1. Introduction
The term ‘smart textiles’ is used to describe those fabrics that, thanks to the integration of specific digital tools, devices, and sensors, show supplementary features which make them sensitive to environmental conditions and/or able to interact with the end-user [
1,
2,
3,
4,
5]. These technologies are recently receiving growing interest for extensive applications in different fields spanning from the safety industry to the healthcare and fashion industry [
6]. A specific class of materials used for the fabrication of smart textiles is chromogenic materials, whereby the change of their color is reversibly induced as a response to an environmental stimulus, which can be thermal, optical, mechanical, electrical, etc. [
7]. In particular, photochromism is a photo-induced modification of the electronic state of the material and it is a phenomenon already used in various commercial products such as sunglasses, packaging, cosmetics, memories, sensors, and displays [
8]. Thus, we saw a potential interest for textile applications to open perspectives towards the development of UV protective and sensing clothes that can find considerable implications in the safety and military industries [
7,
9,
10]. Disparate examples of photochromic fabrics were obtained by using several organic dyes, such as spirooxazines, spiropyrans and naphthopyrans, employed with different kinds of integration processes [
11,
12,
13,
14,
15,
16,
17,
18]. Traditional and innovative dyeing techniques based on supercritical CO
2 were used for the production of photochromic textiles by using organic molecules [
16,
17]. An alternative approach is based on electrospinning, Zheng et al. reported electrospun polycaprolactone fibers doped with photochromic dyes and their subsequent embroidering into commercial fabrics for the development of wearable UV indicators [
14]. Printing techniques such as inkjet and screen printing were also widely used for the development of photochromic textile, by incorporating the dyes into polymeric matrices to obtain printable inks [
11,
12,
13]. The electrostatic layer-by-layer self-assembly method of photochromic microcapsules was also reported for the preparation of photochromic cotton fabric [
15].
Despite this, organic materials are at the same time largely affected by external environmental conditions such as oxygen and pH, hence, microencapsulation processes are usually required to ensure long-time service [
13,
15,
18]. Nevertheless, embedding photochromic dyes into a rigid polymeric matrix often reduces the molecular mobility and therefore slows down the reversible modification responsible for the photochromism in organic molecules. Immobilization of photochromic dyes onto inorganic matrices, before their combination with textiles, might partially overcome those drawbacks and possibly induce enhanced color-exchange properties, stability and comfort [
19,
20,
21].
Inorganic materials represent a valid alternative to organic molecules since they usually report better stability and for that have recently been under investigation for photochromic textiles fabrication [
8,
22,
23,
24,
25]. Fang et al. reported the preparation of cellulose fibers with the incorporation of hackmanite micro-particles for the development of wearable UV sensors [
24]. A photochromic cellulosic fabric was also obtained by screen printing of strontium aluminate pigment and aqueous binder [
8]. Photochromic properties of tungsten-based materials were also exploited for the development of smart chromogenic textiles [
22,
23,
25,
26].
Bao et al. obtained photochromic textiles by a simple hydrogen bonding self-assembly of polyacrylic acid and sodium deca-tungstate [
25]. Wang et al. reported waterborne tungsten-based polyvinyl alcohol (PVA) coating by simply dipping the fiber into a mixture of Na
2WO
4 and PVA and the obtained fibers showed an instantaneous color response after UV irradiation [
23]. Another approach based on dip coating was developed by Ling et al. starting from WO
3 nanomaterials and PVA, obtaining photochromic fibers with a fast and reversible color switch [
22]. Electrospinning is another valid technique employed to obtain fibrous substrates and it was employed by Wei et al. to fabricate fibrous photochromic membranes starting from polyvinylpyrrolidone and WO
3 [
26].
Although different specific mechanisms were proposed in the literature (tungsten bronze formation where the optical transition is associated with the intervalence-charge transfer mechanism or where absorption arises from free or trapped charge carriers) [
27], the photochromic performance in tungsten-based materials is determined by the behavior of optically excited electron-hole pairs [
28]. Thus, by controlling the lifetime and the optical paths of these free charge carriers, the coloration performances can be controlled. For this purpose, it was reported that the photochromic properties of tungsten oxide can be improved by mixing/coupling it with other metal oxides such as TiO
2. Nowadays, consistent experimental pieces of evidence demonstrated the advantages of mixed WO
3-xTiO
2 materials for photochromic applications [
29,
30,
31,
32]. Such performance enhancement can be understood by evaluating the energy levels of the two materials and the formation of a junction at the WO
3-x-TiO
2 interface. In other words, the enhanced photochromic response is a consequence of reduced recombination paths of photogenerated carriers which result in more electrons trapped within the bandgap of the WO
3-x domain, contributing to the coloration process. Additionally, for tungsten oxide-based nanocomposites, polymeric matrix encapsulation proved to be a valid strategy for influencing both coloration and bleaching processes, which depend on concentrations of proton available in the nanocomposites–polymer matrix and on the diffusion rate achievable of generated proton via hydrogen bonding into the matrix [
33]. However, the stronger the interaction between tungsten oxide and the matrix is, the higher and faster the coloration process could be, and the slower the bleaching process would be. Thus, it is a big challenge to make a proper balance and compatibility between the matrix and inorganic nanoparticles to achieve a consistent coloration process followed by rapid bleaching recovery, thus achieving a suitable compromise necessary for any specific practical application.
In this context, we used for the first time, a mixture of WO3-x and TiO2 nanomaterials to obtain photochromic textiles with improved performances compared to bare WO3-x. In particular, we report a low-cost and industrially suitable procedure to functionalize common textiles, through a simple impregnation method, with nanostructured active inks obtained by mixing WO3-x and TiO2 nanocrystals. A systematic study of the effect of the addition of TiO2 nanoparticles on the photochromic response of WO3-x coating textiles was achieved by simply mixing the nanocrystal solutions of the two photoactive materials. Different photochromic performances resulted from variable compositions of photoactive inks used for textile impregnation. Moreover, the photochromic response of the WO3-x:TiO2 blend-coated textile was evaluated in the presence of selected organic solvent and/or embedded in polymeric matrices. Coloration kinetics, operational stability toward environmental chemicals (water and oxygen), and physical stresses of the nano-functionalized and –structured smart textile were also investigated.
2. Materials and Methods
2.1. Materials
All chemicals were used as received without further purification. The following chemicals were purchased from Sigma-Aldrich (Burlington, MA, USA): methanol (MeOH, 99.9%), ethanol (EtOH, 95%), 2-propanol (i-PrOH, 95.5%), hydroxyethylcellulose (HEC, average molecular weight: 380 kDa), starch from corn. Nafion alcoholic solution (D2021CS isopropanol-based 1100 EW at 20%
w/
w) was provided by Ion Power. Fabrics were provided by our industrial partner Klopman SRL made by cotton–polyester 50/50 blend, 270 g/m
3. Water-dispersed sub-stoichiometric WO
3-x nanocrystals (NCs) were synthesized as reported elsewhere [
34]; water-dispersible TiO
2 NCs were prepared according to a previously reported procedure, with minor changes [
35]. A more detailed synthetic procedure of the latter will be exhaustively reported elsewhere. Representative TEM pictures and XRD diffraction patterns of TiO
2 and WO
3-x nanocrystals are reported in
Figure S1a–d, respectively.
2.2. Preparation of Samples and Analysis Method
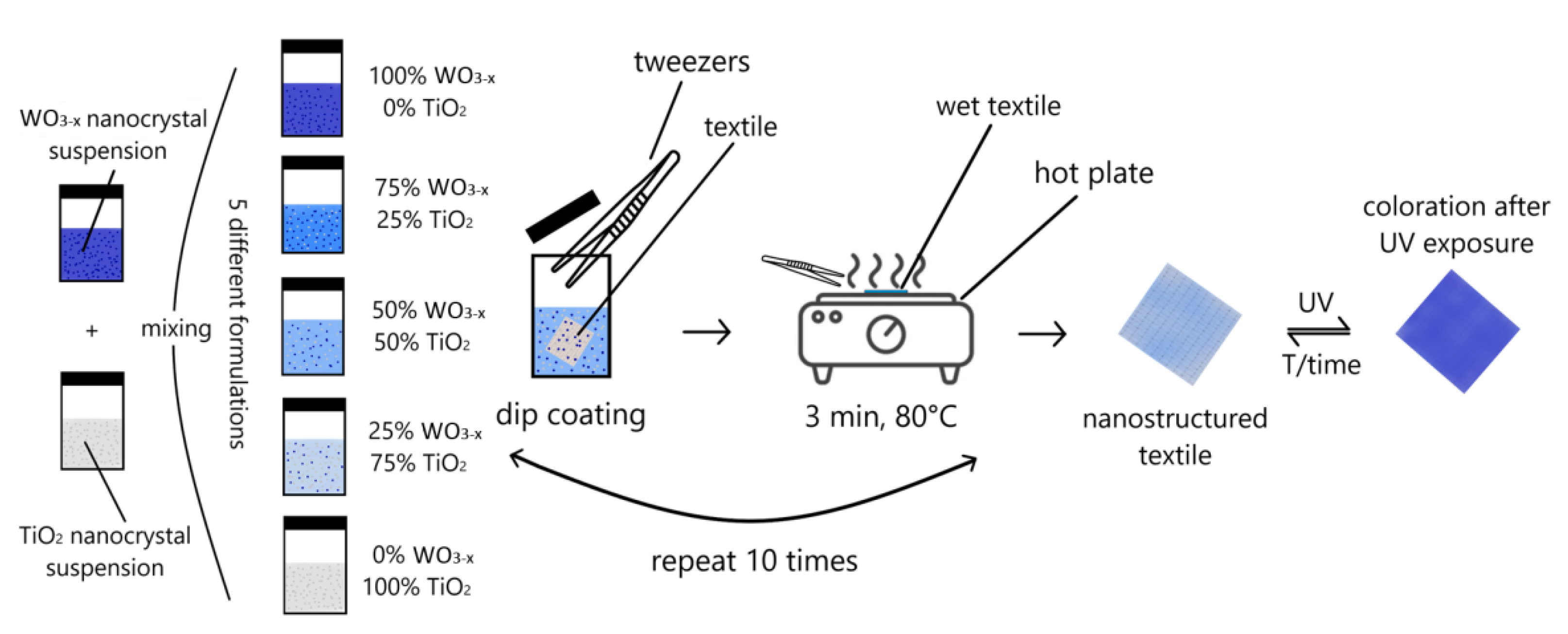
Textile samples were prepared by cutting samples as 1.0 × 2.0 cm in size. NC impregnation was performed by soaking samples in a proper blend of NC solution made by different WO
3-x:TiO
2 ratio content (relative molar compositions of 4:0, 3:1, 2:2, 1:3, 0:4 expressed in WO
3-x% named hereafter, 100%, 75%, 50%, 25% and 0%), for 3 s and then dried on hot plate at 80 °C for 3 min. This process was repeated 10 times for each sample to ensure an adequate loading of nanostructures. The manufacturing of textiles NC functionalization is sketched in
Figure 1. The photochromic properties were evaluated by irradiating samples at 320–400 nm lamp with Bromograph MF 1030 (Nuova Delta Electronica) with different time exposure. Reflectance measurements were performed soon after UV (ultraviolet) exposure with PerkinElmer Lambda 1050 UV/Vis (visible)/NIR (near-infrared) spectrophotometer in a wavelength range between 250 nm and 1000 nm. For optical properties measurements, we evaluated a negligible transmittance (data not shown), thus we acquired reflectance (R) information that can be eventually converted into absorption (Abs) as follow Abs = 1-R. For photochromic measurements with different solvents exposure, 300 μL of solvent were cast on each sample and then exposed to UV radiation. Reflectance spectrum was suddenly recorded ensuring that the textile was still wetted during the measurement. The solvent impregnation process was repeated for each exposure time investigated. Photochromic measurements with starch and HEC were performed as follows: polymers were stirred in hot distilled water (80 °C) until complete powder gelation (concentration 0.05 mg/mL) before being uniformly deposited on samples by spin coating 1 mL of solutions (2000 rpm for 60 s); then, a further step of 5 min at 80 °C was adopted to ensuring complete evaporation of water. Nafion solution was spin-coated (2000 rpm for 60 s) as received without further dilutions, and dried onto the hot plate (80 °C for 5 min). Samples were then exposed to UV irradiation at different time scales and reflectance spectra were suddenly acquired.
SEM morphological investigations were carried out on NC-decorated textiles without any further treatment, with FE-SEM Zeiss Merlin (Oberkochen, Germany) equipped with a GEMINI2 column, Schottky-type electron gun and secondary electron/Inlens detectors; images were recorded at 5 kV.
TEM images of WO3-x and TiO2 nanocrystals were recorded on a JEOL JEM 1011 microscope (Peabody, MA, USA), equipped with a W filament source operating at 100 kV. Samples for TEM analysis were prepared by drop-casting a few drops of dilute nanocrystal solutions onto standard carbon-coated Cu grids, then allowing the solvent to evaporate. The as-dried sample grids were stored at 50 °C overnight before being transferred into the microscope for imaging.
XRD spectra were collected at room temperature using a Bruker D8 Discover diffractometer (Billerica, MA, USA) (operating conditions 40 kV, 40 mA) equipped with a Goebel mirror for copper radiation (λKα1 = 1.540 56 Å, λKα2 = 1.544 39 Å), and a scintillator detector. Samples were deposited onto a silicon zero background substrate. Data were collected in a reflection geometry at a fixed incidence angle of ω = 3° while moving the detector between 5° and 120° with a step size of 0.05°.
CAM 200 (KSV Instruments Ltd., Helsinki Finland) instrument was used to allow static contact angle measurements of all the samples by the sessile drop method (water drop volume—10 µL, time between frames—16 ms).
3. Results
For the functionalization of the hydrophilic component of textiles, with the idea of defining a process extendible to various commercially available fabrics, proper water-dispersible NC solutions were adopted. In the view of industrially accessible processability, scalability, and greener chemistry, water-dispersible inorganic NC solutions of TiO2 and WO3-x were properly synthesized and processed. The chemical synthesis was performed within a hydroalcoholic environment and heated through microwave irradiation, which allows faster and in-core homogeneous heating rates. Both individual inorganic nano-components were generated without the need for the NC surface of a specific organic capping layer that would hinder the direct interaction either between the inorganic NCs with polymeric matrices or with other chemical environments.
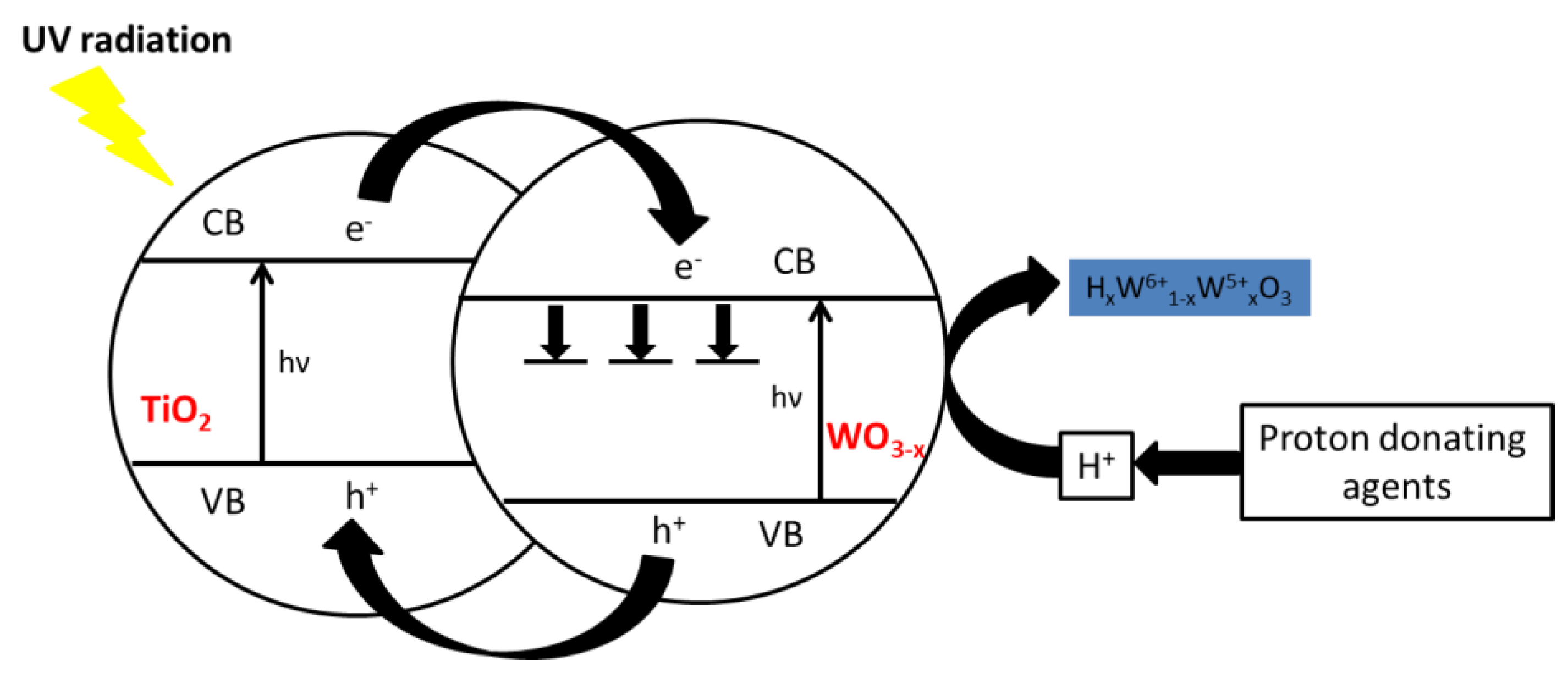
The commonly accepted mechanism for tungsten oxide photochromism claims the formation of H
xW
6+1-xW
5+xO
3 is responsible for the blue color observed upon irradiation [
27]. The combination with TiO
2 would clearly emphasize the coloration mechanism, but to ensure an efficient and homogeneous photochromic behavior an intimate contact between TiO
2 and WO
3-x is a critical issue and, for that, high surface contact between both nanostructured building blocks needs to be guaranteed. The nanocrystalline colloids exhibit opposite surface charges (−39 mV for TiO
2 and +2.8 mV for WO
3-x related to Z-potential measurements performed on individual NC water solutions), which allows an electrostatic attraction between nanosized building blocks, and mutual interaction, therefore, when admixed in the same solution. When fabric samples were dipped into different NC solutions, the expressed photochromism reflects on photochromic textiles.
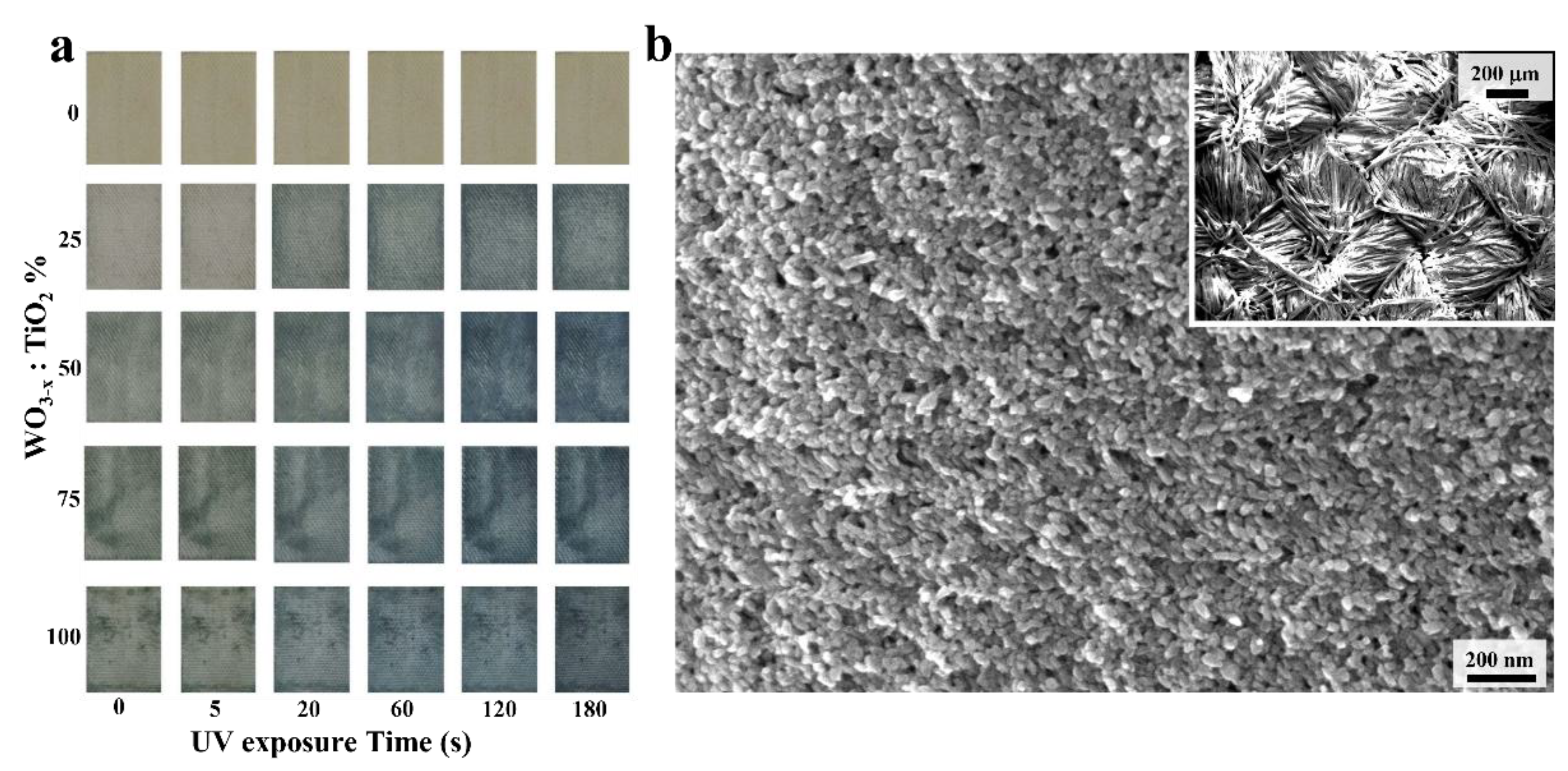
Figure 2a reports a gallery of different pictures recorded on different samples impregnated with different WO
3-x:TiO
2 content (as reported by vertical annotations) and compared with only TiO
2 and WO
3-x impregnation. The relative elemental amounts were characterized and controlled by inductively coupled plasma optical emission spectrometry (ICP-OES, data not shown) to keep constant the overall inorganic amounts deposited on each textile. A first qualitative outcome of the different TiO
2 content on photochromic textiles can be appreciated with distinctive blue intensities obtained at different UV exposure times (as reported by horizontal annotations).
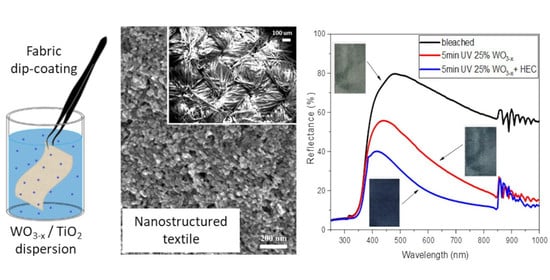
The nanostructured texture covering the pristine textile manufacture was characterized by SEM microscopy.
Figure 2b and
Figure S1e report SEM images recorded on textile functionalized with NC solutions prepared with a WO
3-x:TiO
2 ratio of 25% after hotplate drying, without any other washing step. In particular, the inset of
Figure 2b shows the fabric texture after NC impregnation and the higher magnification SEM images show complete and homogenous nanocrystalline coverage along the fibers (
Figure 2b). In the
Supporting Information, the SEM picture of one single fiber before and after NC impregnation is reported (
Figure S1e,f). The pristine fiber is characterized by a fibrous motif/pattern (
Figure S1f), which is not any more visible after impregnation (
Figure S1e).
To investigate the photochromic textile performances with different WO
3-x:TiO
2 contents and compare them with two bare oxides on coloration and bleaching kinetics, detailed reflectance spectra were measured by a UV/Vis/NIR spectrometer.
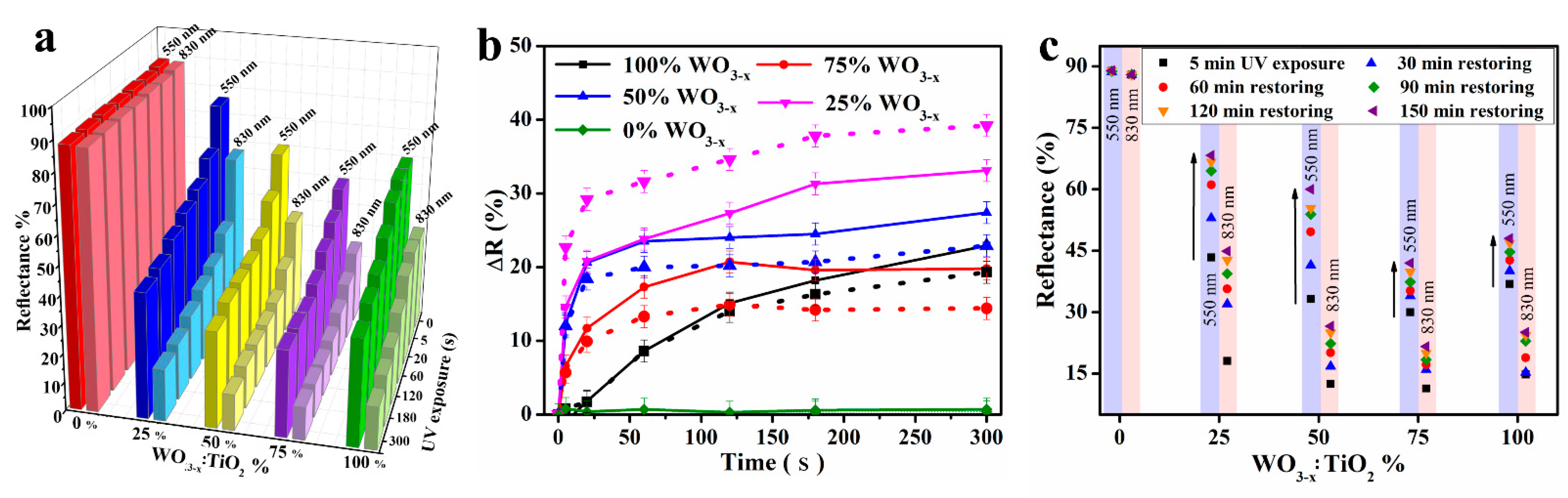
Figure 3a shows the reflectance changing values of NC-based photochromic fabrics of discrete compositions, at different UV exposure times and obtained at the two characteristic wavelengths of 550 nm and 830 nm. The individual reflectance spectra variations for each NC-functionalized photochromic fabric to different compositions are reported in
Figure S2. The reflectance decreased within the initial 30 s and the changing rate became gradually slower with an exponential trend, reaching a plateau (becoming saturated) within 5 min of UV exposure. It is worth underlying that a strong absorption in the IR and Vis regions already within 5 s of UV exposure was achieved only for the WO
3-x:TiO
2 mixture, compared with both pure oxide-coated textiles (TiO
2 alone does not show any photochromic behavior at the irradiate wavelength). The comparison of differences in reflectance between the no-exposure state and different times of UV exposure (ΔR) for all NC compositions are reported in
Figure 3b (solid line for λ = 550 nm and dashed line for λ = 830 nm). It becomes clearer that the presence of TiO
2 increases the coloring kinetics for all mixture compositions compared with pure WO
3-x. Moreover, as the greatest ΔR values were achieved with a WO
3-x:TiO
2 ratio of 1:3 for both wavelengths, we selected this composition for further characterizations, as reported below. The after effect of TiO
2 blending (content) on the bleaching kinetics of the photochromic textiles was evaluated by monitoring, immediately after 5 min of UV exposure, the reflectance decay as a function of time. The representative reflectance values during the bleaching process of WO
3-x:TiO
2 functionalized textiles are reported in
Figure 3c. All samples showed good reversible photochromic properties and TiO
2 blending was also revealed to be beneficial for photochromic recovery. Indeed, fabric samples containing TiO
2 blended with WO
3-x NCs showed a recovery of 80–100% (for λ = 550 nm) and 75–85% (for λ = 830 nm) values unlike samples functionalized with only WO
3-x, which recovered only the 75% and 70% of pristine reflectance values for Vis and IR, respectively (see
Table 1 for specific values). All samples returned to their original bleached state after being stored in the dark (in the air) for about 6 h. Representative reflectance values are summarized in
Table 1 for a more immediate comparison.
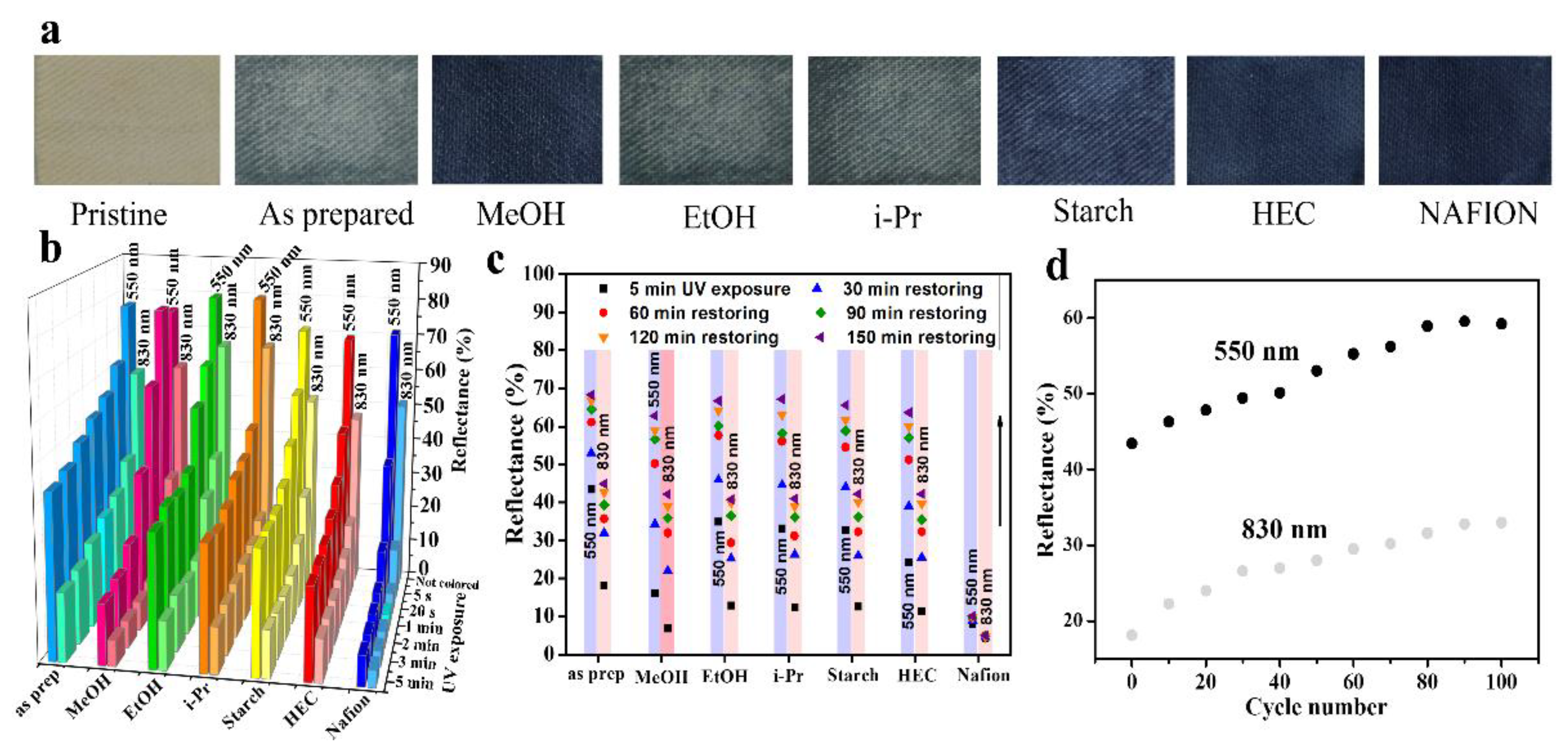
The photochromic responses of the mixed oxide-functionalized textiles were tested in different alcoholic environments. Different photochromic responses or coloration intensities observed were compared in
Figure 4a, while individual reflectance spectra variation recorded on 25% WO
3-x impregnated textiles soaked with different solvents were reported in
Figure S3. For that aim, we selected the textile sample functionalized with WO
3-x:TiO
2 (25% WO
3-x) because of its greater ΔR observed without any additive. We monitored the reflectance as a function of UV time exposure after soaking each sample with 300 μL of selected organic solvent (see experimental details). The largest optical response in the Vis as well as in the NIR was achieved with MeOH, followed by EtOH and i-PrOH, which show also an improvement in coloration sensitivity for both wavelengths compared with pristine samples (NC-coated textile with no alcohol impregnation) (
Figure 4b). Moreover, we used acetone for comparison which did not show any coloration improvement (data not shown). Our interpretation of the increased modulation relates to the protic nature of alcohols compared with the aprotic habit of acetone, while the observed modulation trend (MeOH > EtOH~i-PrOH) could be attributed to the greater acidity of MeOH compared with EtOH and i-PrOH (pKa MeOH ~ 15.5; pKa EtOH ~ 16; pKa i-PrOH ~ 17) or to their intrinsic hole scavenger nature (see Discussion below for a more exhaustive analysis).
Based upon this solvent effect, the following measurements were conducted with a suitable polymeric medium in order to mimic the solvent behavior in a solid-state. As the presence of hydroxyl groups increases the photochromic efficiency [
28] we selected hydroxyl-rich, cheap, non-toxic, and naturally abundant materials as a polymeric matrix. For this purpose we over-coated the NC-functionalized textile with two alternative polysaccharides, taking advantage of structural –OH moieties present in their chemical structures. More specifically, we compared corn starch and hydroxyethylcellulose (HEC). Both of them underwent a gelation process (rather than a full dissolution) when stirred in hot (80 °C) water, while they became badly water-soluble at room temperature, materials suitable in view of greener chemistry since no organic solvents were adopted for polymer overcoating. It is worth saying that HEC displays easier solubility and processability at higher concentrations, which is a not negligible detail in the view of the industrial scalability process. Pictures of different photochromic responses recorded on as described samples are reported in
Figure 4a. The reflectance variations as a function of UV exposure times are compared in
Figure 4b, while individual reflectance spectra variation recorded on 25% WO
3-x-impregnated textiles over-coated with different polymers are reported in
Figure S4. The HEC-coated photochromic textile reported faster and stronger exponential modulation sensitivity than pristine samples as well as compared with EtOH and i-PrOH soaked samples, while slightly reduced compared with MeOH both in the Vis and NIR window with comparable UV exposure time. The starch-coated photochromic textile reported similar behavior as the EtOH and i-PrOH treated textiles. The bleaching kinetics, reported in
Figure 4c, remain mainly influenced by the TiO
2 presence in the inorganic NCs blend and was not substantially inferred by any further over-coating, besides Nafion (see below). To investigate the coloring–bleaching cycles stability, specific experiments were carried out with the HEC over-coated WO
3-x:TiO
2 (25%) textile, by exposing a sample to repeated cycles of 5 min UV irradiation and then keeping it at 75 °C under air on a hot plate to accelerate the bleaching condition. We stressed the sample with 100 cycles and the results are reported in
Figure 4d. The initial reflectance (in the colored state) of each cycle raises by increasing the number of irradiation cycles, ascribable this to a little decline in photochromism. For comparison, we over-coated the NC-functionalized textile with Nafion. Samples reported an extremely fast and intense, though irreversible, modulation for Vis (λ = 550) and NIR (λ = 830) wavelengths. We suppose this to be due to the intrinsic high acidity of sulfonic moieties in the Nafion chemical structure, which could stabilize the chemical structure responsible for the colored state.
Contact angle measurements were performed to evaluate functionalized fabrics’ wettability (
Figure S5). All functionalized fabrics (except the one treated with Nafion) showed high wettability and the water drops were rapidly adsorbed within a few hundred ms. For this reason, it was not possible to evaluate the contact angle. In the case of fabrics coated with starch and HEC, the hydrophilic nature of the two polymers led to an absorption of the drop-in times very similar to those of the pristine fabric. In the case of fabrics treated with nanocrystals only (100% TiO
2 or 100% WO
3-x), the adsorption of the drop is even faster, occurring in less than 100 ms. Only fabric coated with Nafion, a fluorinated polymer, acquired good hydrophobicity, with slightly asymmetrical left–right contact angles due to the surface morphology of the fabric (113° left, 120° right).
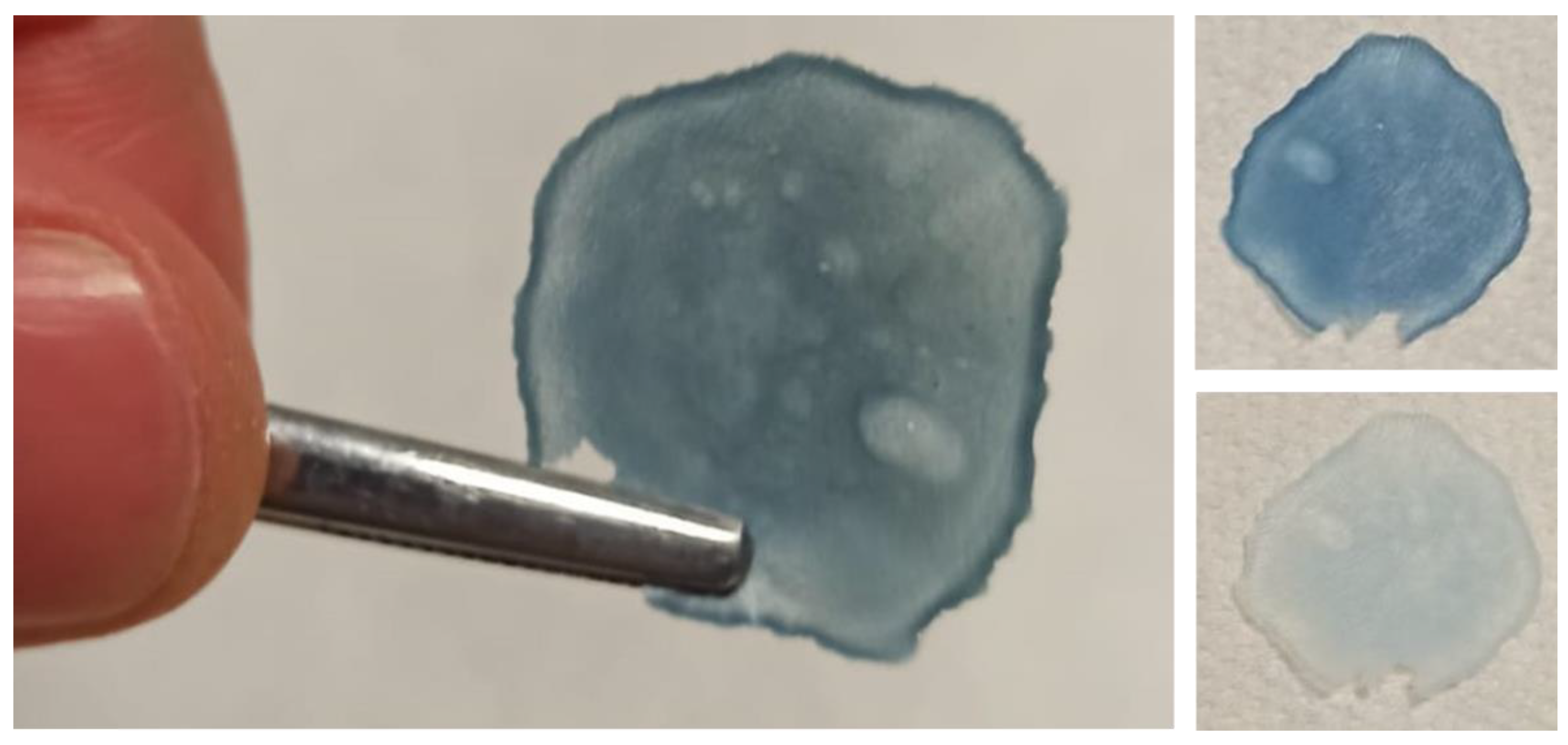
To demonstrate the proof of concept of a free-standing membrane, a prototype was fabricated following the identical experimental procedures described in this study. For that, WO
3-x and TiO
2 solutions (25% WO
3-x) were dispersed in an HEC solution (50 mg/mL), cast onto a hydrophobic substrate, and then dried at 70 °C for 2 h. The as-deposited film could be detached from the substrate and used as a free-standing photochromic membrane. This lab-scale prototype has a dimension of about 3 cm × 3 cm and shows a good color change modulation. Pictures of the bleached and colored states are shown in
Figure 5. This represented just a proof of concept for developing a sticky photochromic membrane to be eventually attached and detached onto any kind of already fabricated textiles.
4. Discussion
The commonly accepted photo-induced coloration mechanism for tungsten oxide, which represents the photo-active optical material for our purpose, can be roughly summarized as follows:
The irradiation of WO3 with UV-light induces the excitation of electrons to the conduction band and the formation of holes in the valence band (Reaction 1). The photogenerated holes can interact with absorbed water molecules, thus inducing the decomposition into protons and oxygen radicals (Reaction 2). Finally, photogenerated electrons react with protons and WO3 with the formation of hydrogen tungsten bronze responsible for the blue color (Reaction 3).
In comparison with pure WO
3 (as well as for its substoichiometric counterpart WO
3-x), as for other reported oxide samples such as MoO
3 (into CdS/WO
3 bi-layers and for MoO
3/TiO
2 composites), the suddenly reported photochromic mechanism for WO
3 is maximized by the presence of TiO
2. Thus, WO
3-x:TiO
2 blends exhibited much stronger photochromic properties to date, an effect mainly ascribable to hetero-interface formation between the two oxides [
28,
36]. WO
3-x and TiO
2 are both n-type semiconductors and can both be excited by UV light, generating holes and electrons (reaction 1). Because of the staggered alignment of the energetics bands, the photogenerated holes can be transferred from the WO
3-x valence band to the TiO
2 valence band and the electrons can transfer from the TiO
2 conduction band to the WO
3-x conduction band. The segregation of photoexcited carriers into two different materials would consequently increase the electron-hole lifetime. The photo-produced hole can weaken the H−O bond of adsorbed water molecules, or other H-O-containing species, and causes the water molecules decomposition into a proton and highly reactive oxygen radicals (reaction 2). While protons diffuse into the lattice, the photogenerated electrons injected into the conduction band of WO
3-x, balanced by proper counterions (i.e., protons), react inducing the reduction of W(VI) to W(V) and generate H
xW
6+1-xW
5+xO
3, (hydrogen tungsten bronze) (reaction 3). Moreover, it was suggested that the coloration in WO
3-x could also be due to electrons trapped at energy levels within the forbidden gap, which can lead to a broad absorption in the IR region when they are excited into higher energy levels [
28]. A representative scheme of photochromism in the WO
3-x:TiO
2 systems is shown in
Figure 6.
Therefore, the photogenerated carriers in WO3-x:TiO2 blends are more effectively separated with respect to pure WO3 and result in improved coloration performances because of the effective interfacial interaction. In particular, the more protons diffuse into the lattice, the more hydrogen tungsten bronze will be formed, and faster and stronger polaronic absorption will be obtained.
The number of available protons depends on the amount and nature of the surface adsorbed solvents such as H
2O, or alcohols. Alcohol can form protons easier because of its greater proton acidity compared to water, therefore, WO
3-x-TiO
2 alcohol-wetted fabrics showed larger photochromic efficiency than as-prepared samples (i.e., only NC-functionalized) where the photochromism is most probably powered by atmospheric water [
35]. Moreover, alcohols (particularly MEOH) are largely used in photocatalytic similar systems for their prerogative of acting as an efficient hole scavenger or, in other words, used as a sacrificial electron donor for the depletion of photogenerated holes, thus they would partially inhibit intrinsic charges quenching.
Therefore, methanol represents the best solvent for improving the performance of the photochromic fabrics, both in terms of acidity and hole scavenger properties.
The nanoscale dimensions of inorganic colloids play also a fundamental role for two main reasons: (i) they decrease the distance of charges transfer, thus increasing the rate of coloration and decreasing the time of de-coloration; (ii) they maximize the surface-assisted hetero-interface interaction between oxides contributing to the higher charge transfer and ion intercalation kinetics, favoring the overall photochromic efficiency.
In the examples of over-coating with polymeric matrices, the –OH moieties in their chemical structure can enhance the number of available protons thus inducing an improvement of photochromic performances (as observed for alcohol impregnated samples), while the polymeric chain and the presence of hydrogen bonds would facilitate the migration of produced H+ and electrons.
Polymers with this prerogative, with an adequate viscosity that would guarantee enough ionic mobility and eventually could show interesting foil-forming potential, would be of great interest for accelerating coloration and bleaching behavior of the tungsten oxide-based hybrid photochromic foil with outstanding cycling stability.
5. Conclusions
In this work, photo-sensitive chromogenic smart textiles were realized with a straightforward industrially practicable approach potentially extendible over a broad range of every hydrophilic fabric. For that, commercial fabrics were functionalized with mixtures of WO3-x and TiO2 nanocrystalline breeds to produce photochromic textiles. Because of nanometric particle size and their peculiar opposite surface charges, high specific surface areas with rich interfacial sites for charge transfer were created and hetero-interfaces were designed through strong electrostatic interactions. Indeed, an enhanced and reversible optical response both in the visible (λ = 550 nm) and infrared (λ = 830 nm) ranges was obtained. Moreover, the photochromic behaviors of nano-functionalized textiles exposed to different solvents and dispersed in several matrices were studied. Analyses of the different coloring and bleaching efficiencies were performed to reach an optimal balance between coloration and discoloration behavior along with consistent cycling stability. Furthermore, by using hydroxyl-rich water-dispersible polysaccharides over-coating the nano-functionalized textiles, an interesting enhancement of the overall photochromic response was generated. Those substrates furnish non-toxic, unpolluted, moldable rigidity, strong adhesion, and solvent resistance platforms for different practical useful textile applications or even photochromic freestanding foils. The high UV sensitivity and the filmogenic nature of the resulting hybrid materials make them important candidates for application in practical UV sensing devices and light-activated smart textiles.