Abstract
Anthraquinone acid dyes are widely used in dyeing polyamide due to their good exhaustion and brightness. While ionic interactions primarily govern dye–fiber bonding, the molecular weight (Mw) of these dyes can significantly influence migration, apparent color strength, and fastness behavior. This study offers comparative insight into how the Mw of structurally similar anthraquinone acid dyes impacts their diffusion, fixation, and functional outcomes (e.g., UV protection) on polyamide 6 fabric, using Acid Blue 260 (Mw~564) and Acid Blue 127:1 (Mw~845) as representative low- and high-Mw dyes. The effects of dye concentration, pH, and temperature on color strength (K/S) were evaluated, migration index and zeta potential were measured, and UV protection factor (UPF) and FTIR analyses were used to assess fabric functionality. Results showed that the lower-Mw dye exhibited higher migration tendency, particularly at increased dye concentrations, while the higher-Mw dye demonstrated greater color strength and superior wash fastness. Additionally, improved UPF ratings were associated with higher-Mw dye due to enhanced light absorption. These findings offer practical insights for optimizing acid dye selection in polyamide coloration to balance color performance and functional attributes.
1. Introduction
Dyes, used to color a wide range of industrial materials including textiles, plastics, and paper, are produced globally at over one million tons per year across approximately 10,000 types [1]. It is estimated that at least 10–15% of dyes are lost in wastewater [2] and harm the environment. There is an utmost requirement to maximize color use during dyeing of textiles, achieving functionality and maintaining appropriate fastness, to reduce dye wastage.
Dyes can be either cationic, anionic, or nonionic, which are suitable for different particular fiber types [3]. Acid dyes are water-soluble anionic dyes commonly applied onto nitrogenous fibers such as polyamide (PA), wool, and silk that include basic groups in their polymer chains [4,5]. Polyamides are a class of synthetic polymers widely used in textiles due to their excellent mechanical strength, abrasion resistance, and dyeability. Among them, polyamide 6 (PA 6) and polyamide 66 (PA 66) are the most common, with applications ranging from activewear (e.g., swimwear and sports garments) to technical textiles (e.g., combat uniforms and protective gear) [6,7]. Although both are structurally similar with recurring amide (–CONH–) linkages, PA 66 has a higher melting point and greater crystallinity, whereas PA 6 exhibits better dye uptake and slightly greater moisture regain, making it more favorable for dyeing applications [8]. These polyamides contain protonated amino groups along the polymer chain, which can form electrostatic interactions with the sulphonated anionic groups of acid dyes [9]. Among the different chemical groups of acid dyes, such as mono azo [10], nitro, triphenylmethane, xanthene, azine, quinoline, ketonimine, anthraquinone [11], and phthalocyanine [12], the anthraquinone group has a relatively higher exhaustion property towards PA fiber and produces deeper shades with uniform appearance and good fastness to light [13,14]. Additionally, their extended conjugated structure can contribute to improved UV protection [15], making them beneficial for functional textile applications where sun exposure is a concern [16].
Over the years, dyeing PA with acid dyes has been gaining tremendous interest, and analysis from different perspectives is available. For instance, Tayebi et al. studied the adsorption of acid dye on PA yarns in the presence of different amounts of Titania and reported the adsorption rates in fibers having varying cross-sectional shapes [17]. Sada et al. investigated the diffusion and adsorption process of acid dye onto PA based on a diffusion model that unites prompt dual adsorption of the Langmuir and the Nernst type [18]. Atherton et al. performed a study with twelve acid dyes to investigate the affinity towards PA by desorption with inorganic anions. They presented an equation that is applicable in general for the relationship between affinity and desorption [19]. Gupta et al. considered both monofilament and multifilament yarns for dyeing with acid dye and examined the roles played by the physical structure of the PA as well as the chemical affinity between the dye and fiber [20]. Moreover, Bell studied the relation between dye diffusion behavior and mechanical properties of PA fiber and explored the effect of temperature, surface area, and the amount of dye absorbed at saturation on the dyeing rate [21]. A further study was reported by Wang et al., in which a hydrogen peroxide–glyoxal redox system was proposed for investigating the kinetics and thermodynamics of dyeing PA fiber, in addition to dyeing transition temperature, activation energies of diffusion, diffusion coefficient, dyeing enthalpy, entropy, and dyeing affinity [22]. Burkinshaw and Son performed a comparative analysis of color strength and fastness properties after frequent washing of acid dyes after dyeing PA fiber [23]. While ionic interactions between acid dyes and PA fibers predominantly govern initial adsorption, the dye molecular structure, including Mw, affects diffusion, substantivity, and leveling properties. Higher Mw dyes typically show slower diffusion and reduced migration due to increased steric bulk, and they also could impact fabric functionality, such as UV protection. Generally, when the molecular weight is high, uneven dyeing can occur, whereas when the molecular weight is low, the wash fastness properties can suffer [9]. Most of the previous studies were performed mainly on the affinity, adsorption, and color fastness properties of PA fibers with different classes of acid dye, with no attempt to identify the impact of the Mw of acid dye on the dyeing and functionality of PA.
Taking account of the possible impact of Mw and the lack of reports in this area, this research aims to explore the effect of Mw on the color strength and fastness properties of acid dyeing on PA, with a parallel insight into the possible influence on UV functionality. The chosen dyes were C I Acid Blue 260 and C I Acid Blue 127:1, both of which have extensive use in actual industrial dyeing. These two anthraquinone dyes were chosen due to similar chromophores but differing molecular weights, allowing isolation of size-related migration and functional effects. Through systematic variation of key dyeing parameters, such as temperature, dye concentration, and pH, this study provides a comparative assessment of their dyeing behavior, migration tendencies, and associated functional benefits, offering practical guidance for selecting dyes based on performance and end-use requirements.
2. Materials and Methods
2.1. Materials
PA 6 single jersey knitted fabric, having 40 Wales per inch and 36 courses per inch and a weight of 160 g per square meter, was selected for this study. Two different acid dyes, C I Acid Blue 260 and C I Acid Blue 127:1, were collected from Orient Chem-Tex Ltd., Dhaka, Bangladesh. The former one was a low-molecular-weight dye (LMD), and the latter one was a high-molecular-weight dye (HMD). The specifications of the selected dyes are listed in Table 1, and the chemical structures of these dyes are shown in Figure 1.

Table 1.
Specifications of C I Acid Blue 260 and C I Acid Blue 127:1 anthraquinone acid dyes.
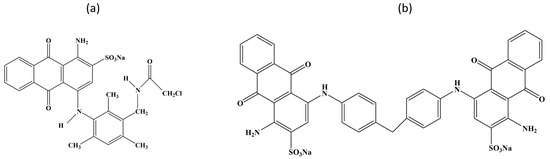
Figure 1.
Chemical structure of (a) Acid Blue 260 and (b) Acid Blue 127:1.
2.2. Dyeing
Dyeing of PA fabrics was carried out at different dye concentrations, pH, and temperature setups in separate baths, as it is mentioned in the next sections. Equilibrium of dyeing was identified with several trials before testing of the samples. The weight of the sample was taken as 3 g for each trial, and a fixed M:L ratio was maintained at 1:6. Dyes were applied as supplied without any further purification. A constant pH condition was maintained by buffering the dye solution, using dilute acetic acid and sodium acetate buffer, and measured using a calibrated pH meter before dyeing. The dyed sample was then washed and dried to take the spectral measurement. The whole dyeing process is illustrated in Figure 2.
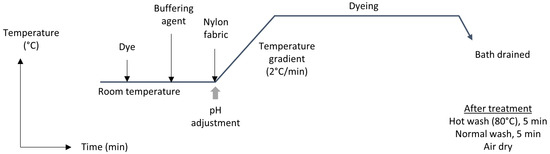
Figure 2.
Dyeing process and post-treatment of PA fabric with anthraquinone acid dyes.
2.3. Spectral Measurement and Saturation Limit Analysis
A reflectance spectrophotometer (Datacolor 650, Lawrenceville, NJ, USA) was used to obtain the reflectance values of LMD- and HMD-dyed samples to identify the wavelength (λmax) of maximum absorption with D65 standard illuminant D65 and 10° observer. All the reflectance measurements were taken by preparing two folds of each dyed specimen in four different places, and their average was taken. Maximum values of color strength (K/S) of minimum reflectance at (λmax) were measured using the Kubelka–Munk equation (Equation (1)):
where K is the absorption coefficient of the dye, S is the scattering coefficient of the dye, and R is the reflectance value at λmax.
2.4. Dynamic Behavior of Color Strength (K/S) at Different Dye-Bath Variants
A fixed dye bath temperature (100 °C) and pH 4 were maintained over a period. Different shade percentages, 1, 2, 3, 4, and 5% OWF (on the weight of fabric), applied from a separate bath and gradual accumulation of dyes into the PA fabric, were measured in terms of color strength (K/S) using reflectance data for individual dye concentration.
To study the effect of temperature, dyeing was carried out for a fixed shade percentage and pH condition within a dyeing temperature range (70, 80, 90, 100, and 110 °C) individually. At every constant temperature, color strength (K/S) was measured after a set interval of time to study the most appropriate temperature at which the dye molecules migrate with maximum uniformity.
To study the effect of pH, different pH condition was maintained as 2, 3, 4, 5, and 6 using buffer for dyeing at 100 °C and 3% (OWF) dye concentration throughout the whole dyeing period. Acidic to neutral media were applied separately, and color strength (K/S) was measured at each time interval to identify the most suitable pH condition for dye transfer.
2.5. Measurement of Dye Migration Index
A dye migration test was performed at each set of temperatures from separate bath conditions [3]. For individual dyes, two separate baths were prepared to contain a pair of samples. One pair of samples was exhausted with dye and salt (sample D1–D2, dye bath in Figure 3), and the other pair was completely exhausted with the same amount of salt concentration (sample B1–B2, blank bath in Figure 3). After 30 min of exhaustion, the exhausted D2 sample was exchanged with B1 in the blank bath and allowed to migrate for another 30 min. The fixation process was carried out with concentrated alkali, and the samples were directly dried without any further wash treatment after being taken out of the bath. The propensity to migration of the dyed samples was investigated at 100 °C and 70 °C from separate dye baths, keeping other parameters constant over a range of shade depths. A color measurement technique was adopted to measure the depth of shade of the test samples of the originally dyed and migrated sample to determine the migration index (MI) at 70 °C and 100 °C, as in the following equation:
where A is the color strength of the migrated sample and B is the color strength of the originally dyed sample. An illustration for the migration test is shown in Figure 3.
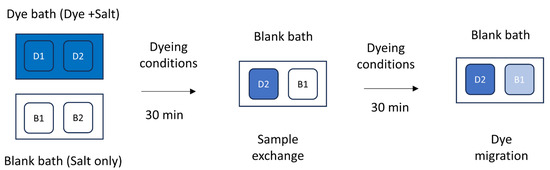
Figure 3.
Schematic diagram of anthraquinone acid dye migration test on PA fabric at 100 °C.
The zeta potential of undyed and dyed PA fabrics was measured using a SurPASS 3 electrokinetic analyzer (Anton Paar, Graz, Austria). The samples were run under a 600–200 mbar pressure cycle. Undyed fabrics were tested from pH 2 to 8, whereas the dyed fabrics were tested at pH 4. Three tests were conducted for each sample to obtain average values.
2.6. Evaluation of UV Protection Factor and Fourier Transform Infrared Spectra
The UV protection factor (UPF) of the fabrics was calculated from the results of the measurement of UVA and UVB transmittance using a UV penetration measurement system (YG902, Fangyuan Instrument, Hefei, China), adopting the AS/NZS 4399 standard. The FTIR spectra were obtained through a Vertex 70 spectrometer (Bruker, Berlin, Germany) using 4 cm−1 scan resolution, and data were normalized from 0 to 1.
2.7. Color Fastness Evaluation
The fastness properties in wet conditions such as wash, water, perspiration, and sublimation fastness were tested for the fabric samples dyed with LMD and HMD using DW multifiber adjacent cloth (SDL Atlas, Shanghai, China) and assessments were performed with grey scales for color change and staining on a rating of 1 to 5, where grade 5 and grade 1 signified to excellent and very poor quality, respectively. Wash fastness properties of the dyed samples were assessed by adopting both color fastness to wash methods AATCC 61 3A and AATCC 61 2A but using altered temperatures, i.e., 60 °C and 38 °C, respectively, for 45 min, using 1993 AATCC Standard Reference Detergent WOB (Labtex, Dhaka, Bangladesh). Color fastness to water was determined according to the ISO 105 E01 method [24]. The ISO 105 E04 standard [25] method was used to test fastness to alkaline perspiration of dyed samples.
3. Results
In this study, the color performance on polyamide was evaluated using K/S values in relation to variable time, dye concentration, and pH. Color fastness in wet conditions (wash, water, and perspiration) and dye migration were assessed through stepwise shade build-up. UPF was measured spectrophotometrically, while FTIR and zeta potential provided structural and surface charge insights, respectively. The results are discussed in the following subsections.
3.1. Saturation Limit Analysis from Color Strength
From the values in Table 2, the dye saturation limit was indicated at 3% shade (OWF) for LMD and 2.5% shade (OWF) for HMD. This is because the increase in color strengths was very negligible after these points. The LMD showed the saturation point at a slightly higher concentration than HMD. It may be justified by Henry and Graham’s diffusion law that smaller-molecular-weight dye particles more easily penetrate into the interior of fibers than HMD, which ultimately leads to higher dye accumulation due to enough inter-molecular free space during absorption [26]. According to Graham’s law, lighter molecules (e.g., LMD) diffuse faster than heavier ones (e.g., HMD) under the same conditions. Brownian motion also favors smaller dye molecules, allowing them to move more randomly and rapidly, explaining why LMD reached equilibrium more quickly (discussed later in Section 3.2.2) [27]. However, even though HMD was slower to diffuse, it probably formed stronger and more stable interactions with PA fibers due to the availability of more functional groups in the structure [28], resulting in greater overall fixation, and hence represented a higher color strength at its saturation point.

Table 2.
Color strength of anthraquinone acid dyes on PA fabric at different initial concentrations.
3.2. Dynamic Behavior of Color Strength (K/S)
3.2.1. Effect of Dye Concentration
The surface plot for the effect of dye concentration on the dynamic behavior of color strength is shown in Figure 4. The profound dye diffusion into the fabric indicated a sharp increase with increasing amounts of dye concentration up to a lower level of shade percentage used. Color strength showed a rapid increase initially with time as well as with higher dye concentrations applied, intensifying the depth. It was observed that for a 400% increment in LMD concentration, K/S increased approximately 170% in 5 min, about 157% in 30 min, and nearly 161% for 120 min of dyeing time. However, for the same increment of HMD concentration, an increase of around 169%, 141%, and 129% was found for 5 min, 30 min, and 120 min, respectively. The maximum K/S value was achieved within the initial five minutes of dyeing for LMD and ten minutes for HMD for all concentrations, individually confirming faster transfer of small dye molecules into the substrate, having a high diffusion coefficient according to Fick’s diffusion law, and which also appeared to level out at a specific shade percentage [22]. The maximum yield of LMD- and HMD-dyed samples was at 3% and 2.5% depth of shade, respectively, indicating the aforementioned saturation level (Table 2).
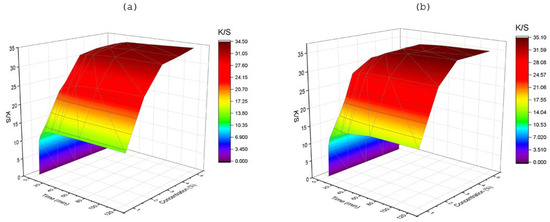
Figure 4.
Effect of dye concentration on color strength (K/S) at different time intervals (t), at 100 °C and pH 4, for (a) LMD and (b) HMD.
3.2.2. Effect of Temperature
The color strength of fabrics gradually improved with the rise in temperature and showed a tendency to reach an equilibrium earlier, above 90 °C for LMD and more than 100 °C for HMD (Figure 5). The enhanced rate of color strength of LMD occurred over 90 °C, whereas the equilibrium K/S approached earlier in comparison to that which was obtained below the range of temperature applied. Due to the smaller molecular size, LMD faced less steric hindrance when diffusing into fiber pores. This probably resulted in faster migration and shorter diffusion paths, allowing it to saturate the fiber surface and interior more rapidly than HMD. The initial slower rate of dye migration at a lower level of temperature indicated to need for higher energy to improve the transfer of HMD. The maximum color yield of fabric was achieved after an initial 20 min of dyeing for HMD and less than 10 min for LMD below 90 °C. In fact, for LMD, a 57% increment in dyeing temperature brought about a 145% increase in K/S in 5 min. However, for 30 min, the increase was only 58%, and for 120 min, it was approximately 43%. In case of HMD, the increment in K/S was near about 138%, 81%, and 70% in 5 min, 30 min, and 120 min, respectively. Because of Brownian motion, an increase in temperature increased the mobility of the dye molecules, which ultimately increased the diffusion rate of dye molecules in the fabric. Additionally, according to Graham’s law, lower-molecular-weight particles diffuse faster than higher-molecular-weight particles with less time, and it is proportional to temperature, which was why LMD showed a higher K/S value in a shorter time than HMD [26]. The improved rate of K/S development with smaller dye molecules was attributed to the promotion of dye relocation into the material at lower energy levels, despite having ultimately lower K/S values than the HMD. The HMD possesses a higher number of ionic groups (-SO3Na) in its chemical structure that are responsible for dye attachment onto PA (Figure 1), which could possibly influence the ultimate color strength achieved.
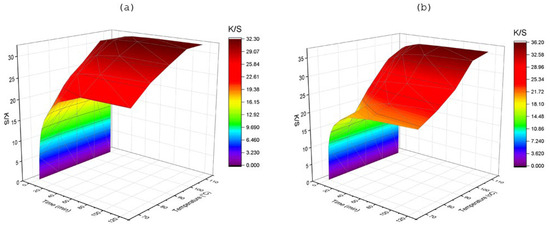
Figure 5.
Effect of temperature on color strength (K/S) at different time intervals (t), with 3% shade and pH 4, for (a) LMD and (b) HMD.
3.2.3. Effect of pH
Varying pH levels, ranging from high to mildly acidic conditions of the dye bath, resulted in different levels of dye strength in PA fabrics, as shown in Figure 6. In case of LMD, when pH was raised from 2 to 6, the K/S value decreased about 28% in 5 min, 22% in 30 min, and 21% in 120 min. However, the K/S value decreased by a smaller amount in the case of HMD under the same conditions (around 9%, 11%, and 12% for 5 min, 30 min, and 120 min, respectively). The dynamic of dye migration was significantly enhanced, and the K/S reached a peak at a certain pH condition. A lower pH condition increases the concentration of hydrogen ions, which elevates the positivity of the polyamide fiber charge in the solution [3]. As the isoelectric point for polyamide fiber appears at pH 3, raising the pH reduces the concentration of positive ions in solution, which deprotonates the carboxyl group and leads the fiber to be neutral or negatively charged, which deaccelerates the interaction with the negatively charged dyestuff [29]. The LMD diffusion was faster around pH 3–4, whereas pH 2–4 assisted the rapid migration of HMD. The degree of transfer of dye into the samples expeditiously reaches near their maximum at the mentioned level. The cause of this may be possessing higher polar groups (as two groups of -NH2 and 2 groups of -SO3Na) in HMD than LMD, as acid dye interacts more rapidly at lower pH with polar end groups (–NH2 and –COOH) in fibers chemically following Lewis acid–base interaction [26]. Despite having a negatively charged surface of polyamide fiber in the board pH range, adsorption of dyestuff still took place because of the attraction force, which is higher than the electrostatic force. However, it imparts a lower level of dye penetration. Therefore, a lower pH condition increased the concentration of hydrogen ions, which elevated the positivity of the polyamide fiber charge in the solution [29].
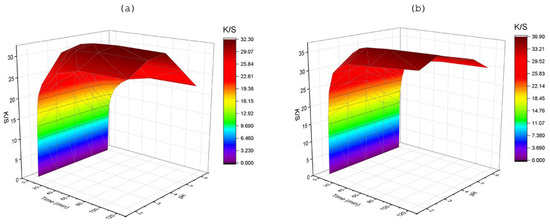
Figure 6.
Effect of pH on color strength (K/S) at different time intervals (t), at 3% shade and 100 °C, for (a) LMD and (b) HMD.
3.3. Dye Migration Index (MI) and Zeta Potential
The results showed (Figure 7a) greater MI at 100 °C than at 70 °C for both dyed fabrics, as a higher temperature increases the rate of desorption in the kinetics stage of migration [30]. The MI at 100 °C was higher, which may lead to the risk of lower wet fastness property at the elevated temperature as a result of promoted thermal migration of dye molecules. The migration level of dye increased more for LMD-dyed material than for HMD-dyed ones, which was assisted by the higher temperature applied in the blank bath because of having a high diffusion coefficient with low affinity of LMD particles. Higher temperatures increase fiber swelling and dye kinetic energy, enhancing the diffusion rate of LMD particles due to their smaller size. At elevated dye concentrations, greater dye availability promotes deeper penetration and higher migration [31]. Both dyes showed a little upward trend of migration indices with the increased dye concentration due to a rise in electrolyte concentration in the migration bath, which influences the diffusion coefficient [30].
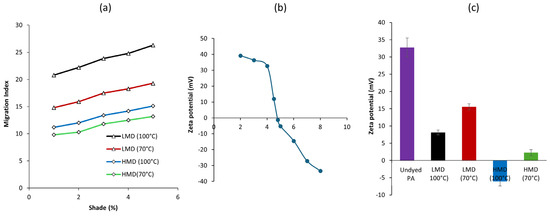
Figure 7.
(a) Migration index of low-molecular-weight (LMD) and high-molecular-weight (HMD) anthraquinone acid dyes on PA fabric, (b) zeta potential of undyed PA fabric at different pH, and (c) zeta potential of the dyed fabric compared to undyed fabric at pH 4.
Zeta potential measurements were performed to assess the surface charge behavior of PA fabrics before and after dyeing at pH 4, representative of actual dyeing environments. The zeta potential of undyed PA (Figure 7b) exhibited a clear dependence on pH, with values transitioning from positive to negative across the tested range (pH 2–8). At acidic pH (e.g., +39.2 mV at pH 2), the surface of PA remained positively charged due to the protonation of terminal amino groups (–NH2 + H+ → –NH3+). As the pH increased, the surface charge decreased steadily and reached zero between pH 4.5 and 4.8, which corresponds to the isoelectric point (IEP) of PA [32]. Beyond this point, deprotonation of functional groups led to a net negative surface charge (e.g., −27.1 mV at pH 7), driven by the presence of residual carboxylic acid or deprotonated amide groups. This electrokinetic behavior is critical for understanding dye–fiber interactions in acid dyeing. At pH < IEP, the positively charged PA surface promotes strong electrostatic attraction with the anionic sulfonate groups of acid dyes, facilitating higher initial dye uptake [33]. During the coloration of polyamide (PA) fibers, hydrolysis of amide linkages may occur under acidic and high-temperature conditions. This reaction results in the formation of new amino groups, which, upon protonation, can serve as additional binding sites for anionic dye molecules [34]. However, at pH values below 2.5, acid-catalyzed hydrolytic degradation becomes significant, compromising the mechanical integrity of the fiber and leading to reduced tensile strength. Moreover, exposing PA fibers to elevated temperatures (e.g., 100 °C) under highly acidic conditions (pH < 3.5) further accelerates hydrolysis [34,35]. Therefore, dyeing under such conditions is not recommended due to the risk of fiber damage. Therefore, maintaining a dye bath pH slightly below the IEP (e.g., 4.0) can provide a balanced environment, optimizing dye uptake and leveling during acid dyeing processes.
The zeta potential behavior of the undyed PA, in relation to the dyed fabrics at pH 4, is shown in Figure 7c. The undyed PA sample exhibited a positive zeta potential of +32.7 mV, at pH 4. This is consistent with the presence of protonated amide groups (–NH3+) on the PA surface. After dyeing, a progressive reduction in zeta potential was observed across all dyed samples, indicating surface modification by the adsorption of anionic dye molecules (sulfonate-containing structures). Samples dyed with LMD showed a shift to +15.5 mV at 70 °C and +8.1 mV at 100 °C, suggesting moderate dye uptake and increased surface coverage at higher temperature. In contrast, fabrics dyed with the HMD displayed a stronger change in surface charge, with values of +2.3 mV at 70 °C and −6.1 mV at 100 °C, indicating more substantial adsorption of negatively charged dye groups. The negative value at 100 °C confirms the dominance of sulfonic acid groups on the fabric surface, likely due to the extensive conjugated structure and better fixation of HMD. Overall, the zeta potential data provide evidence that dye molecular weight and dyeing temperature jointly influence dye–fiber interaction, with HMD showing greater affinity and functional impact on the PA substrate. The zeta potential data were seen as complementary to the dye migration behavior observed in the prior discussion. The fabrics dyed with the LMD retained a more positive surface charge, indicating weaker dye–fiber interactions and a higher tendency for dye migration. In contrast, the HMD reduced the zeta potential to near-zero or negative values, reflecting stronger dye fixation and correspondingly lower migration behavior during and after dyeing.
3.4. UV Protection and FTIR
The UV protection of textiles can be significantly influenced by the fabric’s physical structure. Knitted fabrics tend to have a more open construction and higher porosity than woven fabrics, making them more prone to UV penetration. As noted in previous studies, low-porosity structures offer better inherent UV protection [36,37], and knitted fabrics may therefore require additional UV-absorbing treatments or functional dyes to achieve acceptable performance levels. In this study, the structural influence was controlled by maintaining consistent fabric properties, allowing the specific effect of the dye on UV protection to be isolated and evaluated. The UPF results (Figure 8a) demonstrated that dyeing PA fabric significantly improves its UV protection ability. The undyed PA showed a low UPF (~7), indicating “non-rateable” natural UV shielding. In contrast, HMD dyed at 100 °C showed the highest UPF (~41), qualifying it as an “Excellent” UV protection category (UPF > 40) [37,38]. LMD dyed at 100 °C falls within the “good” category (UPF ~24), lower than the values from HMD. Both dyes showed a lower UPF when dyed at 70 °C, confirming that higher dyeing temperature enhances dye uptake and UV protection. The superior UPF of HMD was likely due to higher UV absorptivity by the extended conjugated aromatic structure [15,39,40]. The extended conjugation in HMD allows them to absorb a broader range of UV radiation, particularly in the UV-A and UV-B regions [41]. This increases the UPF of dyed fabrics, as more UV photons are absorbed by the dye molecules before reaching the skin. However, the results confirmed that both dye structure and process conditions significantly influence the UV-protective functionality of dyed PA fabrics. The FTIR spectra presented in Figure 8b illustrates both the inherent chemical structure of PA and the modifications introduced by acid dyeing. FTIR can identify key functional groups (e.g., –SO3−, –NH, –C=O) associated with dye–fiber interactions. Shifts in N–H or C=O stretching after dyeing suggest hydrogen bonding or ionic interactions. Enhanced absorbance in aromatic or C=C regions for HMD-dyed samples may also correlate with better UV absorption and color retention. Primarily, electrostatic interactions between the anionic sulfonate groups of the dye (–SO3−) and the protonated amine groups (–NH3+) on PA occur at acidic pH [28,31,35]. HMD has more –SO3Na groups, thus a higher ionic character, which increased its affinity to the positively charged sites on PA at acidic pH. Additionally, hydrogen bonding and van der Waals forces can also contribute to overall dye fixation and fastness.
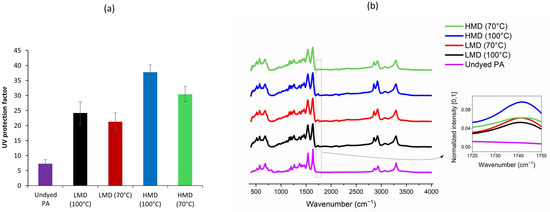
Figure 8.
(a) UV protection factor and (b) FTIR spectra dyed PA with low-molecular-weight (LMD) and high-molecular-weight (HMD) anthraquinone acid dyes compared to undyed PA fabric.
For undyed PA, several characteristic absorption bands were observed, i.e., 3300–3500 cm−1: broad N–H stretching vibrations from amide groups; 2930 cm−1: C–H stretching of aliphatic –CH2– groups; 1630–1650 cm−1: amide I band, primarily C=O stretching of the amide group; 1530–1550 cm−1: amide II band, arising from N–H bending and C–N stretching; 1200–1300 cm−1: C–N stretching vibrations; and 600–750 cm−1: C–H out-of-plane bending modes [42,43]. These bands confirmed the typical chemical backbone of PA 6, composed of repeated amide and methylene units. After dyeing, a new absorbance band appeared in the region of 1720–1750 cm−1, which was not present in the undyed fabric. This band was attributed to C=O stretching of ester or carboxylic acid groups originating from dye molecules [44]. Its presence indicated that dye molecules have been successfully adsorbed or bound to the PA substrate. Notably, this peak was more intense in the fabrics dyed with the high-molecular-weight dye (HMD), especially at 100 °C, compared to the low-molecular-weight dye (LMD). This suggested more abundant chromophores and functional groups that may have contributed to UV absorption. Overall, FTIR analysis validated the incorporation of anthraquinone dye molecules into the PA matrix and highlighted a temperature-dependent increase in surface interaction, consistent with dyeing performance and UV protection trends.
3.5. Color Fastness
The color fastness results of the dyed PA fabric are shown in Table 3. Washing at higher temperatures imparted good to moderate staining properties to both of the acid-dyed substrates. Dye transferred to adjacent cloths is the most dependent upon the affinity of the acid dyes for the fibrous material under wet treatments as well as temperature [29,30]. The HMD exhibited allover better resistance to the action of every washing condition than the LMD, which may be due to its larger size and more extensive conjugated structures, which allow stronger van der Waals and hydrogen bonding interactions with the polyamide backbone. Compared to LMD, HMD is likely to have limited mobility and stronger binding to polyamide via multiple interactions (e.g., hydrogen bonds, van der Waals forces, and dipole–dipole). Its lower solubility and diffusion reduce the tendency to migrate out of the fiber during washing or mechanical action.

Table 3.
Fastness properties of PA fabric dyed with low-molecular-weight (LMD) and high-molecular-weight (HMD) anthraquinone acid dye.
The results of MI were also an indication of the wash fastness behavior of the used acid dyes [30,45].
The results of color change and staining of fastness to water showed the rating “good”. Like the wash fastness, comparatively higher fastness was achieved in this case of the HMD-dyed fabric.
The alkaline perspiration condition was again in much favor of HMD-dyed samples, which resulted in an excellent staining rating overall for all the adjacent cloths. The smaller dye molecules may be closely involved with their responses to the heat treatments, which was assisted by the transfer of a significant amount of dyes to the adjacent substrates [30]. It was noticed that the adjacent multifiber-fabrics received color mostly from the LMD-dyed samples, resulting in poor fastness to dry heat as compared to that of HMD ones.
4. Conclusions
In this study, the influence of anthraquinone acid dye molecular weight on the color strength development, migration behavior, and functional performance of PA 6 fabric was systematically explored under varying process parameters. Although both low- (LMD) and high-molecular-weight dyes (HMD) exhibited similar color strength in the initial dyeing phase, LMD showed a more rapid increase at later stages, particularly under elevated dye concentrations and temperatures. LMD also demonstrated higher migration proneness, especially at increased shade levels, supported by zeta potential data. In contrast, HMD offered superior wash and rub fastness (ratings ~4–5) and contributed to greater UV protection, likely due to its larger conjugated structure, as supported by FTIR spectral analysis. These findings provide valuable insights into optimizing dye selection and processing conditions in PA dyeing applications to achieve desired aesthetic and functional outcomes.
Author Contributions
Conceptualization, N.F. and A.N.M.A.H.; software, N.F. and S.A.S.; validation, N.F.; formal analysis, N.F. and A.N.M.A.H.; investigation, N.F. and M.N.; writing—original draft preparation, N.F.; writing—review and editing, A.N.M.A.H., A.S.M.S., F.S., M.A.I. and S.M.K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Md Nasim was employed by the Liberty Knitwear Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Jóźwiak, T.; Filipkowska, U. The use of rapeseed husks to remove acidic and basic dyes from aquatic solutions. Appl. Sci. 2024, 14, 1174. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Sorption properties of fabricated film from cotton gin trash. Mater. Today Proc. 2020, 31, S221–S226. [Google Scholar] [CrossRef]
- Farzana, N.; Uddin, M.Z.; Haque, M.M.; Haque, A.N.M.A. Dyeability, kinetics and physico-chemical aspects of Bombyx mori muslin silk fabric with bi-functional reactive dyes. J. Nat. Fibers 2020, 17, 986–1000. [Google Scholar] [CrossRef]
- Waring, D.R.; Hallas, G. The Chemistry and Application of Dyes; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Khatri, M.; Hussain, N.; El-Ghazali, S.; Yamamoto, T.; Kobayashi, S.; Khatri, Z.; Ahmed, F.; Kim, I.S. Ultrasonic-assisted dyeing of silk fibroin nanofibers: An energy-efficient coloration at room temperature. Appl. Nanosci. 2020, 10, 917–930. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Cabello-Alvarado, C.J.; Ávila Orta, C.A.; Cadenas-Pliego, G.; Cruz-Ortiz, B. Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior. Materials 2024, 17, 3007. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Naebe, M. Future Textiles; Deakin University: Waurn Ponds, VIC, Australia, 2024; Available online: https://hdl.handle.net/10779/DRO/DU:27221277.v1 (accessed on 19 June 2025).
- Bird, C.L.; Boston, W.S. Theory of Colouration of Textiles; White Rose Press Ltd.: London, UK, 1975. [Google Scholar]
- Carpignano, R.; Savarino, P.; Barni, E.; Viscardi, G.; Baracco, A.; Clementi, S. Dyeing of nylon 66 with disperse dyes. An optimization study. Dye. Pigment. 1989, 10, 23–31. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of Dye and Pigments. In Dyes and Pigments; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–45. [Google Scholar]
- Bae, J.-S.; Park, J.H.; Koh, J.; Kim, S.D. Dyeing and fastness properties of a reactive disperse dye on PET, nylon, silk and N/P fabrics. Fibers Polym. 2006, 7, 174–179. [Google Scholar] [CrossRef]
- Trotman, E.R. Dyeing and Chemical Technology of Textile Fibres; Griffin: London, UK, 1975. [Google Scholar]
- Huang, X.; Ye, W.; Zhuang, J.; Hu, C.; Dong, H.; Lei, B.; Liu, Y. π-Conjugated structure enhances the UV absorption performance of carbon dots and application in the design of light-colored sunglasses. ACS Sustain. Chem. Eng. 2024, 12, 10399–10410. [Google Scholar] [CrossRef]
- Klinkhammer, K.; Weskott, P.; Ratovo, K.; Krieg, M.; Bendt, E.; Mahltig, B. Transmission reduction for UV and IR radiation with dyed lyocell knitted textiles. Appl. Sci. 2023, 13, 5432. [Google Scholar] [CrossRef]
- Tayebi, H.-A.; Yazdanshenas, M.E.; Rashidi, A.; Khajavi, R.; Montazer, M. The Isotherms, Kinetics, and Thermodynamics of Acid Dye on Nylon6 with Different Amounts of Titania and Fiber Cross Sectional Shape. J. Eng. Fibers Fabr. 2015, 10, 155892501501000110. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Ando, T. The Concentration Dependence of the Diffusion Coefficient of Acid Dyes in Nylon. J. Soc. Dye. Colour. 1983, 99, 92–97. [Google Scholar] [CrossRef]
- Atherton, E.; Downey, D.; Peters, R. Studies of the dyeing of nylon with acid dyes: Part I: Measurement of affinity and the mechanism of dyeing. Text. Res. J. 1955, 25, 977–993. [Google Scholar] [CrossRef]
- Gupta, V.; Chavan, R.; Kulkarni, M.; Natarajan, K. Dye-uptake behaviour of nylon-6 filaments and its structural dependence. Color. Technol. 2000, 116, 385–392. [Google Scholar] [CrossRef]
- Bell, J.P. Relation between nylon fiber mechanical properties and dye diffusion behavior. J. Appl. Polym. Sci. 1968, 12, 627–638. [Google Scholar] [CrossRef]
- Wang, H.H.; Wang, C.C.; Kuo, H.J. The kinetics and thermodynamics of nylon 6 fiber dyeing with hydrogen peroxide-glyoxal redox system. J. Appl. Polym. Sci. 2000, 76, 2105–2114. [Google Scholar] [CrossRef]
- Burkinshaw, S.; Son, Y.-A. A comparison of the colour strength and fastness to repeated washing of acid dyes on standard and deep dyeable nylon 6,6. Dye. Pigment. 2006, 70, 156–163. [Google Scholar] [CrossRef]
- ISO 105 E01; Textiles—Tests for Colour Fastness: Colour Fastness to Water. International Organisation for Standardisation: Geneva, Switzerland, 2013.
- ISO 105 E04; Textiles—Tests for Colour Fastness: Colour Fastness to Perspiration. International Organisation for Standardisation: Geneva, Switzerland, 2013.
- Fick, A. On liquid diffusion. J. Membr. Sci. 1995, 100, 33–38. [Google Scholar] [CrossRef]
- Mason, E.; Evans, R.I. Graham’s laws: Simple demonstrations of gases in motion: Part I, Theory. J. Chem. Educ. 1969, 46, 358. [Google Scholar] [CrossRef]
- Remington, W.; Gladding, E. Equilibria in the dyeing of nylon with acid dyes. J. Am. Chem. Soc. 1950, 72, 2553–2559. [Google Scholar] [CrossRef]
- Lewis, G.N. Valence and the Structure of Atoms and Molecules; Chemical Catalog Company, Incorporated: New York, NY, USA, 1923. [Google Scholar]
- Bouloton, J.; Morton, T. The dyeing of cellulosic materials: A review of the physics and chemistry of the dyeing process. J. Soc. Dye. Colour. 1940, 56, 145–159. [Google Scholar] [CrossRef]
- Islam, M.T.; Islam, T.; Islam, T.; Repon, M.R. Synthetic dyes for textile colouration: Process, factors and environmental impact. Text. Leather Rev. 2022, 5, 327–373. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, M.; Ni, P. Size distribution and zeta-potential of polyamide microcapsules. J. Membr. Sci. 1992, 72, 163–169. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Zille, A.; Souto, A.P. Dyeing mechanism and optimization of polyamide 6,6 functionalized with double barrier discharge (DBD) plasma in air. Appl. Surf. Sci. 2014, 293, 177–186. [Google Scholar] [CrossRef]
- Stiegelmaier, E.; Costa, T.C.; Pakuszewski, G.; de Souza, S.A.G.U.; de Souza, A.A.U.; Immich, A.P.S. Enhancing polyamide 6: Acid hydrolysis for functionalization and amino group quantification. Polymer 2024, 298, 126905. [Google Scholar] [CrossRef]
- Bhatt, N.; Daruwalla, E. Studies in the mechanism of dyeing of polyamide fibers with acid dyes. Text. Res. J. 1964, 34, 435–444. [Google Scholar] [CrossRef]
- Reinert, G.; Fuso, F.; Hilfiker, R.; Schmidt, E. UV-protecting properties of textile fabrics and their improvement. Text. Chem. Color. 1997, 29, 36–43. [Google Scholar]
- Tarbuk, A.; Grancarić, A.M.; Šitum, M. Skin cancer and UV protection. AUTEX Res. J. 2016, 16, 19–28. [Google Scholar] [CrossRef]
- Louris, E.; Sfiroera, E.; Priniotakis, G.; Makris, R.; Siemos, H.; Efthymiou, C.; Assimakopoulos, M. Evaluating the ultraviolet protection factor (UPF) of various knit fabric structures. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Lesvos, Greece, 5–7 September 2018; p. 012051. [Google Scholar]
- Saha, B.; Saha, A.; Das, P.; Kakati, A.; Banerjee, A.; Chattopadhyay, P. A comprehensive review of ultraviolet radiation and functionally modified textile fabric with special emphasis on UV protection. Heliyon 2024, 10, e40027. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, C.; Song, K.; Hou, A. Preparation of multi-functional cellulose containing huge conjugated system and its UV-protective and antibacterial property. Carbohydr. Polym. 2014, 114, 392–398. [Google Scholar] [CrossRef]
- Zhang, Y.; Haque, A.N.M.A.; Naebe, M. UV-functional flexible nanocomposite film with high lignin-cellulose nanocrystals content. J. Mater. Res. Technol. 2023, 26, 5990–6000. [Google Scholar] [CrossRef]
- Fayyaz, A.; Asghar, H.; Waqas, M.; Kamal, A.; Al-Onazi, W.A.; Al-Mohaimeed, A.M. Multi-Spectroscopic Characterization of MgO/Nylon (6/6) Polymer: Evaluating the Potential of LIBS and Statistical Methods. Polymers 2023, 15, 3156. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Chen, X.; Gao, Y.; Zhu, B.; He, Y. Effects of polyetheramine on the properties of polyamide 6. J. Polym. Sci. Appl. 2017, 6, 2–10. [Google Scholar]
- Saito, K.; Xu, T.; Ishikita, H. Correlation between C=O stretching vibrational frequency and p K a shift of carboxylic acids. J. Phys. Chem. B 2022, 126, 4999–5006. [Google Scholar] [CrossRef]
- Cegarra, J. Determination of the migratory properties of direct dyes. J. Soc. Dye. Colour. 1957, 73, 375–381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).