Facile Fabrication of Pd-Doped CuO-ZnO Composites for Simultaneous Photodegradation of Anionic and Neutral Dyes
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of CuO-ZnO Nanomaterials
2.3. Synthesis of Pd-Doped CuO-ZnO Nanomaterial
2.4. Catalytic Degradation of Dye Mixture
3. Results and Discussion
3.1. Surface Morphology and Elemental Analysis
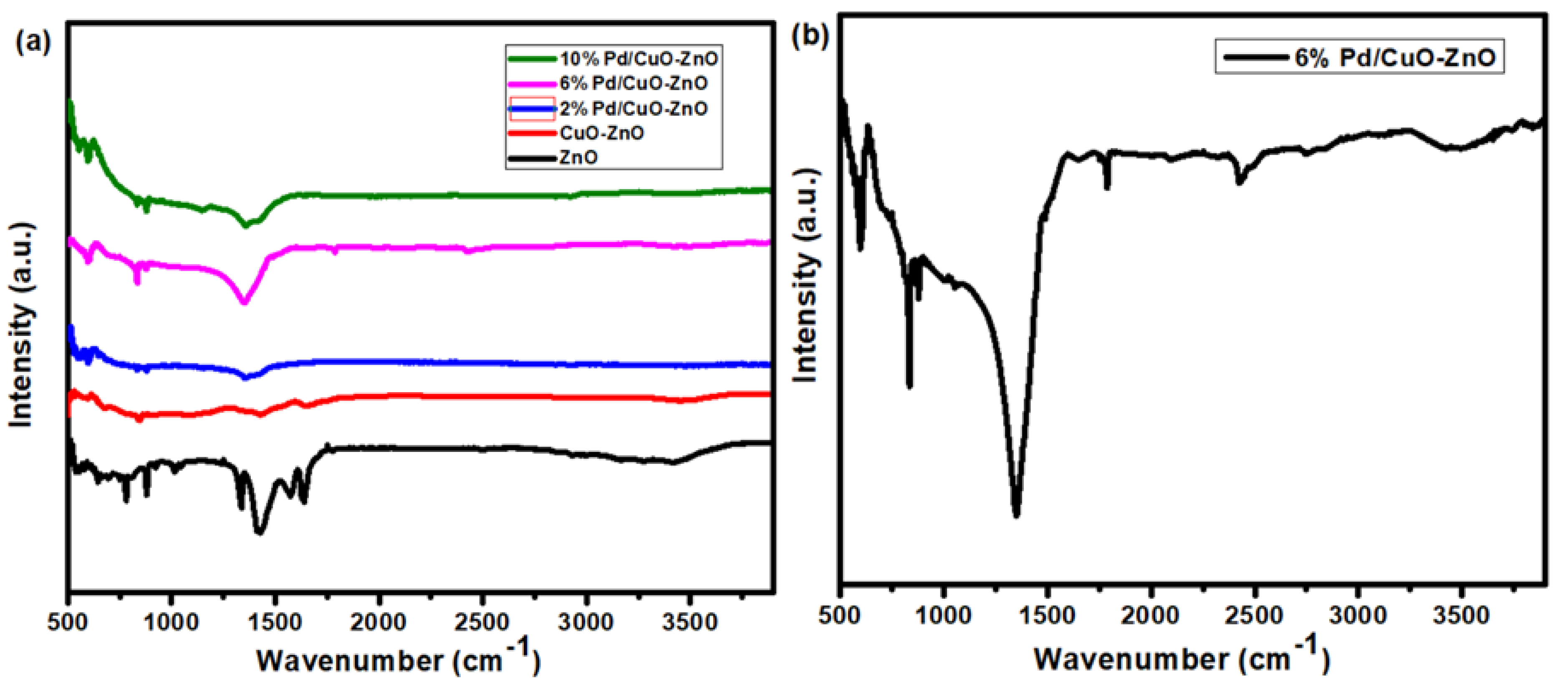
3.2. FT-IR Analysis
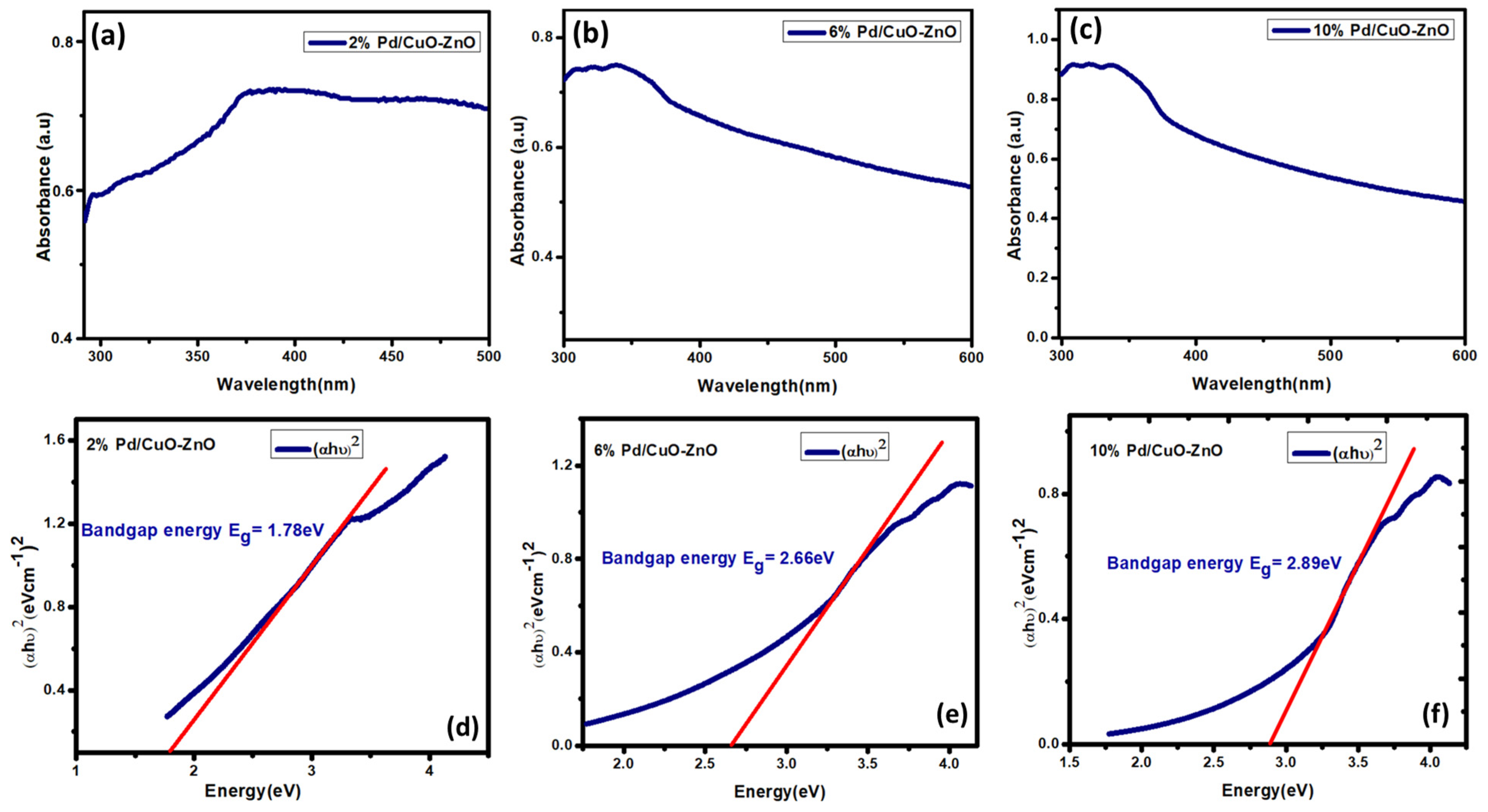
3.3. UV-Visible Analysis
3.4. XRD Analysis
3.5. Photodegradation of Two Dye Mixture by Pd/CuO-ZnO Catalyst
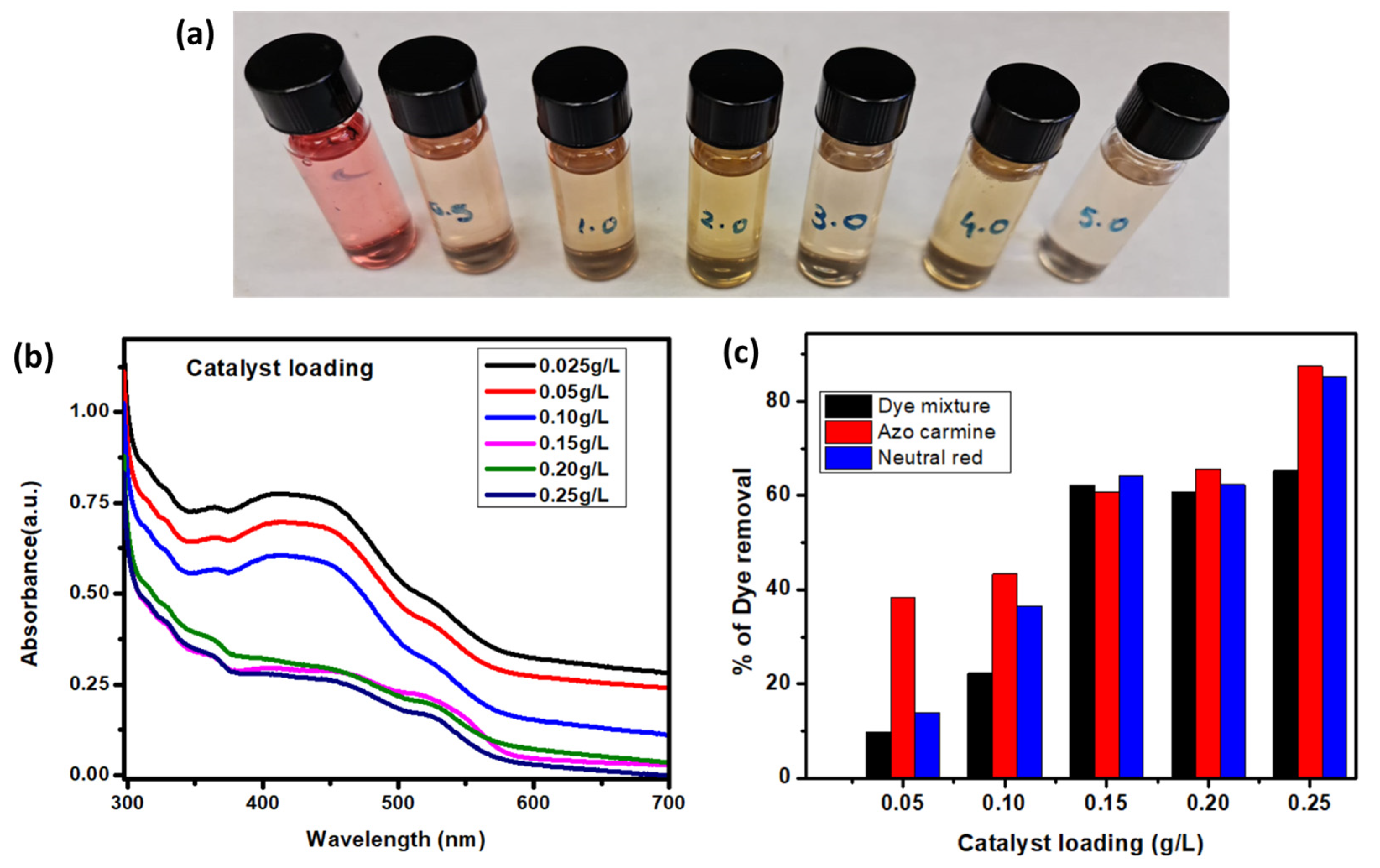
3.5.1. Effect of Catalyst Loading
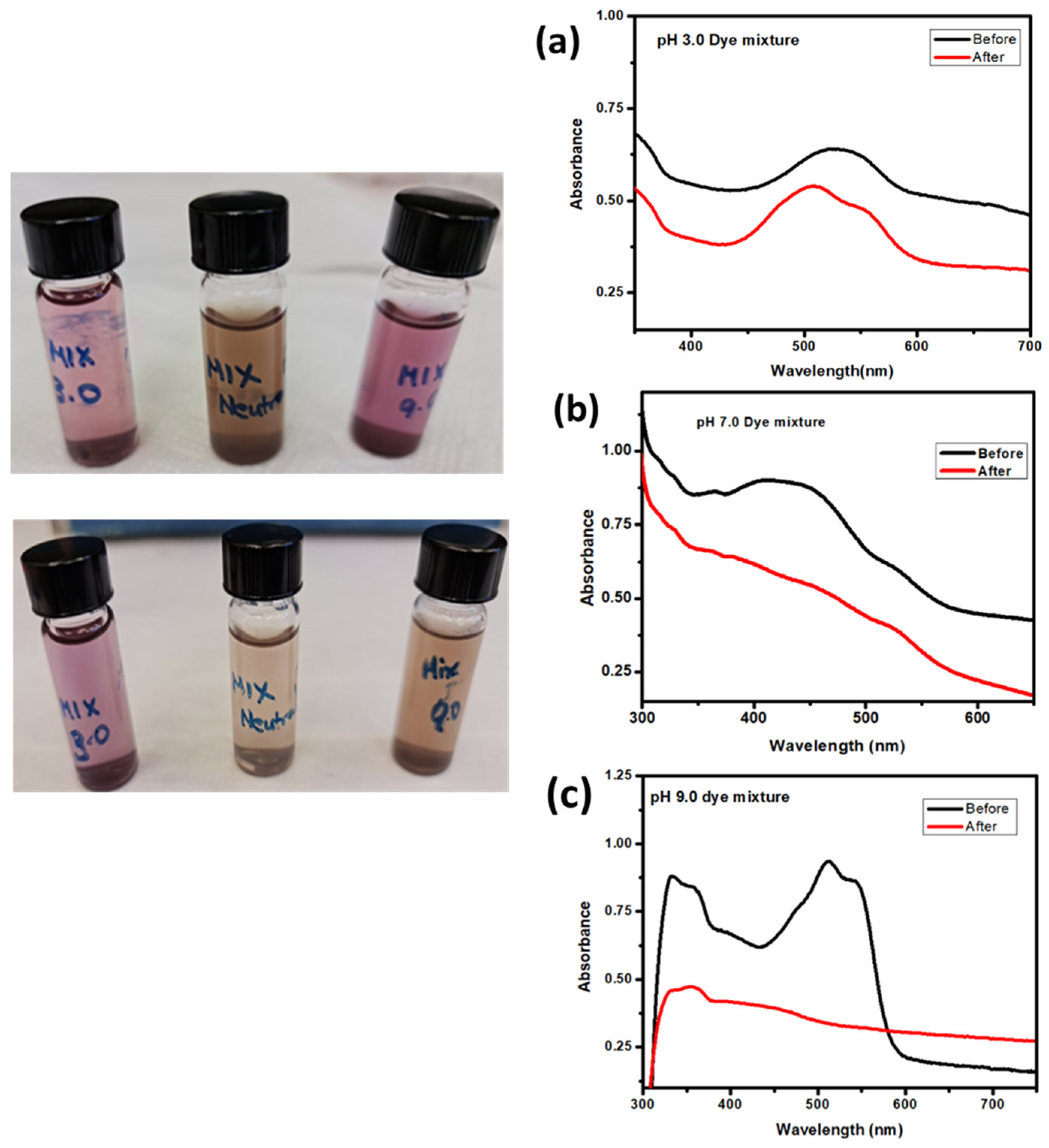
3.5.2. Effect of pH of the Solution
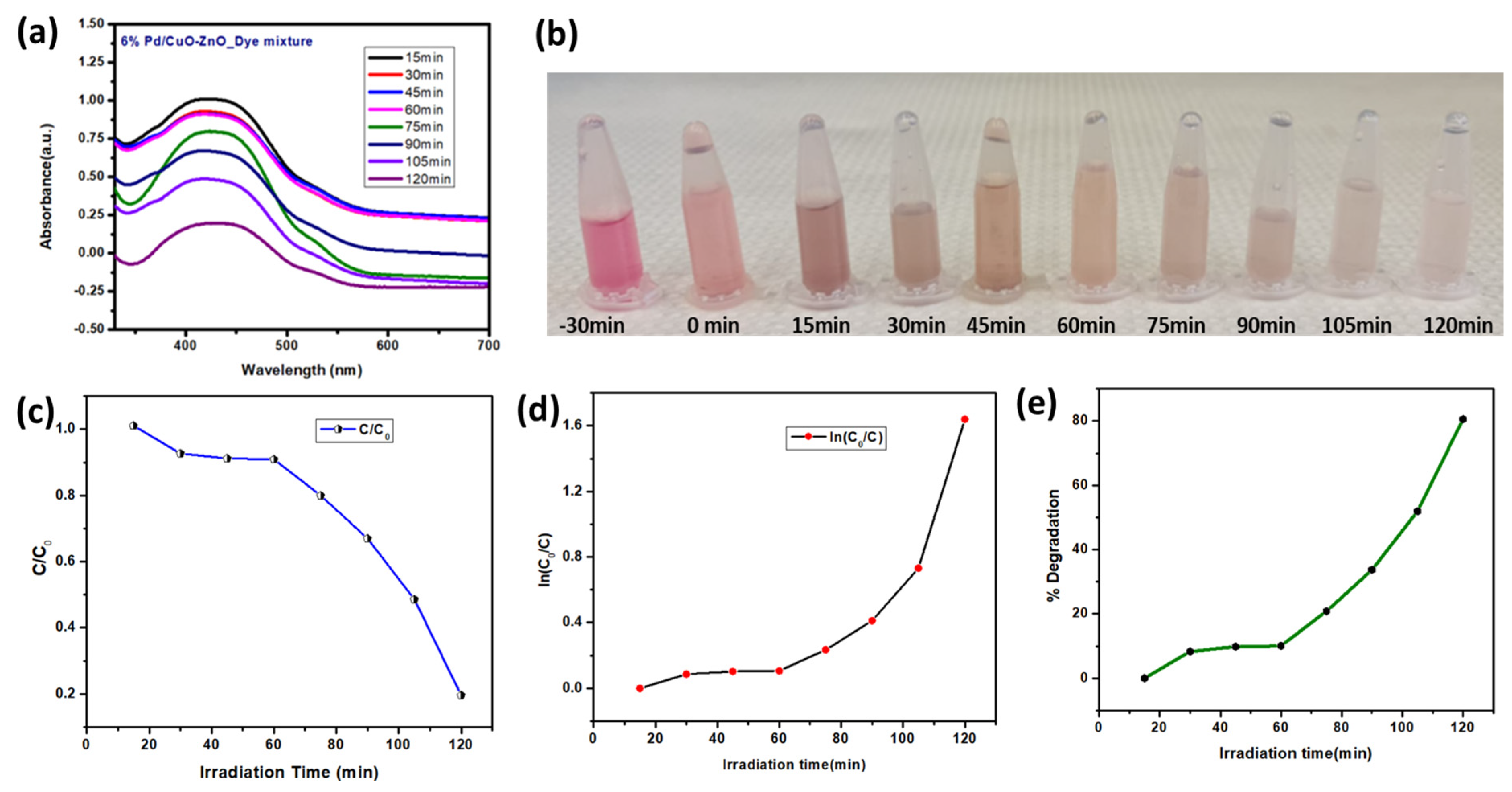
3.5.3. Time Degradation
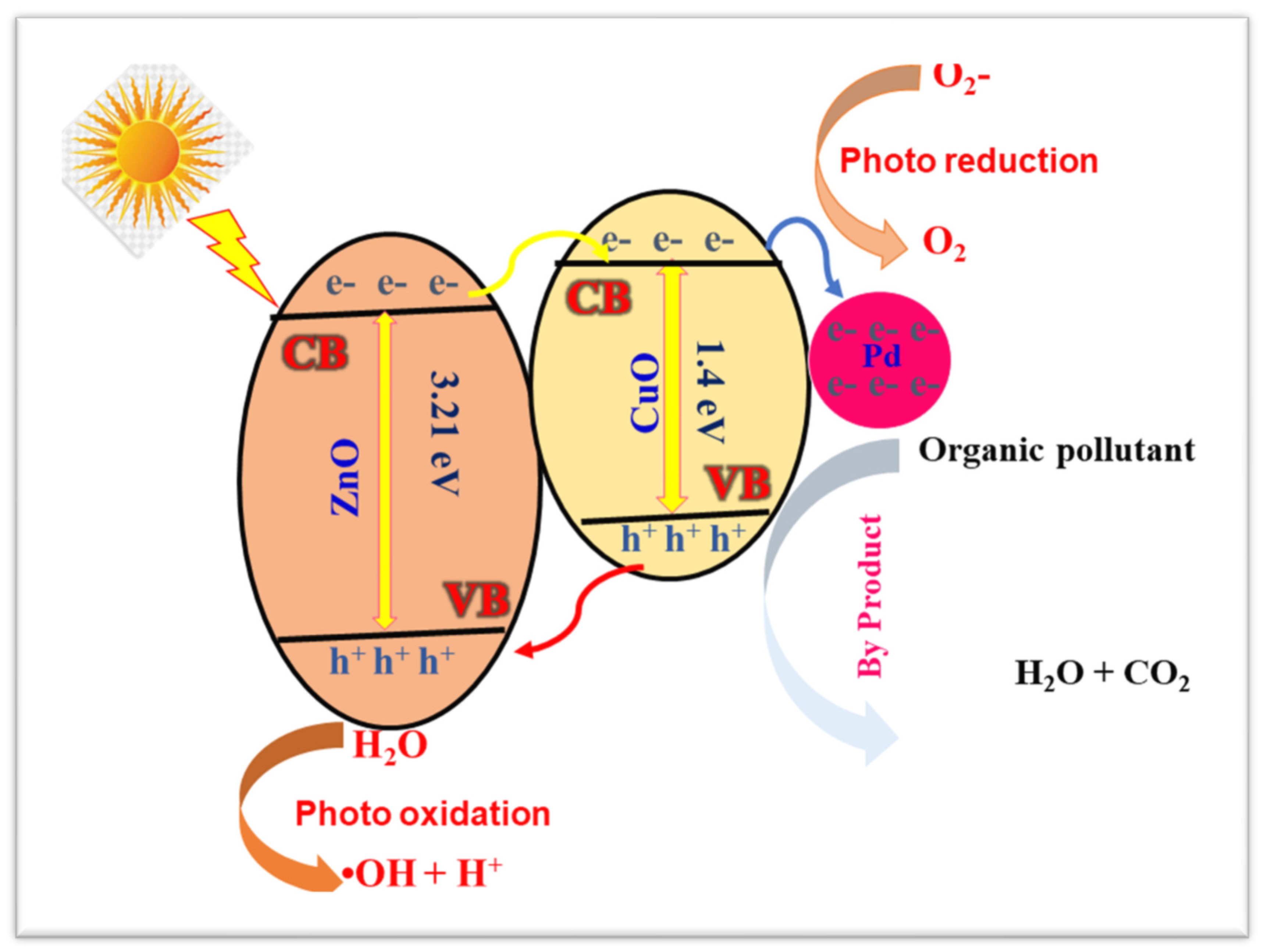
3.6. Possible Microscopic Mechanism of the ZnO/CuO-Pd Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gürses, A.; Güneş, K.; Şahin, E. Removal of dyes and pigments from industrial effluents. In Green Chemistry and Water Remediation: Research and Application; Elsevier: Amsterdam, The Netherlands, 2021; pp. 135–187. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Bonthula, S.; Bonthula, S.R.; Pothu, R.; Srivastava, R.K.; Boddula, R.; Radwan, A.B.; Al-Qahtani, N. Recent Advances in Copper-Based Materials for Sustainable Environmental Applications. Sustain. Chem. 2023, 4, 246–271. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Kumar, A.N.; Shanbhag, V.; Nagaswarupa, H.P.; Boddula, R.; Al-Kahtani, A.A.; Kumar, K.D. Energy storage, sensors, photocatalytic applications of green synthesized ZnO:Fe3+ nanomaterials. Chem. Phys. Impact 2023, 7, 100387. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Ul Haq, A.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.; Sharma, A.; Bin Khamidun, M.H. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Oyetade, J.A.; Machunda, R.L.; Hilonga, A. Photocatalytic degradation of azo dyes in textile wastewater by Polyaniline composite catalyst—A review. Sci. Afr. 2022, 17, e01305. [Google Scholar] [CrossRef]
- Arif, M. Catalytic degradation of azo dyes by bimetallic nanoparticles loaded in smart polymer microgels. RSC Adv. 2023, 13, 3008–3019. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Sharma, M.; Pandey, O.P.; Singh, K. Photocatalytic degradation of azo dyes using Zn-doped and undoped TiO2 nanoparticles. Appl. Phys. A 2014, 116, 371–378. [Google Scholar] [CrossRef]
- Mittal, M.; Sharma, M.; Pandey, O.P. Photocatalytic Studies of Crystal Violet Dye Using Mn Doped and PVP Capped ZnO Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 2725–2733. [Google Scholar] [CrossRef]
- Mohammed Redha, Z.; Abdulla Yusuf, H.; Amin, R.; Bououdina, M. The study of photocatalytic degradation of a commercial azo reactive dye in a simple design reusable miniaturized reactor with interchangeable TiO2 nanofilm. Arab. J. Basic Appl. Sci. 2020, 27, 287–298. [Google Scholar] [CrossRef]
- Ramesh, M. CuO as efficient photo catalyst for photocatalytic decoloration of wastewater containing Azo dyes. Water Pract. Technol. 2021, 16, 1078–1090. [Google Scholar] [CrossRef]
- Abdullah, A.H. Degradation of Methylene Blue Dye by Cuo-BiVO4 Photocatalysts Under Visible Light Irradiation. Malays. J. Anal. Sci. 2016, 20, 1338–1345. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Yu, B.; Peng, Q.; Shen, Y.; Cong, H. Photocatalytic potential of bio-engineered copper nanoparticles synthesized from Ficus carica extract for the degradation of toxic organic dye from waste water: Growth mechanism and study of parameter affecting the degradation performance. Mater. Res. Bull. 2019, 120, 110583. [Google Scholar] [CrossRef]
- Umar, K.; Mfarrej, M.F.B.; Rahman, Q.I.; Zuhaib, M.; Khan, A.; Zia, Q.; Banawas, S.; Nadeem, H.; Khan, M.F.; Ahmad, F. ZnO Nano-swirlings for Azo Dye AR183 photocatalytic degradation and antimycotic activity. Sci. Rep. 2022, 12, 14023. [Google Scholar] [CrossRef]
- El-Sayed, F.; Hussien, M.S.A.; AlAbdulaal, T.H.; Ismail, A.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S.; Khairy, Y.; Ali, T.E.; Ibrahim, M.A. Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures. Crystals 2022, 12, 535. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, L.; Mahmud, S.; Yang, F.; Liu, H. Bio-synthesized palladium nanoparticles using alginate for catalytic degradation of azo-dyes. Chin. J. Chem. Eng. 2020, 28, 1334–1343. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Wang, Z.; Liu, H. Green synthesis of palladium nanoparticles with carboxymethyl cellulose for degradation of azo-dyes. Mater. Chem. Phys. 2017, 187, 133–140. [Google Scholar] [CrossRef]
- Mehta, M.; Sharma, M.; Pathania, K.; Jena, P.K.; Bhushan, I. Degradation of synthetic dyes using nanoparticles: A mini-review. Environ. Sci. Pollut. Res. 2021, 28, 49434–49446. [Google Scholar] [CrossRef]
- Kandasamy, M.; Vasudevan, V.; Thangavelu, P.; Parasuraman, B.; Boddula, R.; Pothu, R.; Shanmugam, P.; Nadesan, K. Exploring prompt photocatalytic degradation of MB dye using Cu 0.5 Co 0.5 WO4/g-C3N4 nanocomposite under visible light irradiation. Emergent Mater. 2024, 7, 987–998. [Google Scholar] [CrossRef]
- Snehal, A.P.; Patel, P.; Manali, D.; Ruparelia, J.; Patel, U.D. Rapid electro-catalytic reduction of azo dyes and phenolic compounds in the presence of metallic palladium. Sep. Purif. Technol. 2021, 254, 117658. [Google Scholar] [CrossRef]
- Tackett, B.M.; Lee, J.H.; Chen, J.G. Electrochemical Conversion of CO2 to Syngas with Palladium-Based Electrocatalysts. Acc. Chem. Res. 2020, 53, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties. Molecules 2022, 27, 3206. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Dahryn Trivedi, A.B. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. J. Chromatogr. Sep. Tech. 2015, 6, 5. [Google Scholar] [CrossRef]
- Salman, S.H.; Khashan, K.S.; Hadi, A.A. Green Synthesis and Characterization of Palladium Nanoparticles by Pulsed Laser Ablation and Their Antibacterial Activity. Metals 2023, 13, 273. [Google Scholar] [CrossRef]
- Patel, M.; Mishra, S.; Verma, R.; Shikha, D. Synthesis of ZnO and CuO nanoparticles via Sol gel method and its characterization by using various technique. Discov. Mater. 2022, 2, 1. [Google Scholar] [CrossRef]
- Ben Elkamel, I.; Hamdaoui, N.; Mezni, A.; Ajjel, R.; Beji, L. Synthesis and characterization of Cu doped ZnO nanoparticles for stable and fast response UV photodetector at low noise current. J. Mater. Sci. Mater. Electron. 2019, 30, 9444–9454. [Google Scholar] [CrossRef]
- Asamoah, R.B.; Yaya, A.; Mensah, B.; Nbalayim, P.; Apalangya, V.; Bensah, Y.D.; Damoah, L.N.W.; Agyei-Tuffour, B.; Dodoo-Arhin, D.; Annan, E. Synthesis and characterization of zinc and copper oxide nanoparticles and their antibacteria activity. Results Mater. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Fawzy, I.M.; Saleh, B.M.; Issa, M.Y.; Bakowsky, U.; Azzazy, H.M.E.-S. Green Synthesis of Platinum and Palladium Nanoparticles Using Peganum harmala L. Seed Alkaloids: Biological and Computational Studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K.; et al. Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria. Nanomaterials 2021, 11, 451. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Qureshi, M.S.; Malik, M.M.; Haque, F.Z. Studies of structural, optical, and electrical properties associated with defects in sodium-doped copper oxide (CuO/Na) nanostructures. J. Mater. Sci. 2018, 53, 8826–8843. [Google Scholar] [CrossRef]
- Celebi, M.; Karakas, K.; Ertas, I.E.; Kaya, M.; Zahmakiran, M. Palladium Nanoparticles Decorated Graphene Oxide: Active and Reusable Nanocatalyst for the Catalytic Reduction of Hexavalent Chromium(VI). ChemistrySelect 2017, 2, 8312–8319. [Google Scholar] [CrossRef]
- Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Goddati, M.; Lee, J.; Sabir, F.K. Green Synthesis of a CuO–ZnO Nanocomposite for Efficient Photodegradation of Methylene Blue and Reduction of 4-Nitrophenol. ACS Omega 2022, 7, 30908–30919. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, S.A. High sensitivity Photoconductive for ZnO:MgO Nanoparticles. Energy Procedia 2019, 157, 355–361. [Google Scholar] [CrossRef]
- Zadeh, F.A.; Bokov, D.O.; Salahdin, O.D.; Abdelbasset, W.K.; Jawad, M.A.; Kadhim, M.M.; Qasim, M.T.; Kzar, H.H.; Al-Gazally, M.E.; Mustafa, Y.F.; et al. Cytotoxicity evaluation of environmentally friendly synthesis Copper/Zinc bimetallic nanoparticles on MCF-7 cancer cells. Rend. Lincei Sci. Fis. Nat. 2022, 33, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, S.; Bakkiyaraj, R.; Shanmugam, P.; Boonyuen, S.; Venugopal, T. Investigation of biological efficacy assessment of cobalt-doped cerium oxide nanocomposites against pathogenic bacteria, fungi, and lung cancer cells. Mater. Chem. Phys. 2024, 321, 129496. [Google Scholar] [CrossRef]
- Li, P.; Zhuang, Z.; Zhang, Z.; Guo, J.; Fang, Z.; Chen, W. Interfacial heterojunction construction by introducing Pd into W18O49 nanowires to promote the visible light-driven photocatalytic degradation of environmental organic pollutants. J. Colloid Interface Sci. 2021, 590, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yi, W.; Yuan, H.; Wu, X.; Li, F. A highly photoactive S, Cu-codoped nano-TiO2 photocatalyst: Synthesis and characterization for enhanced photocatalytic degradation of neutral red. Environ. Prog. Sustain. Energy 2014, 33, 419–429. [Google Scholar] [CrossRef]
- El-Samak, A.A.; Ponnamma, D.; Hassan, M.K.; Al-Maadeed, M.A.A. A stable porous vessel for photocatalytic degradation of Azocarmine G dye. Microporous Mesoporous Mater. 2022, 341, 111994. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Al-Maadeed, A.S.M.A.; Al-Mohannadi, A.M.A.A.; Al-Thani, N. Electrospun PVDF/ZnO Based Composite Fibers for Oil Absorption and Photocatalytic Degradation of Organic Dyes from Waste Water. Fibers Polym. 2022, 23, 1217–1224. [Google Scholar] [CrossRef]
- Montero, J.; Welearegay, T.; Thyr, J.; Stopfel, H.; Dedova, T.; Acik, I.O.; Österlund, L. Copper-zinc oxide heterojunction catalysts exhibiting enhanced photocatalytic activity prepared by a hybrid deposition method. RSC Adv. 2021, 11, 10224–10234. [Google Scholar] [CrossRef]
- Bhapkar, A.R.; Geetha, M.; Jaspal, D.; Gheisari, K.; Laad, M.; Cabibihan, J.-J.; Sadasivuni, K.K.; Bhame, S. Aluminium doped ZnO nanostructures for efficient photodegradation of indigo carmine and azo carmine G in solar irradiation. Appl. Nanosci. 2023, 13, 5777–5793. [Google Scholar] [CrossRef]
- Mu, Z.; Hua, J.; Kumar Tammina, S.; Yang, Y. Visible light photocatalytic activity of Cu, N co-doped carbon dots/Ag3PO4 nanocomposites for neutral red under green LED radiation. Colloids Surf A Physicochem. Eng. Asp. 2019, 578, 123643. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Januário, E.F.D.; Araújo, M.F.; Bergamasco, R.; Vieira, A.M.S. Efficient performance of copper oxide nanoparticles synthesized with pomegranate leaf extract for neutral red dye adsorption. Environ. Prog. Sustain. Energy 2022, 41, e13864. [Google Scholar] [CrossRef]
- Sharma, M.; Poddar, M.; Gupta, Y.; Nigam, S.; Avasthi, D.K.; Adelung, R.; Abolhassani, R.; Fiutowski, J.; Joshi, M.; Mishra, Y.K. Solar light assisted degradation of dyes and adsorption of heavy metal ions from water by CuO–ZnO tetrapodal hybrid nanocomposite. Mater. Today Chem. 2020, 17, 100336. [Google Scholar] [CrossRef]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic degradation of methylene blue using ZnO and 2%Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: A comparative study. SN Appl. Sci. 2019, 1, 1247. [Google Scholar] [CrossRef]
- Akter, S.; Islam, M.S. Effect of additional Fe2+ salt on electrocoagulation process for the degradation of methyl orange dye: An optimization and kinetic study. Heliyon 2022, 8, e10176. [Google Scholar] [CrossRef]
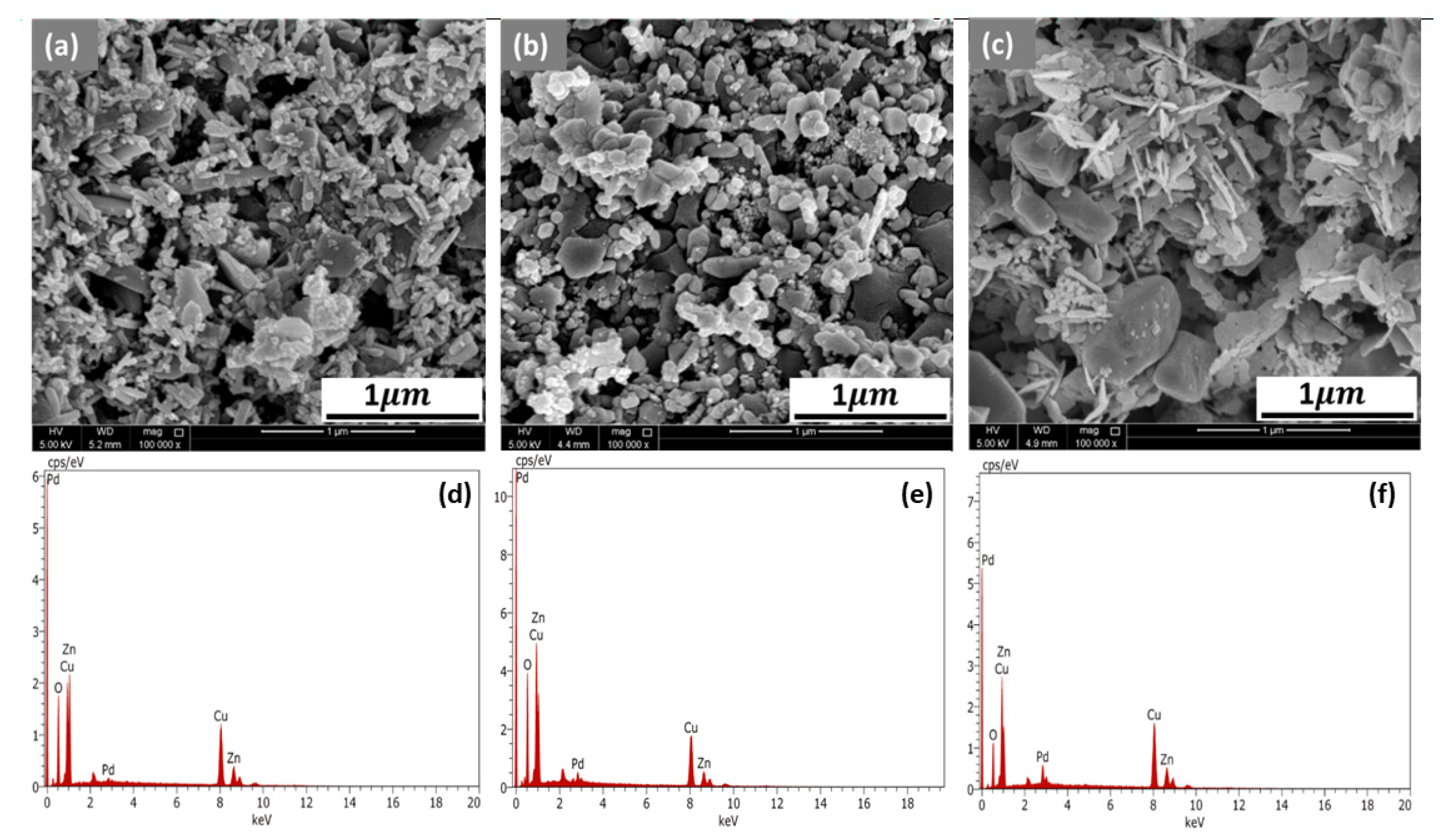

| Samples | Elemental Constituent of the Sample in Weight % | |||
|---|---|---|---|---|
| Pd | Cu | Zn | O | |
| 2%Pd/CuO-ZnO | 0.65 | 55.12 | 21.15 | 23.09 |
| 6%Pd/CuO-ZnO | 2.58 | 50.97 | 16.74 | 29.71 |
| 10%Pd/CuO-ZnO | 5.61 | 58.44 | 22.26 | 13.69 |
| S.No | Nanomaterial Used | Light Source | Synthesis Method | Pollutant | Catalyst Amount | Dye Concentration | Degradation Time | Degradation Efficiency | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pd/W18O49 nanowires | Sunlight | Hydro thermal | Methylene Blue | 10 mg | 15 mg/L | 40 min | 98.4% | 1 February 2021 | [38] |
| Neutral red | 10 mg | 10 mg/L | 40 min | 96.1% | ||||||
| 2 | Sulfur Cu-TiO2 | UV light | Sol-gel | Neutral red | 0.75 g/L | 20 mg/L | 120 min | 98.4% | 1 July 2014 | [39] |
| 3 | PS/ZnO | Sunlight | Electrospun | Azocarmine G | 10 mg | 10 mg/L | 6 h | 95% | 15 June 2022 | [40] |
| 4 | PVDF/ZnO | Sunlight | Electro spinning | Azocarmine | 5 mg | 10 mg/L | 120 min | 85% | 11 April 2022 | [41] |
| Malachite green | 5 mg | 10 mg/L | 240 min | 90% | ||||||
| 5 | KA@CP-S | Sunlight | Hydrothermal | Neutral red | 0.025 mmol | 10 mg/L | 150 min | 72% | 31 October | [42] |
| 6 | Al/ZnO | Sunlight | Auto combustion | Azocarmine | 0.1 g/L | 0.15 g/L | 140 min | 93% | 20 March 2023 | [43] |
| 7 | ZnO | Sunlight | Azocarmine | 0.1 g/L | 0.15 g/L | 140 min | 68.13% | 20 March 2023 | [43] | |
| 8 | Cu,N-CDs/Ag3PO4 | Visible light | Thermolysis | Neutral red | 50 mg | 12 mg/L | 60 min | 95.5% | 3 July 2019 | [44] |
| 9 | CuO | Hydrothermal | Neutral red | 0.4 g/L | 120 min | 96% | 28 March 2022 | [45] | ||
| 10 | CuO-ZnO tetrapodal hybrid | Solar irradiation | Hydrothermal synthesis | BV-3 | 1 g/L | 40 mg/L | 100 min | 86% | 23 July 2020 | [46] |
| RY-145 | 1 g/L | 40 mg/L | 80 min | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonthula, S.; Ibrahim, M.F.; Al-Jaber, A.O.; Al-Siddiqi, A.-D.F.; Pothu, R.; Chowdhury, T.; Siddiqui, Y.; Boddula, R.; Radwan, A.B.; Al-Qahtani, N. Facile Fabrication of Pd-Doped CuO-ZnO Composites for Simultaneous Photodegradation of Anionic and Neutral Dyes. Physchem 2024, 4, 181-196. https://doi.org/10.3390/physchem4030014
Bonthula S, Ibrahim MF, Al-Jaber AO, Al-Siddiqi A-DF, Pothu R, Chowdhury T, Siddiqui Y, Boddula R, Radwan AB, Al-Qahtani N. Facile Fabrication of Pd-Doped CuO-ZnO Composites for Simultaneous Photodegradation of Anionic and Neutral Dyes. Physchem. 2024; 4(3):181-196. https://doi.org/10.3390/physchem4030014
Chicago/Turabian StyleBonthula, Sumalatha, Muna Farah Ibrahim, Aisha Omar Al-Jaber, Al-Dana Faisal Al-Siddiqi, Ramyakrishna Pothu, Tauqeer Chowdhury, Yusuf Siddiqui, Rajender Boddula, Ahmed Bahgat Radwan, and Noora Al-Qahtani. 2024. "Facile Fabrication of Pd-Doped CuO-ZnO Composites for Simultaneous Photodegradation of Anionic and Neutral Dyes" Physchem 4, no. 3: 181-196. https://doi.org/10.3390/physchem4030014
APA StyleBonthula, S., Ibrahim, M. F., Al-Jaber, A. O., Al-Siddiqi, A.-D. F., Pothu, R., Chowdhury, T., Siddiqui, Y., Boddula, R., Radwan, A. B., & Al-Qahtani, N. (2024). Facile Fabrication of Pd-Doped CuO-ZnO Composites for Simultaneous Photodegradation of Anionic and Neutral Dyes. Physchem, 4(3), 181-196. https://doi.org/10.3390/physchem4030014








