Reactions of Graphene Nano-Flakes in Materials Chemistry and Astrophysics
Abstract
:1. Introduction
2. Interaction of Hydrogen Atoms with GNF
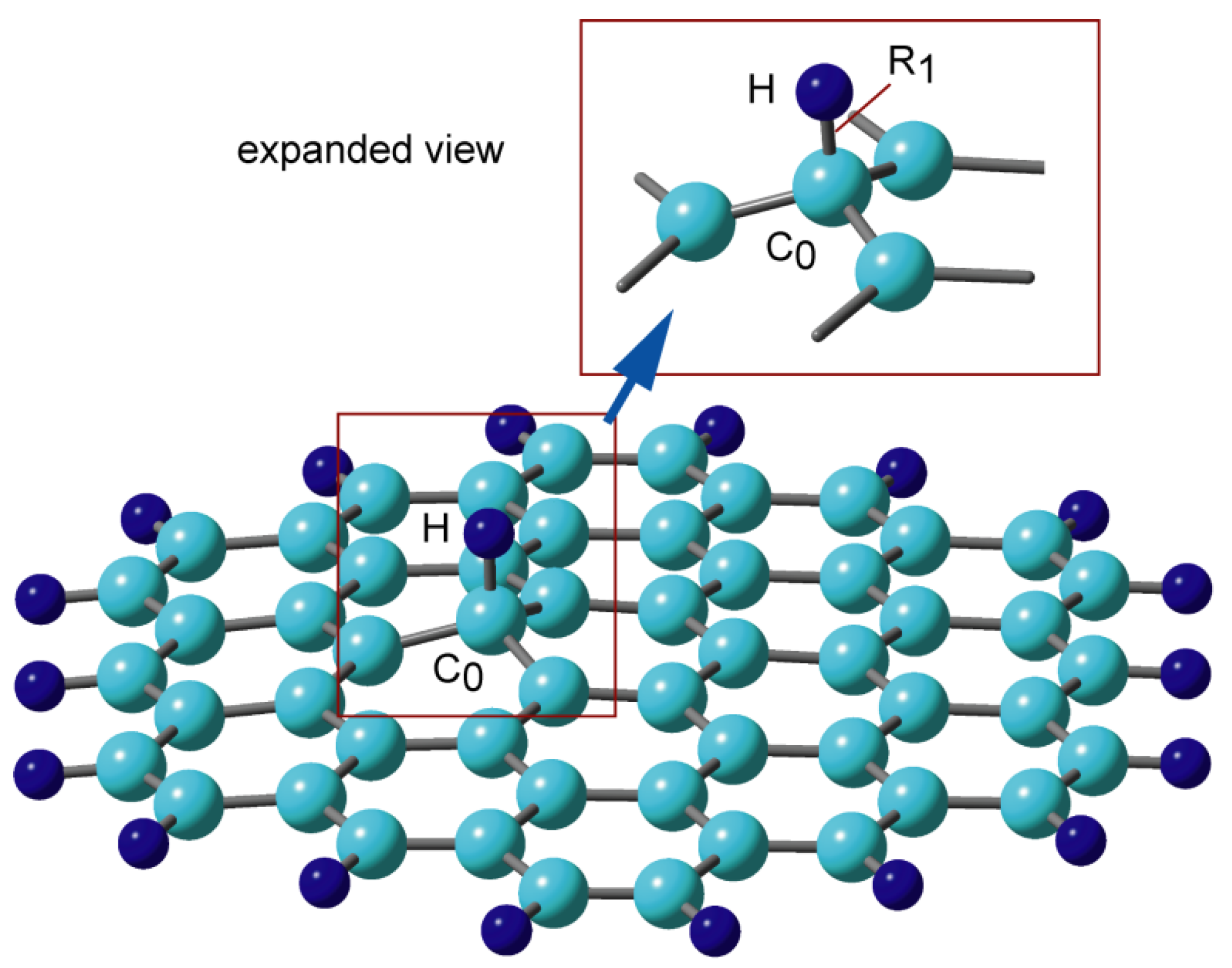
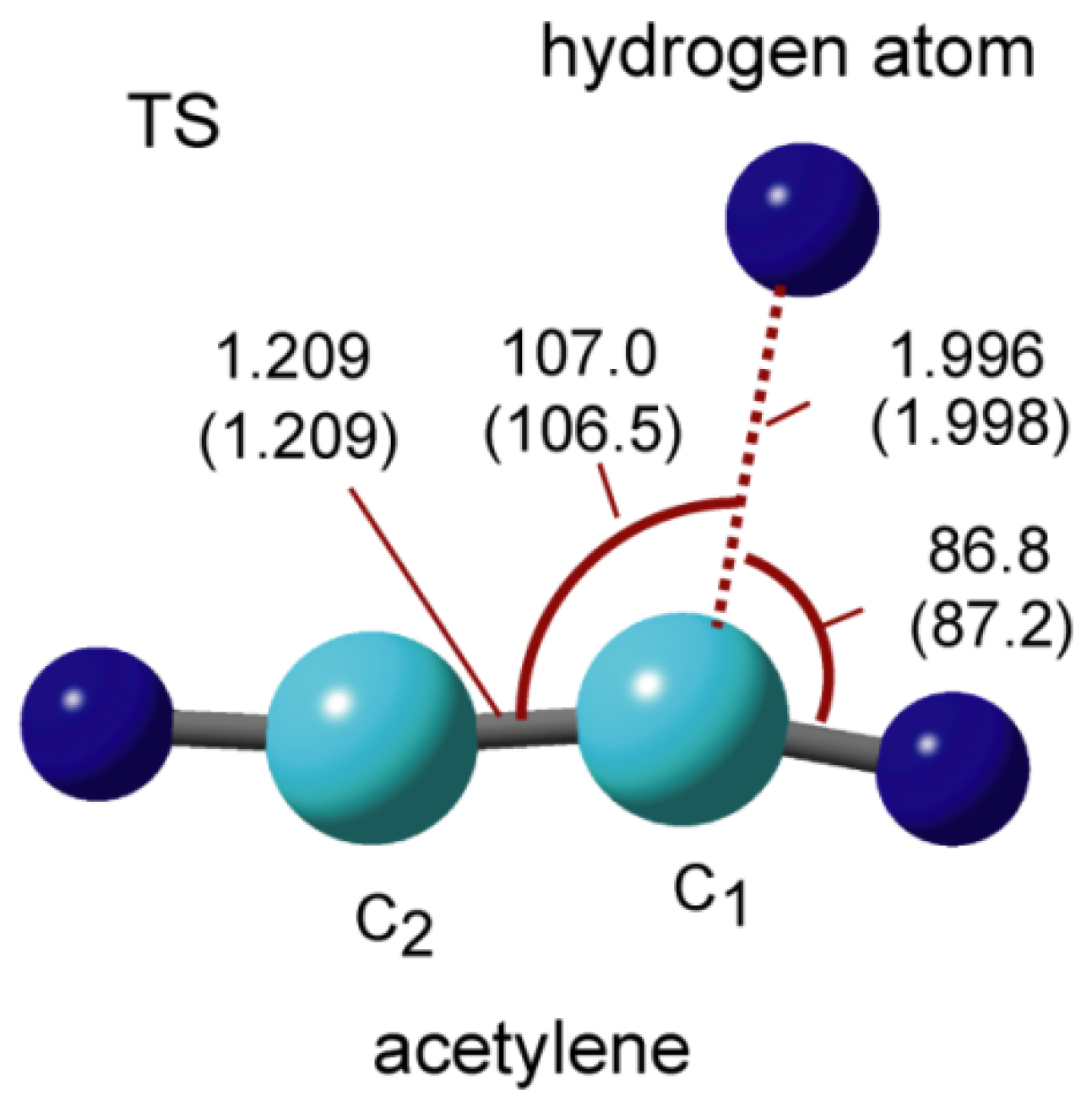
2.1. Bonding Structure of Hydrogen Atoms to GNFs
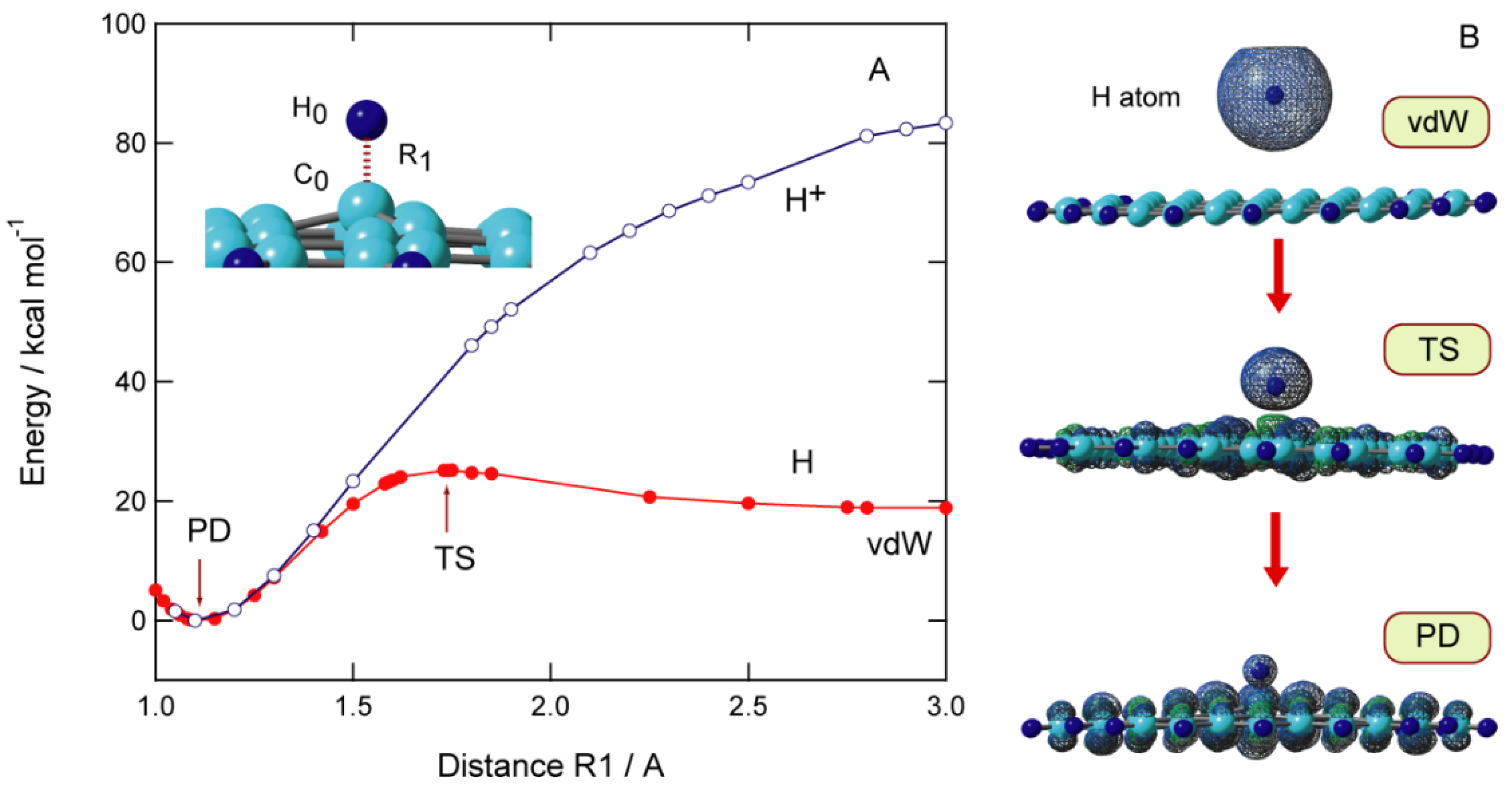
2.2. Potential Energy Curve
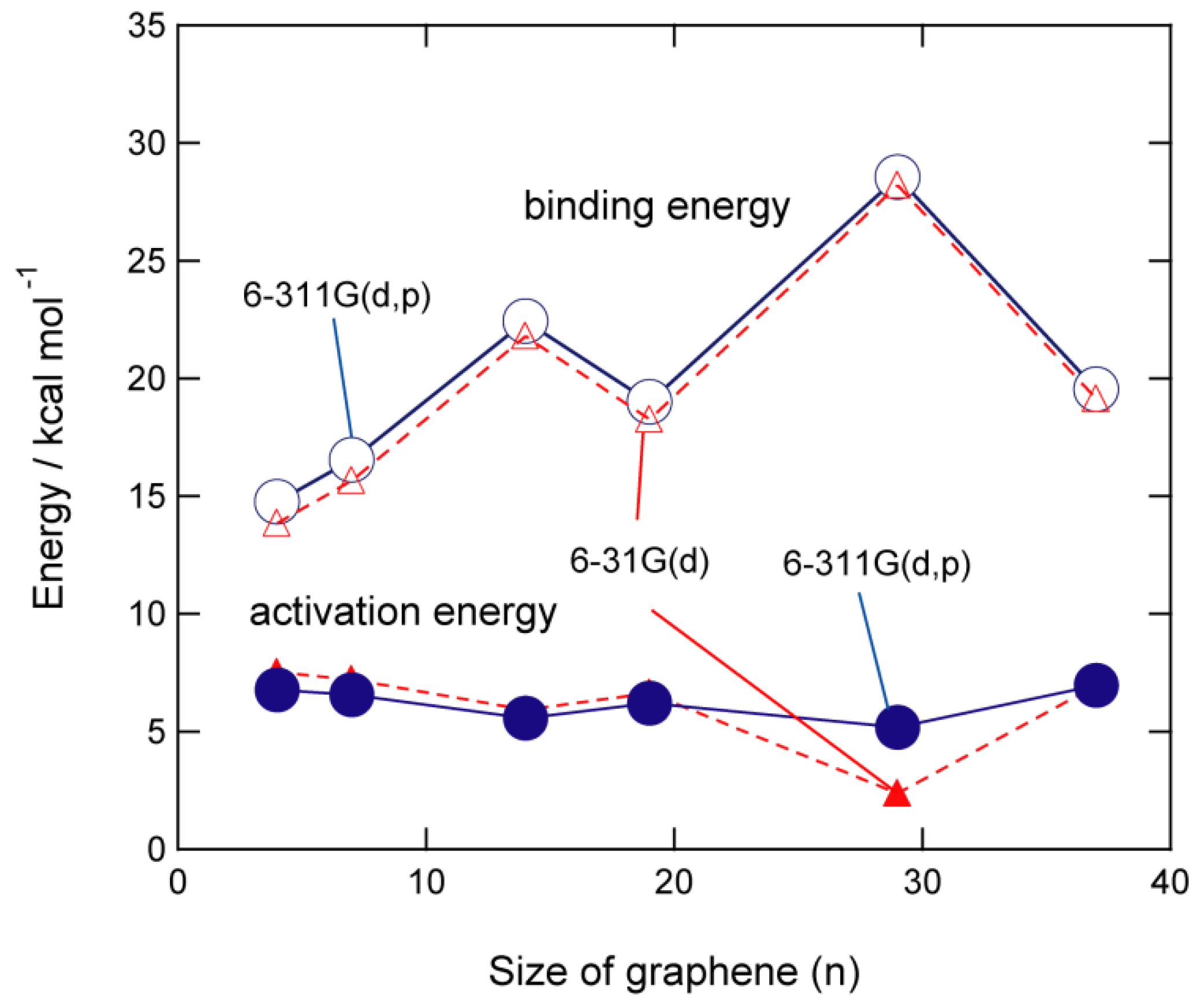
2.3. Activation and Binding Energies
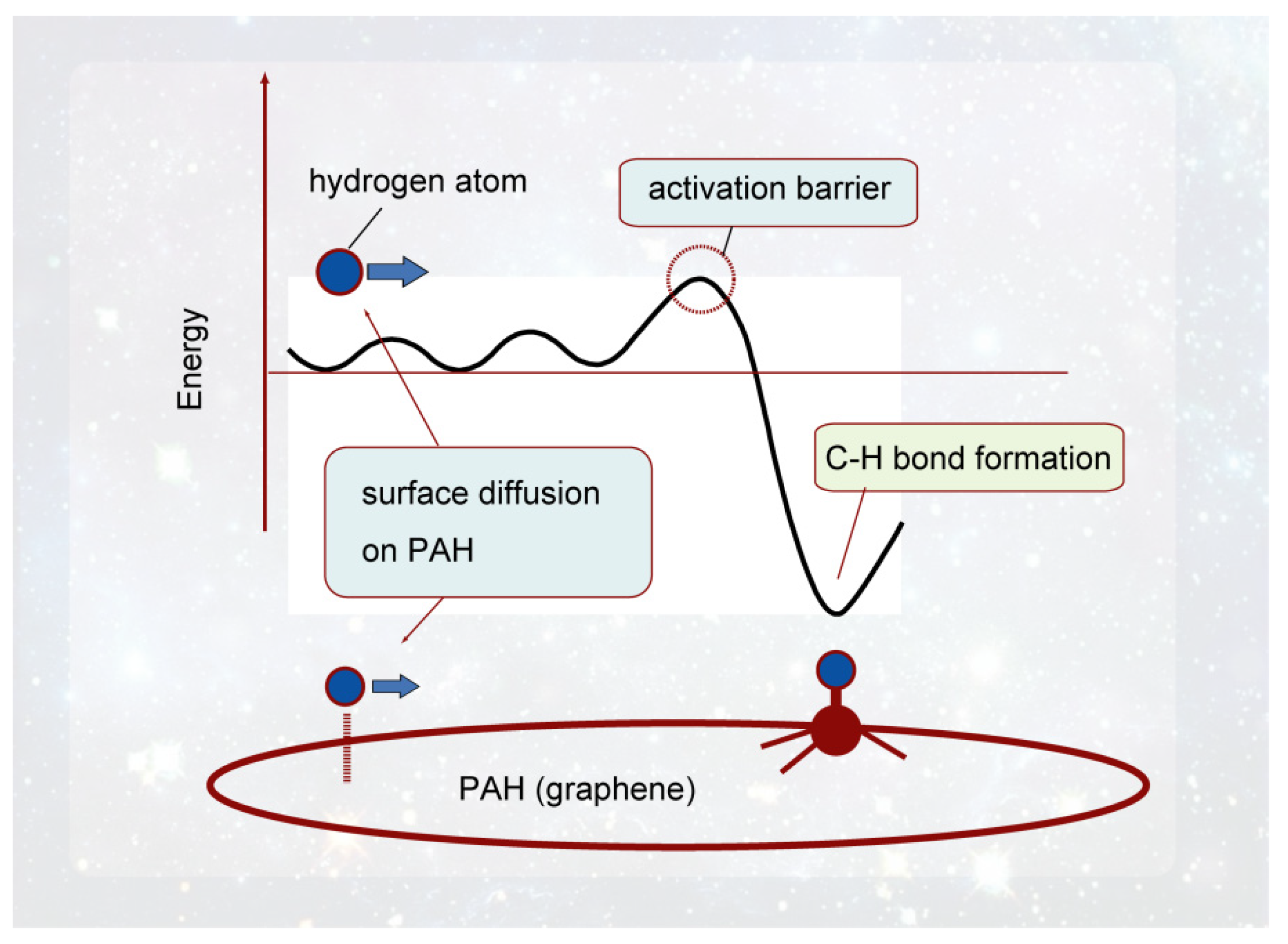
2.4. Behavior of Hydrogen Atoms on GNF (PAH) Surfaces
3. Interaction of GNFs with Methyl Radicals
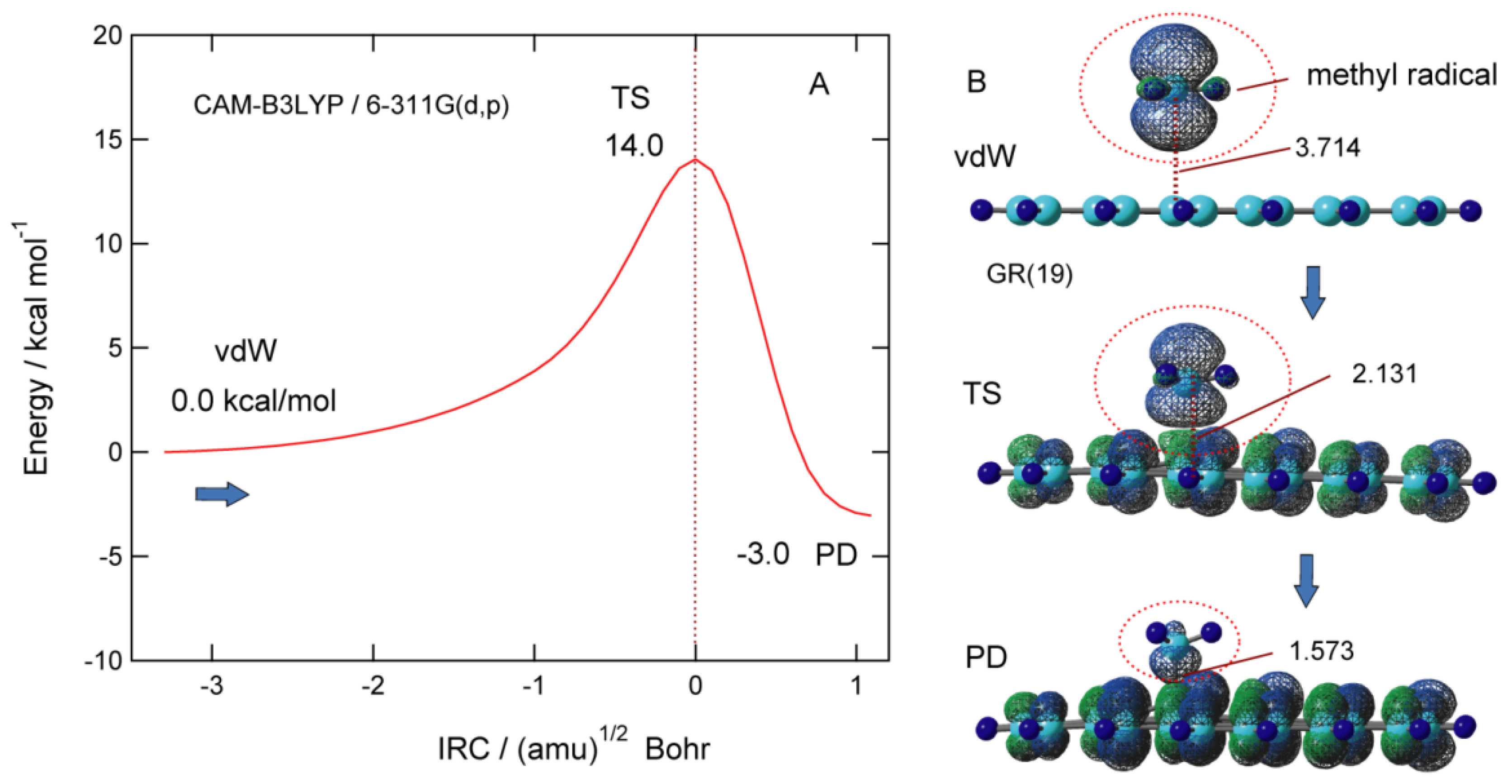
3.1. Binding Structure of Methyl Radical to GNF
3.2. Potential Energy Curve
3.3. Activation and Binding Energies
3.4. Behavior of Methyl Radicals on GNF Surface
3.5. Activation Energies of Alkyl Radicals Addition to GNF
4. Chemical Reaction on GNF Surface
5. Mechanism of Hydrogen Storage in the Graphene Nanoflake Doped by Alkali-Metals
5.1. Structures of the Li Doped-Graphene Nanoflake
5.2. Binding Structures of the Hydrogen Molecules to GNF-Li
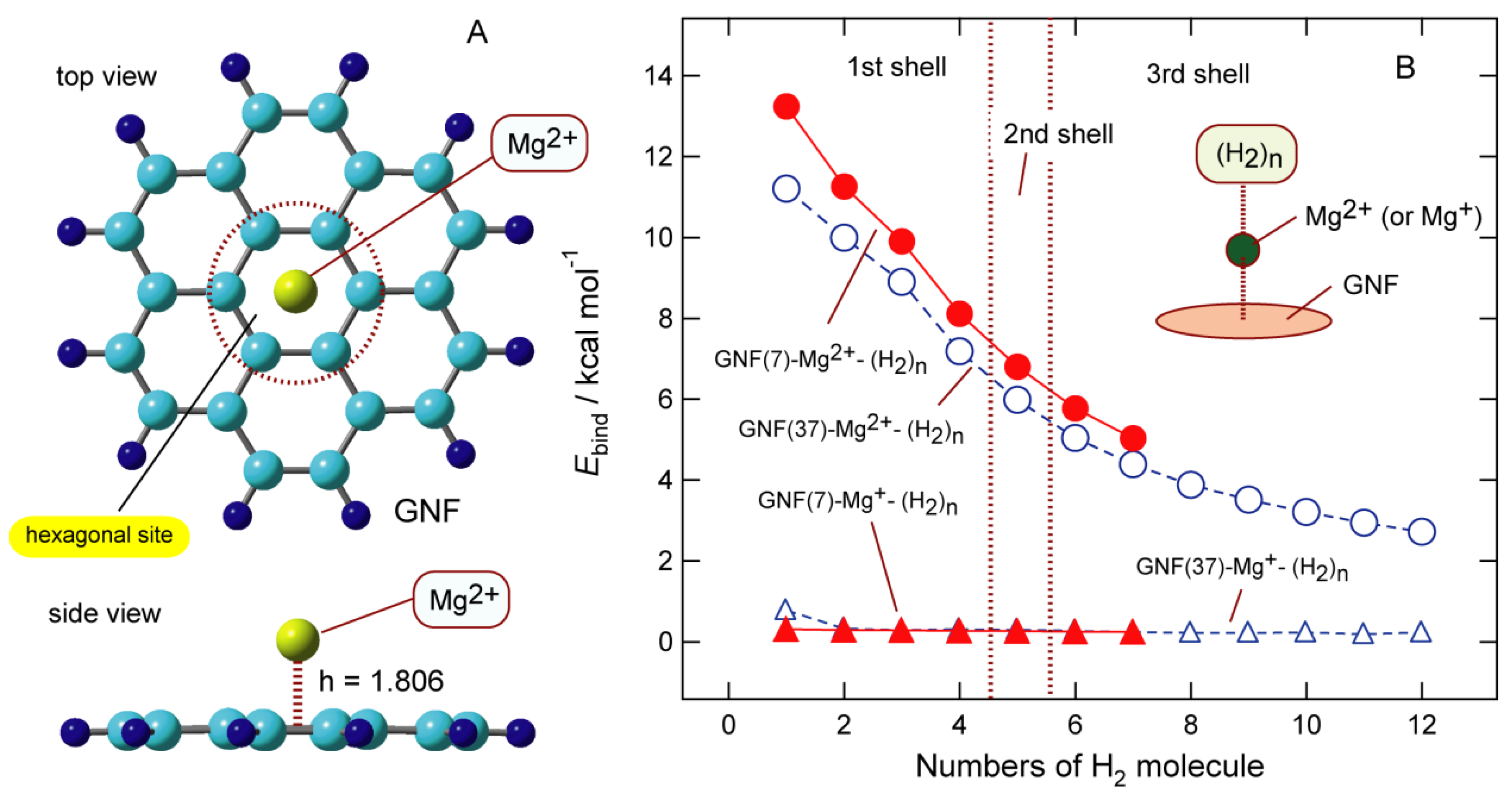
5.3. Binding Energy of H2 to GNF-Li
5.4. Binding Structures of H2 to GNF-Na
5.5. Binding Energies of H2 to GNF-Na
6. Molecular Design of Reversible Hydrogen Storage Device
6.1. Structures of Mg-Doped GNF
6.2. Binding Structures and Energies of H2 to GNF-Mgm+ (m = 1 and 2)
6.3. Electron Capture Dynamics of GNF-Mg2+-H2
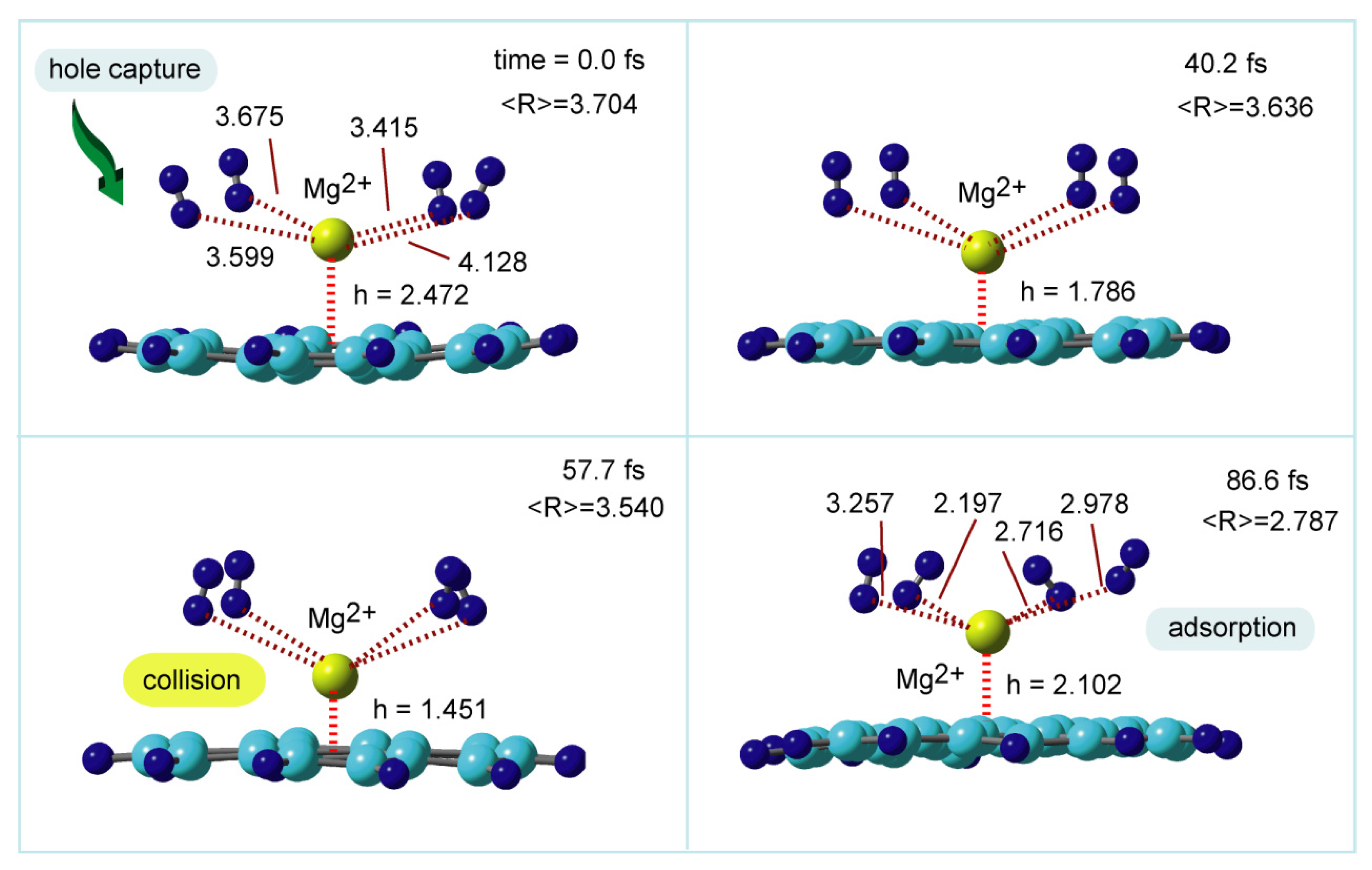
6.4. Hole Capture Dynamics of GNF-Mg+-H2
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, G. Chemical elements in the Universe: Origin and evolution. J. Astrophys. Astr. 2020, 41, 31. [Google Scholar] [CrossRef]
- Phua, Y.Y.; Sakakibara, N.; Ito, T.; Terashima, K. Low Temperature Plasma for Astrochemistry: Toward a Future Understanding with Continuous and Precise Temperature Control. Plasma Fusion Res. 2020, 15, 1506041. [Google Scholar] [CrossRef]
- Kauffman, S.; Jelenfi, D.P.; Vattay, G. Theory of chemical evolution of molecule compositions in the universe, in the Miller-Urey experiment and the mass distribution of interstellar and intergalactic molecules. J. Theor. Biol. 2020, 486, 110097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwek, E.; Arendt, R.G.; Fixsen, D.J.; Sodroski, T.J.; Odegard, N.; Weiland, J.L.; Reach, W.T.; Hauser, M.G.; Kelsall, T.; Moseley, S.H.; et al. Detection and Characterization of Cold Interstellar Dust and Polycyclic Aromatic hydrocarbon Emission, from COBE Observations. Astrophys. J. 1997, 475, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.I.; Onaka, K.; Sakon, I.; Ishihara, D.; Shimonishi, T.; Ohsawa, R.; Bell, A.C. Observational studies on the Near-infrared Unidentified Emission Bands in Galactic H II regions. Astrophys. J. 2014, 784, 53. [Google Scholar] [CrossRef] [Green Version]
- Vats, A.; Pathak, A.; Onaka, T.; Buragohain, M.; Sakon, I.; Endo, I. Theoretical study of infrared spectra of interstellar PAH molecules with N, NH, and NH2 incorporation. Publ. Astron. Soc. Jpn. 2022, 74, 161–174. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Zhang, C.; Chen, Y.; Zhen, J.; Qin, L. Gas-phase laboratory formation of large, astronomically relevant PAH-organic molecule clusters. Astron. Astrophys. 2021, 656, A80. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Hu, X.; Zhang, D.; Chen, Y.; Zhen, J.; Qin, L. Gas-phase laboratory study on PAH/amino-acid cluster cations. MNRAS 2021, 508, 3009–3022. [Google Scholar] [CrossRef]
- Qu, J.; Dai, X.-X.; Cui, J.-S.; Chen, R.-X.; Wang, X.; Lin, Y.-H.; Verduzco, R.; Wang, H.-L. Hierarchical polyaromatic hydrocarbons (PAH) with superior sodium storage properties. J. Mater. Chem. A 2021, 9, 16554–16564. [Google Scholar] [CrossRef]
- Hjertenaes, E.; Andersson, S.; Koch, H. Potential Energy Surfaces and Charge Transfer of PAH-Sodium-PAH Complexes. Chemphyschem 2016, 17, 2908–2915. [Google Scholar] [CrossRef]
- Munoz-Castro, A.; Gomez, T.; MacLeod Carey, D.; Miranda-Rojas, S.; Mendizabal, F.; Zagal, J.H.; Arratia-Perez, R. Surface on Surface. Survey of the Monolayer Gold-Graphene Interaction from Au12 and PAH via Relativistic DFT Calculations. J. Phys. Chem. C 2016, 120, 7358–7364. [Google Scholar] [CrossRef]
- Gloriozov, I.P.; Demyanov, P.I.; Zhulyaev, N.S.; Nechaev, M.S.; Oprunenko, Y.F.; Gam, F.; Saillard, J.-Y.; Kuznetsov, A.E. DFT Investigation of the eta(6) reversible arrow eta(6)-Inter-ring Haptotropic Rearrangement of the Group 8 Metals Complexes [(graphene)MCp](+) (M = Fe, Ru, Os). J. Phys. Chem. A 2021, 125, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, G.R. Atomic and Molecular Hydrogen in Interstellar Space. Space Sci. Rev. 1970, 10, 459–482. [Google Scholar] [CrossRef]
- Sancheza, M.; Ruette, F. Hydrogen atom chemisorption and diffusion on neutral and charged polycyclic aromatic hydrocarbon (PAH) flakes in the interstellar media. Chem. Phys. Lett. 2015, 640, 11–15. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H.-J.; Morokuma, K.; Irle, S. Coupled Cluster and Density Functional Theory Calculations of Atomic Hydrogen Chemisorption on Pyrene and Coronene as Model Systems for Graphene Hydrogenation. J. Phys. Chem. A 2012, 116, 7154–7160. [Google Scholar] [CrossRef]
- Beitollahi, A.; Sheikhaleslami, M.A.S. A novel approach for development of graphene structure in mesoporous carbon of high specific surface area. Carbon 2016, 107, 440–447. [Google Scholar] [CrossRef]
- Mutoh, M.; Abe, S.; Kusaka, T.; Nakamura, M.; Yoshida, Y.; Iida, J.; Hiroto Tachikawa, H. Density Functional Theory (DFT) Study on the Ternary Interaction System of the Fluorinated Ethylene Carbonate, Li+ and Graphene Model. Atoms 2016, 4, 4. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, H. Hydrogen atom addition to the surface of graphene nanoflakes: A density functional theory study. Appl. Surf. Sci. 2017, 396, 1335–1342. [Google Scholar] [CrossRef]
- Feuchtgruber, H.; Helmich, F.P.; van Dishoeck, E.F.; Wright, C.M. Detection of Interstellar CH3. Astrophys. J. 2000, 535, L111–L114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knez, C.; Lacy, J.H.; Evans, N.J., II; van Dishoeck, E.F.; Richter, M.J. High-Resolution Mid-Infrared Spectroscopy of NGC 7538 IRS 1: Probing Chemistry in a Massive Young Stellar Object. Astrophys. J. 2009, 696, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, H. Methyl radical addition to the surface of graphene nanoflakes: A density functional theory study. Surf. Sci. 2019, 679, 196–201. [Google Scholar] [CrossRef]
- Tachikawa, H.; Iyama, T. Mechanism of Hydrogen Storage in the Graphene Nanoflake-Lithium-H2 System. J. Phys. Chem. C 2019, 123, 8709–8716. [Google Scholar] [CrossRef]
- Umadevi, D.; Sastry, G.N. Molecular and Ionic Interaction with Graphene Nanoflakes: A Computational Investigation of CO2, H2O, Li, Mg, Li+, and Mg2+ Interaction with Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. C 2011, 115, 9656–9667. [Google Scholar] [CrossRef]
- Tachikawa, H.; Yi, H.; Iyama, T.; Yamasaki, S.; Azumi, K. Hydrogen Storage Mechanism in Sodium-Based Graphene Nanoflakes: A Density Functional Theory Study. Hydrogen 2022, 3, 43–52. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, W.; Chen, L.; Zhang, X.; Mei, S. Equilibrium Model of a Regional Hydrogen Market with Renewable Energy Based Suppliers and Transportation Costs. Energy 2021, 220, 119608. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Jenn, A.; Fulton, L. Low Carbon Scenario Analysis of a Hydrogen-Based Energy Transition for On-Road Transportation in California. Energies 2021, 14, 7163. [Google Scholar] [CrossRef]
- Lobo, R.; Alvarez, N.; Shanov, V. Hydrogen Nanometrology in Advanced Carbon Nanomaterial Electrodes. Nanomaterials 2021, 11, 1079. [Google Scholar] [CrossRef]
- Lobo, R.; Ribeiro, J.H.F.; Inok, F. Hydrogen Uptake and Release in Carbon Nanotube Electrocatalysts. Nanomaterials 2021, 11, 975. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, X.; Liu, Y.; Zhang, L.; Hu, J.; Gao, M.; Pan, H. A Unique Double-Layered Carbon Nanobowl-Confined Lithium Borohydride for Highly Reversible Hydrogen Storage. Small 2020, 16, 202001963. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Tam, J.; Singh, C.V. A First Principles Study of Hydrogen Storage on Lithium Decorated Two Dimensional Carbon Allotropes. Int. J. Hydrogen Energy 2015, 40, 6128–6136. [Google Scholar] [CrossRef]
- Tachikawa, H.; Izumi, Y.; Iyama, T.; Azumi, K. Molecular Design of a Reversible Hydrogen Storage Device Composed of the Graphene Nanoflake-Magnesium-H2 System. ACS Omega 2021, 6, 7778–7785. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, H. Reaction mechanism of an intracluster SN2 reaction induced by electron capture. Phys. Chem. Chem. Phys. 2022, 24, 3941–3950. [Google Scholar] [CrossRef]
- Tachikawa, H. Reactions of Photoionization-Induced CO-H2O Cluster: Direct Ab Initio Molecular Dynamics Study. ACS Omega 2021, 6, 16688–16695. [Google Scholar] [CrossRef]
- Tachikawa, H. Formation of Hydrogen Peroxide from O-(H2O)n Clusters. J. Phys. Chem. A 2021, 125, 4598–4605. [Google Scholar] [CrossRef]








| Radical | E(TS) | E(PD) | E(vdW) |
|---|---|---|---|
| CH3 | 13.8 | −3.6 | −0.3 |
| Et | 14.7 | 1.1 | −0.6 |
| n-Pr | 15.2 | 1.3 | −0.2 |
| iso-Pr | 17.6 | 8.1 | −0.8 |
| n-Bu | 15.2 | 1.4 | −0.2 |
| sec-Bu | 18.7 | 9.7 | −0.4 |
| tert-Bu | 22.1 | 16.6 | −0.9 |
| iso-Bu | 20.1 | 9.0 | −0.7 |
| F | 0.0 | −29.4 | -- |
| Cl | 0.0 | −6.5 | -- |
| State | Vacuo | on GNF |
|---|---|---|
| RC (H + HCCH) | 0.0 | 0.0 (1.3) |
| vdW(1) | -- | −1.3 (0.0) |
| TS | 1.3 | 0.2 (1.5) |
| vdW(2) | -- | −48.7 (−47.4) |
| PD (H2CCH) | −47.6 | −47.6 (−46.3) |
| Ebind | Height (h) | NPA | |
|---|---|---|---|
| GNF-Li | 17.1 | 1.736 | +0.929 |
| GNF-Li+ | 52.8 | 1.771 | +0.937 |
| GNF-Na | 4.4 | 2.247 | +0.978 |
| GNF-Na+ | 37.5 | 2.288 | +0.979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachikawa, H.; Iyama, T. Reactions of Graphene Nano-Flakes in Materials Chemistry and Astrophysics. Physchem 2022, 2, 145-162. https://doi.org/10.3390/physchem2020011
Tachikawa H, Iyama T. Reactions of Graphene Nano-Flakes in Materials Chemistry and Astrophysics. Physchem. 2022; 2(2):145-162. https://doi.org/10.3390/physchem2020011
Chicago/Turabian StyleTachikawa, Hiroto, and Tetsuji Iyama. 2022. "Reactions of Graphene Nano-Flakes in Materials Chemistry and Astrophysics" Physchem 2, no. 2: 145-162. https://doi.org/10.3390/physchem2020011
APA StyleTachikawa, H., & Iyama, T. (2022). Reactions of Graphene Nano-Flakes in Materials Chemistry and Astrophysics. Physchem, 2(2), 145-162. https://doi.org/10.3390/physchem2020011






