Morphological Engineering of Battery-Type Cobalt Oxide Electrodes for High-Performance Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
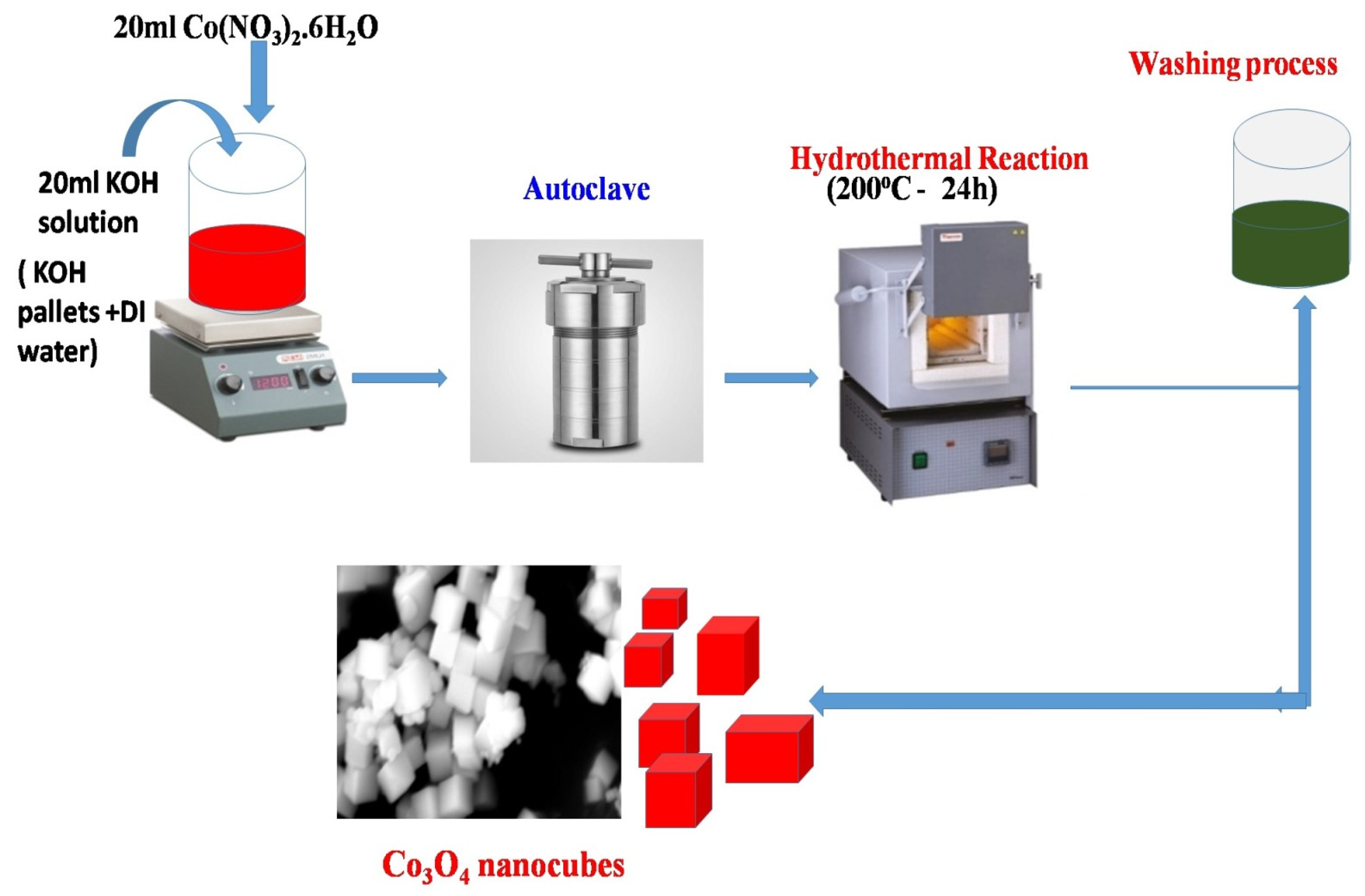
2.2. Synthesis Procedure
2.3. Microstructural and Electrochemical Measurements
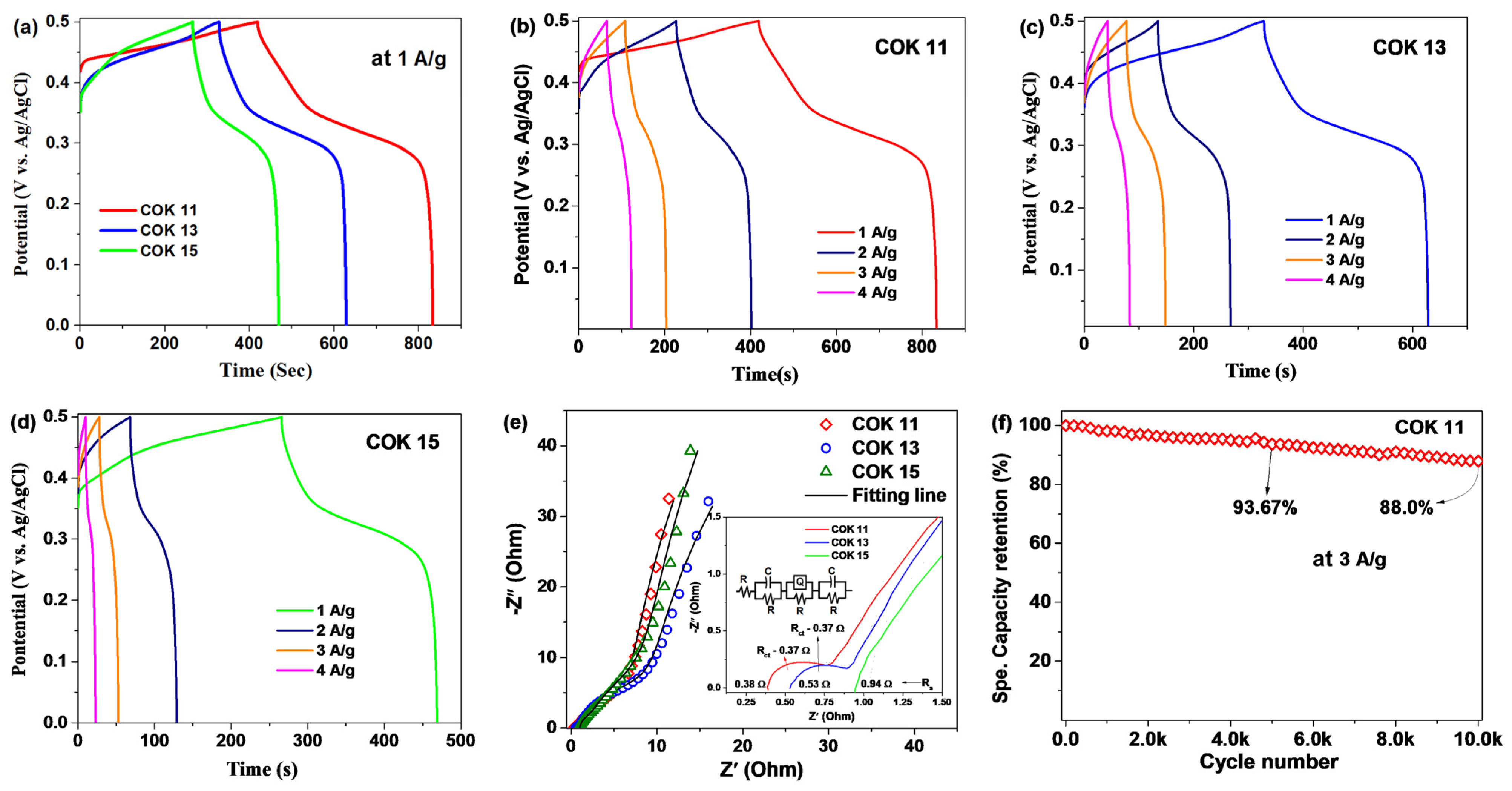
3. Results and Discussion
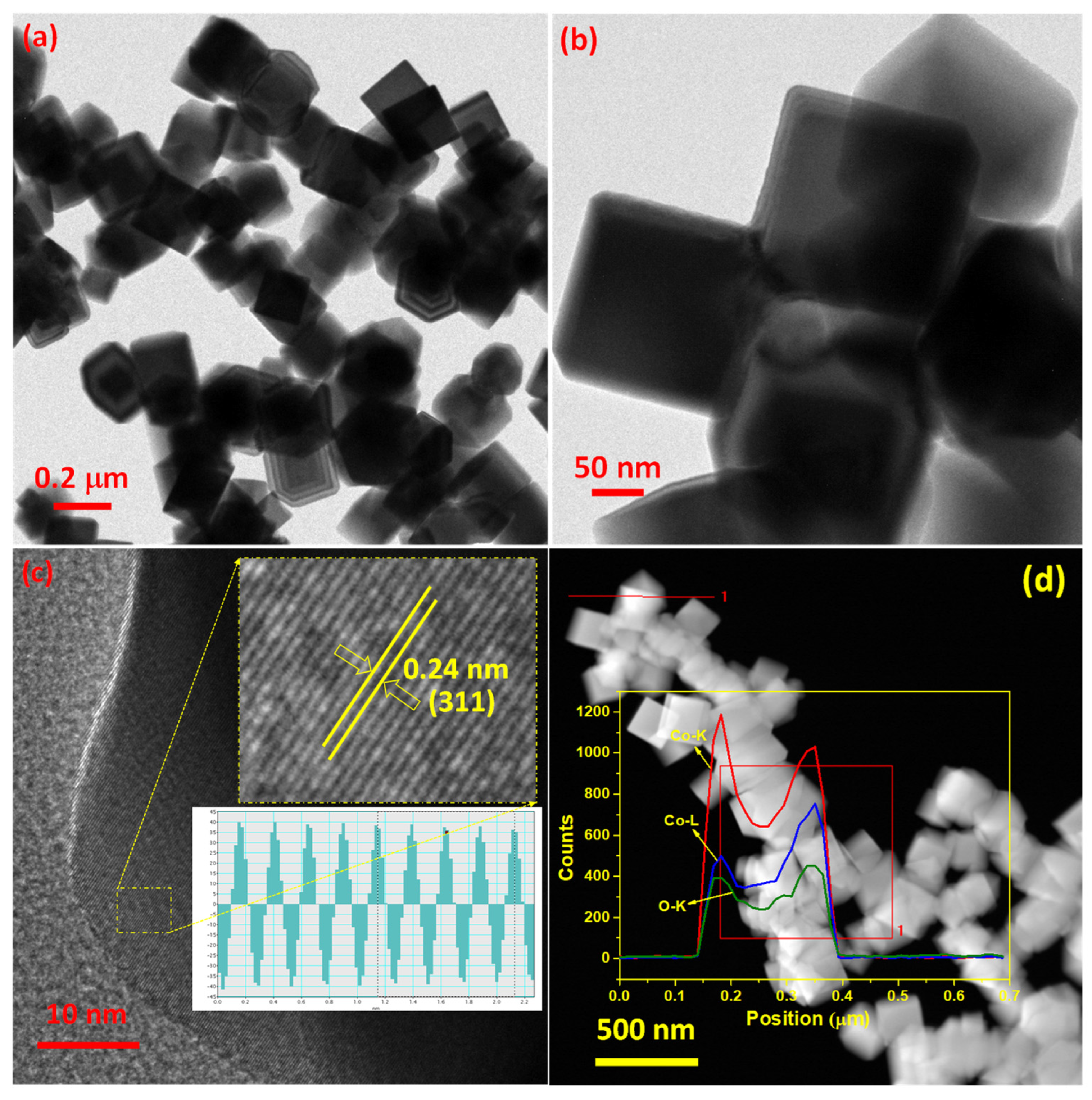
3.1. Morphology and Composition
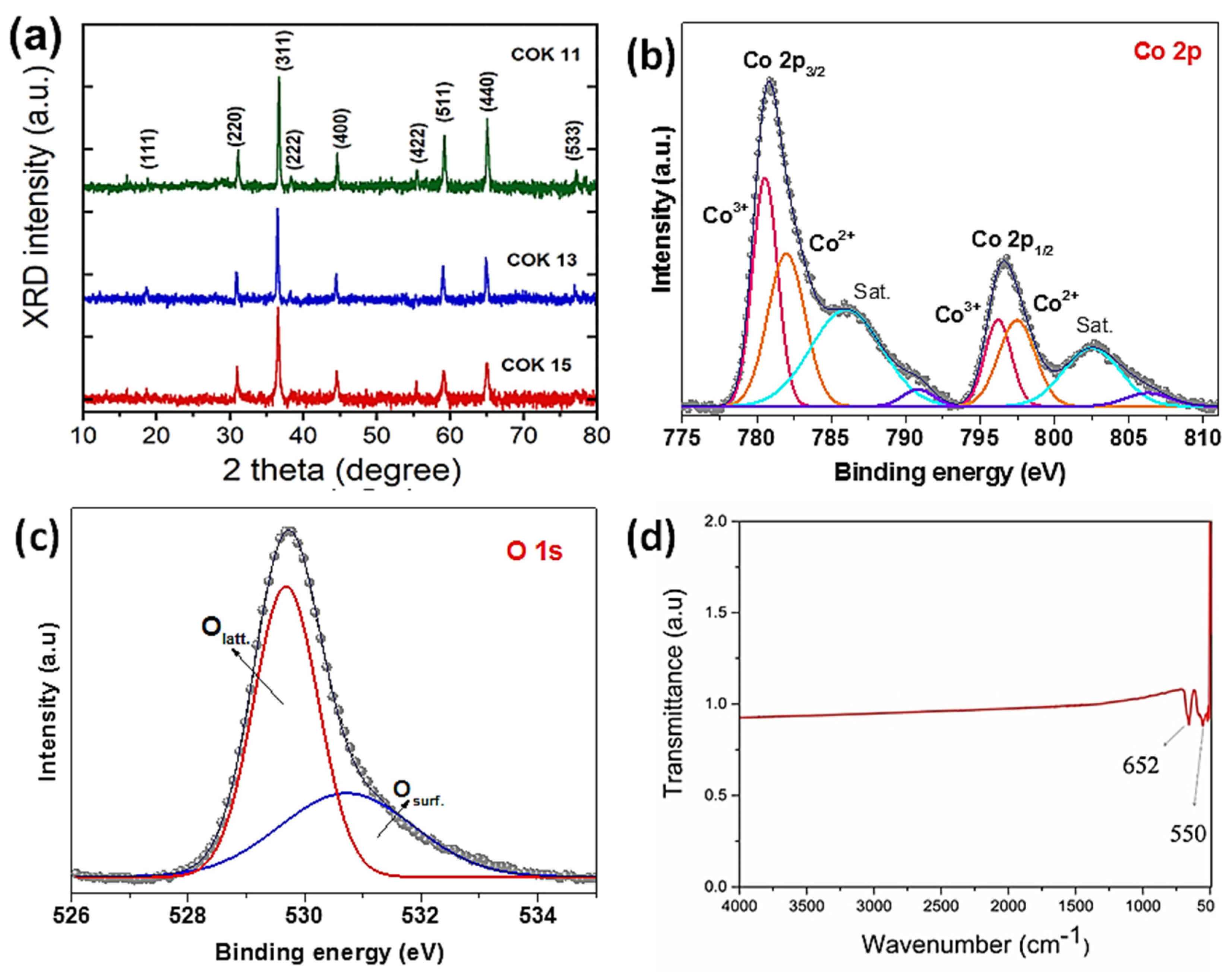
3.2. Structure
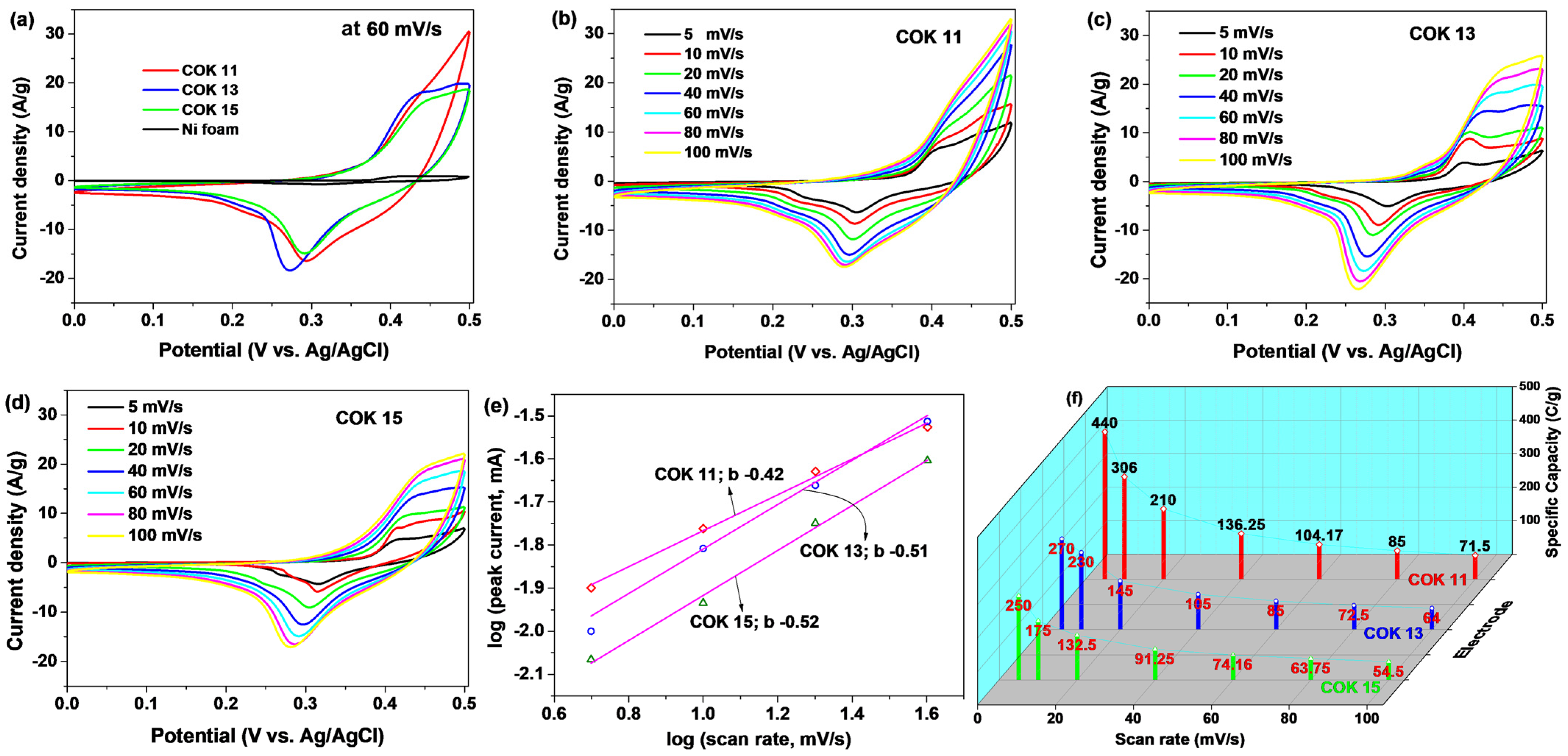
3.3. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-oxide Nanomaterials Synthesis and applications in flexible and wearable sensors. ACS Nanosci. Au 2022, 2, 64–92. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, K.; Bhatnagar, N. Applications of nanomaterials for enhanced performance, and sustainability in energy storage devices: A review. ChemistrySelect 2024, 9, e202400543. [Google Scholar] [CrossRef]
- Singh, P.K.; Kaur, G.A.; Shandilya, M.; Rana, P.; Rai, R.; Mishra, Y.K.; Syväjärvi, M.; Tiwari, A. Trends in piezoelectric nanomaterials towards green energy scavenging nanodevices. Mater. Today Sustain. 2023, 24, 100583. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and characterization of nanomaterials for application in cost-effective electrochemical devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Al-Mahmud, M.Z. A Concise Review of nanoparticles utilized energy storage and conservation. J. Nanomater. 2023, 2023, 5432099. [Google Scholar]
- Pérez Mendoza, A.E.; Schmidt, A.; Zarbin, A.J.G.; Winnischofer, H. Review of nanoscale approaches for tailoring electrode materials for advanced energy storage systems. ACS Appl. Nano Mater. 2024, 7, 23295–23320. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Recent advanced supercapacitor: A review of storage mechanisms, electrode materials, modification, and perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Ngidi, N.P.D.; Koekemoer, A.F.; Ndlela, S.S. Recent advancement in the electrochemical performance of electrochemical capacitors based on biomass-derived porous carbon: A review. J. Energy Storage 2024, 89, 111638. [Google Scholar] [CrossRef]
- Waris, M.S.; Chaudhary, A.H.; Anwer, S.; Sultana, P.P.; Ingole, S.A.A.; Nami, M.Z.; Khan, A. Review on development of carbon-based nanomaterials for energy storage devices: Opportunities and challenges. Energy Fuels 2023, 37, 19433–19460. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent advances in carbon-based electrodes for energy storage and conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef]
- Jayakumar, S.; Santhosh, P.C.; Mohideen, M.M.; Radhamani, A.V. A comprehensive review of metal oxides (RuO2, Co3O4, MnO2 and NiO) for supercapacitor applications and global market trends. J. Alloys Compd. 2024, 976, 173170. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Peng, Y.; Wang, L.; Li, B.; Zheng, G.; Song, M. Application of transition metal (Ni, Co and Zn) oxides based electrode materials for ion-batteries and supercapacitors. Int. J. Electrochem. Sci. 2023, 18, 100233. [Google Scholar] [CrossRef]
- Quispe-Garrido, V.; Cerron-Calle, G.A.; Bazan-Aguilar, A.; Ruiz-Montoya, J.G.; López, E.O.; Baena-Moncada, A.M. Advances in the design and application of transition metal oxide-based supercapacitors. Open Chem. 2021, 19, 709–725. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Ahmmed, A.S.; Lübben, J.F. Review on conductive polymer composites for supercapacitor applications. J. Composites Sci. 2024, 8, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Alcaraz-Espinoza, J.J.; de Melo, C.P.; de Oliveira, H.P. Fabrication of highly flexible hierarchical polypyrrole/carbon nanotube on eggshell membranes for supercapacitors. ACS Omega 2017, 2, 2866–2877. [Google Scholar] [CrossRef]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef]
- Xu, W.; Li, T.-T.; Zheng, Y.-Q. Porous Co3O4 nanoparticles derived from a Co(ii)-cyclohexanehexacarboxylate metal–organic framework and used in a supercapacitor with good cycling stability. RSC Adv. 2016, 6, 86447–86454. [Google Scholar] [CrossRef]
- Wiegmann, T.; Pacheco, I.; Reikowski, F.; Stettner, J.; Qiu, C.; Bouvier, M.; Bertram, M.; Faisal, F.; Brummel, O.; Libuda, J.; et al. Operando identification of the reversible skin layer on Co3O4 as a three-dimensional reaction zone for oxygen evolution. ACS Catal. 2022, 12, 3256–3268. [Google Scholar] [CrossRef]
- Velhal, N.B.; Yun, T.H.; Ahn, J.; Kim, T.; Kim, J.; Yim, C. Tailoring cobalt oxide nanostructures for stable and high-performance energy storage applications. Ceram. Int. 2023, 49, 4889–4897. [Google Scholar] [CrossRef]
- Chen, M.; Ge, Q.; Qi, M.; Liang, X.; Wang, F.; Chen, Q. Cobalt oxides nanorods arrays as advanced electrode for high performance supercapacitor. Surf. Coat. Technol. 2019, 360, 73–77. [Google Scholar] [CrossRef]
- Kunhikrishnan, L.; Shanmugham, R. High electrochemical performance of morphologically controlled cobalt oxide for supercapacitor application. Mater. Character. 2021, 177, 111160. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Cao, J.; Wang, W.; Chen, Z. Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 2010, 56, 732–736. [Google Scholar] [CrossRef]
- More, S.; Joshi, B.; Khadka, A.; Samuel, E.; Il Kim, Y.; Aldalbahi, A.; El-Newehy, M.; Gurav, K.; Lee, H.-S.; Yoon, S.S. Oriented attachment of carbon/cobalt-cobalt oxide nanotubes on manganese-doped carbon nanofibers for flexible symmetric supercapacitors. Appl. Surf. Sci. 2023, 615, 156386. [Google Scholar] [CrossRef]
- Kalpana, S.; Bhat, V.S.; Hegde, G.; Niranjana Prabhu, T.; Anantharamaiah, P.N. Morphology-dependent supercapacitive properties of Co3O4 nanomaterials synthesized via coprecipitation and hydrothermal methods. Inorg. Chem. Commun. 2023, 158, 111458. [Google Scholar] [CrossRef]
- Desai, R.S.; Jadhav, V.S.; Morankar, P.J.; Patil, S.B.; Sadale, S.B.; Pardeshi, S.R.; Lad, D.D.; Patil, P.S.; Jeon, C.-W.; Dalavi, D.S. Hydrothermal synthesis of self-supported hierarchical microflowers of Co3O4 nanowires for potential supercapacitor application. J. Electroanal. Chem. 2025, 976, 118800. [Google Scholar] [CrossRef]
- Wei, G.; Yan, L.; Huang, H.; Yan, F.; Liang, X.; Xu, S.; Lan, Z.; Zhou, W.; Guo, J. The hetero-structured nanoarray construction of Co3O4 nanowires anchored on nanoflakes as a high-performance electrode for supercapacitors. Appl. Surf. Sci. 2021, 538, 147932. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef]
- Lu, C.; Liu, L.; Yang, Y.; Ma, Y.; Luo, Q.; Zhu, M. Recent progress in Co3O4-based nanomaterials for supercapacitors. ChemNanoMat 2023, 9, e202200537. [Google Scholar] [CrossRef]
- Deori, K.; Ujjain, S.K.; Sharma, R.K.; Deka, S. Morphology Controlled Synthesis of Nanoporous Co3O4 nanostructures and their charge storage characteristics in Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10665–10672. [Google Scholar] [CrossRef]
- Shwetha, K.P.; Manjunatha, C.; Sudha Kamath, M.K.; Vinaykumar; Radhika, M.G.R.; Khosla, A. Morphology-controlled synthesis and structural features of ultrafine nanoparticles of Co3O4: An active electrode material for a supercapacitor. Appl. Res. 2022, 1, e202200031. [Google Scholar] [CrossRef]
- Babu, C.R.; Avani, A.V.; Shaji, S.; Anila, E.I. Electrochemical characteristics of Co3O4 nanoparticles synthesized via the hydrothermal approach for supercapacitor applications. J. Solid State Electrochem. 2024, 28, 2203–2210. [Google Scholar] [CrossRef]
- Nare, R.K.; Ramesh, S.; Basavi, P.K.; Kakani, V.; Bathula, C.; Yadav, H.M.; Dhanapal, P.B.; Kotanka, R.K.R.; Pasupuleti, V.R. Sonication-supported synthesis of cobalt oxide assembled on an N-MWCNT composite for electrochemical supercapacitors via three-electrode configuration. Sci. Rep. 2022, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wu, Y.; Chen, H.; Chen, W.; Wang, J.; Tong, Y.; Pei, G.; Shen, Z.; Guan, C. Synthesis of amorphous hydroxyl-rich Co3O4 for flexible high-rate supercapacitor. Chem. Eng. J. 2020, 396, 125364. [Google Scholar] [CrossRef]
- Hao, P.; Peng, B.; Shan, B.-Q.; Yang, T.-Q.; Zhang, K. Comprehensive understanding of the synthesis and formation mechanism of dendritic mesoporous silica nanospheres. Nanoscale Adv. 2020, 2, 1792–1810. [Google Scholar] [CrossRef]
- Quirk, J.; Rothmann, M.; Li, W.; Abou-Ras, D.; McKenna, K.P. Grain boundaries in polycrystalline materials for energy applications: First principles modeling and electron microscopy. Appl. Phys. Rev. 2024, 11, 011308. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Li, Y.; Zhao, M. Porous Co3O4 column as a high-performance Lithium anode material. J. Porous Mater. 2021, 28, 889–894. [Google Scholar] [CrossRef]
- Wei, Z.; Xia, T.; Ma, J.; Feng, W.; Dai, J.; Wang, Q.; Yan, P. Investigation of the lattice expansion for Ni nanoparticles. Mater. Character. 2007, 58, 1019–1024. [Google Scholar] [CrossRef]
- Merum, D.; Nallapureddy, R.R.; Pallavolu, M.R.; Mandal, T.K.; Gutturu, R.R.; Parvin, N.; Banerjee, A.N.; Joo, S.W. Pseudocapacitive performance of freestanding Ni3V2O8 nanosheets for high energy and power density asymmetric supercapacitors. ACS Appl. Energy Mater. 2022, 5, 5561–5578. [Google Scholar] [CrossRef]
- Nasiri, S.; Rabiei, M.; Palevicius, A.; Janusas, G.; Vilkauskas, A.; Nutalapati, V.; Monshi, A. Modified Scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano Trends 2023, 3, 100015. [Google Scholar] [CrossRef]
- Supriya, S.; Das, S.; Senapati, S.; Naik, R. Cu2Te/CoTe nanoparticles with tuneable bandgaps: Implications for photovoltaic and optoelectronic devices. Surf. Interfaces 2024, 44, 103823. [Google Scholar] [CrossRef]
- Adesuji, E.T.; Guardado-Villegas, E.; Fuentes, K.M.; Sánchez-Domínguez, M.; Videa, M. Pt-Co3O4 superstructures by one-pot reduction/precipitation in bicontinuous microemulsion for electrocatalytic oxygen evolution reaction. Catalysts 2020, 10, 1311. [Google Scholar] [CrossRef]
- Cole, K.M.; Kirk, D.W.; Thorpe, S.J. Co3O4 nanoparticles characterized by XPS and UPS. Surf. Sci. Spectra 2021, 28, 014001. [Google Scholar] [CrossRef]
- Daza-Gómez, L.; Pérez Salas, K.Y.; Ruiz-Huerta, L.; García Peña, N.G.; Maturano Rojas, V.; Redón, R. Co3O4 @SiO2 3D monolith catalysts, additive manufactured structures for propane oxidation reaction. ChemistrySelect 2024, 9, e202304849. [Google Scholar] [CrossRef]
- Murugesan, R.A.; Chandar Nagamuthu Raja, K.; Devi, N.; Lin, H.-T.; Huang, C.-C.; Jiang, X.-Y.; Li, Y.-Y.; Arthanareeswaran, G.; Ponvijayakanthan, L.; Jaiswal, N.K.; et al. Development of Ni-doped Co3O4 oxygen evolution catalysts for anion exchange membrane water electrolysis. Int. J. Hydrog. Energy 2024, 72, 677–686. [Google Scholar] [CrossRef]
- Urgunde, A.B.; Kamboj, V.; Kannattil, H.P.; Gupta, R. Layer-by-layer coating of cobalt-based ink for large-scale fabrication of OER electrocatalyst. Energy Technol. 2019, 7, 1900603. [Google Scholar] [CrossRef]
- Makhlouf, S.A.; Bakr, Z.H.; Aly, K.I.; Moustafa, M.S. Structural, electrical and optical properties of Co3O4 nanoparticles. Superlattices Microstruct. 2013, 64, 107–117. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Deraz, N.M.; Abd-Elkader, O.H. Magnetic and characterization studies of CoO/Co3O4 nanocomposite. Processes 2020, 8, 844. [Google Scholar] [CrossRef]
- Mohanty, G.C.; Das, S.; Verma, A. Fabrication of aqueous asymmetric supercapacitor device by using spinel type (FeCoNiCuZn)3O4 high entropy oxide and green carbon derived from plastic wastes. Ceram. Int. 2024, 50, 48938–48947. [Google Scholar] [CrossRef]
- Kalpana, S.; Bhat, V.S.; Hegde, G.; Anantharamaiah, P.N. Exploring the influence of KOH electrolyte concentration on the electrochemical properties of Co3O4-GO nanocomposite. J. Phys. Chem. Solids 2024, 190, 112019. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Gupta, R.K.; Mishra, S.R. Facile synthesis of bio-template tubular MCo2O4 (M = Cr, Mn, Ni) microstructure and its electrochemical performance in aqueous electrolyte. Processes 2020, 8, 343. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshii, K.; Asahi, M.; Chiku, M.; Kitazumi, Y. Cyclic voltammetry Part 2: Surface adsorption, electric double layer, and diffusion layer. Electrochemistry 2022, 90, 102006. [Google Scholar] [CrossRef]
- Merum, D.; Ambadi, L.N.; Mahammad, H.O.; Pallavolu, M.R.; Goddati, M.; Lee, J.; Al-Asbahi, B.A.; Pitcheri, R.; Banerjee, A.N.; Joo, S.W. Direct growth of cobalt-doped nickel vanadate shelf-like architectures on Ni foam electrodes for solid-state alkaline battery. J. Alloys Compd. 2023, 950, 169771. [Google Scholar] [CrossRef]
- Merum, D.; Arla, S.K.; Radhalayam, D.; Tighezza, A.M.; Mooni, S.P.; Joo, S.W. Garland-structured Bi2O2CO3@Ni(OH)2 as a battery-type electrode for high-performance electrochemical energy storage device applications. J. Energy Storage 2024, 99, 113189. [Google Scholar] [CrossRef]
- Thonge, P.N.; Dhas, S.D.; Waghmare, S.D.; Patil, A.H.; Patil, T.M.; Yewale, M.A.; Mendhe, A.C.; Kim, D. Facile hydrothermal synthesis of NiMn2O4/C nanosheets for solid-state asymmetric supercapacitor and electrocatalytic oxygen evolution reaction. ACS Appl. Nano Mater. 2024, 7, 18579–18589. [Google Scholar] [CrossRef]
- Sanayee, M.; Arvand, M. Synthesis and electrochemical properties of nanocubes Mn2SnS3 for high-performance supercapacitors. Sci. Rep. 2023, 13, 20838. [Google Scholar] [CrossRef]
- Merum, D.; Parvin, N.; Vattikuti, S.V.P.; Nallapureddy, R.R.; Pitcheri, R.; Shkir, M.; Manthrammel, M.A.; Banerjee, A.N.; Joo, S.W. Impact of Co-doping on the microstructural and electrochemical features of mesoporous 3D oval–shaped Ni3−xCoxV2O8 electrodes for high-performance hybrid supercapacitors. J. Energy Storage 2023, 61, 106674. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, Y. Sacrificial template synthesis of hollow-structured NiCoP microcubes as novel electrode materials for asymmetric supercapacitors. Dalton Trans. 2022, 51, 16017–16026. [Google Scholar] [CrossRef]
- Sethi, M.; Shenoy, U.S.; Bhat, D.K. A porous graphene–NiFe2O4 nanocomposite with high electrochemical performance and high cycling stability for energy storage applications. Nanoscale Adv. 2020, 2, 4229–4241. [Google Scholar] [CrossRef]
- Gomez Vidales, A.; Sridhar, D.; Meunier, J.L.; Omanovic, S. Nickel oxide on directly grown carbon nanofbers for energy storage applications. J. Appl. Electrochem. 2020, 50, 1217–1229. [Google Scholar] [CrossRef]
- Dhananjaya, M.; Lakshmi Narayana, A.; Guru Prakash, N.; Rosaiah, P.; Hussain, O.M. Intertwining network structured VnO2n+1-CNT/GO nanocomposite electrodes for supercapacitors. Mater. Chem. Phys. 2019, 237, 121825. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy─A tutorial. ACS Measurement Sci. Au. 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, M.; Guru Prakash, N.; Lakshmi Narayana, A.; Hussain, O.M. Electrochemical performance of nanocrystalline vanadium pentoxide thin films grown by RF magnetron sputtering. J. Electronic Mater. 2020, 49, 1922–1934. [Google Scholar] [CrossRef]
- Selvarajan, R.; Vadivel, S.; Saranya, A.; Baraneedharan, P.; Jayavel, R. Facile synthesis of rGO@ CoO nanocomposites electrode material for photocatalytic hydrogen generation and supercapacitor applications. Inorg. Chem. Commun. 2022, 139, 109345. [Google Scholar] [CrossRef]
- Zha, X.; Wu, Z.; Cheng, Z.; Yang, W.; Li, J.; Chen, Y.; He, L.; Zhou, E.; Yang, Y. High performance energy storage electrodes based on 3D Z-CoO/RGO nanostructures for supercapacitor applications. Energy 2021, 220, 119696. [Google Scholar] [CrossRef]
- Al-Jahdaly, B.A.; Abu-Rayyan, A.; Taher, M.M.; Shoueir, K. Phytosynthesis of Co3O4 nanoparticles as the high energy storage material of an activated carbon/Co3O4 symmetric supercapacitor device with excellent cyclic stability based on a Na2SO4 aqueous electrolyte. ACS Omega 2022, 7, 23673–23684. [Google Scholar] [CrossRef]

| Electrode Material | Electrolyte | Specific Capacity/Capacitance (C/g or F/g) | Current Density (A/g) | Cycling Stability | Ref. |
|---|---|---|---|---|---|
| Co3O4 nanorod arrays | 3 M KOH | 154.9 C/g (387.25 F/g) | 1 | 88% after 1000 cycles at 1 A/g | [21] |
| Hexagonal platelet Co3O4 particles | 2 M KOH | 476 F/g | 0.5 | 82% after 2000 cycles at 2.5 A/g | [30] |
| Co3O4 nanoparticles | 2 M KOH | 166 F/g | 0.5 | 90% after 5000 cycles at 5 A/g | [31] |
| Co3O4 nanoparticles | 3 M KOH | 450 F/g | 1 | 88% over 10,000 cycles at 20 A/g | [32] |
| Co3O4@N-MWCNT | 3 M KOH | 225 F/g | 0.5 | 97.8% after 5000 cycles at 0.5 A/g | [33] |
| Hydroxyl-rich Co3O4 | 1 M KOH | 226.1 C/g | 1.3 | 77% after 5000 cycles at 5 mA cm−2 | [34] |
| CoO/rGO nanocomposite | 1 M KOH | 592 F/g | 2 | 90% after 3000 cycles at 5 A/g | [64] |
| ZIF-67-CoO/rGO | 6 M KOH | 275 F/g | 1 | -- | [65] |
| AC/Co3O4 nanoparticles | 1 M Na2SO4 | 182 F/g | 1 | 99.6% over 6000 cycles at 2.5 A/g | [66] |
| COK 11 or cobalt oxide nanocubes | 3 M KOH | 825.6 F/g (412.8 C/g) | 1 | 88% after 10,000 cycles at 3 A/g | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haritha, B.; Deepak, M.; Hussain, O.M.; Julien, C.M. Morphological Engineering of Battery-Type Cobalt Oxide Electrodes for High-Performance Supercapacitors. Physchem 2025, 5, 11. https://doi.org/10.3390/physchem5010011
Haritha B, Deepak M, Hussain OM, Julien CM. Morphological Engineering of Battery-Type Cobalt Oxide Electrodes for High-Performance Supercapacitors. Physchem. 2025; 5(1):11. https://doi.org/10.3390/physchem5010011
Chicago/Turabian StyleHaritha, Boddu, Mudda Deepak, Obili M. Hussain, and Christian M. Julien. 2025. "Morphological Engineering of Battery-Type Cobalt Oxide Electrodes for High-Performance Supercapacitors" Physchem 5, no. 1: 11. https://doi.org/10.3390/physchem5010011
APA StyleHaritha, B., Deepak, M., Hussain, O. M., & Julien, C. M. (2025). Morphological Engineering of Battery-Type Cobalt Oxide Electrodes for High-Performance Supercapacitors. Physchem, 5(1), 11. https://doi.org/10.3390/physchem5010011







