboidal | 5 | [33] |
| Aquilaria malaccensis leaf extract | 800 °C for 2 h | 80 g in 400 mL | 80 °C for 20 min | Sphere tetragonal | 27 | [20] |
| Indigofera tinctoria leaf extract | No calcination, oven dried for 8 h at 60 °C | 5 g in 100 mL | 60 °C for 30 min | Sphere and nanosheets Tetragonal | - | [34] |
| Murraya paniculate (orange jasmine) leave extract | Oven dried at 60 °C | 10 g in 100 mL | 80 °C for 180 min | Sphere and sheets | 3–5 | [35] |
| Photocatalyst | Dye | Bandgap | Light Source (Watts) | Parameters (Dosage, Conc and pH) | Contact Time | Degradation Removal | References |
|---|---|---|---|---|---|---|---|
| SnO2 | CBB MR CBB MR | - | Solar Solar UV lamp UV lamp | 30 mg | 90 min | 2.87% 1.94% 2.87% 0.27% | [21] |
| SnO2 | MB MO RhB | - | 5 mg 10−5 M | 8 min 8 min 6 min | 89% 86% 97% | [22] | |
| SnO2 | CR | - | UV Reactor | 2.5 mg 10−4 M | 45 min | 90% | [23] |
| SnO2 | MB EBT | - | Solar | 8 mg 100 ppm | 300 min | 90% 83% | [24] |
| SnO2 | RY186 | - | Solar (637 W) | 10 mg 40 ppm | 180 min | 90% | [25] |
| SnO2 | MO MB RhB | 4.02 eV 3.95 eV 3.79 eV | Solar and UV lamp | 50 mg 15 ppm | 180 min | 81% 100% 100% | [26] |
| SnO2 | MB MO | 2.68 eV 2.98 eV 3.14 eV | UV lamp (125 Watts) | 20 mg 10 ppm | 30 min 40 min | 99% 98% | [27] |
| SnO2 | CR MB EY | - | UV Lamp | 2.5 × 10−5 2.7 × 10−6 M | 20 min | 2.2 nm 2.2 nm 19 nm | [28] |
| SnO2 | R6G | - | Solar | 20 mg 15 ppm | 390 min | 99% | [6] |
| SnO2 | MB | - | Solar | 20 mg 15 ppm | 240 min | 90% | [29] |
| SnO2 | MB RhB | 3.45 eV | Solar | 50 mg 10 ppm | 200 min | 93% 47% | [30] |
| SnO2 | EB | 3.3 eV | Solar | 2 mg 1.5 × 10−4 M | 90 min | 96% | [31] |
| SnO2 | RhB MB | 3.03 eV, 2.71 eV, 2.61 eV, and 2.41 eV | UV lamp | 50 mg 0.1 g/L pH = 6 | 80 min | 91% 96% | [17] |
| SnO2 QDs | MB MO RhB | 3.80 eV | Solar | 15 mg 1 × 10−4 M | 16 min 26 min 44 min | 94% 87% 93% | [32] |
| SnO2 | MB | 4.17 eV 3.78 eV 4.0 eV | Solar | 10 mg 10−4 M | 240 min | 96% | [37] |
| Psidium Guajava | RY | 3.64 eV | Sunlight | 180 min | 90 | [40] | |
| SnO2 | MB | 3.12 eV | 70 min | 94% | [41] | ||
| SnO2 | V4BSN | 3.6 eV | UV lamp (125 Watts) | 10 mg 25 ppm | 40 min | 100% | [43] |
| SnO2 | RhB | - | Solar UV lamp (125 Watts) | 50 mg 20 ppm | 30 min | 97% 46% | [44] |
| SnO2 | RhB | - | Solar | 100 mg 1 × 10−5 M pH | 90 min | 86% | [45] |
| SnO2 | EBT | - | UV lamp (500 W) | (2.87 × 10−5 M, 0.211 g L−1 pH = 9 | 120 min | 36% | [18] |
| Photocatalyst | Dye | Bandgap | Light Source (Watts) | Parameters (Dosage, Conc and pH) | Contact Time | Degradation Removal | References |
|---|---|---|---|---|---|---|---|
| SnO2/NGO | MB MO | - | Solar | 20 mg 20 ppm | 150 min | 89% 94% | [34] |
| SnO2:Zn@g-C3N4 | MG | 3.22 eV | Solar | 30 mg pH = 9 10 ppm | 100 min | 94% | [35] |
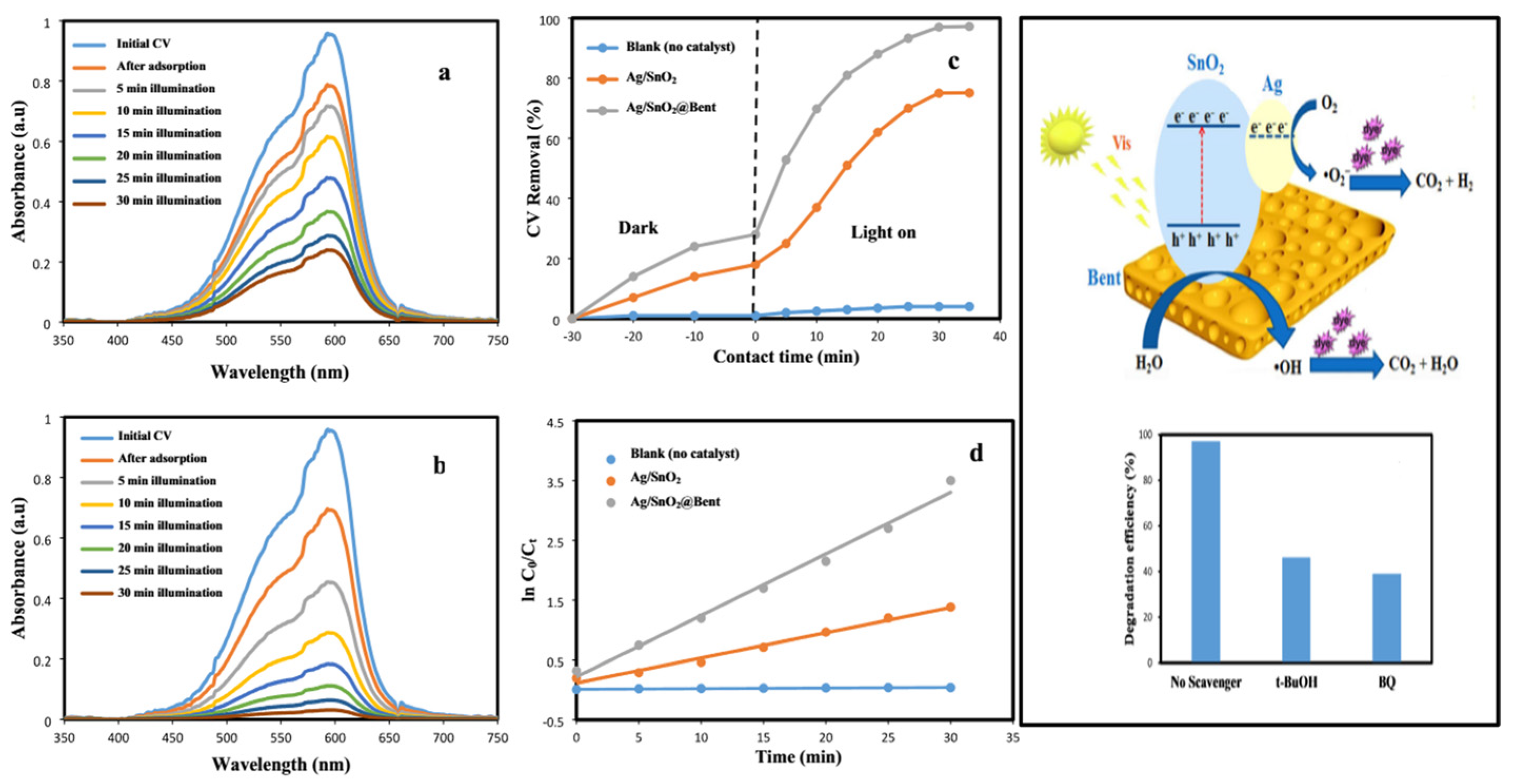
| Ag/SnO2-bento | CV | visible | 1 g/L−1 | 30 min | 97 | [37] | |
| PANI-SnO2 | RhB MO MB MR | 2.68 eV | 100 mg 20 ppm | 84% 92% 60% 90% | [42] | ||
| SnO2/gCN | MB | 2.44 eV | UV lamp (50 W) | 50 mg 10 ppm | 120 min | 99% | [59] |
| Sr:SnO2 | MB CV | 2.43 eV | Solar | 25 mg 10 ppm | 150 min | 83% 68% | [60] |
| Fe3O4/SnO2/Pr+ | MB RhB | 50 W LED | 150 min | 89% 83% | |||
| SnO2/ZnO | MB | UV | 150 min | 88% | [57] | ||
| ZnO/SnO2 | MB MO RhB | Solar sunlight | 55 min 55 min 65 min | 100 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlaule-Glory, L.M.; Hintsho-Mbita, N.C. Recent Progress on Green-Derived Tin Oxide (SnO2) for the Degradation of Textile Dyes: A Review. Textiles 2025, 5, 36. https://doi.org/10.3390/textiles5030036
Mahlaule-Glory LM, Hintsho-Mbita NC. Recent Progress on Green-Derived Tin Oxide (SnO2) for the Degradation of Textile Dyes: A Review. Textiles. 2025; 5(3):36. https://doi.org/10.3390/textiles5030036
Chicago/Turabian StyleMahlaule-Glory, L. M., and N. C. Hintsho-Mbita. 2025. "Recent Progress on Green-Derived Tin Oxide (SnO2) for the Degradation of Textile Dyes: A Review" Textiles 5, no. 3: 36. https://doi.org/10.3390/textiles5030036
APA StyleMahlaule-Glory, L. M., & Hintsho-Mbita, N. C. (2025). Recent Progress on Green-Derived Tin Oxide (SnO2) for the Degradation of Textile Dyes: A Review. Textiles, 5(3), 36. https://doi.org/10.3390/textiles5030036







