Confirmed Wild Reproduction and Distribution Records of Palea steindachneri in Northern Vietnam, with Notes on Sympatric Pelodiscus sp. in Dam-Impacted Habitats
Abstract
1. Introduction
2. Materials and Methods
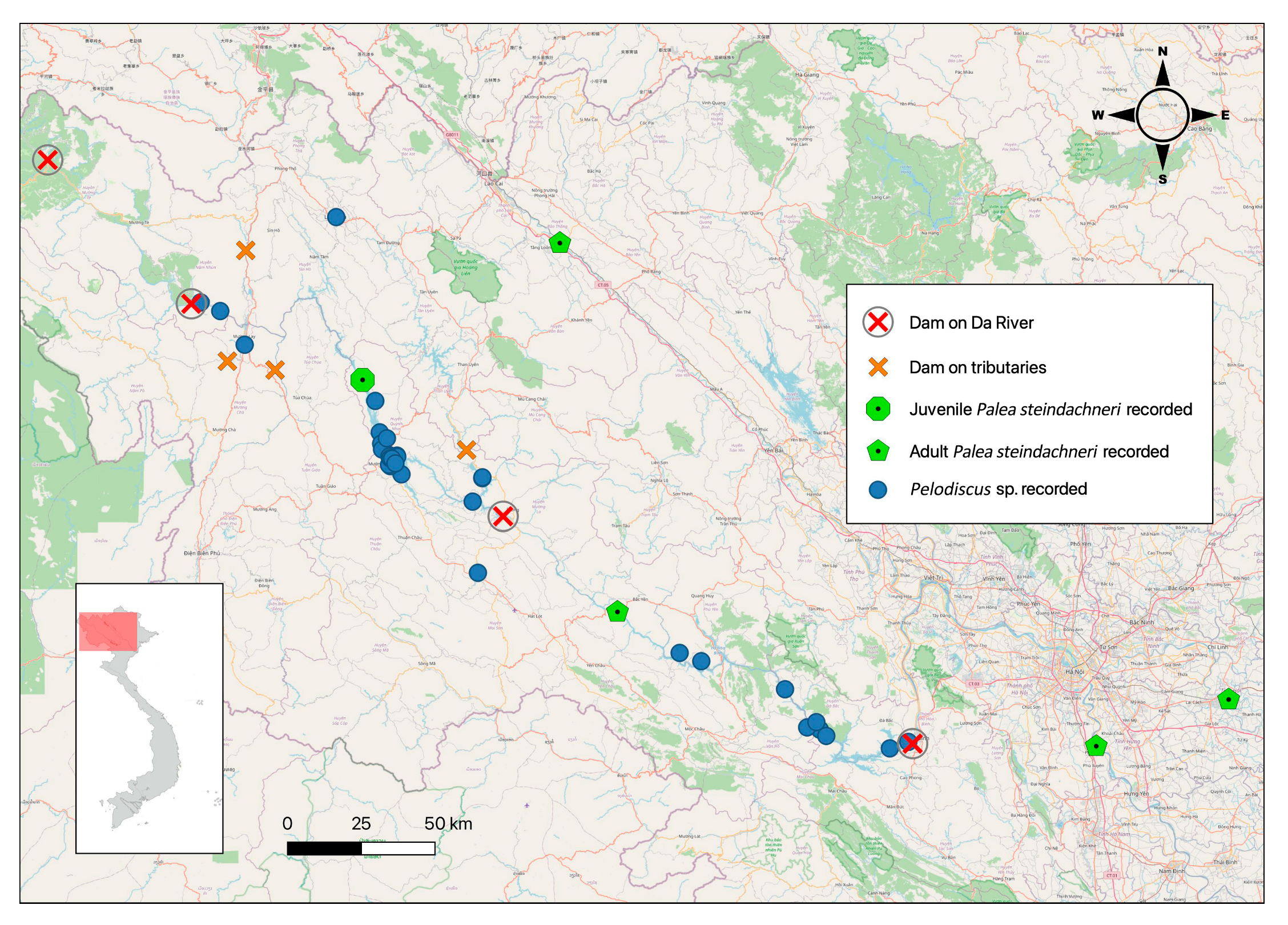
3. Results
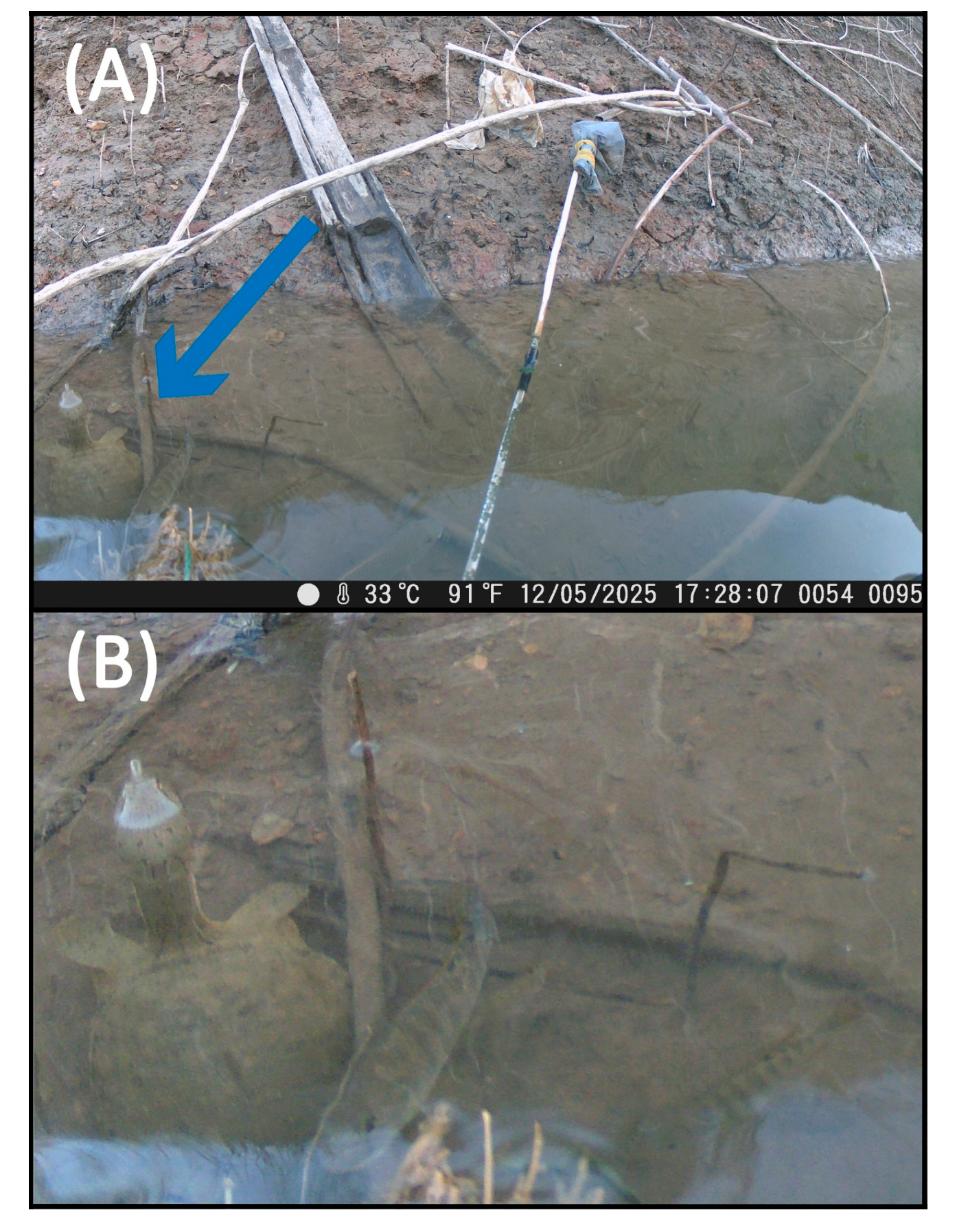
3.1. Palea steindachneri
3.2. Pelodiscus sp.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Dam | River | Province | LAT | LONG | Construction Date | Capacity (MW) | Reservoir Capacity (m3) | High (m) | Length (m) |
|---|---|---|---|---|---|---|---|---|---|
| Hoa Binh | Da River | Hoa Binh | 20.808110 | 105.326619 | 1994–2012 | 1920 | 9.8 billion | 128 | 970 |
| Son La | Da River | Son La | 21.496458 | 103.996610 | 2005–2012 | 2400 | 9.3 billion | 138 | 1000 |
| Huoi Quang | Nam Mu | Lai Chau | 21.696632 | 103.877913 | 2006–2016 | 520 | 184 mil | 104 | 267 |
| Trung Thu | Nam Muc | Dien Bien | 21.938172 | 103.256023 | 2014–2016 | 29.6 | 30.7 mil | 39 | 50 |
| Nam Na 3 | Nam Na | Lai Chau | 22.298428 | 103.160793 | 2014–2018 | 30 | 34.25 mil | 37.5 | 220 |
| Nam He | Nam He | Dien Bien | 21.964770 | 103.101122 | 2010–2014 | 16 | 10.8 mil | 55.5 | 207.5 |
| Lai Chau | Da River | Lai Chau | 22.137730 | 102.983801 | 2011–2016 | 1200 | 1.2 billion | 137 | 613 |
| Pac Ma | Da River | Lai Chau | 22.570238 | 102.517356 | 2016–2019 | 140 | N/A | N/A | 450 |
References
- Rhodin, A.G.; Stanford, C.B.; Van Dijk, P.P.; Eisemberg, C.; Luiselli, L.; Mittermeier, R.A.; Hudson, R.; Horne, B.D.; Goode, E.V.; Kuchling, G.; et al. Global Conservation Status of Turtles and Tortoises (Order Testudines). Chelonian Conserv. Biol. 2018, 17, 135–161. [Google Scholar] [CrossRef]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.; van Dijk, P.P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E.; et al. Turtles and tortoises are in trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef] [PubMed]
- Thirakhupt, K.; Van Dijk, P.P. Species diversity and conservation of the turtles of Western Thailand. Nat. Hist. Bull. Siam Soc. 1994, 42, 209–259. [Google Scholar]
- Ducotterd, C.; Le Duc, O.; Van Pham, T.; Leprince, B.; Bordes, C.; Nghiêm, T.L.; Thu, P.H.; Le, A.T.; Tran, B.Q.; Luu, V.Q.; et al. Previously Unrecorded Invasive Species and the Unsatisfying Knowledge of Turtle Communities in Northern Vietnam. Conservation 2022, 3, 1–13. [Google Scholar] [CrossRef]
- Gray, T.N.E.; Phan, C.; Chan, B.; Pin, C.; Prum, S. Assessing biological value and threats to protected areas in Cambodia using interviews and simple field surveys. Oryx 2011, 46, 544–553. [Google Scholar]
- van Dijk, P.P.; Stuart, B.L.; Rhodin, A.G.J. Asian Turtle Trade: Proceedings of a Workshop on Conservation and Trade of Freshwater Turtles and Tortoises in Asia. Chelonian Res. Monogr. 2000, 2, 1–164. [Google Scholar]
- Das, R.K.; Mondal, R.; Joardar, B.S.; Ray, N. First report of occurrence and conservation status of black softshell turtle, Nilssonia nigricans (Anderson 1875) (Reptilia: Testudines: Trionychidae) in West Bengal, India. Proc. Zool. Soc. 2020, 73, 215–219. [Google Scholar] [CrossRef]
- Le Duc, O.; Van Pham, T.; Leprince, B.; Bordes, C.; Quang, V.L.; Van, O.L.; Thi Tam, A.N.; Luiselli, L. Farming characteristics and the ecology of Palea steindachneri (Trionychidae) in Vietnam. Russ. J. Herpetol. 2020, 27, 97–108. [Google Scholar] [CrossRef]
- Le Duc, O.; Van, T.P.; Leprince, B.; Bordes, C.; Tuan, A.N.; Benansio, J.S.; Pacini, N.; Luu, V.Q.; Luiselli, L. Fishers, dams, and the potential survival of the world’s rarest turtle, Rafetus swinhoei, in two river basins in northern Vietnam. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1074–1087. [Google Scholar] [CrossRef]
- Xiaoyou, H.; Xiaodan, C.; Chen, C.; Xiaoli, L.; Jian, Z.; Quanbo, Q.; Xinping, Z. Conservation status of the Asian giant softshell turtle (Pelochelys cantorii) in China. Chelonian Conserv. Biol. 2019, 18, 68–74. [Google Scholar] [CrossRef]
- De Nardi, L.; Le Duc, O.; Viriyataveekul, S.; Charpentier, L.; Leprince, B.; Walde, A.D.; Luiselli, L. New records of the Critically Endangered Asian Narrow-headed Softshell Turtle, Chitra chitra, in Thailand. Chelonian Conserv. Biol. 2025; in press. [Google Scholar]
- Marchetti, M.P.; Engstrom, T. The conservation paradox of endangered and invasive species. Conserv. Biol. 2016, 30, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Turtle Taxonomy Working Group; Rhodin, A.G.J.; Iverson, J.B.; Bour, R.; Fritz, U.; Georges, A.; Shaffer, H.B.; van Dijk, P.P. Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status (9th Ed.). In Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group; Rhodin, A.G.J., Iverson, J.B., van Dijk, P.P., Stanford, C.B., Goode, E.V., Buhlmann, K.A., Mittermeier, R.A., Eds.; Chelonian Research Monographs; Chelonian Research Foundation: Arlington, VT, USA, 2021; Volume 8, pp. 1–472. [Google Scholar] [CrossRef]
- Radford, C.C. The Endangered Wattle-Necked Softshell Turtle (Palea steindachneri) Throughout the Hawaiian Islands. Master’s Thesis, California State University, Chico, CA, USA, 2011. [Google Scholar]
- Le Duc, O.; Van Pham, T.; Zuklin, T.; Bordes, C.; Leprince, B.; Ducotterd, C.; Quang, V.L.; Luiselli, L. A new locality of presence for the world’s rarest turtle (Rafetus swinhoei) gives new hope for its survival. J. Nat. Conserv. 2020, 55, 125833. [Google Scholar] [CrossRef]
- Farkas, B.; Ziegler, T.; Pham, C.T.; Ong, A.V.; Fritz, U. A new species of Pelodiscus from northeastern Indochina (Testudines, Trionychidae). ZooKeys 2019, 824, 71–86. [Google Scholar] [CrossRef]
- Gong, S.; Fritz, U.; Vamberger, M.; Gao, Y.; Farkas, B. Disentangling the Pelodiscus axenaria complex, with the description of a new Chinese species and neotype designation for P. axenaria (Zhou, Zhang & Fang, 1991). Zootaxa 2022, 5125, 131–143. [Google Scholar] [PubMed]
- Gong, Y.A.; Peng, L.F.; Huang, S.; Lin, Y.F.; Huang, R.Y.; Xu, Y.H.; Yang, D.C.; Nie, L.W. A new species of the genus Pelodiscus Fitzinger, 1835 (Testudines: Trionychidae) from Huangshan, Anhui, China. Zootaxa 2021, 5060, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Le, M.D.; Rödder, D.; Nguyen, T.T.; Nguyen, T.Q.; Ong, A.V.; McCormack, T.E.; Nguyen, T.T.; Ziegler, T. Climatic niche modelling and genetic analyses highlight conservation priorities for the Spotted Softshell Turtle (Pelodiscus variegatus). Nat. Conserv. 2024, 55, 67–82. [Google Scholar] [CrossRef]
- Haitao, S.; Parham, J.F.; Zhiyong, F.; Meiling, H.; Feng, Y. Evidence for the massive scale of turtle farming in China. Oryx 2008, 42, 147–150. [Google Scholar] [CrossRef]
- Fong, J.; Hoang, H.; Li, P.; McCormack, T.; Timmins, R.J.; Wang, L. Palea steindachneri. The IUCN Red List of Threatened Species, 2021. e.T15918A794203. Available online:www.iucnredlist.org (accessed on 28 May 2025). [CrossRef]
- Van Pham, T.; Van Ngoan, H.; Van Dinh, V.; Trung, N.H.; Van Nam, L.; Bieu, L.T.; Nguyen, P.T.H.; Phuong, P.M.; Van Thai, N.; Cuong, T.X.; et al. First natural history observations of the Critically Endangered Wattle-necked softshell turtle Palea steindachneri in its native range. Herpetol. J. 2024, 34, 127–129. [Google Scholar]
- Yen, C.T.; Picco, L.; Dung, B.X.; Bao, T.Q. Hydropower dam and hydrological alteration on the Da River in Son La, Vietnam. J. For. Sci. Technol. 2019, 7, 124–133. [Google Scholar]
- Dao, N. Dam development in Vietnam: The evolution of dam-induced resettlement policy. Water Altern. 2010, 3, 324. [Google Scholar]
- Le Duc, O.; Ducotterd, C.; Bordes, C.; Van Pham, T.; Leprince, B.; Le, A.T.; Luu, V.Q.; Tran, B.Q.; Luiselli, L. Discovering threatened freshwater turtles by an innovative floating camera trap system. Acta Oecologica 2025, 127, 104081. [Google Scholar] [CrossRef]
- Bárcenas-García, A.; Michalski, F.; Morgan, W.H.; Smith, R.K.; Sutherland, W.J.; Gibbs, J.P.; Norris, D. Impacts of dams on freshwater turtles: A global review to identify conservation solutions. Trop. Conserv. Sci. 2022, 15, 19400829221103709. [Google Scholar] [CrossRef]
- Barcenas-Garcıa, A.; Michalski, F.; Gibbs, J.P.; Norris, D. Amazonian run-of-river dam reservoir impacts underestimated: Evidence from a before–after control–impact study of fresh water turtle nesting areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 508–522. [Google Scholar] [CrossRef]
- Bodie, J.R. Stream and riparian management for freshwater turtles. J. Environ. Manag. 2001, 62, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Bondi, C.A.; Marks, S.B. Differences in flow regime influence the seasonal migrations, body size, and body con dition of western pond turtles (Actinemys marmorata) that inhabit perennial and intermittent riverine sites in northern California. Copeia 2013, 1, 142–153. [Google Scholar] [CrossRef]
- Berry, G.; Brown, G.J.; Haden, L.; Jones, R.L.; Pearson, L.; Selman, W. Chutes and Ladders: Drainage Exchange of Map Turtles (Genus Graptemys) Across the Tennessee Tombigbee Waterway in Northeastern Mississippi. Chelonian Conserv. Biol. 2020, 19, 262–267. [Google Scholar] [CrossRef]
- Clark, N.J.; Gordos, M.; Franklin, C. Implications of river damming: The influence of aquatic hypoxia on the diving physiology and behaviour of the endangered Mary River turtle. Anim. Conserv. 2009, 12, 147–154. [Google Scholar] [CrossRef]
- Clark, N.J.; Mills, C.E.; Osborne, N.A.; Neil, K.M. The influence of a new water infrastructure development on the relative abundance of two Australian freshwater turtle species. Aust. J. Zool. 2018, 66, 57–66. [Google Scholar] [CrossRef]
- Luiselli, L. Resource partitioning in freshwater turtle communities: A null model meta-analysis of available data. Acta Oecologica 2008, 34, 80–88. [Google Scholar] [CrossRef]
- Kong, F.; Zhu, Q.; Xiao, F.; Ngwawa, J.M.; Zhang, H.; Haitao, S. Preliminary Diet Analysis of Chinese Soft-shelled Turtle (Pelodiscus sinensis) in the Middle Confines of the Yellow River, Shaanxi Province, China. Pak. J. Zool. 2022, 54, 1489–1491. [Google Scholar] [CrossRef]
- Luiselli, L.; Le Duc, O.; Van, T.P.; Xuan, T.N.; Dang, P.B.; Kuchling, G.; Leprince, B.; Shi, H.T.; McCaskill, L.; Giovacchini, P.; et al. A threat analysis for the world’s most threatened turtle (Rafetus swinhoei). J. Nat. Conserv. 2024, 78, 126577. [Google Scholar] [CrossRef]
- Bui, V.D.; Dinh, Q.V.; Grzegorz, L.; Izabela, N.; Jan, W.; Vi, V.V.; Le Ba, M.; Nguyen, T.L.; Vu, M.T.; Nguyen, T.H.; et al. Analysis of Geomorphic Factors Related to Seismic Activity in Lai Chau Hydropower Reservoir in the Period 2015–2024. VNU J. Sci. Earth Environ. Sci. 2025, 1, 1. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Duc, O.; Trong, M.N.; Leprince, B.; Minh, H.H.; Van, H.T.; Van, S.H.; Luiselli, L. Confirmed Wild Reproduction and Distribution Records of Palea steindachneri in Northern Vietnam, with Notes on Sympatric Pelodiscus sp. in Dam-Impacted Habitats. Conservation 2025, 5, 32. https://doi.org/10.3390/conservation5030032
Le Duc O, Trong MN, Leprince B, Minh HH, Van HT, Van SH, Luiselli L. Confirmed Wild Reproduction and Distribution Records of Palea steindachneri in Northern Vietnam, with Notes on Sympatric Pelodiscus sp. in Dam-Impacted Habitats. Conservation. 2025; 5(3):32. https://doi.org/10.3390/conservation5030032
Chicago/Turabian StyleLe Duc, Olivier, Minh Nguyen Trong, Benjamin Leprince, Hoa Huynh Minh, Hoang Tong Van, Sam Hoang Van, and Luca Luiselli. 2025. "Confirmed Wild Reproduction and Distribution Records of Palea steindachneri in Northern Vietnam, with Notes on Sympatric Pelodiscus sp. in Dam-Impacted Habitats" Conservation 5, no. 3: 32. https://doi.org/10.3390/conservation5030032
APA StyleLe Duc, O., Trong, M. N., Leprince, B., Minh, H. H., Van, H. T., Van, S. H., & Luiselli, L. (2025). Confirmed Wild Reproduction and Distribution Records of Palea steindachneri in Northern Vietnam, with Notes on Sympatric Pelodiscus sp. in Dam-Impacted Habitats. Conservation, 5(3), 32. https://doi.org/10.3390/conservation5030032








