Abstract
Accurate taxonomy is fundamental for assessing extinction risks and implementing conservation strategies. We evaluated the extinction risk of Grindelia atlantica (Asteraceae), endemic to southern Brazil, using the IUCN criteria, and comparing three scenarios of taxonomic accuracy and data availability. Herbaria records and field surveys confirmed the historical existence of five records and currently only two remaining, isolated populations, totaling 633 individuals (513 in Pelotas and Rio Grande; 120 in Jaguarão). Habitat loss and invasive species are the primary threats. Analyses resulted in an Extent of Occurrence of 475.832 km2 and an Area of Occupancy of 36 km2. These findings, coupled with significant population decline, justify the classification as Critically Endangered. The results emphasize the critical role of reliable taxonomy in conservation biology. They demonstrate the impact of a few errors on extinction risk assessments, which can unfold in the misallocation of resources or insufficient protection. This is critical, particularly for endemic species like G. atlantica in the threatened Pampas, one of Brazil’s most degraded biomes and the least represented in preserves. The creation of a conservation unit is proposed as an urgent measure to ensure the survival of this species and its habitat, benefiting other endemic and rare threatened animal and plant species.
1. Introduction
For conservation efforts to be effective, it is essential to gather information about species and their current extinction risk [1]. Studies on extinction risk, such as those utilizing the parameters and criteria established by the IUCN (International Union for Conservation of Nature), play a fundamental role in guiding preservation efforts [2]. However, good taxonomy is crucial for accurately assessing the conservation status of species, especially for lists like the IUCN Red Lists [3], which rely on precise species identification for a correct assessment of extinction risk. Taxonomic confusion can lead to errors in assessing extinction risk, impacting decisions on conservation priorities and resource allocation [4,5]. Poor taxonomic delimitation may result in underestimation or overestimation of threatened species, compromising the effectiveness of conservation strategies [5].
The loss of biodiversity, resulting from climate change and human activities, has been one of the major concerns of the global community in recent decades [6,7,8]. Since the historic Earth Summit of 1992, held in the city of Rio de Janeiro, which established the Convention on Biological Diversity (CBD), nations around the world have committed ambitious goals to slow down the alarming rate of species extinction [9]. Over the years, new objectives and agreements have been established. Notably, we have the United Nations Sustainable Development Goals [10] and reports from the CBD, such as the Kunming–Montreal Global Biodiversity Framework, approved at the 15th Conference of the Parties to the Convention on Biological Diversity [11]. However, after three decades, none of the 20 targets established by the original convention have been fully achieved globally [12], and the loss of species continues at an accelerated rate. It has been highlighted that two out of every five plant species are at risk of extinction, primarily due to anthropogenic actions [13]. Subsequently, the State of the World’s Plants and Fungi 2023 reported that 45% of flowering plant species are potentially threatened with extinction [14]. This is corroborated by evidence indicating that approximately 45% of angiosperms face some degree of threat [15]. Additionally, these authors estimated that three out of four of the world’s undescribed plant species are likely to be at risk of extinction. The alarming patterns of accelerated biodiversity loss indicate that due to ecosystem degradation and climate change, particularly exacerbated by human activities, Earth is facing the sixth mass extinction event [16,17,18,19].
Grindelia Willd. (Asteraceae, Astereae) comprises more than 70 exclusively American species, showing a disjunct distribution, with species in North America and in southern South America [20,21]. There are seven species of the genus in Brazil, five of them restricted to the pampas in the southernmost state of Rio Grande do Sul. Further, Grindelia atlantica Deble & A.S.Oliveira and G. gaucha Deble & A.S.Oliveira are endemic to the state [22,23], with the former limited to the coastal plain and the latter to a few sandy and basaltic rocky outcrops.
Grindelia atlantica, known as “margarida-da-praia” (beach daisy) (Figure 1A), was first described to science with the suggested extinction risk category assessment of Endangered—EN. This threat indication is because the species was described based only on two restricted and separated populations: one in the municipality of Tramandaí, in the northeast Atlantic Ocean shoreline of Rio Grande do Sul, Brazil, and another in Patos lagoon shoreline in Pelotas coastal urban neighborhood of Laranjal, in the southern part of the state [22]. The species was subsequently recognized as Critically Endangered—CR (B1b(iii)) and officially included in the List of Threatened Species of Rio Grande do Sul [24] due to its restricted Area of Occupancy (AOO) (Figure 1B). Ten years since its description, G. atlantica has not undergone a reassessment nor been assessed according to the International Union for Conservation of Nature (IUCN) Red List guidelines and criteria.

Figure 1.
Grindelia atlantica (Asteraceae: Astereae): (A) mature plant in its preserved habitat at Barra Falsa, Rio Grande, Brazil; (B) characteristic decumbent habit in bloom; (C) lateral view of the capitulum; (D) capitulum in front view. Photos by F. Fernandes (A,B) and B. de Souza (C,D).
Thus, this study aimed to assess the extinction risk of G. atlantica according to IUCN criteria using herbarium specimen data to support efforts for the species’ long-term conservation and to discuss the importance of taxonomy in conservation initiatives.
2. Materials and Methods
2.1. Data Collection
Initially, georeferenced data available for the species were compiled by consulting online platforms such as Flora e Funga do Brasil [25], GBIF (Global Biodiversity Information Facility) [26], and speciesLink [27]. In addition, the following Brazilian and Uruguayan herbaria were consulted: BHCB (Universidade Federal de Minas Gerais, Brazil), CEN (Embrapa Recursos Genéticos e Biotecnologia, Brazil), ECT (Embrapa Clima Temperado, Brazil), FLOR (Universidade Federal de Santa Catarina, Brazil), HAS (Secretaria do Meio Ambiente e Infraestrutura do Rio Grande do Sul, Brazil), HDCF (Departamento de Ciências Florestai—Universidade Federal de Santa Maria, Brazil), HUCS (Universidade de Caxias do Sul, Brazil), HURG (Universidade Federal do Rio Grande, Brazil), ICN (Universidade Federal do Rio Grande do Sul, Brazil), MBM (Museu Botânico Municipal—Curitiba, Brazil), MPUC (Pontifícia Universidade Católica do Rio Grande do Sul, Brazil), MVFA (Universidad de la República, Uruguay), MVJB (Museo y Jardín Botánico, Uruguay), MVM (Museo Nacional de Historia Natural, Uruguay), PACA (Instituto Anchietano de Pesquisas—Universidade do Vale do Rio dos Sinos, Brazil), PEL (Universidade Federal de Pelotas, Brazil), R (Museu Nacional, Brazil), RB (Jardim Botânico do Rio de Janeiro, Brazil), SP (Instituto de Pesquisas Ambientais, Brazil), and SPF (Universidade de São Paulo, Brazil) (acronyms according to Thiers [28], continuously updated). Besides the online databases, specimens from the herbaria CORD (Museu Botánico—Córdoba, Argentina), NY (New York Botanical Garden, United States), and SI (Instituto de Botánica Darwinion, Argentina) were also examined (acronyms according to Thiers [28], continuously updated).
Subsequently, the data were processed into a spreadsheet, verifying the accuracy of identifications and population locations. Taxonomic identifications were confirmed through visits to herbaria or via photographs of herbarium samples available online or requested for loan. Field expeditions were carried out in November 2022 to survey and sample populations of G. atlantica across its distribution range, including the municipalities of Tramandaí, Laranjal (Pelotas), and Barra Falsa (Rio Grande), in the Rio Grande do Sul state, Brazil, in December 2024. Each group of individuals was sampled and herbarized, with the records deposited in the ECT herbarium and duplicate samples sent to ICN. Additionally, field expeditions were conducted beyond the known southern and northern limits of occurrence of the two populations in search of new occurrences and in several other areas of the state’s coastal plain.
2.2. Extinction Risk Assessment
Using the original, noncurated data obtained from herbarium records and online databases, a preliminary conservation assessment was conducted to calculate the Extent of Occurrence (EOO) and Area of Occupancy (AOO) of the species. These values were generated using the online platform GeoCAT (Geospatial Conservation Assessment Tool) [29,30].
Subsequently, two additional rounds of analyses were performed applying progressively stricter data curation procedures. In the second scenario, a curated version of the dataset was used, in which georeferencing errors, duplications, and taxonomic inconsistencies were corrected, and additional records from recent fieldwork were included. In the third scenario, a refined dataset was prepared, considering only currently confirmed extant populations, with the exclusion of populations possibly extinct or lacking recent confirmation. For each scenario, new maps were generated, and EOO and AOO recalculated using GeoCAT. Distribution maps were rendered using Quantum GIS version 3.40.3 [31].
The results from all three scenarios were then analyzed and interpreted to assign a preliminary conservation status to the species, following the criteria proposed by the International Union for Conservation of Nature (IUCN) [31]. These analyses consider five main criteria: A (population reduction), B (restricted geographic distribution with evidence of fragmentation, decline, or fluctuations), C (small population size combined with fragmentation, decline, or fluctuations), D (very small population or very restricted distribution), and E (quantitative analysis of extinction risk). Based on these, species are assigned to one of seven IUCN categories: Least Concern (LC), Near Threatened (NT), Vulnerable (VU), Endangered (EN), Critically Endangered (CR), Extinct in the Wild (EW), and Extinct (EX). Criteria A through D were assessed using information from herbarium records and fieldwork, which included population mapping and individual counts. In addition, direct field observations and inferences based on historical distribution data from herbarium collections were incorporated into the assessment.
3. Results
3.1. Data Collection
During the review of herbarium collections and online records, 18 occurrences of G. atlantica were found. Among these, six were reported from different locations in Argentina. Two records were from the city of Tramandaí, in the northern part of the state of Rio Grande do Sul, Brazil; one from Tapes, on the shoreline of the Laguna dos Patos; and nine from the beaches of Laranjal in the municipality of Pelotas and the contiguous locality of Barra Falsa in the municipality of Rio Grande, both in the southern part of the Brazilian state. We found, after taxonomic investigation through photographs of the exsiccates, that the six records from Argentina belonged to another species, which is common in that region, G. argentina Deble & A.S.Oliveira. Additionally, two samples were correctly identified in the exsiccate but were mislabeled in the online system, including one Argentinian record that contained a typographical error in the coordinate data, which displaced its locality significantly. It was observed that the prostrate habit and the shape of the leaves, typical of G. atlantica, did not match the records found (Figure 2).

Figure 2.
Specimens of Grindelia argentina (Asteraceae: Astereae) incorrectly identified as G. atlantica in Argentina. (A–D)—specimen with obovate leaves, characteristic of G. argentina: (A) (CORD109480), (B) (NY3236051), (C) (SI360165), and (D) (SI360166). (E)—specimen incorrectly identified only on the online platform (listed as G. buphthalmoides DC., the species from which G. argentina’s original description was based on [32]) (SI24510). (F)—specimen incorrectly identified only in the online platform, with a handwritten note indicating similarity to G. atlantica (SI212155).
Extensive fieldwork conducted across the coastal plain of southern Rio Grande do Sul and its tributary river basins revealed only one previously undocumented population of G. atlantica, discovered in December 2024 along Estrada da Perdiz, in the municipality of Jaguarão. This inland occurrence significantly expands the known distribution range of the species and stands out as the most important finding of recent surveys. In contrast, despite targeted searches, G. atlantica was not found in Tramandaí, in the northern region of the state, suggesting the species may be locally extinct there. Similarly, the historical record from Tapes, on the shore of the Laguna dos Patos, could not be confirmed. Additional expeditions to similar habitats, both north and south of known populations, did not yield further occurrences.
Currently, confirmed persistent populations of G. atlantica are restricted to three localities in Rio Grande do Sul, Brazil: the coastal urban zone of Laranjal in Pelotas, the contiguous rural area of Barra Falsa in Rio Grande, and the newly recorded site in Jaguarão. During expeditions to Laranjal and Barra Falsa in 2022, a total of 513 individuals were recorded—458 along the contiguous coastal neighborhoods of Balneário dos Prazeres, Barro Duro, and Colônia Z3 in Pelotas, and 55 in the rural zone of Barra Falsa. The Jaguarão population was estimated to comprise 120 individuals.
Based on the data obtained in Supplementary Material [S1] it was possible to carry out a conservation status assessment of G. atlantica under different levels of data accuracy: Scenario 1—expedite analysis with raw data obtained directly from online platforms, without taxonomic validation or the verification of extinct populations; Scenario 2—curated data based on herbarium records confirmed as G. atlantica through taxonomic revision, including new collections from recent fieldwork and populations possibly extinct or not recently confirmed; and Scenario 3—refined and updated data considering only currently confirmed extant populations (Table 1). In Scenario 1, the Extent of Occurrence (EOO) was estimated at 1,039,467.568 km2 and the Area of Occupancy (AOO) at 64 km2 (Figure 3). In Scenario 2, the GeoCAT analysis resulted in considerably lower values, with an EOO of 9681.071 km2 and an AOO of 48 km2 (Figure 4). Finally, in Scenario 3, considering only the current and confirmed populations, the EOO was 475.832 km2 and the AOO was 36 km2 (Figure 5).

Table 1.
Extent of Occurrence (EOO), Area of Occupancy (AOO), and preliminary IUCN conservation status assessments for Grindelia atlantica under three different data accuracy scenarios: (1) raw data obtained directly from online platforms, without taxonomic validation or verification of extinct populations; (2) curated data based on taxonomic verification of herbarium records and inclusion of new field collections, but without excluding populations possibly extinct or not recently confirmed; and (3) refined data considering only current, confirmed extant populations.

Figure 3.
Occurrence records of Grindelia atlantica obtained from online platforms prior to herbaria review, taxonomic validation, and field work verification. Points include both confirmed and unverified records, as well as potentially extinct populations.
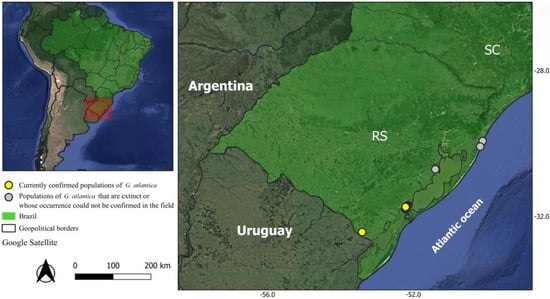
Figure 4.
Occurrence records of Grindelia atlantica confirmed through taxonomic revision of herbarium specimens, including new collections from recent fieldwork and population census. The dataset also includes populations considered possibly extinct or not recently confirmed in Tapes and Tramandaí, Rio Grande do Sul, Brazil.

Figure 5.
Distribution of the last known population of Grindelia atlantica in Laranjal, Pelotas; Barra Falsa, Rio Grande; and Estrada da Perdiz in Jaguarão, Rio Grande do Sul, Brazil.
3.2. Extinction Risk Assessment
The first two data treatments—(1) all records obtained directly from online platforms, and (2) the curated dataset, which excludes populations not identified as G. atlantica and includes those found during this study, including historical records—did not allow for the application of all the IUCN criteria [31] due to the lack of detailed environmental and population data. Therefore, these scenarios were evaluated solely under criterion B (Restricted geographic distribution and showing fragmentation, decline, or fluctuations). In the scenario using all the records obtained directly from online platforms, the species could be classified as Least Concern (LC) due to its large Extent of Occurrence (EOO: 1,039,467.568 km2), and as Endangered (EN) based on its Area of Occupancy (AOO: 64 km2). Using the curated dataset, excluding misidentified populations and including those recorded during this study, the species could be assessed as Vulnerable (VU) based on its EOO (9542.839 km2), and Endangered (EN) based on its AOO (48 km2).
With the cleaned data obtained from herbarium specimens, recent sampling data added, and extinct populations removed, an assessment of the extinction risk of the species was conducted following the parameters of the IUCN [31]. The following results were obtained:
Criterion A (population reduction). Critically Endangered (CR) A2ace + 3ce + 4ace. The species has been categorized as Critically Endangered (CR) due to the extinction of the Tramandaí and Tapes populations, and an estimated reduction of approximately 80% of the total known population in Laranjal, Pelotas, caused by the mowing and landfill of the coastal strip observed in recent years. Additionally, the population in Jaguarão is restricted to a narrow remnant paleodune between the roadside and a soybean plantation. Unplanned urban and agricultural expansion poses a serious threat to the species, such as the removal of native coastal vegetation, its replacement with exotic species, and paving may compromise the few remaining suitable habitats (Figure 6 and Figure 7).

Figure 6.
Grindelia atlantica on the shores of Laguna dos Patos, in Pelotas, Rio Grande do Sul, Brazil. In the background, invasive exotic plant species that occupy the restricted habitat and exclude G. atlantica through competition: (A)—Giant Reed (Arundo donax L.—Poaceae). (B)—Lovegrass (Eragrostis plana Ness—Poaceae). (C)—Palisade grass (Urochloa brizantha A.Rich.) R.D.Webster—Poaceae). Photos by F. Fernandes.

Figure 7.
(A) Grindelia atlantica in a less disturbed habitat at Barra Falsa, Rio Grande, Rio Grande do Sul, Brazil, where individuals can reach up to 2 m in diameter. (B) In most of its distribution along the urban neighborhoods of Laranjal in Pelotas, Rio Grande do Sul, Brazil, G. atlantica grows in a narrow strip of sand between the road and the lagoon. (C) The Pelotas public administration routinely mows or entirely remove the native vegetation along the strip of sand between the road and the lagoon, and after the 2024 floods, road renovations with landfill completely destroyed the dunes where G. atlantica used to occur in the Pontal da Barra sector, Laranjal, Pelotas, Rio Grande do Sul, Brazil. Photos by: F. Fernandes.
Criterion B (restricted geographic distribution and showing fragmentation, decline, or fluctuations). Endangered (EN) B2ab(i,ii,iii,iv),c(i,ii,iii,iv,v). The species has been classified as Endangered (EN) due to its Area of Occupancy (AOO) being less than 500 km2, with an estimated AOO of 36 km2. It occurs in only two locations (subpopulations subject to the same threat pressures), which are approximately 132 km apart. Both locations are subject to anthropogenic pressures, past and present, with a high potential for intensification in the near future.
Criterion C—(small population with fragmentation, decline, or fluctuations). Endangered (EN) C2a(i)b. The species has been classified as Endangered (EN) due to a continuing population decline, following the local extinction of the Tramandaí and Tapes populations. It is currently restricted to two locations. An estimated 513 individuals occur in the contiguous areas of Pelotas and Rio Grande, and about 120 individuals are found in Jaguarão. The largest number occurs in Laranjal, Pelotas, where the population is subject to periodic mowing of the coastal vegetation and occasional landfill. Both remaining locations are affected by anthropogenic pressures, which contribute to significant fluctuations in the number of mature individuals.
Criterion D—(Very small population or very restricted distribution). Vulnerable (VU) D1 + 2. The species has been classified as Vulnerable (VU) because it has fewer than 1000 mature individuals, all in a single location. The entire distribution of the species was surveyed, and 633 individuals were counted.
Criterion E (Quantitative risk of extinction analysis) was not applied in this assessment due to the unavailability of detailed demographic, ecological, and temporal data required to support robust population viability models. The absence of consistent long-term monitoring data, such as birth and mortality rates, recruitment, and age structure, precludes the use of simulation-based approaches, which are fundamental to evaluate extinction probabilities under this criterion.
In extinction risk assessments, the highest degree of threat is considered [31]; therefore, G. atlantica should be regarded as a Critically Endangered species according to criteria A (A2ace + 3ce + 4ace).
4. Discussion
The study on the conservation status of G. atlantica highlights the indispensable role of taxonomic expertise in conservation research, particularly in refining extinction risk assessments by correcting overestimated distribution data. It also confirms that the species faces critical extinction due to habitat alterations, such as urbanization and the invasion of exotic species. During the sampling efforts of this study, the population historically recorded in Tramandaí was not found and is likely locally extinct, possibly due to significant urban landscape changes in the region [33]. Similarly, a historical record from the municipality of Tapes, on the shore of Laguna dos Patos, was not confirmed in the field and may also represent a locally extinct population.
The coastal region of Rio Grande do Sul is relatively well-sampled and easily accessible, allowing inference that the isolated populations historically recorded in Tramandaí and Tapes are likely no longer present. However, the populations at Praia do Laranjal (Pelotas) and Barra Falsa (Rio Grande) remain. Furthermore, during this study, a previously undocumented population of G. atlantica was found in the municipality of Jaguarão, expanding the known distribution of the species and confirming its continued presence in the extreme south of the state.
Currently, the known extant populations of G. atlantica are found along the shoreline of Laranjal beaches in Pelotas, in Barra Falsa in Rio Grande, and in Jaguarão, all in the state of Rio Grande do Sul. The latter two represent the only additional records of the species since its original description [22]. It is worth noting that in the few locations where it has been recorded, G. atlantica always occurs associated with a sandy matrix of distinct grain size compared to the sands found on other oceanic and lagoon beaches of Rio Grande do Sul. The species is consistently found in patches of coarse, ferruginous sand between the beach strip and the marshes, growing in soil deposits related to the Pleistocene Barrier II [34], which restricts its ecological niche to small dune relics along the shoreline and coastal plain.
The availability of online databases dedicated to biodiversity and the growth of citizen science platforms represent a revolution in accessing vast volumes of scientific data. Those data can be used in various fields such as determining species distribution [35], evolutionary studies [36], and biogeography [37], as well as modeling the distribution of disease vectors [38], invasive species [39], and conservation efforts [40]. Moreover, it is essential to recognize the crucial role of herbaria in documenting and preserving biological diversity. Criterion B, in particular, relies heavily on data from previous collections deposited in herbaria. It is imperative not only to conduct new collections but also to digitize herbaria, as these biological collections serve as a cornerstone for robust conservation studies [41]. However, to ensure the quality and reliability of analyses, the responsible use of these data is essential, given potential issues such as spatial bias, imperfect detection, and incomplete and uneven sampling in different species distribution areas [35]. Furthermore, challenges related to taxonomic identification and potential typing errors during online data provision may arise. This situation was illustrated in the findings of this study, where some samples were incorrectly identified not only in the herbarium specimens, but also on online platforms.
As urgent as extinction risk assessments, it is imperative to devote proper attention to the data processing stages. This care becomes crucial since analyses conducted directly with online platform data may contain errors, as evidenced in this study. To ensure accuracy, it is necessary to revisit populations, ensure their ongoing existence, and verify taxonomic identifications, especially for more challenging taxa. With an EOO of 1 million km2, automated or careless evaluation could mistakenly indicate that G. atlantica is widely distributed; however, the species is poorly sampled or has a sparse distribution. This scenario reinforces a pattern observed in previous studies [4,5], showing that deficient taxonomy can lead to serious misunderstandings in conservation assessments, as also demonstrated here. This reinforces the urgent need for more taxonomists actively engaged in conservation.
In the context of the habitat of G. atlantica, one of the main challenges faced is the presence of invasive species, which compete directly with it and eliminate it through competition. Along with habitat loss, the invasion of exotic species represents one of the main threats to native flora [42]. This invasion causes a reduction in species richness in the invaded ecosystem [43], which shows that it not only represents a threat to G. atlantica but also to the entire regional biodiversity.
According to the Thematic Report on Invasive Alien Species, Biodiversity, and Ecosystem Services by the Brazilian Platform on Biodiversity and Ecosystem Services [44], among the primary causes of biodiversity loss, biological invasions are the most neglected in Brazilian public management. A study on the impacts of invasive species in Brazil reveals that 1003 records of ecological and economic impacts caused by 239 different invasive species were documented from 1981 to 2022. Of these records, 970 were considered negative, highlighting the predominance of harmful impacts of invasive species in the country. These data underscore the severity of the invasive species problem and the urgency of actions to mitigate their effects on Brazilian biodiversity and ecosystems [44,45].
Furthermore, the habitat faces increasing pressure from unplanned urban expansion, agricultural encroachment, landfilling, and frequent mowing, all of which are detrimental to the remaining populations of G. atlantica and to the integrity of the dune systems near the lagoon where it occurs. As has already been demonstrated [46], the constant removal of native vegetation through mowing can lead to habitat loss, decreased biodiversity, and increased species vulnerability to extinction, besides favoring invasive exotic species.
Therefore, it is crucial to implement sustainable management practices that avoid excessive mowing and protect vulnerable plant communities, such as G. atlantica, and the associated coastal ecosystems. It is important to highlight that these dunes on the lagoon shore are supposed to be protected by municipal, state, and federal environmental laws, as they are considered Permanent Preservation Areas (APP) under Brazilian law. The region’s status as an APP is even included in the city’s master plan [47]. Permanent Preservation Areas are defined and receive full protection under the Brazilian Native Vegetation Protection Law [48], the State Environmental Code [49], and the Municipal Environmental Code [50], and should not be mown. Additionally, the region is part of an ecotourism interest area called the “Laguna dos Patos shoreline”, as defined in Municipal Law [51], and should be preserved and protected, including from alteration caused by urban development.
Our findings indicate that the greatest challenges faced by G. atlantica are caused by the mismanagement of human activities: the advancement of urbanization, frequent mowing, and the invasion of exotic species. All these characteristics have deeper origins, reflecting historical neglect of native flora and grassland environments. The term “Botanical Blindness” was originally proposed [52] and later reinterpreted as “Botanical Unawareness” [53,54] to avoid the use of an ableist expression. It refers to a set of perspectives and attitudes that result in a lack of recognition of the importance of plants in the biosphere and in daily life. This unawareness includes difficulty in appreciating the unique aesthetic and biological aspects of native plants, as well as the perception that plants are inferior compared to animals and do not deserve equivalent attention [52]. One consequence of this unawareness is the historical neglect of plants in conservation efforts, with assessments primarily focused on woody species and those with economic value [13]. This pattern has also contributed to a disproportionate representation of perennial woody and useful species in the Red List [3], while species endemic to a single botanical country remain largely underrepresented [13]. In grassland environments, such as those in the South American Pampas, this trend of unawareness and neglect is even more pronounced. The Pampa is one of the most degraded and least protected environments in Brazil [55,56]. Over the last 36 years, the Pampa has suffered the most significant proportional loss of native vegetation in relation to its total coverage, and currently has only 3% of its area protected [57]. Despite this, the Brazilian Pampa harbors remarkable biodiversity, with approximately 12,500 species of plants, fungi, and animals, representing 9% of Brazil’s total biodiversity, even though it occupies only 2% of the national territory [58]. The need for studies on the extinction risk of wild flora, including species like G. atlantica with unknown or unexplored economic potential, is evident. The urgency of action is clear; without it, we risk irreversible losses that go far beyond economic potential. Preserving grassland environments, historically neglected, is essential to protect biodiversity and maintain ecological balance [59,60].
Grindelia atlantica finds one of its last refuges on the beach of Pontal da Barra do Laranjal, a highly biodiverse region where the Pampa meets the Atlantic Forest. This area harbors the largest remaining population of the species and is the focus of efforts by various institutions to establish a conservation unit [61,62]. The recognition of the species as Critically Endangered globally adds another argument in favor of the conservation of the entire area, even making it a flagship species. Additionally, in a study involving mammals, it was demonstrated that they serve as tools for raising public awareness, as the general public tends to show greater interest in species assigned a threat status [63]. Furthermore, efforts focused on the conservation of this threatened species can be used as a “flagship event” to promote the protection of the entire region. This specific event, whether it would be a species protection campaign, a monitoring program, or a habitat restoration action, can act as a catalyst to raise awareness and mobilize conservation efforts on a larger scale [64]. The effective promotion of wildlife conservation can be achieved through the strategic use of conservation efforts, media, and public relations professionals, focusing on communication and community engagement [65]. Similar approaches can be applied to highlight the importance of protecting G. atlantica and its habitat in Pontal da Barra do Laranjal. This coordinated effort can not only safeguard the species but also serve as a rallying point for broader conservation actions in the region, ultimately contributing to the preservation of its unique biodiversity and ecosystems. For instance, the protection of G. atlantica would simultaneously benefit various other threatened or endemic species of flora and fauna that share the same habitat. A total of 42 species in the Pontal da Barra region are currently classified as threatened to varying degrees. Among them are fauna such as the glass lizard (Ophiodes enso Entiauspe-Neto et al., 2017), the annual killifish (Austrolebias wolterstorffi Ahl, 1924), and Geoffroy’s cat (Leopardus geoffroyi d’Orbigny & Gervais, 1844), as well as flora including Hippeastrum breviflorum Herb. (bog lily), Habenaria dutraei Schltr. (terrestrial orchid), and Noticastrum malmei Zardini (dune daisy) [66].
Beyond being Earth’s most singular characteristic, the diversity of life forms on the planet is essential for maintaining ecosystems and ecological functions upon which humans themselves depend [67,68]. The consequences of biodiversity loss go far beyond the most dramatic impacts, such as altered rainfall patterns and the loss of pollinators [69]. The destruction of ecosystems and species extinction imply the loss of genes that could be used in crop improvement programs, the discovery of compounds that could be developed into medicines [67,70,71], and impact food security [72]. Moreover, ecosystem destruction is directly related to the emergence of infectious diseases and epidemics [73,74], making biodiversity conservation a crucial issue for public health as well [75].
Between April and May 2024, the state of Rio Grande do Sul experienced one of the largest and most damaging floods in its history, caused by an extreme weather event that affected 96% of municipalities and led to the declaration of a state of emergency [76,77]. The situation was exacerbated by the El Niño phenomenon, which is historically associated with periods of intense rainfall in Rio Grande do Sul [78], as seen in 1941, 1982, and 2023. However, the severity and frequency of recent disasters can also be attributed to more intense climatic events and unregulated urbanization near watersheds, which disrupts the hydrological cycle and amplifies environmental impacts [79]. This highlights the urgent need for nature-based adaptation measures, such as the restoration of native vegetation along riverbanks and floodplains, which can reduce soil erosion, slow surface runoff, and enhance the resilience of ecosystems and communities to extreme weather events [76].
The municipalities of Pelotas and Rio Grande were also severely affected by the unprecedented floods, with water levels reaching 3.3 m above the flood level, just 20 cm less than the most pessimistic simulation [80]. The populations of G. atlantica were severely impacted, with almost their entire distribution in the region area submerged (Figure 8). After this, fewer plants survived and were observed by researchers after the waters receded. However, in an emergency response, the Pelotas city government, while attempting to repair the road connecting the city to the urban settlement of Pontal da Barra, ended up removing the entire habitat of the species in that region. Additionally, populations in the Barra Falsa region of Rio Grande were recovering very slowly, and it is unknown how resilient these populations will be if the extreme events become regular. With the recent extreme events, only depleted populations remain, highlighting the impact of climate change and the increasing frequency of extreme events on species survival [8], and underscoring the urgency of actions to save G. atlantica from extinction.
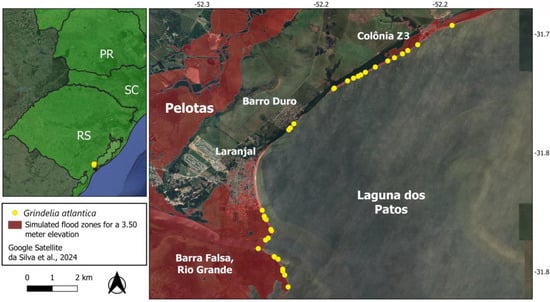
Figure 8.
Distribution of Grindelia atlantica in Laranjal, Pelotas, and Barra Falsa, Rio Grande, Rio Grande do Sul, Brazil, with the projected flood area at an elevation of 3.5 m in red [80]. The represented region recorded an elevation of 3.3 m, the exact maximum level the region experienced during the last catastrophic floods that occurred in 2024.
5. Conclusions
Based on our results, it is evident that the inclusion of G. atlantica in the IUCN Red List as “Critically Endangered (CR)” is justified by criterion A. However, more than a mere designation, these results indicate the imminent disappearance of this emblematic species if no action is taken. Conservation measures are urgently needed not only to save G. atlantica from extinction but also to protect the biodiversity of the entire region. In this context, the creation of a conservation unit in the Pontal da Barra do Laranjal region emerges as a vital and indispensable strategy to ensure the survival of G. atlantica and to preserve the precious local ecosystem. Moreover, ex situ conservation strategies, such as the maintenance of germplasm banks and the cultivation of individuals in botanical gardens, must be strengthened as complementary actions to in situ efforts. These approaches not only ensure genetic preservation but also serve as living repositories for future reintroduction or reinforcement programs. In addition, the concept of conservation through use should be embraced by encouraging the cultivation of G. atlantica in public green spaces, urban landscaping, and home gardens. Such initiatives help increase awareness, promote local engagement, and foster a culture of appreciation and care for native flora.
This is not just a protective measure but a crucial opportunity to prevent the disappearance of G. atlantica from becoming a sad symbol of environmental neglect or a belated “flagship event” that finally draws attention to the conservation of the region’s biodiversity. While the Pontal da Barra region harbors the largest known population of G. atlantica, the population identified in Jaguarão must not be overlooked. Its existence reinforces the urgency of integrated conservation efforts that consider the species’ full distribution range.
Furthermore, these findings underscore the importance of rigorous taxonomic research in conservation science, emphasizing the need to monitor, reassess, and carefully manage both field-confirmed and digitally recorded populations, and also to train the next generation of taxonomists. Such approaches are fundamental in our race against time to safeguard what remains of our natural world.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/conservation5030036/s1, Spreadsheet containing raw data based on georeferenced information obtained from digital platforms, as well as from collections made during field expeditions and visits to herbaria for specimens initially identified as Grindelia atlantica. It includes the following fields: species, country, state/province, municipality, locality, latitude, longitude, collection date, collector, collection number, herbarium, and collection code.
Author Contributions
Conceptualization, F.F., J.I. and G.H.; methodology, F.F., J.I., T.T.d.S.-C. and G.H.; validation, J.I., T.T.d.S.-C. and G.H.; formal analysis, F.F.; investigation, F.F., J.I., T.T.d.S.-C. and G.H.; resources, J.I., T.T.d.S.-C. and G.H.; data curation, F.F.; writing—original draft preparation, F.F.; writing—review and editing, J.I., T.T.d.S.-C. and G.H.; visualization, F.F.; supervision, T.T.d.S.-C. and G.H.; project administration, F.F.; funding acquisition, F.F., J.I., T.T.d.S.-C. and G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundo Brasileiro para a Biodiversidade (FunBio, Conservando o Futuro scholarship) and by a CNPq research scholarship granted to the first author. T.T. Souza-Chies received funding from CNPq (306807/2020-3). G. Heiden received support from LinnéSys 2021, WoRMS Philanthropy Grants 2022, FAPERGS (24/2551-0001284-2), and CNPq (403618/2024-0, 444807/2024-1, 312897/2025-1). J. Iganci was funded by CNPq (311847/2021-8, 403808/2021-9).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available in the Supplementary Materials. They can also be found on the platforms mentioned in the Methods section: Flora e Funga do Brasil, SpeciesLink, and GBIF.
Acknowledgments
FF would like to thank Taciane Schöder and Juliene Lopes Costa for their assistance during the field expeditions, and Giovanni Nachtigall Mauricio for his support in the development of this work. We are also grateful to Camila Leal Bonilha, Secretary of Environmental Quality of Pelotas, for her commitment to protecting the margarida-da-praia in the city of Pelotas, and to Mateus Henrique Schenkel, who reported the occurrence of the species in Jaguarão.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IUCN | International Union for Conservation of Nature |
| CBD | Convention on Biological Diversity |
| GBIF | Global Biodiversity Information |
| EOO | Extent of Occurrence |
| AOO | Area of Occupancy |
| GeoCAT | Geospatial Conservation Assessment Tool |
| APP | Permanent Preservation Areas |
References
- Le Breton, T.D.; Zimmer, H.; Gallagher, R.; Cox, M.P.G.; Allen, S.; Auld, T. Using IUCN criteria to perform rapid assessments of at-risk taxa. Biodivers. Conserv. 2019, 28, 863–883. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef] [PubMed]
- IUCN—International Union for Conservation of Nature. Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 13 June 2025).
- Mace, G.M. The role of taxonomy in species conservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Ely, C.V.; Bordignon, S.A.L.; Trevisan, R.; Boldrini, I.I. Implications of poor taxonomy in conservation. J. Nat. Conserv. 2017, 36, 10–13. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef]
- CDB—Convention on Biological Diversity. United Nations. Available online: https://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 13 June 2025).
- United Nations. Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 13 June 2025).
- CDB—Convention on Biological Diversity. Kunming-Montreal Global Biodiversity Framework; Secretariat of the CBD: Montreal, QC, Canada, 2022; pp. 1–15. Available online: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (accessed on 13 June 2025).
- CDB—Convention on Biological Diversity. Global Biodiversity Outlook 5; Secretariat of the CBD: Montreal, QC, Canada, 2020; Available online: https://www.cbd.int/gbo5 (accessed on 13 June 2025).
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Antonelli, A.; Fry, C.; Smith, R.J.; Eden, J.; Govaerts, R.H.A.; Kersey, P.; Nic Lughadha, E.; Onstein, R.E.; Simmonds, M.S.J.; Zizka, A.; et al. State of the World’s Plants and Fungi 2023, 1st ed.; Royal Botanic Gardens, Kew: London, UK, 2023. [Google Scholar] [CrossRef]
- Bachman, S.P.; Brown, M.J.; Nic Lughadha, E.; Leão, T.C.; Walker, B. Extinction risk predictions for the world’s flowering plants to support their conservation. New Phytol. 2024, 242, 797–808. [Google Scholar] [CrossRef]
- Cafaro, P. Three ways to think about the sixth mass extinction. Biol. Conserv. 2015, 192, 387–393. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H.; Bouchet, P.; Fontaine, B. The Sixth Mass Extinction: Fact, fiction or speculation? Biol. Rev. 2022, 97, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Zelinka, J. How many species will Earth lose to climate change? Glob. Change Biol. 2024, 30, e17125. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, A.; Tortosa, R.D. Revisión de las especies sudamericanas de Grindelia (Asteraceae: Astereae). Kurtziana 1999, 27, 327–359. [Google Scholar]
- Bartoli, A.; Tortosa, R.D. Revision of the North American species of Grindelia (Asteraceae). Ann. Mo. Bot. Gard. 2012, 98, 447–513. [Google Scholar] [CrossRef]
- Deble, L.P.; Oliveira-Deble, A.S. Novelties in Grindelia (Asteraceae: Astereae) from South America. Bonplandia 2010, 19, 47–57. [Google Scholar]
- Fernandes, F.; Borges, R.A.X.; Sancho, G.; Heiden, G. Grindelia. In Flora e Funga do Brasil; Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB5331 (accessed on 13 June 2025).
- Rio Grande do Sul. Decreto Estadual nº 52.109, de 1 de Dezembro de 2014. Declara as espécies da flora nativa ameaçadas de extinção no Estado do Rio Grande do Sul. In Diário Oficial do Estado do Rio Grande do Sul; Rio Grande do Sul: Porto Alegre, Brazil, 2014; pp. 1–34. [Google Scholar]
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 13 June 2025).
- GBIF.org. GBIF Home Page. Available online: https://www.gbif.org (accessed on 13 June 2025).
- INCT—Instituto Nacional de Ciência e Tecnologia. Specieslink. Herbário Virtual da Flora e dos Fungos—HVFF. Available online: https://specieslink.net/search/ (accessed on 13 June 2025).
- Thiers, B. Index Herbariorum. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 13 June 2025).
- Bachman, S.; Moat, J.; Hill, A.W.; Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- GeoCAT. Available online: http://geocat.kew.org/ (accessed on 7 January 2025).
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria, version 16; 2024. Available online: https://www.iucnredlist.org/resources/redlistguidelines (accessed on 13 June 2025).
- Deble, L.P.; Sabatino, M. Rehabilitation of Grindelia argentina (Asteraceae: Astereae) and updates on its geographic range. Balduinia 2022, 70, 26–31. [Google Scholar] [CrossRef]
- Strohaecker, T.M. A urbanização no Rio Grande do Sul. In Rio Grande do Sul: Paisagens e Territórios em Transformação; Verdum, R., Basso, L.A., Suertegaray, D.M.A., Eds.; Editora da UFRGS: Porto Alegre, Brazil, 2012; pp. 187–209. [Google Scholar]
- Almeida, F.T.; Dillenburg, S.R.; Clerot, L.C.P. Estratigrafia e evolução da barreira holocênica do Rio Grande do Sul no trecho Tramandaí-Cidreira. Bol. Parana. Geociênc. 2005, 57, 57–73. [Google Scholar] [CrossRef][Green Version]
- Chowdhury, S.; Fuller, R.A.; Ahmed, S.; Alam, S.; Callaghan, C.T.; Das, P.; Correia, R.A.; Di Marco, M.; Di Minin, E.; Jarić, I. Using social media records to inform conservation planning. Conserv. Biol. 2024, 38, e14161. [Google Scholar] [CrossRef]
- López-Delgado, J.; Meirmans, P.G. History or demography? Determining the drivers of genetic variation in North American plants. Mol. Ecol. 2022, 31, 1951–1962. [Google Scholar] [CrossRef]
- Yu, H.; Deane, D.C.; Zhang, Y.; Li, S.; Miao, S.; Xie, G.; Yin, X.; Favre, A. Integrating multiple indices of geobiodiversity reveals a series of regional species-rich areas worthy of conservation in the region of the Qinghai-Tibet Plateau. Biol. Conserv. 2021, 261, 109238. [Google Scholar] [CrossRef]
- Dario, M.A.; Maranhão, P.H.C.; Santos, G.Q.; Rocha, M.M.; Falqueto, A.; Silva, L.F.C.F.; Jansen, A.M.; Xavier, S.C.C. Environmental influence on Triatoma vitticeps occurrence and Trypanosoma cruzi infection in the Atlantic Forest of south-eastern Brazil. Geospat. Health 2021, 16, 997. [Google Scholar] [CrossRef]
- Pouteau, R.; Thuiller, W.; Hobohm, C.; Brunel, C.; Conn, B.J.; Dawson, W.; de Sá Dechoum, M.; Ebel, A.L.; Essl, F.; Fragman-Sapir, O.; et al. Climate and socio-economic factors explain differences between observed and expected naturalization patterns of European plants around the world. Glob. Ecol. Biogeogr. 2021, 30, 1514–1531. [Google Scholar] [CrossRef]
- Goettsch, B.; Urquiza-Haas, T.; Koleff, P.; Acevedo Gasman, F.; Aguilar-Meléndez, A.; Alavez, V.; Alejandre-Iturbide, G.; Aragón Cuevas, F.; Azurdia Pérez, C.; Carr, J.A.; et al. Extinction risk of Mesoamerican crop wild relatives. Plants People Planet 2021, 3, 775–795. [Google Scholar] [CrossRef]
- Rivers, M.C.; Taylor, L.; Brummitt, N.A.; Meagher, T.R.; Roberts, D.L.; Nic Lughadha, E. How many herbarium specimens are needed to detect threatened species? Biol. Conserv. 2011, 144, 2541–2547. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness: A global meta-analysis. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar] [CrossRef]
- BPBES—Plataforma Brasileira de Biodiversidade e Serviços Ecossistêmicos. Relatório Temático Sobre Espécies Exóticas Invasoras, Biodiversidade e Serviços Ecossistêmicos; BPBES: Campinas, Brasil, 2024; 297p, Available online: https://www.bpbes.net.br/produtos/relatorios-e-diagnosticos/ (accessed on 15 June 2025).
- Sampaio Franco, A.C.; da Rocha, R.M.; Pivello, V.R.; Magalhães, A.L.B.; de Castro, C.F.; da Cruz Neto, C.C.; da Silva Matos, D.M.; Brown, G.G.; Heringer, G.; Saulino, H.H.L.; et al. Dataset of the impacts of invasive alien species in Brazil. Ecol. Res. 2024, 39, 380–390. [Google Scholar] [CrossRef]
- Hernandez, S.; Duce, S.; Sheaves, M.; Murray, N.; Adams, V.M. Predicting the impacts of clearing on vegetation communities: A model-based approach for identifying conservation priorities in Queensland, Australia. Australas. J. Environ. Manag. 2024, 31, 40–63. [Google Scholar] [CrossRef]
- Pelotas. Lei Municipal nº 6636, de 03 de Outubro de 2018. Altera a Lei Municipal nº 5502, de 11 de Setembro de 2008, Que Dispõe Sobre o Plano Diretor de Pelotas, e dá Outras Providências. Diário Oficial de Pelotas, 04 de Outubro de 2018. Available online: https://sapl.pelotas.rs.leg.br/norma/2680 (accessed on 15 June 2025).
- Brasil. Lei nº 12.651, de 25 de Maio de 2012. Dispõe Sobre a Proteção Da Vegetação Nativa; Altera as Leis nºs 6.938, de 31 de Agosto de 1981, 9.393, de 19 de Dezembro de 1996, e 11.428, de 22 de Dezembro de 2006; Revoga as Leis nºs 4.771, de 15 de Setembro de 1965, e 7.754, de 14 de Abril de 1989, e a Medida Provisória nº 2.166-67, de 24 de Agosto de 2001; e dá Outras Providências. Diário Oficial da União, Brasília, 26 de Maio de 2012. Available online: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12651.htm (accessed on 15 June 2025).
- Rio Grande do Sul. Lei Estadual nº 15.434, de 09 de Janeiro de 2020. Institui o Código Estadual do Meio Ambiente do Estado do Rio Grande do Sul. Diário Oficial do Estado, Porto Alegre, 10 de Janeiro de 2020. Available online: https://www.pge.rs.gov.br/upload/arquivos/202001/10084233-doe-ultimo-10012020.pdf (accessed on 15 June 2025).
- Pelotas. Lei Municipal nº 4594, de 20 de Outubro de 2000. Institui o Código do Meio Ambiente do Município de Pelotas, e dá Outras Providências. Diário Oficial de Pelotas, 21 de Outubro de 2000. Available online: https://sapl.pelotas.rs.leg.br/norma/998?display (accessed on 15 June 2025).
- Pelotas. Lei Municipal nº 4392, de 05 de Julho de 1999. Declara Como Área de Interesse Ecoturístico a “Orla da Laguna Dos Patos” no Município de Pelotas, Nos Termos Do Artigo 258 da L.O.M. e dá Outras Providências. Diário Oficial de Pelotas, 6 de Julho de 1999. Available online: https://sapl.pelotas.rs.leg.br/norma/732?display (accessed on 15 June 2025).
- Wandersee, J.H.; Schussler, E.E. Toward a theory of plant blindness. Plant Sci. Bull. 2002, 47, 2–9. [Google Scholar]
- McDonough MacKenzie, C.; Kuebbing, S.; Barak, R.S.; Bletz, M.; Dudney, J.; McGill, B.M.; Nocco, M.A.; Young, T.; Tonietto, R.K. We do not want to “cure plant blindness” we want to grow plant love. Plants People Planet 2019, 1, 139–141. [Google Scholar] [CrossRef]
- Ursi, S.; Salatino, A. É tempo de superar termos capacitistas no ensino de biologia: “Impercepção botânica” como alternativa para “cegueira botânica”. Bol. Bot. Univ. São Paulo 2022, 39, 1–4. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Venticinque, E.M. Biodiversity conservation gaps in Brazil: A role for systematic conservation planning. Perspect. Ecol. Conserv. 2018, 16, 61–67. [Google Scholar] [CrossRef]
- Overbeck, G.E.; Toma, T.S.P.; Silveira-Filho, R.R.; Dechoum, M.S.; Fonsêca, N.C.; Grelle, C.E.V.; Guimarães, A.F.; Negreiros, D.; Nunes, A.V.; Oliveira, H.F.M.; et al. Brazil’s natural grasslands under attack. Science 2024, 384, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Projeto MapBiomas. Coleção 8 da Série Anual de Mapas de Cobertura e Uso da Terra do Brasil. Available online: https://brasil.mapbiomas.org/produtos/?category=maps (accessed on 15 June 2025).
- Andrade, B.O.; Dröse, W.; Aguiar, C.A.; Aires, E.T.; Alvares, D.J.; Barbieri, R.L.; Carvalho, C.J.B.; Bartz, M.; Becker, F.G.; Bencke, G.A.; et al. 12,500+ and counting: Biodiversity of the Brazilian Pampa. Front. Biogeogr. 2023, 15, e59288. [Google Scholar] [CrossRef]
- Overbeck, G.E.; Vélez-Martin, E.; Scarano, F.R.; Lewinsohn, T.M.; Fonseca, C.R.; Meyer, S.T.; Müller, S.C.; Ceotto, P.; Dadalt, L.; Durigan, G.; et al. Conservation in Brazil needs to include non-forest ecosystems. Divers. Distrib. 2015, 21, 1455–1460. [Google Scholar] [CrossRef]
- Ribeiro, S.; Moreira, L.F.B.; Overbeck, G.E.; Maltchik, L. Protected Areas of the Pampa biome presented land use incompatible with conservation purposes. J. Land Use Sci. 2021, 16, 260–272. [Google Scholar] [CrossRef]
- Maurício, G.N. A importância ambiental da área do Pontal da Barra/várzea do canal São Gonçalo, Pelotas (RS): Justificativas para a implantação de uma unidade de conservação. Cad. CIM 2017, 1, 36–60. [Google Scholar] [CrossRef][Green Version]
- Barcellos, S. Fundamentação Técnico-Científica para a Criação da Unidade de Conservação Pontal da Barra do Laranjal, Pelotas, RS, 1st ed.; UFPel: Pelotas, Brazil, 2019. [Google Scholar]
- Van Huynh, A. Effect of IUCN Red List category on public attention to mammals. Conserv. Biol. 2023, 37, e14050. [Google Scholar] [CrossRef]
- Jarić, I.; Crowley, S.L.; Veríssimo, D.; Jeschke, J.M. Flagship events and biodiversity conservation. Trends Ecol. Evol. 2024, 39, 106–108. [Google Scholar] [CrossRef]
- Gessa, S.J.; Tayeebwa, W.; Tumwesigye, C.; Rothman, J.M. The role of public relations in wildlife conservation: Examples from Uganda. Trop. Conserv. Sci. 2024, 17, 19400829241233471. [Google Scholar] [CrossRef]
- Maurício, G.N.; Cheffe, M.M.; Hefler, S.M.; Venzke, T.S.L.; Matzenauer, W.; Salazar, E. Importância biológica. In Fundamentação técnico-Científica para a Criação da Unidade de Conservação Pontal da Barra do Laranjal, Pelotas, RS; Barcellos, S.C.B., Ed.; Universidade Federal de Pelotas: Pelotas, Brazil, 2019; pp. 17–30. [Google Scholar]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. (Eds.) Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, 1st ed.; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Naman, C.B.; Leber, C.A.; Gerwick, W.H. Modern natural products drug discovery and its relevance to biodiversity conservation. In Microbial Resources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 103–120. [Google Scholar] [CrossRef]
- Dannenberg, P.; Braun, B.; Greiner, C.; Follmann, A.; Haug, M.; Hargo Yuwono, P.S.; Stetter, M.; Widlok, T.; Kopriva, S. Eight arguments why biodiversity is important to safeguard food security. Plants People Planet 2024, 6, 604–610. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Ziliotto, M.; Chies, J.A.B. Protect Brazil’s overlooked Pampa biome. Science 2022, 377, 720. [Google Scholar] [CrossRef]
- Pinter, A.; Prist, P.R.; Marrelli, M.T. Biodiversity and public health interface. Biota Neotrop. 2022, 22, e20221372. [Google Scholar] [CrossRef]
- Gautier, A.; Gardon, S.; Déprés, C. The emergence of the Biodiversity/Health nexus: Making biodiversity a health issue. Rev. Agric. Food Environ. Stud. 2023, 104, 27–46. [Google Scholar] [CrossRef]
- Pillar, V.; Overbeck, G.E. Learning from a climate disaster: The catastrophic floods in southern Brazil. Science 2024, 385, eadr8356. [Google Scholar] [CrossRef]
- Rocha, R.P.; Reboita, M.S.; Crespo, N.M. Análise do evento extremo de precipitação ocorrido no Rio Grande do Sul entre abril e maio de 2024. J. Health NPEPS 2024, 9, e12603. [Google Scholar] [CrossRef]
- Bruick, Z.S.; Rasmussen, K.L.; Rowe, A.K.; McMurdie, L.A. Characteristics of intense convection in subtropical South America as influenced by El Niño-Southern Oscillation. Mon. Weather Rev. 2019, 147, 1947–1966. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Ziliotto, M.; Kulmann-Leal, B.; Chies, J.A.B. Environmental challenges in Southern Brazil: Impacts of pollution and extreme weather events on biodiversity and human health. Int. J. Environ. Res. Public Health 2025, 22, 305. [Google Scholar] [CrossRef]
- da Silva, T.S.; Rocha, F.A.; da Silva, D.F.; Lenhard, J.C.; Sfreddo, G.A.; de Aquino, J.N.; Prestes, L.D.; Gezatt, J.N.; Gandra, T.; Gianuca, K.S.; et al. Base de Dados da Inundação na Região da Lagoa dos Patos em Maio de 2024; OSF: Peoria, IL, USA, 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).