From Waste to Value: Advances in Recycling Textile-Based PET Fabrics
Abstract
1. Introduction
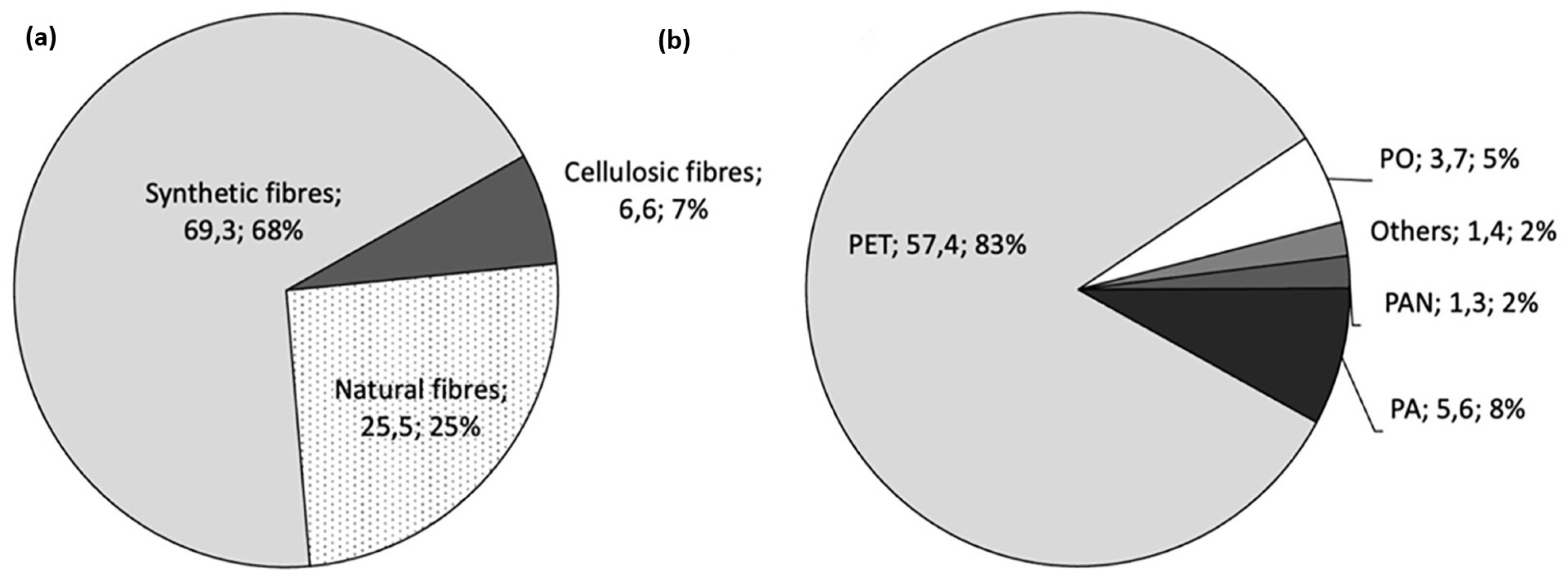
2. The Global Textile Market
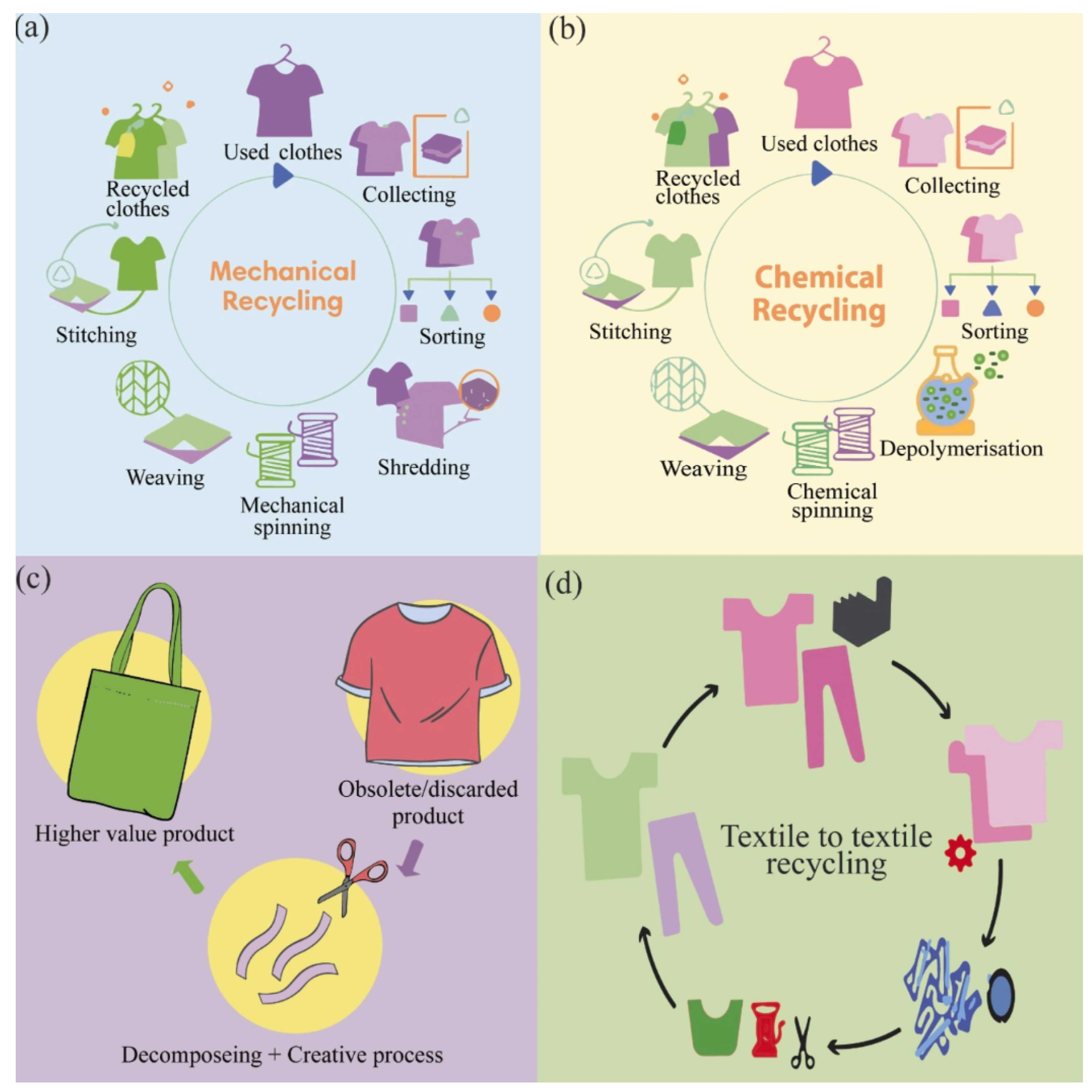
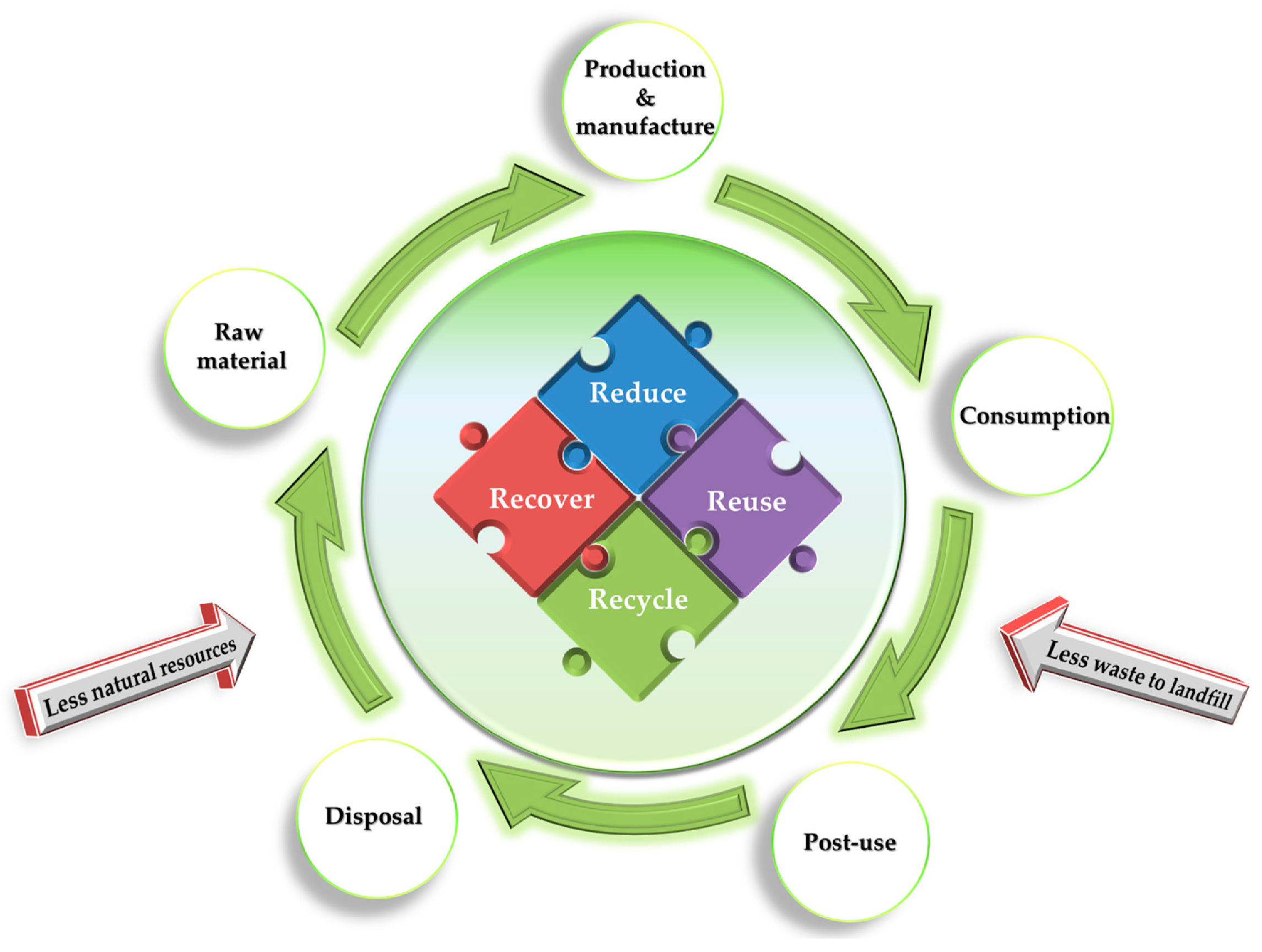
3. Recycling Process
3.1. Mechanical Recycling
Advantages and Disadvantages
3.2. Chemical Recycling
Advantages and Disadvantages
3.3. Enzymatic Recycling
Advantages and Disadvantages
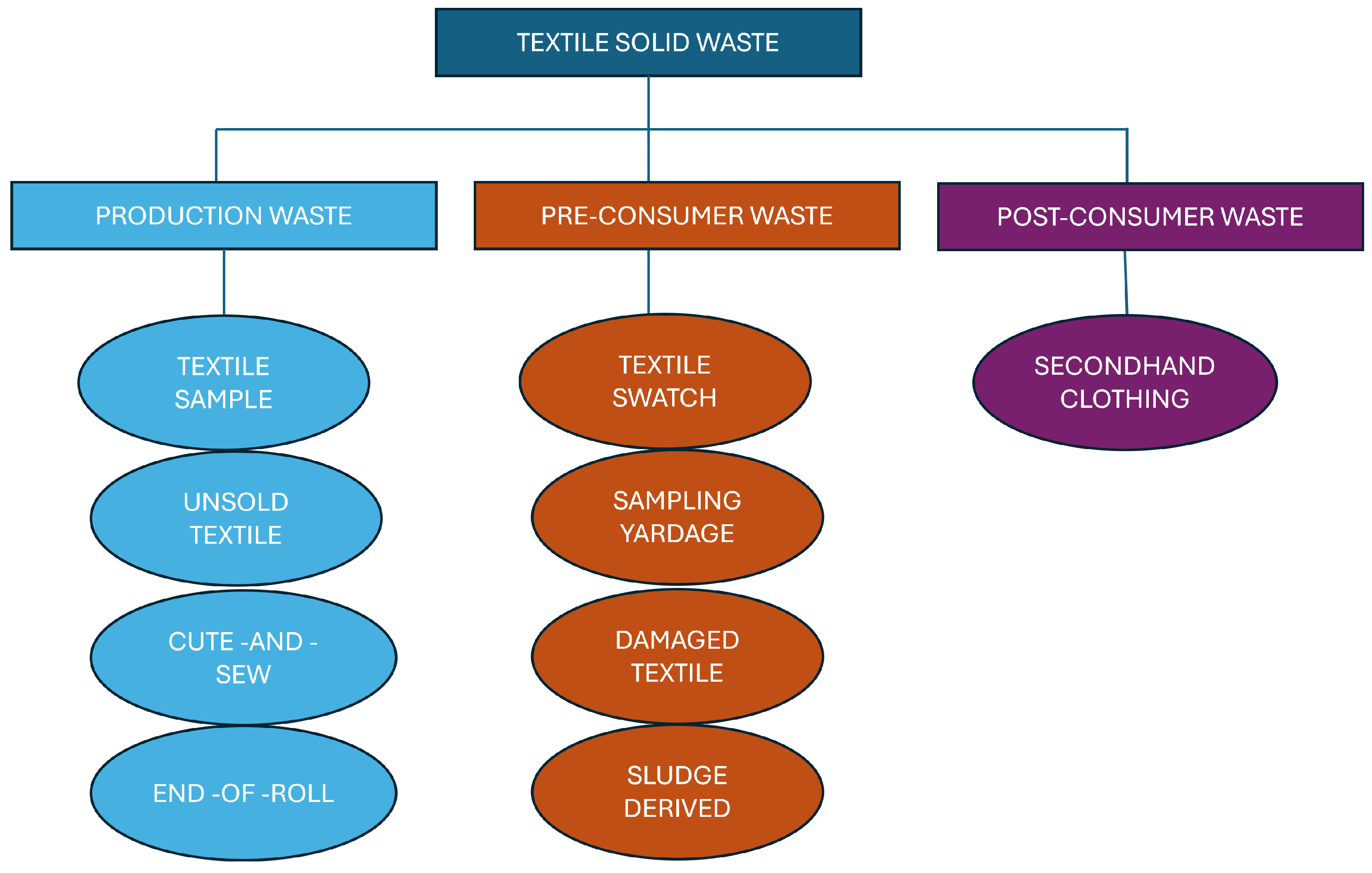
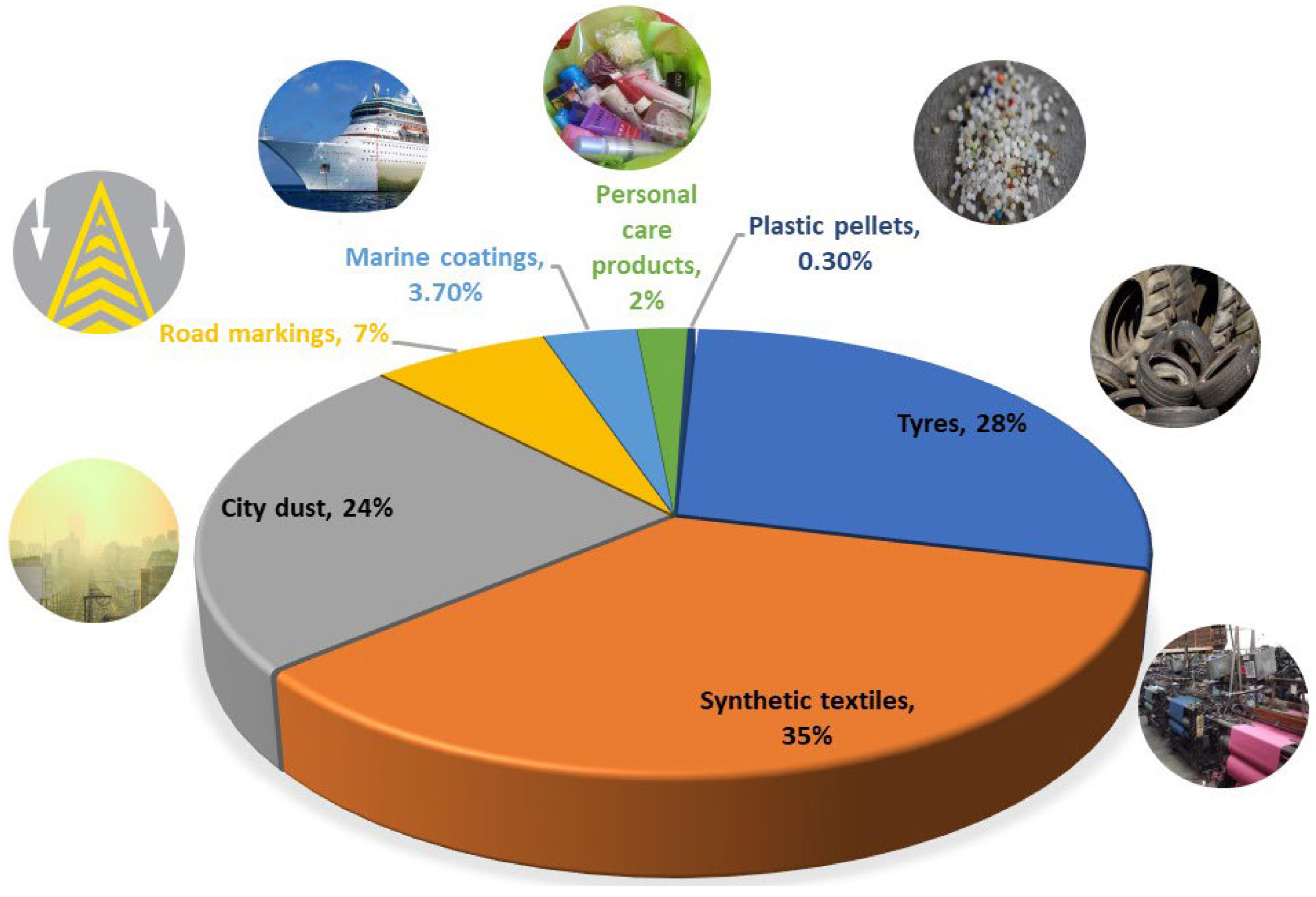
4. Solid Waste from Textile Industry
4.1. Production Waste
4.2. Pre-Consumer Waste
4.3. Post-Consumer Waste
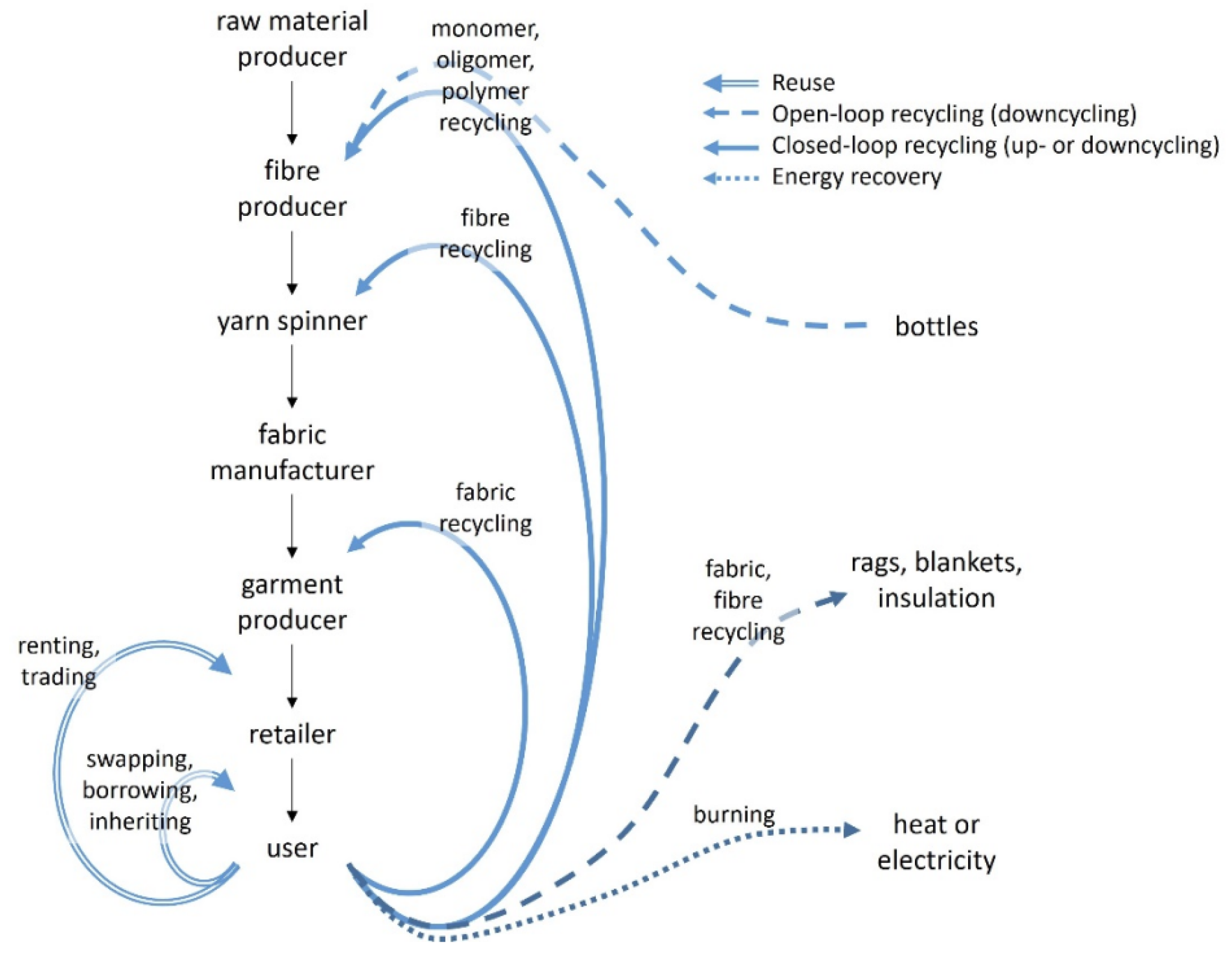
5. Textile-to-Textile Recycling for PET
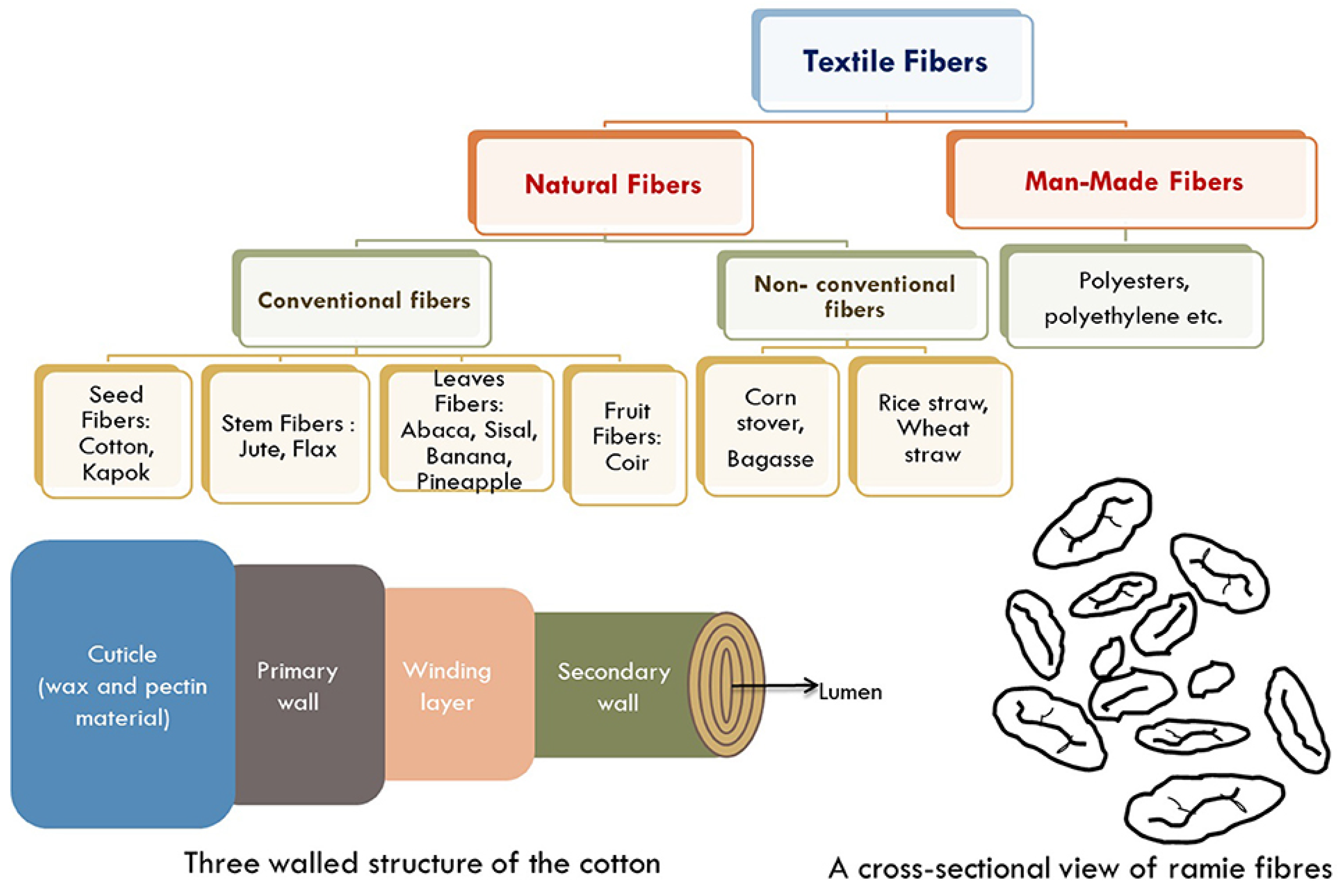
Fiber Regeneration from Textile Waste
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piribauer, B.; Bartl, A.; Ipsmiller, W. Enzymatic textile recycling–best practices and outlook. Waste Manag. Res. 2021, 39, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Aus, R.; Moora, H.; Vihma, M.; Unt, R.; Kiisa, M.; Kapur, S. Designing for circular fashion: Integrating upcycling into conventional garment manufacturing processes. Fash. Text. 2021, 8, 34. [Google Scholar] [CrossRef]
- Dissanayake, D.; Weerasinghe, D. Fabric waste recycling: A systematic review of methods, applications, and challenges. Mater. Circ. Econ. 2021, 3, 24. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A review on textile recycling practices and challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Pensupa, N.; Leu, S.Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Wang, H.; Lin, C.S.K. Recent trends in sustainable textile waste recycling methods: Current situation and future prospects. In Chemistry and Chemical Technologies in Waste Valorization; Springer: Cham, Switzerland, 2018; pp. 189–228. [Google Scholar]
- Abbas-Abadi, M.S.; Tomme, B.; Goshayeshi, B.; Mynko, O.; Wang, Y.; Roy, S.; Kumar, R.; Baruah, B.; De Clerck, K.; De Meester, S.; et al. Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types. Polymers 2025, 17, 628. [Google Scholar] [CrossRef]
- Enking, J.; Becker, A.; Schu, G.; Gausmann, M.; Cucurachi, S.; Tukker, A.; Gries, T. Recycling processes of polyester-containing textile waste–A review. Resour. Conserv. Recycl. 2025, 219, 108256. [Google Scholar] [CrossRef]
- Dev, B.; Rahman, M.A.; Tazrin, T.; Islam, M.S.; Datta, A.; Rahman, M.Z. Investigation of Mechanical Properties of Nonwoven Recycled Cotton/PET Fiber-Reinforced Polyester Hybrid Composites. Macromol. Mater. Eng. 2024, 309, 2400020. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M.; Kulikowska, D. The growing problem of textile waste generation—The current state of textile waste management. Energies 2024, 17, 1528. [Google Scholar] [CrossRef]
- Mu, B.; Yang, Y. Complete separation of colorants from polymeric materials for cost-effective recycling of waste textiles. Chem. Eng. J. 2022, 427, 131570. [Google Scholar] [CrossRef]
- Subramanian, K.; Chopra, S.S.; Cakin, E.; Li, X.; Lin, C.S.K. Environmental life cycle assessment of textile bio-recycling–valorizing cotton-polyester textile waste to pet fiber and glucose syrup. Resour. Conserv. Recycl. 2020, 161, 104989. [Google Scholar] [CrossRef]
- Hu, Y.; Du, C.; Leu, S.Y.; Jing, H.; Li, X.; Lin, C.S.K. Valorisation of textile waste by fungal solid state fermentation: An example of circular waste-based biorefinery. Resour. Conserv. Recycl. 2018, 129, 27–35. [Google Scholar] [CrossRef]
- Wani, N.A.; Mishra, U. Lifecycle assessment and electro-spinning technique for a sustainable fiber bottle production system with controllable waste and wastewater treatment. J. Clean. Prod. 2024, 443, 141026. [Google Scholar] [CrossRef]
- Kahoush, M.; Kadi, N. Towards sustainable textile sector: Fractionation and separation of cotton/polyester fibers from blended textile waste. Sustain. Mater. Technol. 2022, 34, e00513. [Google Scholar] [CrossRef]
- Yu, J.; Bai, L.; Feng, Z.; Chen, L.; Xu, S.; Wang, Y. Waste treats waste: Facile fabrication of porous adsorbents from recycled PET and sodium alginate for efficient dye removal. Chemosphere 2024, 355, 141738. [Google Scholar] [CrossRef]
- Samak, N.A.; Jia, Y.; Sharshar, M.M.; Mu, T.; Yang, M.; Peh, S.; Xing, J. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int. 2020, 145, 106144. [Google Scholar] [CrossRef]
- Djapovic, M.; Milivojevic, D.; Ilic-Tomic, T.; Lješević, M.; Nikolaivits, E.; Topakas, E.; Maslak, V.; Nikodinovic-Runic, J. Synthesis and characterization of polyethylene terephthalate (PET) precursors and potential degradation products: Toxicity study and application in discovery of novel PETases. Chemosphere 2021, 275, 130005. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Majumdar, A.; Shukla, S.; Singh, A.A.; Arora, S. Circular fashion: Properties of fabrics made from mechanically recycled poly-ethylene terephthalate (PET) bottles. Resour. Conserv. Recycl. 2020, 161, 104915. [Google Scholar] [CrossRef]
- Shukla, S.; Harad, A.M.; Jawale, L.S. Recycling of waste PET into useful textile auxiliaries. Waste Manag. 2008, 28, 51–56. [Google Scholar] [CrossRef]
- Grămadă, A.M.; Stoica, A.-E.; Niculescu, A.-G.; Bîrcă, A.C.; Vasile, B.Ș.; Holban, A.M.; Mihaiescu, T.; Șerban, A.I.; Ciceu, A.; Balta, C.; et al. Zinc Oxide-Loaded Recycled PET Nanofibers for Applications in Healthcare and Biomedical Devices. Polymers 2025, 17, 45. [Google Scholar] [CrossRef]
- Bian, X.; Xia, G.; Xin, J.H.; Jiang, S.; Ma, K. Applications of waste polyethylene terephthalate (PET) based nanostructured materials: A review. Chemosphere 2024, 350, 141076. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.S.; Kim, S.H. Dyeing and antibacterial properties of chemically recycled PET thermal-bonded nonwovens dyed with Terminalia chebula dye. Polymers 2020, 12, 1675. [Google Scholar] [CrossRef]
- Sadeghi, B.; Marfavi, Y.; AliAkbari, R.; Kowsari, E.; Borbor Ajdari, F.; Ramakrishna, S. Recent studies on recycled PET fibers: Production and applications: A review. Mater. Circ. Econ. 2021, 3, 4. [Google Scholar] [CrossRef]
- El Darai, T.; Ter-Halle, A.; Blanzat, M.; Despras, G.; Sartor, V.; Bordeau, G.; Lattes, A.; Franceschi, S.; Cassel, S.; Chouini-Lalanne, N.; et al. Chemical recycling of polyester textile wastes: Shifting towards sustainability. Green Chem. 2024, 26, 6857–6885. [Google Scholar] [CrossRef]
- Joseph, T.M.; Azat, S.; Ahmadi, Z.; Jazani, O.M.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethylene terephthalate (PET) recycling: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef]
- Boschmeier, E.; Ipsmiller, W.; Bartl, A. Market assessment to improve fibre recycling within the EU textile sector. Waste Manag. Res. 2024, 42, 135–145. [Google Scholar] [CrossRef]
- Vuorinen, J. Chemical Recycling of Polycotton Textiles: Fractionation of Cotton by TEMPO-Mediated Oxidation. Master’s Thesis, Aalto University, Espoo, Finland, 2024. [Google Scholar]
- Gergely, A. The Production of Polyethylene Terephthalate Nanofibers by Electrospinning with Minimum Amount of Trifluoroacetic Acid. Biomed. J. Sci. Tech. Res. 2020, 29, 22399–22401. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun polymer nanofibers: Processing, properties, and applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh-Mamaqani, M.; Razzaghi, D.; Roghani-Mamaqani, H.; Babaie, A.; Rezaei, M.; Hoogenboom, R.; Salami-Kalajahi, M. Photo-responsive electrospun polymer nanofibers: Mechanisms, properties, and applications. Prog. Mater. Sci. 2024, 146, 101312. [Google Scholar] [CrossRef]
- Esmaeili, E.; Deymeh, F.; Rounaghi, S.A. Synthesis and characterization of the electrospun fibers prepared from waste polymeric materials. Int. J. Nano Dimens. 2017, 8, 171–181. [Google Scholar]
- Grumezescu, A.M.; Stoica, A.E.; Dima-Bălcescu, M.Ș.; Chircov, C.; Gharbia, S.; Baltă, C.; Roșu, M.; Herman, H.; Holban, A.M.; Ficai, A.; et al. Electrospun polyethylene terephthalate nanofibers loaded with silver nanoparticles: Novel approach in anti-infective therapy. J. Clin. Med. 2019, 8, 1039. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The history of electrospinning: Past, present, and future developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Stoica, A.E.; Bîrcă, A.C.; Mihaiescu, D.E.; Grumezescu, A.M.; Ficai, A.; Herman, H.; Cornel, B.; Roșu, M.; Gharbia, S.; Holban, A.M.; et al. Biocompatibility and Antimicrobial Profile of Acid Usnic-Loaded Electrospun Recycled Polyethylene Terephthalate (PET)—Magnetite Nanofibers. Polymers 2023, 15, 3282. [Google Scholar] [CrossRef] [PubMed]
- Strain, I.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A 2015, 3, 1632–1640. [Google Scholar] [CrossRef]
- Naksuwan, P.; Sasithorn, N.; Komárek, M.; Salačová, J.; Militkỳ, J. Recycled poly (ethylene terephthalate) fibers from PET bottle via electro spinning. In Proceedings of the Workshop Světlanka 2015, Rokytnice nad Jizerou, Czech Republic, 22–25 September 2015. [Google Scholar]
- Hossain, M.T.; Shahid, M.A.; Ali, A. Development of nanofibrous membrane from recycled polyethene terephthalate bottle by electrospinning. OpenNano 2022, 8, 100089. [Google Scholar] [CrossRef]
- Mehdi, M.; Mahar, F.K.; Qureshi, U.A.; Khatri, M.; Khatri, Z.; Ahmed, F.; Kim, I.S. Preparation of colored recycled polyethylene terephthalate nanofibers from waste bottles: Physicochemical studies. Adv. Polym. Technol. 2018, 37, 2820–2827. [Google Scholar] [CrossRef]
- Kijeńska-Gawrońska, E.; Wiercińska, K.; Bil, M. The dependence of the properties of recycled PET electrospun mats on the origin of the material used for their fabrication. Polymers 2022, 14, 2881. [Google Scholar] [CrossRef]
- Keßler, L.; Matlin, S.A.; Kümmerer, K. The contribution of material circularity to sustainability—Recycling and reuse of textiles. Curr. Opin. Green Sustain. Chem. 2021, 32, 100535. [Google Scholar] [CrossRef]
- Ribul, M.; Lanot, A.; Pisapia, C.T.; Purnell, P.; McQueen-Mason, S.J.; Baurley, S. Mechanical, chemical, biological: Moving towards closed-loop bio-based recycling in a circular economy of sustainable textiles. J. Clean. Prod. 2021, 326, 129325. [Google Scholar] [CrossRef]
- Stefan, D.; Bosomoiu, M.; Stefan, M. Methods for Natural and Synthetic Polymers Recovery from Textile Waste. Polymers 2022, 14, 3939. [Google Scholar] [CrossRef]
- Solis, M.; Huygens, D.; Tonini, D.; Astrup, T.F. Management of textile waste in Europe: An environmental and a socio-economic assessment of current and future scenarios. Resour. Conserv. Recycl. 2024, 207, 107693. [Google Scholar] [CrossRef]
- Atkar, A.; Pabba, M.; Sekhar, S.C.; Sridhar, S. Current limitations and challenges in the global textile sector. In Fundamentals of Natural Fibres and Textiles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 741–764. [Google Scholar]
- Xujayev, I. Development Trends of the Global Textile Industry: A Comprehensive Analysis. Sci. J. Actuar. Financ. Account. 2024, 4, 165–170. [Google Scholar]
- Kim, E.H.; Lee, H. Comprehensive Review of Textile Waste Recycling: From Origins to Innovations. Fibers Polym. 2025, 26, 1449–1464. [Google Scholar] [CrossRef]
- Diriba, M.; Ghadai, S.K.; Misra, S.N. Ethiopia as a newly emerging global textile centre: A review. Korea 2019, 11, 132. [Google Scholar]
- Fahmy, A.E.; Mohammed, M.A.; Hassabo, A.G. Recycling and sustainability innovations in the textile industry. J. Text. Color. Polym. Sci. 2025, 22, 45–52. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Wang, B.; Li, P.; Zhu, J.; Ma, S. Closed-loop chemical recycling of thermosetting polymers and their applications: A review. Green Chem. 2022, 24, 5691–5708. [Google Scholar] [CrossRef]
- Ghosal, K.; Nayak, C. Recent advances in chemical recycling of polyethylene terephthalate waste into value added products for sustainable coating solutions–hope vs. hype. Mater. Adv. 2022, 3, 1974–1992. [Google Scholar] [CrossRef]
- Ambrus, M.; Mucsi, G. Open-loop recycling of end-of-life textiles as geopolymer fibre reinforcement. Waste Manag. Res. 2024, 42, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, S.; Thakur, A.; Tamakuwala, D.; Kumar, V.V.; Ramakrishna, S.; Chandran, S. Sustainable recycling of polymers: A comprehensive review. Polym. Bull. 2024, 81, 9569–9610. [Google Scholar] [CrossRef]
- Beghetto, V.; Sole, R.; Buranello, C.; Al-Abkal, M.; Facchin, M. Recent advancements in plastic packaging recycling: A mini-review. Materials 2021, 14, 4782. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Schwarz, A.; Ligthart, T.; Bizarro, D.G.; De Wild, P.; Vreugdenhil, B.; van Harmelen, T. Plastic recycling in a circular economy; determining environmental performance through an LCA matrix model approach. Waste Manag. 2021, 121, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Nikles, D.E.; Farahat, M.S. New motivation for the depolymerization products derived from poly (ethylene terephthalate)(PET) waste: A review. Macromol. Mater. Eng. 2005, 290, 13–30. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; Van Harmelen, T.; De Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Gao, B.; Sun, Y.; Lu, Q.; Qin, J.; Chen, M.; Li, X.; Yao, C.; Mao, L. Catalyst-Free Upcycling of Poly (ethylene terephthalate)(PET) Waste into Degradable PET-Based Engineering Plastics via the Solvothermal Method. ACS Sustain. Chem. Eng. 2025, 13, 4758–4767. [Google Scholar] [CrossRef]
- Jia, Z.; Gao, L.; Qin, L.; Yin, J. Chemical recycling of PET to value-added products. RSC Sustain. 2023, 1, 2135–2147. [Google Scholar] [CrossRef]
- Hossain, M.T.; Shahid, M.A.; Limon, M.G.M.; Hossain, I.; Mahmud, N. Techniques, applications, and challenges in textiles for sustainable future. J. Open Innov. Technol. Mark. Complex. 2024, 10, 100230. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Gbadeyan, O.J.; Sithole, B.; Chunilall, V. Recent advances in recycling technologies for waste textile fabrics: A review. Text. Res. J. 2024, 94, 508–529. [Google Scholar] [CrossRef]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Thachnatharen, N.; Shahabuddin, S.; Sridewi, N. The waste management of polyethylene terephthalate (PET) plastic waste: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1127, 012002. [Google Scholar] [CrossRef]
- Bezeraj, E.; Debrie, S.; Arraez, F.J.; Reyes, P.; Van Steenberge, P.H.; D’hooge, D.R.; Edeleva, M. State-of-the-art of industrial PET mechanical recycling: Technologies, impact of contamination and guidelines for decision-making. RSC Sustain. 2025, 3, 1996–2047. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for circular fashion: The logic behind recycling options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Moreno-Marrodán, C.; Brandi, F.; Barbaro, P.; Liguori, F. Advances in catalytic chemical recycling of synthetic textiles. Green Chem. 2024, 26, 11832–11859. [Google Scholar] [CrossRef]
- Altun, S.; Ulcay, Y. Improvement of waste recycling in PET fiber production. J. Polym. Environ. 2004, 12, 231–237. [Google Scholar] [CrossRef]
- Boondaeng, A.; Keabpimai, J.; Srichola, P.; Vaithanomsat, P.; Trakunjae, C.; Niyomvong, N. Optimization of textile waste blends of cotton and PET by enzymatic hydrolysis with reusable chemical pretreatment. Polymers 2023, 15, 1964. [Google Scholar] [CrossRef] [PubMed]
- Zanela, T.M.P.; Muniz, E.C.; Almeida, C.A.P. Chemical recycling of poly (ethylene terephthalate)(PET) by alkaline hydrolysis and catalyzed glycolysis. Orbital Electron. J. Chem. 2018, 10, 226–233. [Google Scholar]
- Lamberti, F. Studies on the Chemical Recycling of Poly (Lactic Acid) via Alcoholysis. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2023. [Google Scholar]
- Viana, M.E.; Riul, A.; Carvalho, G.M.; Rubira, A.F.; Muniz, E.C. Chemical recycling of PET by catalyzed glycolysis: Kinetics of the heterogeneous reaction. Chem. Eng. J. 2011, 173, 210–219. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Martínez-Vila, S.; Riera-Malgosa, L.; Prieto-Fuentes, R.; Duran-Serra, A.; Carrillo-Navarrete, F. Chemical recycling of polyester fabrics by alkaline hydrolysis using alcohols as cosolvents. Sustain. Chem. Pharm. 2025, 43, 101891. [Google Scholar] [CrossRef]
- Shukla, S.; Harad, A.M.; Jawale, L.S. Chemical recycling of PET waste into hydrophobic textile dyestuffs. Polym. Degrad. Stab. 2009, 94, 604–609. [Google Scholar] [CrossRef]
- Ndagano, U.N.; Cahill, L.; Smullen, C.; Gaughran, J.; Kelleher, S.M. The Current State-of-the-Art of the Processes Involved in the Chemical Recycling of Textile Waste. Molecules 2025, 30, 299. [Google Scholar] [CrossRef]
- Bharadwaj, C.; Purbey, R.; Bora, D.; Chetia, P.; Maheswari R, U.; Duarah, R.; Dutta, K.; Sadiku, E.R.; Varaprasad, K.; Jayaramudu, J.; et al. A Review on Sustainable PET Recycling: Strategies and Trends. Mater. Today Sustain. 2024, 27, 100936. [Google Scholar] [CrossRef]
- Lanz, I.E.; Laborda, E.; Chaine, C.; Blecua, M. A Mapping of Textile Waste Recycling Technologies in Europe and Spain. Textiles 2024, 4, 359–390. [Google Scholar] [CrossRef]
- Klaimy, S.; Lamonier, J.F.; Casetta, M.; Heymans, S.; Duquesne, S. Recycling of plastic waste using flash pyrolysis–Effect of mixture composition. Polym. Degrad. Stab. 2021, 187, 109540. [Google Scholar] [CrossRef]
- Ng, K.W.J.; Yu, E.; Hu, C.P.; Liang, Y.N.; Periasamy, K.; Chen, H.; Hu, X. Continuous Rapid Depolymerization Process to Upcycle Polyethylene Terephthalate into Polyols. ACS Sustain. Chem. Eng. 2025, 13, 4170–4181. [Google Scholar] [CrossRef]
- Tanguy, A.; Laforest, V. Does Textile Recycling Reduce Environmental Impact? A Probabilistic and Parametric Analysis for a Case of Open-Loop Recycling. In Novel Sustainable Alternative Approaches for the Textiles and Fashion Industry; Springer: Berlin/Heidelberg, Germany, 2023; pp. 75–92. [Google Scholar]
- El Achkar, E.; Frigerio, N. Production and changeover control of textile and PET recycling. J. Clean. Prod. 2024, 474, 143609. [Google Scholar] [CrossRef]
- Gritsch, S.M.; Mihalyi, S.; Bartl, A.; Ipsmiller, W.; Jenull-Halver, U.; Putz, R.F.; Quartinello, F.; Guebitz, G.M. Closing the cycle: Enzymatic recovery of high purity glucose and polyester from textile blends. Resour. Conserv. Recycl. 2023, 188, 106701. [Google Scholar] [CrossRef]
- Egan, J.; Wang, S.; Shen, J.; Baars, O.; Moxley, G.; Salmon, S. Enzymatic textile fiber separation for sustainable waste processing. Resour. Environ. Sustain. 2023, 13, 100118. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Almdal, K.; Meyer, A.S. Significance of poly (ethylene terephthalate) (PET) substrate crystallinity on enzymatic degradation. New Biotechnol. 2023, 78, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Boschmeier, E.; Mehanni, D.; Sedlmayr, V.L.; Vetyukov, Y.; Mihalyi, S.; Quartinello, F.; Guebitz, G.M.; Bartl, A. Recovery of pure PET from wool/PET/elastane textile waste through step-wise enzymatic and chemical processing. Waste Manag. Res. 2024, 43, 0734242X241276089. [Google Scholar] [CrossRef]
- Yalcin-Enis, I.; Kucukali-Ozturk, M.; Sezgin, H. Risks and management of textile waste. In Nanoscience and Biotechnology for Environmental Applications; Springer: Cham, Switzerland, 2019; pp. 29–53. [Google Scholar]
- Sanjrani, M.A.; Gang, X.; Mirza, S.N.A. A review on textile solid waste management: Disposal and recycling. Waste Manag. Res. 2025, 43, 522–539. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M. Sustainable management of textile solid waste materials: The progress and prospects. Mater. Today Proc. 2022, 62, 3320–3324. [Google Scholar] [CrossRef]
- Patel, M.; Sahu, A.; Rajak, R. Solid waste management in textile industry. In Handbook of Solid Waste Management: Sustainability through Circular Economy; Springer: Singapore, 2022; pp. 1225–1256. [Google Scholar]
- McCauley, E.; Jestratijevic, I. Exploring the business case for textile-to-textile recycling using post-consumer waste in the US: Challenges and opportunities. Sustainability 2023, 15, 1473. [Google Scholar] [CrossRef]
- Vaishali, M.; Gopal, S.; Sreeram, K.J. A facile approach towards recycling of polyurethane coated PET fabrics. RSC Sustain. 2024, 2, 2324–2334. [Google Scholar] [CrossRef]
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Huang, X.; Tan, Y.; Huang, J.; Zhu, G.; Yin, R.; Tao, X.; Tian, X. Industrialization of open- and closed-loop waste textiles recycling towards sustainability: A review. J. Clean. Prod. 2024, 436, 140676. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, S.K. Eco-Sustainability of the Textile Production: Waste Recovery and Current Recycling in the Composites World. Dogo Rangsang Res. J. 2021, 14, 1916–1919. [Google Scholar]
- Sharma, N.; Allardyce, B.; Rajkhowa, R.; Adholeya, A.; Agrawal, R. A substantial role of agro-textiles in agricultural applications. Front. Plant Sci. 2022, 13, 895740. [Google Scholar] [CrossRef] [PubMed]
- Tamta, M. Textile Fibers: Production, Classification, Properties, Morphology and Care. In Basics of Community Science; Elite Publishing House: New Delhi, India, 2024; p. 110. [Google Scholar]
- Luo, L.B.; Chen, R.; Lian, Y.X.; Wu, W.J.; Zhang, J.H.; Fu, C.X.; Sun, X.L.; Xiao, L.R. Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement. Polymers 2024, 16, 1052. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Sun, X.L.; Chen, Q.; Qian, Q. Recycled poly (ethylene terephthalate) from waste textiles with improved thermal and rheological properties by chain extension. Polymers 2022, 14, 510. [Google Scholar] [CrossRef]
- Aydemir, H.; Demiryürek, O. The effect of electrospinning parameters on morphology and diameter of polyethylene terephthalate (PET) and recycled polyethylene terephthalate (r-PET) nanofibers. J. Text. Inst. 2023, 114, 1443–1454. [Google Scholar] [CrossRef]
- Stanescu, M.D. State of the art of post-consumer textile waste upcycling to reach the zero waste milestone. Environ. Sci. Pollut. Res. 2021, 28, 14253–14270. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty years of microplastic pollution research—What have we learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Zinchenko, A. Conversion of waste bottle PET to magnetic microparticles adsorbent for dye-simulated wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 108055. [Google Scholar] [CrossRef]
- Mu, B.; Yu, X.; Shao, Y.; McBride, L.; Hidalgo, H.; Yang, Y. Complete recycling of polymers and dyes from polyester/cotton blended textiles via cost-effective and destruction-minimized dissolution, swelling, precipitation, and separation. Resour. Conserv. Recycl. 2023, 199, 107275. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1. [Google Scholar] [CrossRef]
- Nandi, S.; Mahish, S.S.; Das, S.K.; Datta, M.; Nath, D. A review of various recycling methods of PET waste: An avenue to circularity. Polym.-Plast. Technol. Mater. 2023, 62, 1663–1683. [Google Scholar] [CrossRef]
- Enache, A.C.; Grecu, I.; Samoila, P. Polyethylene terephthalate (PET) recycled by catalytic glycolysis: A bridge toward circular economy principles. Materials 2024, 17, 2991. [Google Scholar] [CrossRef]
- Muthu, S.S. Environmental Footprints of Recycled Polyester; Springer: Singapore, 2020. [Google Scholar]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.S. Possibility routes for textile recycling technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef]
- Mu, B.; Shao, Y.; McBride, L.; Hidalgo, H.; Yang, Y. Rapid fiber-to-fiber recycling of poly (ethylene terephthalate) and its dye from waste textiles without damaging their chemical structures. Resour. Conserv. Recycl. 2023, 197, 107102. [Google Scholar] [CrossRef]
- Fei, X.; Freeman, H.S.; Hinks, D. Toward closed loop recycling of polyester fabric: Step 1. decolorization using sodium formaldehyde sulfoxylate. J. Clean. Prod. 2020, 254, 120027. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Roungpaisan, N.; Srisawat, N.; Rungruangkitkrai, N.; Chartvivatpornchai, N.; Boonyarit, J.; Kittikorn, T.; Chollakup, R. Effect of recycling PET fabric and bottle grade on r-PET fiber structure. Polymers 2023, 15, 2330. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Deng, Y.; Ye, F.; Zhang, C.; Hu, X.; Liu, Y.; Zhang, D. Recycling and reutilizing polymer waste via electrospun micro/nanofibers: A review. Nanomaterials 2022, 12, 1663. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Hinestroza, J.P.; Uyar, T. Structural investigation on electrospun nanofibers from postconsumer polyester textiles and PET bottles. ACS Appl. Polym. Mater. 2023, 5, 7298–7307. [Google Scholar] [CrossRef]
- Ko, Y.; Hinestroza, J.P.; Uyar, T. Electrospun nanofibrous membranes from discarded polyester textiles for oil sorption. ACS Appl. Polym. Mater. 2024, 6, 8798–8810. [Google Scholar] [CrossRef]
- Nakanishi, M.; Chan, K.; Zinchenko, A. Upcycling of waste PET fibers and fabrics via surface engineering: Surface aminolysis-assisted functionalization with catalytic nanoparticles. Chem. Eng. J. 2025, 511, 161839. [Google Scholar] [CrossRef]
- Santomasi, G.; Todaro, F.; Petrella, A.; Notarnicola, M.; Thoden van Velzen, E.U. Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content. Recycling 2024, 9, 93. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohtaram, F.; Fojan, P. From Waste to Value: Advances in Recycling Textile-Based PET Fabrics. Textiles 2025, 5, 24. https://doi.org/10.3390/textiles5030024
Mohtaram F, Fojan P. From Waste to Value: Advances in Recycling Textile-Based PET Fabrics. Textiles. 2025; 5(3):24. https://doi.org/10.3390/textiles5030024
Chicago/Turabian StyleMohtaram, Fatemeh, and Peter Fojan. 2025. "From Waste to Value: Advances in Recycling Textile-Based PET Fabrics" Textiles 5, no. 3: 24. https://doi.org/10.3390/textiles5030024
APA StyleMohtaram, F., & Fojan, P. (2025). From Waste to Value: Advances in Recycling Textile-Based PET Fabrics. Textiles, 5(3), 24. https://doi.org/10.3390/textiles5030024







