Temperature-Induced Phase Transformations in Tutton Salt K2Cu(SO4)2(H2O)6: Thermoanalytical Studies Combined with Powder X-Ray Diffraction
Abstract
1. Introduction
2. Materials and Methods
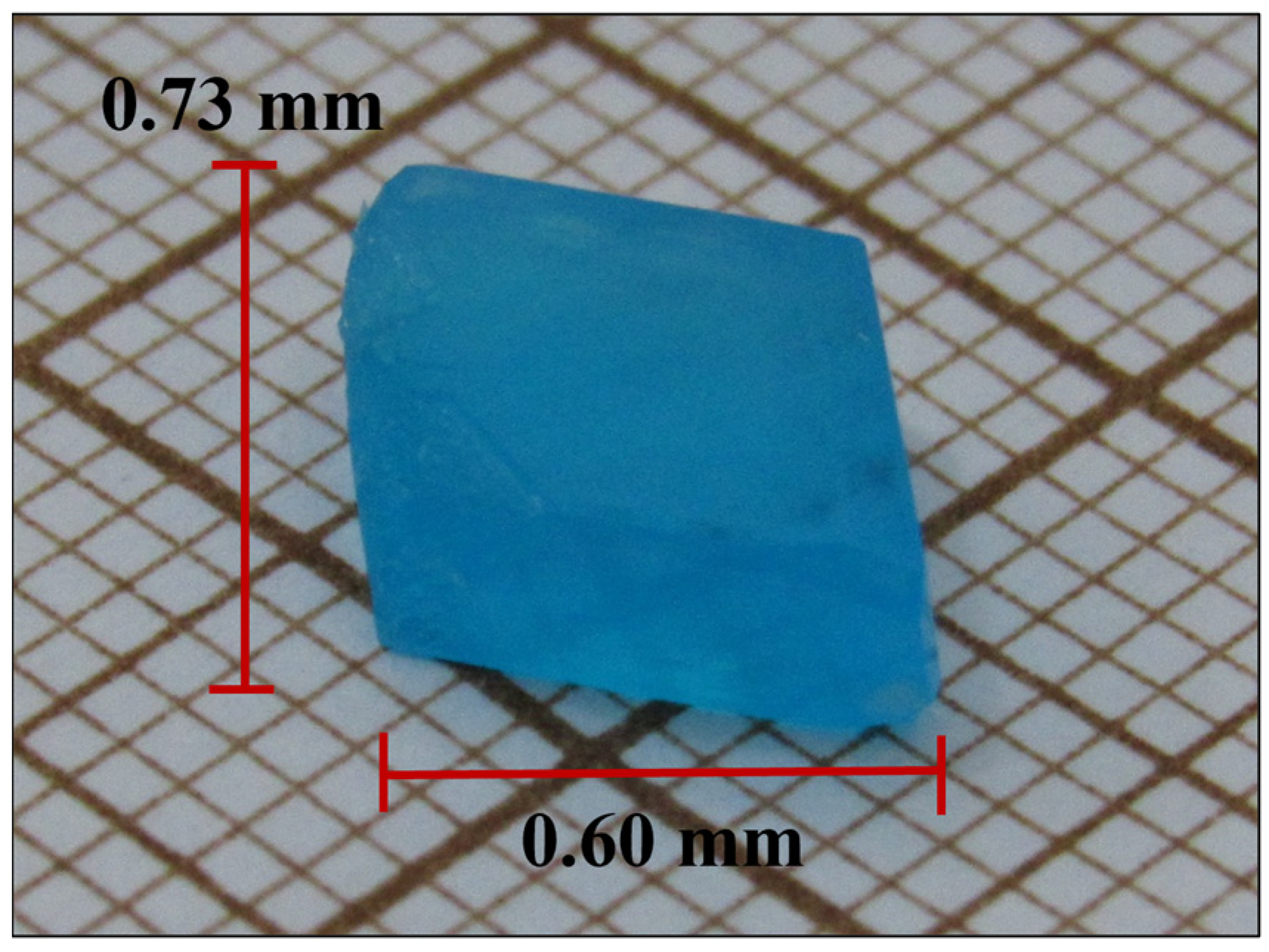
2.1. Crystal Growth
2.2. Characterization Techniques
3. Results and Discussion
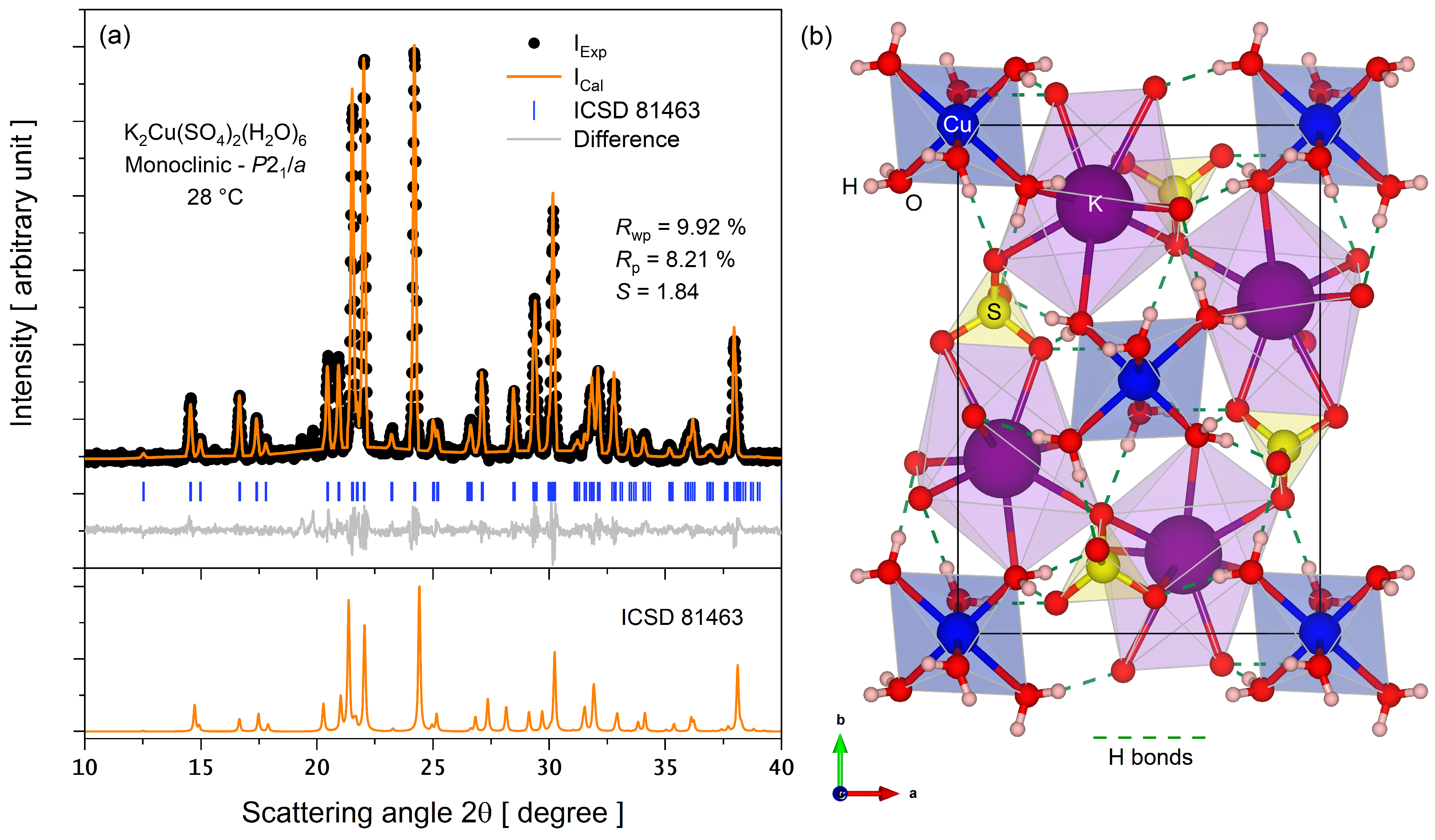
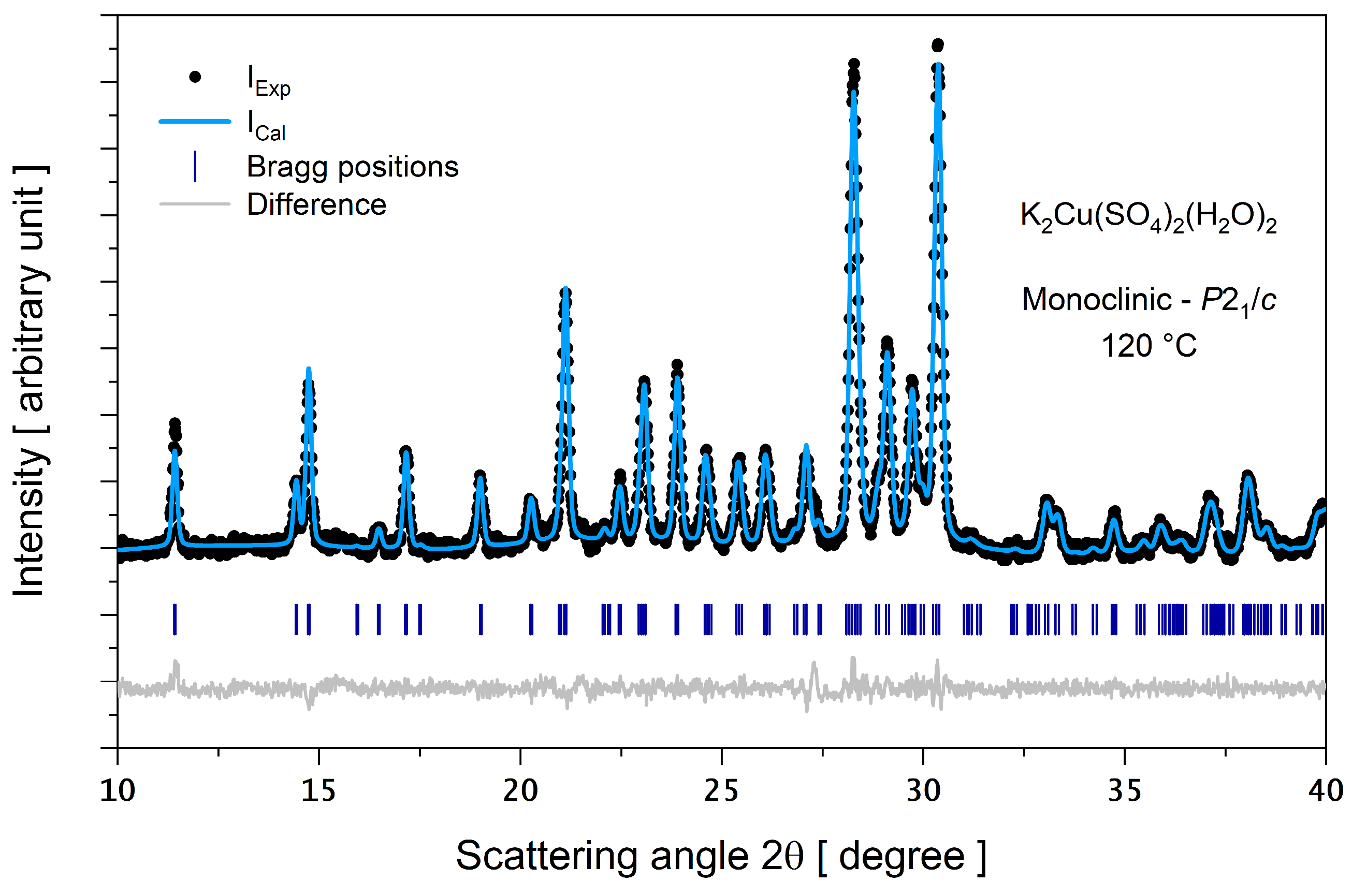
3.1. Crystal Phase Identification and Molecular Structure
3.2. Crystal Surface and Elemental Analysis
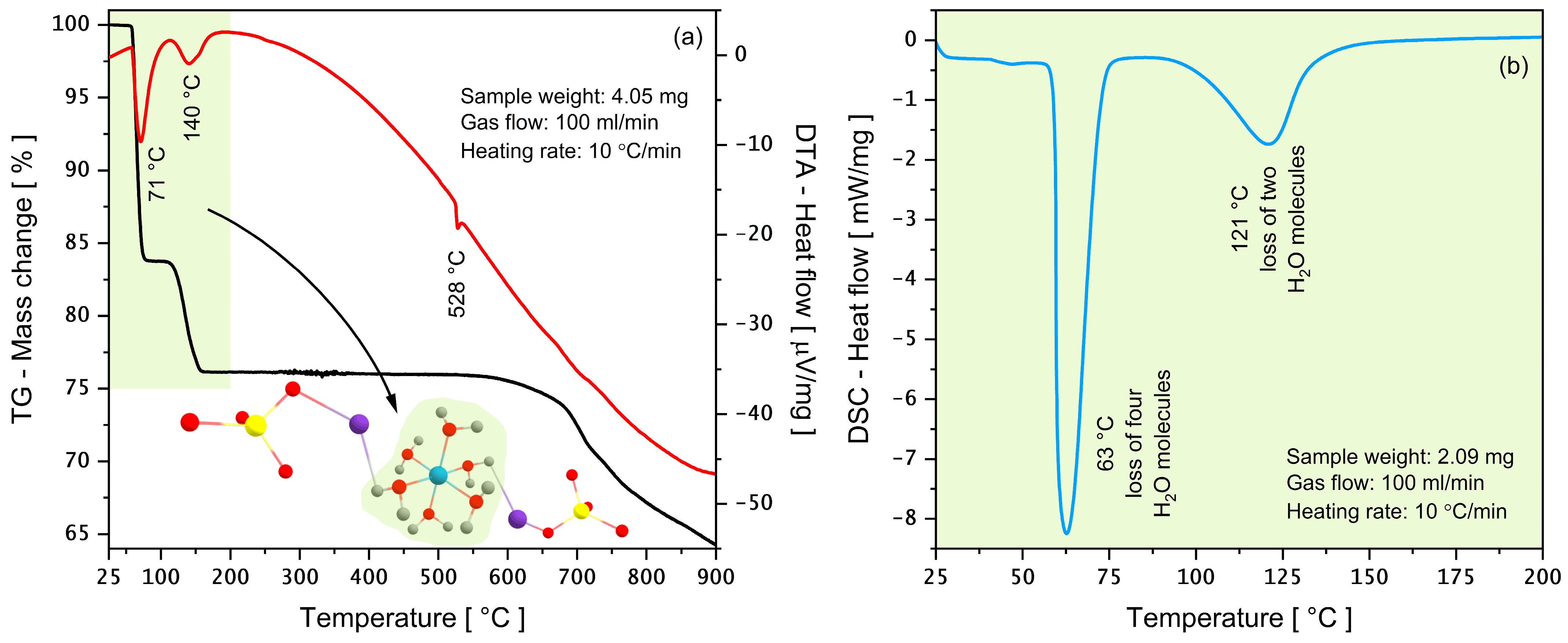
3.3. Thermal Properties via TG-DTA and DSC
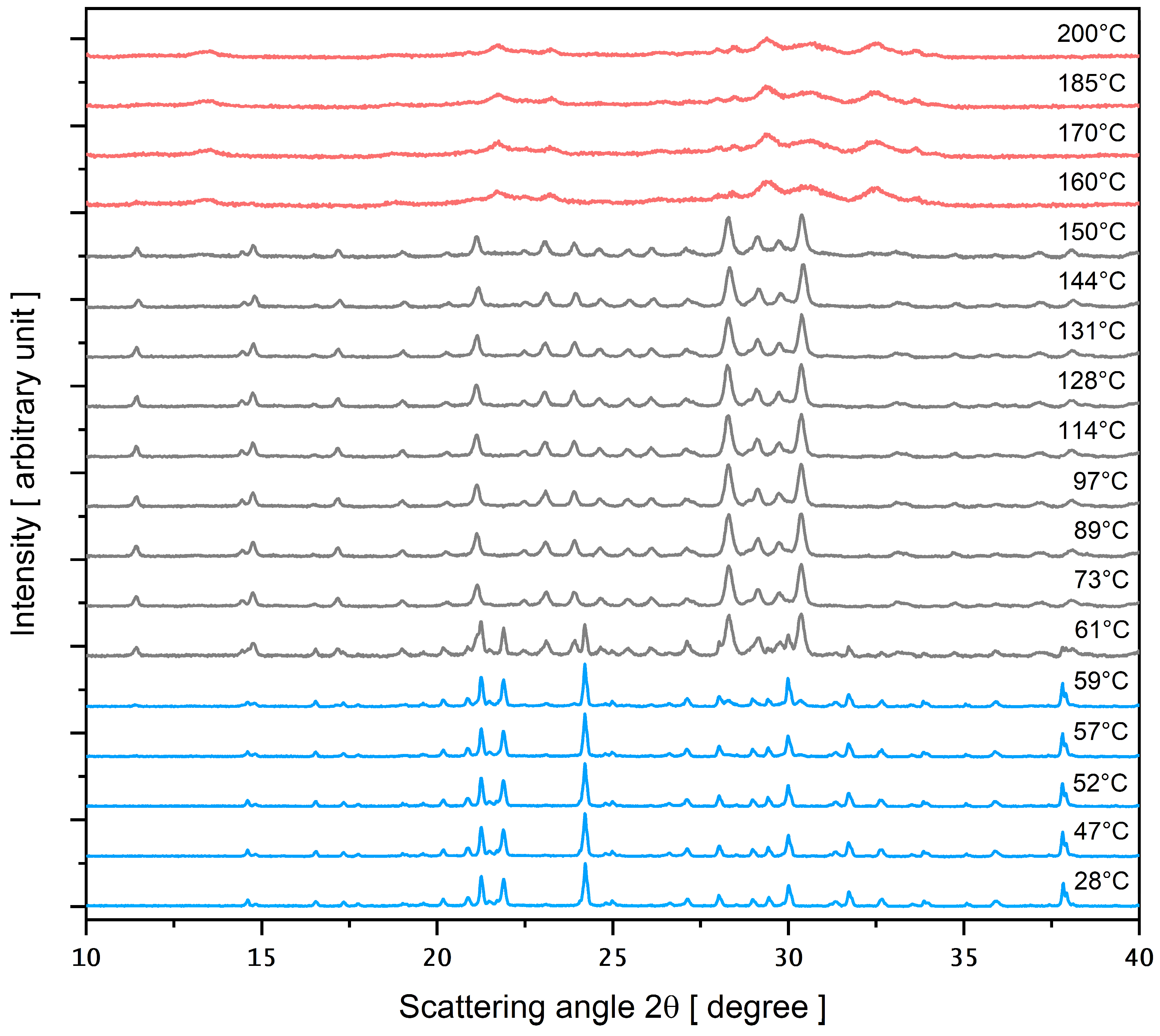
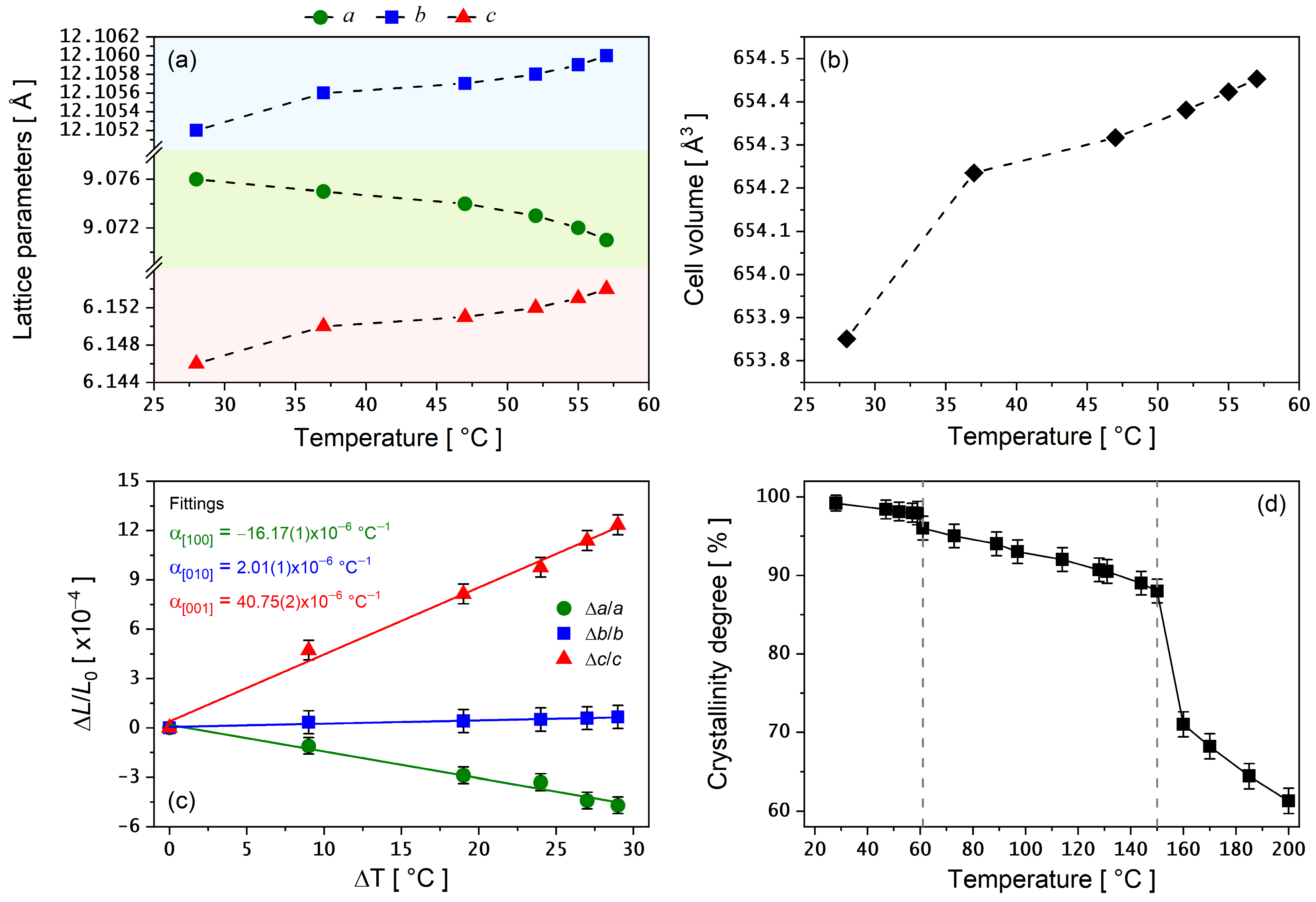
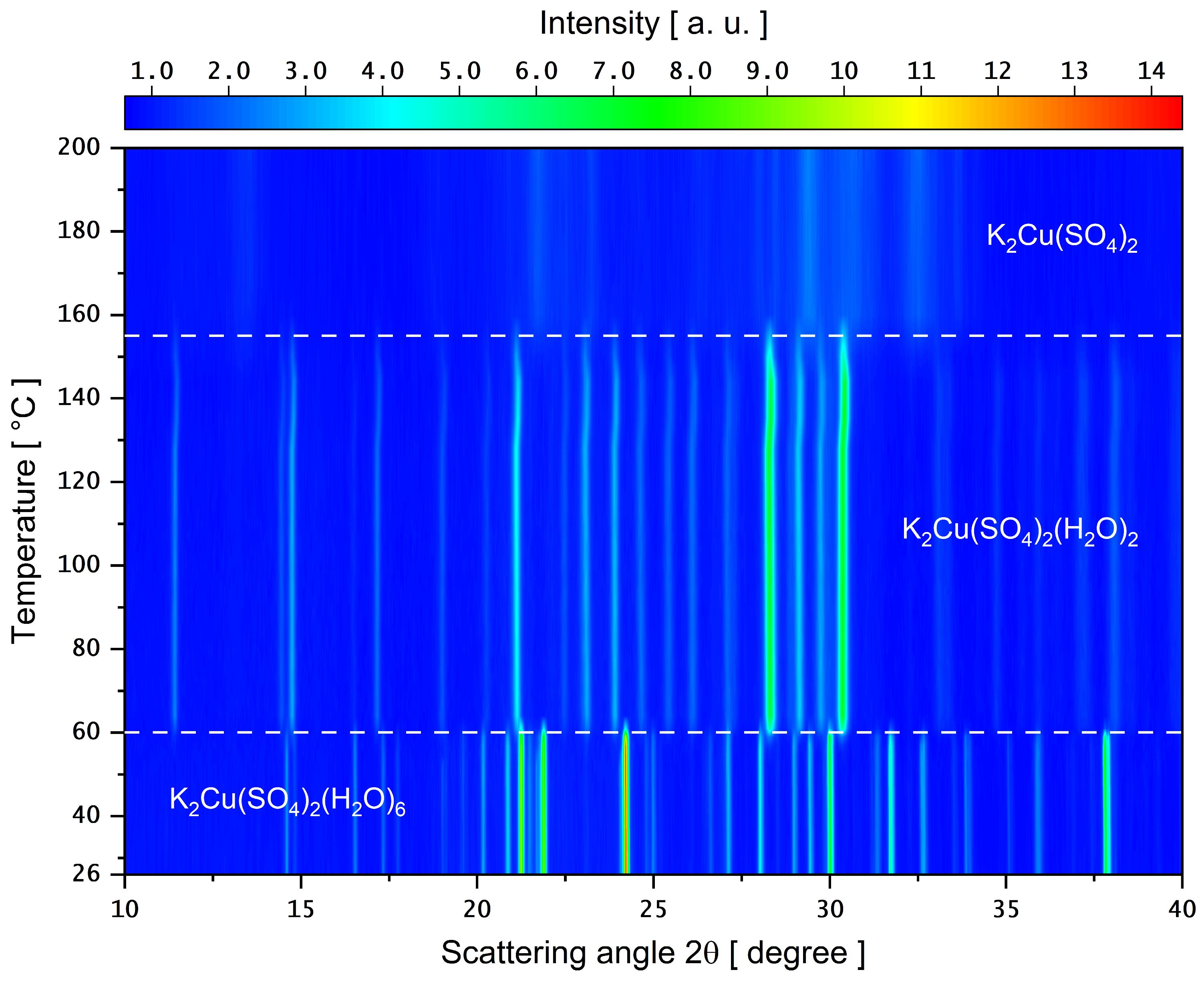
3.4. PXRD as a Function of Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganesh, G.; Ramadoss, A.; Kannan, P.S.; Subbiahpandi, A. Crystal Growth, Structural, Thermal, and Dielectric Characterization of Tutton Salt (NH4)2Fe(SO4)2·6H2O Crystals. J. Therm. Anal. Calorim. 2013, 112, 547–554. [Google Scholar] [CrossRef]
- Morales, A.; Cooper, N.; Reisner, B.A.; DeVore, T.C. Enthalpies of Formation and Standard Entropies for Some Potassium Tutton Salts. Chem. Thermodyn. Therm. Anal. 2022, 8, 1–8. [Google Scholar] [CrossRef]
- Lopes, J.B.d.O.; Neto, J.G.d.O.; Santos, A.O.d.; Lang, R. Evaluation of Mixed Tutton Salts (NH4K)MII(SO4)2(H2O)6, (MII = Co or Ni) and Their Corresponding Langbeinite Phases as Thermochemical Heat Storage Materials: Dehydration/Hydration Behavior and Reversibility of Structural Phase Transformation. J. Energy Storage 2019, 64, 371–378. [Google Scholar] [CrossRef]
- Kooijman, W.; Kok, D.J.; Blijlevens, M.A.R.; Meekes, H.; Vlieg, E. Screening Double Salt Sulfate Hydrates for Application in Thermochemical Heat Storage. J. Energy Storage 2022, 55, 105459. [Google Scholar] [CrossRef]
- Ait Ousaleh, H.; Sair, S.; Zaki, A.; Younes, A.; Faik, A.; El Bouari, A. Advanced Experimental Investigation of Double Hydrated Salts and Their Composite for Improved Cycling Stability and Metal Compatibility for Long-Term Heat Storage Technologies. Renew. Energy 2020, 162, 447–457. [Google Scholar] [CrossRef]
- Smith, J.; Weinberger, P.; Werner, A. Dehydration Performance of a Novel Solid Solution Library of Mixed Tutton Salts as Thermochemical Heat Storage Materials. J. Energy Storage 2024, 78, 110003. [Google Scholar] [CrossRef]
- De Oliveira, G.; Carvalho, J.D.O.; Marques, J.V.; Façanha, P.F.; Adenilson, O.; Lang, R. Tutton K2Zn(SO4)2(H2O)6 Salt: Structural-Vibrational Properties as a Function of Temperature and Ab Initio Calculations. Spectrochim. Acta Part. A Mol. Spectrosc. 2024, 306, 123611. [Google Scholar] [CrossRef]
- Lim, A.R. Thermodynamic Properties and Phase Transitions of Tutton Salt (NH4)2Co(SO4)2·6H2O Crystals. J. Therm. Anal. Calorim. 2012, 109, 1619–1623. [Google Scholar] [CrossRef]
- De Vroomen, A.C.; Lijphart, E.E.; Poulis, N.J. Electron Spin-Lattice Relaxation of Cu++ in Zinc Ammonium Tutton Salt. Physica 1970, 47, 458–484. [Google Scholar] [CrossRef]
- Ballirano, P.; Belardi, G. Rietveld Refinement of the Tutton Salt K2[Fe(H2O)6](SO4)2. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, 60–62. [Google Scholar] [CrossRef]
- Ghosh, S.; Oliveira, M.; Pacheco, T.S.; Perpétuo, G.J.; Franco, C.J. Growth and Characterization of Ammonium Nickel-Cobalt Sulfate Tuttonʹs Salt for UV Light Applications. J. Cryst. Growth 2018, 487, 104–115. [Google Scholar] [CrossRef]
- Ghosh, S.; Ullah, S.; de Mendonça, J.P.A.; Moura, L.G.; Menezes, M.G.; Flôres, L.S.; Pacheco, T.S.; de Oliveira, L.F.C.; Sato, F.; Ferreira, S.O. Electronic Properties and Vibrational Spectra of (NH4)2M″(SO4)2 6H2O (M″ = Ni, Cu) Tutton’s Salt: DFT and Experimental Study. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.R.; Kim, S.H. Structural and Thermodynamic Properties of Tutton Salt K2Zn(SO4)2·6H2O. J. Therm. Anal. Calorim. 2016, 123, 371–376. [Google Scholar] [CrossRef]
- Narasimhulu, K.V.; Rao, J.L. A Single Crystal EPR Study of VO2+ Ions Doped in Cs2Co(SO4)2.6H2O Tutton Salt. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 1997, 53, 2605–2613. [Google Scholar] [CrossRef]
- Abu El-Fadl, A.; Nashaat, A.M. Growth, Structural, and Spectral Characterizations of Potassium and Ammonium Zinc Sulfate Hydrate Single Crystals. Appl. Phys. A Mater. Sci. Process. 2017, 123, 1–11. [Google Scholar] [CrossRef]
- Ran Lim, A.; Lee, J.H. 23Na and 87Rb Relaxation Study of the Structural Phase Transitions in the Tutton Salts Na2Zn(SO4)2·6H2O and Rb2Zn(SO4)2·6H2O Single Crystals. Phys. Status Solidi Basic. Res. 2010, 247, 1242–1246. [Google Scholar] [CrossRef]
- Souamti, A.; Zayani, L.; Lozano-Gorrín, A.D.; Ben Hassen Chehimi, D.; Morales Palomino, J. Synthesis, Characterization and Thermal Behavior of New Rare Earth Ion-Doped Picromerite-Type Tutton’s Salts. J. Therm. Anal. Calorim. 2017, 128, 1001–1008. [Google Scholar] [CrossRef]
- Ballirano, P.; Belardi, G. Rietveld Refinement of the Tutton’s Salt Rb2[Cu(H2O)6](SO4)2 from Parallel-Beam X-Ray Powder Diffraction Data. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, 56–58. [Google Scholar] [CrossRef]
- Ballirano, P.; Belardi, G.; Bosi, F. Redetermination of the Tutton’s Salt Cs2[Cu(H2O)6](SO4)2. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, 351–359. [Google Scholar] [CrossRef]
- Peets, D.C.; Avdeev, M.; Rahn, M.C.; Pabst, F.; Granovsky, S.; Stötzer, M.; Inosov, D.S. Crystal Growth, Structure, and Noninteracting Quantum Spins in Cyanochroite, K2Cu(SO4)2·6H2O. ACS Omega 2022, 7, 5139–5145. [Google Scholar] [CrossRef]
- Maslen, E.N.; Watson, K.J.; Moore, F.H. Crystal Structure and Electron Density of Diammonium Hexaaquacopper(II) Sulfate. Acta Crystallogr. Sect. B 1988, 44, 102–107. [Google Scholar] [CrossRef]
- Bosi, F.; Belardi, G.; Ballirano, P. Structural features in Tutton's salts K2[M2+(H2O)6](SO4)2, with M2+ = Mg, Fe, Co, Ni, Cu, and Zn. Am. Mineral. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Rauw, W.; Ahsbahs, H.; Hitchman, M.A.; Lukin, S.; Reinen, D.; Schultz, A.J.; Simmons, C.J.; Stratemeier, H. Pressure Dependence of the Crystal Structures and EPR Spectra of Potassium Hexaaquacopper(II) Sulfate and Deuterated Ammonium Hexaaquacopper(II) Sulfate. Inorg. Chem. 1996, 35, 1902–1911. [Google Scholar] [CrossRef]
- Augustyniak, M.A.; Usachev, A.E. The Host Lattice Influence on the Jahn-Teller Effect of the Cu(H2O)62+ Complex Studied by EPR in K2Zn(SO4)2 6H2O and (NH4)2Zn(SO4)2 6H2O Tutton Salt Crystals. J. Phys. Condens. Matter 1999, 11, 4391–4400. [Google Scholar] [CrossRef]
- Silver, B.L.; Getz, D. ESR of Cu2 + (H2O)6. II. A Quantitative Study of the Dynamic Jahn-Teller Effect in Copper-Doped Zinc Tutton’s Salt. J. Chem. Phys. 1974, 61, 638–650. [Google Scholar] [CrossRef]
- Morales, A.C.; Cooper, N.D.; Reisner, B.A.; DeVore, T.C. Variable Temperature PXRD Investigation of the Phase Changes during the Dehydration of Potassium Tutton Salts. J. Therm. Anal. Calorim. 2018, 132, 1523–1534. [Google Scholar] [CrossRef]
- Abdulwahab, A.M.; Gaffar, M.A.; Abu El-Fadl, A. Crystal Growth, Structure, and Thermal Analysis of K₂Cu(SO₄)₂·6H₂O Tutton Salt. Thamar Univ. J. Nat. Appl. Sci. 2024, 9, 48–52. [Google Scholar] [CrossRef]
- Mccusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld Refinement Guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a Graphical User Interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Oliveira Neto, J.G.; Lang, R.; Rodrigues, J.A.O.; Gutiérrez, C.E.O.; Murillo, M.A.R.; Sousa, F.F.; Silva Filho, J.G.; Santos, A.O. Kröhnkite-Type K2Mn(SO4)2(H2O)2 Double Salt: Synthesis, Structure, and Properties. J. Mater. Sci. 2022, 57, 8195–8210. [Google Scholar] [CrossRef]
- Souamti, A.; Zayani, L.; Palomino, J.M.; Cruz-Yusta, M.; Vicente, C.P.; Hassen-Chehimi, D. Ben Synthesis, Characterization and Thermal Analysis of K2M(SO4)2·6H2O (M = Mg, Co, Cu). J. Therm. Anal. Calorim. 2015, 122, 929–936. [Google Scholar] [CrossRef]
- De Oliveira, J.G.; Jailton, N.; Kamila, R.V.; Luiz, R.A.; Silva, F.L.; Lage, M.R.; Stoyanov, S.R.; De Sousa, F.F.; Lang, R.; Adenilson, O. Tutton Salt (NH4)2Zn(SO4)2(H2O)6: Thermostructural, Spectroscopic, Hirshfeld Surface, and DFT Investigations. J. Mol. Model. 2024, 30, 339. [Google Scholar] [CrossRef] [PubMed]
- Diniz, R.M.C.S.; Nogueira, C.E.S.; Santos, C.C.; Sinfrônio, F.S.M.; de Sousa, F.F.; de Menezes, A.S. Structural, Vibrational and Thermal Studies on Bis(L-Glutaminato)Copper(II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.O.; Oliveira Neto, J.G.; Santos, C.C.; Nogueira, C.E.S.; de Sousa, F.F.; de Menezes, A.S.; dos Santos, A.O. Phase Changes of Tris(Glycinato)Chromium(III) Monohydrate Crystal Systematically Studied by Thermal Analyses, XRPD, FTIR, and Raman Combined with Ab Initio Calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120883. [Google Scholar] [CrossRef]
- De Menezes, A.S.; Dos Santos, A.O.; Almeida, J.M.A.; Sasaki, J.M.; Cardoso, L.P. Piezoelectric Coefficients of L-Histidine Hydrochloride Monohydrate Obtained by Synchrotron x-Ray Renninger Scanning. J. Phys. Condens. Matter 2007, 19, 106218. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; Van De Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A Program for Crystal Structure Determination from Powder Diffraction Data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Bail, A.L.; Duroy, H.; FourQuet, J.L. Ab-Initio Structure Determination of Lisbwo6 by X-Ray Powder Diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Oliveira Neto, J.G.; Cavalcante, L.A.; Gomes, E.S.; Dos Santos, A.O.; Sousa, F.F.; Mendes, F.; Macêdo, A.A.M. Crystalline Films of L-Threonine Complexed with Copper (II) Dispersed in a Galactomannan Solution: A Structural, Vibrational, and Thermal Study. Polym. Eng. Sci. 2020, 60, 71–77. [Google Scholar] [CrossRef]
- Ferreira Júnior, R.S.; Moura, G.M.; Pereira, A.C.; Ribeiro, P.R.d.S.; da Silva, L.M.; dos Santos, A.O. Time and Temperature Induced Phase Transformation in L-Isoleucine Hydrochloride Monohydrated Crystal. Cryst. Res. Technol. 2016, 51, 738–741. [Google Scholar] [CrossRef]

| TG | Tpeak DTA [°C] | Tpeak DSC [°C] | Molecular Fragment | Chemical Reactions | |||
|---|---|---|---|---|---|---|---|
| Temp. Range [°C] | Weight Loss [%] | Weight Loss [mg] | Molar Mass [g∙mol−1] | ||||
| 58–100 | 16.51 | 0.592 | “72.06” *72.98* | 71 | 63 | 4∙H2O | K2Cu(SO4)2(H2O)6 → K2Cu(SO4)2(H2O)2 + 4∙H2O↑ |
| 110–175 | 8.09 | 0.290 | “36.02” *35.75* | 140 | 121 | 2∙H2O | K2Cu(SO4)2(H2O)2 → K2Cu(SO4)2 + 2∙H2O↑ |
| 175–600 | - | - | - | 528 | - | - | K2Cu(SO4)2 → β-K2SO4 + Cu(SO4) → α-K2SO4 + Cu(SO4) |
| 600–900 | 8.40 | 0.301 | “441.96” *437.12* | - | - | inorganic compounds | sulfates decomposition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Neto, J.G.; Santos, R.S.; Abreu, K.R.; da Silva, L.M.; Lang, R.; dos Santos, A.O. Temperature-Induced Phase Transformations in Tutton Salt K2Cu(SO4)2(H2O)6: Thermoanalytical Studies Combined with Powder X-Ray Diffraction. Physchem 2024, 4, 458-469. https://doi.org/10.3390/physchem4040032
de Oliveira Neto JG, Santos RS, Abreu KR, da Silva LM, Lang R, dos Santos AO. Temperature-Induced Phase Transformations in Tutton Salt K2Cu(SO4)2(H2O)6: Thermoanalytical Studies Combined with Powder X-Ray Diffraction. Physchem. 2024; 4(4):458-469. https://doi.org/10.3390/physchem4040032
Chicago/Turabian Stylede Oliveira Neto, João G., Ronilson S. Santos, Kamila R. Abreu, Luzeli M. da Silva, Rossano Lang, and Adenilson O. dos Santos. 2024. "Temperature-Induced Phase Transformations in Tutton Salt K2Cu(SO4)2(H2O)6: Thermoanalytical Studies Combined with Powder X-Ray Diffraction" Physchem 4, no. 4: 458-469. https://doi.org/10.3390/physchem4040032
APA Stylede Oliveira Neto, J. G., Santos, R. S., Abreu, K. R., da Silva, L. M., Lang, R., & dos Santos, A. O. (2024). Temperature-Induced Phase Transformations in Tutton Salt K2Cu(SO4)2(H2O)6: Thermoanalytical Studies Combined with Powder X-Ray Diffraction. Physchem, 4(4), 458-469. https://doi.org/10.3390/physchem4040032







