Physicochemical Properties of Supramolecular Complexes Formed Between Cyclodextrin and Rice Bran-Derived Komecosanol
Abstract
1. Introduction
2. Materials and Methods
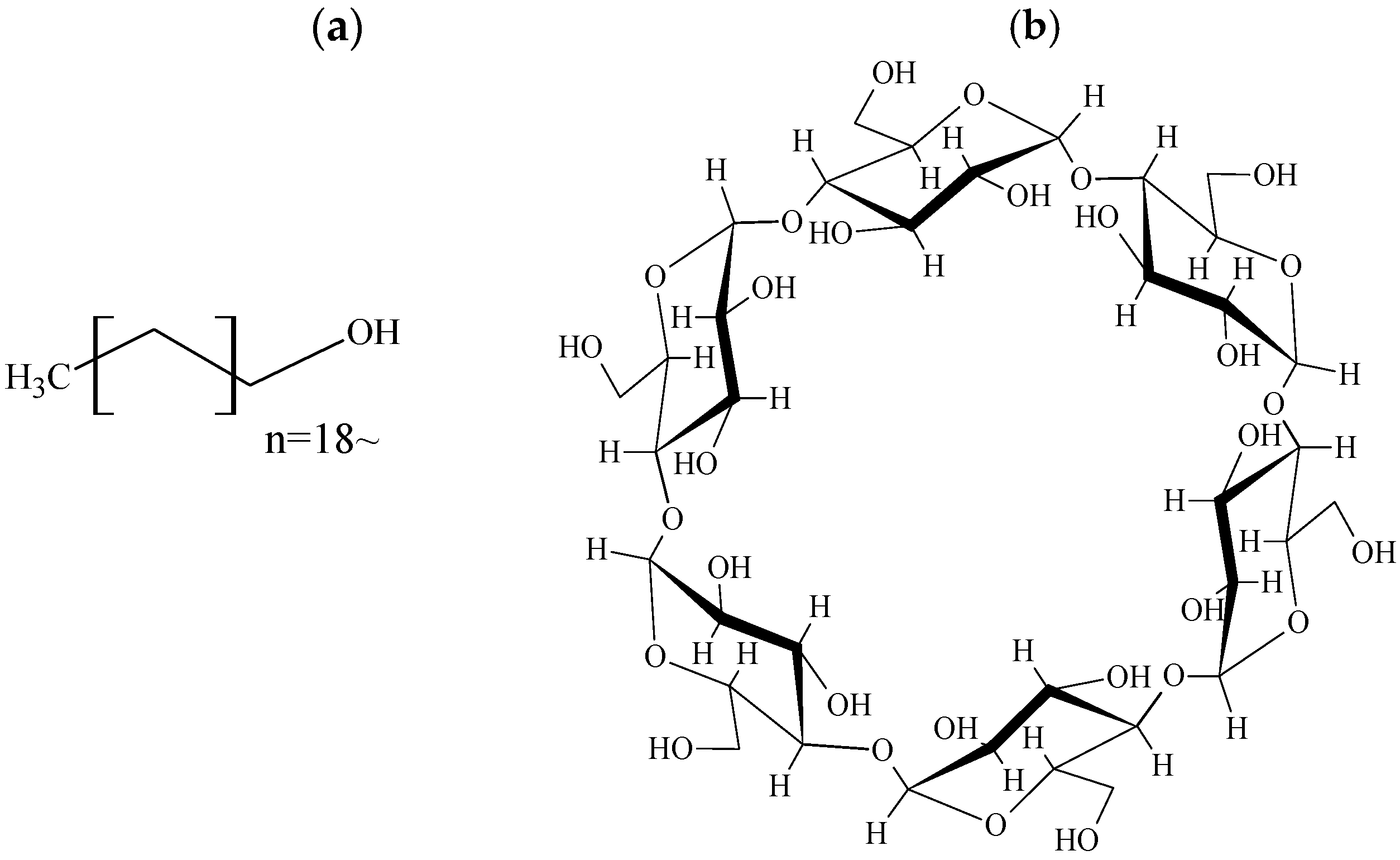
2.1. Materials
2.2. Preparation of Physical Mixtures (PMs) and 3D Ground Mixtures (3DGMs)
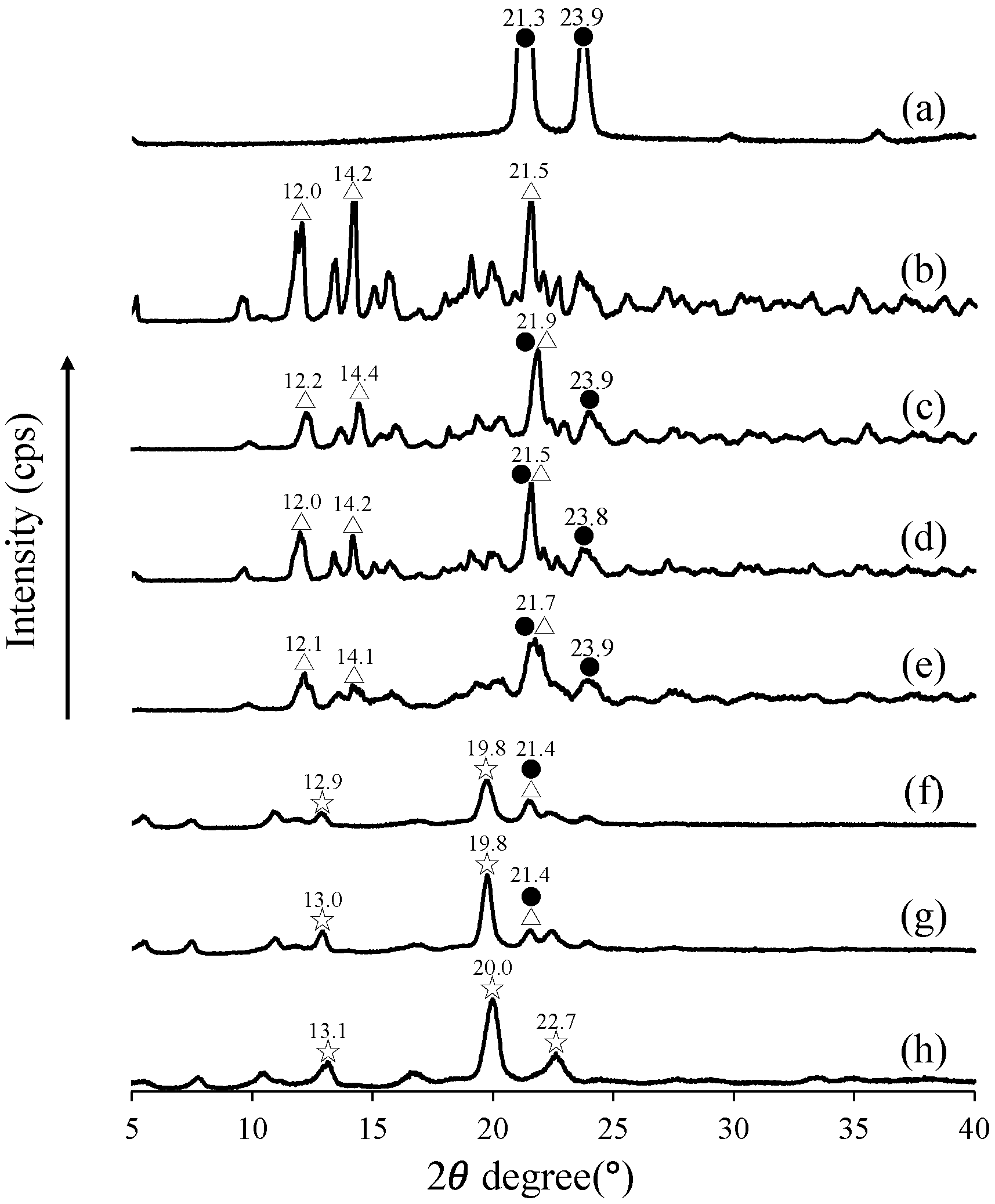
2.3. Powder X-Ray Diffraction (PXRD) Analysis
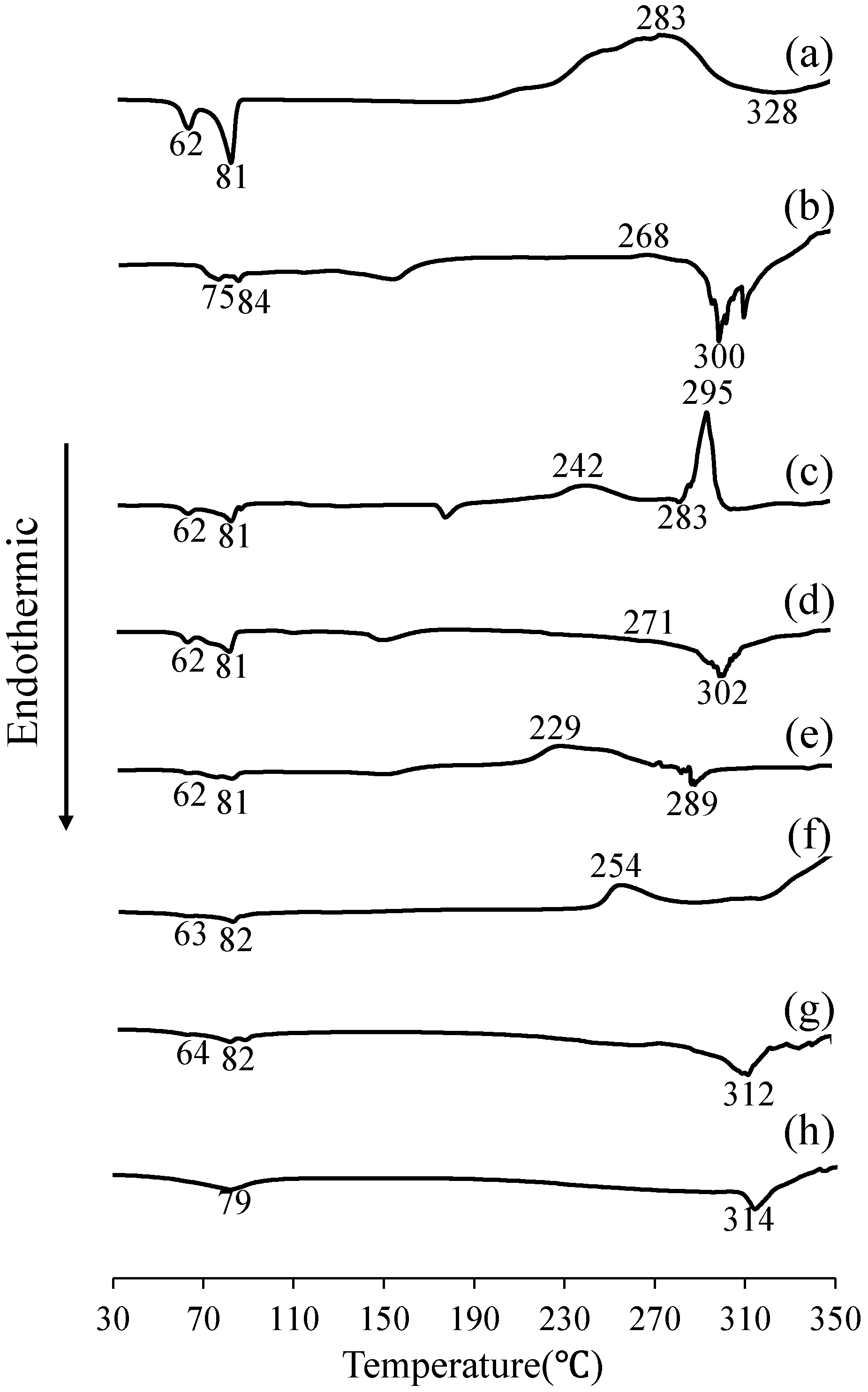
2.4. Differential Scanning Calorimetry (DSC)
2.5. Near-Infrared (NIR) Absorption Spectroscopy
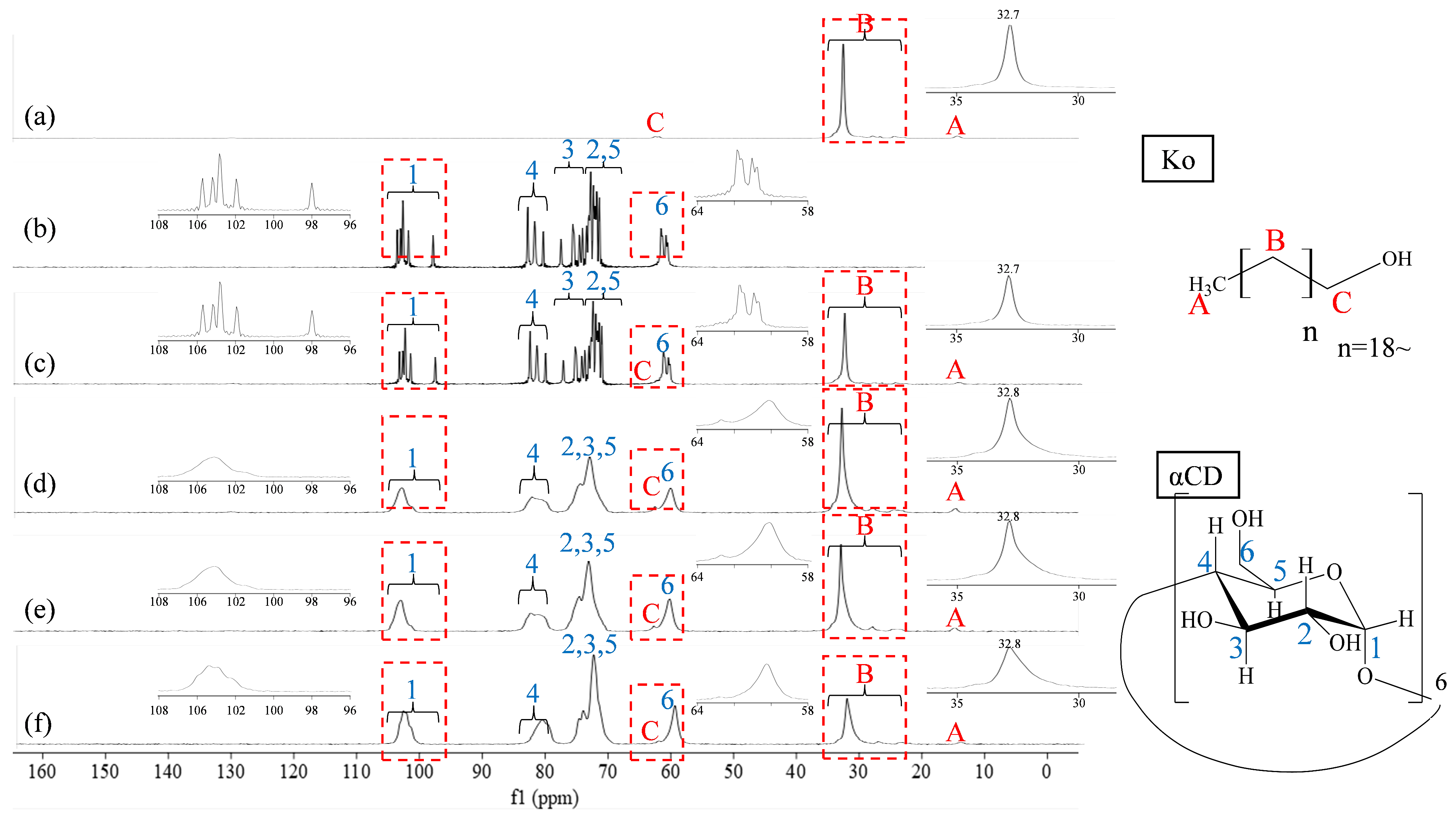
2.6. 13C Solid-State Nuclear Magnetic Resonance (NMR) Spectroscopy
2.6.1. 13C CP/MAS NMR Spectra Acquisition
2.6.2. T1 Relaxation Measurements via Saturation Recovery
2.7. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Evaluation of the Crystalline State via PXRD Analysis
3.2. Evaluation of Thermal Behavior Using DSC
3.3. Evaluation of Intermolecular Interactions Using NIR Spectroscopy
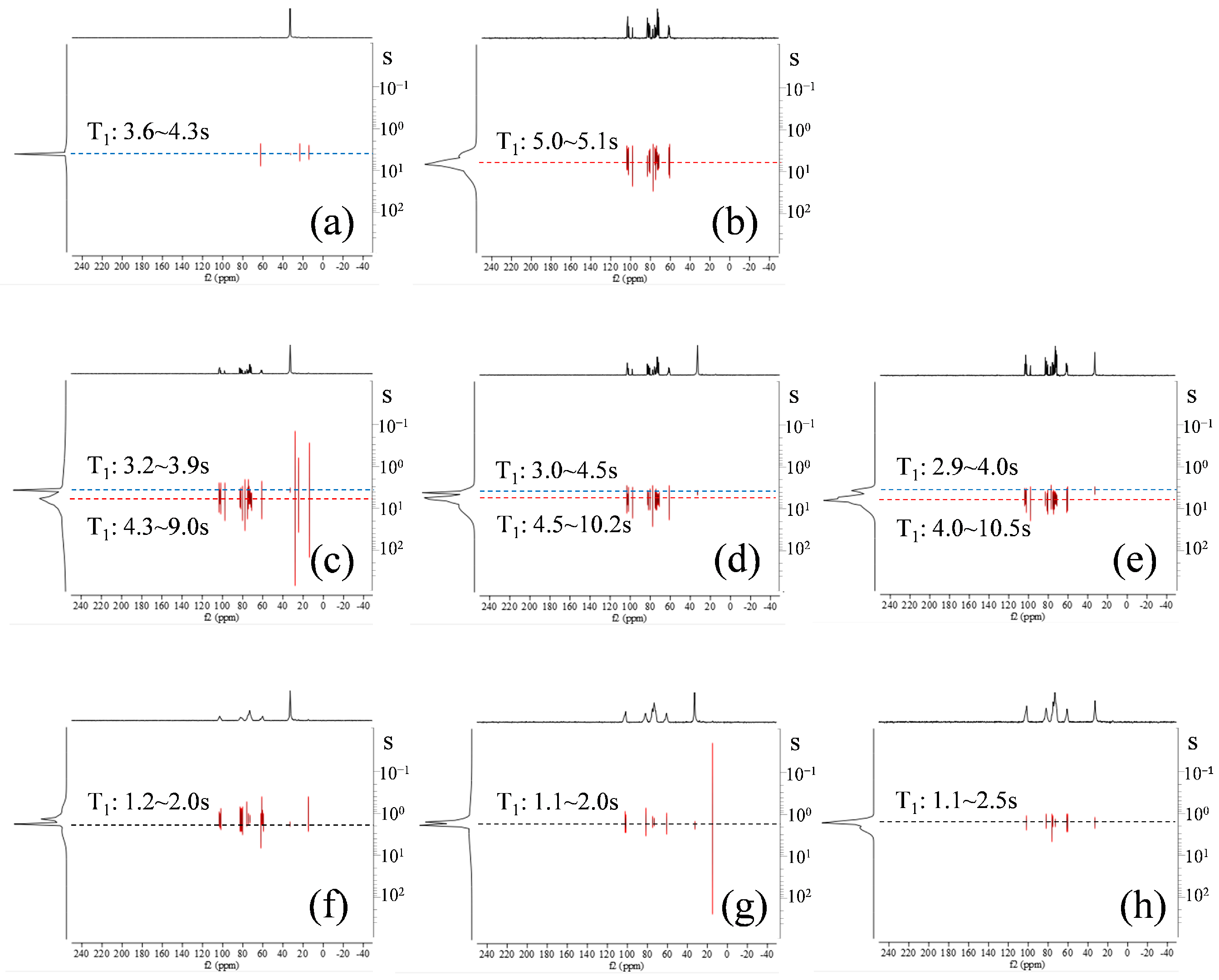
3.4. Evaluation of the Relative Positional Relationship Using 13C CP/MAS NMR Spectroscopy
3.5. T1-NMR Measurement of Ko/αCD Complexes
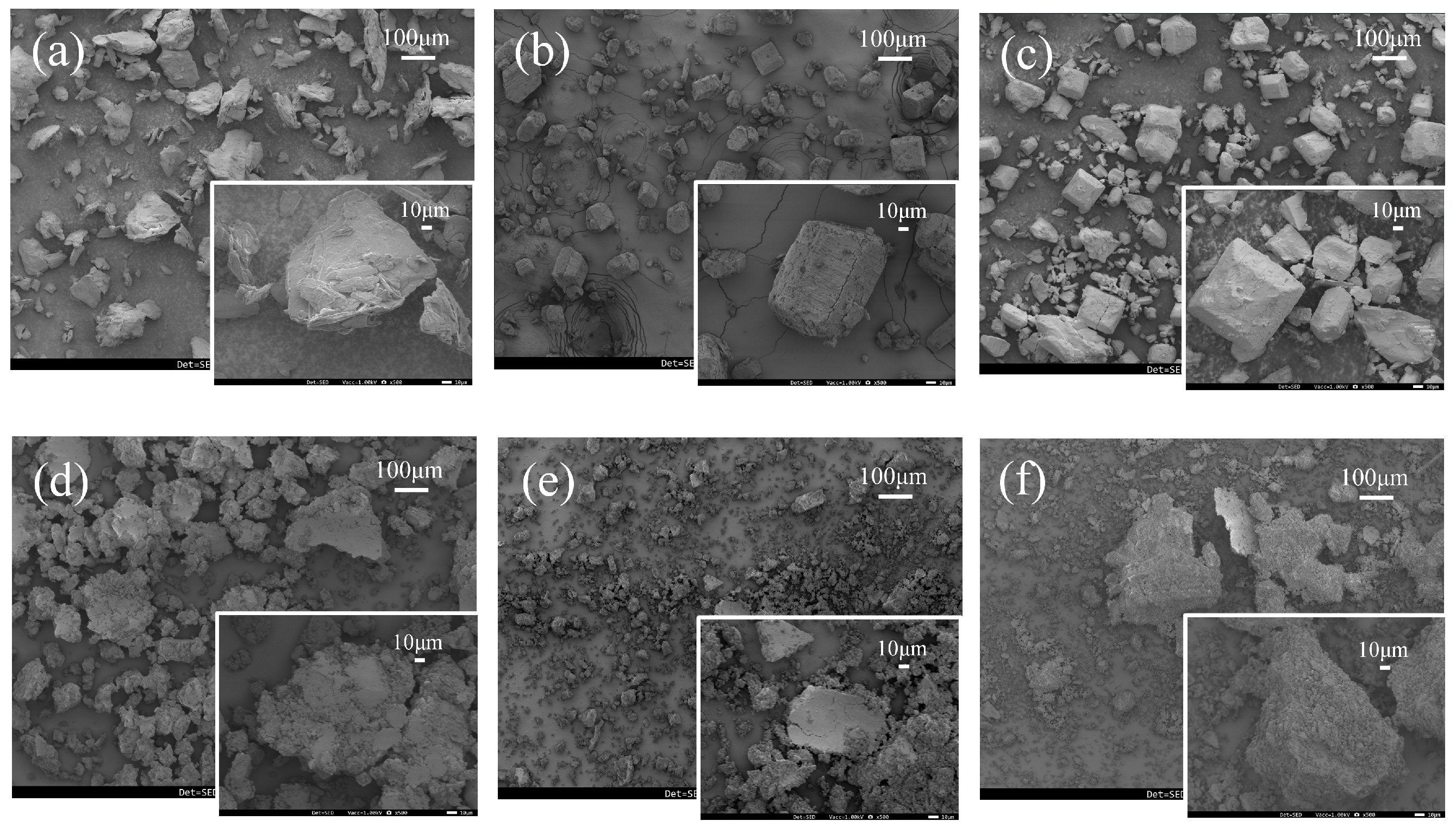
3.6. Evaluation of Morphological Properties via SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ko | Komecosanol |
| PM | Physical mixture |
| 3DGM | 3D ground mixture |
| PXRD | Powder X-ray diffraction |
| DSC | Differential scanning calorimetry |
| NIR | Near-infrared |
| NMR | Nuclear magnetic resonance |
| SEM | Scanning electron microscopy |
| DDS | Drug delivery system |
| CD | Cyclodextrin |
| PEG | Polyethylene glycol |
| CP/MAS | Cross-polarization magic angle spinning |
References
- Tufail, T.; Ain, H.B.U.; Chen, J.; Virk, M.S.; Ahmed, Z.; Ashraf, J.; Shahid, N.U.A.; Xu, B. Contemporary views of the extraction, health benefits, and industrial integration of rice bran oil: A prominent ingredient for holistic human health. Foods 2024, 13, 1305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanaka, T.; Hoshina, M.; Tanabe, S.; Sakai, K.; Ohtsubo, S.; Taniguchi, M. Production of D-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour. Technol. 2006, 97, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Scott, S.D.; Pekas, E.J.; Lee, J.-G.; Park, S.-Y. Improvement of lipids and reduction of oxidative stress with octacosanol after Taekwondo training. Int. J. Sports Physiol. Perform. 2019, 14, 1297–1303. [Google Scholar] [CrossRef]
- Kaushik, M.K.; Aritake, K.; Takeuchi, A.; Yanagisawa, M.; Urade, Y. Octacosanol restores stress-affected sleep in mice by alleviating stress. Sci. Rep. 2017, 7, 8892. [Google Scholar] [CrossRef] [PubMed]
- Crespo, N.; Illnait, J.; Mas, R.; Fernandez, L.; Fernandez, J.; Castano, G. Comparative study of the efficacy and tolerability of policosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int. J. Clin. Pharmacol. Res. 1999, 19, 117–127. [Google Scholar] [PubMed]
- Ohkubo, T.; Ito, T.; Hibino, H. Feasibility study on the use of policosanol to reduce blood acetaldehyde levels in ethanol-administered humans. Alcohol Clin. Exp. Res. 2009, 33, 1407–1412. [Google Scholar] [CrossRef][Green Version]
- Cho, K.H.; Kim, J.E.; Komatsu, T.; Uehara, Y. Protection of liver functions and improvement of kidney functions by twelve weeks consumption of Cuban policosanol (Raydel®) with a decrease of glycated hemoglobin and blood pressure from a randomized, placebo-controlled, and double-blinded study with healthy and middle-aged Japanese participants. Life 2023, 13, 1319. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Cyclodextrins: A versatile tool for drug delivery systems. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Stevens, D.A. Itraconazole in cyclodextrin solution. Pharmacotherapy 1999, 19, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Inoue, Y.; Ikeda, N.; Murata, I.; Kanamoto, I. Improvement of stability due to a cyclamen aldehyde/β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1215, 128161. [Google Scholar] [CrossRef]
- Tachikawa, R.; Saito, H.; Moteki, H.; Kimura, M.; Kitagishi, H.; Arce, F., Jr.; See, G.L.; Tanikawa, T. Preparation, characterization, and in vitro evaluation of inclusion complexes formed between S-allylcysteine and cyclodextrins. ACS Omega 2022, 7, 1064–1073. [Google Scholar] [CrossRef]
- Ikeda, N.; Inoue, Y.; Ogata, Y.; Murata, I.; Meiyan, X.; Takayama, J.; Sakamoto, T.; Okazaki, M.; Kanamoto, I. Improvement of the solubility and evaluation of the physical properties of an inclusion complex formed by a new ferulic acid derivative and γ-cyclodextrin. ACS Omega 2020, 5, 12073–12080. [Google Scholar] [CrossRef]
- Ezawa, T.; Inoue, Y.; Murata, I.; Takao, K.; Sugita, Y.; Kanamoto, I. Characterization of the dissolution behavior of piperine/cyclodextrins inclusion complexes. AAPS PharmSciTech 2018, 19, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Osada, M.; Murata, I.; Kobata, K.; Kanamoto, I. Evaluation of solubility characteristics of a hybrid complex of components of soy. ACS Omega 2019, 4, 8632–8640. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kamachi, M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules 1990, 23, 2821–2823. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The polyrotaxane gel: A topological gel by figure-of-eight cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Molero, G.; Liu, C.; Zhu, Z.; Chen, Q.; Peterson, S.R.; Kolluru, P.V.; Sue, H.J.; Uenuma, S.; Mayumi, K.; Ito, K. Fracture behavior of polyrotaxane-toughened poly(methyl methacrylate). Langmuir 2022, 38, 2335–2345. [Google Scholar] [CrossRef]
- Wang, X.-S.; Kim, H.-K.; Fujita, Y.; Sudo, A.; Nishida, H.; Endo, T. Relaxation and Reinforcing Effects of Polyrotaxane in an Epoxy Resin Matrix. Macromolecules 2006, 39, 1046–1052. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Controlled Delivery of Ibuprofen from Poly(vinyl alcohol)-Poly(ethylene glycol) Interpenetrating Polymeric Network Hydrogels. J. Pharm. Anal. 2019, 9, 108–116. [Google Scholar] [CrossRef]
- Ooya, T.; Yui, N. Multivalent interactions between biotin–polyrotaxane conjugates and streptavidin as a model of new targeting for transporters. J. Control Release 2002, 80, 219–228. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Harada, A.; Okada, M.; Kawaguchi, Y. Formation of Self-assembled Tubular Structures by Mixing Cyclodextrin and Polymers without Solvents. Chem. Lett. 2005, 34, 542–543. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Liu, F.; He, Y.; Wang, L. Discrimination of β-cyclodextrin inclusion complexes using near-infrared spectroscopy and chemometrics. Anal. Chim. Acta 2011, 689, 75–81. [Google Scholar] [CrossRef]
- Chierotti, M.R.; Gobetto, R. Solid-State NMR Studies of Weak Interactions in Supramolecular Systems. Chem. Commun. 2008, 1621–1634. [Google Scholar] [CrossRef]
- Fadler, R.E.; Flood, A.H. Rigidity and Flexibility in Rotaxanes and Their Relatives; On Being Stubborn and Easy-Going. Front. Chem. 2022, 10, 856173. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Preparation and properties of inclusion complexes of polyethylene glycol with alpha-cyclodextrin. Macromolecules 1993, 26, 5698–5703. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Hayakawa, Y.; Nishida, M. Solid-State Nuclear Magnetic Resonance (NMR) and Nuclear Magnetic Relaxation Time Analyses of Molecular Mobility and Compatibility of Plasticized Polyhydroxyalkanoates (PHA) Copolymers. Polymers 2018, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K. Recent Aspects of Pharmaceutical Application of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 3–7. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; Wang, X.; Yi, C.; Su, S. pH-Responsive Pseudorotaxane between Comblike PEO-Grafted Triblock Polymer and α-Cyclodextrin. Colloid Polym. Sci. 2014, 292, 3243–3249. [Google Scholar] [CrossRef]
- Lyon, A.P.; Banton, N.J.; Macartney, D.H. Kinetics of the Self-Assembly of α-Cyclodextrin [2]Pseudorotaxanes with Polymethylene Threads Bearing Quaternary Ammonium and Phosphonium End Groups. Can. J. Chem. 1998, 76, 666–674. [Google Scholar] [CrossRef]
- Castelletto, V.; Kowalczyk, R.M.; Seitsonen, J.; Hamley, I.W. Tuning the solution self-assembly of a peptide-PEG (polyethylene glycol) conjugate with α-cyclodextrin. ChemBioChem 2023, 24, e202200743. [Google Scholar] [CrossRef]
- Tuntipopipat, S.; Tajarati, S.; Nesterenko, P.N.; Paull, B. Inclusion Complexes of Policosanol with β-Cyclodextrin and Its Derivatives. J. Pharm. Sci. 2006, 95, 1825–1834. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchimura, M.; Ohtsu, A.; Tomita, J.; Ishida, Y.; Nakata, D.; Terao, K.; Inoue, Y. Physicochemical Properties of Supramolecular Complexes Formed Between Cyclodextrin and Rice Bran-Derived Komecosanol. Physchem 2025, 5, 34. https://doi.org/10.3390/physchem5030034
Uchimura M, Ohtsu A, Tomita J, Ishida Y, Nakata D, Terao K, Inoue Y. Physicochemical Properties of Supramolecular Complexes Formed Between Cyclodextrin and Rice Bran-Derived Komecosanol. Physchem. 2025; 5(3):34. https://doi.org/10.3390/physchem5030034
Chicago/Turabian StyleUchimura, Mione, Akiteru Ohtsu, Junki Tomita, Yoshiyuki Ishida, Daisuke Nakata, Keiji Terao, and Yutaka Inoue. 2025. "Physicochemical Properties of Supramolecular Complexes Formed Between Cyclodextrin and Rice Bran-Derived Komecosanol" Physchem 5, no. 3: 34. https://doi.org/10.3390/physchem5030034
APA StyleUchimura, M., Ohtsu, A., Tomita, J., Ishida, Y., Nakata, D., Terao, K., & Inoue, Y. (2025). Physicochemical Properties of Supramolecular Complexes Formed Between Cyclodextrin and Rice Bran-Derived Komecosanol. Physchem, 5(3), 34. https://doi.org/10.3390/physchem5030034






