Synthesis and Characterization of Eco-Friendly Nanocomposites Using Galactomannan and Organomodified Montmorillonite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Purification of Biopolymer
2.3. Synthesis of Galactomannan/Organomodified Montmorillonite Nanocomposites
2.4. In Vitro Antioxidant Assay
2.5. Characterization Methods
3. Results and Discussion
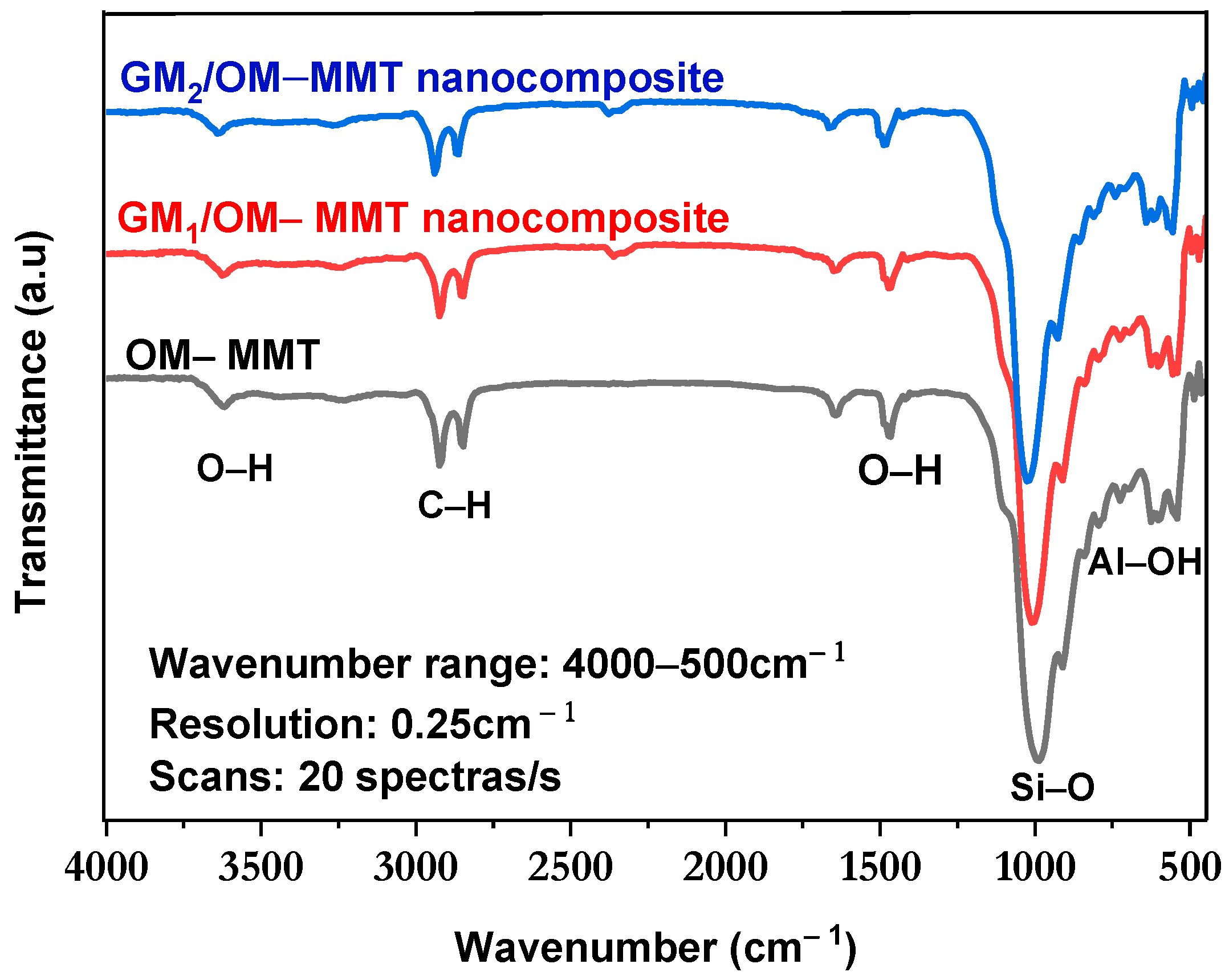
3.1. FT-IR Analysis
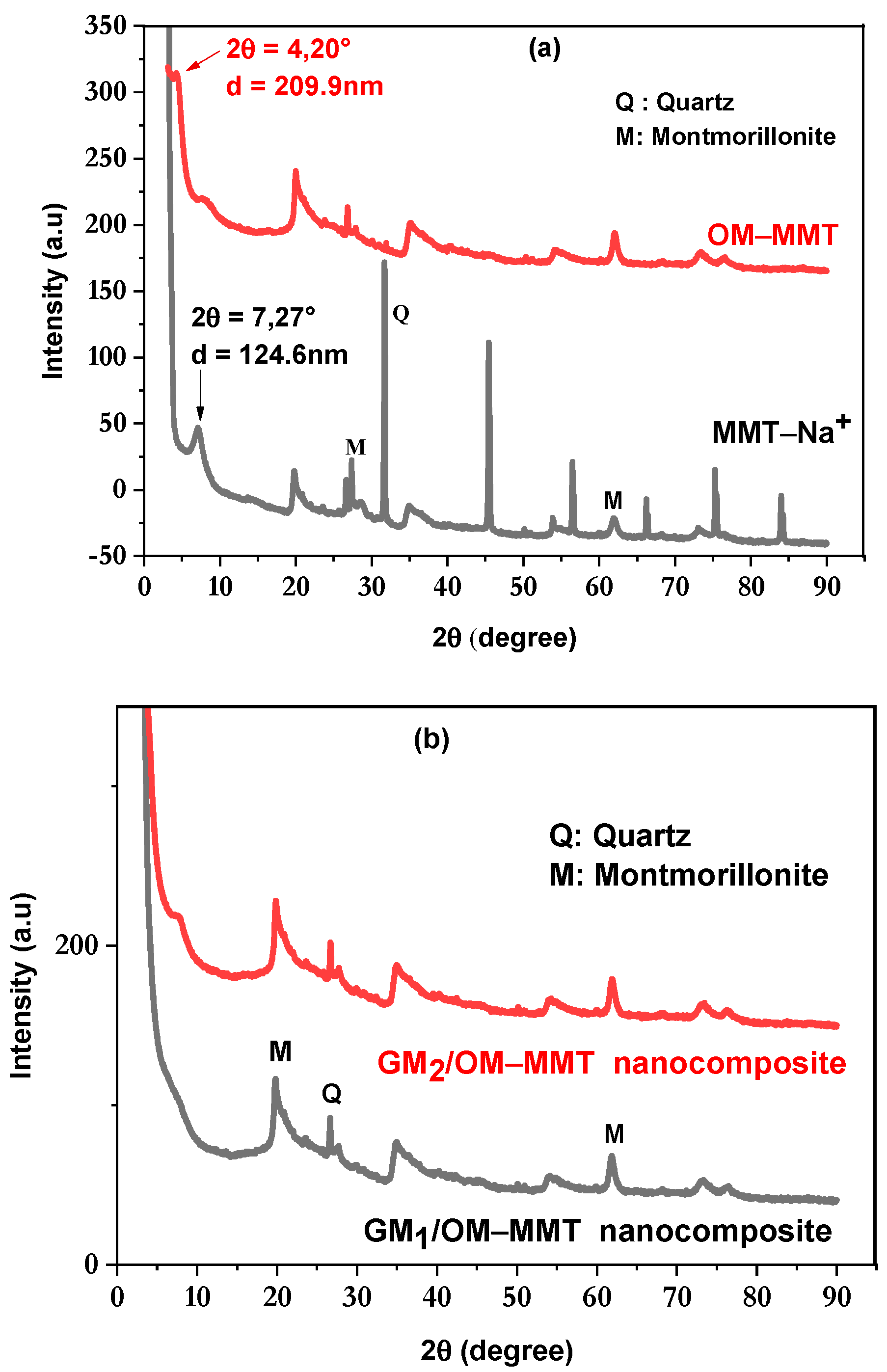
3.2. X-Ray Diffraction (XRD)
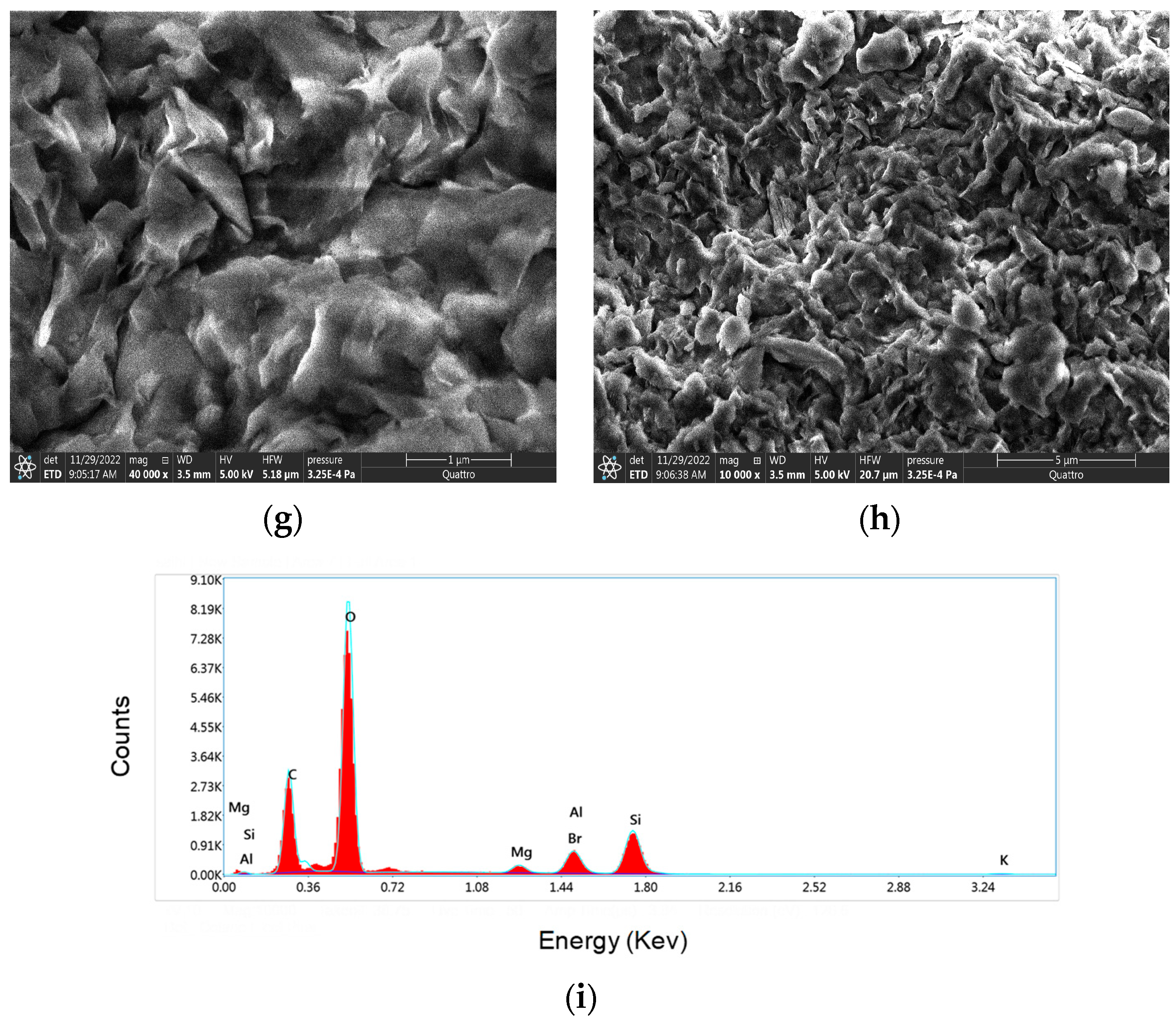
3.3. Morphological Investigation of Bionanocomposites and EDX Analysis
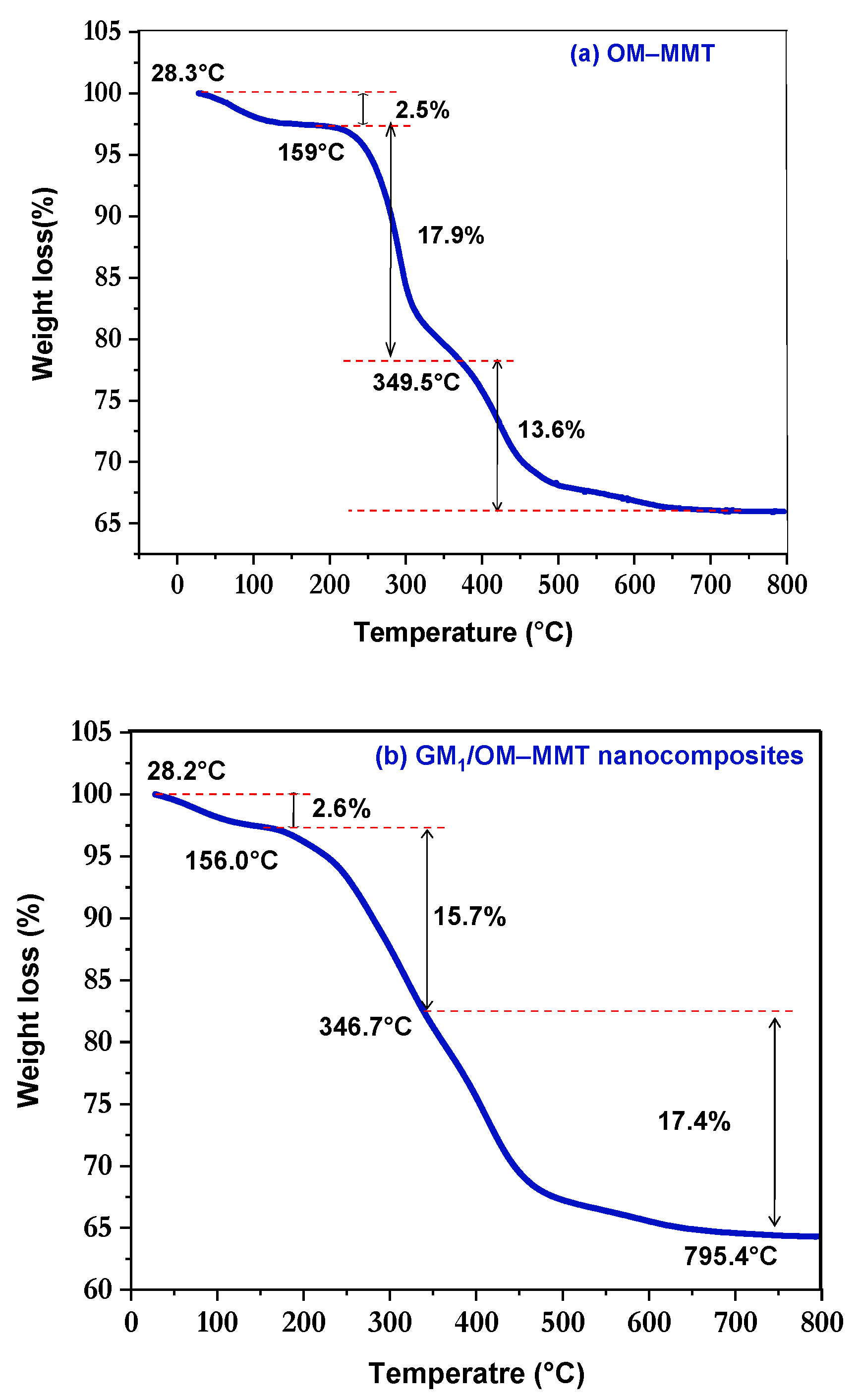
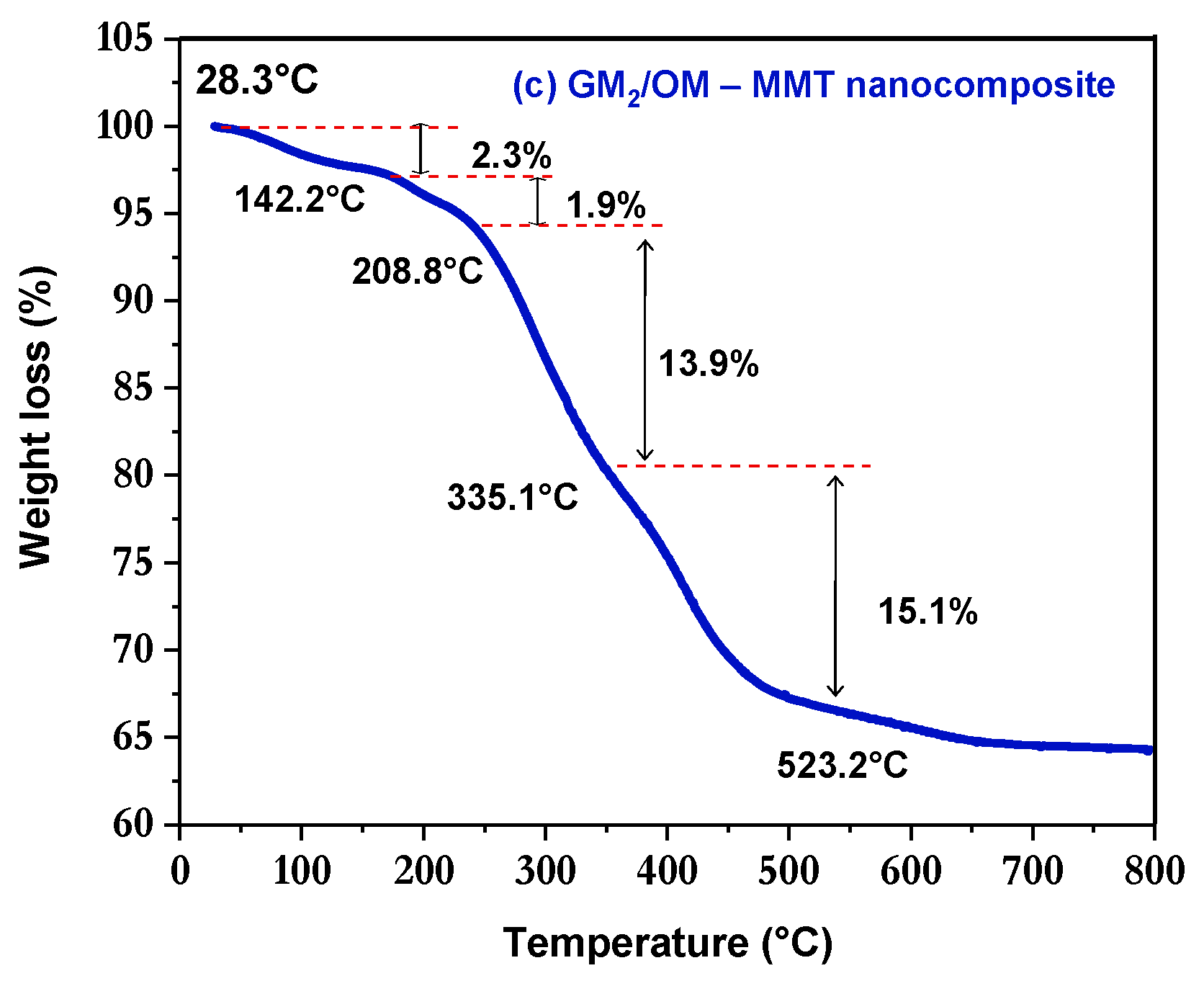
3.4. Thermal Stability
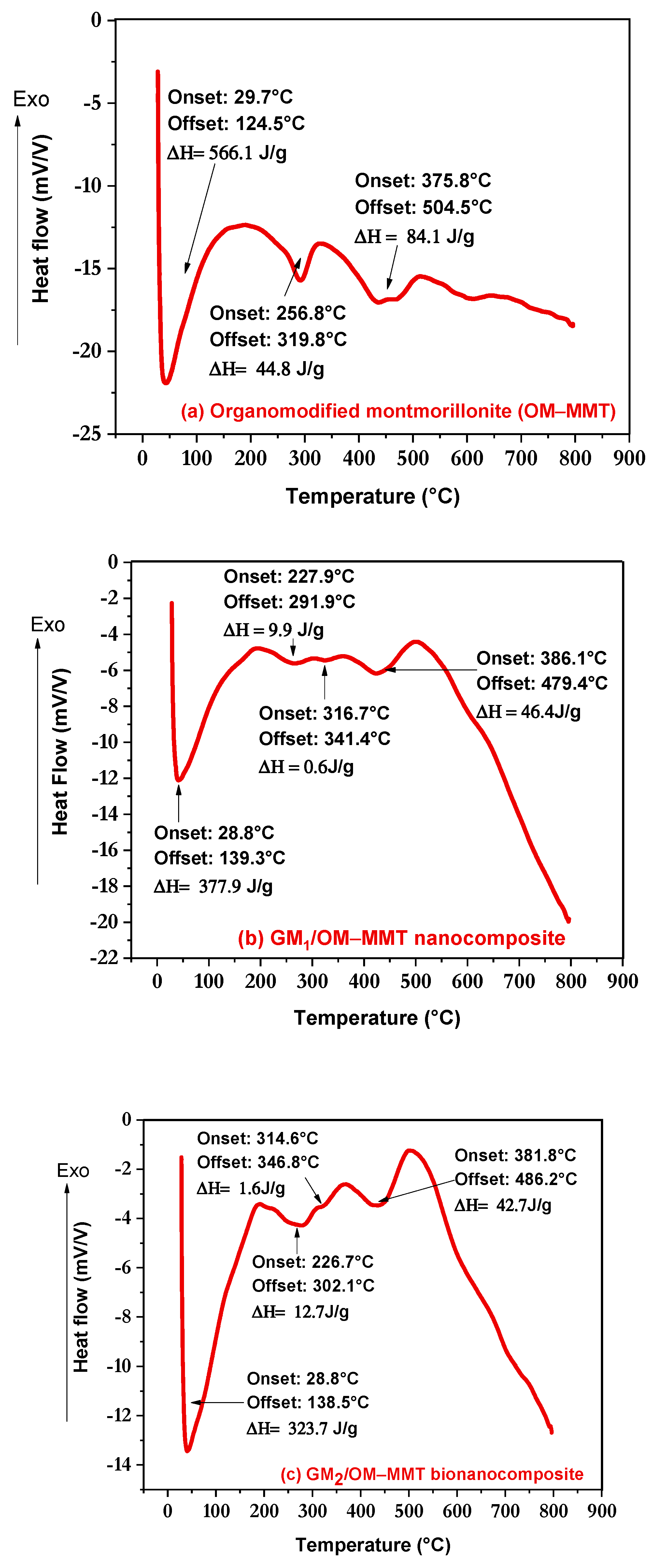
3.5. DSC Analysis
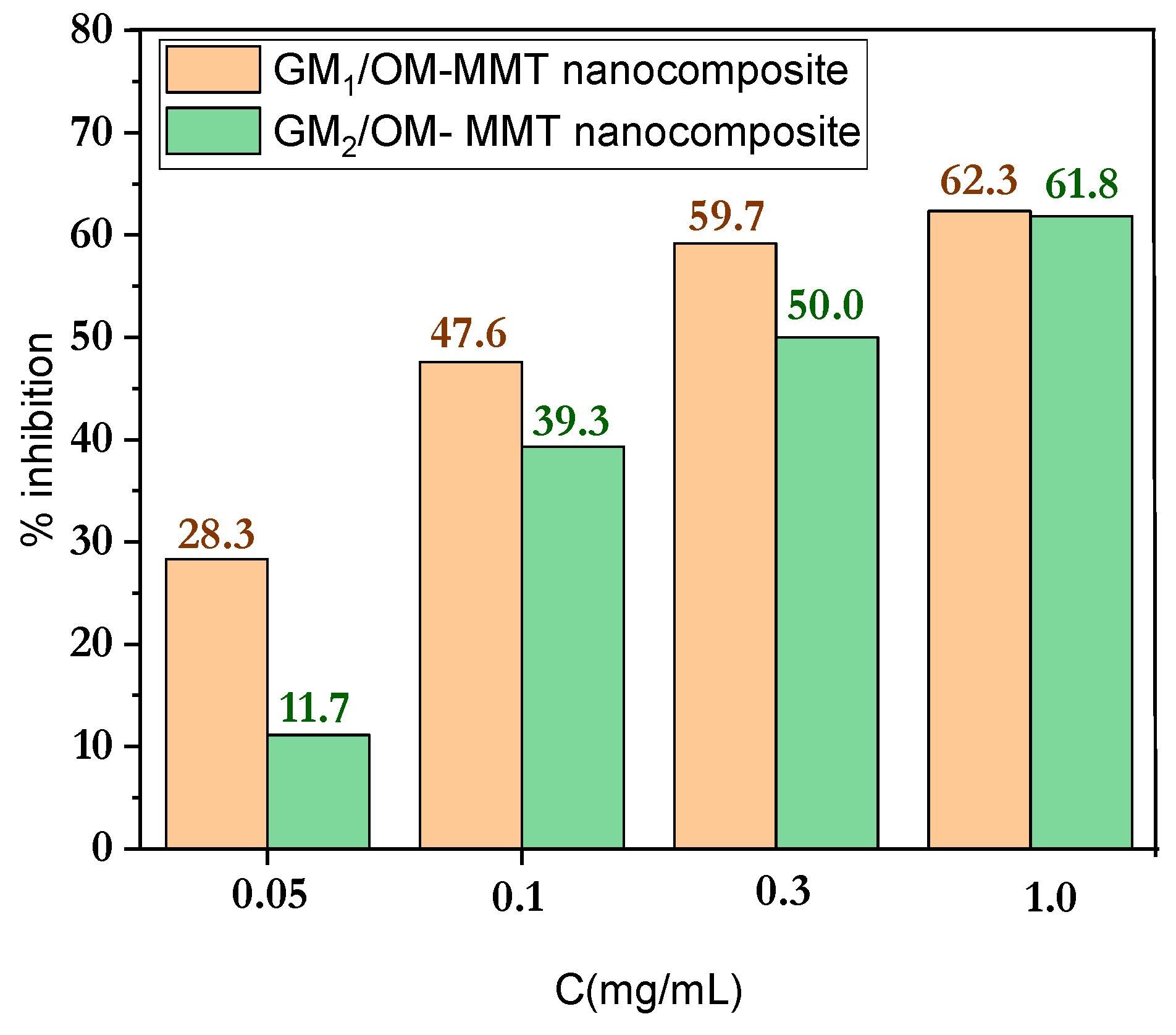
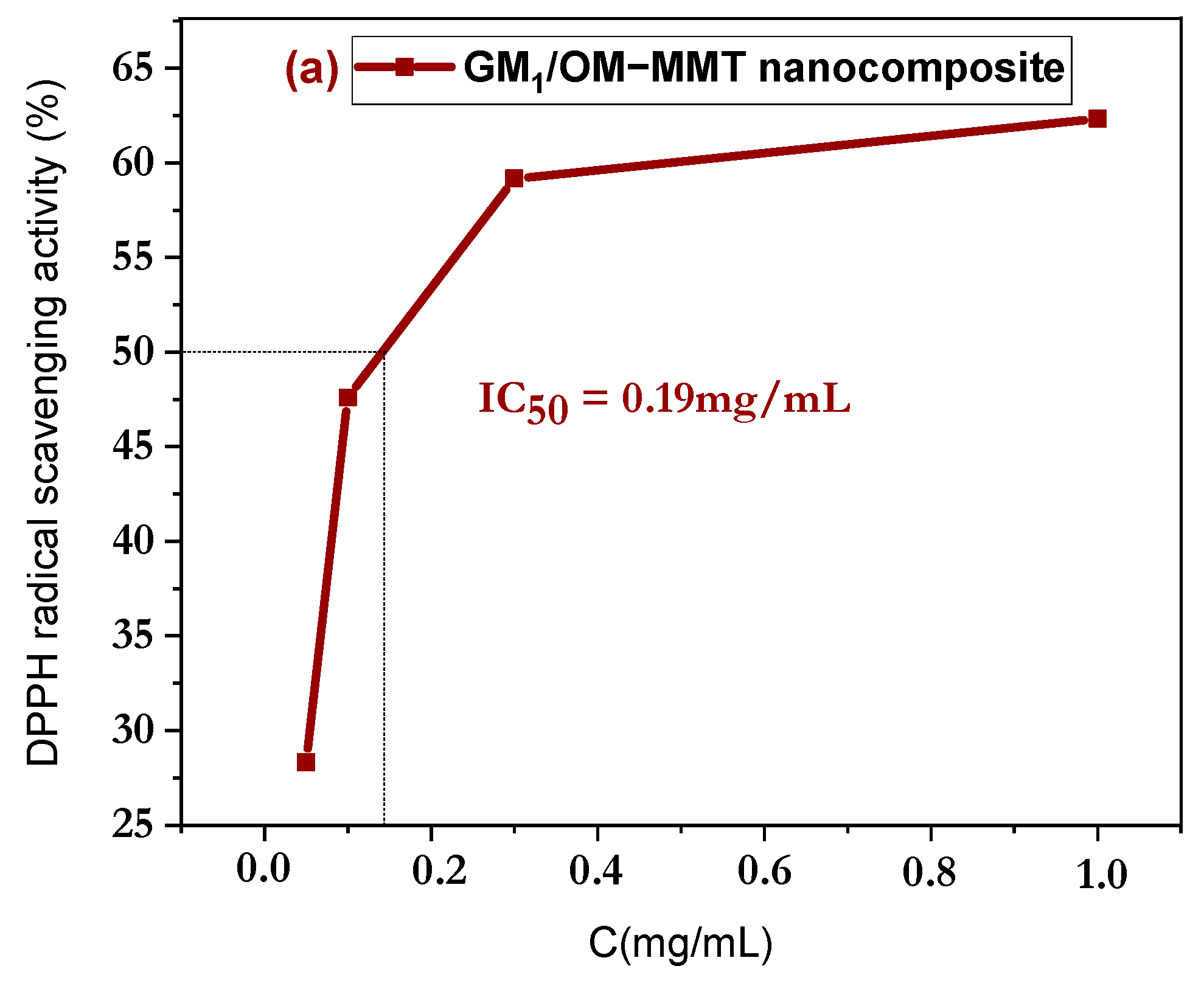
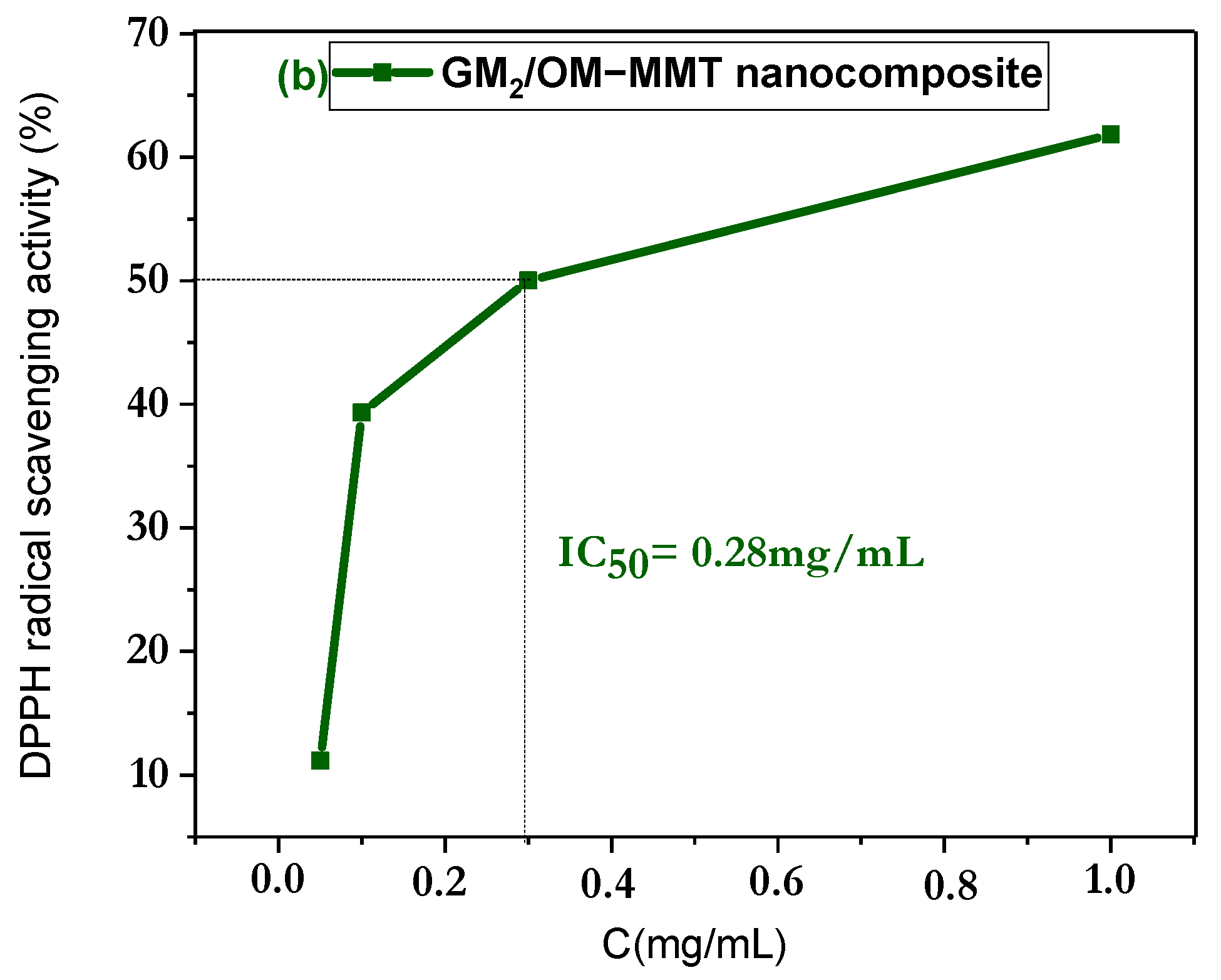
3.6. In Vitro Antioxidant Discussion
3.7. DPPH Radical Scavenging Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| GM | Galactomannan |
| OM-MMT | Organomodified montmorillonite |
| DPPHD | Phenyl-2-picryhydrazyl |
| IC50 | Half-maximal inhibitory concentration |
References
- Rajendra, K.K.; Anshuman, S. Recent Developments in Bio-nanocomposites: A Review. Res. J. Nanosci. Eng. 2018, 2, 1–4. [Google Scholar]
- Zhang, Y.P.; Wang, X.; Shen, Y.; Thakur, K.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Preparation and Characterization of Bio-Nanocomposites Film of Chitosan and Montmorillonite Incorporated with Ginger Essential Oil and Its Application in Chilled Beef Preservation. Antibiotics 2021, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Proença, L.B.; Righetto, G.M.; da Cunha Camargo, I.L.B.; Branciforti, M.C. Poly(acid lactic)-montmorillonite clay bionanocomposites loaded with tea tree oil for application in antibacterial wound healing. Hybrid Adv. 2024, 6, 100201. [Google Scholar] [CrossRef]
- Krzykowska, B.; Uram, Ł.; Frącz, W.; Kovářová, M.; Sedlařík, V.; Hanusova, D.; Kisiel, M.; Paciorek-Sadowska, J.; Borowicz, M.; Zarzyka, I. Polymer Bionanocomposites Based on a P3BH/Polyurethane Matrix with Organomodified Montmorillonite-Mechanical and Thermal Properties, Biodegradability, and Cytotoxicity. Polymers 2024, 16, 2681. [Google Scholar] [CrossRef]
- Botta, L.; La Mantia, F.P.; Mistretta, M.C.; Oliveri, A.; Arrigo, R.; Malucelli, G. Structure-property relationships in bionanocomposites for pipe extrusion applications. Polymers 2021, 13, 782. [Google Scholar] [CrossRef]
- Lizundia, E.; Armentano, I.; Luzi, F.; Bertoglio, F.; Restivo, E.; Visai, L.; Torre, L.; Puglia, D. Synergic Effect of Nanolignin and Metal Oxide Nanoparticles into Poly(L-lactide) Bionanocomposites: Material Properties, Antioxidant Activity, and Antibacterial Performance. ACS Appl. Bio Mater. 2020, 3, 5263–5274. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Ophthalmol. Clin. Res. 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Akbar, M.U.; Athar, M.M.; Bhatti, I.A.; Bhatti, H.N.; Khosa, M.K.; Zia, K.M. Chapter17—Biomedical applications of bionanocomposites. In Bionanocomposites, Green Synthesis and Applications Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 457–483. [Google Scholar] [CrossRef]
- Joshi, G.; Yadav, K.S. Chapter 2—Applications of bioresins and biopolymers derived from natural resources as composites in drug delivery. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 21–34. [Google Scholar]
- Reddy, K.; Mohan, G.K.; Satla, S.; Gaikwad, S. Natural polysaccharides: Versatile excipients for controlled drug delivery systems. Asian J. Pharm. Sci. 2011, 6, 275. [Google Scholar]
- Ozen, I.; Bahtiyari, I.M.; Haji, A.; ul Islam, S.; Wang, X. Properties of galactomannans and their textile-related applications—A concise Review. Int. J. Biol. Macromol. 2023, 227, 1001–1014. [Google Scholar] [CrossRef]
- Bouziane, G.; Henni, A.; Bouricha, M.; Boual, Z.; Belkhalfa, H.; Bachari, K.; Didi Ould El Hadj, M. A new galactomannan extracted from the seeds of Astragalus gombiformis Pomel (Fabaceae) and its utilization in the biosynthesis of silver nanoparticles. Process Biochem. 2024, 136, 48–59. [Google Scholar] [CrossRef]
- Pandey, J.; Singh, B.D.; Khanam, H.; Tiwari, B.; Azaz, T.; Singh, R. Cassia fistula galactomannan stabilized copper nanocatalyst as an efficient, recyclable heterogeneous catalyst for the fast clickable [3+2] Huisgen cycloadditions in water. Int. J. Biol. Macromol. 2024, 255, 128098. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Kaur, G. Leucaena leucocephala (Lam.) galactomannan nanoparticles: Optimization and characterization for ocular delivery in glaucoma treatment. Int. J. Biol. Macromol. 2019, 139, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Kapoor, V.P. Seed galactomannans: Overview. Chem. Biodivers. 2005, 2, 295–317. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Ramakrishna, G.; Srivastava, H.; Gaikwad, K. A comprehensive review on leguminous galactomannans: Structural analysis, functional properties, biosynthesis process and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 443–465. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Amid, B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, W.; Feng, C.; Zhu, L.; Ji, L.; Wang, K.; Jiang, J. Study on structure and properties of galactomannan and enzyme changes during fenugreek seeds germination. Carbohydr. Polym. 2024, 327, 121653. [Google Scholar] [CrossRef]
- Wang, W.; McConaghy, A.M.; Tetley, L.; Uchegbu, I.F. Controls on polymer molecular weight may be used to control the size of palmitoyl glycol chitosan polymeric vesicles. Langmuir 2001, 17, 631–636. [Google Scholar] [CrossRef]
- Joseph, M.M.; Aravind, S.; Varghese, S.; Mini, S.; Sreelekha, T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar]
- Varghese, S.; Joseph, M.M.; Aravind, S.; Unnikrishnan, B.; Sreelekha, T. The inhibitory effect of anti-tumor polysaccharide from Punica granatum on metastasis. Int. J. Biol. Macromol. 2017, 103, 1000–1010. [Google Scholar] [CrossRef]
- Nalbantova, V.; Benbassat, N.; Delattre, C. Fenugreek Galactomannan and Its Versatile Applications. Polysaccharides 2024, 5, 478–492. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J.; Naikwadi, N.N.; Variya, B.C. Galactomannan: A versatile biodegradable seed polysaccharide. Int. J. Biol. Macromol. 2013, 60, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Shahbuddin, M.; Shahbuddin, D.; Bullock, A.J.; Ibrahim, H.; Rimmer, S.; MacNeil, S. High molecular weight plant heteropolysaccharides stimulate fibroblasts but inhibit keratinocytes. Carbohydr Res. 2013, 375, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; Barros, W.; Santos, G.R.C.; Correia, M.T.S.; Mourão, P.A.S.; Teixeira, J.A.; Carneiro-Da-Cunha, M.G. Characterization and rheological study of the galactomannan extracted from seeds of Cassia Grandis. Carbohydr. Polym. 2014, 104, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Rocha, C.; Ribeiro, C.; Maia, J.M.; Gonalves, M.P.; Vicente, A.A. Rheological characterization of carrageenan/galactomannan and xanthan/galactomannan gels: Comparison of galactomannans from non-traditional sources with conventional galactomannans. Carbohydr. Polym. 2011, 83, 392–399. [Google Scholar] [CrossRef]
- Sittikijyothin, W.; Torres, D.; Gonçalves, M.P. Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohydr. Polym. 2005, 59, 339–350. [Google Scholar] [CrossRef]
- Rao, P.S.; Ghosh, T.P.; Krishna, S. Extraction and purification of tamarind seed polysaccharide. J. Sci. Ind. Res. 1946, 4, 705. [Google Scholar]
- Sajjadi, M.; Baran, N.Y.; Baran, T.; Nasrollahzadeh, M.; Tahsili, M.R.; Shokouhimehr, M. Palladium nanoparticles stabilized on a novel Schiff base modified Unye bentonite: Highly stable, reusable and efficient nanocatalyst for treating wastewater contaminants and inactivating pathogenic microbes. Sep. Purif. Technol. 2020, 237, 116383. [Google Scholar] [CrossRef]
- Khelifi, A.; Bouberka, Z.; Bentaleb, K.; Hamani, H.; Derrich, Z. Removal of, 2, 4-DCP from wastewater by CTAB/bentonite using one-step and two-step methods: A comparative study. Chem. Eng. J. 2009, 164, 345–354. [Google Scholar] [CrossRef]
- Lepluart, L. Nanocomposites, Epoxyde, Amine, Montmorillonite: Role of Interactions on The Formation, Morphology at Different Levels of Scale and Mechanical Properties of The Networks. Ph.D. Thesis, National Institute of Applied Sciences, Lyon, France, 2002. [Google Scholar]
- Ponomarev, N.; Repo, E.; Srivastava, V.; Sillanpää, M. Green thermal-assisted synthesis and characterization of novel cellulose-Mg(OH)2 nanocomposite in PEG/NaOH solvent. Carbohydr. Polym. 2017, 176, 327–335. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food. Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Fronza, P.; Batista, M.J.P.A.; Franca, A.S.; Oliveira, L.S. Bionanocomposite Based on Cassava Waste Starch, Locust Bean Galactomannan, and Cassava Waste Cellulose Nanofibers. Foods 2024, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Bin Ahmad, M.; Zargar, M.; Wan Yunus, W.M.Z.; Ibrahim, N.A.; Shabanzadeh, P.; Moghaddam, M.G. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomed. 2011, 6, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Q. PP/clay nanocompo-sites prepared by grafting melt Intercalation. Polymers 2001, 42, 10013–10019. [Google Scholar] [CrossRef]
- Mark, Q.G.; Xinzhong, H.; Changlu, W.; Lianzhong, A. Polysaccharides: Stucture and solibility. Solibility Polysacch. 2017, 2, 8–21. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Angeli, L.; Morozova, K.; Scampicchio, M. A kinetic-based stopped-fow DPPH• method. Sci. Rep. 2023, 13, 7621. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
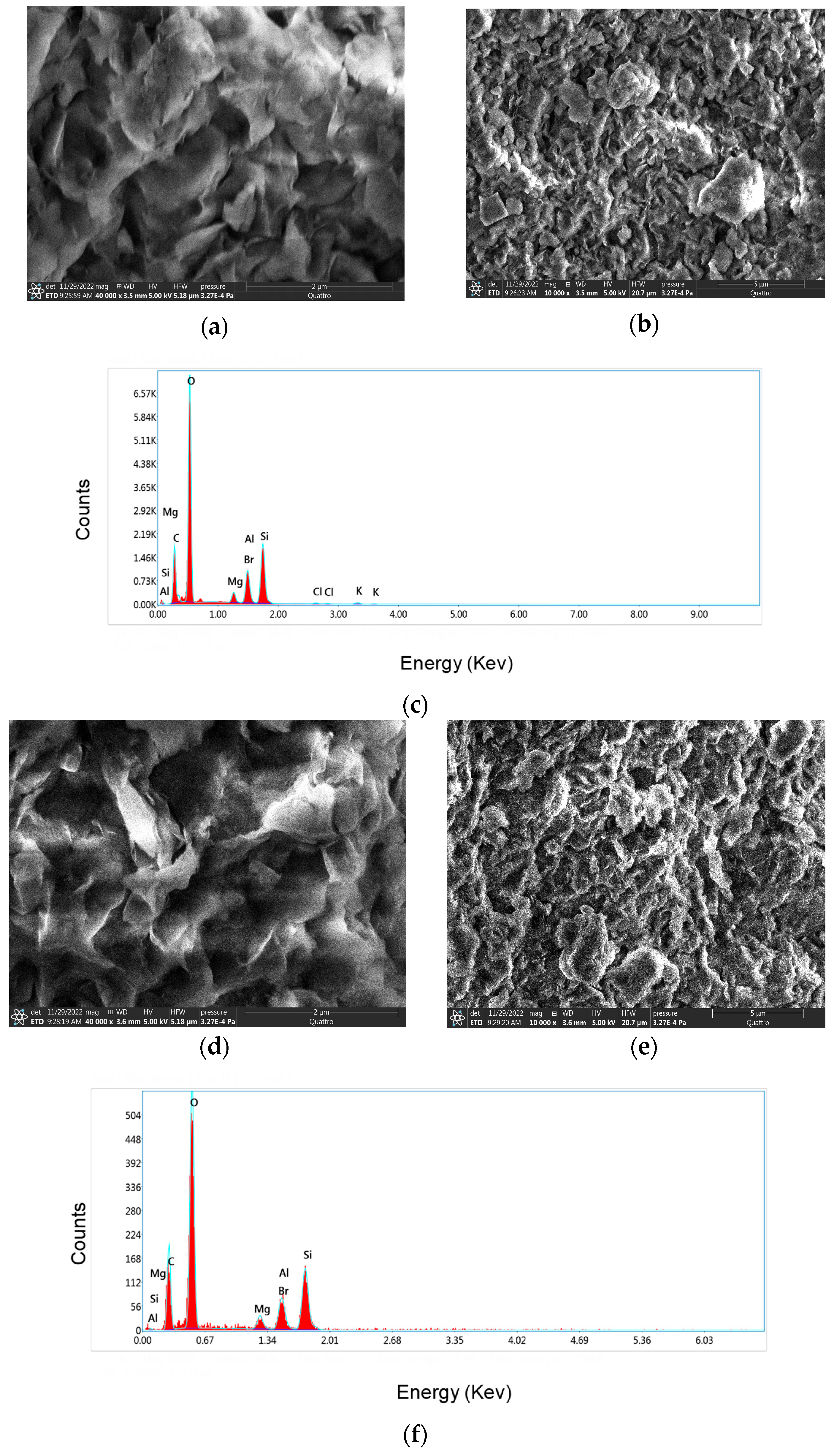

| (a) OM-MMT | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | CK | OK | MgK | AlK | SiK | ClK | KK | Br L | |||||||||
| Weight(%) | 19.9 | 51.8 | 2.9 | 4.6 | 15.7 | 0.4 | 0.1 | 4.5 | |||||||||
| Atomic(%) | 28.8 | 55.8 | 1.5 | 2.9 | 9.6 | 0.2 | 0.4 | 0.9 | |||||||||
| (b) GM1/OM-MMT nanocomposite | |||||||||||||||||
| Element | CK | OK | MgK | AlK | SiK | Br L | |||||||||||
| Weight(%) | 29.2 | 48.4 | 2.8 | 3.2 | 12.8 | 4.1 | |||||||||||
| Atomic(%) | 39.4 | 49.0 | 1.4 | 1.9 | 7.4 | 0.8 | |||||||||||
| (c) GM2/OM-MMT nanocomposite | |||||||||||||||||
| Element | CK | OK | MgK | AlK | SiK | KK | Br L | ||||||||||
| Weight(%) | 25.9 | 56.2 | 1.5 | 3.5 | 10.3 | 0.6 | 1.9 | ||||||||||
| Atomic(%) | 34.5 | 55.9 | 0.9 | 2.1 | 5.8 | 0.2 | 0.4 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saihi, R.; Souli, L.; Chabira, S.F.; Derouiche, Y.; Maschke, U. Synthesis and Characterization of Eco-Friendly Nanocomposites Using Galactomannan and Organomodified Montmorillonite. Physchem 2025, 5, 7. https://doi.org/10.3390/physchem5010007
Saihi R, Souli L, Chabira SF, Derouiche Y, Maschke U. Synthesis and Characterization of Eco-Friendly Nanocomposites Using Galactomannan and Organomodified Montmorillonite. Physchem. 2025; 5(1):7. https://doi.org/10.3390/physchem5010007
Chicago/Turabian StyleSaihi, Razika, Lahcene Souli, Salem Fouad Chabira, Yazid Derouiche, and Ulrich Maschke. 2025. "Synthesis and Characterization of Eco-Friendly Nanocomposites Using Galactomannan and Organomodified Montmorillonite" Physchem 5, no. 1: 7. https://doi.org/10.3390/physchem5010007
APA StyleSaihi, R., Souli, L., Chabira, S. F., Derouiche, Y., & Maschke, U. (2025). Synthesis and Characterization of Eco-Friendly Nanocomposites Using Galactomannan and Organomodified Montmorillonite. Physchem, 5(1), 7. https://doi.org/10.3390/physchem5010007







