Abstract
The electrokinetic properties of materials give useful insight into the behavior of surfaces in contact with liquids and other compounds and their quantification is a powerful tool to predict their behavior during further processing and application, especially in textile materials. In this work, we perform a comparative analysis of influence of the two most common selective oxidative protocols for viscose (regenerated cellulose) fabrics on subsequent functionalization with chitosan, and cellulose fabrics’ electrokinetic properties, zeta potential in a pH range of approx. 3–10, and isoelectric point (IEP). For oxidation before deposition of chitosan, sodium periodate and 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO) were used. The content of functional groups in oxidized cellulose fabric (carboxyl and carbonyl groups) was determined by titration methods, while amino functional groups’ availability in samples with chitosan was determined using the CI acid orange 7 dye absorption method. This study reveals that the periodate oxidation (PO) of cellulose is more effective for binding chitosan onto material, which gave rise to higher availability of amino groups onto cellulose/chitosan material, which also influenced the shift in zeta potential curve towards positive values at a pH below 5. Analysis of a relationship between zeta potential increase at pH 4.4 and amino groups’ amount measured using absorption of CI acid orange 7 dye at pH 4.4 revealed dependency that can be fitted linearly or exponentially, with the latter providing the better fit (R2 = 0.75).
1. Introduction
Oxidation of cellulose is a very common procedure for modification of cellulosic material [1]. In the textile sector, oxidation is usually performed to remove impurities and organic contaminants from textile material, but also to functionalize the surface of fibers, and introduce new functionalities. Cellulose, as a polysaccharide with three hydroxyl (OH) groups on each anhydroglucose unit (AGU), is very susceptible to oxidation and transformation of these groups to other moieties. The reactivity of OH groups is high, and cellulose can be transformed into many other derivatives [2,3,4,5]. In oxidized cellulose, OH groups are oxidized to carbonyl (aldehyde) and/or carboxyl groups depending on the oxidative agent used and conditions of oxidation.
Among various derivatives, oxycellulose stands out due to its very special properties, being bioresorbable, biocompatible, and even hemostatic (depending on the degree of oxidation DO), and can be further functionalized because of carboxyl and carbonyl groups, which are reactive and can be considered as “chemical hooks” to further bind additional compounds [1].
Several highly effective protocols have been established for cellulose oxidation, by using agents that can be classified into two main groups, selective and non-selective [6]. Selective agents target specific OH groups (primary or secondary), while non-selective agents can oxidize primary and/or secondary OH to various degrees, depending on the processing conditions (concentration, pH, and temperature). Naturally, selective oxidative agents are easier to control whereby final product concentration (quantity of functional groups) can be easily predicted, while side reactions can be minimized. The degree of oxidation influences the reactivity and solubility of the derivative and can be tailored according to the final application. From the aspect of selective oxidation for cellulose functionalization, periodate oxidation (PO) and 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO) oxidation in particular stand out [6].
Periodate oxidation is most frequently performed utilizing sodium periodate [7,8,9,10] or potassium periodate [11,12,13]. The final product is dialdehyde cellulose [7,9,14,15,16] whereby OH groups on the C2 and C3 position of AGU are transformed to aldehydes, which is accompanied by ring cleavage that usually does not lead to damage of cellulose macromolecules [17,18]. These aldehyde groups on the C2 and C3 atoms in AGU unit of cellulose can serve as binding sites to form a Schiff’s base with amino groups, as has been shown, for example, in oxidized cellulose with immobilized enzyme [18].
Opposite to periodate oxidation (PO), during TEMPO oxidation, the OH group on C6 is transformed to the COOH group. In traditional TEMPO oxidation, a TEMPO/NaBr/NaClO system in water is used, at room temperature and pH 10. Unfortunately, this approach of TEMPO oxidation under basic conditions leads to a significant depolymerization of cellulose macromolecules [19]. The alternative approach, a TEMPO/NaClO/NaClO2 system in water at elevated temperatures and weakly acidic or neutral pH, offers the possibility to avoid the β-elimination reaction that takes place in an alkaline environment and depolymerization, and it is receiving increased research attention [20]. Regardless of the applied approach (TEMPO/NaBr/NaClO system in water at room temperature and pH 10 or TEMPO/NaClO/NaClO2 system in water at elevated temperatures and weakly acidic or neutral pH), TEMPO oxidation provides cellulose with COOH groups on C6 position, while a certain number of aldehyde groups can also be present depending on the degree of oxidation. This has the consequence of changing the sorption properties of cellulose and its reactivity. The introduction of acidic carboxyl groups in cellulose can lead to an increase in sorption properties and wettability and also increased reactivity of carboxylated cellulose. On another hand, significant inclusion of aldehyde groups into cellulose during periodate oxidation, and specifically viscose as regenerated cellulose fiber, can lead to a decrease in moisture sorption and water retention [10,21] since hydrophilic OH groups are being converted to less polar aldehyde moieties.
Changes in functional groups and sorption properties can also have an impact on the electrokinetic properties. The electrokinetic properties, isoelectric point (IEP), and zeta potential value are important for predicting the behavior of the surface of material in a contact with the liquids [22]. It is also useful to monitor the changes during oxidation of cellulose and the addition of various compounds [10,23]. Swelling of fibers and improvement of their interaction with water have been known to cause a reduction in zeta potential in the alkaline pH region, so called the zeta plateau region [24]. Furthermore, reportedly, in viscose oxidized with periodate and further converted to dicarboxyl cellulose, the curve becomes more distorted with the increase in COOH groups in it [10].
Frequently, oxidation of cellulose is accompanied by further functionalization, to obtain functional fibers with different properties, most frequently antimicrobial. In this sense, the most frequent route for cellulose functionalization is oxidation and the addition of a compound that has special properties, such as, for example, antimicrobial and sorption properties [25,26,27,28]. Chitosan is a compound of choice to incorporate into cellulose material, since it is biodegradable, natural, and a biocompatible polymer [29,30,31]. It is considered that due to its positive surface charge in contact with liquids, chitosan can interact with the cell walls of bacteria and disrupt their growth thus providing an antimicrobial effect [31]. Also, chitosan as an efficient adsorbent can provide cellulose the functionality of improved sorption of various aqueous pollutants [32,33,34,35,36]. Therefore, it would be beneficial to elucidate the influence of pretreatments and chitosan addition onto cellulose fibers’ zeta potential at certain pH. Previously, it has been shown that the addition of chitosan can cause a shift in zeta potential–pH curve of viscose fabric and that TEMPO oxidation causes changes in zeta potential [37]; furthermore, periodate oxidation can also cause the difference in zeta potential [10] depending on the oxidation parameters. But from using different oxidative protocols, and at different conditions, as well as different conditions for chitosan impregnation, it is hard to conclude what influence the certain type of oxidation and chitosan addition on the zeta potential of cellulose fibers has.
In the presented work, we perform a comparative analysis of the influence of carbonyl and carboxyl group content on the electrokinetic properties of viscose (regenerated cellulose) oxidized using two protocols, periodate and TEMPO oxidation, tailored in such a way that samples contain increased total group content, but also carbonyl and carboxyl group content separately. The periodate oxidation serves to transform the hydroxyl groups of cellulose to aldehyde, and introduced aldehyde groups on C2 and C3 carbons in the glucopyranose ring could serve as binding sites for amino groups of chitosan. On another hand, TEMPO oxidation can also improve sorption properties and provide a certain amount of aldehyde groups as well; therefore, the comparison between these two oxidation approaches for the ability to further bind chitosan can be useful for choosing the right protocol to prepare cellulose for chitosan functionalization. In this work, we analyze the deposition of chitosan onto viscose oxidized with both protocols, and we study which oxidative protocol produces viscose that has a higher affinity toward chitosan. This affinity towards chitosan and ultimately production of functionalized cellulose with positively charged groups at a certain pH is assessed through the determination of amino functional group availability in functionalized viscose. Finally, we assess the influence of all these functionalization protocols (oxidation followed by chitosan deposition) on the change in zeta potential, which could help predict properties of functionalized cellulose fibers in contact with other compounds (such as dyes, proteins, enzymes, etc.). In this work, we have tested the dye absorption (CI acid orange 7) of functionalized cellulose with chitosan to assess the availability of amino groups originating from chitosan and, at the same time, to establish if the adsorption of the dye at a certain pH is in correlation with the change in zeta potential of functionalized viscose at that same pH.
2. Materials and Methods
2.1. Materials
Sodium phosphate dibasic (Sigma Aldrich, Saint Louis, MO, USA, p.a. grade), monopotassium phosphate (Carlo Erba, Emmendingen, Germany, p.a. grade), sodium chlorite (Sigma Aldrich, p.a. grade), 13% sodium hypochlorite (Sigma Aldrich, p.a. grade), TEMPO (Sigma Aldrich, p.a. grade), sodium periodate (Acros Organic, Geel, Belgium, 99% p.a.), glacial acetic acid (ZorkaPharm, Šabac, Serbia), sodium acetate (Centrohem, Stara Pazova, Serbia), Orange II Sodium salt (CI acid orange 7, Carl Roth, Karlsruhe, Germany), chitosan (powder, low molecular weight, Sigma Aldrich), acetone (Sigma Aldrich, reagent grade, 99.5%), sodium hydroxide (pellets), 0.1 M HCl solution and 0.1 M NaOH, KCl, calcium acetate (Centrohem, Serbia), phenolphthalein (Kemika, Zagreb, Croatia), standard buffer solutions pH 4, pH 7 (Reagecon, Shannon, Ireland), and pH 10 (Hanna Instruments, Leighton Buzzard, England) were used for experiments as received, without further purification.
Viscose (regenerated cellulose-CV) fabric was provided by IGR Agence, Slovenia and used in experiments. The cellulose fabric structure was plain weave, surface mass—80 g m−2, yarn fineness (warp/weft)—9.6/9.9 tex, warp/weft count—40/35 cm−1 [38]. The chemical composition of viscose was determined using methods described in the literature [39,40]. Briefly, the content of α-cellulose (95.1%) was determined as a difference between starting and residual mass of fabric after treating the fabric with 10% NaOH, while the content of hemicelluloses (4.9%) was determined after treating the fabric with 17.5% NaOH quantitatively by titration of the filtrate.
2.2. Methods
2.2.1. Oxidations
Periodate oxidation was implemented according to the method described in the literature [10,18]. Briefly, viscose fabric (10 g) was oxidized in the dark in a shaker, at room temperature, using 0.4% (w/v) NaIO4 in an acetic (pH 4.0) buffer with material-to-liquid ratio 1:50, w/v. The oxidation with periodate lasted for 60, 120, and 240 min, and quenching the oxidation was performed by washing the sample with 10-fold volume of ice-cold (0–5 °C) distilled water.
TEMPO oxidation was performed according to the method described in the literature [37,41,42]. The oxidation was conducted using 10 g of viscose material, under stirring, at 60 °C, using TEMPO (20 mg TEMPO/g viscose fabric), sodium chlorite (50 mg NaClO2/g viscose fabric), and sodium hypochlorite solution (2.5 mmol NaClO/g viscose fabric) in 0.05 M sodium phosphate (pH 7.0) buffer with material-to-liquid ratio 1:50, w/v. The oxidation with TEMPO lasted for 2.5, 5, and 10 min, aimed at introducing similar content of aldehyde groups in samples, as with periodate oxidation, and to obtain increasing content of total amount of functional groups. At the end of designated time, quenching the oxidation was performed with 5 mL of ethanol of room temperature. TEMPO-oxidized viscose fabric was thoroughly washed with distilled water by filtration, then with ethanol, and finally dried at room temperature.
2.2.2. Dissolution and Deposition of Chitosan onto Viscose
Chitosan (CS) was prepared as 1% solution in 2% acetic acid, and chitosan deposition was performed following a method described previously [38]. Briefly, chitosan was dissolved in acetic acid at 60 °C during total of 120 min, until clear solution was obtained.
Viscose fabric (6 g) was added to chitosan solution (1:50 material-to-liquid ratio) and after 60 min at 60 °C, samples were washed with cold distilled water (10–15 °C), and dried in an oven at 60 °C for 30 min.
2.2.3. Moisture Sorption
Moisture sorption was determined using Sartorius Infrared moisture analyzer MA35. For each sample, moisture sorption was determined in duplicate.
2.2.4. Determination of Carboxyl Group Content
Standard volumetric calcium acetate method was used for carboxyl group measurement [10,43]. Briefly, viscose is first treated with 0.01 M HCl for 60 min and after that time 50 mL of water and 30 mL of 0.25 M Ca(CH3COO)2 are added to samples. After 120 min of reaction, an aliquot of 30 mL is titrated with 0.01 M NaOH using phenolphthalein. The carboxyl group content is calculated using Equation (1):
where c(NaOH) is the concentration of NaOH, V(NaOH) is the volume of NaOH used for titration, and ms is the weight of absolutely dry sample (g).
The volumetric test was performed in triplicate. The standard deviation between measurements was up to 5%.
2.2.5. Determination of Carbonyl Group Content
The carbonyl groups in cellulose were determined following a method described previously [43] whereby first carbonyl groups are selectively oxidized to carboxyl using NaClO2, and the carboxyl group content was determined volumetrically as described above. The carbonyl group content is calculated by subtracting the carboxyl content in the sample before and after conversion with chlorite.
2.2.6. CI Acid Orange 7 Test for Amino Groups of Chitosan
CI acid orange 7 test was performed according to the procedure described in the literature [44,45] with slight modification. The adsorption of dye CI acid orange 7 was evaluated by determining the dye concentration in solutions at pH 4.4 after 3 h of contact with the samples, using a UV-1700 PharmaSpec spectrophotometer, SHIMADZU (JA).
2.2.7. Zeta Potential Measurement
The zeta potential of viscose fabric was determined by the streaming potential method using a SurPASS Electrokinetic Analyzer (Anton Paar GmbH, Graz, Austria). Sample was placed in the cylindrical cell, thereby forming a permeable plug. The reproducible packing density of the fabric plug was maintained by monitoring the sample size and weight and controlling the sample compression in the cylindrical cell. Before measurement, samples were pre-swelled in distilled water for 30 min. The electrolyte solution was 1 mM KCl while the initial pH was adjusted to pH 10 with NaOH. Changes in the pH (from pH 10 to pH 3) were achieved by automatic titrations with 0.05 M HCl. Since the ionic strength of an electrolyte solution during measurement in a low pH region can interfere with the zeta potential [46], measurements were carried down to pH 3. Consequently, isoelectric points (IEP) lower than 3 were determined by extrapolation of experimental data. The average value of zeta potential was calculated based on four measurements and standard deviation was up to 5%.
3. Results
3.1. Carbonyl and Carboxyl Group Content in Oxidized Samples
Oxidation of cellulose is performed to change functional groups of viscose fibers, introduce aldehyde groups, promote better binding of chitosan through aldehyde groups, increase total functional group content, and improve sorption properties. These functional groups can influence many properties of materials but also can serve as binding sites for further functionalization. In this work, we designed experiments to achieve a certain degree of similarity between differently oxidized samples, even though the oxidation used is completely different, in terms that they transform OH groups at different positions, as stated in the Introduction section. This was achieved by varying the time of oxidations, while other parameters used within the same oxidation were kept constant. As can be seen in Table 1, for periodate oxidation (PO), a much longer time was used compared to TEMPO oxidation.

Table 1.
Sample marks with conditions of oxidation.
As is known, TEMPO oxidation that is carried out in a TEMPO/NaClO/NaClO2 system in water at elevated temperatures (60 °C) and weakly acidic or neutral pH is very intensive and requires careful design of oxidation parameters, especially since it tends to lead to a high speed of conversion and degradation. However, for short oxidation time, cellulose degradation is minor [37]; therefore, the major changes to the cellulose fibers are reflected in changes in the functional group. In Figure 1, the functional groups’ content in differently oxidized cellulose fibers is presented.
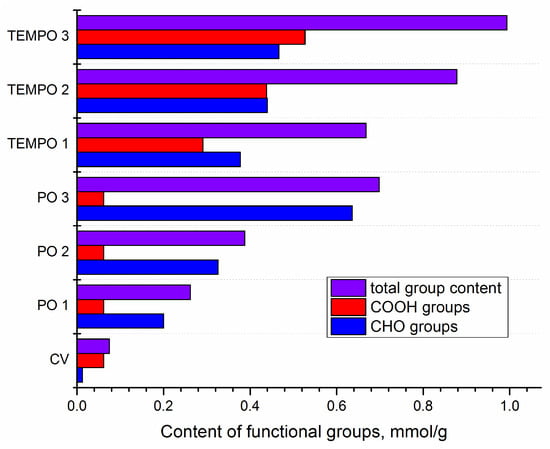
Figure 1.
Content of carbonyl (CHO), carboxyl (COOH), and total content of functional groups in differently oxidized viscose (cellulose) fibers using TEMPO or periodate oxidation (PO).
As can be seen, various contents of functional groups are introduced by varying oxidation parameters (agent and time). The most interesting result is that the total group content in TEMPO-oxidized viscose for only 2.5 min is almost the same as in periodate-oxidized cellulose for 240 min, i.e., 4 h (samples PO 3 and TEMPO 1). Also, the percentage of carbonyl groups in PO 3 and TEMPO 1 is 91.1% and 56.5% of the total group content, respectively. By increasing the duration of TEMPO oxidation, the carboxyl group content increases significantly (1.5× increase for each time step) while carbonyl group content remains almost the same, i.e., changes only slightly.
In periodate-oxidized samples, the situation with type of group is the opposite; carboxyl group content does not change but only carbonyls do. In terms of only carbonyl group content, similar results are obtained by using TEMPO oxidation for 2.5 min or periodate oxidation for 120 min (samples TEMPO 1 and PO 2, respectively). If we look at those results from the aspect of only the amount of functional groups, we could conclude that TEMPO-oxidized fibers could be more suitable for binding chitosan (since it is assumed that chitosan will react with carbonyl groups to form a Schiff’s base with aldehyde moiety in oxidized cellulose as one of the possible binding mechanisms [37,38,47]).
3.2. Amino Group Content in Samples Functionalized with Chitosan
After oxidation, chitosan was added to all samples. To quantitively assess the availability of amino groups of chitosan on cellulose fibers, we determined the content of amino groups via absorption of acid orange dye at pH 4.4, according to a modified protocol [44], and the results are presented in Figure 2.
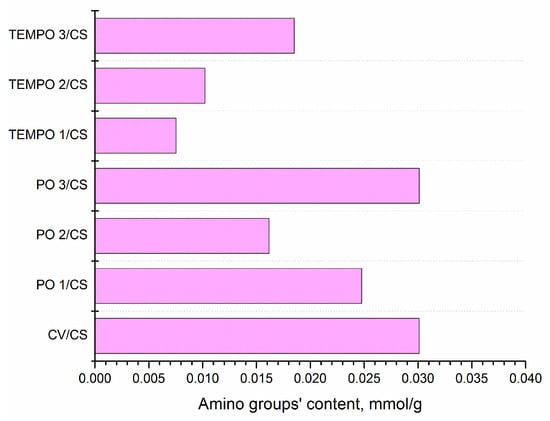
Figure 2.
Content of available amino groups in differently oxidized viscose fibers using TEMPO or periodate oxidation (PO) and functionalized with 1% chitosan solution.
As can be seen from Figure 2, untreated viscose has almost the same amount of available amino groups as the one oxidized with periodate during 240 min, while other samples have a lower amount of available amino groups compared with the starting material. The reason for this could come from two facts, which are not mutually exclusive. The first fact is that introduced functional groups (carboxyl or carbonyl) in cellulose help immobilize the amino groups of chitosan [47], and that is the reason why we cannot detect available amino groups with dye absorption tests. In other words, amino groups of chitosan are involved in electrostatic and covalent interactions with introduced functional groups (carboxyl and carbonyl) in cellulose [47] and cannot be detected by dyeing test. The second fact is that cellulose–chitosan interactions also involve the hydroxyl groups of cellulose and chitosan [38], which participate in hydrogen bonding as one of the dominant mechanisms in cellulose–chitosan interactions. After hydrogen bonding between the hydroxyl groups of cellulose and chitosan, the amino groups of chitosan remain available. If the content of carboxyl and carbonyl groups is increased through oxidation (and at the same time hydroxyl groups’ content decreases), the hydrogen bonding switches to the formation of electrostatic and covalent interactions, which results in a lower amount of available amino groups of chitosan [48]. Nevertheless, cellulose and chitosan most probably react through various mechanisms at the same time, but which one will be dominant depends on the amount but also availability of functional groups.
Another interesting result should be highlighted, which is the decrease in amino group content in TEMPO-oxidized cellulose fibers, as seen in Figure 2, accompanied by the significant decrease in moisture sorption when using short times of oxidation, 2.5 and 5 min, and the addition of chitosan (Figure 3b). This could be a direct consequence of the fast conversion of OH groups in cellulose and the interaction of groups of cellulose and chitosan.
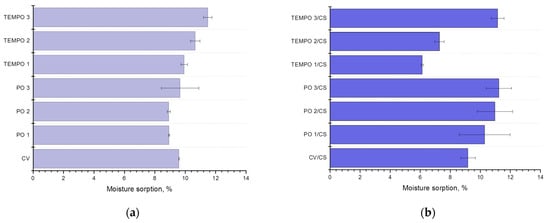
Figure 3.
Moisture sorption of differently oxidized (TEMPO or periodate oxidation (PO)) (a) and chitosan (CS) functionalized cellulose materials (b).
After oxidation and the addition of chitosan, moisture sorption of periodate-oxidized viscose gradually increases with the increase in oxidation time, while in TEMPO-oxidized and chitosan functionalized samples, moisture sorption is decreased by almost 30% compared to pristine viscose but reaches a high value after 10 min of oxidation and chitosan functionalization. This high increase can be a consequence of carboxyl group content in oxidized cellulose, especially using the longest treatment time. Regarding moisture sorption, it is obvious that when cellulose is functionalized with chitosan, functional groups from both components contribute to overall interactions with moisture [27].
3.3. Zeta Potential Analysis of Oxidized and Functionalized Samples
In the Introduction, it was stated that zeta potential can be a powerful tool to predict materials’ behavior at a certain pH through possible electrostatic interactions that depend on the electrostatic attraction between oppositely charged surfaces. Therefore, it is useful to study the zeta potential of the surfaces that are functionalized with chitosan to predict the behavior at a certain pH. Figure 4 represents zeta potential curves for viscose oxidized with periodate (a) and TEMPO-oxidized samples (b). Obtained curves were similar as previously reported for both periodate- and TEMPO-oxidized cotton and viscose (natural and regenerated cellulose) [10,23].
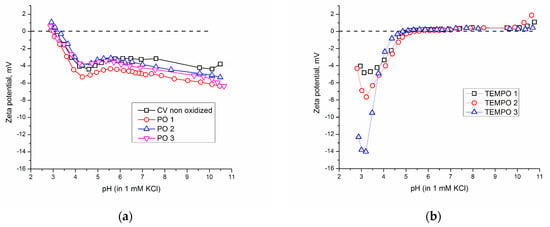
Figure 4.
Zeta potential as a function of pH in 1 mM KCl electrolyte solution for samples oxidized with (a) periodate (PO) and (b) TEMPO.
In periodate-oxidized samples, the zeta potential curve is shifting towards more negative values compared to untreated viscose. This shift in the zeta potential curve at alkali pH can be a consequence of the introduction of less hydrophilic aldehyde groups than hydroxyl groups [24]. The shift in the zeta potential curve towards more negative values of zeta potential at alkaline pH can be an indicator of a decreasing trend of hydrophilicity in materials. Opposite to this, it is also known that the shift in the zeta potential curve towards more negative values of zeta potential at alkaline pH indicates an increase in hydrophilicity in samples [24].
In Figure 4b, the zeta potential curves of TEMPO-oxidized viscose samples are significantly distorted towards 0 mV (0 mV is presented in the graph as a dashed line), as reported previously [10,23], and at pH higher than 5 even have positive values. Because the zeta potential values at alkaline pH should be more negative in the case of higher carboxyl group density at the viscose surface, the obtained less negative zeta potential values for the TEMPO-oxidized viscose fabrics, compared to CV, can be explained by three reasons [37]. The first reason is that deprotonated carboxyl groups can prevent the dissociation of neighboring carboxyl groups by electrostatic repulsion. The second reason is that TEMPO-oxidized viscose fabrics have higher hydrophilicity and swelling than CV. Namely, with increased swelling, the ratio between the maximum negative and plateau value of the zeta potential (ζmax/ζplateau) increases [46]. And finally, the third reason is related to the phenomenon called charge reversal, which is attributed to strongly adsorbed sodium ions [49], present in TEMPO-oxidized viscose fabrics as counter-ions to carboxyl groups.
Related to IEP, after TEMPO oxidation, IEP decreases with increasing time of oxidation, from pH 3.1 for untreated viscose to pH 2 for the TEMPO 3-oxidized sample. Considering that the measurement of zeta potential was performed from pH 3 to pH 10, the values of zeta potential below pH 3 are obtained by extrapolation of experimental values. This shift in IEP toward an acidic value is a confirmation of the introduction of carboxyl groups that have acidic nature. Contrary to this, in periodate-oxidized samples, IEP is not significantly affected by oxidation; it remains around pH 3 ± 0.2, which proves that aldehyde groups cannot contribute to the acidic surface charge in cellulose.
The addition of chitosan, which has a positive pH range around pH 7 and below, had a significant influence and caused a shift in zeta potential curves of oxidized samples as well as isoelectric points (Figure 5), as reported previously in oxidized cellulose [37]. The change in IEP after coating/addition of chitosan has been reported as well for inorganic Fe3O4 nanoparticles coated with chitosan [50] and in packaging foils from synthetic polymers [51].
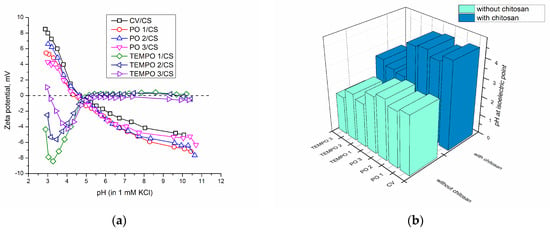
Figure 5.
(a) Zeta potential as a function of pH in 1 mM KCl electrolyte solution for samples functionalized with chitosan; (b) isoelectric point (IEP) of samples with and without chitosan oxidized with periodate (PO) and TEMPO.
In this work, chitosan caused a shift in the zeta potential curves of untreated (non-oxidized viscose) and periodate-oxidized samples, and their IEPs are approx. at pH 4.5 ± 0.2, which is within measurement error. It appears that pretreatment with periodate does not influence the IEP and that IEP of periodate oxidized and non-oxidized viscose with deposited chitosan are similar. On another hand, the addition of chitosan onto TEMPO-oxidized viscose caused the decrease in the maximum negative value of the zeta potential, especially in the TEMPO 3 sample (oxidized using the longest time, 10 min) from approx. −14 mv to approx. −4 mV. Moreover, as seen in Figure 5b, the longer the TEMPO oxidation and addition of chitosan, the higher the IEP, which, in the case of these samples, is around pH 2.8 ± 0.2, compared to only TEMPO-oxidized, whose IEP was between pH 2 and pH 2.5.
If we consider results that present a total shift in IEP towards higher pH and if the aim is the shift in IEP (and overall zeta potential curve shift), periodate oxidation as pretreatment may be superior to TEMPO oxidation. If we compare TEMPO 1 and PO 2 samples with the same amount of carbonyl groups, and if we observe the amount of chitosan through the amount of available amino groups (Figure 2), it is obvious that the affinity of periodate-oxidized viscose towards chitosan is higher. Sample PO 2 also gives the highest shift in IEP after the addition of chitosan, which means that it not only bonded more chitosan after oxidation but also that groups in chitosan are more available, giving rise to IEP.
The question remains, is it possible to predict exactly how much the zeta potential curve will shift after a certain amount of chitosan is added to cellulose? In an attempt to bring us closer to the answer, we plotted the measured zeta potential at pH 4.4 with results of available amino groups from the test with CI acid orange 7 dye (Figure 6). Undoubtedly, the value of zeta potential at a certain pH increases as the number of available amino groups obtained by the dye test increases as well.
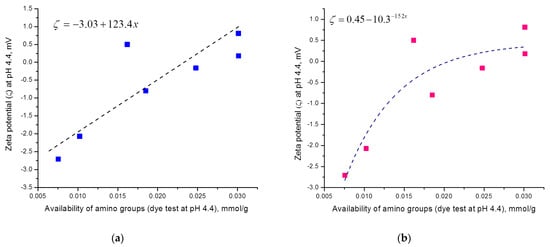
Figure 6.
Increase in zeta potential as a function of increase in available amino groups determined by dye sorption method (a) linear fit of experimental data, (b) exponential fit of experimental data.
This increase can be fitted linear (Figure 6a) or exponential (Figure 6b), being that exponential increase is better fitted (R2 = 0.75 for exponential, R2 = 0.65 for linear) and this correlation is more probable.
We must, however, underline that in this work, a limited data set is present, but for future work, a series of samples with increasing amounts of chitosan, or even with increasing amounts of available functional groups, should be prepared, and whether the zeta potential curve is giving appropriate signal to these changes should be evaluated. From the results presented here, it can be concluded that zeta potential analysis is a very sensitive technique capable of detecting even the smallest changes in the surface chemistry, and it is in good agreement with the dye test using CI acid orange for available amino groups.
4. Conclusions
In this work, viscose (regenerated cellulose) fabric was oxidized with two oxidation protocols, using periodate and TEMPO. These two oxidations are based on completely different reactions, which lead to the inclusion of functional groups in cellulose on different carbons in the anhydroglucose unit (AGU). Periodate oxidation transforms OH groups at the C2 and C3 position of AGU to aldehydes while TEMPO oxidation leads to C6 OH group transformation to carboxyl via an aldehyde intermediate. Two sets of samples were created in such a way as to represent the increasing content of functional groups and to have similar magnitude of carbonyl groups, but on different positions in cellulose macromolecules. Further functionalization of oxidized cellulose was performed in order to introduce chitosan and its amino functional groups on viscose fibers’ surfaces.
The experimental results revealed that even though TEMPO-oxidized samples have the same or an even higher number of carbonyl (aldehyde) groups as well as total number of groups (carbonyl + carboxyl), the periodate-oxidized viscose sample binds chitosan in such a way that the sample has a higher content of available amino groups, which was assessed through CI acid orange dye adsorption (type of dye that has affinity towards chitosan and amino groups but not towards cellulose) of chitosan/viscose materials.
Zeta potential studies revealed that periodate-oxidized samples have more negative zeta potential values, which is probably a consequence of the introduction of less polar carbonyl moieties in the viscose fibers, while TEMPO-oxidized samples have distorted zeta potential curves, typical for hydrophilic cellulose fibers with acidic functional groups. The addition of chitosan shifted the IEP of the periodate-oxidized sample, while those of the TEMPO-oxidized sample were increased slightly, but still below pristine CV. The correlation was established between the content of available amino groups obtained by the dye test at pH 4.4 of cellulose samples and zeta potential measured at pH 4.4. Experimental values were fitted using linear and exponential functions, which gave a better fit with the exponential function (R2 = 0.75). It is proposed that more data should be evaluated in the described way, with the preparation of materials with a greater range of content of available amino groups and at various pH values, which will be the objective of future work.
For the potential applications of functionalized cellulose, and for prediction of a certain electrostatic behavior of the surface, the zeta potential presents a powerful analytical technique. Future work will focus on expanding the data range of samples to also include a wider range of different functional groups and to confirm the correlation between zeta potential analysis and dye adsorption, as representative of electrostatically based interactions between cellulose surfaces and other compounds.
Author Contributions
Conceptualization, A.K. and M.K. (Mirjana Kostić); Formal analysis, A.K. and M.K. (Matea Korica); Funding acquisition, A.K. and M.K. (Mirjana Kostić); Methodology, A.K., M.K. (Matea Korica) and M.K. (Mirjana Kostić); Project administration, A.K.; Supervision, M.K. (Mirjana Kostić); Validation, A.K., M.K. (Matea Korica) and M.K. (Mirjana Kostić); Visualization, A.K., M.K. (Matea Korica) and M.K. (Mirjana Kostić); Writing—original draft, A.K.; Writing—review and editing, M.K. (Matea Korica) and M.K. (Mirjana Kostić). All authors have read and agreed to the published version of the manuscript.
Funding
M.K. (Mirjana Kostić) and M.K. (Matea Korica) acknowledge the funding by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract No. 451-03-47/2023-01/200287 and Contract No. 451-03-47/2023-01/200135).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are contained within the article.
Acknowledgments
A.K. would like to acknowledge the CONEX-Plus program and Universidad Carlos III de Madrid in Spain, as well as the European Commission through the Marie-Sklodowska Curie COFUND Action (Grant Agreement No 801538).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose—Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry Volume 2 Functionalization of Cellulose; Wiley-VCH: Weinheim, Germany, 1998; Volume 2, ISBN 3527294899. [Google Scholar]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and surface modification of cellulose bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Heise, K.; Koso, T.; King, A.W.T.; Nypelö, T.; Penttila, P.; Tardy, B.L.; Beaumont, M. Spatioselective surface chemistry for the production of functional and chemically anisotropic nanocellulose colloids. J. Mater. Chem. A 2022, 10, 23413–23432. [Google Scholar] [CrossRef]
- Duceas, I.A.; Tanasa, F.; Coseri, S. Selective oxidation of cellulose—A multitask platform with significant environmental impact. Materials 2022, 15, 5076. [Google Scholar] [CrossRef]
- Xie, F.; Fardim, P.; Van den Mooter, G. Porous soluble dialdehyde cellulose beads: A new carrier for the formulation of poorly water-soluble drugs. Int. J. Pharm. 2022, 615, 121491. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, X.; Li, M.; Zhao, X.; Zeng, X.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Stability of soluble dialdehyde cellulose and the formation of hollow microspheres: Optimization and characterization. ACS Sustain. Chem. Eng. 2019, 7, 2151–2159. [Google Scholar] [CrossRef]
- Kokol, V.; Novak, S.; Kononenko, V.; Kos, M.; Vivod, V.; Gunde-Cimerman, N.; Drobne, D. Antibacterial and degradation properties of dialdehyded and aminohexamethylated nanocelluloses. Carbohydr. Polym. 2023, 311, 120603. [Google Scholar] [CrossRef] [PubMed]
- Kramar, A.; Ivanovska, A.; Kostić, M. Regenerated cellulose fiber functionalization by two-step oxidation using sodium periodate and sodium chlorite—Impact on the structure and sorption properties. Fibers Polym. 2021, 22, 2177–2186. [Google Scholar] [CrossRef]
- Mangovska, B.; Toshikj, E.; Tarbuk, A.; Grgic, K.; Jordanov, I. Influence of different oxidizing systems on cellulose oxidation level: Introduced groups versus degradation model. Cellulose 2019, 4, 777–794. [Google Scholar] [CrossRef]
- Vu, H.T.; Phan, M.T.D.; Tran, U.T.T.; Nguyen, G.D.; Duong, V.B.; Tran, D.B. N(4)-Morpholinothiosemicarbazide-modified cellulose: Synthesis, structure, kinetics, thermodynamics, and Ni (II) removal studies. ACS Omega 2020, 5, 15229–15239. [Google Scholar] [CrossRef]
- Akl, M.A.; Hashem, M.A.; Ismail, M.A.; Abdelgalil, D.A. Novel diaminoguanidine functionalized cellulose: Synthesis, characterization, adsorption characteristics and application for ICP-AES determination of copper(II), mercury(II), lead(II) and cadmium(II) from aqueous solutions. BMC Chem. 2022, 16, 65. [Google Scholar] [CrossRef]
- Hell, S.; Ohkawa, K.; Amer, H.; Potthast, A.; Rosenau, T. “Dialdehyde cellulose” nanofibers by electrospinning as polyvinyl alcohol blends: Manufacture and product characterization. J. Wood Chem. Technol. 2018, 38, 96–110. [Google Scholar] [CrossRef]
- Kim, U.J.; Wada, M.; Kuga, S. Solubilization of dialdehyde cellulose by hot water. Carbohydr. Polym. 2004, 56, 7–10. [Google Scholar] [CrossRef]
- Plappert, S.F.; Quraishi, S.; Pircher, N.; Mikkonen, K.S.; Veigel, S.; Klinger, K.M.; Potthast, A.; Rosenau, T.; Liebner, F.W. Transparent, flexible, and strong 2,3-dialdehyde cellulose films with high oxygen barrier properties. Biomacromolecules 2018, 19, 2969–2978. [Google Scholar] [CrossRef]
- Potthast, A.; Kostic, M.; Schiehser, S.; Kosma, P.; Rosenau, T. Studies on oxidative modifications of cellulose in the periodate system: Molecular weight distribution and carbonyl group profiles. Holzforschung 2007, 61, 662–667. [Google Scholar] [CrossRef]
- Nikolic, T.; Milanovic, J.; Kramar, A.; Petronijevic, Z.; Milenkovic, L.; Kostic, M. Preparation of cellulosic fibers with biological activity by immobilization of trypsin on periodate oxidized viscose fibers. Cellulose 2014, 21, 1369–1380. [Google Scholar] [CrossRef]
- Milanovic, J.; Schiehser, S.; Potthast, A.; Kostic, M. Stability of TEMPO-oxidized cotton fibers during natural aging. Carbohydr. Polym. 2020, 230, 115587. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Nikolic, T.; Hejnrih, T.; Kramar, A.; Petronijevic, Z.; Kostic, M. Influence of periodate oxidation on sorption properties of viscose yarn. Cellul. Chem. Technol. 2018, 52, 459–467. [Google Scholar]
- Bellmann, C.; Caspari, A.; Albrecht, V.; Loan Doan, T.T.; Mader, E.; Luxbacher, T.; Kohl, R. Electrokinetic properties of natural fibres. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 267, 19–23. [Google Scholar] [CrossRef]
- Tarbuk, A.; Grgi, K.; Daniel, T.; Dimitrovski, D.; Dimova, V.; Jordanov, I. Monitoring of cellulose oxidation level by electrokinetic phenomena and numeric prediction model. Cellulose 2020, 6, 3107–3119. [Google Scholar] [CrossRef]
- Stana-Kleinschek, K.; Ribitsch, V.; Kreze, T.; Fras, L. Determination of the adsorption character of cellulose fibres using surface tension and surface charge. Mater. Res. Innov. 2002, 6, 13–18. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Vähä-Nissi, M.; Harlin, A. Biopolymer films and coatings in packaging applications—A review of recent developments. Mater. Sci. Appl. 2014, 05, 708–718. [Google Scholar] [CrossRef]
- Tanpichai, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Chitosan coating for the preparation of multilayer coated paper for food-contact packaging: Wettability, mechanical properties, and overall migration. Int. J. Biol. Macromol. 2022, 213, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Pratama, A.; Sebayang, F.; Nasution, R.B. Antibacterial properties of biofilm schiff base derived from dialdehyde cellulose and chitosan. Indones. J. Chem. 2019, 19, 405–412. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Peršin, Z.; Šauperl, O.; Rudolf, A.; Kostić, M. Medical textiles based on viscose rayon fabrics coated with chitosan-encapsulated iodine: Antibacterial and antioxidant properties. Text. Res. J. 2018, 88, 2519–2531. [Google Scholar] [CrossRef]
- Strnad, S.; Sauperl, O.; Fras-Zemljic, L. Cellulose fibres funcionalised by chitosan: Characterization and application. In Biopolymers; Elnashar, M., Ed.; IntechOpen: London, UK, 2010; pp. 181–200. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. In Functional Chitosan; Springer: Berlin/Heidelberg, Germany, 2020; pp. 457–489. [Google Scholar] [CrossRef]
- Abdul, S.; Muhammad, M.; Saddique, T.; Ali, Z.; Majeed, M.I. Fabrication of cellulose—Mediated chitosan adsorbent beads and their surface chemical characterization. Polym. Bull. 2020, 77, 183–196. [Google Scholar] [CrossRef]
- Gandavadi, D.; Sundarrajan, S.; Ramakrishna, S. Bio-based nanofibers involved in wastewater treatment. Macromol. Mater. Eng. 2019, 304, 1900345. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Li, J.; Wang, L.; Jin, W.; Liu, J.; Pei, Y.; Tang, K. Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution. Int. J. Biol. Macromol. 2018, 107, 283–289. [Google Scholar] [CrossRef]
- Monvisade, P.; Siriphannon, P. Chitosan intercalated montmorillonite: Preparation, characterization and cationic dye adsorption. Appl. Clay Sci. 2009, 42, 427–431. [Google Scholar] [CrossRef]
- Gopi, S.; Pius, A.; Kargl, R.; Kleinschek, K.S.; Thomas, S. Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym. Bull. 2019, 76, 1557–1571. [Google Scholar] [CrossRef]
- Korica, M.; Peršin, Z.; Trifunovic, S.; Mihajlovski, K.; Nikolic, T.; Maletic, S.; Zemljic, L.F.; Kostic, M.M. Influence of different pretreatments on the antibacterial properties of chitosan functionalized viscose fabric: TEMPO oxidation and coating with TEMPO oxidized cellulose nanofibrils. Materials 2019, 12, 3144. [Google Scholar] [CrossRef]
- Kramar, A.D.; Ilic-Tomic, T.R.; Lađarević, J.M.; Nikodinovic-Runic, J.B.; Kostic, M.M. Halochromic cellulose textile obtained via dyeing with biocolorant isolated from Streptomyces sp. strain NP4. Cellulose 2021, 28, 8771–8784. [Google Scholar] [CrossRef]
- Arce, C.; Llano, T.; Garcıa, P.; Coz, A. Technical and environmental improvement of the bleaching sequence of dissolving pulp for fibre production. Cellulose 2020, 27, 4079–4090. [Google Scholar] [CrossRef]
- Woodings, C. Regenerated Cellulose Fibres; Woodhead Publishing Limited CRC: Boca Raton, FL, USA, 2001; ISBN 0849311470. [Google Scholar]
- Errokh, A.; Ferreira, A.M.; Conceicao, D.S.; Vieira Ferreira, L.F.; Botelho do Rego, A.M.; Rei Vilar, M.; Boufi, S. Controlled growth of Cu2O nanoparticles bound to cotton fibres. Carbohydr. Polym. 2016, 141, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, T.; Korica, M.; Milanovic, J.; Kramar, A.; Petronijevic, Z.; Kostic, M. TEMPO-oxidized cotton as a substrate for trypsin immobilization: Impact of functional groups on proteolytic activity and stability. Cellulose 2017, 24, 1863–1875. [Google Scholar] [CrossRef]
- Fras Zemljič, L.; Strnad, S.; Šauperl, O.; Stana-Kleinschek, K. Characterization of amino groups for cotton fibers coated with chitosan. Text. Res. J. 2009, 79, 219–226. [Google Scholar] [CrossRef]
- Sauperl, O.; Tompa, J.; Volmajer-Valh, J. Influence of the temperature on the efficiency of cellulose treatment using copolymer chitosan-eugenol. J. Eng. Fibers Fabr. 2014, 9, 107–114. [Google Scholar] [CrossRef]
- Luxbacher, T. The ZETA Guide: Principles of the Streaming Potential Technique; Anton Paar GmbH: Graz, Austria, 2014. [Google Scholar]
- Kim, U.J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydr. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Korica, M.; Fras Zemljič, L.; Bračič, M.; Kargl, R.; Spirk, S.; Reishofer, D.; Mihajlovski, K.; Kostić, M. Novel protein-repellent and antimicrobial polysaccharide multilayer thin films. Holzforschung 2019, 73, 93–103. [Google Scholar] [CrossRef]
- Reischl, M.; Stana-Kleinschek, K.; Ribitsch, V. Electrokinetic investigations of oriented cellulose polymers. Macromol. Symp. 2006, 244, 31–47. [Google Scholar] [CrossRef]
- Chapa González, C.; Navarro Arriaga, J.U.; García Casillas, P.E. Physicochemical properties of chitosan–magnetite nanocomposites obtained with different pH. Polym. Polym. Compos. 2021, 29, S1009–S1016. [Google Scholar] [CrossRef]
- Zemljic, L.F.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical characterization of packaging foils coated by chitosan and polyphenols colloidal formulations. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).