Abstract
All-atom molecular dynamics (MD) simulations are employed to compare the lower critical solution temperature (LCST) behaviors of poly(N-isopropylacrylamide) (PNIPAM) and elastin-like polypeptides (ELPs) with the canonical Val-Pro-Gly-Val-Gly ((VPGVG)n) sequence over a range of temperatures from 280 K to 380 K. Our simulations suggest that the structure of proximal water dictates the conformation of both the (VPGVG)n ELPs and PNIPAM chains. Specifically, the LCST transition in ELPs can be attributed to a combination of thermal disruption of the network of the proximal water near both hydrophilic and hydrophobic groups in the backbone and side-chain of (VPGVG)n, resulting in a reduction in solvent accessible surface area (SASA). This is accompanied with an increase in the secondary structure above its LCST. In the case of PNIPAM, the LCST transition is a result of a combination of a reduction in the hydrophobic SASA primarily due to the contributions of isopropyl side-chain and less to the backbone and the formation of intra-chain hydrogen bonds between the amide groups on the side-chain above its LCST.
1. Introduction
Thermoresponsive polymers, such as poly(N-isopropylacrylamide) (PNIPAM), and biomacromolecules, such as elastin-like polypeptides (ELP), undergo liquid–liquid phase separation into polymer-rich phase and water-rich phases upon heating to above their lower critical solution temperature (LCST) [1]. For example, the LCST of (PNIPAM)n is about ~305 K [1,2,3,4], and LCST of ELPs is between ~300 K to ~400 K [5,6,7,8,9]. Unlike PNIPAM, LCST of ELPs with the canonical Val-Pro-Gly-Val-Gly ((VPGVG)n) sequence depends on its molecular weight [10,11,12,13,14,15,16,17]. Many experimental and computational studies have investigated the origin and mechanism of LCST transition in ELPs [5,18,19,20,21,22,23,24,25,26,27] and in PNIPAM and its architectures [3,4,28,29,30,31,32,33,34,35,36]. For PNIPAM, the LCST transition is attributed to the breaking of a cage-like structure of water molecules around its hydrophobic groups due to the gain in the entropy at a temperature greater than its LCST [4,37,38,39,40,41]. With an increase in temperature, the entropy term dominates the enthalpy of hydrogen bonds between polar groups of PNIPAM and water molecules, which leads to a more stable globular conformation of PNIPAM above its LCST. Indeed, coil–globule transition of PNIPAM investigated by Fourier transform infrared (FTIR) spectroscopy shows that in a globular conformation, only 13% of the C=O groups are dehydrated and form intra- or inter-chain hydrogen bonding, while 87% of C=O groups remain hydrogen bonded with water even in this globular state [42]. Maeda et al. revealed partial dehydration of PNIPAM chains resulting in formation of intra- and inter-chain hydrogen bonding between N–H groups in globular conformation [42,43,44,45]. In the case of (VPGVG)n ELPs, the LCST phase behavior is observed as a collective phenomenon that originates from the correlated gradual changes in a single polypeptide structure and the abrupt change in structure and properties of water around the peptide. This has been attributed to a competition between peptide−peptide and peptide−water interactions [23,25,26,27]. Specifically, simulation studies show that the molecular collapse of ELPs happens as a gradual transition in a well-defined temperature region, indicating a shift from extended to contracted structures with increasing temperature [22]. These aforementioned studies on PNIPAM and (VPGVG)n ELPs suggests that their LCST mechanisms could be different. Investigating the nature of their LCST behaviors is important for the design of new soft materials with controllable functionality that can be used in a variety of applications including tissue engineering, electrophoresis, and drug delivery [46,47,48,49].
The differences in the structural properties of PNIPAM and (VPGVG)n ELPs may affect their LCST. In PNIPAM, the hydrophilic entities are located on the side-chain and mainly consist of C=O and N–H groups. The hydrophobic groups including –CH2– and isopropyl groups are located on the backbone and side chain of PNIPAM, respectively (Figure S1). The addition of salt-ions, methanol, ionic liquids, etc., to the solution of PNIPAM alters the interactions between solvent molecules and between the solvent and PNIPAM, which leads to the change in its LCST [3,6,28,33,50]. Experimentally, however, the LCST of PNIPAM is known to be independent of its molecular weight [51]. The LCST of PNIPAM can also be modified by co-polymerizing the PNIPAM chain with hydrophobic and/or hydrophilic co-monomers [52,53,54,55]. For example, co-polymerization of PNIPAM with hydrophilic monomers increases its LCST, whereas the introduction of hydrophobic monomers decreases the LCST of PNIPAM [56].
In (VPGVG)n ELPs, the hydrophobic and hydrophilic groups of ELPs are located on its side-chain and backbone, respectively. The LCST of (VPGVG)n ELPs can be tuned by controlling the salt–ion concentration [57] and it strongly depends on the molecular weight of ELPs [17,23,27]. Moreover, change in the position and sequence of amino acids in ELPs alters its LCST [16,26,58]. For example, the LCST of (VPGVG)n ELPs was increased by substituting hydrophobic Valine in its fourth position with Alanine and Glycine [10]. A change in sequence order results in a significant difference in the dynamics of the residues, such as valines, and differences in overall compaction due to the change in rotational degrees of freedom within the sequence in (VPGVG)18 and (VGPVG)18 [26].
Despite several similarities, there are many differences in the structural characteristics of PNIPAM and (VPGVG)n ELPs. Hydrophilic groups are located on the side-chain of PNIPAM and on the backbone of ELP. ELPs are biopolymers, with sequence directionality and the ability to form various secondary structures. After undergoing LCST (VPGVG)n ELPs are known to form more β-turns and β-sheets [16,20,27]. In contrast, PNIPAM does not have the propensity to form any secondary structures. Unlike PNIPAM, the LCST of ELPs depends on their chain length [17]. In addition, the LCST of (VPGVG)n ELPs is also observed to be more sensitive to the salt–ion concentration as compared with that of PNIPAM [57]. However, the molecular origin of the observed differences in temperature-induced transitions of PNIPAM and ELPs are yet to be fully understood.
Lack of structural data on various stable and metastable states during the inverse temperature transitions limits the understanding of the mechanisms of LCST inverse temperature transition in both (VPGVG)n ELPs and PNIPAM [21,59,60,61]. Moreover, the dynamic structure of the solvent near these polymer chains is difficult to characterize, irrespective of the conformation of the polymer chain, using existing experimental tools. In the present study, we employ all-atom (AA) molecular dynamics (MD) simulations to examine and compare the inverse temperature transitions behavior of single PNIPAM chains versus single (VPGVG)n ELP chains with 18 and 90 monomers (18-mer and 90-mer), in the temperature range of ~280 K to ~380 K. A systematic and careful examinations of structural and dynamical properties of macromolecules and water from the simulation trajectories has been performed. Specifically, we calculated and compared temperature-induced changes in radius of gyration (Rg), water radial distribution function (RDF) in the vicinity of the macromolecules, solvent accessible surface areas (SASA) and dehydration ratio based on hydrogen bonding analysis. To the best of our knowledge, this is the first AA MD study that systematically probes and compares the structures of PNIPAM and (VPGVG)n ELPs as they undergo inverse temperature transition.
2. Computational Materials and Methods
2.1. Force Field
AMBER [62,63] simulation package was used to conduct AA MD simulations of a single chain of PNIPAM and a (VPGVG)n ELP in explicit TIP3P [64] water. Figure 1 shows the structures of PNIPAM and (VPGVG)18 ELPs, which were represented with the ff99SB force-field [63] for proteins. For MD simulations of a single PNIPAM chain, in the presence of an explicit TIP3P [64] water model, the GAFF force-field was employed. For PNIPAM, to identify the appropriate set of partial charges, we have conducted simulations of PNIPAM with four different partial charges above and below its LCST. These partial charges were obtained from (a) the restrained electrostatic potential (RESP) method [65], (b) the electrostatic potential (ESP) method [65], (c) polymer consistent force-field (PCFF) parameters from reference [37] and (d) Original PCFF force-field [66] (Figure S2). Based on the initial analysis of the Rg of PNIPAM chains conducted at 290 K and 350 K, we chose partial charges calculated by the RESP method (Figure S3). For more details, the schematic and partial charges for both PNIPAM and ELPs are shown in Figure S1 and Table S1.
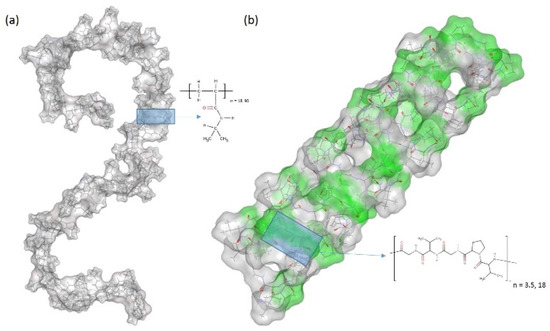
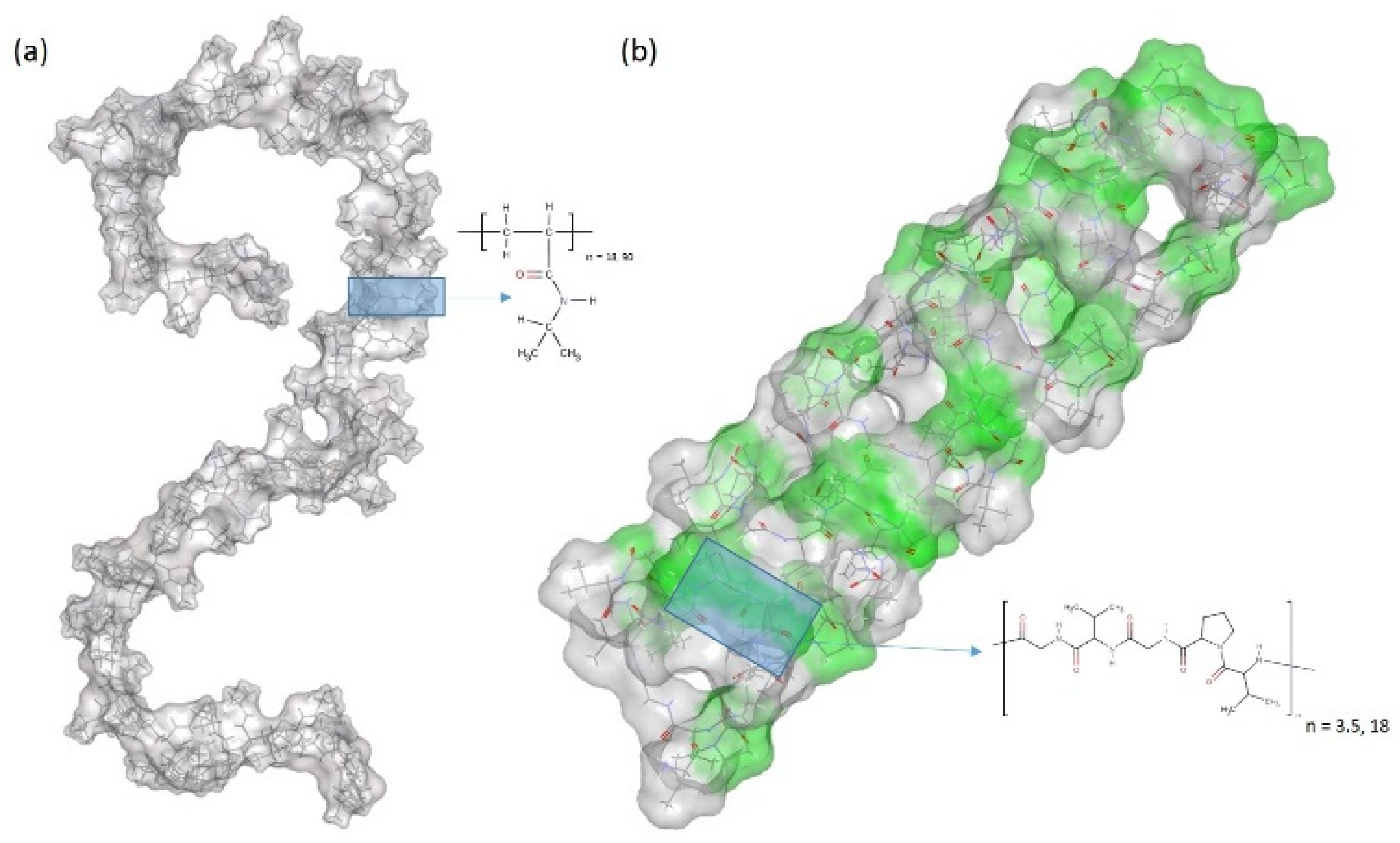
Figure 1.
Initial structures of (a) a single chain of PNIPAM and (b) a β-spiral model of (VPGVG)18 ELPs, where the β-turns are colored in green and the coils are colored in gray. Details of schematic structures are showed in supporting information. Blue shaded boxes and schematic chemical drawings represent the structure of (a) PNIPAM monomer and (b) VPGVG pentamer.
2.2. Initial Structure Generation
Initial structures of atomistic atactic [67] PNIPAM chains with 18-mer and 90-mer were generated using a polymer modeler from nanoHUB [68]. First, a monomer PNIPAM unit was built; then, by specifying the degree of polymerization and tacticity, a PNIPAM chain with the desired length was built. The bond length of ~1.53 Å between the –CH2– group of the first monomer and the carbon atom of the –CH– group of the second monomer was chosen based on the C–C distance defined by the ff12SB force-field. Finally, the PNIPAM chain was subjected to an energy minimization cycle based on the conjugate gradient algorithm and 5000 steps of NVT at ~300 K and 1 fs timestep. The equilibrated chains of PNIPAM were then solvated with TIP3P water molecules, and the size of the box (55 Å × 53 Å × 65 Å for 18-mer and 100 Å × 95 Å × 114 Å for 90-mer) was chosen to be large enough so that no polymer–polymer interactions through periodic boundary conditions can occur. For short-chain PNIPAM (18-mer), simulations were performed at 280 K, 290 K, 300 K, 310 K, 320 K, 330 K, 340 K, 350 K and 360 K. Simulations with long-chain (90-mer) PNIPAM were performed at 280 K, 290 K, 300 K, 305 K, 310 K, 320 K, 330 K, 340 K, 350 K, 360 K, 370 K and 380 K.
For ELP Chain, Urry’s β-spiral model [5,69,70] was used as the initial configuration for (VPGVG)n ELPs of desired chain lengths and was solvated with the TIP3P water model [64]. The size of the box (47 Å × 48 Å × 41 Å for 18-mer and 72 Å × 78 Å × 69 Å for 90-mer) was chosen to be large enough so that no peptide–peptide interactions through periodic boundary conditions can occur. For short ELP chains, temperatures at 290 K, 300 K, 305 K, 310 K, 320 K, 325 K, 340 K, 350 K and 375 K were chosen for MD simulations. For 90-mer ELP chain, simulations were performed at temperatures of 290 K, 300 K, 305 K, 310 K, 320 K, 325 K, 330 K, 335 K, 340 K and 350 K. Note, these temperatures were chosen based on the experimental study of LCST phase transition of (VPGVG)n ELPs in aqueous solution.
2.3. MD Simulation Details
Similar simulation protocols have been utilized for (VPGVG)n ELPs, and details of the simulations procedures were described in our previous study [27]. Briefly, every system was equilibrated in three stages, starting from the energy minimization using the steepest descent method for 10,000 steps and then the conjugate gradient method. This was followed by gradual heating of the simulation box from 10 K to the assigned temperature for 400 ps using NVT. The particle mesh Ewald (PME) summation was used to calculate long-range electrostatic interactions, and the non-bonded interactions were truncated at a 9 Å cutoff along with a 0.00001 tolerance for Ewald convergence [71]. The Berendsen thermostat was used to maintain the temperature [72]. The SHAKE algorithm was used to constrain bonds involving hydrogen atoms [73]. NPT MD production simulations were conducted for ~100 ns with a 2 fs time step at an assigned temperature. Note, analysis of time autocorrelation function of polymer backbone suggested a relaxation time of less than 40 ns (See Figure S4a–d). Thus, only the last 40 ns of the trajectories were considered for statistical analysis.
2.4. Analysis Methods
The changes in Rg over time were used to analyze the structural evolution of polymer chains using the following equation:
where M is the mass of group, is the center of mass position of the group, and the sum is over all the atoms in the groups.
To probe the local ordering of water molecules located close to the polymer chains below and above the LCST, RDFs were calculated. Specifically, we calculated the RDFs for hydrophilic groups (C=O and >N–H) on the polymer chains with oxygen of water (ow). In addition, for PNIPAM, RDFs for carbon atoms from the >CH– group in the backbone and the C=O group of PNIPAM with water oxygen were calculated. For (VPGVG)n ELPs, RDFs were calculated for the alpha carbon and carbon atoms from the C=O group in the backbone with water oxygen.
The LCST is also associated with the dehydration of polymer chains [74,75]. Experimentally, both PNIPAM and (VPGVG)n ELPs show dehydration when the temperature rises from below to above their LCST [9,45,74,75,76,77]. This dehydration not only changes the structure of the water molecules near the polymer but can also significantly modify the hydrogen bond network between polymer and water and between polymer and polymer. In our studies, we use criteria for two atoms to be hydrogen bonded if bond length Å and O…H–O angle . In the present study, distance between donor and acceptor atoms of polymer and water was defined based on the first peak in the RDF of the hydrophilic groups of polymers with water. The first hydration shell was defined based on the first minimum in the RDF of hydrophilic groups of the polymer and oxygen of water. The stability of the hydrogen bonds was described by defining a hydrogen bonding time (HBT) in our simulations through lifetime analysis. The total hydrogen bonding time was defined as the total number of time steps of existence of a specific acceptor–donor bond pair within the last 40 ns of simulations. We also examine the dehydration behavior of both ELPs and PNIPAMs through defining the dehydration ratio, which is the ratio of the number of intra-chain hydrogen bonds (npp) to total hydrogen bonding (npp + npw) that forms between polymer and polymer, and between polymer and water (npw).
The solvent accessible surface area (SASA) is strongly related to the hydrophobic interactions in both biomolecule and synthetic polymer systems [78,79,80]. The folding of the polypeptides is driven by the hydrophobic interactions that essentially minimize the SASA of the nonpolar solutes [27]. Similarly, in thermo-sensitive polymers such as PNIPAM, since the driving force for the LCST transition of polymer chains is to minimize the hydrophobic surface in contact with water, SASA should decrease [79]. SASA calculations were conducted using the LCPO algorithm proposed by Weiser et al. [81] for both hydrophilic and hydrophobic groups of PNIPAM and ELPs.
3. Results and Discussion
3.1. Temperature Dependence of Radius of Gyration (Rg) of Macromolecules
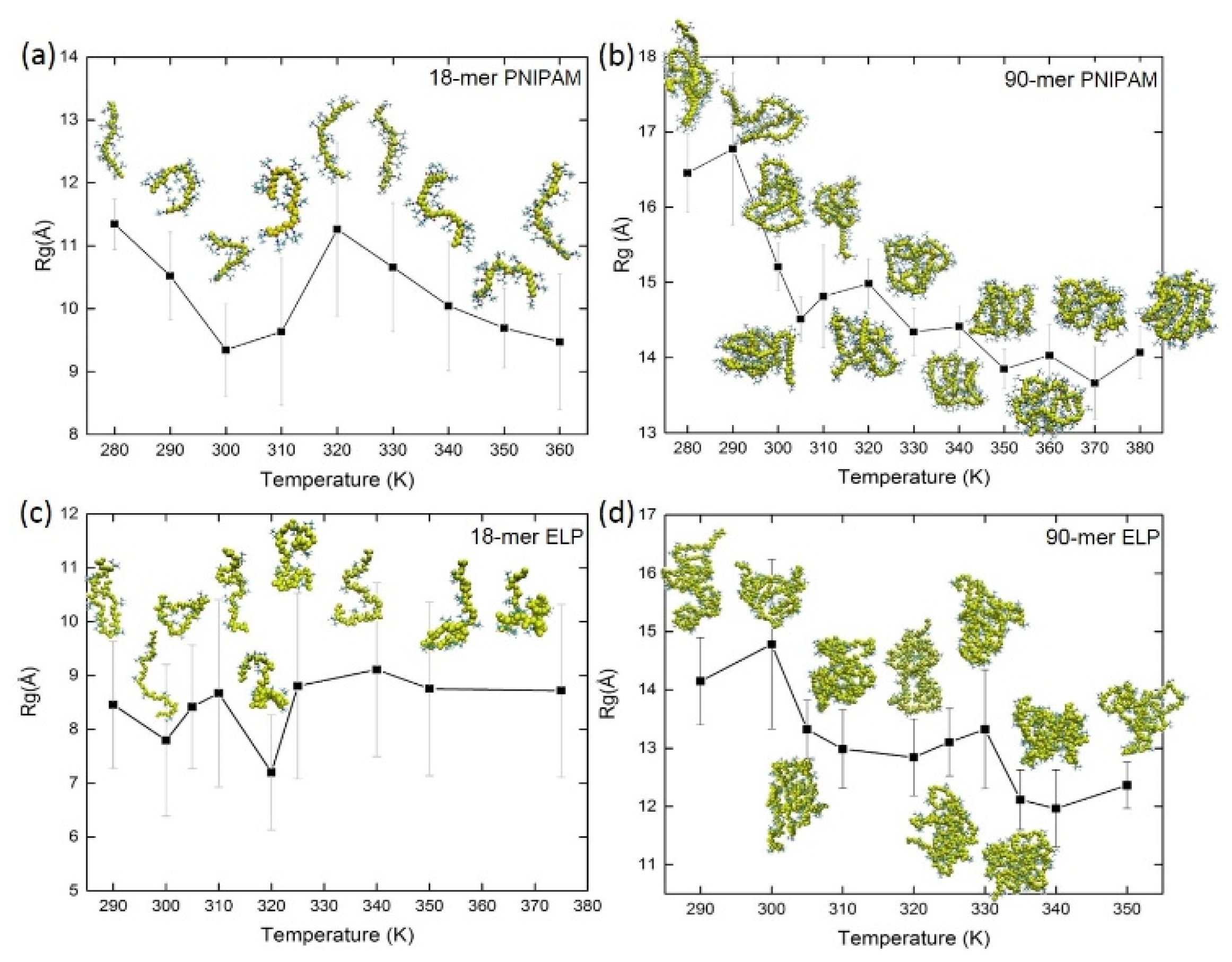
The compactness of the structure of macromolecules can be described by its Rg value. To study the effect of temperature on the compactness of the structure of polymer and biopolymer chains as a function of length, we calculated Rg across simulated temperatures (Figure 2, Table S2). We found that the mean value of Rg for short 18-mer PNIPAM chains decreases from 11.3 ± 0.4 Å to 9.6 ± 1.1 Å when the temperature is raised from 280 K to 310 K. Similar folding of 18-mer of PNIPAM above its LCST has been reported in a previous study by Tucker et al. [82]. Interestingly, as we increase the temperature above 310 K, the Rg value of PNIPAM 18-mer increases due to the thermal fluctuations. In the case of short (VPGVG)n ELPs, the value of Rg oscillates around the mean value of their Rg at all studied temperatures. For example, the mean value of Rg of (VPGVG)n ELPs at ~290 K is 8.4 ± 1.1 Å and it fluctuates between 7.8 ± 1.4 Å to 8.7 ± 1.6 Å when the temperature is raised from ~300 K to ~375 K. This implies that the shorter chains of (VPGVG)n ELPs do not exhibit LCST transition in the simulated temperature range.
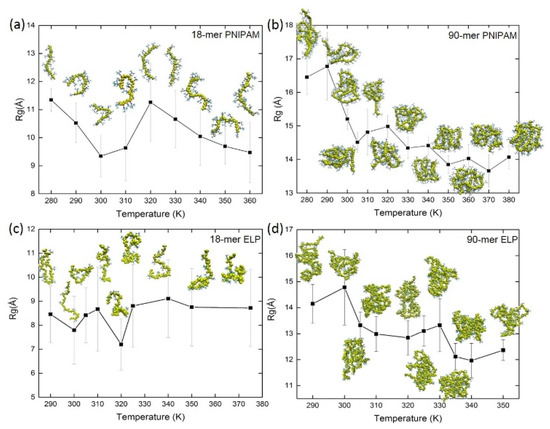
Figure 2.
The radius of gyration and simulation snapshots as a function of temperature for PNIPAM (a) 18-mer and (b) 90-mer and ELPs (c) 18-mer and (d) 90-mer.
The mean value of Rg for a random coil of a longer 90-mer of PNIPAM decreases gradually from 16.4 ± 0.5 Å to 14.0 ± 0.3 Å when temperature is raised from below to above its LCST, namely above ~305 K (Figure 2b). The mean value of Rg for 90-mer PNIPAM at ~280 K and ~290 K is 16.4 ± 0.5 Å and 16.7 ± 1.0 Å, respectively. As the temperature is raised close to the experimental LCST of PNIPAM, namely at ~305 K, the mean value of Rg decreases to 14.5 ± 0.3 Å. For temperatures between ~310 K and ~380 K, the Rg value fluctuates at ~14 Å. This suggests that the 90-mer PNIPAM behaves as expected around LCST. In the case of 90-mer ELPs the Rg value decreases gradually from 14.1 ± 0.7 Å to 12.3 ± 0.4 Å as temperature is increased from ~290 K to ~350 K. In the temperature range from ~300 K to ~330 K, the value of Rg fluctuates between ~13 Å to ~14 Å. When the temperature is raised above ~335 K, the value of Rg drops to 12.1 ± 0.5 Å. This implies that the LCST of 90-mer of ELP could be between 330 K and 340 K.
3.2. Solvation Shell
3.2.1. Analysis of Hydrogen Bond
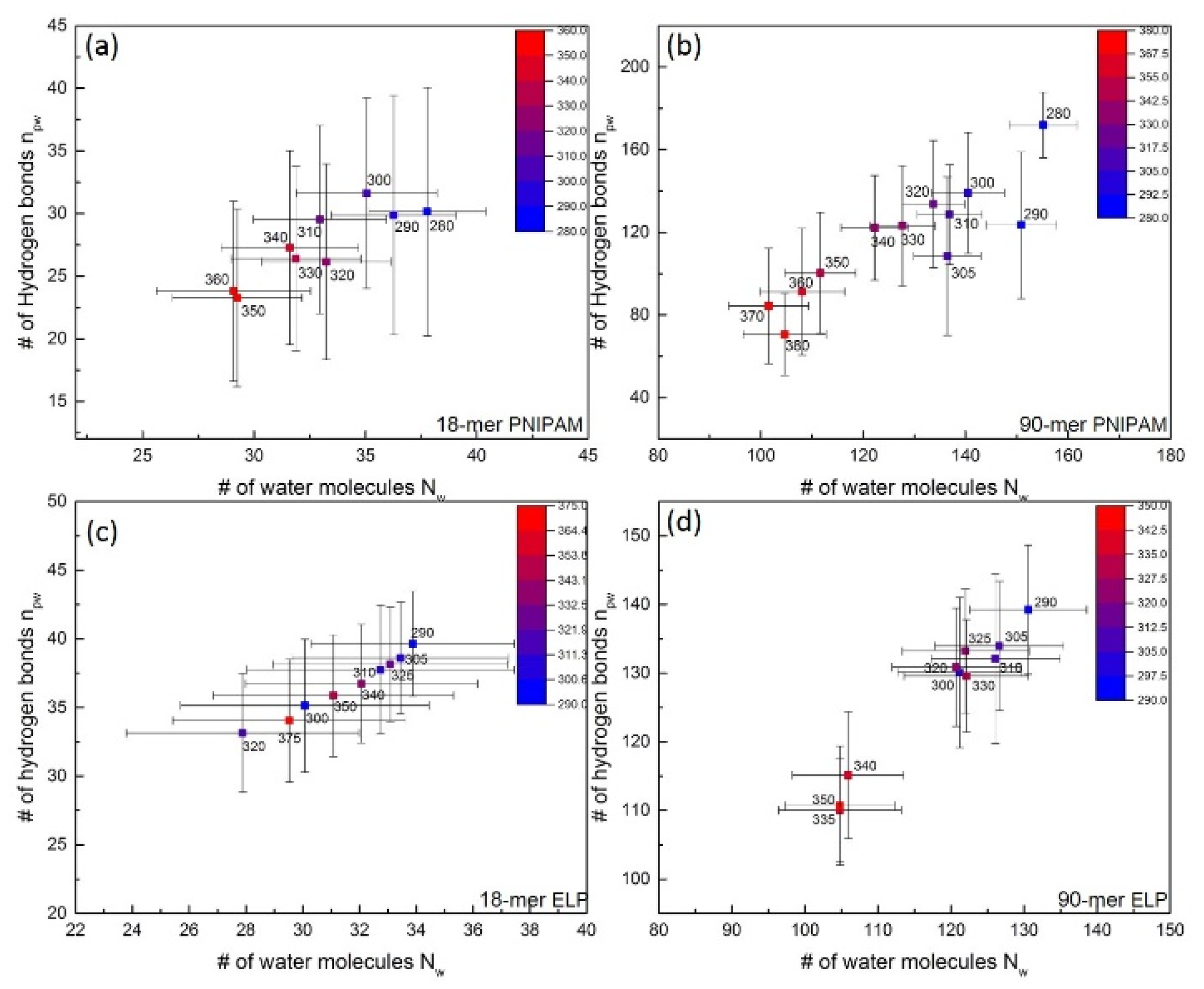
Previous experimental and simulation studies suggest that the LCST transition can significantly alter the hydrogen bonding between polymer and water. To investigate the effect of LCST on the structure of hydration water and establish a hydrogen bonding network between polymer and water, we analyzed simulation trajectories for both PNIPAM and (VPGVG)n ELPs at all studied temperatures using the geometric criteria defined in Section 2.4. In particular, we examine the relationship between hydrogen bonds between polymer and water (npw) and the number of water molecules (Nw) present in the first hydration shell. The correlation between Nw and npw for 18-mer and 90-mer PNIPAM and water molecules is shown in Figure 3a,b, respectively. The relationship between Nw and npw for 18-mer and 90-mer (VPGVG)n ELPs and water molecules is shown in Figure 3c,d, respectively.

Figure 3.
Number of hydrogen bonds between polymer and water (npw) and number of water molecules (Nw) within first hydration shell of hydrophilic group of polymers as a function of temperatures. (a,b) show the npw and Nw correlation of 18-mer and 90-mer of PNIPAM, respectively. (c,d) give the relationship of npw and Nw for 18-mer (GVG(VPGVG)3) and 90-mer ((VPGVG)18) of ELP, respectively.
For the 90-mer PNIPAM, Nw decreases from ~155 to ~101 and npw decreases from ~172 to ~70 when temperature is increased from ~280 K to ~380 K (Figure 3b). Interestingly, we find that close to the LCST of PNIPAM (~305 K), both Nw and npw oscillate at 135 ± 6.5 and 125 ± 30, respectively. This suggests that above the LCST of PNIPAM, the number of water molecules near its hydrophilic groups does not change significantly. Similar observations have been reported in previous studies where the number of water molecules present near hydrophilic groups of 30-mer of PNIPAM were similar both below and above the LCST of PNIPAM [37]. However, as can be seen in Figure 3b, when temperature is raised to above ~340 K, a distinct cluster with a reduced number of Nw and npw was observed. This suggests that the increase in temperature to above ~340 K disrupts the structure of water near the hydrophilic groups of PNIPAM.
In the case of 90-mer (VPGVG)n ELPs, two distinct clusters, one above and one below ~330 K, were observed (Figure 3d). In particular, below ~330 K, the values of Nw and npw were 124 ± 8.6 and 132 ± 9.7, respectively. On the other hand, above ~330 K both Nw and npw decrease to 105 ± 7.8 and 112 ± 8.5, respectively. In our previous study, the LCST of 90-mer of ELP was observed at 332.5 ± 2.5 K [27]. In addition, our study of 50-mer, 90-mer, and 150-mer of (VPGVG)n ELPs showed a similar abrupt change in Nw and npw below the LCST as compared to above their respective LCSTs [23]. Such an abrupt change in Nw and npw indicates that the interactions between the hydrophilic groups in the backbone of (VPGVG)n ELPs and water are strongly dependent on the temperature. This might lead to a significantly different structure of water near the backbone of (VPGVG)n ELPs below its LCST as compared to above its LCST.
The comparison of Nw and npw of 90-mers PNIPAM and (VPGVG)n ELPs suggests that the abrupt change in hydration of the hydrophilic groups of (VPGVG)n ELPs is more prominent as compared to PNIPAM above their LCST. This could be because the hydrophilic groups of PNIPAM are shielded by the isopropyl groups in the side-chain of PNIPAM [39]. The shielding might keep water molecules isolated from the bulk solvent and might allow hydration water to reside for longer time [39]. On the other hand, the hydrophilic groups in (VPGVG)n ELPs are located on its backbone. This might facilitate the reduction in the number of water molecules near (VPGVG)n ELPs above the LCST as compared to below its LCST. This reduction in the number of water molecules can further assist (VPGVG)n ELPs to form a stronger hydrogen bonding network above the LCST.
The relationship between Nw and npw for short PNIPAM and ELP chains, as shown in Figure 3a,c, do not exhibit distinct clusters. In the case of a short PNIPAM chain, Nw decreases from 37 ± 2 to 29 ± 3 and npw decreases from 30 ± 10 to 23 ± 7 when temperature is increased from ~280 K to ~360 K. Similar to 90-mer PNIPAM, the decrease in both Nw and npw is observed above ~340 K. This further supports the notion that interactions between PNIPAM and water are disrupted above ~340 K.
The Nw for 18-mer (VPGVG)n ELPs decreases from 33.8 ± 3.5 to 29.5 ± 4 and the npw decreases from 39.6 ± 3.8 to 34 ± 4.5 when temperature is increased from ~290 K to ~375 K. However, similar to those of 90-mer (VPGVG)n ELPs, abrupt changes in Nw and npw below the LCST as compared to above their LCST were not observed. This suggests that the structural arrangement of the water molecules near the backbone of 18-mer (VPGVG)n ELPs does not change significantly with the increase in temperature.
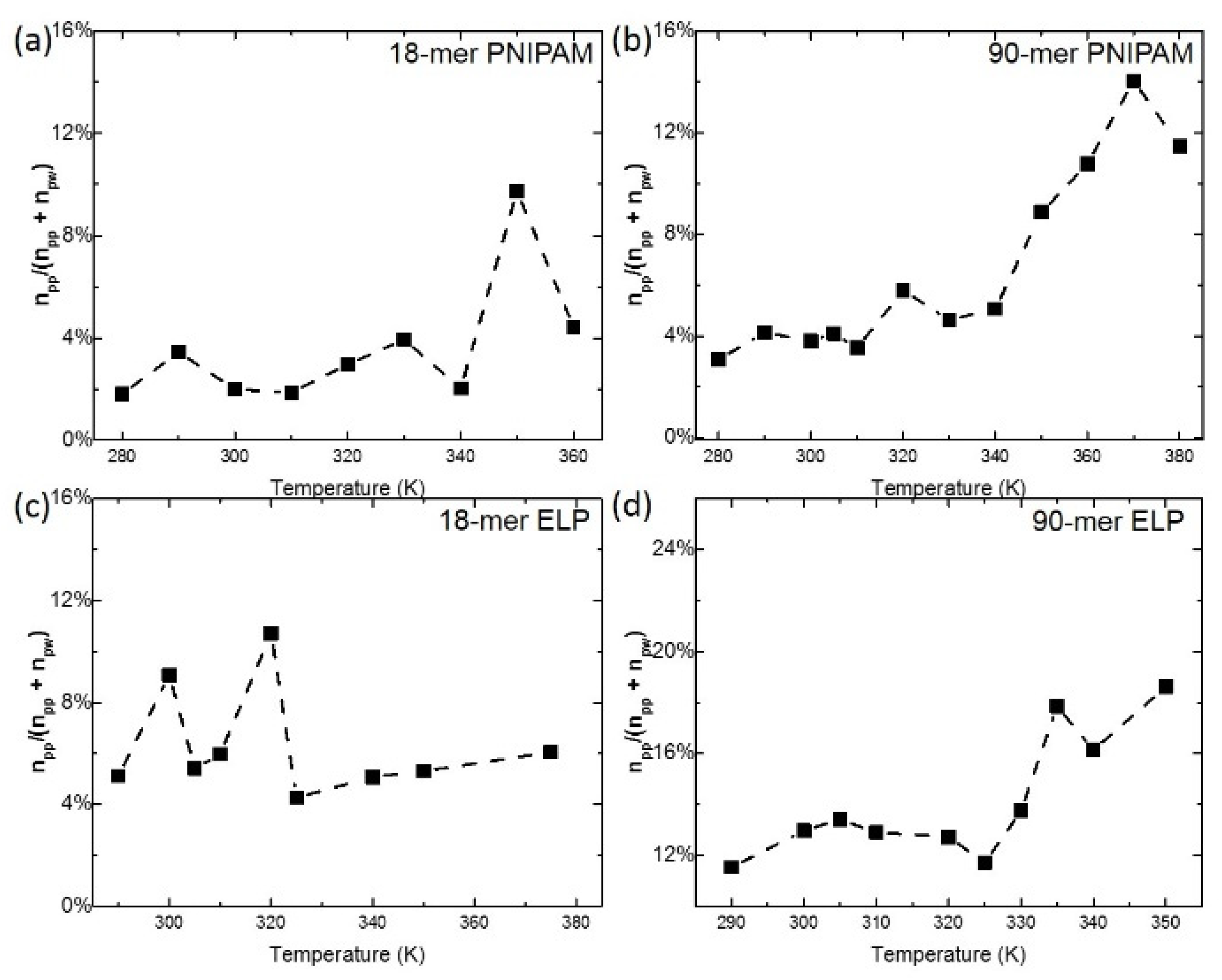
One mechanistic hypothesis of coil–globule transition, as mentioned by Maeda et al. [42], is that the polymer chain is dehydrated above its LCST and hydrophobic interaction between isopropyl groups induces the collapse of the chain. To explore the hydration state of the polymer chain, especially the interaction between amide groups, we use the ratio between npp and (npw + npp) as a measurement of the dehydration behavior during the coil–globule transition (Figure 4). The temporal dehydration ratio (black curves) of 18-mer ELP and 18-mer PNPIAM fluctuate around the average ratio (roughly 4% for 18-mer PNIPAM and 6% for 18-mer ELP), as can be seen in Figure 4a,c. This implies no significant change in dehydration. However, for longer chains, an abrupt transition in dehydration during the coil–globule transition was observed (Figure 4b,d). For 90-mer PNIPAM, the dehydration ratio increases from 4% to above 12%, which is accompanying the threefold increase in intra-chain hydrogen bonding. For 90-mer ELPs, the dehydration ratio increases from 12% to above 17% around the LCST of 90-mer of (VPGVG)n ELPs at 332.5 ± 2.5 K. Thus, the dehydration ratio shows a partial dehydration during coil–globule transition for both 90-mer ELPs and PNIPAM, which agree well with the change in hydration states described in Maeda et al.’s paper and almost step-like behavior of vibrational frequency and peak area observed by Futscher et al. [42,43,44,45].

Figure 4.
Dehydration ratio, defined as ratio between number of polymer-polymer hydrogen bonds (npp) and total hydrogen bonds (npw + npp). (a,b) show the dehydration ratio of 18-mer and 90-mer of PNIPAM, respectively. (c,d) show the dehydration ratio of 18-mer (GVG(VPGVG)3) and 90-mer ((VPGVG)18) of ELP, respectively.
3.2.2. Water Distribution
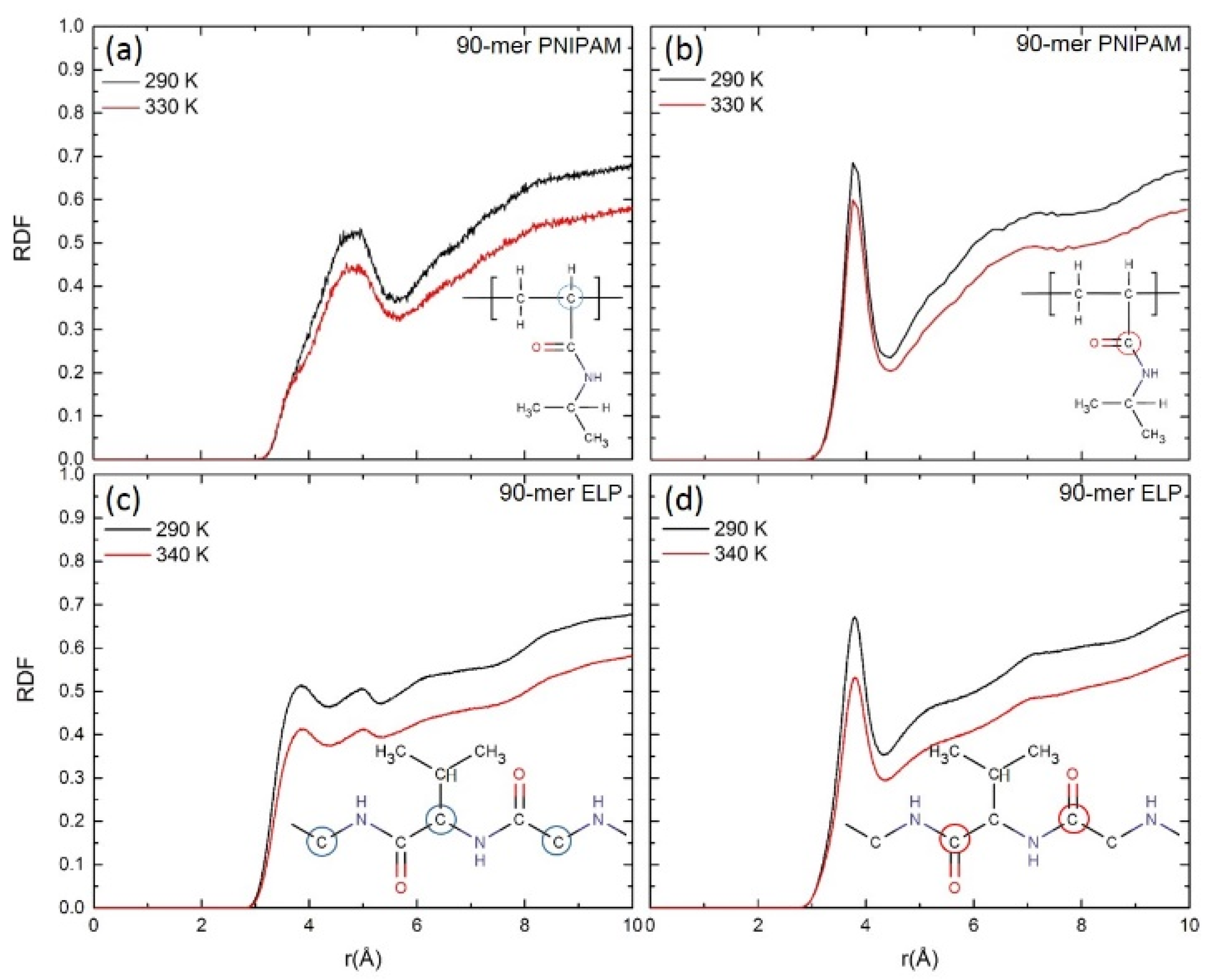
To further probe the effect of LCST transition on the interactions of PNIPAM and (VPGVG)n ELPs with water and the structure of water molecules in their first hydration shell, we calculated the radial distribution function (RDF) for polymer and water. In the supporting information, Figure S5a–d show the RDFs for hydrophilic groups of short (18-mer) and long (90-mer) chains of PNIPAM and (VPGVG)n ELPs with oxygen of water at all simulated temperatures. The RDFs for both short and long chains of PNIPAM and ELPs shows well-defined first, second, and third neighbor peaks at ~2.0, ~2.7, and ~3.8 Å, respectively.
The comparison of Figure S5a,c suggests that 18-mer of PNIPAM and (VPGVG)n ELPs are more solvated as compared to the 90-mer of PNIPAM and (VPGVG)n ELPs in Figure S5b,d, and suggests a similar structural arrangement of water near short chains of PNIPAM and (VPGVG)n ELPs both below and above the LCST. An increase in temperature leads to a gradual loss of local hydration layers, as indicated by the decrease in the heights of the RDF peaks at higher temperatures. However, the difference in the peak height for 90-mer PNIPAM is slightly more prominent as compared to 18-mer PNIPAM. Interestingly, in the case of 90-mer (VPGVG)n ELPs, a slight gap in the RDF peak intensities between the temperatures of ~330 K and ~335 K can be observed (Figure S5d). This gap corresponds to the same temperature window where the change in the conformational properties of (VPGVG)n ELPs has occurred (Figure 2d). This observation suggests a different structural arrangement of water molecules near the 90-mer (VPGVG)n ELPs at two temperatures (namely, ~290 K and ~340 K) and supports the existence of the LCST of the 90-mer of (VPGVG)n ELPs between ~330 to ~335 K [27].
We next compare the RDFs for carbon atoms in the backbone and carbonyl group of both 90-mer PNIPAM and 90-mer (VPGVG)n ELPs to the oxygen atoms of water. RDFs are also calculated for carbon atoms in the backbone and carbonyl group of both PNIPAM and (VPGVG)n ELPs with the oxygen of water and isopropyl groups in the sidechain with the oxygen of water at all simulated temperatures are reported in Figures S6–S8 of the supporting information. Figure 5a,b depict the RDFs of carbon atoms in the >CH- group in the backbone and carbonyl group of 90-mer of PNIPAM with the oxygen atom of water at ~290 K and ~330 K, respectively. Figure 5c,d show the RDFs for alpha carbon and carbon atoms from the C=O group in the backbone of 90-mer of (VPGVG)n ELPs with oxygen atoms of water at ~290 K and ~340 K, respectively. In Figure 5a, for 90-mer PNIPAM at ~330 K, the first peak’s height and width reduces significantly as compared to ~290 K. In the case of 90-mer (VPGVG)n ELPs, as shown in Figure 5c, the first and second peak height also decreases significantly at ~340 K as compared to ~290 K. This suggests that the ordering of water in the first hydration shell near the backbone of both PNIPAM and (VPGVG)n ELPs at ~330/340 K is significantly reduced as compared to ~290 K. In Figure 5b, a reduction in the peak can be observed at ~3.8 Å for the RDF of carbonyl group of long-chain PNIPAM at ~330 K as compared to ~290 K. For 90-mer ELP (Figure 5d), the first peak height for the carbonyl group decreases significantly at ~340 K as compared to ~290 K. Additionally, the overall height for the second (at ~5.2 Å) and third (at ~7.0 Å) shoulder decreases at ~340 K. Both RDFs of long chains of PNIPAM and ELPs show a reduction in the first peak, but (VPGVG)n ELPs show a more prominent reduction. This suggests that at ~340 K, the structure of water near the carbonyl group of 90-mer of (VPGVG)n ELPs has changed more considerably as compared to ~290 K.
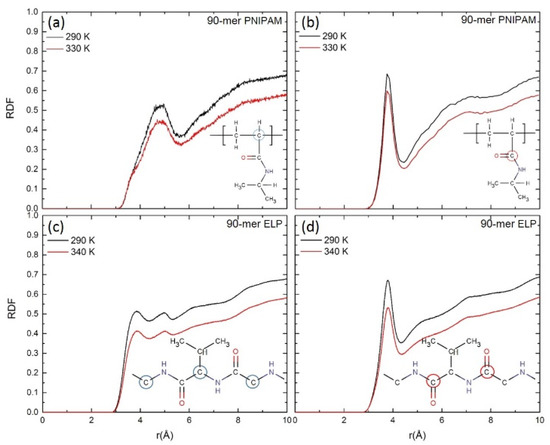
Figure 5.
Radial distribution functions (RDF) for (a,c) the backbone carbon atoms (circled atoms on chemical drawings) and (c,d) carbonyl groups of (a,b) PNIPAM and (c,d) ELP with the oxygen of water.
We then analyze the water molecules in the first hydration shell around the alpha carbon of the backbone of 90-mer (VPGVG)n ELPs. The average number of water molecules per alpha carbon in the first hydration shell are shown in Table 1 for valine, proline and glycine residues, respectively. All residues show a reduction in water molecules above LCST, with proline residues showing the most changes. This explains the multiple RDF peaks shown in Figure 5c that could be due to the different contribution of water molecules around the alpha carbon of valine, proline and glycine residues.

Table 1.
Number of water molecules in first hydration shell at both 290 and 340 K.
3.2.3. Solvent Accessible Surface Area (SASA)
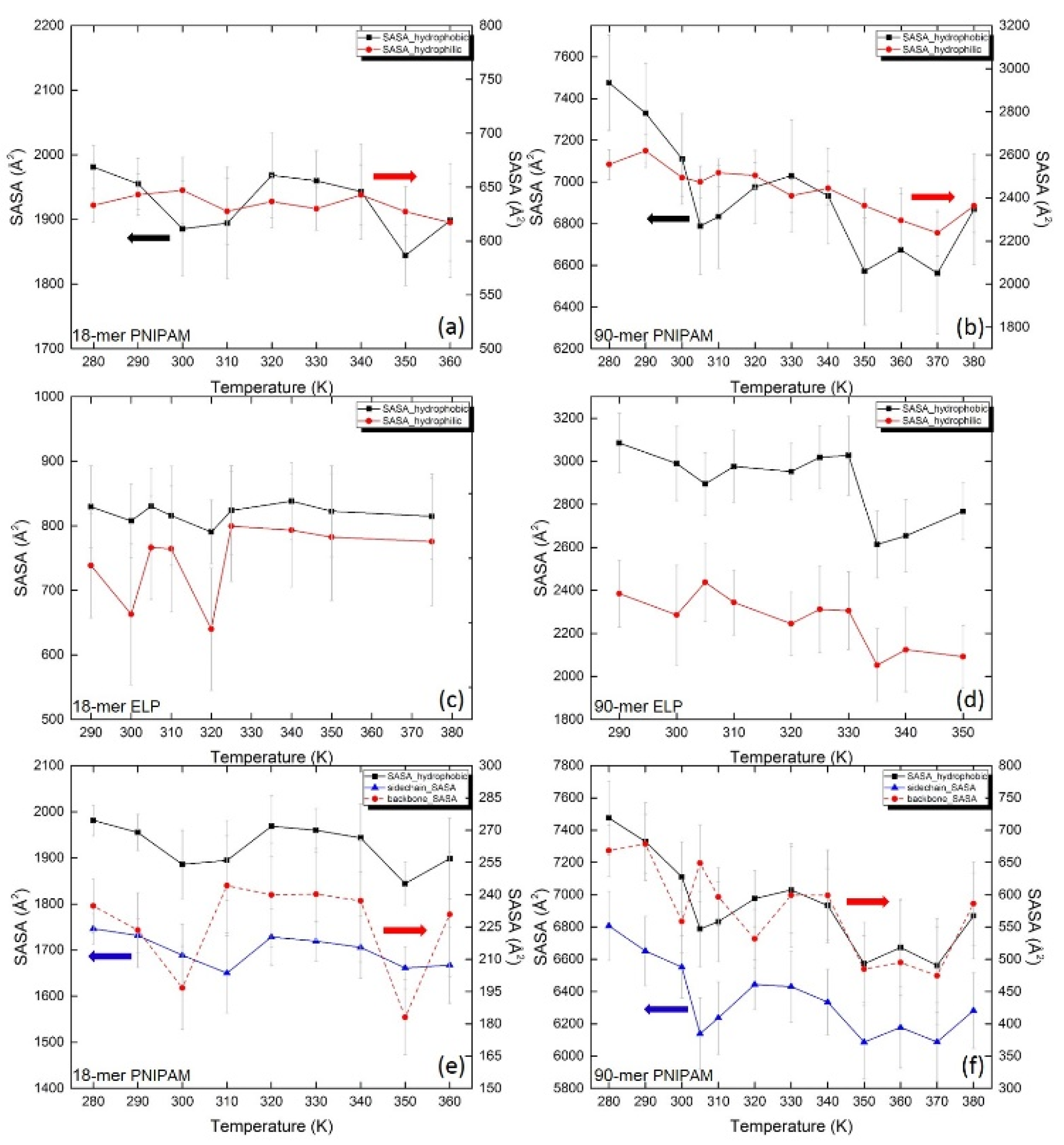
To explore the effect of temperature on water accessibility for both PNIPAM and ELPs, we calculated SASA for hydrophilic (red curves) and hydrophobic (black curves) groups (Figure 6a,b). It can be clearly seen from the red curve in Figure 6a that the SASA of hydrophilic groups for the 18-mer PNIPAM fluctuates around a mean value of 635 Å2 for all temperatures. The SASA of hydrophobic groups (black curve of Figure 6a) decreases slightly with the temperature increase and fluctuates around 1940 Å2 at all temperatures. This is not surprising since RDF shows a similar structural arrangement of water near the short chains of PNIPAM for below and above the LCST. However, in Figure 6b, the SASA of the hydrophilic groups for 90-mer PNIPAM decreases gradually from 2555 Å2 to 2363 Å2 when temperature increases from ~280 K to ~380 K. Interestingly, the hydrophilic SASA does not show any significant change around the transition temperature of PNIPAM, ~305 K. This implies that an increase in temperature does not significantly affect the SASA of the hydrophilic groups of PNIPAM. This is consistent with the previous studies where the numbers of water molecules in the first hydration shell of PNIPAM both below and above the LCST were found to be the same [37]. In addition, in the present study, our hydrogen bond analysis suggests that the Nw and npw are similar for below and above the LCST of 90-mer PNIPAM. The hydrophobic SASA of 90-mer PNIPAM decreases from 7475 Å2 to 6833 Å2 when the temperature is raised from ~280 K to ~310 K. The observed decrease in the SASA of the hydrophobic groups of PNIPAM around LCST can be attributed to the dehydration of PNIPAM chains.

Figure 6.
Solvent accessible surface area (SASA) for hydrophilic groups (red) and hydrophobic groups (black) at different temperatures of PNIPAM (a) 18-mer and (b) 90-mer and of ELP (c) 18-mer (GVG(VPGVG)3) and (d) 90-mer ((VPGVG)18). Contribution of SASA of hydrophobic groups of PNIPAM (e) 18-mer and (f) 90-mer, with (black) overall SASA of hydrophobic groups, (red dash) SASA of backbone, and (blue) SASA from isopropyl moieties in the side chain.
In Figure 6c,d, the SASAs for hydrophilic and hydrophobic groups of short and long chains of (VPGVG)n ELPs are shown as a function of temperature. We find that for short 18-mer ELP chains, SASAs of both the hydrophobic and hydrophilic groups fluctuate around a mean value of 800 Å2 (Figure 6c). In contrast, in the case of 90-mer ELPs, there is a substantial difference between hydrophobic and hydrophilic SASA; both SASAs decrease with the temperature increase and drop at around 335 K. The abrupt decrease in the SASAs of both hydrophilic and hydrophobic groups can be seen in the temperature range from ~330 K to ~335 K, which coincides with the same temperature window attributed to the changes in the hydrogen bond network and the conformation of 90-mer (VPGVG)n ELPs. This finding further supports the notion that the LCST of 90-mer (VPGVG)n ELPs is predicted to be at 332.5 ± 2.5 K [23].
Since hydrophilic groups of PNIPAM do not substantially contribute to the change in SASA with temperature, we next examine the contributions of hydrophobic groups located on the side chains and the backbone to SASA (Figure 6e,f). The isopropyl hydrophobic groups on the side-chain shows the most significant reduction in SASA with an increase in temperature from 6800 to 6100 Å2, compared to the SASA of the hydrophobic groups in the backbone, which decreases from 670 to 475 Å2.This trend is true for both 18-mer and 90-mer PNIPAM. This shows that the dehydration of the isopropyl groups located on the side chain plays an important role in determining the LCST transition in PNIPAM, which agrees with previous observations that the local environment around the PNIPAM isopropyl domains changes around LCST [83,84].
The analysis of SASA shown in Figure 6 indicates that the origin of the LCST transition in both (VPGVG)n ELPs and PNIPAM is associated with the decrease in SASA. In particular, the SASA of both hydrophilic and hydrophobic groups of 90-mer (VPGVG)n ELPs decreases when temperature increases above ~330 K. However, in the case of 90-mer PNIPAM, a significant decrease in SASA of hydrophobic groups is observed with an increase in temperature. Moreover, in PNIPAM, the dehydration of side-chains drives the LCST transition. The LCST transition in (VPGVG)n ELPs can be associated with the dehydration of both hydrophilic and hydrophobic groups. Moreover, the decrease in the SASA near hydrophilic groups in the backbone of (VPGVG)n ELPs could facilitate the formation of β-sheets [27]. This implies that the structural mechanism of the LCST transition in (VPGVG)n ELPs may be different than that of PNIPAM.
3.3. Analysis of Hydrogen Bonds between Polymer and Polymer
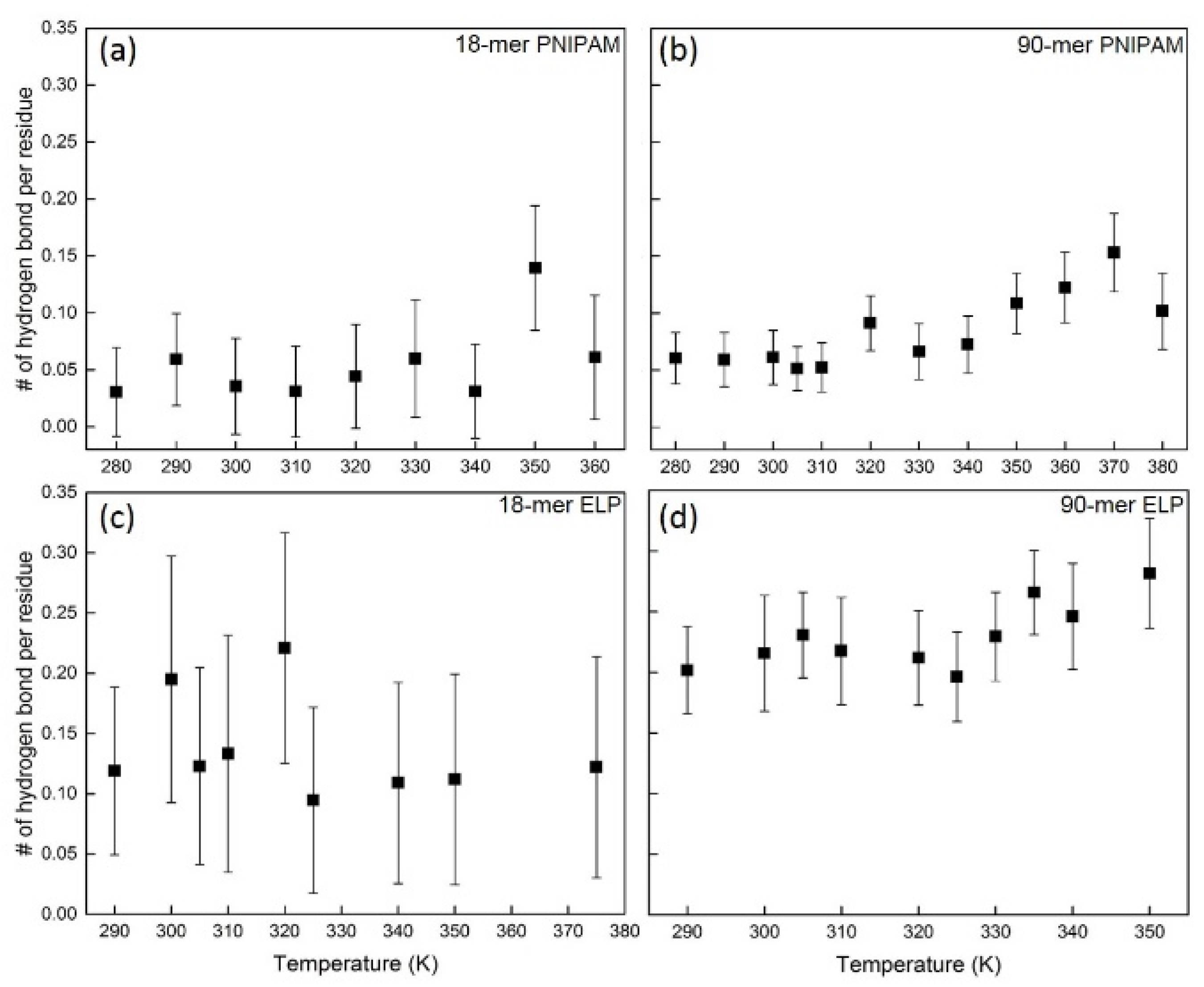
We then explored the effect of solvation dynamics, chain length, temperature, and LCST transition in (VPGVG)n ELPs and PNIPAM on intra-molecular interactions. In the case of PNIPAM, we have calculated the hydrogen bonds between the hydrophilic groups of PNIPAM side-chains (C=O and >N–H). Figure 7a,b show the hydrogen bonds between polymer and polymer for short and long chains, respectively, at all simulated temperatures. For both 18-mer and 90-mer PNIPAM, the number of hydrogen bonds between polymer and polymer increase above the LCST of PNIPAM (temperature >> ~310 K). This could be due to the fact that the structure of water near hydrophilic groups of 18-mer and 90-mer PNIPAM changes slightly as the temperature is raised above its LCST (Figure S5). The increase in the extent of polymer–polymer hydrogen bonds may stabilize polymer conformation above its inverse temperature transition.

Figure 7.
Hydrogen bonds between hydrophilic groups (C=O and >N–H) within polymer as a function of temperature for (a) 18-mer PNIPAM and (b) 90-mer PNIPAM. Number of intra-peptide hydrogen bonds per residues as a function of temperature for (c) 18-mer and (d) 90-mer ELP, GVG(VPGVG)3 and (VPGVG)18, respectively.
Figure 7c,d show the number of peptide–peptide hydrogen bonds per amino acid in both short- and long-chain (VPGVG)n ELPs, respectively. In the case of short-chain (VPGVG)n ELPs, as can be seen in Figure 7c, there is no significant change in the number of hydrogen bonds at all simulated temperatures. This could be because the extent of solvation does not change for short chains of (VPGVG)n ELPs at all simulated temperature, which prohibits hydrogen bond formation between (VPGVG)n ELPs. In the case of 90-mer (VPGVG)n ELPs, a significant increase in the intra-peptide hydrogen bonds can be observed at ~350 K. This suggests that due to structural rearrangement in the first and second hydration water shell near the backbone of (VPGVG)n ELPs above their LCST (T ≥ ~335 K), the number of peptide–peptide hydrogen bonds has increased. This increase in intra-peptide hydrogen bonds may stabilize the structure of (VPGVG)n ELPs above its LCST. Overall, the contribution of intra-peptide hydration and the strength of intra-molecular peptide interaction become accentuated in a critical temperature window (i.e., at around ~330 K to ~335 K).
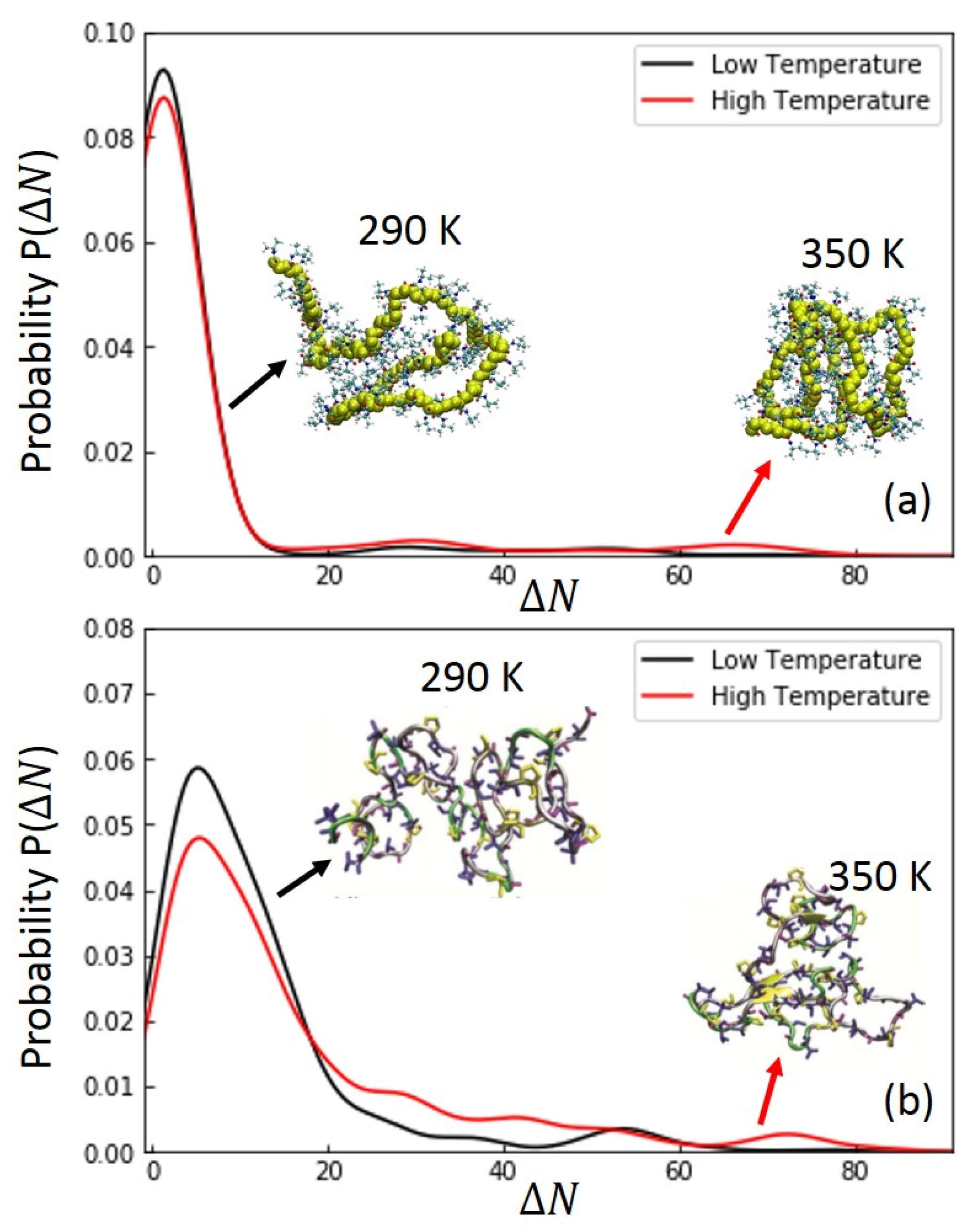
The probability distributions as a function of residue-distance (∆N) for intra-chain hydrogen bonding time (HBT) are calculated for ELPs and PNIPAM at 290 K (below LCST) and 350 K (above LCST) (Figure 8). Residue distance, ∆N, is defined as the number of residues that separate the acceptor–donor pair of hydrogen bonding. Probability distribution curves are averaged over temperatures below and above LCST separately (Figure S9). The profiles of 90-mer ELPs at low temperatures show a higher peak at short ∆N residue distance as compared to that probability distribution of HBT at high temperatures (Figure 8b) roughly above LCST, but they also show less distribution probability of hydrogen bonding time for a larger ∆N of acceptor–donor pairs. This indicates denser and more stable hydrogen bonds at a longer residue distance (∆N) and temperatures above LCST for ELPs. Together with increasing in hydrogen bonding time at a larger ∆N, the snapshot of ELPs at ~350 K shows a more compact structure as compared to the snapshot at ~290 K. In the case of 90-mer PNIPAMs, as shown in Figure 8a, the probability distribution profile of ∆N shows an increase in the extent of intra-chain hydrogen bonding time as a function of temperature as in ELPs, above and below the LCST of PNIPAM. The majority of hydrogen bonding times are for shorter residue distance ∆Ns. In Figure S9b, when temperature is above 305 K, the probability profile shows clear minor peaks for the larger ∆N. This indicates an increase in the extent of hydrogen bonds at longer residue distances (∆Ns) above its LCST. As shown in the snapshot of PNIPAM in Figure 8a, at ~350 K, a globular or spherical PNIPAM structure is prominent above LCST. The increase in intra-chain hydrogen bonding time for a larger ∆N helps to stabilize the compact structures for both 90-mer ELPs and PNIPAM above its inverse temperature transition.

Figure 8.
Hydrogen bonding time (HBT) probability distribution as function of residue distance (∆N) for (a) 90-mer PNIPAM and (b) 90-mer ELPs, respectively for temperatures below LCST (black curve) and above LCST (red curve). For ELP, the high temperature curve is averaged for simulations at temperatures above 325 K. For 90-mer PNIPAM, the high temperature curve is averaged for simulations at temperatures above 310 K. Final simulation snapshots of 90-mer PNIPAM and ELP are shown for ~290 K and ~350 K.
4. Conclusions
All-atom molecular dynamics (MD) simulations of elastin-like polypeptides (ELPs) and poly(N-isopropylacrylamide) (PNIPAM) in aqueous solutions were conducted to investigate the LCST transition and solvation dynamics. Single chains of ELPs with a Val-Pro-Gly-Val-Gly (VPGVG) sequence and PNIPAM with 18 and 90 monomer units (18-mer and 90-mer) were studied at a range of temperatures from ~280 K to ~380 K. The analysis of simulation trajectories revealed that the value of the radius of gyration (Rg) for short 18-mer (VPGVG)n ELPs fluctuates around the average value, suggesting a lack of inverse temperature transition. The simulations of 18-mer PNIPAM may imply the presence of LCST through the decrease in the value of Rg and the presence of PNIPAM chain configurations with multiple folds when the temperature is raised above ~305 K. In the case of 90-mer of PNIPAM, a significant decrease in the Rg value was observed upon increasing the temperature above their respective LCSTs. A gradual transition to a reduced radius of gyration was observed for 90-mer (VPGVG)n ELPs, which is also described as smooth shifts of structural transitions under the single-molecule scale from Tarakanova et al. [22]. This decrease in the Rg suggests a presence of compact structures of (VPGVG)n ELPs and PNIPAM above their LCST as compared to below their LCST.
The extent of the hydrogen bonds between polymer and water (npw) and the number of water molecules (Nw) in the first hydration shell for 18-mer (VPGVG)n ELPs and PNIPAM are similar for below and above LCST. Similarly, for 90-mer PNIPAM, both npw and Nw do not show any significant changes when temperature is raised above their LCST. This indicates that the structure of water near the hydrophilic groups of 18-mer of (VPGVG)n ELPs and 18-mer and 90-mer of PNIPAM does not change significantly in response to LCST transition. However, in the case of 90-mer (VPGVG)n ELPs, two different clusters of npw and Nw can be observed below and above their LCST. This suggests that the structure of water near the hydrophilic groups of 90-mer (VPGVG)n ELPs changes when the temperature is raised above their LCST. Abrupt transitions in partial dehydration along the polymer chain between amide groups were observed for both 90-mer PNIPAM and 90-mer (VPGVG)n ELPs. This localized transition during dehydration might not translated to the abrupt coil collapse, which supports a collective phenomenon that originates from the correlated gradual changes in a single polypeptide.
The analysis of RDF for hydrophilic and hydrophobic atoms in 18-mer of (VPGVG)n ELPs and PNIPAM with water molecules and calculations of solvent accessible surface area (SASA) indicates that the structure of water in the first hydration shell of the 18-mer is similar below and above the LCST. In the case of 90-mer (VPGVG)n ELPs and PNIPAM, the structure of water near the polymer changes significantly as compared to below the LCST. Raising the temperature above the LCST of 90-mer (VPGVG)n ELPs results in a decrease in SASA near its hydrophilic and hydrophobic groups, which indicates significant rearrangements of water in the first hydration shell of 90-mer (VPGVG)n ELPs. On the other hand, the SASA of the hydrophobic groups of 90-mer PNIPAM decreases significantly above its LCST, which suggests the decrease in the extent of hydration of its hydrophobic groups. More specifically, in the case of 90-mer PNIPAM, the dehydration of the hydrophobic groups in the side-chain of PNIPAM, namely the isopropyl group, drives the LCST transition. This implies that the mechanism of the LCST transition in (VPGVG)n ELPs may be different than PNIPAM.
The analysis of hydrogen bonds between polymer and polymer suggests that for 18-mer of ELPs and PNIPAM, there is no significant difference in the extent of hydrogen bonding above their LCST as compared to below the LCST. This suggests a lack of LCST inverse temperature transition in the 18-mer of both ELPs and PNIPAM. In contrast, the 90-mer (VPGVG)n ELPs and PNIPAM show an increase in the extent of hydrogen bonds above their LCST. This increase in the extent of hydrogen bonding can be attributed to the LCST inverse temperature transition in the 90-mer of (VPGVG)n ELPs and PNIPAM. As shown in the probability distribution of intra-chain hydrogen bonding time as a function of ∆N, when temperature increases, higher hydrogen bonding time distribution probability at a longer residue distance(∆N) is associated with the smaller Rg and implies a more stable and compact PNIPAM and ELP structures above LCST. Based on our simulations, the LCST inverse temperature transition in 90-mer ELPs can be attributed to a combination of thermal disruption of the network of the proximal water near both the backbone and side chain of (VPGVG)n ELPs, a reduction in the SASA of its hydrophilic and hydrophobic groups, accompanied by increase in the secondary structure and abrupt transition in dehydration along the backbone above its LCST. In the case of 90-mer of PNIPAM, the LCST inverse temperature transition is the result of the combination of a reduction in the SASA of its hydrophobic groups, the formation of intra-chain hydrogen bonds together with the partial dehydration of sidechain groups above its LCST. Our study provides new insights toward the atomistic mechanism of the LCST transition and solvation dynamics in (VPGVG)n ELPs and PNIPAM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/physchem2010005/s1, Figure S1: Chemical structure of PNIPAM monomer and (VPGVG) ELPs.; Figure S2: Partial charges calculated using different methods or different force field; Figure S3: Radius of gyration as a function of various partial charges; Table S1: Partial charges from AMBER force field; Figure S4: Orientational relaxation of backbone carbon-carbon bonds; Figures S5–S8: Radial distribution functions between PNIPAM and ELP and water; Figure S9: Hydrogen bonding time probability distribution for PNIPAM and ELP; Table S2: Radius of gyration for PNIPAM and ELP. References [85,86] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Y.G.Y., N.K.L. and S.A.D.; methodology, Y.X., N.K.L., A.S., S.A.D. and Y.G.Y.; analysis, Y.X., N.K.L. and A.S.; writing—original draft preparation, Y.X., N.K.L. and S.A.D.; writing—review and editing, Y.G.Y. and S.A.D.; supervision, Y.G.Y.; funding acquisition, Y.G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSF’s Research Triangle MRSEC (DMR-1121107). The computer support was provided by the High Performance Computing Center at North Carolina State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cheng, H.; Shen, L.; Wu, C. LLS and FTIR Studies on the Hysteresis in Association and Dissociation of Poly(N-isopropylacrylamide) Chains in Water. Macromolecules 2006, 39, 2325–2329. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C. Light-Scattering Study of Coil-to-Globule Transition of a Poly(N-isopropylacrylamide) Chain in Deuterated Water. Macromolecules 1999, 32, 4299–4301. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X. Globule-to-Coil Transition of a Single Homopolymer Chain in Solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef] [Green Version]
- Schild, H.G. Poly (N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Urry, D.W. Physical Chemistry of Biological Free Energy Transduction As Demonstrated by Elastic Protein-Based Polymers. J. Phys. Chem. B 1997, 101, 11007–11028. [Google Scholar] [CrossRef]
- Urry, D.W. Elastic Biomolecular Machines. Sci. Am. 1995, 272, 64–69. [Google Scholar] [CrossRef]
- Martino, M.; Perri, T.; Tamburro, A.M. Biopolymers and biomaterials based on elastomeric proteins. Macromol. Biosci. 2002, 2, 319–328. [Google Scholar] [CrossRef]
- Urry, D.W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 1992, 57, 23–57. [Google Scholar] [CrossRef]
- Urry, D.W.; Trapane, T.L.; Prasad, K.U. Phase-structure transitions of the elastin polypentapeptide-water system within the framework of composition-temperature studies. Biopolymers 1985, 24, 2345–2356. [Google Scholar] [CrossRef]
- Urry, D.W.; Luan, C.H.; Parker, T.M.; Gowda, D.C.; Prasad, K.U.; Reid, M.C.; Safavy, A. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J. Am. Chem. Soc. 1991, 113, 4346–4348. [Google Scholar] [CrossRef]
- Meyer, D.E.; Chilkoti, A. Genetically Encoded Synthesis of Protein-Based Polymers with Precisely Specified Molecular Weight and Sequence by Recursive Directional Ligation: Examples from the Elastin-like Polypeptide System. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, B.; Pepe, A.; Crudele, M.; Belloy, N.; Baud, S.; Dauchez, M. Tuning self-assembly in elastin-derived peptides. Soft Matter 2015, 11, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, C.; Ravindra, R.; Ludolph, B.; Winter, R. Characterization of the temperature-and pressure-induced inverse and reentrant transition of the minimum elastin-like polypeptide GVG (VPGVG) by DSC, PPC, CD, and FT-IR spectroscopy. Biophys. J. 2004, 86, 1385–1392. [Google Scholar] [CrossRef]
- Ma, X.; Sun, C.; Huang, J.; Boutis, G.S. Thermal Hysteresis in the Backbone and Side-Chain Dynamics of the Elastin Mimetic Peptide [VPGVG]3 Revealed by 2H NMR. J. Phys. Chem. B 2011, 116, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosalie, L.M.; áde Wolf, F.A.; ávan Hest, J.C.M. Elastin-like polypeptides of different molecular weights show independent transition temperatures when mixed. Soft Matter 2009, 5, 4305–4310. [Google Scholar]
- Nuhn, H.; Klok, H.-A. Secondary Structure Formation and LCST Behavior of Short Elastin-Like Peptides. Biomacromolecules 2008, 9, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkoti, A. Quantification of the Effects of Chain Length and Concentration on the Thermal Behavior of Elastin-like Polypeptides. Biomacromolecules 2004, 5, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. On the molecular mechanisms of elastin coacervation and coacervate calcification. Faraday Discuss. Chem. Soc. 1976, 61, 205–212. [Google Scholar] [CrossRef]
- Urry, D.; Long, M.; Cox, B.; Ohnishi, T.; Mitchell, L.; Jacobs, M. The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates. Biochim. Biophys. Acta (BBA)—Protein Struct. 1974, 371, 597–602. [Google Scholar] [CrossRef]
- Luan, C.H.; Urry, D.W. Solvent deuteration enhancement of hydrophobicity: DSC study of the inverse temperature transition of elastin-based polypeptides. J. Phys. Chem. 1991, 95, 7896–7900. [Google Scholar] [CrossRef]
- Pepe, A.; Armenante, M.R.; Bochicchio, B.; Tamburro, A.M. Formation of nanostructures by self-assembly of an elastin peptide. Soft Matter 2008, 5, 104–113. [Google Scholar] [CrossRef]
- Tarakanova, A.; Huang, W.; Weiss, A.; Kaplan, D.L.; Buehler, M.J. Computational smart polymer design based on elastin protein mutability. Biomaterials 2017, 127, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Li, N.; Yingling, Y.; Hall, C.K. LCST Behavior is Manifested in a Single Molecule: Elastin-Like polypeptide (VPGVG)n. Biomacromolecules 2015, 17, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, F.G.; Li, N.K.; Roberts, S.; Weber, P.; Dzuricky, M.; Weitzhandler, I.; Yingling, Y.G.; Chilkoti, A. Intrinsically disordered proteins access a range of hysteretic phase separation behaviors. Sci. Adv. 2019, 5, eaax5177. [Google Scholar] [CrossRef] [Green Version]
- Li, N.K.; Xie, Y.; Yingling, Y.G. Insights into Structure and Aggregation Behavior of Elastin-like Polypeptide Coacervates: All-Atom Molecular Dynamics Simulations. J. Phys. Chem. B 2021, 125, 8627–8635. [Google Scholar] [CrossRef] [PubMed]
- Li, N.K.; Roberts, S.; Quiroz, F.G.; Chilkoti, A.; Yingling, Y.G. Sequence Directionality Dramatically Affects LCST Behavior of Elastin-Like Polypeptides. Biomacromolecules 2018, 19, 2496–2505. [Google Scholar] [CrossRef]
- Li, N.; Quiroz, F.G.; Hall, C.; Chilkoti, A.; Yingling, Y.G. Molecular Description of the LCST Behavior of an Elastin-Like Polypeptide. Biomacromolecules 2014, 15, 3522–3530. [Google Scholar] [CrossRef]
- Grabowski, C.A.; Mukhopadhyay, A. Contraction and Reswelling of a Polymer Chain Near the Critical Point of a Binary Liquid Mixture. Phys. Rev. Lett. 2007, 98, 207801. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, A.X.; Wu, C. Comparison of the Coil-to-Globule and the Globule-to-Coil Transitions of a Single Poly(N-isopropylacrylamide) Homopolymer Chain in Water. Macromolecules 1998, 31, 2972–2976. [Google Scholar] [CrossRef]
- Furyk, S.; Zhang, Y.; Ortiz-Acosta, D.; Cremer, P.S.; Bergbreiter, D.E. Effects of end group polarity and molecular weight on the lower critical solution temperature of poly(N-isopropylacrylamide). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1492–1501. [Google Scholar] [CrossRef]
- Tong, Z.; Zeng, F.; Zheng, X.; Sato, T. Inverse Molecular Weight Dependence of Cloud Points for Aqueous Poly(N-isopropylacrylamide) Solutions. Macromolecules 1999, 32, 4488–4490. [Google Scholar] [CrossRef]
- Pamies, R.; Zhu, K.; Kjøniksen, A.-L.; Nyström, B. Thermal response of low molecular weight poly-(N-isopropylacrylamide) polymers in aqueous solution. Polym. Bull. 2009, 62, 487–502. [Google Scholar] [CrossRef]
- Du, H.; Wickramasinghe, R.; Qian, X. Effects of Salt on the Lower Critical Solution Temperature of Poly (N-Isopropylacrylamide). J. Phys. Chem. B 2010, 114, 16594–16604. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Yang, H.; Ma, J.; Cheng, R. Solvation Behaviors of N-Isopropylacrylamide in Water/Methanol Mixtures Revealed by Molecular Dynamics Simulations. J. Phys. Chem. B 2010, 114, 8652–8658. [Google Scholar] [CrossRef]
- Ortiz de Solorzano, I.; Bejagam, K.K.; An, Y.; Singh, S.K.; Deshmukh, S.A. Solvation dynamics of N-substituted acrylamide polymers and the importance for phase transition behavior. Soft Matter 2020, 16, 1582–1593. [Google Scholar] [CrossRef]
- Bejagam, K.K.; Singh, S.K.; Ahn, R.; Deshmukh, S.A. Unraveling the Conformations of Backbone and Side Chains in Thermosensitive Bottlebrush Polymers. Macromolecules 2019, 52, 9398–9408. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Suthar, K.; Mancini, D.C. Role of Solvation Dynamics and Local Ordering of Water in Inducing Conformational Transitions in Poly(N-isopropylacrylamide) Oligomers through the LCST. J. Phys. Chem. B 2012, 116, 2651–2663. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Mancini, D.C. Vibrational Spectra of Proximal Water in a Thermo-Sensitive Polymer Undergoing Conformational Transition Across the Lower Critical Solution Temperature. J. Phys. Chem. B 2012, 116, 5501–5515. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Li, Z.; Kamath, G.; Suthar, K.J.; Sankaranarayanan, S.K.; Mancini, D.C. Atomistic insights into solvation dynamics and conformational transformation in thermo-sensitive and non-thermo-sensitive oligomers. Polymer 2013, 54, 210–222. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Kamath, G.; Suthar, K.J.; Mancini, D.C.; Sankaranarayanan, S.K.R.S. Non-equilibrium effects evidenced by vibrational spectra during the coil-to-globule transition in poly(N-isopropylacrylamide) subjected to an ultrafast heating–cooling cycle. Soft Matter 2013, 10, 1462–1480. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.; Mancini, D.C. Atomic scale characterization of the conformational dynamics of a thermo-sensitive and a non-thermo-sensitive oligomer using vibrational spectra obtained from molecular dynamics. Polymer 2012, 53, 1306–1320. [Google Scholar] [CrossRef]
- Maeda, Y.; Higuchi, T.; Ikeda, I. Change in Hydration State during the Coil−Globule Transition of Aqueous Solutions of Poly(N-isopropylacrylamide) as Evidenced by FTIR Spectroscopy. Langmuir 2000, 16, 7503–7509. [Google Scholar] [CrossRef]
- Yamauchi, H.; Maeda, Y. LCST and UCST Behavior of Poly(N-isopropylacrylamide) in DMSO/Water Mixed Solvents Studied by IR and Micro-Raman Spectroscopy. J. Phys. Chem. B 2007, 111, 12964–12968. [Google Scholar] [CrossRef] [PubMed]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Changes in the Hydration States of Poly(N-alkylacrylamide)s during Their Phase Transitions in Water Observed by FTIR Spectroscopy. Macromolecules 2001, 34, 1391–1399. [Google Scholar] [CrossRef]
- Lue, S.J.; Chen, C.-H.; Shih, C.-M. Tuning of Lower Critical Solution Temperature (LCST) of Poly(N-Isopropylacrylamide-co-Acrylic acid) Hydrogels. J. Macromol. Sci. Part B 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Prog. Polym. Sci. 2002, 27, 1165–1193. [Google Scholar] [CrossRef]
- Ohya, S.; Nakayama, Y.; Matsuda, T. Thermoresponsive Artificial Extracellular Matrix for Tissue Engineering: Hyaluronic Acid Bioconjugated with Poly(N-isopropylacrylamide) Grafts. Biomacromolecules 2001, 2, 856–863. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Ahmed, Z.; Gooding, E.A.; Pimenov, K.V.; Wang, L.; Asher, S.A. UV Resonance Raman Determination of Molecular Mechanism of Poly(N-isopropylacrylamide) Volume Phase Transition. J. Phys. Chem. B 2009, 113, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Gangemi, F.; Longhi, G.; Abbate, S.; Lebon, F.; Cordone, R.; Ghilardi, G.P.; Fornili, S.L. Molecular Dynamics Simulation of Aqueous Solutions of 26-Unit Segments of p(NIPAAm) and of p(NIPAAm) “Doped” with Amino Acid Based Comonomers. J. Phys. Chem. B 2008, 112, 11896–11906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Qian, X. Molecular dynamics simulations of PNIPAM-co -PEGMA copolymer hydrophilic to hydrophobic transition in NaCl solution. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1112–1122. [Google Scholar] [CrossRef]
- de Oliveira, T.E.; Mukherji, D.; Kremer, K.; Netz, P.A. Effects of stereochemistry and copolymerization on the LCST of PNIP. Am. J. Chem. Phys. 2017, 146, 034904. [Google Scholar] [CrossRef]
- Singh, R.; Deshmukh, S.A.; Kamath, G.; Sankaranarayanan, S.K.R.S.; Balasubramanian, G. Controlling the aqueous solubility of PNIPAM with hydrophobic molecular units. Comput. Mater. Sci. 2017, 126, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Meyer, D.; Shin, B.; Kong, G.; Dewhirst, M.; Chilkoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef]
- Quiroz, F.G.; Chilkoti, A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 2015, 14, 1164–1171. [Google Scholar] [CrossRef]
- Urry, D.; Khaled, M.; Rapaka, R.; Okamoto, K. Nuclear overhauser enhancement evidence for inverse temperature dependence of hydrophobic side chain proximity in the polytetrapeptide of tropoelastin. Biochem. Biophys. Res. Commun. 1977, 79, 700–706. [Google Scholar] [CrossRef]
- Asakura, T.; Ashida, J.; Ohgo, K. Conformational Characterization of (Val-Pro-Gly-Val-Gly)6 with 13C Solid State NMR. Polym. J. 2003, 35, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Isailovic, D.; McMillan, R.; Conticello, V. Structure of an elastin-mimetic polypeptide by solid-state NMR chemical shift analysis. Biopolymers 2003, 70, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Berryman, J.; Betz, R.M.; Cerutti, D.S.; Cheatham Iii, T.E.; Darden, T.A.; Kollman, P.A. AMBER 2015; University of California: San Francisco, CA, USA, 2015. [Google Scholar]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E. Amber 11; University of California: San Francisco, CA, USA, 2011. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Vanquelef, E.; Simon, S.; Marquant, G.; Garcia, E.; Klimerak, G.; Delepine, J.C.; Cieplak, P.; Dupradeau, F.-Y. RED Server: A web service for deriving RESP and ESP charges and building force field libraries for new molecules and molecular fragments. Nucleic Acids Res. 2011, 39, W511–W517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Mumby, S.J.; Maple, J.R.; Hagler, A.T. An ab Initio CFF93 All-Atom Force Field for Polycarbonates. J. Am. Chem. Soc. 1994, 116, 2978–2987. [Google Scholar] [CrossRef]
- Hirano, T.; Okumura, Y.; Kitajima, H.; Seno, M.; Sato, T. Dual roles of alkyl alcohols as syndiotactic-specificity inducers and accelerators in the radical polymerization of N-isopropylacrylamide and some properties of syndiotactic poly(N-isopropylacrylamide). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4450–4460. [Google Scholar] [CrossRef]
- Haley, B.P.; Wilson, N.; Li, C.; Arguelles, A.; Jaramillo, E.; Strachan, E. Polymer Modeler; Network for Computational Nanotechnology, Purdue University: West Lafayette, IN, USA, 2016. [Google Scholar] [CrossRef]
- Urry, D.W. Molecular Machines: How Motion and Other Functions of Living Organisms Can Result from Reversible Chemical Changes. Angew. Chem. Int. Ed. 1993, 32, 819–841. [Google Scholar] [CrossRef]
- Urry, D.W.; Trapane, T.L.; Sugano, H.; Prasad, K.U. Sequential polypeptides of elastin: Cyclic conformational correlates of the linear polypentapeptide. J. Am. Chem. Soc. 1981, 103, 2080–2089. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Shikata, T. Contrary Hydration Behavior of N-Isopropylacrylamide to its Polymer, P(NIPAm), with a Lower Critical Solution Temperature. J. Phys. Chem. B 2007, 111, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Shikata, T. Hydration and Dynamic Behavior of Poly(N-isopropylacrylamide)s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, J.C.; Alonso, M.; Pérez, T.; Herguedas, M.M. Differential scanning calorimetry study of the hydrophobic hydration of the elastin-based polypentapeptide, poly(VPGVG), from deficiency to excess of water. Biopolymers 2000, 54, 282–288. [Google Scholar] [CrossRef]
- Cho, Y.; Sagle, L.B.; Iimura, S.; Zhang, Y.; Kherb, J.; Chilkoti, A.; Scholtz, J.M.; Cremer, P.S. Hydrogen Bonding of β-Turn Structure Is Stabilized in D2O. J. Am. Chem. Soc. 2009, 131, 15188–15193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, M.; Maeda, Y.; Kitano, H. Effect of Hydrophobicity of Amino Acids on the Structure of Water. J. Phys. Chem. B 1997, 101, 7022–7026. [Google Scholar] [CrossRef]
- Abbott, L.J.; Tucker, A.K.; Stevens, M.J. Single Chain Structure of a Poly(N-isopropylacrylamide) Surfactant in Water. J. Phys. Chem. B 2015, 119, 3837–3845. [Google Scholar] [CrossRef]
- Kauzmann, W. Some Factors in the Interpretation of Protein Denaturation. Adv. Protein Chem. 1959, 14, 1–63. [Google Scholar] [CrossRef]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]
- Tucker, A.K.; Stevens, M.J. Study of the Polymer Length Dependence of the Single Chain Transition Temperature in Syndiotactic Poly(N-isopropylacrylamide) Oligomers in Water. Macromolecules 2012, 45, 6697–6703. [Google Scholar] [CrossRef]
- Pelton, R. Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J. Colloid Interface Sci. 2010, 348, 673–674. [Google Scholar] [CrossRef]
- Galbraith, M.L.; Madura, J.D. Identifying trends in hydration behavior for modifications to the hydrophobicity of poly(N-isopropylacrylamide). J. Mol. Graph. Model. 2017, 78, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.F.; Theodorou, D.N. Molecular Dynamics Simulation of a Glassy Polymer Surface. Macromolecules 1991, 24, 6283–6294. [Google Scholar] [CrossRef]
- Weber, T.A.; Helfand, E. Time-correlation functions from computer simulations of polymers. J. Phys.Chem. 1983, 87, 2881–2889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).