The Surprising Role of Endogenous Calcium Carbonate in Crab Shell-Mediated Biosorption of Pb (II)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion

3.1. Scanning Electron Microscopy (SEM)
3.2. Batch Experiments
3.2.1. High Lead Concentration (100 mg/L)
3.2.2. Low Lead Concentration (10 mg/L)
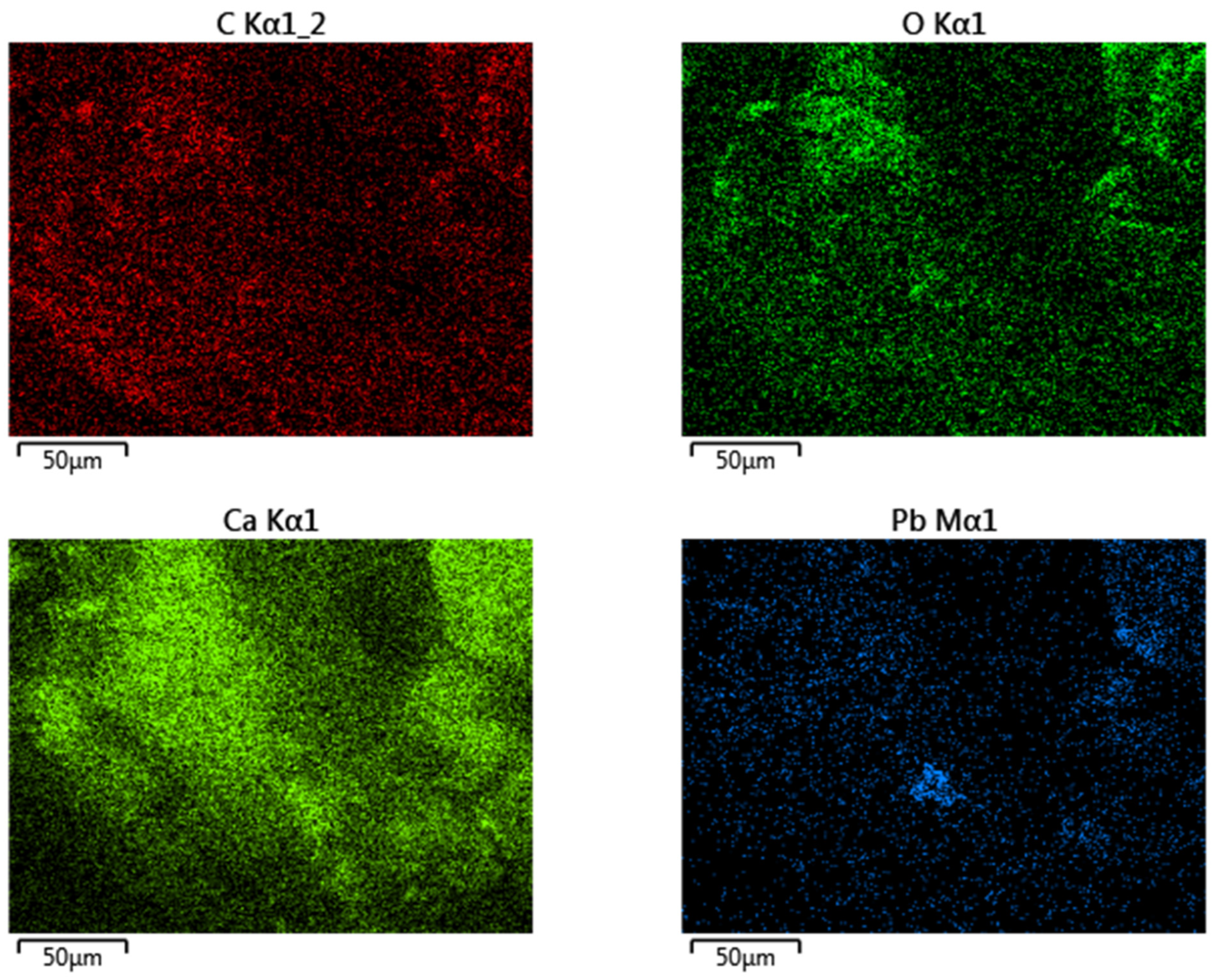
3.3. Energy-Dispersive X-ray Spectroscopy (EDS)
3.4. Adsorption Isotherms
3.5. Kinetic Studies
3.6. BET Surface Area
3.7. Additional Extraction Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fomina, M.; Gadd, G.M. Biosorption: Current Perspectives on Concept, Definition and Application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation Technologies for Heavy Metal Contaminated Groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water: Presence, Removal and Safety; Sharma, S.K., Ed.; The Royal Society of Chemistry: London, UK, 2015; pp. 1–24. [Google Scholar] [CrossRef]
- Shah, G.M.; Umm-e-aiman; Imran, M.; Bakhat, H.F.; Hammad, H.M.; Ahmad, I.; Rabbani, F.; Khan, Z.U.H. Kinetics and equilibrium study of lead bio-sorption from contaminated water by compost and biogas residues. Int. J. Environ. Sci. Technol. 2019, 16, 3839–3850. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Human Rights Watch. Bangladesh: 20 Million Drink Arsenic-Laced Water. Available online: https://www.hrw.org/news/2016/04/06/bangladesh-20-million-drink-arsenic-laced-water (accessed on 17 April 2017).
- World Health Organization. Mercury and Health. Available online: http://www.who.int/mediacentre/factsheets/fs361/en/ (accessed on 17 April 2017).
- Xu, M.; McKay, G. Removal of Heavy Metals, Lead, Cadmium, and Zinc, Using Adsorption Processes by Cost-Effective Adsorbents. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Avila, H.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 109–138. [Google Scholar] [CrossRef]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on Potential Applications of Biomass for the Separation of Heavy Metals from Water and Wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for Heavy Metals Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. The Past, Present, and Future Trends of Biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Comparisons of Low-Cost Adsorbents for Treating Wastewaters Laden with Heavy Metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Zuluaga, C.; Jameel, H.; Gonzalez, R.W.; Lucia, L. Crustacean Shell-Based Biosorption Water Remediation Platforms: Status and Perspectives. J. Environ. Manag. 2019, 231, 757–762. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Aklog, Y.F.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Protein/CaCo3/Chitin Nanofiber Complex Prepared from Crab Shells by Simple Mechanical Treatment and Its Effect on Plant Growth. Int. J. Mol. Sci. 2016, 17, 1600. [Google Scholar] [CrossRef]
- Gagnon, B.; Berrouard, S. Effects of Several Organic Fertilizers on Growth of Greenhouse Tomato Transplants. Can. J. Plant Sci. 1994, 74, 167–168. [Google Scholar] [CrossRef]
- Spinelli, J.; Lehman, L.; Wieg, D. Composition, Processing, and Utilization of Red Crab (Pleuroncodes Planipes) as an Aquacultural Feed Ingredient. J. Fish. Res. Board Can. 1974, 31, 1025–1029. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, J.M.; Yang, J.W. Micro Precipitation of Lead on the Surface of Crab Shell Particles. Process Biochem. 1997, 32, 671–677. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of Chitin Extraction from Shrimp Shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sagheer, F.A.; AAl-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Rinaudo, M.; Chaabouni, M.; Jellouli, K.; Nasri, M. Optimization of Proteins and Minerals Removal from Shrimp Shells to Produce Highly Acetylated Chitin. Int. J. Biol. Macromol. 2016, 84, 246–253. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Synthesis, Characterization and Cr(VI) Uptake Studies of Polypyrrole Functionalized Chitin. Synth. Met. 2014, 198, 181–187. [Google Scholar] [CrossRef]
- Kousalya, G.N.; Gandhi, M.R.; Meenakshi, S. Preparation of Modified Chitin for the Removal of Chromium(VI). Bioremediat. J. 2010, 14, 208–218. [Google Scholar] [CrossRef]
- Jeon, C. Adsorption Characteristics of Waste Crab Shells for Silver Ions in Industrial Wastewater. Korean J. Chem. Eng. 2014, 31, 446–451. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium Carbonate Phase Analysis Using XRD and FT-Raman Spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Kawano, J.; Shimobayashi, N.; Miyake, A.; Kitamura, M. Precipitation Diagram of Calcium Carbonate Polymorphs: Its Construction and Significance. J. Phys. Condens. Matter 2009, 21, 425102. [Google Scholar] [CrossRef] [PubMed]
- Knidri, H.; El Khalfaouy, R.; El Laajeb, A.; Addaou, A.; Lahsini, A. Eco-Friendly Extraction and Characterization of Chitin and Chitosan from the Shrimp Shell Waste via Microwave Irradiation. Process Saf. Environ. Prot. 2014, 4, 395–405. [Google Scholar] [CrossRef]
- Julkapli, N.; Ahmad, Z.; Akil, H.M. HX-Ray Diffraction Studies of Cross Linked Chitosan With Different Cross Linking Agents For Waste Water Treatment Application. AIP Conf. Proc. 2010, 1202, 106–111. [Google Scholar] [CrossRef]
- Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous effluents by rapeseed biomass, Irina Morosanu. New Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Lin, A.Y.-M.; McKittrick, J.; Meyers, M.A. Structure and Mechanical Properties of Crab Exoskeletons. Acta Biomater. 2008, 4, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Van, H.T.; Nguyen, V.D.; Vigneswaran, S.; Nguyen, T.V.; Nguyen, L.H.; Nguyen, X.H.; Rinklebe, J.; Nguyen, T.H.; Tran, H.N. Characteristics and Mechanisms of Cadmium Adsorption onto Biogenic Aragonite Shells-Derived Biosorbent: Batch and Column Studies. J. Environ. Manag. 2018, 241, 535–548. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Chemical Modification of Chitin with Polypyrrole for the Uptake of Pb(II) and Cd(II) Ions. Int. J. Biol. Macromol. 2015, 78, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, C.L.; Lucia, L.A.; Jameel, H.; Gonzalez, R. Marine Exoskeleton-Based Biosorption of Heavy Metals: Performance Cost Analysis. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 28 October–2 November 2018. [Google Scholar]
- Zuluaga, C.L.; Lucia, L.A.; Jameel, H.; Gonzalez, R. Dynamics and kinetics of marine exoskeleton-based heavy metal biosorption and desorption. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2018; Volume 255. [Google Scholar]







| Regression Parameter | Langmuir Isotherm | Freundlich Isotherm |
|---|---|---|
| 0.32 | N/A | |
| (mg/g) | 384.60 | N/A |
| 0.01 | N/A | |
| N/A | 0.89 | |
| N/A | 7.76 | |
| N/A | 1.56 |
| Pseudo-First-Order Fit | Pseudo-Second-Order Fit | |||
|---|---|---|---|---|
| Slope | R2 | qe | k2 | R2 |
| −0.0008 | 0.9429 | 77.69 | 0.001 | 0.9936 |
| Material Characteristics | Surface Area (m2/g) |
|---|---|
| Crab Shell 40 mesh | 28.7 |
| Crab Shell No Protein (No Protein) | 49.1 |
| Crab Shell No Mineral (No CaCO3) | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Londoño-Zuluaga, C.; Jameel, H.; Gonzalez, R.W.; Yang, G.; Lucia, L. The Surprising Role of Endogenous Calcium Carbonate in Crab Shell-Mediated Biosorption of Pb (II). Physchem 2024, 4, 167-180. https://doi.org/10.3390/physchem4020013
Londoño-Zuluaga C, Jameel H, Gonzalez RW, Yang G, Lucia L. The Surprising Role of Endogenous Calcium Carbonate in Crab Shell-Mediated Biosorption of Pb (II). Physchem. 2024; 4(2):167-180. https://doi.org/10.3390/physchem4020013
Chicago/Turabian StyleLondoño-Zuluaga, Carolina, Hasan Jameel, Ronalds W. Gonzalez, Guihua Yang, and Lucian Lucia. 2024. "The Surprising Role of Endogenous Calcium Carbonate in Crab Shell-Mediated Biosorption of Pb (II)" Physchem 4, no. 2: 167-180. https://doi.org/10.3390/physchem4020013
APA StyleLondoño-Zuluaga, C., Jameel, H., Gonzalez, R. W., Yang, G., & Lucia, L. (2024). The Surprising Role of Endogenous Calcium Carbonate in Crab Shell-Mediated Biosorption of Pb (II). Physchem, 4(2), 167-180. https://doi.org/10.3390/physchem4020013









