Effect of Organic Acid Mixtures on the Extraction Efficiency, Physicochemical, and Thermal Properties of Pigskin Gelatin and Resulting Films
Abstract
1. Introduction
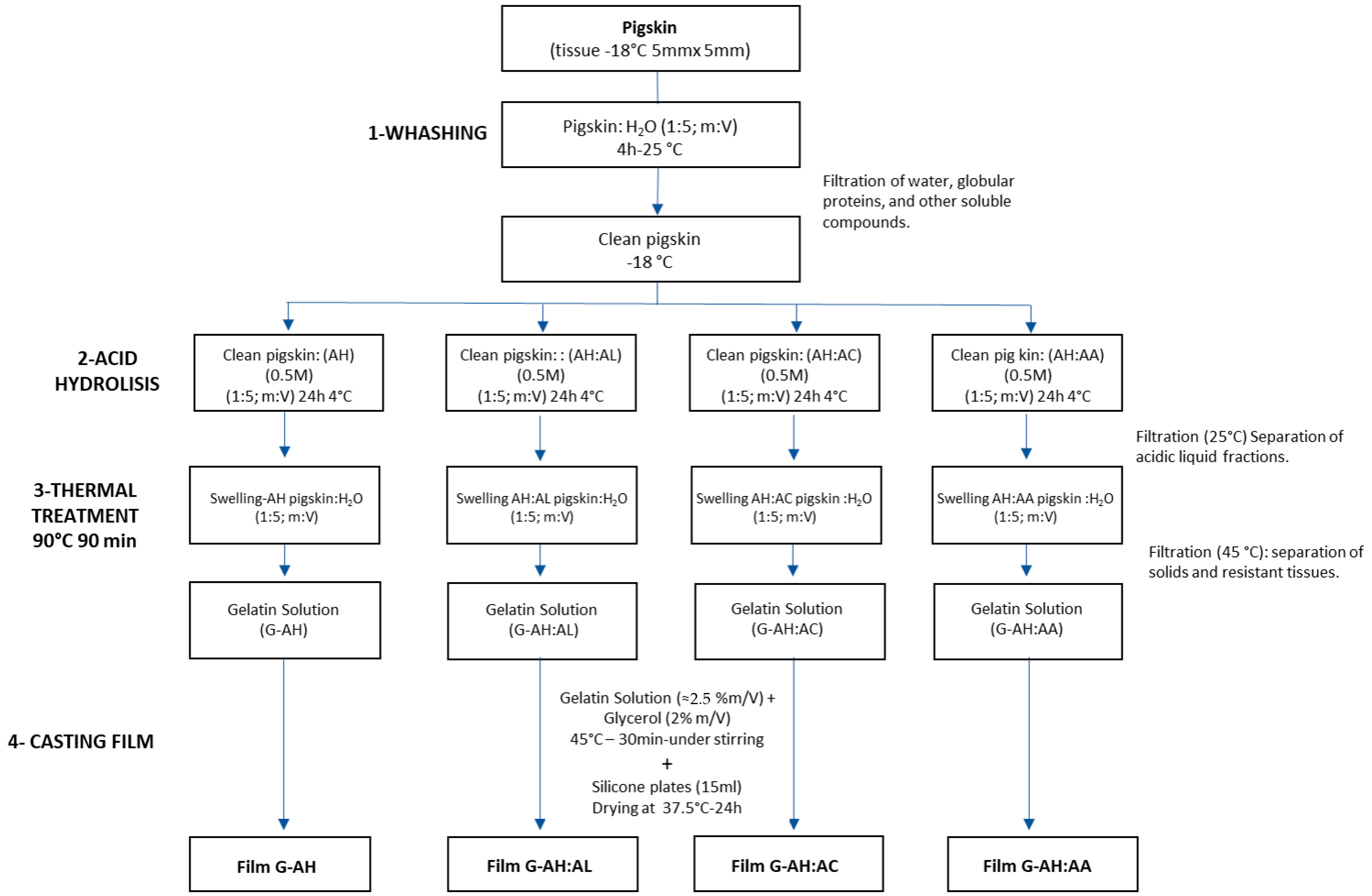
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Gelatin Extraction by Acid Treatment
2.2.1. pH Analysis
2.2.2. Degree of Pigskin Swelling
2.2.3. Quantification of Total Hydroxyproline [Hyp]
2.2.4. Extraction Performance
2.3. Preparation of Gelatin Films
2.3.1. Moisture Content of Gelatin Films
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Color Analysis of Gelatin Films
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Benjakul, S.; Nalinanon, S.; Sumpavapol, P. Gelatin from Lizardfish (Saurida tumbil) Skin: Properties as Affected by Extraction Conditions. Food Hydrocoll. 2010, 24, 616–622. [Google Scholar]
- See, S.F.; Ghassem, M.; Mamot, S.; Babji, A.S. Effect of Different Pretreatment on Functional Properties of African Catfish (Clarias gariepinus) Skin Gelatin. J. Food Sci. Technol. 2015, 52, 753–760. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of Gelatin from the Skin of Unicorn Leatherjacket (Aluterus monoceros) as Influenced by Extraction Conditions. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Roussenova, M.; MacNab, M.; Murray, B.S. Effect of Gelatin Type and Bloom Strength on Gelation Behavior and Rheology. Food Hydrocoll. 2014, 35, 575–584. [Google Scholar]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin Structure and Composition Linked to Functional Properties: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1143. [Google Scholar]
- Alipal, J.; Jamilah, B.; Hashim, D.M.; Sahilah, A.M. Effects of Different Drying Methods on the Properties of Fish Gelatin Films. Food Hydrocoll. 2021, 112, 106267. [Google Scholar]
- Noor, N.Q.I.M.; Razali, R.S.; Ismail, N.K.; Ramli, R.A.; Razali, U.H.M.; Bahauddin, A.R.; Zaharudin, N.; Rozzamri, A.; Bakar, J.; Shaarani, S.M. Application of Green Technology in Gelatin Extraction: A Review. Processes 2021, 9, 2227. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Xia, W. Preparation and Characterization of Gelatin from Tilapia Skin Using Combined Pretreatments. Food Biosci. 2020, 35, 100580. [Google Scholar]
- Yang, Z.-X.; Sha, X.-M.; Wang, H.; Fang, T.; Shu, S.; Tu, Z.-C. Effect of acid pretreatments with various acid types on gelling properties and identification characteristics of pigskin gelatin. Food Chem. X 2025, 26, 102211. [Google Scholar] [CrossRef] [PubMed]
- Sompie, M.; Surtijono, S.E.; Pontoh, J.H.W.; Lontaan, N.N. The Effects of Acetic Acid Concentration and Extraction Temperature on Physical and Chemical Properties of Pigskin Gelatin. Procedia Food Sci. 2015, 3, 383–388. [Google Scholar] [CrossRef]
- Mishra, B.P.; Maheswarappa, N.B.; Rao, B.E.; Banerjee, R.; Mallika, E.N.; Rao, T.S.; Nath, D.N.; Prasad, M.G.; Belore, B.M.; Dasoja, S.; et al. Extraction And Characterization Of Pig Skin Gelatin Compared To Commercial Porcine Gelatin. J. Meat Sci. 2023, 18, 36–42. [Google Scholar] [CrossRef]
- Velázquez, L.; Sanchez, D.; Latorre, M.E. Pigskin Treatment Using Different Food-Grade-Acids: Effects on The Physicochemical Characteristics of The By-Products. Food Sci. J. 2023, 5, 103–115. [Google Scholar] [CrossRef]
- Lestari, N.; Manalu, L.P.; Hidayat, T.; Junaidi, L.; Hartanto, E.S.; Rienoviar; Saputra, S.H.; Zulham, A.; Mala, D.M.; Dewi, C.; et al. The effect of citric and acetic acid treatment on gelatin production from catfish skin. BIO Web Conf. 2024, 87, 03004. [Google Scholar] [CrossRef]
- Bergman, M.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar] [PubMed] [PubMed Central]
- Liu, Z.; Basem, A.; Mostafa, L.; Jasim, D.J.; Al-Rubaye, A.H.; Salahshour, S.; Hekmatifar, M.; Esmaeili, S. Investigating the effect of pH on the swelling process, mechanical and thermal attributes of polyacrylamide hydrogel structure: A molecular dynamics study. Case Stud. Therm. Eng. 2024, 55, 104148. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L. Essential role of isoelectric point of skin/leather in leather processing. J. Leather Sci. Eng. 2022, 4, 25. [Google Scholar] [CrossRef]
- Andrei, W.; Quan, H.; Brown, K.A.; Williams, A.; Proud, W.G.; Meyers, M.A. Tensile behavior and structural characterization of pig dermis. Acta Biomater. 2019, 86, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.M.; Padmaprakashan, A.; Pradeep, A.; Paul, P.T.; Mannuthy, R.J.; Mathew, S. A Review on Fish Skin-Derived Gelatin: Elucidating the Gelatin Peptides—Preparation, Bioactivity, Mechanistic Insights, and Strategies for Stability Improvement. Foods 2024, 13, 2793. [Google Scholar] [CrossRef]
- Yang, Y.L.; Leone, L.M.; Kaufman, L.J. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys. J. 2009, 97, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Goudie, K.J.; McCreath, S.J.; Parkinson, J.A.; Davidson, C.M.; Liggat, J.J. Investigation of the influence of pH on the properties and morphology of gelatin hydrogels. J. Polym. Sci. 2023, 61, 2316. [Google Scholar] [CrossRef]
- Koli, J.; Basu, S.; Nayak, B.; Patange, S.B.; Pagarkar, A.; Gudipati, V. Effect of pH and ionic strength on functional properties of fish gelatin in comparison to mammalian gelatin. Fish. Technol. 2013, 50, 126–132. [Google Scholar]
- Slade, L.; Levine, H. Polymer-chemical properties of gelatin in foods. In Advances in Meat Research, Vol. 4: Collagen as a Food; Pearson, T.R., Dutson, A.M., Bailey, A., Eds.; AVI Publishing: Westport, CT, USA, 1987; pp. 283–311. [Google Scholar]
- Sobral, P.J.D.A.; Habitante, A.M.Q.B. Phase transitions of pigskin gelatin. Food Hydrocoll. 2001, 15, 377–382. [Google Scholar] [CrossRef]
- Mukherjee, I.; Rosolen, M. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J. Therm. Anal. Calorim. 2013, 114, 1161–1166. [Google Scholar] [CrossRef]
- Nicolaï, B.; Barrio, M.; Tamarit, J.; Céolin, R.; Rietveld, I. Thermal expansion of L-ascorbic acid. Eur. Phys. J. Spec. Top. 2017, 226, 905–912. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Paper | Acid Treatment | Thermal Treatment | Results Reported | Notes |
|---|---|---|---|---|
| Sompie et al. [11] | Acetic acid (0.5 M) cc. 2, 4, and 6% (v/v) 24 h | 3 h—50, 55, and 60 °C | Yield, protein gelatin. pH; viscosity strength. | Acid solution neutralized (pH 6) before thermal treatment. |
| Mishra et al. [12] | Acetic acid (0.5 M) 24 h | 6 h—60 °C | Yield, hydroxyproline, pH, proximate composition; sensory; water activity. | Acid solution neutralized (pH 7) before thermal treatment. |
| Velázquez et al. [13] | Acetic, citric, lactic, ascorbic acid (0.5 M) 24 h 4 °C | 90 min 85–90 °C | Yields, Hyp content, film color, and thermal properties. | Thermal treatment without solution neutralization. |
| Yang et al. [10] | Acetic acid, citric acid HCl, H2SO4 (0.2 M) 4 h—60 °C | 12 h—60 °C | Gel strength; MW distribution, peptides analysis. | Acid solution neutralized before thermal treatment. |
| Treatment | [H+] * | Acid Solution (0.5 M) pH | PSSwelling | Gelatin Solution pH | mg Hyp.ml−1 G Solution |
|---|---|---|---|---|---|
| AH (100:0) | 2.96 × 10−3 | 2.55 ± 0.07 a | 3.02 ± 0.07 c | 4.13 ± 0.04 a | 2.81 ± 0.57 |
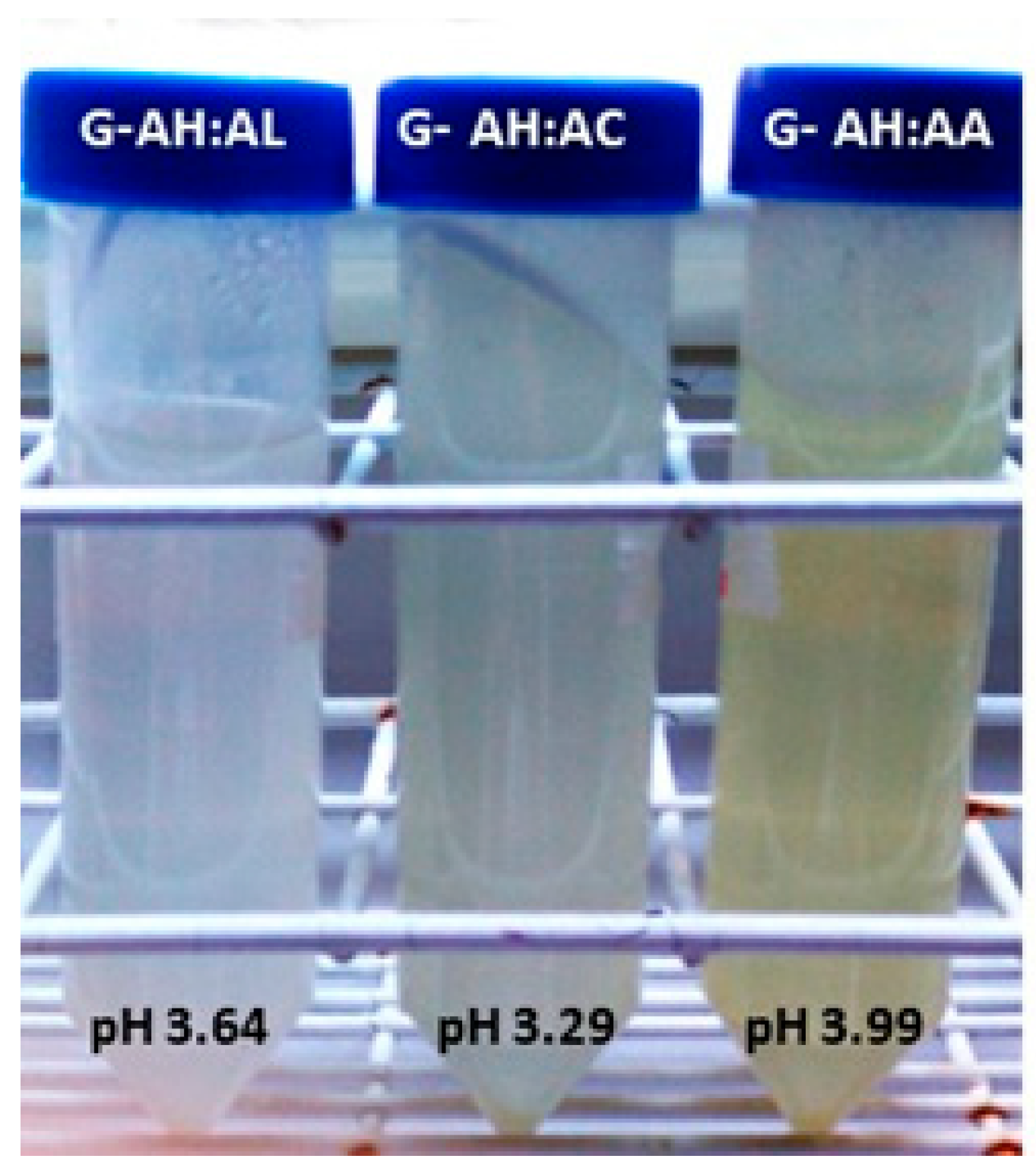
| AH:AL (75:25) | 8.82 × 10−3 | 2.12 ± 0.01 b | 4.06 ± 0.16 b | 3.64 ± 0.02 b | 2.50 ± 0.15 |
| AH:AC(75:25) | 3.05 × 10−2 | 1.74 ± 0.01 c | 3.55 ± 0.32 ab | 3.29 ± 0.02 c | 2.61 ± 0.16 |
| AH:AA(75:25) | 6.52 × 10−3 | 2.31 ± 0.01 d | 3.40 ± 0.13 a | 3.99 ± 0.01 d | 2.63 ± 0.27 |
| Treatment | mg HypG. g−1 PS (d.b.) | %YG |
|---|---|---|
| AH (100:0) | 57.11 ± 8.24 | 75.1 ± 10.8 |
| AH:AL (75:25) | 55.70 ± 3.79 | 73.2 ± 4.89 |
| AH:AC (75:25) | 55.63 ± 4.49 | 73.1 ± 5.90 |
| AH:AA (75:25) | 55.75 ± 1.36 | 73.3 ± 1.70 |
| p-value | 0.9704 | 0.7081 |
| Pearson Parameters | pH Acid Solutions | %YG | [Hyp] | Tg | Tm | ∆H | m.o. | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|---|
| r | 0.980 | 0.100 | 0.082 | 0.621 | −0.780 | −0.023 | 0.510 | −0.280 | 0.330 | 0.340 |
| 95% confidence interval | 0.90 to 1.0 | −0.50 to 0.64 | −0.37 to 0.51 | −0.074 to 0.91 | −0.94 to −0.34 | −0.64 to 0.62 | −0.52 to 0.93 | −0.64 to 0.18 | −0.13 to 0.68 | −0.12 to 0.68 |
| R squared | 0.96 | 0.01 | 0.0068 | 0.39 | 0.61 | 0.00053 | 0.26 | 0.08 | 0.11 | 0.11 |
| p-value | ||||||||||
| P-(two-tailed) | <0.0001 | 0.7515 | 0.7302 | 0.0744 | 0.0045 | 0.9499 | 0.3034 | 0.2269 | 0.1491 | 0.1472 |
| P-summary value | s | ns | ns | ns | s | ns | ns | ns | ns | ns |
| (α = 0.05) | Yes | No | No | No | Yes | No | No | No | No | No |
| Treatment | [H+]G | %m.c. * (g H2O.100 g−1) | Tg * (°C) | Tm * (°C) | ΔH * (J.g−1) |
|---|---|---|---|---|---|
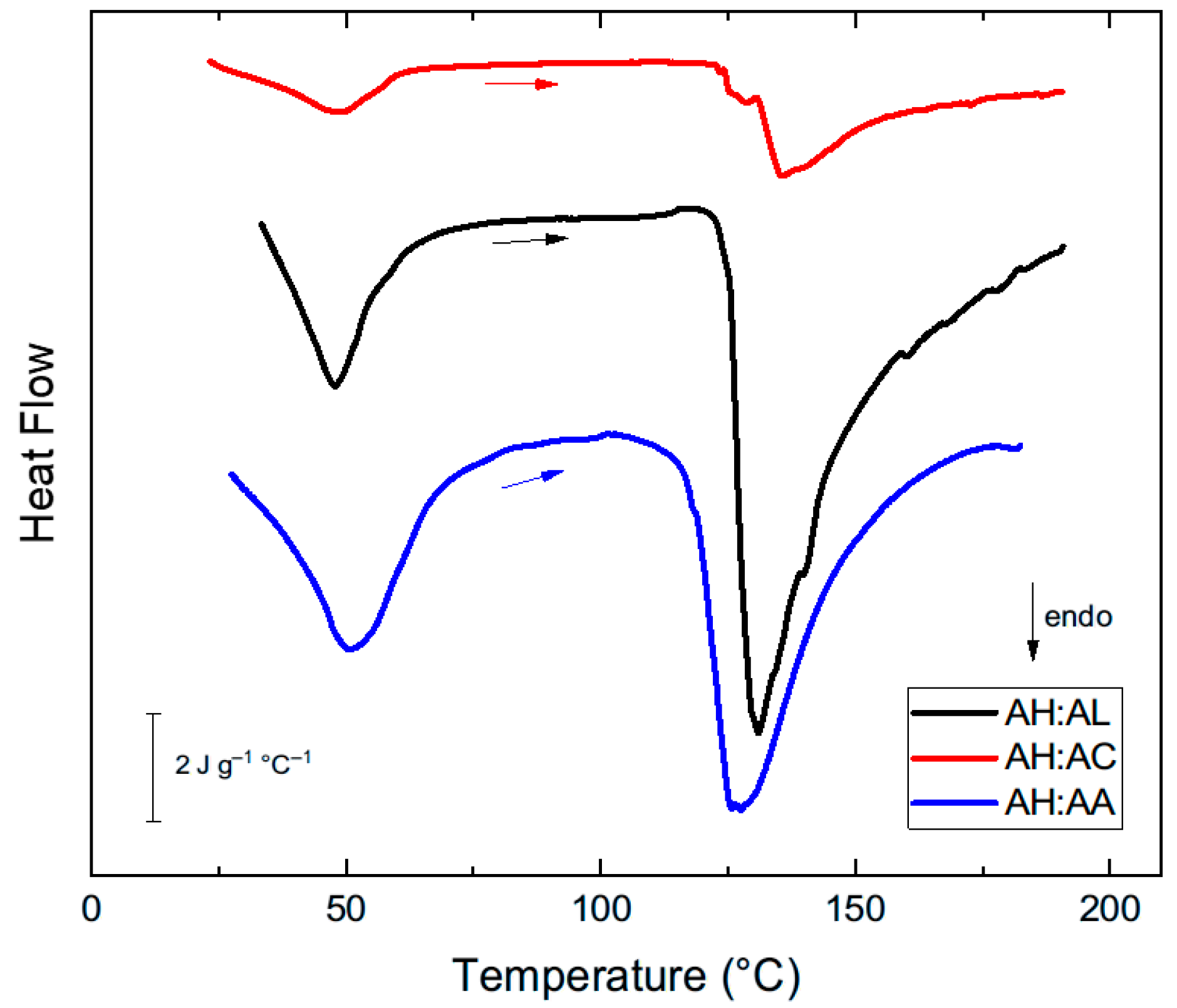
| AH:AL (75:25) | 2.29 × 10−4 | 10.47 ± 0.60 | 48.6 ± 0.5 a | 131.1 ± 0.9 ab | 180 ± 14.6 b |
| AH:AC (75:25) | 5.12 × 10−4 | 11.79 ± 0.56 | 49.1 ± 1.5 ab | 139.0 ± 3.2 a | 56.7 ± 23.4 a |
| AH:AA (75:25) | 1.02 × 10−4 | 13.29 ± 0.13 | 51.0 ± 0.2 b | 123.9 ± 5.0 b | 130 ± 31.1 ab |
| p-value | <0.001 | 0.0822 | 0.0367 | 0.0239 | 0.0038 |
| Treatment | L* | a* | b* | Films |
|---|---|---|---|---|
| AH:AL (75:25) | 84.7 ± 0.43 a | −1.64 ± 0.02 a | 11.6 ± 0.83 a |  |
| AH:AC (75:25) | 85.0 ± 1.33 a | −1.29 ± 0.28 a | 9.58 ± 2.50 a |  |
| AH:AA (75:25) | 74,7 ± 1.74 b | 3.59 ± 1.27 b | 45.2 ± 3.79 c |  |
| p-value | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velazquez, D.E.; Latorre, M.E. Effect of Organic Acid Mixtures on the Extraction Efficiency, Physicochemical, and Thermal Properties of Pigskin Gelatin and Resulting Films. Physchem 2025, 5, 38. https://doi.org/10.3390/physchem5030038
Velazquez DE, Latorre ME. Effect of Organic Acid Mixtures on the Extraction Efficiency, Physicochemical, and Thermal Properties of Pigskin Gelatin and Resulting Films. Physchem. 2025; 5(3):38. https://doi.org/10.3390/physchem5030038
Chicago/Turabian StyleVelazquez, Diego Ezequiel, and María Emilia Latorre. 2025. "Effect of Organic Acid Mixtures on the Extraction Efficiency, Physicochemical, and Thermal Properties of Pigskin Gelatin and Resulting Films" Physchem 5, no. 3: 38. https://doi.org/10.3390/physchem5030038
APA StyleVelazquez, D. E., & Latorre, M. E. (2025). Effect of Organic Acid Mixtures on the Extraction Efficiency, Physicochemical, and Thermal Properties of Pigskin Gelatin and Resulting Films. Physchem, 5(3), 38. https://doi.org/10.3390/physchem5030038






