A Comparison between the Lower Critical Solution Temperature Behavior of Polymers and Biomacromolecules
Abstract
:1. Introduction
2. Computational Materials and Methods
2.1. Force Field
2.2. Initial Structure Generation
2.3. MD Simulation Details
2.4. Analysis Methods
3. Results and Discussion
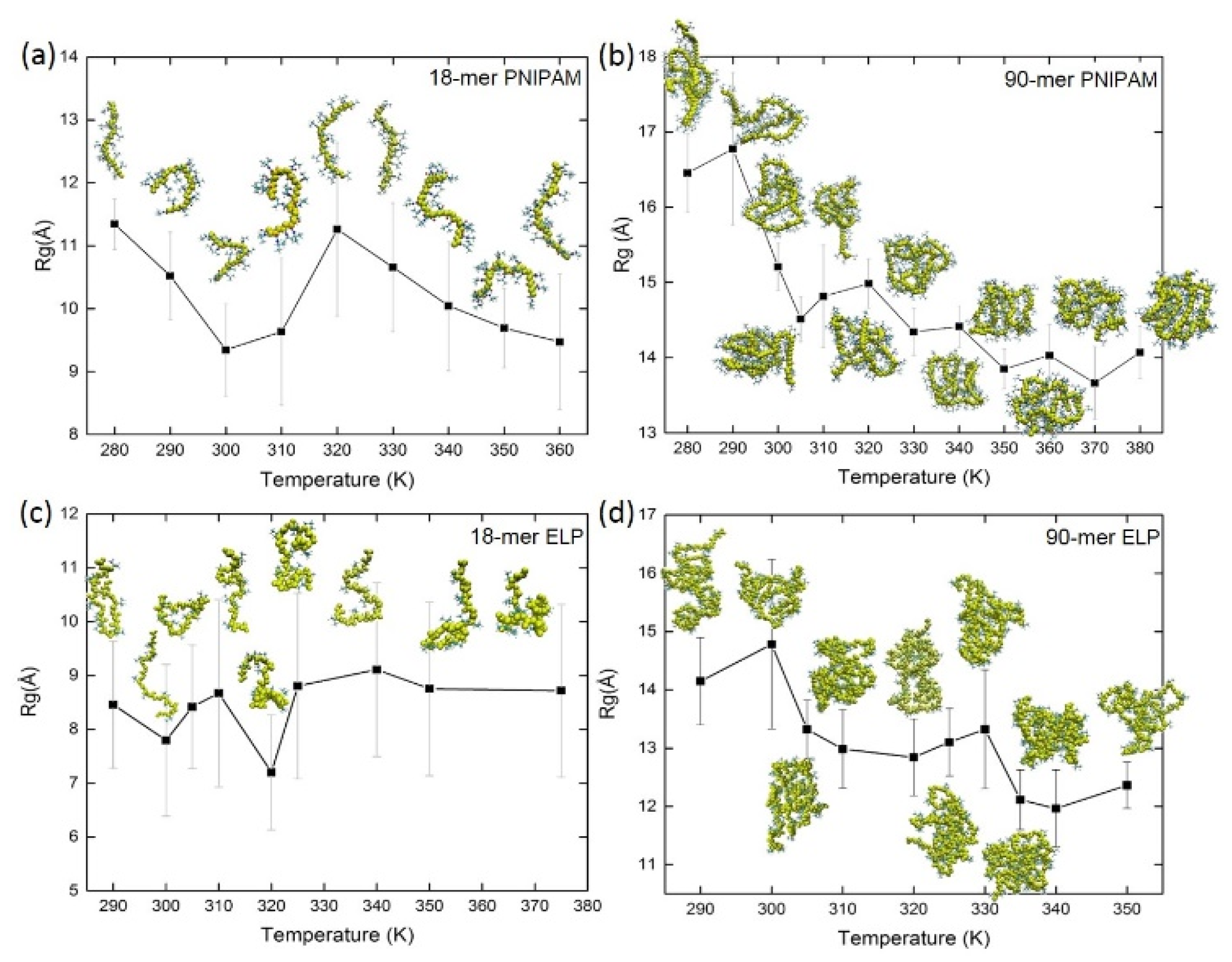
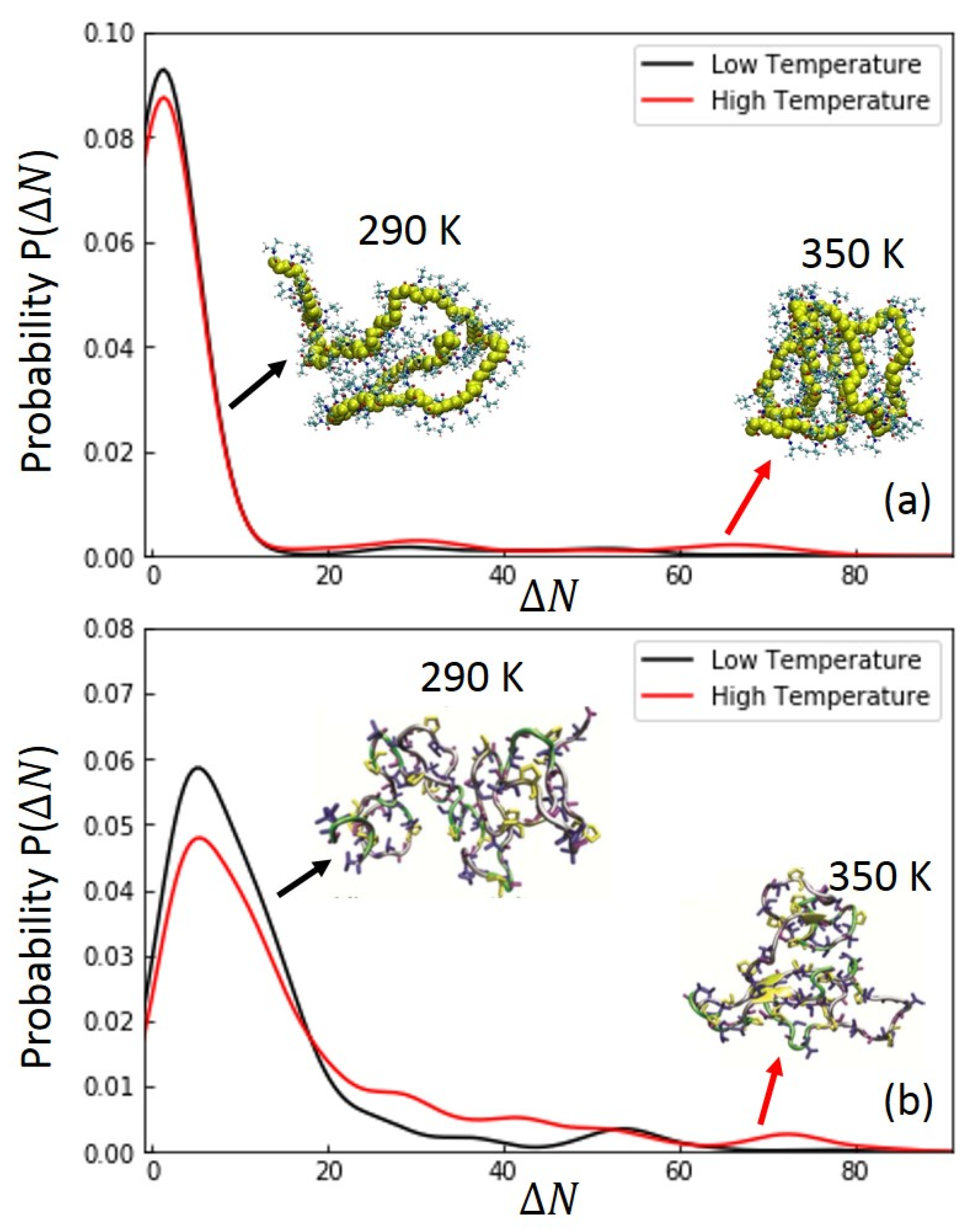
3.1. Temperature Dependence of Radius of Gyration (Rg) of Macromolecules
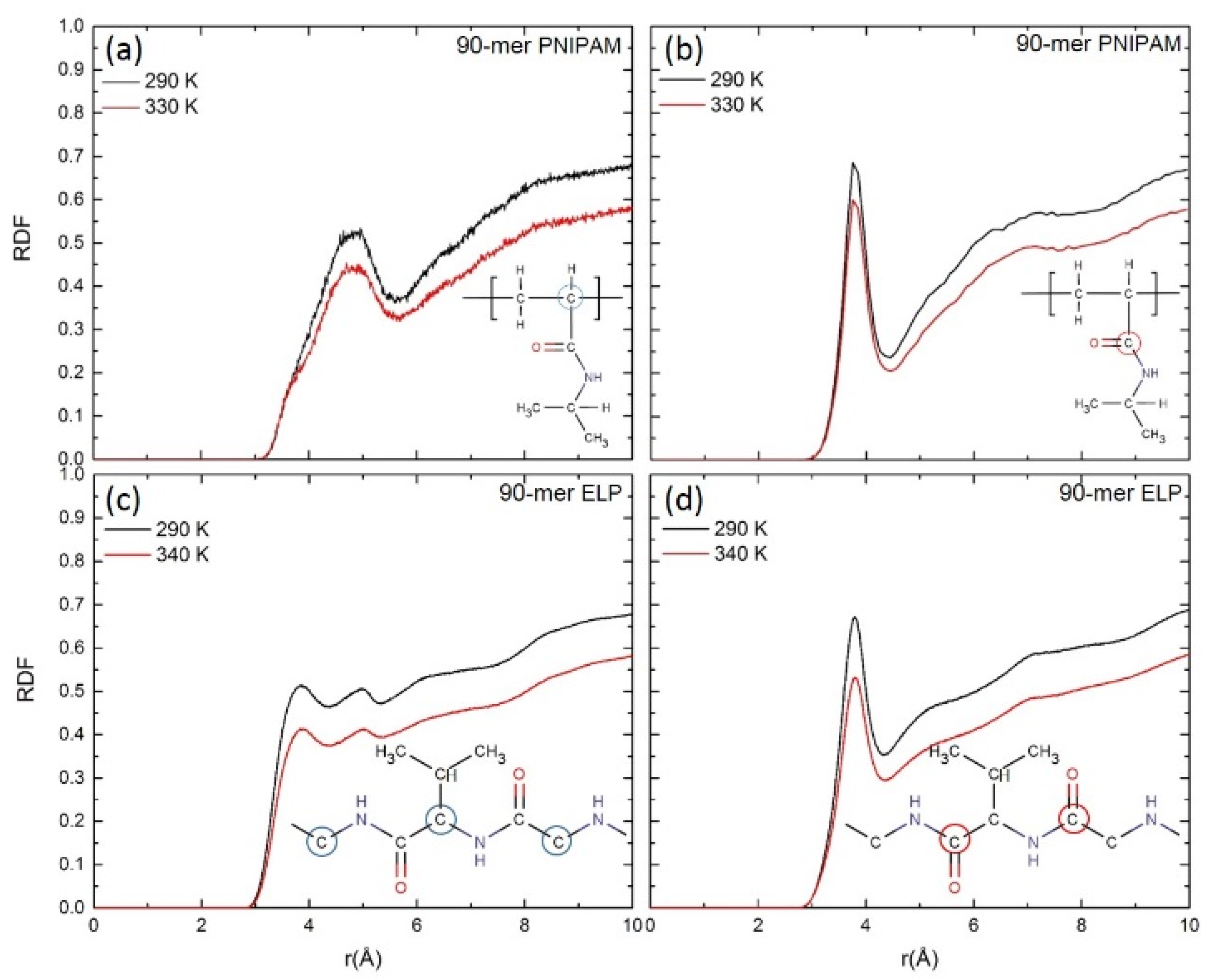
3.2. Solvation Shell
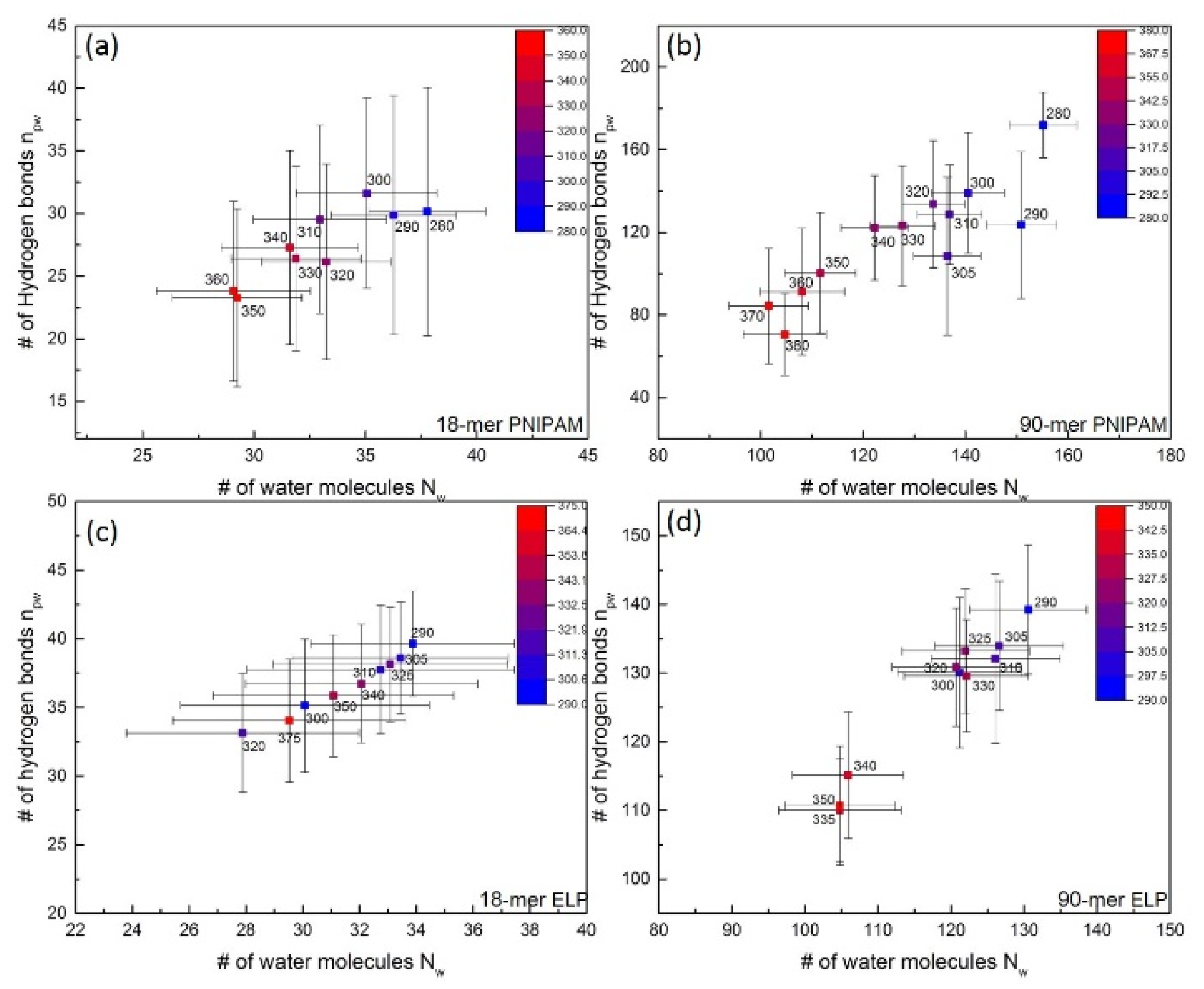
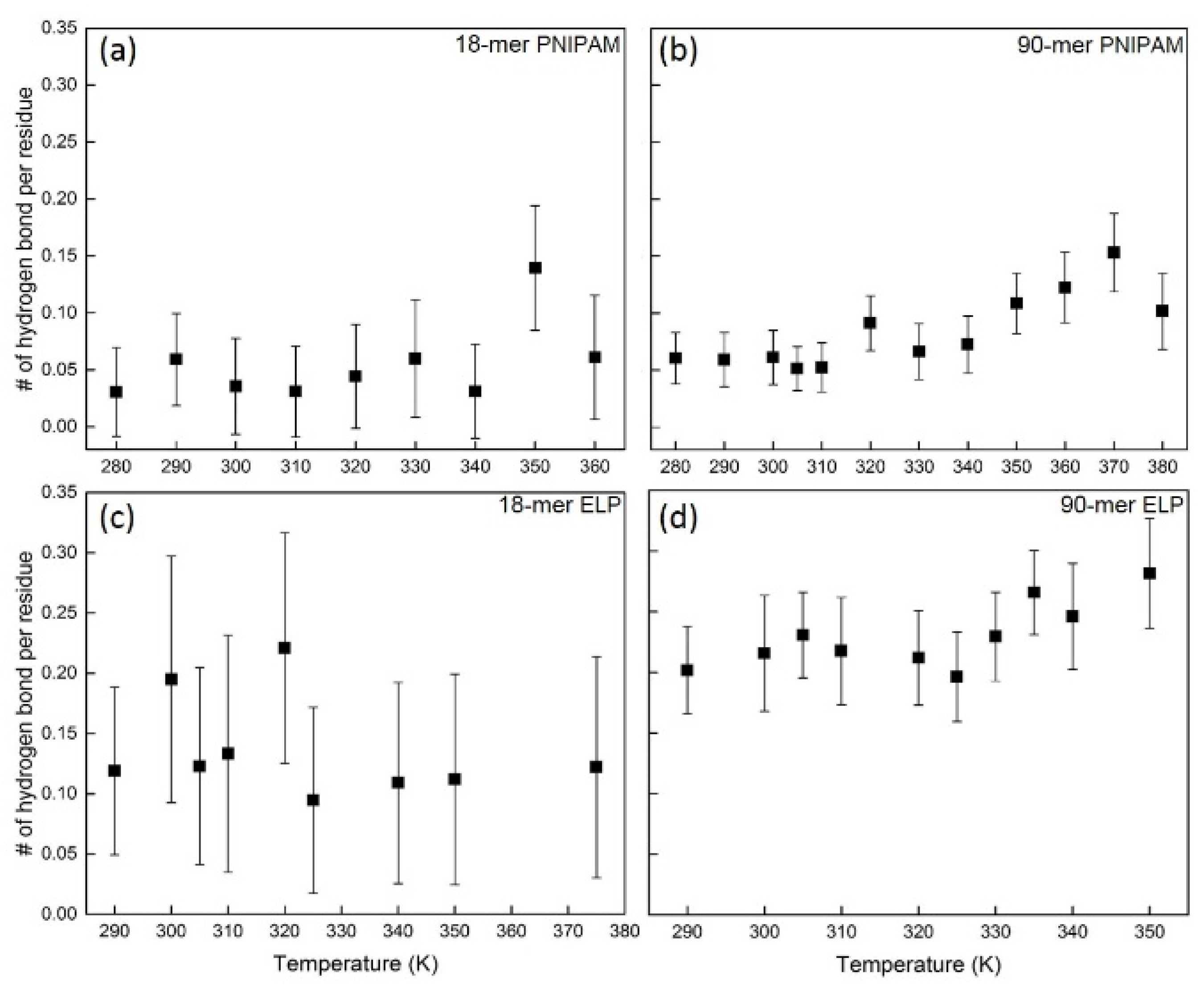
3.2.1. Analysis of Hydrogen Bond
3.2.2. Water Distribution
3.2.3. Solvent Accessible Surface Area (SASA)
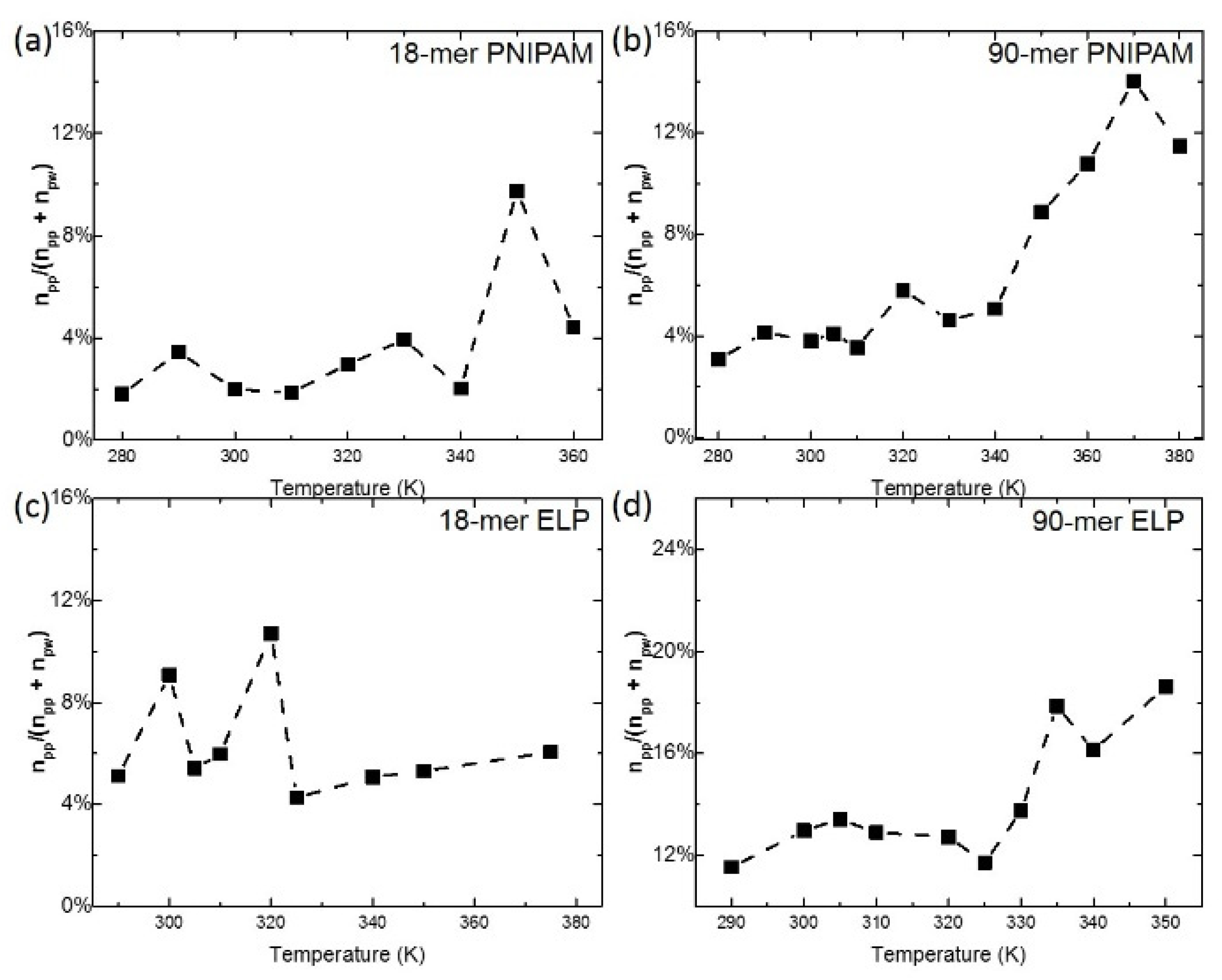
3.3. Analysis of Hydrogen Bonds between Polymer and Polymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.; Shen, L.; Wu, C. LLS and FTIR Studies on the Hysteresis in Association and Dissociation of Poly(N-isopropylacrylamide) Chains in Water. Macromolecules 2006, 39, 2325–2329. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C. Light-Scattering Study of Coil-to-Globule Transition of a Poly(N-isopropylacrylamide) Chain in Deuterated Water. Macromolecules 1999, 32, 4299–4301. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X. Globule-to-Coil Transition of a Single Homopolymer Chain in Solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef] [Green Version]
- Schild, H.G. Poly (N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Urry, D.W. Physical Chemistry of Biological Free Energy Transduction As Demonstrated by Elastic Protein-Based Polymers. J. Phys. Chem. B 1997, 101, 11007–11028. [Google Scholar] [CrossRef]
- Urry, D.W. Elastic Biomolecular Machines. Sci. Am. 1995, 272, 64–69. [Google Scholar] [CrossRef]
- Martino, M.; Perri, T.; Tamburro, A.M. Biopolymers and biomaterials based on elastomeric proteins. Macromol. Biosci. 2002, 2, 319–328. [Google Scholar] [CrossRef]
- Urry, D.W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 1992, 57, 23–57. [Google Scholar] [CrossRef]
- Urry, D.W.; Trapane, T.L.; Prasad, K.U. Phase-structure transitions of the elastin polypentapeptide-water system within the framework of composition-temperature studies. Biopolymers 1985, 24, 2345–2356. [Google Scholar] [CrossRef]
- Urry, D.W.; Luan, C.H.; Parker, T.M.; Gowda, D.C.; Prasad, K.U.; Reid, M.C.; Safavy, A. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J. Am. Chem. Soc. 1991, 113, 4346–4348. [Google Scholar] [CrossRef]
- Meyer, D.E.; Chilkoti, A. Genetically Encoded Synthesis of Protein-Based Polymers with Precisely Specified Molecular Weight and Sequence by Recursive Directional Ligation: Examples from the Elastin-like Polypeptide System. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, B.; Pepe, A.; Crudele, M.; Belloy, N.; Baud, S.; Dauchez, M. Tuning self-assembly in elastin-derived peptides. Soft Matter 2015, 11, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, C.; Ravindra, R.; Ludolph, B.; Winter, R. Characterization of the temperature-and pressure-induced inverse and reentrant transition of the minimum elastin-like polypeptide GVG (VPGVG) by DSC, PPC, CD, and FT-IR spectroscopy. Biophys. J. 2004, 86, 1385–1392. [Google Scholar] [CrossRef]
- Ma, X.; Sun, C.; Huang, J.; Boutis, G.S. Thermal Hysteresis in the Backbone and Side-Chain Dynamics of the Elastin Mimetic Peptide [VPGVG]3 Revealed by 2H NMR. J. Phys. Chem. B 2011, 116, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosalie, L.M.; áde Wolf, F.A.; ávan Hest, J.C.M. Elastin-like polypeptides of different molecular weights show independent transition temperatures when mixed. Soft Matter 2009, 5, 4305–4310. [Google Scholar]
- Nuhn, H.; Klok, H.-A. Secondary Structure Formation and LCST Behavior of Short Elastin-Like Peptides. Biomacromolecules 2008, 9, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkoti, A. Quantification of the Effects of Chain Length and Concentration on the Thermal Behavior of Elastin-like Polypeptides. Biomacromolecules 2004, 5, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. On the molecular mechanisms of elastin coacervation and coacervate calcification. Faraday Discuss. Chem. Soc. 1976, 61, 205–212. [Google Scholar] [CrossRef]
- Urry, D.; Long, M.; Cox, B.; Ohnishi, T.; Mitchell, L.; Jacobs, M. The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates. Biochim. Biophys. Acta (BBA)—Protein Struct. 1974, 371, 597–602. [Google Scholar] [CrossRef]
- Luan, C.H.; Urry, D.W. Solvent deuteration enhancement of hydrophobicity: DSC study of the inverse temperature transition of elastin-based polypeptides. J. Phys. Chem. 1991, 95, 7896–7900. [Google Scholar] [CrossRef]
- Pepe, A.; Armenante, M.R.; Bochicchio, B.; Tamburro, A.M. Formation of nanostructures by self-assembly of an elastin peptide. Soft Matter 2008, 5, 104–113. [Google Scholar] [CrossRef]
- Tarakanova, A.; Huang, W.; Weiss, A.; Kaplan, D.L.; Buehler, M.J. Computational smart polymer design based on elastin protein mutability. Biomaterials 2017, 127, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Li, N.; Yingling, Y.; Hall, C.K. LCST Behavior is Manifested in a Single Molecule: Elastin-Like polypeptide (VPGVG)n. Biomacromolecules 2015, 17, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, F.G.; Li, N.K.; Roberts, S.; Weber, P.; Dzuricky, M.; Weitzhandler, I.; Yingling, Y.G.; Chilkoti, A. Intrinsically disordered proteins access a range of hysteretic phase separation behaviors. Sci. Adv. 2019, 5, eaax5177. [Google Scholar] [CrossRef] [Green Version]
- Li, N.K.; Xie, Y.; Yingling, Y.G. Insights into Structure and Aggregation Behavior of Elastin-like Polypeptide Coacervates: All-Atom Molecular Dynamics Simulations. J. Phys. Chem. B 2021, 125, 8627–8635. [Google Scholar] [CrossRef] [PubMed]
- Li, N.K.; Roberts, S.; Quiroz, F.G.; Chilkoti, A.; Yingling, Y.G. Sequence Directionality Dramatically Affects LCST Behavior of Elastin-Like Polypeptides. Biomacromolecules 2018, 19, 2496–2505. [Google Scholar] [CrossRef]
- Li, N.; Quiroz, F.G.; Hall, C.; Chilkoti, A.; Yingling, Y.G. Molecular Description of the LCST Behavior of an Elastin-Like Polypeptide. Biomacromolecules 2014, 15, 3522–3530. [Google Scholar] [CrossRef]
- Grabowski, C.A.; Mukhopadhyay, A. Contraction and Reswelling of a Polymer Chain Near the Critical Point of a Binary Liquid Mixture. Phys. Rev. Lett. 2007, 98, 207801. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, A.X.; Wu, C. Comparison of the Coil-to-Globule and the Globule-to-Coil Transitions of a Single Poly(N-isopropylacrylamide) Homopolymer Chain in Water. Macromolecules 1998, 31, 2972–2976. [Google Scholar] [CrossRef]
- Furyk, S.; Zhang, Y.; Ortiz-Acosta, D.; Cremer, P.S.; Bergbreiter, D.E. Effects of end group polarity and molecular weight on the lower critical solution temperature of poly(N-isopropylacrylamide). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1492–1501. [Google Scholar] [CrossRef]
- Tong, Z.; Zeng, F.; Zheng, X.; Sato, T. Inverse Molecular Weight Dependence of Cloud Points for Aqueous Poly(N-isopropylacrylamide) Solutions. Macromolecules 1999, 32, 4488–4490. [Google Scholar] [CrossRef]
- Pamies, R.; Zhu, K.; Kjøniksen, A.-L.; Nyström, B. Thermal response of low molecular weight poly-(N-isopropylacrylamide) polymers in aqueous solution. Polym. Bull. 2009, 62, 487–502. [Google Scholar] [CrossRef]
- Du, H.; Wickramasinghe, R.; Qian, X. Effects of Salt on the Lower Critical Solution Temperature of Poly (N-Isopropylacrylamide). J. Phys. Chem. B 2010, 114, 16594–16604. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Yang, H.; Ma, J.; Cheng, R. Solvation Behaviors of N-Isopropylacrylamide in Water/Methanol Mixtures Revealed by Molecular Dynamics Simulations. J. Phys. Chem. B 2010, 114, 8652–8658. [Google Scholar] [CrossRef]
- Ortiz de Solorzano, I.; Bejagam, K.K.; An, Y.; Singh, S.K.; Deshmukh, S.A. Solvation dynamics of N-substituted acrylamide polymers and the importance for phase transition behavior. Soft Matter 2020, 16, 1582–1593. [Google Scholar] [CrossRef]
- Bejagam, K.K.; Singh, S.K.; Ahn, R.; Deshmukh, S.A. Unraveling the Conformations of Backbone and Side Chains in Thermosensitive Bottlebrush Polymers. Macromolecules 2019, 52, 9398–9408. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Suthar, K.; Mancini, D.C. Role of Solvation Dynamics and Local Ordering of Water in Inducing Conformational Transitions in Poly(N-isopropylacrylamide) Oligomers through the LCST. J. Phys. Chem. B 2012, 116, 2651–2663. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Mancini, D.C. Vibrational Spectra of Proximal Water in a Thermo-Sensitive Polymer Undergoing Conformational Transition Across the Lower Critical Solution Temperature. J. Phys. Chem. B 2012, 116, 5501–5515. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Li, Z.; Kamath, G.; Suthar, K.J.; Sankaranarayanan, S.K.; Mancini, D.C. Atomistic insights into solvation dynamics and conformational transformation in thermo-sensitive and non-thermo-sensitive oligomers. Polymer 2013, 54, 210–222. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Kamath, G.; Suthar, K.J.; Mancini, D.C.; Sankaranarayanan, S.K.R.S. Non-equilibrium effects evidenced by vibrational spectra during the coil-to-globule transition in poly(N-isopropylacrylamide) subjected to an ultrafast heating–cooling cycle. Soft Matter 2013, 10, 1462–1480. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.; Mancini, D.C. Atomic scale characterization of the conformational dynamics of a thermo-sensitive and a non-thermo-sensitive oligomer using vibrational spectra obtained from molecular dynamics. Polymer 2012, 53, 1306–1320. [Google Scholar] [CrossRef]
- Maeda, Y.; Higuchi, T.; Ikeda, I. Change in Hydration State during the Coil−Globule Transition of Aqueous Solutions of Poly(N-isopropylacrylamide) as Evidenced by FTIR Spectroscopy. Langmuir 2000, 16, 7503–7509. [Google Scholar] [CrossRef]
- Yamauchi, H.; Maeda, Y. LCST and UCST Behavior of Poly(N-isopropylacrylamide) in DMSO/Water Mixed Solvents Studied by IR and Micro-Raman Spectroscopy. J. Phys. Chem. B 2007, 111, 12964–12968. [Google Scholar] [CrossRef] [PubMed]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Changes in the Hydration States of Poly(N-alkylacrylamide)s during Their Phase Transitions in Water Observed by FTIR Spectroscopy. Macromolecules 2001, 34, 1391–1399. [Google Scholar] [CrossRef]
- Lue, S.J.; Chen, C.-H.; Shih, C.-M. Tuning of Lower Critical Solution Temperature (LCST) of Poly(N-Isopropylacrylamide-co-Acrylic acid) Hydrogels. J. Macromol. Sci. Part B 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Prog. Polym. Sci. 2002, 27, 1165–1193. [Google Scholar] [CrossRef]
- Ohya, S.; Nakayama, Y.; Matsuda, T. Thermoresponsive Artificial Extracellular Matrix for Tissue Engineering: Hyaluronic Acid Bioconjugated with Poly(N-isopropylacrylamide) Grafts. Biomacromolecules 2001, 2, 856–863. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Ahmed, Z.; Gooding, E.A.; Pimenov, K.V.; Wang, L.; Asher, S.A. UV Resonance Raman Determination of Molecular Mechanism of Poly(N-isopropylacrylamide) Volume Phase Transition. J. Phys. Chem. B 2009, 113, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Gangemi, F.; Longhi, G.; Abbate, S.; Lebon, F.; Cordone, R.; Ghilardi, G.P.; Fornili, S.L. Molecular Dynamics Simulation of Aqueous Solutions of 26-Unit Segments of p(NIPAAm) and of p(NIPAAm) “Doped” with Amino Acid Based Comonomers. J. Phys. Chem. B 2008, 112, 11896–11906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Qian, X. Molecular dynamics simulations of PNIPAM-co -PEGMA copolymer hydrophilic to hydrophobic transition in NaCl solution. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1112–1122. [Google Scholar] [CrossRef]
- de Oliveira, T.E.; Mukherji, D.; Kremer, K.; Netz, P.A. Effects of stereochemistry and copolymerization on the LCST of PNIP. Am. J. Chem. Phys. 2017, 146, 034904. [Google Scholar] [CrossRef]
- Singh, R.; Deshmukh, S.A.; Kamath, G.; Sankaranarayanan, S.K.R.S.; Balasubramanian, G. Controlling the aqueous solubility of PNIPAM with hydrophobic molecular units. Comput. Mater. Sci. 2017, 126, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Meyer, D.; Shin, B.; Kong, G.; Dewhirst, M.; Chilkoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef]
- Quiroz, F.G.; Chilkoti, A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 2015, 14, 1164–1171. [Google Scholar] [CrossRef]
- Urry, D.; Khaled, M.; Rapaka, R.; Okamoto, K. Nuclear overhauser enhancement evidence for inverse temperature dependence of hydrophobic side chain proximity in the polytetrapeptide of tropoelastin. Biochem. Biophys. Res. Commun. 1977, 79, 700–706. [Google Scholar] [CrossRef]
- Asakura, T.; Ashida, J.; Ohgo, K. Conformational Characterization of (Val-Pro-Gly-Val-Gly)6 with 13C Solid State NMR. Polym. J. 2003, 35, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Isailovic, D.; McMillan, R.; Conticello, V. Structure of an elastin-mimetic polypeptide by solid-state NMR chemical shift analysis. Biopolymers 2003, 70, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Berryman, J.; Betz, R.M.; Cerutti, D.S.; Cheatham Iii, T.E.; Darden, T.A.; Kollman, P.A. AMBER 2015; University of California: San Francisco, CA, USA, 2015. [Google Scholar]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E. Amber 11; University of California: San Francisco, CA, USA, 2011. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Vanquelef, E.; Simon, S.; Marquant, G.; Garcia, E.; Klimerak, G.; Delepine, J.C.; Cieplak, P.; Dupradeau, F.-Y. RED Server: A web service for deriving RESP and ESP charges and building force field libraries for new molecules and molecular fragments. Nucleic Acids Res. 2011, 39, W511–W517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Mumby, S.J.; Maple, J.R.; Hagler, A.T. An ab Initio CFF93 All-Atom Force Field for Polycarbonates. J. Am. Chem. Soc. 1994, 116, 2978–2987. [Google Scholar] [CrossRef]
- Hirano, T.; Okumura, Y.; Kitajima, H.; Seno, M.; Sato, T. Dual roles of alkyl alcohols as syndiotactic-specificity inducers and accelerators in the radical polymerization of N-isopropylacrylamide and some properties of syndiotactic poly(N-isopropylacrylamide). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4450–4460. [Google Scholar] [CrossRef]
- Haley, B.P.; Wilson, N.; Li, C.; Arguelles, A.; Jaramillo, E.; Strachan, E. Polymer Modeler; Network for Computational Nanotechnology, Purdue University: West Lafayette, IN, USA, 2016. [Google Scholar] [CrossRef]
- Urry, D.W. Molecular Machines: How Motion and Other Functions of Living Organisms Can Result from Reversible Chemical Changes. Angew. Chem. Int. Ed. 1993, 32, 819–841. [Google Scholar] [CrossRef]
- Urry, D.W.; Trapane, T.L.; Sugano, H.; Prasad, K.U. Sequential polypeptides of elastin: Cyclic conformational correlates of the linear polypentapeptide. J. Am. Chem. Soc. 1981, 103, 2080–2089. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Shikata, T. Contrary Hydration Behavior of N-Isopropylacrylamide to its Polymer, P(NIPAm), with a Lower Critical Solution Temperature. J. Phys. Chem. B 2007, 111, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Shikata, T. Hydration and Dynamic Behavior of Poly(N-isopropylacrylamide)s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, J.C.; Alonso, M.; Pérez, T.; Herguedas, M.M. Differential scanning calorimetry study of the hydrophobic hydration of the elastin-based polypentapeptide, poly(VPGVG), from deficiency to excess of water. Biopolymers 2000, 54, 282–288. [Google Scholar] [CrossRef]
- Cho, Y.; Sagle, L.B.; Iimura, S.; Zhang, Y.; Kherb, J.; Chilkoti, A.; Scholtz, J.M.; Cremer, P.S. Hydrogen Bonding of β-Turn Structure Is Stabilized in D2O. J. Am. Chem. Soc. 2009, 131, 15188–15193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, M.; Maeda, Y.; Kitano, H. Effect of Hydrophobicity of Amino Acids on the Structure of Water. J. Phys. Chem. B 1997, 101, 7022–7026. [Google Scholar] [CrossRef]
- Abbott, L.J.; Tucker, A.K.; Stevens, M.J. Single Chain Structure of a Poly(N-isopropylacrylamide) Surfactant in Water. J. Phys. Chem. B 2015, 119, 3837–3845. [Google Scholar] [CrossRef]
- Kauzmann, W. Some Factors in the Interpretation of Protein Denaturation. Adv. Protein Chem. 1959, 14, 1–63. [Google Scholar] [CrossRef]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]
- Tucker, A.K.; Stevens, M.J. Study of the Polymer Length Dependence of the Single Chain Transition Temperature in Syndiotactic Poly(N-isopropylacrylamide) Oligomers in Water. Macromolecules 2012, 45, 6697–6703. [Google Scholar] [CrossRef]
- Pelton, R. Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J. Colloid Interface Sci. 2010, 348, 673–674. [Google Scholar] [CrossRef]
- Galbraith, M.L.; Madura, J.D. Identifying trends in hydration behavior for modifications to the hydrophobicity of poly(N-isopropylacrylamide). J. Mol. Graph. Model. 2017, 78, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.F.; Theodorou, D.N. Molecular Dynamics Simulation of a Glassy Polymer Surface. Macromolecules 1991, 24, 6283–6294. [Google Scholar] [CrossRef]
- Weber, T.A.; Helfand, E. Time-correlation functions from computer simulations of polymers. J. Phys.Chem. 1983, 87, 2881–2889. [Google Scholar] [CrossRef]


| Temp (K) | Water/Val | Water/Pro | Water/Gly |
|---|---|---|---|
| 290 | ~3.3 ± 0.2 | ~4.3 ± 0.3 | ~5.7 ± 0.4 |
| 340 | ~2.9 ± 0.3 | ~3.0 ± 0.4 | ~4.7 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Li, N.K.; Singh, A.; Deshmukh, S.A.; Yingling, Y.G. A Comparison between the Lower Critical Solution Temperature Behavior of Polymers and Biomacromolecules. Physchem 2022, 2, 52-71. https://doi.org/10.3390/physchem2010005
Xie Y, Li NK, Singh A, Deshmukh SA, Yingling YG. A Comparison between the Lower Critical Solution Temperature Behavior of Polymers and Biomacromolecules. Physchem. 2022; 2(1):52-71. https://doi.org/10.3390/physchem2010005
Chicago/Turabian StyleXie, Yuxin, Nan K. Li, Abhishek Singh, Sanket A. Deshmukh, and Yaroslava G. Yingling. 2022. "A Comparison between the Lower Critical Solution Temperature Behavior of Polymers and Biomacromolecules" Physchem 2, no. 1: 52-71. https://doi.org/10.3390/physchem2010005
APA StyleXie, Y., Li, N. K., Singh, A., Deshmukh, S. A., & Yingling, Y. G. (2022). A Comparison between the Lower Critical Solution Temperature Behavior of Polymers and Biomacromolecules. Physchem, 2(1), 52-71. https://doi.org/10.3390/physchem2010005








