Abstract
Saprotrophic and lignocellulolytic fungi from tropical areas especially represent a promising yet relatively underexplored frontier for both taxonomy and applied research. This makes ex situ conservation through culture collections of paramount importance. Here, 10 lignocellulolytic strains isolated from the State of São Paulo (Brazil) and deposited in the Brazilian Culture Collection (now CCIBt) were identified through the ITS region. In order to prevent accidental losses, these strains have been shared with the collection of the University of Milano—Department of Food, Environmental and Nutritional Sciences (DeFENS), as well as the MicUNIPV Fungal Research Culture Collection—University of Pavia (Italy). Most of the fungal species in the examined set exhibit a neotropical distribution, while 3 out of 10 are nowadays recognized as subcosmopolitan despite their prevalence in the neotropical area. One holotropical, one cosmopolitan and one holarctic species are also present. Based on the literature, 8 out of the 10 characterized species are known to produce psilocybin (e.g., Psilocybe cubensis and Candolleomyces candolleanus) and/or enzymes with potential applications in environmental and medical biotechnology (e.g., Lentinus crinitus). All 10 strains were described for their micro- and macro-characteristics; their growth rate was evaluated and culture pictures provided. Taxonomic and nomenclatural controversies concerning Candolleomyces candolleanus, Cubamyces lactineus and Pycnoporus sanguineus are discussed.
1. Introduction
Fungal culture collections have been increasing their importance as tools for conservation, studies and applied research in mycology [1,2,3,4]. In fact, they allow storage, preservation and the on-demand reproduction of fungal organisms at different developmental stages depending on the species and the experimental protocol. Since 1980, the World Federation for Culture Collections (WFCC) [5] has been committed to providing standardized protocols and guidelines for existing and upcoming culture collections. The WFCC network currently includes 104 partners worldwide, focusing on fungi, other microbes or both. Regarding the European collections, the Microbial Resource Research Infrastructure (MIRRI) project [6] integrates contributions from over 50 official partners including Biological Resource Centers (mBRCs), culture collections and several research institutes across 10 European countries. Moreover, each official partner can also collect additional resources from non-official partners, such as the collections of local institutions or private collections. Draining samples from the territory allows for an increase in territorial representativeness, also enhancing the role of contributors otherwise prevented from accessing these resources. Strains are thus a resource that allows a representation of diversity and a fundamental tool for the ex situ conservation of species. Since one of the main causes of diversity loss is habitat degradation, preserving species ex situ is a method to avoid resource loss for studies and research, and possibly allows us to reintroduce them in situ [7].
Strains to be applied in any experimental application must be identified by both morphology and molecular barcoding; in fungi, and in Agaricomycetes in particular, the latter is mainly based on the ITS region (Internal Transcribed Spacer of nuclear rDNA), which has been suggested to successfully discriminate the wide majority of taxa. Even when insufficient, per se, the ITS region is usually regarded as the first stepstone marker for concatenation in the multilocus approach [8]. However, such a double check does not exclude the need for the comprehensive analysis of further aspects that contribute to disambiguating the strain identification, such as ecology (host, possible trophic mode, phytoclimate in growth locality), distribution (geographic location of sampling localities), nomenclatural issues, potential misinterpretation in newly generated and previously deposited sequences in repositories and other pitfalls, such as possible species complexes and polyphyletic taxa [9].
Strains maintained in pure culture are representative of diversity at both the interspecific and intraspecific level, since each one introduces an unpredictable component of variability that makes it unique in terms of growth profiles (e.g., at different temperatures), biomass yield in selected conditions, the secretion and/or synthesis of target metabolites, and so forth [10]. Consequently, obtaining as many strains as possible even within the same species is crucial for adequately representing the diverse features of the species itself. Ideally, at least in culturable Basidiomycota, both monokaryon from basidiospore germination and dikaryon e.g., from the context (i.e., the internal pulp) should be achieved to fully display the morphological and metabolic features of each species. Monokaryon strains are mainly functional in mating tests (both for taxonomy and applied purposes). On the other hand, dikaryon strains are ready-to-use for all experiments, implying an advanced substrate degradation and metabolic changes finalized to sexual reproduction, that is, changes in mycelial morphology, primordia production and basidiomata.
Fungal biogeography has not been investigated as deeply as in plants [11], and the concept of ecotype also remains poorly defined, both in terms of ecological issues and in terms of the valorization of metabolic potential in selected strains. These are two sides of the same coin: the ecotype concept has been often argued to be vague, since the isolation barriers to mating are incomplete and therefore poorly support the search for adaptation evidence. Analogously, the environmental constraints driving the ecotype evolution could be unclear and mistaken, with random genetic drift in allopatric conditions or along divergence clines. This is why the comparison of conspecific strains, as well as congeneric, from different regions of an apparently unitary distribution area can shed light on the variability spectrum.
Tropical regions, broadly defined as neotropical and paleotropical kingdoms in phytogeography [12], represent the new frontier of mycology, offering extensive and unexplored resources from both taxonomy and technology perspectives. Tropical samples have been revealing new challenges for taxonomy and species concepts, particularly in many wood decay fungi, as extensively reported in the very wide literature devoted to neotropical and African polypores by Ryvarden and coauthors [13,14], as well as by Decock and Amalfi [15,16,17]. This has been contributing to sweeping away many obsolete approximations in species chorology by achieving new molecular and ecological data, such as with Hymenochaetaceae Donk, which are often impossible to disentangle on a merely phenetic basis.
On the other hand, strains adapted to an aseasonal climate may be easier to manage for several applied purposes, as they are expected to require fewer adjustments in temperature and humidity to simulate often unpredictable internal clocks [18]. This characteristic could increase the reproducibility of growth/metabolic processes in cultivation batches, therefore favoring strain selection for biotechnological applications.
The aim of this work is to present and basically characterize a set of fungal strains collected from 1992 to 1994 in different areas of São Paulo State (Brazil) [19] and shared by the Culture Collection CCB (Colecao de Culturas de Basidiomicetos) of the Instituto de Botanica, São Paulo (Brazil)—now CCIBt (Collection of Algae, Cyanobacteria and Fungi Cultures) and the culture collection of the University of Milano-Department of Food, Environmental and Nutritional Sciences (DeFENS). The strains are also preserved in MicUNIPV, the fungal research culture collection of the Department of Earth and Environmental Sciences of University of Pavia (Italy), and are accessible for further investigation across various disciplines. This work therefore remarks on (a) how the conservation value of culture collections increases by introducing morphological and geographical diversity in the strain set; and (b) how important multiple storage is, since sharing the strain set has allowed us to preserve it entirely despite accidental losses occurring in the respective collections.
2. Materials and Methods
2.1. Sampling and Isolation
The full strain set is listed in Table 1, along with the identification codes [19,20]. Please note that all the requirements by the new Brazilian legislation on access to biodiversity (Law 13,123/15 and Decree 8772/16) have been formally addressed [21].

Table 1.
Fungal isolates in the study. All the strains were isolated from the State of São Paulo (Brazil). § = strain code in the culture collection of DeFENS (Department of Food, Environmental and Nutritional Sciences, Università degli Studi di Milano); * = strain code in CCB (Coleção de Culturas de Basidiomycetes); CCIBt = Collection of Algae, Cyanobacteria and Fungi Cultures. Please note that CCB no longer exists and its collections are now part of CCIBt.
All strains were maintained on 5 cm plates containing MEA modified as follows (g/L in distilled water): glucose 20, malt extract 20, soybean peptone 1 and agar 15. The inoculum was placed by depositing around 1 cm2 of an older (max 2 months) solid culture plate on the surface, using a sterile lancet. The plates were subsequently incubated at 25 °C in the dark. Once mycelium completely covered the surface of the solid culture, the plates were sealed with Parafilm and stored at 4 °C in both the UniMI-DeFENS, University of Milano (Italy) and MicUNIPV Fungal Research Culture Collection of DSTA, University of Pavia (Italy). The strains are refreshed every 2 months. All strains are preserved at 4 °C; at least one clone per strain was also transferred to aqueous glycerol medium [4].
2.2. Identification
Morphological characteristics of mycelia were checked in the DSTA-UNIPV Laboratory of Mycology using a Nikon LABOPHOT-2 microscope.
In order to achieve biomass for molecular identification, mycelia were grown in ME 2% broth and incubated at 25 °C, then washed with sterilized distilled water and lyophilized; about 20 mg per sample was used for extraction of total genomic DNA. Extraction was obtained by means of Macherey-Nagel Nucleospin Plant II Kit, based on the manufacturer’s instructions. A protocol that uses a modified CTAB was applied.
PCR was conducted by means of a DreamTaq Green Master Mix and ITS1-ITS4 primers (Thermofisher, Waltham, MA, USA) in a MJ Mini Biorad thermocycler (Biorad, Berkeley, CA, USA) set as follows: starting denaturation at 95 °C for 5 min; 35 cycles including 30 s denaturation at 95 °C, 45 s annealing at 50 °C and 1 min elongation at 72 °C; and final elongation at 72 °C for 10 min. Amplified DNA was purified using ExoSAP-IT™ PCR Product Cleanup Reagent based on the manufacturer’s instructions. Next, 1% agarose gel was stained with SYBR™ Safe DNA Gel Stain—Thermo Fisher Scientific, and imaging was obtained using Gel Doc (Biorad, Berkeley, CA, USA) [3].
Sanger sequencing was commissioned to Macrogen (Milano, Italy), and sequences were edited by Sequencher 5.0, then analyzed using both Mycobank Molecular ID [22] and NCBI BLAST (highly similar sequences—Megablast) [23] to cross-check the identification parameters and sources. Only identities ≥97% were considered valid. The ITS sequences were deposited in GenBank NCBI [24], and accessions are reported in Table 1.
The main environmental features of sampling localities were retrieved from the inventory reported by Instituto Brasileiro de Geografia e Estatística–IBGE (2025) [25] and Sano et al. [26]. Based on this, the Assis sampling area belongs to the Cerrado biome, and the other sampling localities belong to the Atlantic Rainforest biome, despite the fact that limited data are available for the highly anthropized surroundings of São Paulo, as well as for Ribeirão Grande. In summary, the Assis sampling area belongs to the Cerrado biome and Vão do Paranã ecoregion, which is classified as a high-environmental-liability area; the precipitation range is 1400–1600 mm y−1. Assis is located on a Ferralsol plateau with an average altitude of 528 m and 4.8% average slope.
2.3. Distribution
Distribution was assessed based on a cross-check of different sources, namely GBIF record maps [27]. Indications of geographic origin are reported in sequences deposited in GenBank; records are in the Neotropical Fungi archive [28]. Other scientific literature is contextually cited in the following paragraphs.
The biogeographic nomenclature recalls the proposal by Carta et al. [29] for phytogeographic subkingdoms and regions.
2.4. Culture Characterization
In order to perform culture description and evaluate growth rate, fresh cultures were prepared to obtain actively growing mycelium. Three replicates per strain were set by inoculating them in Petri dishes (90 mm diameter) and placing the agar plug at the edge of the dish. Edge inoculation was preferred in order to measure colony growth for longer times compared to central inoculation. Petri dishes were incubated at 25 °C in the dark until complete colonization or up to 6 weeks. Mycelium growth was quantified everyday by measuring the colony radius (i.e., distance between colony edge and the side of the inoculated agar plug) with a calliper (0.1 mm resolution). Growth rate was calculated as the average growth per day (mm/day) among the three replicates [4,30].
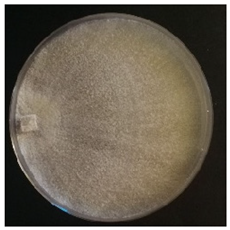
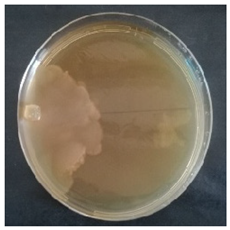
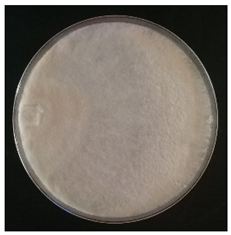
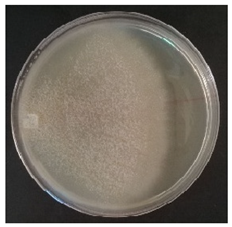
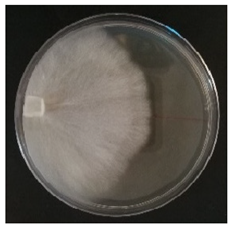
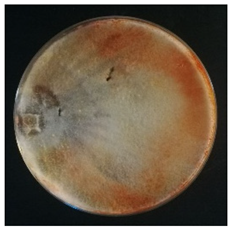
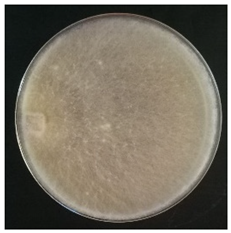
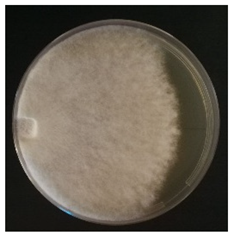
Culture characteristics were described as the macro- and microscopical features (i.e., colony color, aspect of aerial mycelium, reverse color clamps and hyphal structures). Samples were observed in lacto-fuchsin using a Nikon LABOPHOT-2 (Nikon, Minato, Tokyo, Japan) microscope and a Zeiss Stemi 2000-C (Zeiss, Oberkochen, Germany) stereomicroscope. Culture characteristics are reported as code numbers corresponding to defined characteristics (Table 2), as already proposed in Buratti et al. [30] and Cartabia et al. [4].

Table 2.
Macro- and micro-morphological features and corresponding codes.
3. Results
The overall set of isolated and identified strains is reported in Table 3, together with some featuring references about distribution and properties. Nomenclature follows Index Fungorum [31] and Mycobank [32], if not otherwise specified. Consistently with the isolation method described above, which is from context or subicular hyphae, all the strains are to be considered dikaryotic and potentially able to produce basidiomata.

Table 3.
Features and references of the characterized species. Information and references are reported concerning application aspects only.
Strains’ macro- and micro-culture characteristics, growth rate and colony morphology are reported in Table 4. Notes about particular non-coded features and references to pre-existing descriptions are reported as well. Among the species listed in Table 4, 6 out of 15 do not yet have a description of their culture characteristics in the literature. Stereum gausapatum was found to be the species with the highest average growth rate (average of 0.69 mm/day), while the one with the lowest was Gloeophyllum striatum (average of 0.1 mm/day).

Table 4.
Mycelium and colony features of the 15 isolated species. Numerical codes for culture characteristics refer to morphological features reported in Table 2.
4. Discussion
Based on the Index Fungorum [31], the most represented orders in the present strain set are Polyporales Gäum, including five species from three different families (Cerrenaceae Miettinen, Justo & Hibbett, Polyporaceae Fr. ex Corda and Incrustoporiaceae Jülich); and Agaricales Underw., including four species from two families (Psathyrellaceae Vilgalys, Moncalvo & Redhead and Galeropsidaceae Singer.). Typical wood-related taxa in Russulales Kreisel ex P.M. Kirk, P.F. Cannon & J.C. David are represented by Stereum Hill ex Pers. As shown in Table 5, the strains belong to species poorly represented or not represented in the major international culture collections.

Table 5.
Number of strains of the examined species preserved in some of the major culture collections in the world. CBS: Centraal Bureau voor Schimmelcultures [68]; MUT: Mycotheca Universitatis Taurinensis [69]; MUCL: Mycotheque de l’Université catholique de Louvain [70]; LE-BIN/VKM: Basidiomycetes Culture Collection Komarov Botanical Institute RAS [71]; CABI: CABI Bioscience [72].
As a whole, species in Psathyrellaceae cover the widest distribution of this selection of strains, including neotropical, subcosmopolitan and cosmopolitan species. Polyporaceae in the present set include neotropical and subcosmopolitan species only, whereas Cerrenaceae and Gloeophyllaceae include only one holotropical and one neotropical strain, respectively. Other strains in Stereaceae are to be considered native to the holarctic. At the order level, Polyporales include the most neotropical species.
According to Index Fungorum and Mycobank, Candolleomyces candolleanus is considered the current name and obligatory synonym of Psathyrella candolleana (Fr.) Maire; however, many sequences are still deposited in the databanks as P. candolleana, generating confusion or leading to an underestimate of the sequence set actually available for analyses. Based on the available references, this species is apparently cosmopolitan and displays an adaptability to different climates in different biogeographic regions of the world.
Though unlikely, C. caperata [syn. Coriolopsis caperata (Berk.) Murrill] is related to tropical climates in the neotropical and African regions, where it is widespread. Increasing the number of strains in culture collections is thus functional for exploring the diversity in such apparent disjunctions. Whether the examined species sensu stricto is truly cosmopolitan, or whether it is a species complex to disentangle, each population may have, in fact, evolved adaptations to local environmental constraints, and this may reflect different metabolic peculiarities to be addressed and characterized in cultivation and biotechnological applications.
According to both Mycobank and Index Fungorum, the species name Cubamyces lactineus has been replacing Leiotrametes lactinea (Berk.) Welti & Courtec. and Trametes lactinea (Berk.) Sacc., which are now both declassed to synonyms. At the genus level, analyses of the ITS region suggest a substantial synonymy between Leiotrametes Welti & Courtec. and Cubamyces Murrill; in this light, the C. lactineus clade should be segregated from other Asian lineages [73]. Based on the same reference, the C. lactineus clade also includes sequences of C. cubensis (Mont.) Murrill, and the latter, of course, were described based on neotropical specimens [74,75]. Interestingly, Mycobank only accepts C. cubensis, while Index Fungorum indicates Trametes cubensis (Mont.) Sacc. as the current name. However, the same mentioned work by Lucking et al. [76] suggests that C. cubensis phylogeny cannot be conclusively resolved based solely on the ITS region, as the sequences are mostly split into several clades. Such an inconsistency of the ITS-only approach was also found in Vlasák and Kout [73], where T. lactinea was found to be widespread in Florida, at the edge of the neotropical subkingdom. It should be highlighted that C. lactineus can be discriminated from C. cubensis/T. cubensis through some significant morphological features, as recently remarked by Zmitrovich et al. [77]. Therefore, as observed by Justo et Hibbett [78], the phylogenetic relationships among neotropical strains still appear unclear, although C. cubensis may be synonymized with C. lactineus. Considering both the morphology and ITS similarities, the strain investigated in this study has been attributed to C. lactineus, despite the ambiguity arising from comparison sequences from both the Mycobank Molecular ID and BLAST [22,23]. Finally, such a complex taxonomical debate indicates how important it is to preserve the different expression of local strains, particularly when the true distribution area of the species is uncertain and/or relatively small.
Pycnoporus P. Karst., a genus related to Cubamyces/Leiotrametes, is also involved in the general revision of these taxa; based on the 5-marker concatenation by Justo et Hibbett [78], they all share the same clade. While Mycobank accepts the species P. sanguineus, Index Fungorum indicates Fabisporus sanguineus (L.) Zmitr. as the current name. Trametes sanguinea (L.) Lloyd and T. sanguinea Corner are to be considered obsolete synonyms of F. sanguineus/P. sanguineus based on both Mycobank and Index Fungorum [31,32]. The clear morphological characteristics [79] both in basidiomata and the culture are consistent with the high ITS support. In this case, one can notice that several strains are detained by European culture collections and presumably reflect a prevalence of European origin; in the present work, extra-European strains represent extra-sources of variability and bioactivity potential.
Stereum gausapatum assessment was also critical, since the available data strongly suggest a holarctic distribution, with only a few scattered records from South America. The strain examined in the present study displays culture characteristics consistent with those outlined by Stalpers [66] for S. gausapatum, thereby confirming the basidiome morphological identification. The ITS sequence exhibits 100% and 98.52% similarity with two sequences of Stereum sp. obtained from Hevea brasiliensis by Martin et al. [80], and it shows an over >97% similarity with a few S. gausapatum sequences. Notably, both the morphology and ecology are strikingly discriminant in this case with respect to S. sanguinolentum and exclude the debated American species S. ostrea (Blume & T. Nees) Fr. and its possible complex.
The current and potential applications of the characterized species have been reviewed in Table 3, and it is noteworthy that all the species are culturable on easily reproducible, low-cost substrates, namely wood, litter and manure.
Fungal species in neotropical areas represent a relatively underexplored field with a great potential for applied purposes; either they are regarded or not regarded as being native to the neotropical range. Metabolic features are, in fact, expected to be affected by both the climate conditions and the segregation of different populations. Fungal strains have a great potential for addressing some of the world’s most pressing issues, such as food security, energy insecurity, human medicine and environmental sustainability. The strains studied in the present research can thus be considered an incipient resource for potential applications, including but not limited to bioremediation, bioactivity, nutraceuticals [81,82,83] and novel foods [84,85]. Within the strain set, it is of major concern that two strains—from C. candolleanus and P. cubensis, respectively—belong to species ascertained to synthetize psilocybin, and one more strain belongs to a species [P. antillarum, formerly known as Psilocybe antillarum (Fr.) Dennis] suggested to synthetize psilocybin as well. The literature is contradictory in reporting the presence of psilocybin and related psychoactive compounds in P. antillarum: Allen et Merlin [86,87] is the only reference about psilocyn, psilocybin and baeocystin (<0.01%), and was disproved by Guzmán et al. [88], whereas Dulay et al. [53] proved the production of alkaloids by P. antillarum but did not investigate their exact identity and concentration.
Most species in the examined set have been reported for antioxidant and antimicrobial bioactivities, followed by antiproliferative and enzymatic properties potentially useful for degrading persistent organic pollutants (POPs). These species therefore show at least two desirable features for potential biotechnological and nutraceutical applications. They are also good candidates for process scalability, as they can be cultivated in lignocellulosic substrates, possibly including wastes and residues to be valorized according to the principles of circular economy [2,18,89,90]. Any application potential, however, is closely linked to the strain-specific culture characteristics, which is why growth tests and morphological descriptions were carried out. As far as possible, it was possible to observe that almost all the 10 strains showed a certain consistency in morphology between the replicates. The variability of growth rates within the same strain is also very low in most cases. This is particularly important in order to select strains that are fast-growing but are also consistent in doing so. For example, both S. gausapatum and P. sanguineus have a high growth rate, but the former has a high variability while the latter has a low one, which is why it could be chosen instead of the former for process scalability. Taking the same example, however, the P. sanguineus pigmentation could be a negative feature in some applications. These examples stress the importance of knowing the culture features of the strains both from the perspective of their ex situ conservation and applicability. An added value of this small collection is the fact that 4 out of 10 strains had not yet been described for culture morphologies. This group, in particular, consists of strains belonging to species with a predominantly neotropical distribution, with the exception of C. candolleanus, which is cosmopolitan.
In addition to the great importance of culture collections in maintaining fungal strains for biotechnological application, it is also necessary to emphasize their importance in the ex situ preservation of these species. The 10 strains in the present study come from areas of the State of São Paulo (Brazil), which was originally covered by the Cerrado and Atlantic Rainforest biomes. The original vegetation of the State has been greatly reduced, and currently the Atlantic Rainforest biome has 32.6% of its original area of occurrence, with remnants, while the Cerrado biome has only 3.0% of its original area of occurrence preserved in this State [91,92]. Therefore, the ex situ preservation of these species is of fundamental importance.
Finally, some of the species reported in this study are almost completely absent in major European fungal culture collections: C. caperata (1 strain available), C. lactineus (1 strain available), L. crinitus (1 to 3 strains available), P. antillarum (zero strains available) and T. villosa (2 strains available) [68,69,70,71,72].
5. Conclusions
Culture collections are fundamental tools for several research topics and applications in mycology, since they allow us to indefinitely reproduce pure material. This manuscript allowed us to certify the identity of a set of 15 strains in a pure culture from the State of São Paulo (Brazil), shared by the UniMI-Department of Food, Environmental and Nutritional Sciences, MicUNIPV and CCIBt, to prevent an accidental loss and jointly plan further bioprospecting.
Fungal diversity in neotropical regions represents a hot frontier in mycology, due to its poorly explored potential. The Brazilian strains in the newly established collection could be therefore included in applied studies, e.g., to test their bioactivity or enzymatic activity spectrum, as well as in phylogenetic reconstructions in comparison to European strains.
Author Contributions
Conceptualization, C.E.G., E.S., G.V. and M.R.; methodology, C.E.G., S.B. and L.G.; validation, C.E.G., C.P. and M.L.G.; formal analysis, C.E.G., S.B., L.G. and V.M.V.V.; investigation, C.E.G., S.B. and L.G.; resources, C.E.G., C.P., E.S. and M.R.; data curation, C.E.G.; writing—original draft preparation, C.E.G., L.G. and S.B.; writing—review and editing, E.S., P.R., G.V., A.d.M.G., V.M.V.V. and M.R.; visualization, C.E.G., S.B. and L.G.; supervision, E.S., P.R., G.V. and M.R.; project administration, G.V., A.d.M.G. and M.R.; funding acquisition, C.E.G. and G.V. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is part of the project NODES, which has received funding from the MUR-M4C2 1.5 of PNRR funded by the European Union-NextGenerationEU (Grant agreement no. ECS00000036). The APC was funded by Ministero dell’Università e della Ricerca, grant number IR0000005, through the project entitled “Strengthening the MIRRI Italian Research Infrastructure for Sustainable Bioscience and Bioeconomy” (SUS-MIRRI.IT).
Data Availability Statement
The ITS sequences are deposited in GenBank NCBI (https://www.ncbi.nlm.nih.gov/genbank; accessed on 5 February 2025).
Acknowledgments
The authors are grateful to Rebecca Michela Baiguera and Anthea Desiderio for their collaboration in molecular analyses and maintenance of the fungal culture collection. The authors acknowledged Rosana Maziero for her preliminar work on fungal strain collection and maintenance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Varese, G.C.; Angelini, P.; Bencivenga, M.; Buzzini, P.; Donnini, D.; Gargano, M.L.; Maggi, O.; Pecoraro, L.; Persiani, A.M.; Savino, E.; et al. Ex situ conservation and exploitation of fungi in Italy. Plant Biosyst. 2011, 145, 997–1005. [Google Scholar] [CrossRef]
- Gargano, M.L. Mycotheca of edible and medicinal mushrooms at herbarium SAF as a potential source of nutraceuticals and cultivated mushrooms. Int. J. Med. Mushrooms 2018, 20, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Girometta, C.E.; Bernicchia, A.; Baiguera, R.M.; Bracco, F.; Buratti, S.; Cartabia, M.; Picco, A.M.; Savino, E. An Italian research culture collection of wood decay fungi. Diversity 2020, 12, 58. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Baiguera, R.M.; Buratti, S.; Babbini, S.; Bernicchia, A.; Savino, E. Lignicolous fungi collected in Northern Italy: Identification and morphological description of isolates. Diversity 2022, 14, 413. [Google Scholar] [CrossRef]
- WFCC: World Federation for Culture Collections. Available online: https://wfcc.info/ (accessed on 18 November 2023).
- MIRRI: Microbial Resource Research Infrastructure. Available online: https://www.mirri.org/ (accessed on 18 November 2023).
- Mueller, G.M.; Cunha, K.M.; May, T.W.; Allen, J.L.; Westrip, J.R.; Canteiro, C.; Costa-Rezende, D.H.; Drechsler-Santos, E.R.; Vasco-Palacios, A.M.; Ainsworth, A.M.; et al. What do the first 597 Global Fungal Red List assessments tell us about the threat status of fungi? Diversity 2022, 14, 736. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; White, M.M. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Ryberg, M.; Kristiansson, E.; Abarenkov, K.; Larsson, K.H.; Kõljalg, U. Taxonomic reliability of DNA sequences in public sequence databases: A fungal perspective. PLoS ONE 2006, 1, e59. [Google Scholar] [CrossRef]
- Dresch, P.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 4. [Google Scholar] [CrossRef]
- Peay, K.G.; Bidartondo, M.I.; Arnold, A.E. Not every fungus is everywhere: Scaling to the biogeography of fungal–plant interactions across roots, shoots and ecosystems. New Phytol. 2010, 185, 878–882. [Google Scholar] [CrossRef]
- Ubaldi, D. Guida Allo Studio Della Flora e Della Vegetazione; CLUEB: Bologna, Italy, 2012. [Google Scholar]
- Ryvarden, L. African polypores—A review. Belg. J. Bot. 1998, 131, 150–155. [Google Scholar]
- Ryvarden, L. Studies in Neotropical polypores 43. Some new species from tropical America. Synop. Fungorum 2016, 35, 43–47. [Google Scholar]
- Decock, C.; Figueroa, S.H.; Robledo, G.; Castillo, G. Fomitiporia punctata (Basidiomycota, Hymenochaetales) and its presumed taxonomic synonyms in America: Taxonomy and phylogeny of some species from tropical/subtropical areas. Mycologia 2007, 99, 733–752. [Google Scholar] [CrossRef]
- Decock, C.A.; Ryvarden, L.; Amalfi, M. Niveoporofomes (Basidiomycota, Fomitopsidaceae) in Tropical Africa: Two additions from Afromontane forests, Niveoporofomes oboensis sp. nov. and N. widdringtoniae comb. nov. and N. globosporus comb. nov. from the Neotropics. Mycol. Prog. 2022, 21, 29. [Google Scholar] [CrossRef]
- Amalfi, M.; Yombiyeni, P.; Decock, C. Fomitiporia in sub-Saharan Africa: Morphology and multigene phylogenetic analysis support three new species from the Guineo-Congolian rainforest. Mycologia 2010, 102, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: Berkeley, CA, USA, 2011. [Google Scholar]
- Okino, L.K.; Gomes Machado, K.M.; Fabris, C.; Ramos Bononi, V.L. Ligninolytic activity of tropical rainforest basidiomycetes. World J. Microbiol. Biotechnol. 2000, 16, 889–893. [Google Scholar] [CrossRef]
- Maziero, R.; Cavazzoni, V.; Ramos Bononi, V.L. Screening of basidiomycetes for the production of exopolysaccharide and biomass in submerged culture. Rev. Microbiol. 1999, 30, 77–84. [Google Scholar] [CrossRef]
- Da Silva, M.; Ribeiri de Oliveira, D. The new Brazilian legislation on access to the biodiversity (Law 13,123/15 and Decree 8772/16). Braz. J. Microbiol. 2018, 49, 1–4. [Google Scholar] [CrossRef]
- Mycobank Molecular ID. Available online: https://www.mycobank.org/Pairwise_alignment (accessed on 18 November 2024).
- BLAST: Basic Local Alignment Search Tool—NCBI. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 17 June 2025).
- GenBank NCBI. Available online: https://www.ncbi.nlm.nih.gov/genbank (accessed on 17 June 2025).
- IBGE: Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br/en/home-eng.html?lang=en-GB (accessed on 21 June 2025).
- Sano, E.E.; Rodrigues, A.A.; Martins, E.S.; Bettiol, G.M.; Bustamante, M.M.; Bezerra, A.S.; Couto, A.C., Jr.; Vasconcelos, V.; Schüler, J.; Bolfe, E.L. Cerrado ecoregions: A spatial framework to assess and prioritize Brazilian savanna environmental diversity for conservation. J. Environ. Manag. 2019, 232, 818–828. [Google Scholar] [CrossRef]
- GBIF: Global Biodiversity Information Facility. Available online: https://www.gbif.org/occurrence/download/0104897-230530130749713 (accessed on 17 June 2025).
- Neotropical Fungi Archive. Available online: https://www.neotropicalfungi.com/ (accessed on 17 June 2025).
- Carta, A.; Peruzzi, L.; Ramìrez-Barahona, S. A global phylogenetic regionalization of vascular plants reveals a deep split between Gondwanan and Laurasian biotas. New Phytol. 2022, 233, 1494–1504. [Google Scholar] [CrossRef]
- Buratti, S.; Girometta, C.E.; Savino, E.; Gorjón, S.P. An example of the conservation of wood decay fungi: The new research culture collection of corticioid and polyporoid strains of the University of Salamanca (Spain). Forests 2023, 14, 2029. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: www.indexfungorum.org (accessed on 17 June 2025).
- Mycobank Database. Available online: www.mycobank.org (accessed on 17 June 2025).
- Koike, Y.; Wada, K.; Kusano, G.; Nozoe, S.; Yokoyama, K. Isolation of psilocybin from Psilocybe argentipes and its determination in specimens of some mushrooms. J. Nat. Prod. 1981, 44, 362–365. [Google Scholar] [CrossRef]
- Liu, Y.P.; Dai, Q.; Wang, W.X.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Psathyrins: Antibacterial diterpenoids from Psathyrella candolleana. J. Nat. Prod. 2020, 83, 1725–1729. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.X.; Li, Z.H.; Feng, T.; Liu, J.K. Psathyrellins A–E, antibacterial guanacastane diterpenoids from mushroom Psathyrella candolleana. Nat. Prod. Bioprospect 2021, 11, 447–452. [Google Scholar] [CrossRef]
- Karaltı, İ.; Eraslan, E.C.; Sarıdoğan, B.G.Ö.; Akata, I.; Sevindik, M. Total antioxidant, antimicrobial, antiproliferative potentials and element contents of wild mushroom Candolleomyces candolleanus (Agaricomycetes) from Turkey. Int. J. Med. Mushrooms 2022, 24, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Al-Habib, M.N.; Holliday, J.; Aladahmy, M.S. Psathyrella candolleana and Agaricus bisporus extracts provide protection against DNA oxidative damage induced by doxorubicin. Int. J. Med. Mushrooms 2018, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Sivanandhan, S.; Ganesan, P.; David, R.H.A.; Paulraj, M.G.; Ignacimuthu, S. Mosquitocidal activity of the pale brittle stem mushroom, Psathyrella candolleana (Agaricomycetes), against three vector mosquitoes. Int. J. Med. Mushrooms 2019, 21, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M.; Wurster, M.; Kreisel, H.; Lindequist, U. Antimicrobial, cytotoxic and antioxidant activity of selected basidiomycetes from Yemen. Pharmazie 2005, 60, 776–780. [Google Scholar]
- Santos, G.S.; Peters, L.P.; Carvalho, C.M. Study of antibacterial activity of Amazonian Agaricomycetes mushrooms from Brazil. Int. J. Med. Mushrooms 2020, 22, 573–580. [Google Scholar] [CrossRef]
- Dona, M.P.S.M.; Deraniyagala, A.S.; Wijesinghe, P.; Attanayake, R.N. Molecular phylogeny of wood decay fungi of hardwood and their ability to produce laccase that correlates with triphenylmethane dye decolorization. BioRxiv 2019, 648147. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, J.; Zheng, H.; Li, M.; Huang, F. Biological Characters of Cubamyces lactineus. Acta Edulis Fungi 2022, 29, 51–58. [Google Scholar]
- He, N.; Tian, L.; Zhai, X.; Zhang, X.; Zhao, Y. Composition characterization, antioxidant capacities and anti-proliferative effects of the polysaccharides isolated from Trametes lactinea (Berk.). Pat. Int. J. Biol. Macromol. 2018, 115, 114–123. [Google Scholar] [CrossRef]
- Hao, J.; Ye, L.; Meng, G.; Song, Y.; Fu, J.; Wu, X. The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics. Food Sci. Hum. Wellness 2021, 10, 480–489. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, N.; Wang, J.; Luo, H.; He, H.; Ding, M.; Deng, W.; Zou, K. The H+/K+-ATPase inhibitory activities of Trametenolic acid B from Trametes lactinea (Berk.) Pat, and its effects on gastric cancer cells. Fitoterapia 2013, 89, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Awala, S.I.; Oyetayo, V.O. Assessment of free radical scavenging potentials of extracts of Trametes lactinea collected from Akure. Res. J. Phytochem. 2016, 10, 10–20. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; He, H.; She, X.; He, Y.; Li, S.; Liu, L.; Luo, T.; Huang, N.; Luo, H.; et al. Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 2019, 112, 108692. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Felczak, A.; Nowak, M.; Kowalczyk, A.; Piwoński, I.; Lisowska, K. Antimicrobial activity and toxicological risk assessment of silver nanoparticles synthesized using an eco-friendly method with Gloeophyllum striatum. J. Hazard. Mater. 2021, 418, 126316. [Google Scholar] [CrossRef]
- Faria, M.G.I.; Avelino, K.V.; Philadelpho, B.O.; dos Santos Bomfim, R.; do Valle, J.S.; Júnior, A.C.G.; Dragunski, D.C.; de Souza Ferreira, E.; de Souza, C.O.; Ferreira Ribeiro, C.D.; et al. Lithium bioaccumulation in Lentinus crinitus mycelia grown in media with different lithium sources and pH values. Environ. Sci. Pollut. Res. 2022, 29, 87519–87526. [Google Scholar] [CrossRef]
- Bertéli, M.B.D.; Barros, L.; Reis, F.S.; Ferreira, I.C.; Glamočlija, J.; Soković, M.; Silveira do Valle, J.; Linde, G.A.; Ruiz, S.P.; Colauto, N.B. Antimicrobial activity, chemical composition and cytotoxicity of Lentinus crinitus basidiocarp. Food Funct. 2021, 12, 6780–6792. [Google Scholar] [CrossRef]
- Bertéli, M.B.; Oliveira Filho, O.B.; Freitas, J.D.; Bortolucci, W.C.; Silva, G.R.; Gazim, Z.C.; Lívero, F.A.R.; Lovato, E.C.W.; Silveira do Valle, J.; Linde, G.A.; et al. Lentinus crinitus basidiocarp stipe and pileus: Chemical composition, cytotoxicity and antioxidant activity. Eur. Food Res. Technol. 2021, 247, 1355–1366. [Google Scholar] [CrossRef]
- López-Legarda, X.; Arboleda-Echavarría, C.; Parra-Saldívar, R.; Rostro-Alanis, M.; Alzate, J.F.; Villa-Pulgarín, J.A.; Segura-Sánchez, F. Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. Int. J. Biol. Macromol. 2020, 164, 3133–3144. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Cabalar, A.C.; De Roxas, M.J.B.; Concepcion, J.M.P.; Cruz, N.E.; Esmeralda, M.; Jimenez, N.; Aguilar, J.C.; De Guzman, E.J.; Santiago, J.Q.; et al. Proximate composition and antioxidant activity of Panaeolus antillarium, a wild coprophilous mushroom. Curr. Res. Environ. Appl. 2015, 5, 52–59. [Google Scholar] [CrossRef]
- Gotvaldová, K.; Hájková, K.; Borovička, J.; Jurok, R.; Cihlářová, P.; Kuchař, M. Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis. Drug Test. Anal. 2021, 13, 439–446. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.; Eloff, J.N. Effects and safety of Psilocybe cubensis and Panaeolus cyanescens magic mushroom extracts on endothelin-1-induced hypertrophy and cell injury in cardiomyocytes. Sci. Rep. 2020, 10, 22314. [Google Scholar] [CrossRef]
- Zulfadhly, Z.; Mashitah, M.D.; Bhatia, S. Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ. Pollut. 2001, 112, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, M.; Yang, J.; Li, F.; Wang, Y.; Feng, K.; Wang, S.; Lin, N.; Wang, Y.; Yang, B. Structural characterization of a polysaccharide from Trametes sanguinea Lloyd with immune-enhancing activity via activation of TLR4. Int. J. Biol. Macromol. 2022, 206, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, M.; Zhu, Z.; Zhang, J.; Cheng, G.; Lin, N.; Zhao, H.; Yang, B. Structural characterization and tumor microvascular inhibition activity of total polysaccharide from Trametes sanguinea Lloyd. Chem. Biodivers. 2022, 19, e202100765. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, B.; Huang, L.; Chen, Y.; Zhao, H.; Zhu, Z. Cardioprotective effect of crude polysaccharide fermented by Trametes sanguinea Lyoyd on doxorubicin-induced myocardial injury mice. BMC Pharmacol. Toxicol. 2023, 24, 1. [Google Scholar]
- Jović, J.; Buntić, A.; Radovanović, N.; Petrović, B.; Mojović, L. Lignin-degrading abilities of novel autochthonous fungal isolates Trametes hirsuta F13 and Stereum gausapatum F28. Food Technol. Biotechnol. 2018, 56, 354. [Google Scholar] [CrossRef]
- Ortiz-Monsalve, S.; Valente, P.; Poll, E.; Jaramillo-García, V.; Henriques, J.A.P.; Gutterres, M. Biodecolourization and biodetoxification of dye-containing wastewaters from leather dyeing by the native fungal strain Trametes villosa SCS-10. Biochem. Eng. J. 2019, 141, 19–28. [Google Scholar] [CrossRef]
- Ferreira-Silva, V.; Gusmão, N.B.D.; Gibertoni, T.B.; Silva, L.A.D.O.D. Trametes lactinea and T. villosa collected in Brazil are able to discolor indigo carmine. Acta Bot. Brasilica 2022, 36, 2021abb0356. [Google Scholar] [CrossRef]
- Stalpers, J.A. Identification of wood-inhabiting fungi in pure culture. In Studies in Mycology; CABI: Oxfordshire, UK, 1978; Volume 16, 248p. [Google Scholar]
- Bakshi, B.K.; Sehgal, H.S.; Singh, B. Culture diagnosis of Indian Polyporaceae I. Genus Polyporus. Indian For. Rec. 1969, 2, 205–244. [Google Scholar]
- Motato-Vásquez, V.; Pires, R.M.; Vitali, V.M.V.; Gugliotta, A.D.M. Cultural and ligninolytic activity studies of some polypores (Basidiomycota) from brazilian Atlantic Forest, São Paulo State, Brazil. Hoehnea 2016, 43, 289–300. [Google Scholar] [CrossRef][Green Version]
- Nobles, M.K. Studies in wood-inhabiting Hymenomycetes III. Stereum pini and species of Peniophora sect. coloratae on conifers in Canada. Can. J. Bot. 1956, 34, 104–130. [Google Scholar]
- Nakasone, K.K. Culture studies and identification of wood-inhabiting Corticiaceae and selected Hymenomycetes from North America. Mycol. Mem. 1990, 15, 1–412. [Google Scholar]
- CBS-KNAW Collections—Westerdijk Institute. Available online: https://wi.knaw.nl/Collection (accessed on 17 June 2025).
- Mut-Mycotheca Universitatis Taurinensis. Available online: https://www.tucc-database.unito.it/mut (accessed on 17 June 2025).
- BCCM/MUCL Agro-Food & Environmental Fungal Collection. Available online: https://bccm.belspo.be/about-us/bccm-mucl (accessed on 17 June 2025).
- LE-BIN Strains—All-Russian Collection of Microorganisms—VKM. Available online: http://www.vkm.ru/eCatalogue/LE-BIN.htm (accessed on 17 June 2025).
- CABI Bioscience. Available online: https://form.jotform.com/232901487583059 (accessed on 28 April 2025).
- Vlasák, J.; Kout, J. Tropical Trametes lactinea is widely distributed in the Eastern USA. Mycotaxon 2011, 115, 271–279. [Google Scholar] [CrossRef]
- Murrill, W.A. Some Philippine Polyporaceae. Bull. Torrey Bot. Club 1907, 34, 465–481. [Google Scholar] [CrossRef]
- Murrill, W.A. The Polyporaceae of North America: XIII. The described species of Bjerkandera, Trametes, and Coriolus. Bull. Torrey. Bot. Club. 1905, 32, 633–656. [Google Scholar] [CrossRef]
- Lücking, R.; Truong, B.V.; Huong, D.T.; Le, N.H.; Nguyen, Q.D.; Nguyen, V.D.; Von Raab-Straube, E.; Bollendorff, S.; Kim Govers, K.; Di Vincenzo, V. Caveats of fungal barcoding: A case study in Trametes s.lat. (Basidiomycota: Polyporales) in Vietnam reveals multiple issues with mislabelled reference sequences and calls for third-party annotations. Willdenowia 2020, 50, 383–403. [Google Scholar] [CrossRef]
- Zmitrovich, I.V.; Ezhov, O.N.; Wasser, S.P. A survey of species of genus Trametes Fr. (higher Basidiomycetes) with estimation of their medicinal source potential. Int. J. Med. Mushrooms 2012, 14, 307–319. [Google Scholar] [CrossRef]
- Justo, A.; Hibbett, D.S. Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 2011, 60, 1567–1583. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Villegas, E.; Rodriguez, A.; Acosta-Urdapilleta, M.L.; O’Donovan, A.; Diaz-Godinez, G. Mycosphere essay 11: Fungi of Pycnoporus: Morphological and molecular identification, worldwide distribution and biotechnological potential. Mycosphere 2016, 7, 1500. [Google Scholar] [CrossRef]
- Martin, R.; Gazis, R.; Skaltsas, D.; Chaverri, P.; Hibbett, D. Unexpected diversity of basidiomycetous endophytes in sapwood and leaves of Hevea. Mycologia 2015, 107, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Musatti, A.; Ficara, E.; Mapelli, C.; Sambusiti, C.; Rollini, M. Use of solid digeState for lignocellulolytic enzymes production through submerged fungal fermentation. J. Environ. Manag. 2017, 199, 1–6. [Google Scholar] [CrossRef]
- Bellucci, M.; Marazzi, F.; Fornaroli, R.; Musatti, A.; Turolla, A.; Visigalli, S.; Bargna, M.; Bergna, G.; Canziani, R.; Mezzanotte, V.; et al. Assessment of anammox, microalgae and white-rot fungi-based processes for the treatment of textile wastewater. PLoS ONE 2021, 16, e0247452. [Google Scholar] [CrossRef] [PubMed]
- Castorina, G.; Cappa, C.; Negrini, N.; Criscuoli, F.; Casiraghi, M.C.; Marti, A.; Rollini, M.; Consonni, G.; Erba, D. Characterization and nutritional valorization of agriculture waste corncobs from Italian maize landraces through the growth of medicinal mushrooms. Sci. Rep. 2023, 13, 21148. [Google Scholar] [CrossRef]
- Goppa, L.; Spano, M.; Baiguera, R.M.; Cartabia, M.; Rossi, P.; Mannina, L.; Savino, E. NMR-Based characterization of wood decay fungi as promising novel foods: Abortiporus biennis, Fomitopsis iberica and Stereum hirsutum mycelia as case studies. Foods 2023, 12, 2507. [Google Scholar] [CrossRef]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of metabolites in Italian Hericium erinaceus mycelium, primordium, and sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Merlin, M.D. Psychoactive mushroom use in Koh Samui and Koh Pha-Ngan. J. Ethnopharmacol. 1992, 35, 205–228. [Google Scholar] [CrossRef]
- Merlin, M.D.; Allen, J.W. Species identification and chemical analysis of psychoactive fungi in the Hawaiian Islands. J. Ethnopharmacol. 1993, 40, 21–40. [Google Scholar] [CrossRef]
- Guzmán, G.; Allen, J.W.; Gartz, J. A worldwide geographical distribution of the neurotropic fungi, analysis and discussion. Annali Civ. Mus. Rovereto 2000, 14, 189–270. [Google Scholar]
- Grimm, D.; Wösten, H.A. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Nalon, M.A.; Matsukuma, C.K.; Pavão, M.; Ivanauskas, N.M.; Kanashiro, M.M. Inventário da Cobertura Vegetal Nativa do Estado de São Paulo. São Paulo: SIMA/IPA 2022. Available online: https://indd.adobe.com/view/a5aba10f-0090-4109-ac1c-944c8260b1ff (accessed on 17 June 2025).
- Dib, V.; Nalon, M.A.; Amazonas, N.T.; Vidal, C.Y.; Ortiz-Rodríguez, I.A.; Daněk, J.; Formis de Oliveira, M.; Alberti, P.; da Silva, A.; Salomão Precinoto, R.; et al. Drivers of change in biodiversity and ecosystem services in the Cantareira System Protected Area: A prospective analysis of the implementation of public policies. Biota Neotrop. 2020, 20, e20190915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).