Increasing the Probability of Obtaining Intergrown Mixtures of Nanostructured Manganese Oxide Phases Under Solvothermal Conditions by Mixing Additives with Weak and Strong Chelating Natures
Abstract
1. Introduction
2. Materials and Methods
2.1. MnxOy Materials Synthesis
2.2. Characterization
3. Results and Discussion
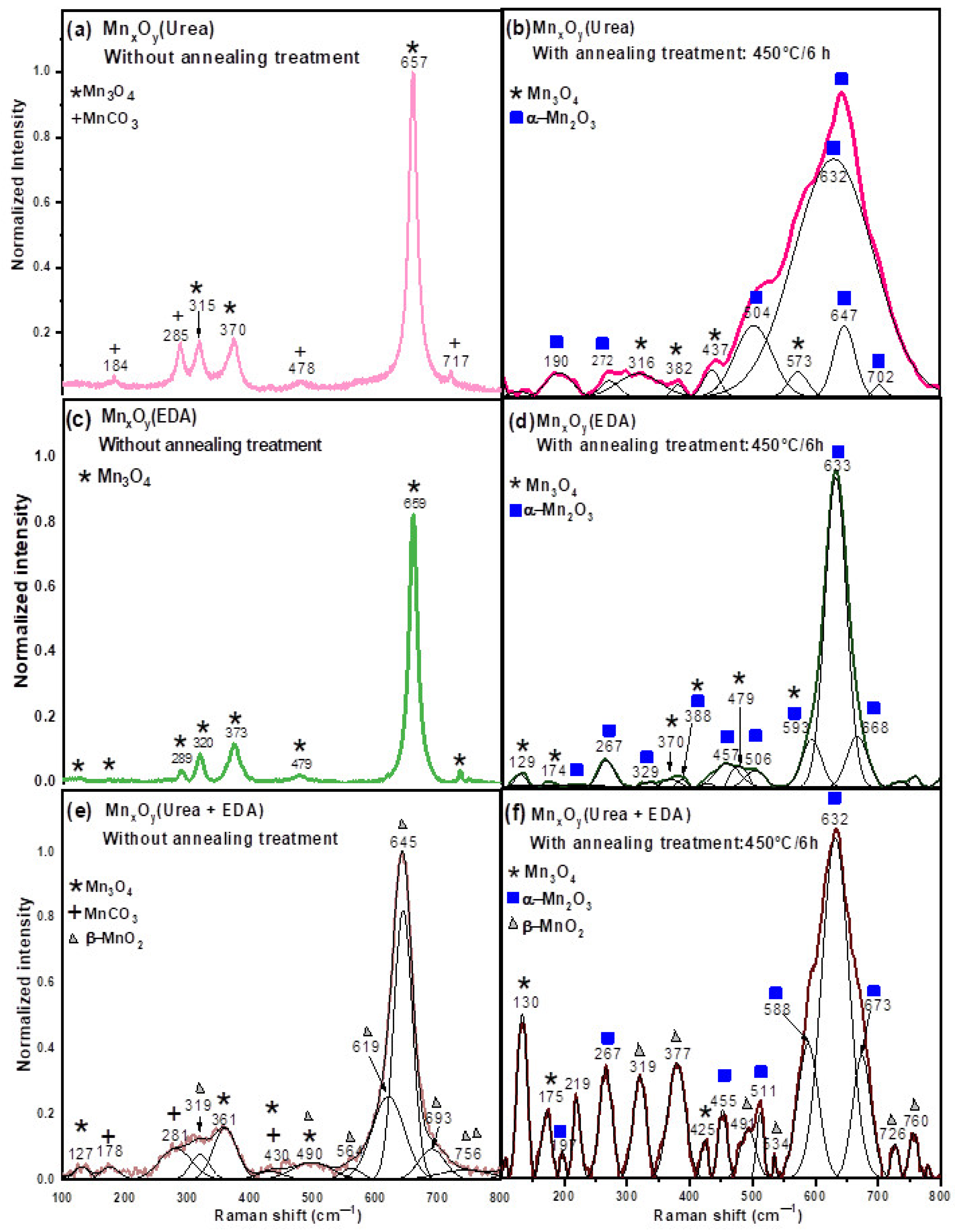
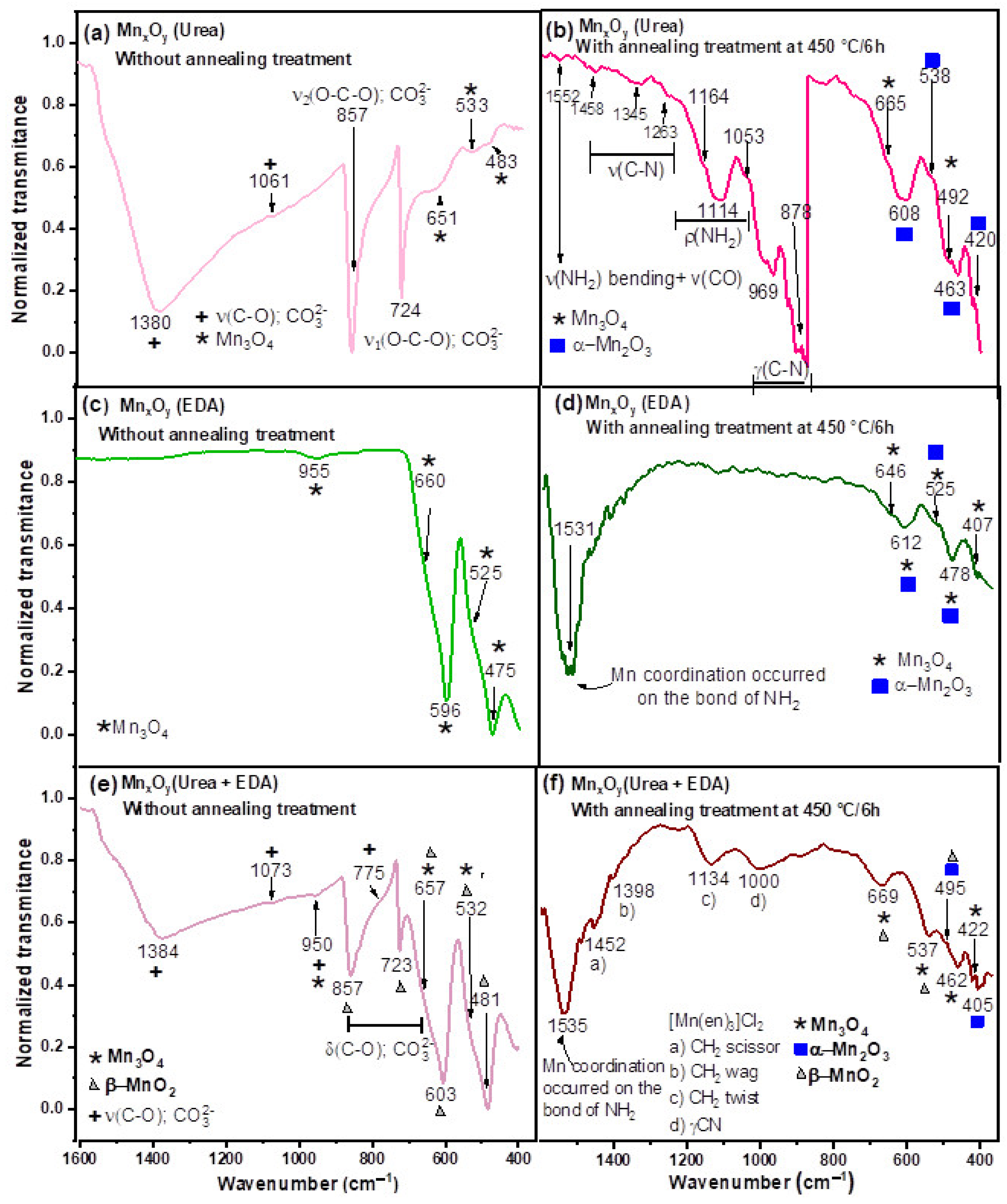
- (a)
- Chemical reactions using urea as a reaction medium
- An aqueous solution was prepared with manganese (II) chloride and deionized water, which forms manganese ions, according to the reaction [133]:MnCl2 + H2O → Mn2+ + 2Cl−
- Afterward, an aqueous solution of urea was prepared separately, and then both solutions were mixed and transferred to a Teflon container and then to a stainless-steel autoclave. This resulting solution could form manganese carbonate as a decomposition product, according to the chemical reactions [134,135,136]:
- (b)
- Chemical reactions using EDA as a reaction medium
- (c)
- Chemical reactions using the urea–EDA system as a reaction medium
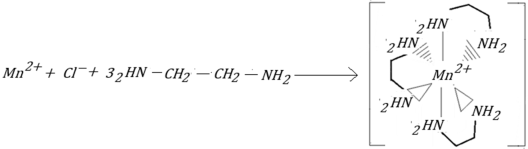
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calegaro, M.L.; Lima, F.H.B.; Ticianelli, E.A. Oxygen reduction reaction on nanosized manganese oxide particles dispersed on carbon in alkaline solutions. J. Power Sources 2006, 158, 735–739. [Google Scholar] [CrossRef]
- Bezdička, P.; Grygar, T.; Klápŝtê, B.; Vondrák, J. MnOx:C composites as electrode materials. I. Synthesis, XRD and cyclic volammetric investigation. Electrochim. Acta 1999, 45, 913–920. [Google Scholar] [CrossRef]
- Roche, I.; Scott, K. Carbon-supported manganese oxide nanoparticles as electrocatalysts for oxygen reduction reaction (ORR). J. Appl. Electrochem. 2009, 39, 197–204. [Google Scholar] [CrossRef]
- Ghos, S.; Kar, P.; Bhandary, N.; Basu, S.; Maiyalagan, T.; Sardar, S.; Kumar Pal, S. Reduced graphene oxide supported hierarchical flower like manganese oxide as efficient electrocatalysts toward reduction and evolution of oxygen. Int. J. Hydrogen Energy 2017, 42, 4111–4122. [Google Scholar] [CrossRef]
- Ryabova, A.S.; Bonnefont, A.; Zagrebin, P.; Poux, T.; Paria Sena, R.; Hadermann, J.; Abakumov, A.M.; Kéranguéven, G.; Istomin, S.Y.; Antipov, E.V.; et al. Study of hydrogen peroxide reactions on manganese oxides as a tool to decode the oxygen reduction reaction mechanism. Chem. ElectroChem 2016, 3, 1667–1677. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, L.; Yan, M.; Han, C.; He, L.; Hercule, K.M.; Niu, C.; Yuan, Z.; Xu, W.; Qu, L.; et al. Manganese oxide/carbon yolk-shell nanorod anodes for high-capacity lithium batteries. Nano Lett. 2015, 15, 738–744. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as electrode materials for energy storage. J. Nanomater. 2017, 7, 396. [Google Scholar] [CrossRef]
- Hashema, A.M.; Abdel-Latif, A.M.; Abuzeid, H.M.; Abbas, H.M.; Ehrenberg, H.; Farag, R.S.; Mauger, A.; Julien, C.M. Improvement of the electrochemical performance of nanosized α-MnO2 used as cathode material for Li-batteries by Sn-doping. J. Alloys Compd. 2011, 509, 9669–9674. [Google Scholar] [CrossRef]
- Delmondo, L.; Salvador, G.P.; Muñoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Gerosa, M.; Massaglia, G.; Chiodoni, A.; Quaglio, M. Nanostructured MnxOy for oxygen reduction reaction (ORR) catalysts. Appl. Surf. Sci. 2016, 388, 631–639. [Google Scholar] [CrossRef]
- Augustin, M.; Fenske, D.; Bardenhagen, I.; Westphal, A.; Knipper, M.; Plaggenborg, T.; Kolny-Olesiak, J.; Parisi, J. Manganese oxide phases and morphologies: A study on calcination temperature and atmospheric dependence. Beilstein J. Nanotechnol. 2015, 6, 47–59. [Google Scholar] [CrossRef]
- Ghosh, S.; Kar, P.; Bhandary, N.; Basu, S.; Sardar, S.; Maiyalagan, T.; Majumdar, D.; Bhattacharya, S.K.; Bhaumik, A.; Lemmens, P.; et al. Microwave-assisted synthesis of porous Mn2O3 nanoballs as bifunctional electrocatalyst for oxygen reduction and evolution reaction. Catal. Sci. Technol. 2016, 6, 1417–1429. [Google Scholar] [CrossRef]
- Luo, X.F.; Wang, J.; Liang, Z.S.; Chen, S.Z.; Liu, Z.L.; Xu, C.W. Manganese oxide with different morphology as efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 7151–7157. [Google Scholar] [CrossRef]
- Bhandary, N.; Ingole, P.P.; Basu, S. Electrosynthesis of Mn-Fe oxide nanopetals on carbon paper as bi-functional electrocatalyst for oxygen reduction and oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 3165–3171. [Google Scholar] [CrossRef]
- Prasetya, P.; Awaluddin, A.; Muhdarina, M.; Saputra, E.; Linggawati, A.; Nurhayati, N.; Fudholi, A. Solvothermal synthesis of α-MnO2 and Mn2O3 for efficient catalytic dye degradation. Case Stud. Chem. Environ. Eng. 2025, 12, 101242. [Google Scholar] [CrossRef]
- Deng, B.; Huang, H. Hydrothermal synthesis and characterization of Mn2O3 nanowires. Adv. Mat. Res. 2014, 1033, 1040–1043. [Google Scholar]
- Wang, X.; Li, Y. Selected-control hydrothermal synthesis of α-and β-MnO2 single crystal nanowires. J. Am. Chem. Soc. 2002, 124, 2880–2881. [Google Scholar] [CrossRef]
- Pang, S.C.; Chin, S.F.; Ling, C.Y. Controlled synthesis of manganese dioxide nanostructures via a facile hydrothermal route. J. Nanomater. 2012, 2012, 607870. [Google Scholar] [CrossRef]
- Taliakos, M.; Katsoulakou, E.; Terzis, A.; Raptopoulou, C.; Cordopatis, P.; Manessi-Zoupa, E. The dipeptide H-Aib-l-Ala-OH ligand in copper(II) chemistry: Variation of product identity as a function of pH. Inorg. Chem. Commun. 2005, 8, 1085–1089. [Google Scholar] [CrossRef]
- Qian, C.; Liu, G.; Okamura, T.; Huang, Y.; Sun, W.; Ueyama, N. Structure modulation of metal–organic frameworks via reaction pH: Self-assembly of a new carboxylate containing ligand N-(3-carboxyphenyl)iminodiacetic acid with cadmium(II) and cobalt(II) salts. Polyhedron 2008, 27, 812–820. [Google Scholar]
- Deng, Z.; Li, L.; Li, Y. Novel inorganic-organic-layered structures: Crystallographic understanding of both phase and morphology formations of one-dimensional CdE (E=S, Se, Te) nanorods in ethylenediamine. Inorg. Chem. 2003, 42, 2331–2341. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Qiao, T.; Hu, X.Y. Preparation of Mn3O4 nanocrystallites by low-temperature solvothermal treatment of γ-MnOOH nanowires. J. Solid. State Chem. 2004, 177, 4093–4097. [Google Scholar] [CrossRef]
- Hazarika, K.K.; Goswami, C.; Saikia, H.; Borah, B.J.; Bharali, P. Cubic Mn2O3 nanoparticles on carbon as bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Mol. Catal. 2018, 451, 153–160. [Google Scholar]
- Qiu, G.; Huang, H.; Dharmarathna, S.; Benbow, E.; Stafford, L.; Suib, S.L. Hydrothermal synthesis of manganese oxide nanomaterials and their catalytic and electrochemical properties. Chem. Mater. 2011, 23, 3892–3901. [Google Scholar] [CrossRef]
- Sawant, V.A.; Gotpagar, S.N.; Yamgar, B.A.; Sawant, S.K.; Kankariya, R.D.; Chavan, S.S. Characterization and electrochemical studies of Mn(II), Co(II), Ni(II) and Cu(II) complexes with 2-mercapto-3-substituted-quinazolin-4-one and 1,10-phenanthroline or ethylenediamine as ligands. Spectrochim. Acta Part. 2009, 72, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Dollé, M.; Patoux, S.; Doeff, M.M. Layered manganese oxide intergrowth electrodes for rechargeable lithium batteries. 1. Substitution with Co or Ni. Chem. Mater. 2005, 17, 1036–1043. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Zhu, Y.; Jin, S.; Wang, Y.; Qian, Y. LiNi0.5Mn1.5O4 nanostructures with two-phase intergrowth as enhanced cathodes for lithium-ion batteries. Electrochim. Acta 2014, 121, 253–257. [Google Scholar] [CrossRef]
- Smith, R.L.; Sławiński, W.A.; Lind, A.; Wragg, D.S.; Cavka, J.H.; Arstad, B.; Fjellvåg, H.; Attfield, M.P.; Akporiaye, D.; Anderson, M.W. Nanoporous intergrowths: How crystal growth dictates phase composition and hierarchical structure in the CHA/AEI system. Chem. Mater. 2015, 27, 4205–4215. [Google Scholar] [CrossRef]
- Porta, A.; Visconti, C.G.; Castoldi, L.; Matarrese, R.; Jeong-Potter, C.; Farrauto, R.; Lietti, L. Ru-Ba synergistic effect in dual functioning materials for cyclic CO2 capture and methanation. Appl. Catal. 2021, 283, 119654. [Google Scholar] [CrossRef]
- Charenton, J.C.; Strobel, P. Experimental evidence of the ramsdellite-rutile intergrowth model in electrolytic γ-MnO2. J. Solid. State Chem. 1988, 77, 33–39. [Google Scholar] [CrossRef]
- Alemayehu, M.B.; Ta, K.; Falmbigl, M.; Johnson, D.C. Structure, stability, and properties of the intergrowth compounds ([SnSe]1+δ)m(NbSe2)n, where m = n = 1–20. J. Am. Chem. Soc. 2015, 137, 4831–4839. [Google Scholar] [CrossRef]
- Galliez, K.; Deniard, P.; Petit, P.; Lambertin, D.; Bart, F.; Jobic, S. Modelling and quantification of intergrowth in γ-MnO2 by laboratory pair distribution function analysis. J. Appl. Crystallogr. 2014, 47, 552–560. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, P.; Maitra, A. Size-dependent catalytic behavior of platinum nanoparticles on the hexacyanoferrate(III)/thiosulfate redox reaction. J. Colloid. Interface Sci. 2003, 265, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Song, J.; Zhu, T.; Xu, W. Structure-activity relationship of manganese oxide catalysts for the catalytic oxidation of (chloro)-VOCs. Catalysts 2019, 9, 726. [Google Scholar] [CrossRef]
- Patoux, S.; Dollé, M.; Doeff, M.M. Layered manganese oxide intergrowth electrodes for rechargeable lithium batteries. 2. Substitution with Al. Chem. Mater. 2005, 17, 1044–1054. [Google Scholar] [CrossRef]
- Paweł, B.; Paweł, S.; Paweł, B.; Wacław, M.; Andrzej, K. Demonstration of the influence of specific surface area on reaction rate in heterogeneous catalysis. J. Chem. Educ. 2021, 98, 935–940. [Google Scholar] [CrossRef]
- Dey, S.; Kumar, V.V.P. The performance of highly active manganese oxide catalysts for ambient conditions carbon monoxide oxidation. Curr. Opin. Green Sustain. Chem. 2020, 3, 100012–100026. [Google Scholar] [CrossRef]
- Alan Palmer, R.; Chin-Lan Yang, M.; Hempel, J.C. Electronic Structure of the Tris(ethylenediamine) manganese(II) Ion. Circular and Linear Dichroism and Electron Paramagnetic Resonance Spectra of Mn(en)3(NO3)2. Inorg. Chem. 1978, 17, 1200–1203. [Google Scholar] [CrossRef]
- Kaduk, J.A.; Billinge, S.J.L.; Dinnebier, R.E.; Henderson, N.; Madsen, I.; Černý, R.; Leoni, M.; Lutterotti, L.; Thakral, S.; Chateigner, D. Powder diffraction. Nat. Rev. Methods Primers 2021, 1, 77–81. [Google Scholar] [CrossRef]
- Suescun, L. International Tables for Crystallography, Volume H, Powder Diffraction. J. Appl. Crystallogr. 2021, 54, 710–713. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Hupp, B.N.; Donovan, J.J. Quantitative mineralogy for facies definition in the Marcellus Shale (Appalachian Basin, USA) using XRD-XRF integration. Sediment. Geol. 2018, 371, 16–31. [Google Scholar] [CrossRef]
- Marques, C.F.; Olhero, S.; Abrantes, J.C.C.; Marote, A.; Ferreira, S.; Vieira, S.I.; Ferreira, J.M.F. Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Hübschen, G.; Altpeter, I.; Tschuncky, R.; Herrmann, H.-G.J. X-ray diffraction (XRD) techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Woodhead Publishing: Sawston, UK, 2016; pp. 81–124. [Google Scholar]
- Bratoev, B.; Doykov, I.; Ninov, J.; Lenchev, A. Pozzolanic activity assessment of calcined clays with complex minerals content. Adv. Cem. Res. 2018, 30, 103–112. [Google Scholar] [CrossRef]
- Qinyuan, H.; Chunjian, W.; Quan, S. Quantitative Deviation of Nanocrystals Using the RIR Method in X-ray Diffraction (XRD). Nanomaterials 2022, 12, 2320. [Google Scholar] [CrossRef]
- Wolfgang, B.; Quiroga-González, E.; Kienle, L.; Duppel, V.; Lee, D.-K.; Janek, J. In-CuInS2 nanocomposite film prepared by pulsed laser deposition using a single source precursor. Solid State Sci. 2010, 12, 1953–1959. [Google Scholar]
- Scardi, P. IUCr Commission on Powder Diffraction News; International Union of Crystallography: Chester, UK, 2000; pp. 24–27. [Google Scholar]
- Madsen, I.C.; Scarlett, N.V.; Cranswick, L.M.; Lwin, T. Outcomes of the International Union of Crystallography Commission on Powder Diffraction Round Robin on Quantitative Phase Analysis: Samples 1a to 1h. J. Appl. Cryst. 2001, 34, 409–426. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Madsen, I.C.; Cranswick, L.M.; Lwin, T.; Groleau, E.; Stephenson, G.; Aylmore, M.; Agron-Olshina, N. Outcomes of the International Union of Crystallography 20. Commission on Powder Diffraction Round Robin on Quantitative Phase Analysis: Samples 2, 3, 4, synthetic bauxite, natural granodiorite and pharmaceuticals. J. Appl. Cryst. 2002, 35, 383–400. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld method, International Union of Crystallography; Oxford University Press: New York, NY, USA, 1993; pp. 298–300. [Google Scholar]
- Bish, D.L.; Post, J.E. Modern Powder Diffraction, Reviews in Mineralogy Vol 20; Mineralogical Society of America: Washington, DC, USA, 1989; pp. 1–10. [Google Scholar]
- Etter, M.; Müller, M.; Hanfland, M.; Dinnebier, R.E. Possibilities and limitations of parametric Rietveld refinement on high pressure data: The case study of LaFeO. Z. Kristallogr. 2014, 229, 246–258. [Google Scholar]
- Rietveld, H.M. The Rietveld method. Phys. Scr. 2014, 89, 098002. [Google Scholar] [CrossRef]
- Toby, B.H. A simple solution to the Rietveld refinement recipe problem. J. Appl. Cryst. 2024, 57, 175–180. [Google Scholar] [CrossRef]
- León-Reina, L.; García-Maté, M.; Álvarez-Pinazo, G.; Santacruz, I.; Vallcorba, O.; De la Torre Aranda, M.A. Accuracy in Rietveld quantitative phase analysis: A comparative study of strictly monochromatic Mo and Cu radiations. J. Appl. Crystallogr. 2016, 12, 722–735. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louërd, D.; Scardie, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: London, UK, 2001; pp. 359–374. [Google Scholar]
- Namigata, H.; Watanabe, K.; Welling, T.A.J.; Suga, K.; Nagao, D. Colloidal photonic crystals with tunable reflection wavelengths or intensities derived from their reconfigurable structures. Colloids Interface Sci. Commun. 2024, 62, 100806. [Google Scholar] [CrossRef]
- Ermrich, M.; Opper, D. X-Ray Powder Diffraction: XRD for the Analyst Getting Acquainted with the Principles; PANalytical B.V.: Almelo, The Netherlands, 2011; pp. 58–72. [Google Scholar]
- Waseda, Y.; Matsubara, E.; Shinoda, K. X-Ray Diffraction Crystallography Introduction, Examples and Solved Problems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 107–179. [Google Scholar]
- Kacher, J.; Landon, C.; Adams, B.L.; Fullwood, D. Bragg’s Law diffraction simulations for electron backscatter diffraction analysis. Ultramicroscopy 2009, 109, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Shuijin Lei, Zhihong Liang, Lang Zhou, Kaibin Tang, Synthesis and morphological control of MnCO3 and Mn(OH)2 by a complex homogeneous precipitation method. Mater. Chem. Phys. 2009, 113, 445–450. [CrossRef]
- Regulski, M.; Przeniosło, R.; Sosnowska, I.; Hohlwein, D.; Schneider, R. Neutron diffraction study of the magnetic structure of α-Mn2O3. J. Alloys Compd. 2004, 362, 236–240. [Google Scholar] [CrossRef]
- Guo, L.W.; Peng, D.L.; Makino, H.; Inaba, K.; Ko, H.J.; Sumiyama, K.; Yao, T. Structural and magnetic properties of Mn3O4 films grown on MgO(001) substrates by plasma-assisted MBE. J. Magn. Magn. Mater. 2000, 213, 321–325. [Google Scholar] [CrossRef]
- Gangwar, D.; Rath, C. Structural, optical, and magnetic properties of α- and β-MnO2 nanorods. Appl. Surf. Sci. 2021, 557, 149693. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; John Wiley & Sons, Inc.: New York, NY, USA, 1996; pp. 287–317. [Google Scholar]
- Klug, H.P.; Alexander, L.E. Crystallite size and lattice strains from line broadening. In X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1974; pp. 618–708. [Google Scholar]
- Askeland, D.R.; Wendelin, J.W.; Bhattacharya, D.K.; Raj, P.; Peralta Rosales, C.L. Ciencia e Ingeniería de Materiales, 7th ed.; Cengage Learning: Mexico City, Mexico, 2017; pp. 50–58. [Google Scholar]
- Nasiri, S.; Rabiei, M.; Palevicius, A.; Janusas, G.; Vilkauskas, A.; Nutalapati, V.; Monshi, A. Modified scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano Trends 2023, 3, 100015. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles-A comparative study. Mater. Chem. Phys. 2019, 239, 122021. [Google Scholar] [CrossRef]
- Jacob, R.; Isac, J. X-ray diffraction line profile analysis of Ba0.6Sr0.4FexTi(1-x)O3-8, (x = 0.4). Int. J. Chem. Stud. 2015, 2, 12–21. [Google Scholar]
- Warren, B.E.; Averbach, B.L. The separation of cold-work distortion and particle size broadening in X-ray patterns. J. Appl. Phys. 1952, 23, 497. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Bernard, M.C.; Hugot-Le Goff, A.; Thi, B.V.; Cordoba de Torresi, S. Electrochromic reactions in manganese oxides: I. Raman analysis. J. Electrochem. Soc. 1993, 140, 3065–3070. [Google Scholar] [CrossRef]
- Zhao, C.; Li, H.; Jiang, J.; He, Y.; Liang, W. Phase transition and vibration properties of MnCO3 at high pressure and high temperature by Raman spectroscopy. High-Press. Res. 2018, 38, 212–223. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzinib, M. From greenhouse gas to feedstock: Formation of ammonium carbamate from CO2 and NH3 in organic solvents and its catalytic conversion into urea under mild conditions. Green Chem. 2011, 13, 1267–1274. [Google Scholar] [CrossRef]
- Mavis, B.; Akinc, M. Kinetics of urea decomposition in the presence of transition metal ions: Ni2+. J. Am. Ceram. Soc. 2006, 89, 471–477. [Google Scholar] [CrossRef]
- Al-Hazmi, G.H.; Alibrahim, K.A.; Refat, M.S.; Ibrahim, O.B.; Adam, A.M.A.; Shakya, S. A new simple route for synthesis of cadmium(II), zinc(II), cobalt(II), and manganese(II) carbonates using urea as a cheap precursor and theoretical investigation. Bull. Chem. Soc. Ethiop. 2022, 36, 363–372. [Google Scholar] [CrossRef]
- Shim, S.H.; LaBounty, D.; Duffy, T.S. Raman spectra of bixbyite, Mn2O3, up to 40 GPa. Phys. Chem. Miner. 2011, 38, 685–691. [Google Scholar] [CrossRef]
- White, W.B.; Keramidas, V.G. Vibrational spectra of oxides with the C-Type rare earth oxide structure. Spectrochim. Acta 1972, 28, 501–509. [Google Scholar] [CrossRef]
- Kim, M.; Chen, M.; Wang, X.; Nelson, C.S.; Budakian, R.; Abbamonte, P.; Cooper, S.L. Pressure and field tuning the magnetostructural phases of Mn3O4 Raman scattering and x-ray diffraction studies. Phys. Rev. 2011, 84, 174424. [Google Scholar] [CrossRef]
- Xu, H.Y.; Xu, S.H.; Li, X.D.; Wang, H.; Yan, H. Chemical bath deposition of hausmannite Mn3O4 thin films. Appl. Surf. Sci. 2006, 252, 4091–4096. [Google Scholar] [CrossRef]
- Han, Y.F.; Chen, L.W.; Ramesh, K.; Widjaja, E.; Chilukoti, S.; Surjami, I.K.; Chen, J. Kinetic and spectroscopic study of methane combustion over α-Mn2O3 nanocrystal catalysts. J. Catal. 2008, 253, 261–268. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. A comparison study on Raman scattering properties of α- and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef]
- Shah, H.U.; Wang, F.; Toufiq, A.M.; Ali, S.; Haq Khan, Z.U.; Li, Y.; Hu, J.; He, K. Electrochemical properties of controlled size Mn3O4 nanoparticles for supercapacitor applications. J. Nanosci. Nanotechnol. 2018, 18, 719–724. [Google Scholar] [CrossRef]
- Nam, K.; Kim, M.G.; Kim, K. In situ Mn K-edge X-ray absorption spectroscopy studies of electrodeposited manganese oxide films for electrochemical capacitors. J. Phys. Chem. C 2007, 111, 749–758. [Google Scholar] [CrossRef]
- Lee, H.K.; Sakemi, D.; Selyanchyn, R.; Lee, C.G.; Lee, S.W. Titania nanocoating on MnCO3 microspheres via liquid-phase deposition for fabrication of template-assisted core−shell- and hollow-structured composites. Appl. Mater. Interfaces 2014, 6, 57–64. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim. Acta A 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Chen, Z.W.; Lai, J.K.L.; Shek, C.H. Influence of grain size on the vibrational properties in Mn2O3 nanocrystals. J. Non. Cryst. Solids 2006, 352, 3285–3289. [Google Scholar] [CrossRef]
- Dong, Y.; Li, K.; Jiang, P.; Wang, G.; Miao, H.; Zhang, J.; Zhang, C. Simple hydrothermal preparation of α-, β-, and γ-MnO2 and phase sensitivity in catalytic ozonation. RSC Adv. 2014, 4, 39167–39173. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Pasupulety, L.; Kumari, K. Thermochemistry of manganese oxides in reactive gas atmospheres: Probing redox compositions in the decomposition course MnO2→MnO. Thermochim. Acta 1997, 303, 171–181. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Rangan, S.; Lemal, M.; Guyomard, D. Study of structural defects in γ-MnO2 by Raman spectroscopy. J. Raman Spectrosc. 2002, 33, 223–228. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Hercules, D.M. Surface spectroscopic characterization of manganese/aluminum oxide catalysts. J. Phys. Chem. 1984, 88, 4922–4929. [Google Scholar] [CrossRef]
- Oenema, J.; Harmel, J.; Pérez Vélez, R.; Meijerink, M.J.; Eijsvogel, W.; Poursaeidesfahani, A.; Vlugt, T.J.H.; Zečević, J.; Jong, K.P. Influence of nanoscale intimacy and zeolite micropore size on the performance of bifunctional catalysts for n-heptane hydroisomerization. ACS Catal. 2020, 10, 14245–14257. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, L.; Zhao, Y.; Liu, M.; Zhang, W.; Wu, J.; Lai, X.; Fan, G.; Bi, J.; Gao, D. 3D flower-like MnCO3 microcrystals: Evolution mechanisms of morphology and enhanced electrochemical performances. Electrochim. Acta 2017, 251, 119–128. [Google Scholar] [CrossRef]
- Chalmers, J.M.; Griffiths, P.R. Handbook of Vibrational Spectroscopy; Wiley: West Sussex, UK, 2002; pp. 110–112. [Google Scholar]
- Kim, Y.; Caumon, M.C.; Barres, O.; Sall, A.; Cauzid, J. Identification and composition of carbonate minerals of the calcite structure by Raman and infrared spectroscopies using portable devices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119980–119992. [Google Scholar] [CrossRef] [PubMed]
- Rutt, H.N.; Nicola, J.H. Raman spectra of carbonates of calcite structure. J. Phys. C Solid State Phys. 1974, 7, 4522–4528. [Google Scholar] [CrossRef]
- Todorova, S.; Naydenov, A.; Kolev, H.; Ivanov, G.; Ganguly, A.; Mondal, S.; Saha, S.; Ganguli, A.K. Reaction kinetics and mechanism of complete methane oxidation on Pd/Mn2O3 catalyst. React. Kinet. Mech. Catal. 2018, 123, 585–605. [Google Scholar] [CrossRef]
- Penland, R.B.; Mizushima, S.; Curran, C.; Quagliano, J.V. Infrared Absorption Spectra of Inorganic Coordination Complexes. X. Studies of Some Metal-Urea Complexes. J. Am. Chem. Soc. 1957, 79, 1575–1578. [Google Scholar] [CrossRef]
- Barbier, J.P.; Hugel, R. Coordination of manganese(II): High spin complexes with urea. Inorg. Chim. Acta 1974, 10, 93–96. [Google Scholar] [CrossRef]
- Lupin, M.S.; Peters, G.E. Thermal decomposition of aluminum, iron and manganese complexes of urea. Thermochim. Acta 1984, 73, 79–87. [Google Scholar] [CrossRef]
- Chen, Z.W.; Lai, J.K.L.; Shek, C.H. Nucleation site and mechanism leading to growth of bulk-quantity Mn3O4 nanorods. Appl. Phys. Lett. 2005, 86, 181911. [Google Scholar] [CrossRef]
- Srivastava, G.; Dalela, S.; Kumar, S.; Choudhary, B.L.; Alvi, P.A. Structural and Raman studies of MnO2 and Mn2O3 nanoparticles. Mater. Today Proc. 2023, 79, 169–171. [Google Scholar] [CrossRef]
- Jansi Rani, B.; Ravina, M.; Ravi, G.; Ravichandran, S.; Ganesh, V.; Yuvakkumar, R. Synthesis and characterization of hausmannite (Mn3O4) nanostructures. Surf. Interfaces 2018, 11, 28–36. [Google Scholar] [CrossRef]
- Kirillov, S.A.; Aleksandrova, V.S.; Lisnycha, T.V.; Dzanashvili, D.I.; Khainakov, S.A.; García, J.R.; Visloguzova, N.M.; Pendelyuk, O.I. Oxidation of synthetic hausmannite (Mn3O4) to manganite (MnOOH). J. Mol. Struct. 2009, 928, 89–94. [Google Scholar] [CrossRef]
- Gabelica, Z. Vibrational studies of metal-ethylenediamine thiosulfates-I. Infrared and Raman spectra of the tris-ethylenediamine thiosulfates M11(en)3S2O2(M11= Zn, Cd, Fe, Ni, Co, Mn) and some of their N-deuterated analogues. Spectrochim. Acta 1976, 32, 327–336. [Google Scholar] [CrossRef]
- Yang, W.; Wang, C.; Arrighi, V. An organic silver complex conductive ink using both decomposition and self-reduction mechanisms in film formation. J. Mater. Sci. Mater. Electron. 2018, 29, 2771–2783. [Google Scholar] [CrossRef]
- Sharrouf, M.; Awad, R.; Roumié, M.; Marhaba, S. Structural, optical and room temperature magnetic study of Mn2O3 nanoparticles. Mater. Sci. Appl. 2015, 6, 850–859. [Google Scholar] [CrossRef]
- Shaik, M.R.; Syed, R.; Adil, S.F.; Kuniyil, M.; Khan, M.; Alqahtani, M.S.; Shaik, J.P.; Siddiqui, M.R.H.; Al-Warthan, A.; Sharaf, M.A.F.; et al. Mn3O4 nanoparticles: Synthesis, characterization and their antimicrobial and anticancer activity against A549 and MCF-7 cell lines. Saudi J. Biol. Sci. 2021, 28, 1196–1202. [Google Scholar] [CrossRef]
- Kong, Y.; Jiao, R.; Zeng, S.; Cui, C.; Li, H.; Xu, S.; Wang, L. Study on the synthesis of Mn3O4 nanooctahedrons and their performance for lithium ion batteries. Nanomaterials 2020, 10, 367. [Google Scholar] [CrossRef]
- Buciuman, F.; Patcas, F.; Craciun, R.; Zahn, D.R.T. Vibrational spectroscopy of bulk and supported manganese oxides. Phys. Chem. Chem. Phys. 1999, 1, 185–190. [Google Scholar] [CrossRef]
- Sannasi, V.; Subbian, K. Influence of moringa oleifera gum on two polymorphs synthesis of MnO2 and evaluation of the pseudo-capacitance activity. J. Mater. Sci. Mater. Electron. 2020, 31, 17120–17132. [Google Scholar] [CrossRef]
- Ananth, M.V.; Pethkar, S.; Dakshinamurthi, K. Distortion of MnO6 octahedra and electrochemical activity of Nstutite-based MnO2 polymorphs for alkaline electrolytes—An FTIR study. J. Power Sources 1998, 75, 78–282. [Google Scholar] [CrossRef]
- Boulard, E.; Goncharov, A.F.; Blanchard, M.; Mao, W.L. Pressure-induced phase transition in MnCO3 and its implications on the deep carbon cycle. J. Geophys. Res. Solid Earth 2015, 120, 3991–4679. [Google Scholar] [CrossRef]
- Stradella, L.; Argentero, M. A study of the thermal decomposition of urea, of related compounds and thiourea using DSC and TG-EGA. Termochimica Acta 1993, 219, 315–323. [Google Scholar] [CrossRef]
- Theophanides, T.; Harvey, P.D. Structural and spectroscopic properties of metal-urea complex. Coord. Chem. Rev. 1987, 76, 237–264. [Google Scholar] [CrossRef]
- Bennett, A.M.A.; Foulds, G.A.; Thornton, D.A.; Watkins, G.M. The infrared spectra of ethylenediamine complexes--ll. Tris-, his- and mono(ethylenediamine) complexes of metal(ll) halides. Spectrochim. Acta 1990, 46, 13–22. [Google Scholar] [CrossRef]
- Srivastava, P.C.; Singh, B.N.; Aravindakshan, C.; Banerji, K.C. Studies on the structure and thermal behaviour of the Mn-urea complex. Thermochim. Acta 1983, 71, 227–236. [Google Scholar] [CrossRef]
- Shena, X.; Ji, Z.; Miao, H.; Yang, J.; Chen, K. Hydrothermal synthesis of MnCO3 nanorods and their thermal transformation into Mn2O3 and Mn3O4 nanorods with a single crystalline structure. J. Alloys Compd. 2011, 509, 5672–5676. [Google Scholar] [CrossRef]
- Astam, A.; İnanç, C.T. Hydrothermal synthesis of MnCO3 thin film and its conversion to Mn-oxides by annealing in different atmospheres. J. Mater. Sci. Mater. Electron. 2023, 34, 2078. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M. Lattice dynamics of manganese oxides and their intercalated compounds. In New Trends in Intercalation Compounds for Energy Storage; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 235–252. [Google Scholar]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M. Synthesis of Mn3O4 nanoparticles by thermal decomposition of a [bis(salicylidiminato)manganese(II)] complex. Polyhedron 2008, 27, 3467–3471. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, M.; Liu, Z.H.; Ooi, K. IR spectra of manganese oxides with either layered or tunnel structures. Spectrochim. Acta A 2007, 67, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Bose, V.C.; Biju, V. Optical, electrical and magnetic properties of nanostructured Mn3O4 synthesized through a facile chemical route. Physica E Low Dimens. Syst. Nanostruct. 2015, 66, 24–32. [Google Scholar] [CrossRef]
- Lan, D.; Qin, M.; Yang, R.; Wu, H.; Jia, Z.; Kou, K.; Wu, G.; Fan, Y.; Fu, Q.; Zhang, F. Synthesis, characterization and microwave transparent properties of Mn3O4 microspheres. J. Mater. Sci. Mater. Electron. 2019, 30, 8771–8776. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, J.; Li, Y.; Zhao, Y.; Ma, D.; Li, L. Mild aqueous synthesis of octahedral Mn3O4 nanocrystals with varied oxidation states. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 42–47. [Google Scholar] [CrossRef]
- Bose, V.C.; Biju, V. Structure, cation valence states and electrochemical properties of nanostructured Mn3O4. Mater. Sci. Semicond. Process 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Ozutsumi, K.; Taguchi, Y.; Kawashima, T. Thermodynamics of formation of urea complexes with manganese(II), nickel(II) and zinc(II) ions in N,N-dimethylformamide. Talanta 1995, 42, 535–541. [Google Scholar] [CrossRef]
- Stojceva Radovanovic, B.C.; Premovic, P.I. Thermal behaviour of Cu(II)-urea complex. J. Therm. Anal. 1992, 38, 715–719. [Google Scholar] [CrossRef]
- Bennett, A.M.A.; Foulds, G.A.; Thornton, D.A. The IR spectra of ethylenediamine complexes-I. The tris (ethylenediamine) complexes of first transition series metal(II) sulphates. Spectrochim. Acta 1989, 45, 219–233. [Google Scholar] [CrossRef]
- Gammons, C.H.; Seward, T.M. Stability of manganese (II) chloride complexes from 25 to 300 °C. Geochim. Cosmochim. Acta 1996, 60, 4295–4311. [Google Scholar] [CrossRef]
- Beddie, C.; Webster, C.E.; Hall, M.B. Urea decomposition facilitated by a urease model complex: A theoretical investigation. Dalton Trans. 2005, 21, 3542–3551. [Google Scholar] [CrossRef]
- Yang, L.X.; Zhu, Y.J.; Tong, H.; Wang, W.W. Submicrocubes and highly oriented assemblies of MnCO3 synthesized by ultrasound agitation method and their thermal transformation to nanoporous Mn2O3. Ultrason. Sonochem. 2007, 14, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Al-Qahtani, M.M. Unusual route for preparation of manganese(II), cobalt(II), zinc(II) and cadmium(II) carbonate compounds: Synthesis and spectroscopic characterizations. Bull. Mater. Sci. 2011, 34, 853–857. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Liang, H.; Yang, H.; Yang, Y. Facile synthesis of MnCO3 hollow dumbbells and their conversion to manganese oxide. Mater. Lett. 2010, 64, 2060–2063. [Google Scholar] [CrossRef]
- Tischer, S.; Börnhorst, M.; Amsler, J.; Schochb, G.; Deutschmann, O. Thermodynamics and reaction mechanism of urea decomposition. Phys. Chem. Chem. Phys. 2019, 21, 16785–16797. [Google Scholar] [CrossRef]
- Shaw, W.H.R.; Bordeaux, J.J. The Decomposition of urea in aqueous media. J. Am. Chem. Soc. 1955, 77, 4729–4733. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Liu, Z.; Zhao, Y.; Lai, X.; Bi, J.; Gao, D. In-situ N-doped MnCO3 anode material via one-step solvothermal synthesis: Doping mechanisms and enhanced electrochemical performances. J. Chem. Eng. 2020, 383, 123161. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.d.A.A.; Jobbagy, M.; Candal, R.J.; Regazzoni, A.E.; Blesa, M.A. Synthesis of metal oxide particles from aqueous media: The homogeneous alkalinization method. J. Dispers. Sci. Technol. 1998, 19, 207–228. [Google Scholar] [CrossRef]
- Musić, S.; Ristić, M.; Popović, S. Synthesis and microstructure of porous Mn-oxides. J. Mol. Struct. 2009, 924, 243–247. [Google Scholar] [CrossRef]
- Zemieche, A.; Chetibi, L.; Hamana, D.; Achour, S.; Pagot, G.; Noto, V.D. Synthesis and phase transformation study of nanostructured manganese oxide polymorphs. J. Cryst. Growth 2024, 633, 127661. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhou, Y.; Su, Z.; Yao, L.; Cao, J.; Jiang, L.; Hu, G.; Kong, M.; Yang, J.; et al. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3–SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Atmane, I.; Sobti, N.; Chetibi, L.; Dimitrova, A.; Zerkout, S.; Achour, S. Defective graphite and its decoration with copper oxide nanoparticles synthesized with olive leaf extract for electrochemical water splitting. J. Inorg. Organometal. Polym. Mater. 2019, 29, 132–143. [Google Scholar] [CrossRef]
- Earnshaw, A.; Larkworthy, L.F.; Patel, K.C. Chromium(II) chemistry. Part V. Ethylenediamine complexes. J. Chem. Soc. A Inorg. Phys. Theor. A 1969, 1339–1343. [Google Scholar] [CrossRef]
- Leussing, D.L. Complex Formation between Manganese(II), Nickel(II), and Zinc(II) ions and some symmetrically substituted ethylenediamines: The use of E and δH values in assessing inductive and steric effects. Inorg. Chem. 1963, 2, 77–82. [Google Scholar] [CrossRef]
- Mellan, T.A.; Maenetja, K.P.; Ngoepe, P.E.; Woodley, S.M.; Catlow, C.R.A.; Grau- Crespo, R. Lithium and oxygen adsorption at the β-MnO2 (110) surface. J. Mater. Chem. 2013, 1, 14879–14887. [Google Scholar] [CrossRef]
- Paoletti, P. Formation of metal complexes with ethylenediamine: A critical survey of equilibrium constants, enthalpy and entropy values. Pure Appi. Chem. 1984, 56, 491–522. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fašmon-Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Scientific Opinion on the safety and efficacy of a feed additive consisting of manganese chelate of ethylenediamine for all animal species (Zinpro Animal Nutrition (Europe), Inc.). EFSA J. 2021, 19, 6468–6485. [Google Scholar]
- Jackson, T.B.; Edwards, J.O. Coordination compounds of labile metals with ethylenimine. J. Am. Chem. Soc. 1961, 83, 355–360. [Google Scholar] [CrossRef]
- Sharnin, V.A. Thermochemistry of formation of copper (II) ethylenediamine complexes and solvation of reagents in aqueous organic solvents. J. Therm. Anal. 1995, 45, 721–728. [Google Scholar] [CrossRef]
- Segoviano-Garfias, J.J.N.; Zanor, G.A.; Ávila-Ramos, F. Solution equilibria formation of manganese(II) complexes with ethylenediamine, 1,3-propanediamine and 1,4-butanediamine in methanol. Molbank 2022, 2022, M1367. [Google Scholar] [CrossRef]
- Sordelet, D.; Akinc, M. Preparation of spherical, monosized yttrium oxide precursor particles. J. Colloid Interface Sci. 1988, 122, 47–59. [Google Scholar] [CrossRef]
- Aiken, B.; Hsu, W.P.; Matijevic, E. Preparation and properties of monodispersed colloidal particles of lanthanide compounds: III. Yttrium (III) and mixed yttrium (III)/cerium (III) systems. J. Am. Ceram. Soc. 1988, 71, 845–853. [Google Scholar] [CrossRef]
- Mustapha, A.; Shehu, A.; Ahmed, A. Synthesis of Mn(II) and Fe(II) Complexes with ethylenediamine and acetylacetonate ligands. J. Nat. Sci. Res. 2014, 4, 65–66. [Google Scholar]
- Douglas, B.E.; Alexander, J.J. Conceptos y Modelos de Química Inorgánica; Reverté: Barcelona, Spain, 1994. [Google Scholar]
- Pudukudy, M.; Yaakob, Z.; Rajendran, R. Facile synthesis of mesoporous α-Mn2O3 microspheres via morphology conserved thermal decomposition of MnCO3 microspheres. Mater. Lett. 2014, 136, 85–89. [Google Scholar] [CrossRef]
- Lei, S.; Tang, K.; Fang, Z.; Liu, Q.; Zheng, H. Preparation of α-Mn2O3 and MnO from thermal decomposition of MnCO3 and control of morphology. Mater. Lett. 2006, 60, 53–56. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Xie, T. Preparation of high-purity γ-Mn3O4 by decomposing MnCO3 from low-grade rhodochrosite ore. Miner. Process. Extr. Metall. 2015, 124, 132–136. [Google Scholar] [CrossRef]
- Roche, I.; Chaînet, E.; Chatenet, M.; Vondrák, J. Carbon-supported manganese oxide nanoparticles as electrocatalysts for the oxygen reduction reaction (ORR) in alkaline medium: Physical characterizations and ORR mechanism. J. Phys. Chem. 2007, 111, 1434–1443. [Google Scholar] [CrossRef]
- Vondrák, J.; Klápštˇe, B.; Velická, J.; Sedlăríková, M.; Reitera, J.; Roche, I.; Chainet, E.; Fauvarque, J.F.; Chatenet, M. Electrochemical activity of manganese oxide/carbon-based electrocatalysts. J. New Mat. Electrochem. Syst. 2005, 8, 209–212. [Google Scholar]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]


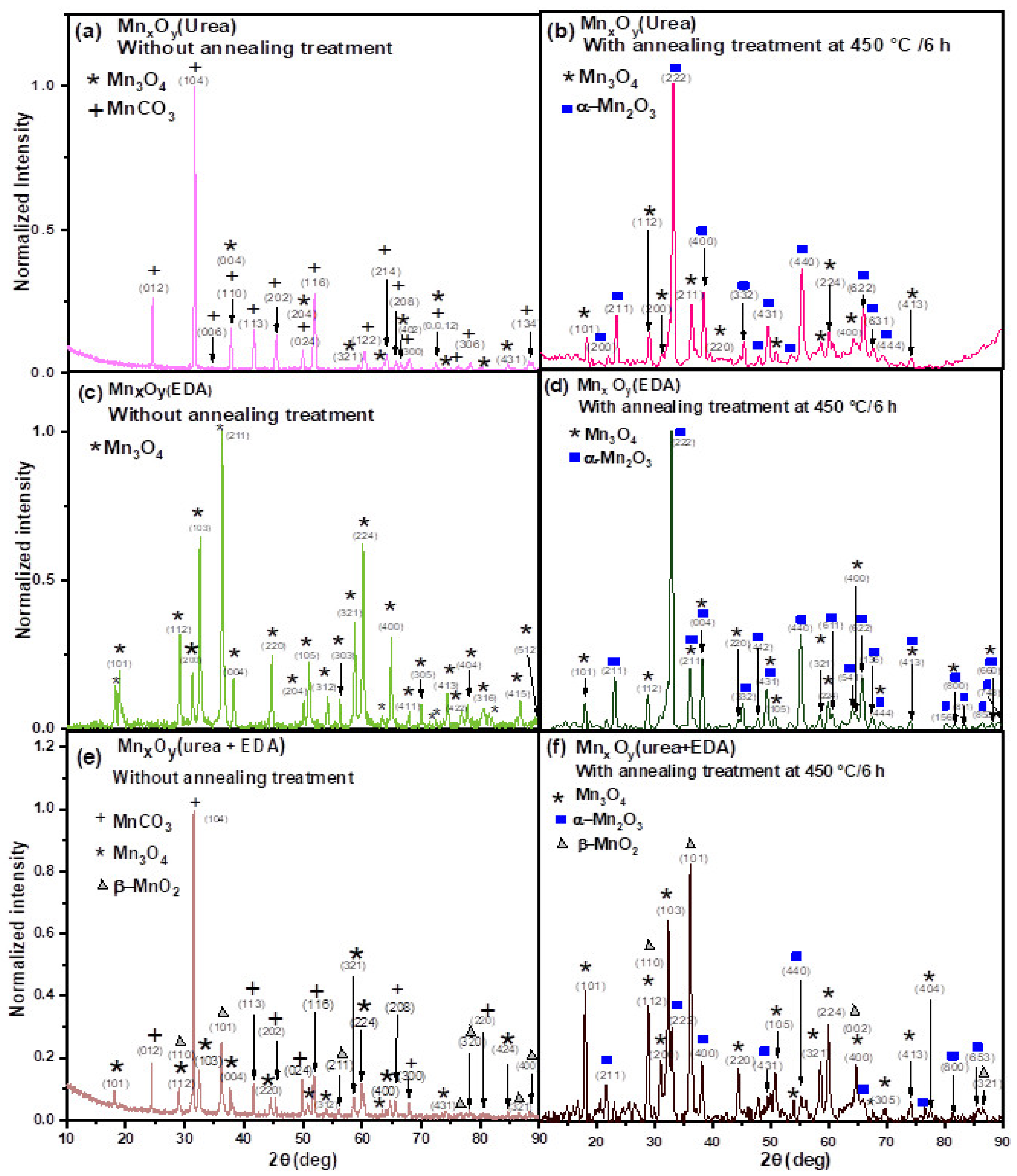
| Sample | Compound | Lattice Parameters (Å) | c/a | Volume (Å3) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Without annealing treatment | ||||||
| Urea | MnCO3 | 4.77 | 4.77 | 15.7 | 3.29 | 308.7 |
| Mn3O4 | 5.62 | 5.62 | 9.99 | 1.78 | 315.3 | |
| EDA | Mn3O4 | 5.58 | 5.58 | 9.48 | 1.7 | 294.7 |
| Urea+EDA | MnCO3 | 4.78 | 4.78 | 14.1 | 2.94 | 277.8 |
| Mn3O4 | 5.57 | 5.57 | 10.5 | 1.89 | 328.7 | |
| β-MnO2 | 4.28 | 4.28 | 3.04 | 0.71 | 55.7 | |
| With annealing treatment: 450 °C/6 h | ||||||
| Urea | α-Mn2O3 | 9.4 | 9.4 | 9.4 | 1 | 831.1 |
| Mn3O4 | 5.69 | 5.69 | 10.1 | 1.78 | 328.6 | |
| EDA | α-Mn2O3 | 9.39 | 9.39 | 9.39 | 1 | 827.16 |
| Mn3O4 | 5.57 | 5.57 | 10.2 | 1.82 | 315.2 | |
| Urea+EDA | α-Mn2O3 | 9.43 | 9.43 | 9.43 | 1 | 838.3 |
| Mn3O4 | 5.58 | 5.58 | 10.4 | 1.86 | 324.6 | |
| β-MnO2 | 3.97 | 3.97 | 3.16 | 0.79 | 49.9 | |
| Reference work | ||||||
| MnCO3 [63] | 4.77 | 4.77 | 15.6 | 3.278 | 308.17 | |
| α-Mn2O3 [64] | 9.41 | 9.41 | 9.41 | 1 | 833.2 | |
| Mn3O4 [65] | 5.75 | 5.75 | 9.42 | 1.64 | 269.71 | |
| β-MnO2 [66] | 4.4 | 4.4 | 2.88 | 0.65 | 55.8 | |
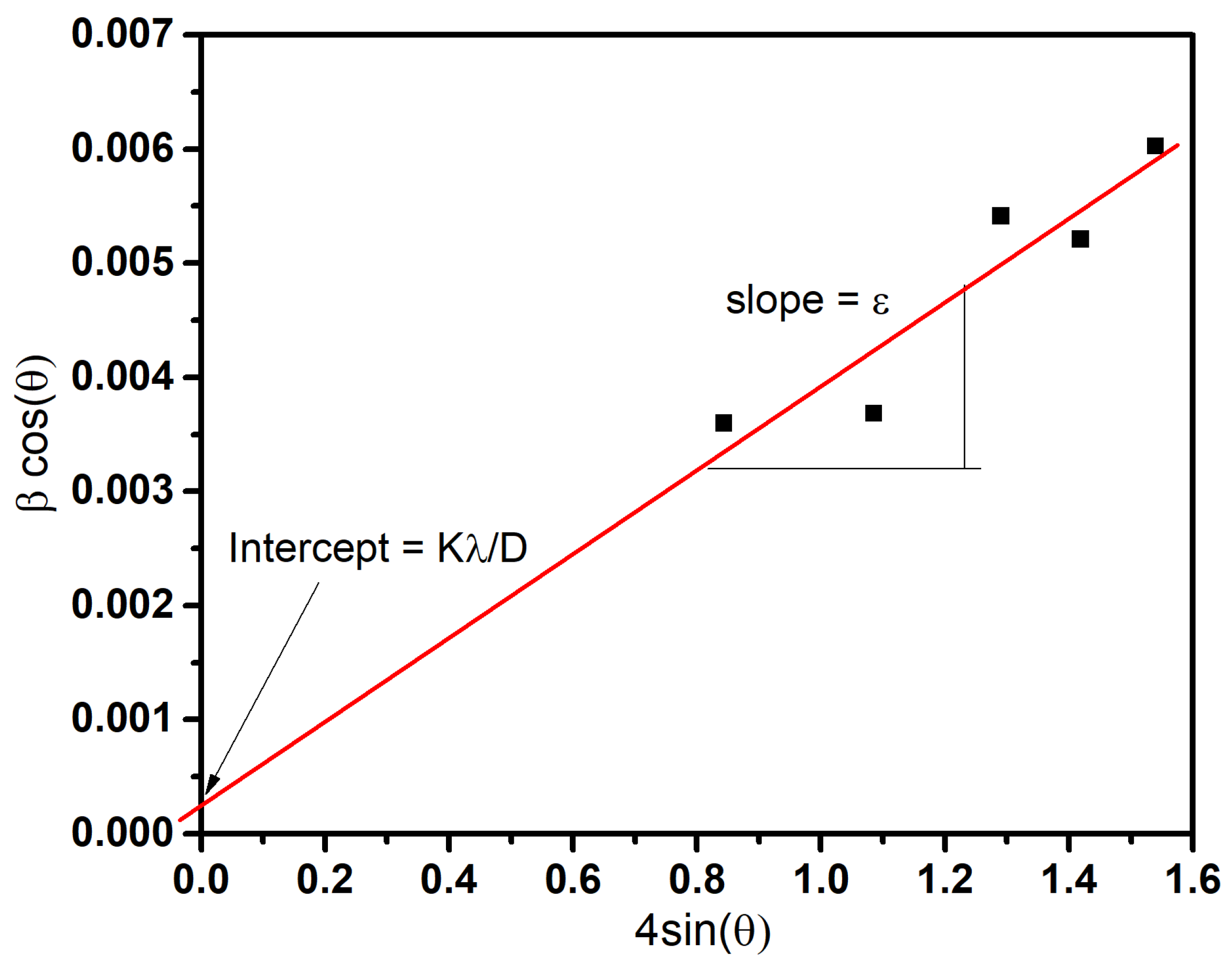
| Sample | Compound | Crystal Symmetry | (hkl) | Crystal Size Sherrer Equation | Crystal Size Williamson–Hall | Lattice Strain |
|---|---|---|---|---|---|---|
| (nm) | (nm) | |||||
| Without annealing treatment | ||||||
| Urea | MnCO3 | Hexagonal (R-3c) | 104 | 40 | 42.4 | 0.0037 |
| Mn3O4 | Tetragonal (I41/amd) | 413 | 19.8 | 24.8 | 0.004 | |
| EDA | Mn3O4 | Tetragonal (I41/amd) | 103 | 26.8 | - | - |
| Urea+EDA | MnCO3 | Hexagonal (R-3c) | 104 | 39.7 | 34.8 | 0.0028 |
| Mn3O4 | Tetragonal (I41/amd) | 103 | 22.7 | 16.5 | 0.005 | |
| β-MnO2 | Tetragonal (P42/mnm) | 101 | 29.6 | 29.9 | 0.002 | |
| With annealing treatment: 450 °C/6 h | ||||||
| Urea | α-Mn2O3 | Cubic (Ia3) | 222 | 17.2 | - | - |
| Mn3O4 | Tetragonal (I41/amd) | 211 | 16.5 | 17.9 | 0.0023 | |
| EDA | α-Mn2O3 | Cubic (Ia3) | 222 | 16.8 | - | - |
| Mn3O4 | Tetragonal (I41/amd) | 112 | 18.1 | - | - | |
| Urea+EDA | α-Mn2O3 | Cubic (Ia3) | 222 | 15.5 | - | - |
| Mn3O4 | Tetragonal (I41/amd) | 103 | 21.8 | - | - | |
| β-MnO2 | Tetragonal (P42/mnm) | 101 | 23.8 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Reyna, M.L.; Sánchez-Mora, E.; Quiroga-González, E. Increasing the Probability of Obtaining Intergrown Mixtures of Nanostructured Manganese Oxide Phases Under Solvothermal Conditions by Mixing Additives with Weak and Strong Chelating Natures. Physchem 2025, 5, 35. https://doi.org/10.3390/physchem5030035
Barrios-Reyna ML, Sánchez-Mora E, Quiroga-González E. Increasing the Probability of Obtaining Intergrown Mixtures of Nanostructured Manganese Oxide Phases Under Solvothermal Conditions by Mixing Additives with Weak and Strong Chelating Natures. Physchem. 2025; 5(3):35. https://doi.org/10.3390/physchem5030035
Chicago/Turabian StyleBarrios-Reyna, María Lizbeth, Enrique Sánchez-Mora, and Enrique Quiroga-González. 2025. "Increasing the Probability of Obtaining Intergrown Mixtures of Nanostructured Manganese Oxide Phases Under Solvothermal Conditions by Mixing Additives with Weak and Strong Chelating Natures" Physchem 5, no. 3: 35. https://doi.org/10.3390/physchem5030035
APA StyleBarrios-Reyna, M. L., Sánchez-Mora, E., & Quiroga-González, E. (2025). Increasing the Probability of Obtaining Intergrown Mixtures of Nanostructured Manganese Oxide Phases Under Solvothermal Conditions by Mixing Additives with Weak and Strong Chelating Natures. Physchem, 5(3), 35. https://doi.org/10.3390/physchem5030035







