Abstract
Increasing the germination percentages from chemical and/or mechanical processes in native species is vital for the conservation of the species. The objective was to evaluate the effect of different treatments on the germination process of species belonging to the tropical deciduous forest (TDF) in a forest nursery. Eight species of trees and shrubs reported to be native to the TDF were selected; the seeds were collected from various sites of the state of Queretaro, Mexico. A randomized design of four replicates with 25 seeds was used, for which three pre-germinative scarification treatments were evaluated for each species: (1) thermal scarification (TS), (2) mechanical scarification (MS), and (3) chemical scarification (CS). Germination was considered as the protrusion of cotyledons onto the substrate and was recorded every 48 h for 60 days. A significant difference was observed between treatments (p ≤ 0.05). The mechanical treatment (MS) obtained the highest germination percentage and the least variation, in contrast to the thermal, chemical, and control treatments, which presented the lowest amount of germinated seeds. The MS treatment was the most effective for all the species studied because it allowed for 100% of the germination to be achieved.
1. Introduction
Species inhabiting tropical forests are subject to disturbances that are mainly anthropic in origin. This type of vegetation hosts more than 75% of the biodiversity and occupies about 7% of the Earth’s surface. It hosts two-thirds of the world’s floral diversity and has a global distribution ranging from 0.5 to 1100 million hectares [1,2].
In Mexico, the tropical deciduous forest (TDF) is defined as a group of forests that occur mainly in warm climates and are dominated by trees that lose their leaves during the dry season (this season is different in each region of the Mexican territory). This type of ecosystem hosts a great diversity of plants, vertebrates, and insects [3]. The plant families that best represent the TDF in some regions of Mexico and Central America are the Fabaceae and Euphorbiaceae [4,5].
It is estimated that before human intervention, the TDF covered up to 30% of the national territory [6], but today, it occupies an area of 226,898 km2, which is equivalent to 11.7% of the territory [7]. Consequently, it is one of the ecosystems that most of the human population inhabits. Furthermore, about 25% of the world’s population depends on the ecosystem services that the TDF provides, such as carbon storage, soil conservation, high biodiversity, and extraction of fuelwood and non-timber products, in addition to the intensive use caused by extensive livestock production systems and overgrazing. Due to the aforementioned and given that the greatest threat to this ecosystem is land use change, several studies have suggested that conservation of the TDF should be a priority [1,6,8,9].
There are concerns about the small number of studies conducted in comparison to other wet temperate ecosystems [8,10,11]. In addition to anthropogenic pressures, the disruption of hydrological cycles caused by climate change has led to prolonged droughts and desertification, which has contributed to the degradation of this ecosystem type, as the high tree density, enormous diversity, and high endemism of these forests are due to its topography and marked seasonality [10,11]. Additionally, the TDF is greatly undervalued due to its low visual appeal during the dry season, which consequently, negatively impacts the interest in its conservation, as well asnd its ecological value not being recognized by society [6].
Substantial efforts have been made to restore tropical forests [12]; however, to date, public TDF conservation strategies have been insufficient relative to deforestation rates and compared to other vegetation types. Natural regeneration is undoubtedly the most effective alternative, but it has been shown to be limited by recruitment of individual seeds in the soil due to herbivory, dormancy, and disturbance [13]. Restoration efforts must be well planned to make the best decision regarding which species to use and the type of plant structure to work with. Additionally, there are insufficient studies regarding the phenology and adaptive characteristics of individual species in the TDF, making it difficult to develop appropriate conservation strategies [14,15,16].
In order to achieve successful conservation and restoration programs, it is important to understand the ecology of succession of these forests, specifically secondary succession processes [17]. The process of succession in the TDF is poorly understood, and further investigation of its regeneration pathways is required [9]. Nevertheless, the introduction of native trees that make up the canopy of the TDF can contribute to the regeneration of secondary forests by facilitating the regeneration of local species of woody plants [18].
An important characteristic of the species that contribute to the regeneration of the disturbed TDF is their high capacity for colonizing sites that are in very poor condition, which makes them inherently resilient. Moreover, even though the rapid recovery of the tree population has occasionally been noted after a disturbance, the recovery of floristic structure and composition is slow [19].
Restoration activities with native species are not limited to wild areas; the restoration of urban areas is also important because 56% of the world’s population lives in cities [20]. The increase in restored urban areas has many important benefits from a social perspective. It has been proven that the interaction between people and nature helps to reduce stress and promotes positive mood and good cognitive functioning [21,22,23,24]. These positive effects are referred to as “restorers,” and people tend to attribute them to natural areas rather than urban areas without nature [25]. In terms of ecological benefits, the use of native species in restored areas has been shown to be beneficial compared to introduced species, because soil conditions favor the establishment of native species [26].
Consequently, conservation strategies using native species help us to combat the accelerated conversion of wild areas to urban areas, thus promoting the protection of wild vegetation patches.
There is evidence to support that in order to achieve a good outcome in reforestation, it is very important to select plant structures that favor rapid adaptability [27]. Additionally, the ecological contribution of seeds is well supported, but there is a lack of emphasis on the importance of their functional role in the ecosystem, which at present, is considered to be minor [28,29]. The physicochemical properties of seeds, which provide unique characteristics to the seeds and the plants, are determined by the environmental conditions surrounding their progenitor [29]. It is important to keep these properties in mind while developing restoration strategies in order to make it possible for productive forests to continually adapt to site conditions [30]. Therefore, several studies have proposed the evaluation of seed characteristics and their behavior under laboratory and field conditions [16,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47].
Additionally, successful reforestation depends greatly on the nursery phase, which must produce plants with high adaptability and survival. In turn, this depends largely on the characteristics of the germplasm and the procedures used for reproduction [48]. Nonetheless, the seeds of some species become dormant during their maturation, where physical dormancy is one of the most common [49]; however, this kind of dormancy prevents water uptake and thus germination [50]. As a result, this makes the propagation of some TDF species difficult, requiring the use of pregerminative treatments during the nursery phase.
Applied to the seed before germination, these pregerminative treatments contribute to the success of plant production as they lead to embryonic development, promoting germination. These treatments vary depending on the type of dormancy of the seed, and several authors have reported their effectiveness in TDF species [32,33,51,52].
Seed scarification is a pregerminative treatment that consists of physical damage to the seed coat in order to allow imbibition [53]. Among the most commonly used are heat or thermal scarification (TS), chemical scarification (CS), and mechanical scarification (MS). For TS, the seeds are exposed to high temperatures by dipping them in boiling water. On the other hand, CS consists of the use of acids to corrode the seed coat. However, the most direct method is MS, which consists of the removal of a portion of the seed coat or simply the creation of small scars on the surface of the seed [53]. Pregermination treatments are among the most important activities in a forest nursery, and the use of the most adequate treatment for each species ensures the success of plant production [32,48,54]. Although propagation protocols have been published for TDF species of trees and shrubs, most of them were conducted in vitro under controlled conditions and the species included were not representative of the great diversity found in the TDF [32,33,36,38,39,40,42,43,44,45,47,51,52].
Forest nurseries that are dedicated to mass propagation are crucial for the success of afforestation and reforestation programs in the country. They are also considered to be the starting point for the reversal of the degradation of natural resources, which can be a low-cost method for improving the quality of life of the human population [55]. In their endeavor of producing healthy and vigorous plants, forest nurseries search for management strategies that fit each species and create the ideal conditions for their proper development [56]. Due to the aforementioned, the present study analyzed the effect of three pregerminative treatments on the seeds of eight native TDF plant species with the aim of evaluating their effectiveness for breaking seed dormancy.
Selected Species
Eight species of trees and shrubs reported to be native to the TDF [57] were selected, all of which belong to the Fabaceae family, except for Dodonaea viscosa, which belongs to the Sapindaceae family (Figure 1).

Figure 1.
Seeds of the selected species: (A) Albizia occidentalis, (B) Dodonaea viscosa, (C) Leucaena esculenta, (D) Lysiloma divaricatum, (E) Prosopis laevigata, (F) Senna septemtrionalis, (G) Vachellia farnesiana, and (H) Vachellia pennatula.
Albizia occidentalis Brandegee (Fabaceae), also known as “parotilla”, is an endemic tree species of the TDF [34] that occurs at altitudes from sea level to 2400 masl [58]. It is well adapted to lateritic alluvial soils and sandy areas [59]. Its seeds are brown, ovoid to elliptical, with a hard seed coat, and they have a pleurogram and an areola. They have an orthodox behavior and remain viable several years after their collection. They are widely distributed in Mexico, from Baja California, through the Pacific slope, to Central America.
Dodonaea viscosa (L.) Jacq (Sapindaceae) is an evergreen woody shrub that adapts to impacted soils, survives with very little water, and is found in temperate, arid, tropical, and subtropical environments, as well as in ecological transition areas [60]. It can be found from sea level to 2600 masl. Its seeds are small, black, and lenticular [61], with an orthodox behavior and physical dormancy. Seeds of this species exhibit physical dormancy when collected in Hawaii and New Zealand [51], but no dormancy in seeds collected in Mexico City [35]. It is commonly used as an ornament in gardens [60].
Leucaena esculenta (Moc. Et Sessé ex Dc.) Benth. (Fabaceae) is a tree species of 3 to 20 m tall that grows in poor soils (regosol, calcareous), and it has been found from 1250 to 1880 masl [36]. It is distributed in the highlands and the Pacific Slope of Mexico. It has ovoid seeds with a dormant embryo; it presents orthodox behavior and significantly loses viability after a year [36]. The wood from this tree is used to build rural buildings, and as firewood and charcoal, with an excellent calorific value of 18,600 kJ/kg [62,63].
Lysiloma divaricatum (Jacq.) J.F. Macbr (Fabaceae) is a tree of up to 18 m tall that occurs naturally from the south of the United States to Central America, at altitudes between 600 and 2300 masl [64]. The seeds are compressed, elliptical to ovoid in shape, and dark brown to black in color [65]; it presents orthodox behavior without dormancy and with high viability (>50%) after two years of dry storage [36]. It is used as a noble wood for construction, flooring, furniture, firewood, and charcoal, and in some places, it is planted in combination with coffee plantations [64].
Prosopis laevigata (Humb. & Bonpl. ex Willd.) M.C. Johnst. (Fabaceae) is a tree species that grows up to 12 m tall and is widespread from Texas in the United States to Chiapas in Mexico, from a sea level up to 2050 masl [64]. It is common in plains and lowlands with deep soils [66]. The seeds show orthodox behavior with a seed coat-imposed dormancy and high viability (>60%) even after 50 years of dry storage [38]. It is used to obtain various goods and services, such as firewood, rubber, and construction materials [67]. The seeds can also have a rhomboid shape, a rough coat, and be light brown in color [37,68].
Senna septemtrionalis (Viv.) H.S. Irwin & Barneby (Fabaceae) is an herb or shrub, and rarely grows to be a tree of up to 4 m high. It is native to Mexico and Central America, and can be found distributed along the Sierra Madre Oriental (in Mexico) and through the mountains of Guatemala, Honduras, Costa Rica, and Nicaragua (in Central America), from 1000 to 1950 masl [69]. It has been reported to grow in very poor leptosol soil [39,70] and it has flattened, olive-colored, and glossy seeds of about 3.6–4.9 mm in length; it is of orthodox behavior with a physical dormancy and high viability (>50%) after two years of storage at room temperature [39]. It is commonly used as a remedy for pain and inflammation [71,72]
Vachellia farnesiana (L.) Wight & Arn. (Fabaceae) is a shrub or tree of 2 to 5 m tall, with a characteristic smell of honey when it flowers. It is native to Mexico and has been reported in 29 states. It is usually found along low slopes covered with scrub, at altitudes that range from sea level to 2400 masl. It is used as fodder, as well as an ornamental plant [73]. It grows in different types of soils but grows better in calcareous clay soils. Its obovoid seeds are rounded at the base and apex, and have a hard seed coat with a smooth, dark brown-colored cover [40]; it is of orthodox behavior with physical dormancy [41]. These seeds can be stored for a long time and are able to stay viable for up to 151 years at room temperature [74].
Vachellia pennatula (Schltdl. & Cham.) Seigler & Ebinger (Fabaceae) is a shrub or tree that reaches a height of up to 12 m and a diameter of up to 30 cm. It is reported to grow on andosols, acrosols, and regosols at mid-elevation sites (below 1500 masl). They are distributed throughout 26 states in Mexico and play an important role as timber for rural buildings [75]. It has oblong seeds that are laterally compressed, of about 8–9 mm in length and 5–6 mm wide, and slightly shiny and red colored, with a smooth and woody consistency. Their orthodox behavior allows them to stay viable when stored at room temperature [76].
2. Materials and Methods
The seeds were collected from different sites within fragments of a TDF, located in the state of Queretaro: (1) La Barreta (20°48′59.90″ N 100°31′13.10″ W) (D. viscosa and S. septemtrionalis), (2) Mompaní (20°41′50.62″ N 100°30′35.69″ W) (P. lavigata), and (3) Juriquilla (20°42′0.61″ N 100°26′26.51″ W) (A. occidentalis, L. divaricatum, L. esculenta, V. farnesiana, and V. pennatula) (Figure 2).
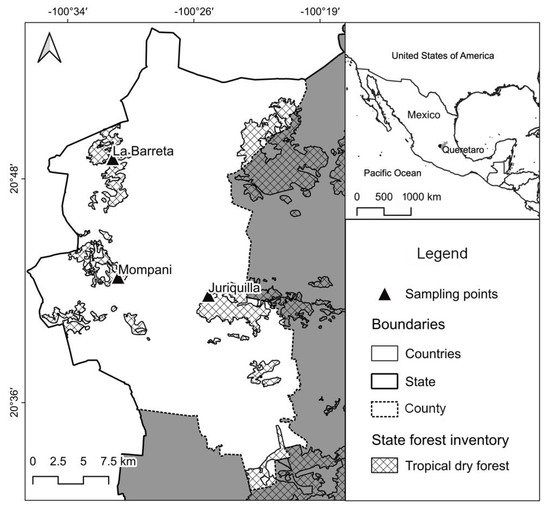
Figure 2.
Seed collection sites based on location of the donor trees within remnant areas of a TDF in Queretaro, Mexico.
Collection of seeds was performed once every 15 days, from January to December of 2020, at the three aforementioned sites. In order to characterize the donor trees, four phenotypic criteria were considered: apparent good phytosanitary status, minimum distance of 100 m between donor trees (of the same species), and presence of abundant and ripe fruits.
After harvesting, the fruits were cleaned with water and stored in airtight and sterile glass containers at 5 °C in a refrigerator (Torrey©, model R14L, Mexico City, Mexico). The experiments were carried out between April and June of 2021 at the forest nursery of the Natural Sciences Faculty of the Autonomous University of Queretaro (20°42′1.7″ N and 100°26′36.7” W). During the study, the nursery registered a mean temperature of 22.49 °C and a relative humidity of 53.76%. These were measured and registered using a USB data logger (LASCAR, model EL-USB-1-LCD, Salisbury, UK).
The study consisted of a randomized design of four replicates with 25 seeds each, per species. We consider the sample size used in this study to be appropriate, as it has been shown that for species with low seed production, a small sample size is sufficiently robust to draw statistical inferences [77,78]. Germination was considered as the protrusion of cotyledons onto the substrate (emergence) [79] and was recorded every 48 h for 60 days.
2.1. Pre-Germinative Treatments
Seeds were carefully selected based on criteria such as: (a) no perforations of any kind, (b) no color change, which might indicate fungal or insect invasion, and (c) maximum size according to the species.
Three treatments of pre-germination scarification were evaluated (Table 1): (1) thermal scarification (TS), (2) mechanical scarification (MS), and (3) chemical scarification (CS). After the treatments, the seeds were soaked in running water for 24 h. The temperatures (TS) and times of immersion (CS and TS) were based on specific literature for the selected species that report the use of temperatures between 65 °C and 100 °C [32,36,39,42,45,51,80]. For CS, sulfuric acid was used at different concentrations and immersion times for the same species [42,44,47,81].

Table 1.
Pre-germinative treatment conditions for chemical and thermal scarification by species.
- (1)
- For thermal scarification (TS), the seeds were immersed in water at a temperature between 70 °C and 94 °C.
- (2)
- The mechanical scarification (MS) consisted of the removal of a portion of 1 mm (or 0.5 mm in the case of D. viscosa) of the seed using diagonal cut pliers (Truper, model PM-CD4, Mexico City, Mexico). The size of the removed portion was dependent upon the total size of the seed and thickness of the seed coat.
- (3)
- For chemical scarification (CS), the seeds were immersed in 98% sulfuric acid for 20 to 45 min with constant manual agitation.
Planting was carried out manually in Styrofoam seedbeds with alveoli of 5 cm in diameter and 20 cm deep, which were previously sterilized with a 2% solution of commercial sodium hypochlorite. The seedbeds were then placed in plastic tents (Figure 3). The substrate used consisted of a 3:1:1 mixture of peatmoss, vermiculite, and perlite. Irrigation was carried out at least every 48 h (field capacity), except for when the substrate had an excessive loss of water.

Figure 3.
(Left) Seedbeds with emerging seedlings of V. farnesiana. (Right) Plastic tents.
2.2. Statistical Analysis
The germination percentages were subjected to an arcsine angular transformation in order to normalize the data and stabilize the variances.
Once normalized, multiple media analyses were run with a 95% confidence level. Subsequently, the comparison between treatments was performed using an HSD Tukey test. SPSS v.25 software by IBM was used for all statistical analyses.
3. Results
3.1. Treatments
A significant difference was observed between treatments (p ≤ 0.05). The MS obtained the highest germination percentage and the least variation, elevating the absolute germination (Figure 4), in contrast to the Control and TS which presented the lowest number of germinated seeds. The CS treatment was intermediate.
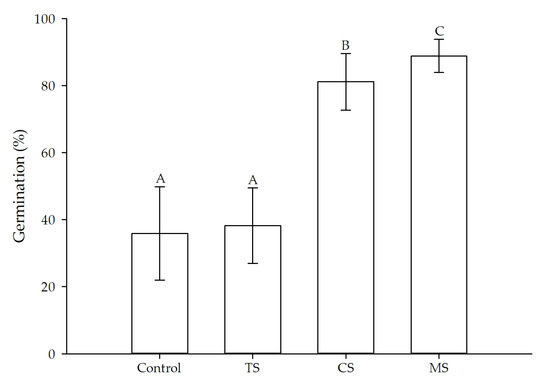
Figure 4.
Germination percentages by treatment. Diferrent letters express significant differences between treatments (p ≤ 0.05). (TS) thermal scarification, (CS) chemical scarification, (MS) mechanical scarification. Error bars represent standard error.
3.2. Species
In the control group, L. divaricatum and A. occidentalis obtained the highest germination percentages with 98% and 88%, respectively (Figure 2 and Figure 5), while the species with the lowest germination were V. farnesiana, V. pennatula, and S. septemtrionalis, which obtained 5%, 2%, and 3%, respectively, during the entire study (Table 2).
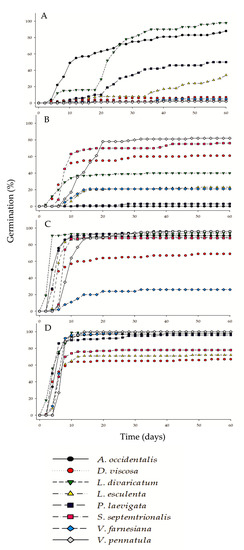
Figure 5.
Cumulative seed germination (germination percentage vs. days). (A) Control; (B) Thermal Scarification; (C) Chemical Scarification; and (D) Mechanical Scarification.

Table 2.
Germination percentage and standard deviation of the eight species with respect to each pre-germinative treatment. (TS) thermal scarification, (CS) chemical scarification, (MS) mechanical scarification.
In thermal scarification, the best responding species were V. pennatula (82%) and S. septentrionalis (76%). On the contrary, P. laevigata obtained only 3% and A. occidentalis obtained 0% germination. Overall, chemical scarification gave the best results, where P. laevigata and V. pennatula obtained 96% germination, while L. divaricatum obtained 94%, and the lowest percentage was for V. farnesiana with 26%. This treatment had the highest germination for D. viscosa, L. esculenta, and S. septemtrionalis.
Finally, mechanical scarification was the only treatment that achieved 100% germination in three species (L. divaricatum, V. farnesiana, and V. pennatula); however, D. viscosa obtained the lowest value at 67%. In addition, it can be observed that in MS, all species reached their highest germination level 10 days after the start of the treatment. Furthermore, a large number of seeds germinated within the same period for CS, except for D. viscosa and V. farnesiana. For TS, lower germination rates were observed on average compared to the other treatments, as well as different behaviors for each species.
4. Discussion
Firstly, it is important to highlight that the seeds used in this study were those of larger sizes. This practice is common in forest nurseries because it helps to maintain uniformity in seedling size [82]. Nevertheless, this practice affects the genetic diversity of the seed lot, which should be taken into account in future studies with native species of the TDF.
In the present study, and according to the overall results, P. laevigata with CS achieved 96% of germinated seeds, which is different from the results reported by Sobrevilla-Solis [42], where they estimated 2.5% germination. These differences may be caused by modification of the pre-germination process, where they immersed the seeds in H2SO4 for 5 min, in contrast to the 20 to 45 min of this study. Sobrevilla-Solis [42] also believe that mechanical treatments such as seed abrasion and liquefaction over different time periods provide better results than thermal and/or chemical treatments. For their part, Rivas-Medina [37] agrees that mechanical treatments (mixing the seed at different periods) achieve the best germination rates, with an average of 53%. The 24-h soaking period previous to the treatment could explain the differences in the estimated germination percentages among the different studies.
In the case of L. divaricatum and A. occidentalis, there was little literature prescribing treatment of these species before germination; however, in the present study, high germination rates were estimated (88% and 98%, respectively), so we assume a lower degree of physical dormancy compared to other Fabaceae, as described in previous studies [43,83].
The control group of L. esculenta also obtained a significantly lower germination percentage than both the chemical and mechanical scarification treatments. The same was observed in the TS treatment, so we conclude that there is a negative effect of the thermal scarification treatment, as discussed by Cervantes-Gutierrez [36], where a reduction of viability was reported for the same treatment.
Lysiloma divaricatum and A. occidentalis responded negatively to the TS treatment and had the lowest percentages within each species. V. farnesiana, A. occidentalis, L. esculenta, and P. laevigata had low germination rates (less than 50%). This behavior is consistent with the data of Tadros et al. [32], whose tests showed a negative interaction between immersion in hot water and subsequent immersion in water at room temperature for 24 or more hours. Therefore, the authors of the present work do not recommend this treatment to break dormancy in the species mentioned above.
Prosopis laevigata did not respond positively to the TS treatment, obtaining only 3% germination. We assume that the high water temperature (94 °C) was responsible for this result. Cervantes et al. [43] indicate that immersion in water at 65 °C for 4 min allows for 20% to 25% germination after 25 days, so we consider that using high temperatures when treating these species is not appropriate; it is better to use temperatures below the boiling point and longer periods of time.
This was not the case for V. pennatula, as it obtained 82% germination, which was significantly lower than the other two treatments (CS and MS). Cervantes-Gutierrez [36] achieved a germination of 33% with boiling water for 10 min, so we assume a possible negative effect due to the high water temperature.
In the case of A. occidentalis, no studies on germination by hot water treatments have been reported so far, but our results indicate that it is susceptible to high temperatures. S. septemtrionalis responded positively to thermal scarification and was significantly superior to the control. Seeds that were subjected to TS reacted differently in all species, possibly due to the great variability in efficacy depending on the device used (oven or hot water), temperature, and duration of treatment [33].
The percentages obtained by Teketay [52] were close to 100%; however, his experiments were conducted in Petri dishes and the seeds were dipped in coffee filter paper, so these conditions could be the cause of this difference. D. viscosa also responded positively to this treatment, although it did not reach the high percentages reported by Baskin et al. [51], which were close to 100%. In this study, high temperatures (100 °C) and short durations (3 to 60 s) were used, so we assume that this species responds positively to high temperatures and short durations, although lower temperatures (80 °C) may increase the germination percentage.
As mentioned in the results, chemical scarification was the most effective treatment for D. viscosa (69%), L. esculenta (89%), and S. septemtrionalis (88%), as it significantly increased germination rates compared to the other two treatments and the control group. Overall, this treatment raised the absolute germination rate.
However, in the case of V. farnesiana, we obtained a relatively low percentage (26%); similarly, Rivas-Medina [37] reported low percentages (less than 50%) using the same method. Two factors may have caused this difference, their experiments were conducted at a constant temperature and they did not soak the seeds for 24 h as in the present study.
In general, mechanical scarification was the treatment that achieved the highest germination rates and also significantly increased the germination rate, except for S. septemtrionalis. We are of the opinion that this treatment can be harmful to the seeds but requires a considerable amount of time compared to the other methods used.
The germination percentages of the species studied show that their germination ability is not affected even in a suburban environment. These species can adapt to stress and must be considered as a source of genetic and biological diversity in reforestation projects.
5. Conclusions
The TS treatment is suitable for D. viscosa, S. septemtrionalis, and V. pennatula because of the large number of seeds that can be treated at the same time; additionally, the use of sulfuric acid does not require long periods of time for MS treatment. For the species that responded negatively to this treatment (A. occidentalis, V. farnesiana, and L. esculenta), further tests are needed to determine the optimal temperatures and times.
The MS treatment is the most effective for all the species studied, as it allows 100% of germination to be achieved and significantly increases the germination rate. However, it is the most time consuming when applied on a large scale and small seeds can be damaged.
Lysiloma divaricatum and A. occidentalis do not require pre-germinative treatment. We consider adequately soaking the seeds in water at room temperature for 24 h before sowing.
Author Contributions
Conceptualization, J.D.J.-V. and V.H.C.-S.; methodology H.L.-S., D.G.-G., R.A.T.-B. and A.C.-V.; software, J.D.J.-V.; Validation, V.H.C.-S., D.G.-G. and J.D.J.-V.; writing—original draft preparation, J.D.J.-V., V.H.C.-S. and D.G.-G. writing—review and editing, V.H.C.-S. and J.D.J.-V. All authors have read and agreed to the published version of the manuscript.
Funding
ENGIE S.A.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schröder, J.M.; Rodríguez, L.P.; Günter, S. Research trends: Tropical dry forests: The neglected research agenda? For. Policy Econ. 2020, 122, 102333. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Forests 2018-Forest Pathways to Sustainable Development; FAO: Rome, Italy, 2018; pp. i–xi. Available online: https://www.fao.org/publications/card/en/c/I9535EN/ (accessed on 30 March 2023).
- Portillo-Quintero, C.; Sanchez-Azofeifa, A.; Calvo-Alvarado, J.; Quesada, M.; do Espirito Santo, M.M. The role of tropical dry forests for biodiversity, carbon and water conservation in the neotropics: Lessons learned and opportunities for its sustainable management. Reg. Environ. Change 2015, 15, 1039–1049. [Google Scholar] [CrossRef]
- Gillespie, T.W.; Grijalva, A.; Farris, C.N. Diversity, composition, and structure of tropical dry forests in Central America. Plant Ecol. 2000, 147, 37–47. [Google Scholar] [CrossRef]
- Harker, M.L.; Hernndez-López, L.; Muñiz-Castro, M.A. Flora del bosque tropical caducifolio en una zona con suelos yesosos y calcáreos de Colima, México. Acta Bot. Mex. 2021, 128, 2448–7589. [Google Scholar] [CrossRef]
- Meave, J.; Romero-Romero, M.; Salas-Morales, S.; Pérez-García, E.; Gallardo-Cruz, J. Diversidad, amenazas y oportunidades para la conservación del bosque tropical caducifolio en el estado de Oaxaca, México. Ecosistemas 2012, 21, 85–100. Available online: https://www.revistaecosistemas.net/index.php/ecosistemas/article/view/29 (accessed on 2 February 2023).
- INEGI. Cobertura de Uso Del Suelo y Vegetación Serie VI; Mexico. 2017. Available online: https://www.inegi.org.mx/temas/usosuelo/#descargas (accessed on 20 March 2023).
- FAO. Resilient Livelihoods—Disaster Risk Reduction for Food and Nutrition Security; FAO: Rome, Italy, 2013; pp. 2–4. Available online: https://www.fao.org/fsnforum/resources/reports-and-briefs/resilient-livelihoods-disaster-risk-reduction-food-and-nutrition (accessed on 19 January 2023).
- Vieira, D.L.M.; Scariot, A. Principles of natural regeneration of tropical dry forests for restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar] [CrossRef]
- FAO. Trees, Forests and Land Use in Drylands: The First Global Assessment—Full Report; FAO: Rome, Italy, 2019; pp. 49–51. Available online: https://www.fao.org/documents/card/en/c/ca7148en/ (accessed on 19 January 2023).
- Stan, K.; Sanchez-Azofeifa, A. Tropical Dry Forest Diversity, Climatic Response, and Resilience in a Changing Climate. Forests 2019, 10, 443. [Google Scholar] [CrossRef]
- Méndez-Toribio, M.; Martínez-Garza, C.; Ceccon, E.; Guariguata, M.R. La Restauración de Ecosistemas Terrestres en México. Estado actual, Necesidades y Oportunidades; Documentos Ocasionales; Centro para la Investigación Forestal Internacional: Bogor, Indonesia, 2018; pp. 1–7. Available online: https://www.cifor.org/knowledge/publication/6853/ (accessed on 12 December 2022).
- Álvarez-Aquino, C.; Williams-Linera, G. Seedling survival and growth of tree species: Site condition and seasonality in tropical dry forest restoration. Bot. Sci. 2012, 90, 341–351. [Google Scholar] [CrossRef]
- Pezzini, F.F.; Ranieri, B.D.; Brandão, D.O.; Fernandes, G.W.; Quesada, M.; Espírito-Santo, M.M.; Jacobi, C.M. Changes in tree phenology along natural regeneration in a seasonally dry tropical forest. Plant Biosyst. 2014, 148, 965–974. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alía, R.; González-Martínez, S.C.; Hänninen, H.; Kremer, A.; Lefèvre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—Evidence from tree populations. Glob. Change Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef]
- Luna-Nieves, A.L.; Meave, J.A.; Morellato, L.P.C.; Ibarra-Manríquez, G. Reproductive phenology of useful Seasonally Dry Tropical Forest trees: Guiding patterns for seed collection and plant propagation in nurseries. For. Ecol. Manag. 2017, 393, 52–62. [Google Scholar] [CrossRef]
- Quesada, M. Succession and management of tropical dry forests in the Americas: Review and new perspectives. For. Ecol. Manag. 2009, 258, 1014–1024. [Google Scholar] [CrossRef]
- Derroire, G.; Tigabu, M.; Odén, P.C.; Healey, J.R. The Effects of Established Trees on Woody Regeneration during Secondary Succession in Tropical Dry Forests. Biotropica 2016, 48, 290–300. [Google Scholar] [CrossRef]
- Gordillo-Ruiz, M.C.; Pérez-Farrera, M.A.; Castillo-Santiago, M. Estructura y composición arbórea del bosque tropical caducifolio secundario en la Depresión Central, Chiapas, México. Madera Bosques 2020, 26, e2632055. [Google Scholar] [CrossRef]
- Understanding Poverty: Urban Development. Available online: https://www.worldbank.org/en/topic/urbandevelopment (accessed on 31 March 2023).
- Park, B.J.; Furuya, K.; Kasetani, T.; Takayama, N.; Kagawa, T.; Miyazaki, Y. Relationship between psychological responses and physical environments in forest settings. Landsc. Urban Plan. 2011, 102, 24–32. [Google Scholar] [CrossRef]
- Tyrväinen, L.; Ojala, A.; Korpela, K.; Lanki, T.; Tsunetsugu, Y.; Kagawa, T. The influence of urban green environments on stress relief measures: A field experiment. J. Environ. Psychol. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Castell, C. Naturaleza y salud: Una Alianza Necesaria. Gac. Sanit. 2020, 34, 194–196. [Google Scholar] [CrossRef]
- Coppel, G.; Wüstemann, H. The impact of urban green space on health in Berlin, Germany: Empirical findings and implications for urban planning. Landsc. Urban Plan. 2017, 167, 410–418. [Google Scholar] [CrossRef]
- Martínez-Soto, J.; Montero-López, M.; Córdova-Vázquez, A. Restauración Psicológica y Naturaleza Urbana: Algunas Implicaciones Para La Salud Mental. Salud Ment. 2014, 37, 217–224. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-33252014000300005 (accessed on 1 April 2023). [CrossRef]
- Kowarik, I. Novel Urban Ecosystems, Biodiversity, and Conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Kleyer, M.; Minden, V. Why functional ecology should consider all plant organs: An allocation-based perspective. Basic Appl. Ecol. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Grubb, P.J. The maintenance of species-Richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschold, P. Seed germination traints can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Schleicher, A.; Biedermann, R.; Kleyer, M. Dispersal traits determine plant response to habitat connectivity in an urban landscape. Landsc. Ecol. 2011, 26, 529–540. [Google Scholar] [CrossRef]
- Sánchez-Gómez, A.; Rosendo-Ponce, A.; Vargas-Romero, J.M.; Rosales-Martínez, F.; Platas-Rosado, D.E.; Becerril-Pérez, D.M. Energía germinativa en guaje (Leucaena leucocephala cv. cunningham) con diferentes métodos de escarificación de la semilla. Agrociencia 2018, 52, 863–874. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952018000600863 (accessed on 12 December 2022).
- Tadros, M.J.; Samarah, N.H.; Alqudah, A.M. Effect of different pre-sowing seed treatments on the germination of Leucaena leucocephala (Lam.) and Acacia farnesiana (L.). New For. 2011, 42, 397–407. [Google Scholar] [CrossRef]
- Vargas, G.H.; Velásquez, L.R.S.; Aragón, F. Tratamientos pregerminativos en cuatro especies arbóreas de uso forrajero de la selva baja caducifolia de la Sierra de Manantlán. For. Ver. 2001, 3, 9–15. Available online: https://www.redalyc.org/articulo.oa?id=49730102 (accessed on 8 March 2023).
- Encino-Ruiz, L.; Linding-Cisneros, R.; Gomez-Romero, M.; Blanco-García, A. Desempeño de tres especies arbóreas del bosque tropical caducifolio en un ensayo de restauración ecológica. Bot. Sci. 2013, 91, 107–114. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-42982013000100009 (accessed on 12 December 2022). [CrossRef]
- Benítez-Rodríguez, L.; Gamboa-de Buen, A.; Sánchez-Coronado, M.E.; Alvarado-López, S.; Soriano, D.; Méndez, I.; Vázquez-Santana, S.; Carabias-Lillo, J.; Mendoza, A.; Orozco-Segovia, A. Effects of seed burial on germination, protein mobilisation and seedling survival in Dodonaea viscosa. Plant. Biol. 2014, 16, 732–739. [Google Scholar] [CrossRef]
- Cervantes-Gutierrez, M.G. La reforestación en la Montaña de Guerrero: Una Estrategia Alternativa con Leguminosas Nativas. Master’s Thesis, UNAM, Mexico City, Mexico, 1996. Available online: https://repositorio.unam.mx/contenidos/la-reforestacion-en-la-montana-de-guerrero-una-estrategia-alternativa-con-leguminosas-353687?c=a88jq3&d=false&q=*:*&i=1&v=1&t=search_0&as=0 (accessed on 9 December 2022).
- Rivas-Medina, G.; Cervantes, G.G.; Castro, C.M.V.; Cohen, I.S.; Díaz, J.V. Morfología y escarificación de la semilla de mezquite, huizache y ahuehuete. Téc. Pecu. Méx. 2005, 43, 441–448. Available online: https://www.redalyc.org/articulo.oa?id=61343314 (accessed on 9 December 2022).
- Ffolliott, P.F.; Thames, J.L. Collection, Handling, Storage and Pre-Treatment of Prosopis Seeds in Latin America, 1st ed.; Food & Agriculture Organization of the United Nations: Tucson, AZ, USA, 1983; pp. 2–33. Available online: https://www.fao.org/3/Q2180E/Q2180E00.htm#TOC (accessed on 1 February 2023).
- Coronado-Perez, B. Efecto de dos Tratamientos Pregerminativos en el Crecimiento de Senna septemtrionalis Bajo Diferentes Condiciones de Estrés Hídrico. Bachelor’s Thesis, UNAM, Tlalnepantla de Baz, Mexico, 2018. [Google Scholar]
- De Melo, P.A.F.R.; Silva, K.B.; Alves, E.U.; de Medeiros, R.L.S.; Neto, A.P.A.; Pinto, K.M.S.; Leite, W.S.; Matos, V.P. Morphological analysis of fruits, seeds, and seedling germination Acacia farnesiana (L.) Willd. Afr. J. Agric. 2016, 11, 2912–2919. [Google Scholar] [CrossRef]
- Jayasuriya, K.G.; Wijetunga, A.S.; Baskin, J.M.; Baskin, C.C. Seed dormancy and storage behaviour in tropical Fabaceae: A study of 100 species from Sri Lanka. Seed Sci. Res. 2013, 23, 257–269. [Google Scholar] [CrossRef]
- Sobrevilla-Solís, J.A.; López-Herrera, M.; López-Escamilla, A.L.; Romero-Bautista, L. Evaluación de diferentes tratamientos pregerminativos y osmóticos en la germinación de semillas Prosopis laevigata (Humb. y Bonpl. Ex Willd) MC Johnston. In Estudios Científicos en el Estado de Hidalgo y Zonas Aledañas; Pulido-Flores, G., Monks, S., Eds.; University of Nebraska–Lincoln Libraries: Lincoln, NE, USA, 2013; Volume 2, pp. 83–95. Available online: https://digitalcommons.unl.edu/zeabook/16 (accessed on 11 December 2022).
- Cervantes, M.; Ceccon, E.; Bonfil, C. Germination of stored seeds of four tree species from the tropical dry forest of Morelos, Mexico. Bot. Sci. 2014, 92, 281–287. [Google Scholar] [CrossRef]
- Al-Namazi, A.A.; Al-Ammari, B.S.; Davy, A.J.; Al-Turki, T.A. Seed dormancy and germination in Dodonaea viscosa (Sapindaceae) from south-western Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 2420–2424. [Google Scholar] [CrossRef]
- Silva-Farías, M.A. Ensayos de Procedencias y Características de las Semillas y la Madera de Albizia plurijuga (Standl.) Britt. et Rose. Master’s Thesis, Universidad Michoacana de San Nicolás Hidalgo, Michoacan, Mexico, 2012. [Google Scholar]
- Sepúlveda-Ríos, O. Técnicas de pre germinación en leucaena (Leucaena leucocephala w.) y botón de oro (Tithonia diversifolia g.) como forrajeras con potencial para alimentación animal. Bachelor’s Thesis, Universidad Francisco de Paula Santander, Norte de Santander, Colombia, 2020. Available online: https://repositorio.ufps.edu.co/handle/ufps/4370 (accessed on 11 December 2022).
- Mendonça, A.J.T.; Silva, M.C.C.; Berto, F.H.R.; Gondim, A.R.D.O.; Medeiros, M.N.V.; Lins, W.L. Superação de dormência em sementes de Leucaena leucocephala (Lam.) de Wit. com métodos físicos e químicos. Rev. Verde Agro. Des. Sust 2020, 15, 325–329. [Google Scholar] [CrossRef]
- Rueda-Sánchez, A.; Benavides-Solorio, J.D.; Saenz-Reyez, J.T.; Muñoz Flores, H.J.; Prieto-Ruiz, J.Á.; Orozco-Gutiérrez, G. Calidad de planta producida en los viveros forestales de Nayarit. Rev. Mex. Cienc. For. 2014, 5, 58–73. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11322014000200005&lng=es&tlng=es (accessed on 10 December 2022). [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 1998; pp. 27–42. [Google Scholar]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Baskin, J.M.; Davis, B.H.; Baskin, C.C.; Gleason, S.M.; Cordell, S. Physical dormancy in seeds of Dodonaea viscosa (Sapindales, Sapindaceae) from Hawaii. Seed Sci. Res. 2004, 14, 81–90. [Google Scholar] [CrossRef]
- Teketay, D. The effect of different pre-sowing seed treatments, temperature and light on the germination of five Senna species from Ethiopia. New For. 1996, 11, 155–171. Available online: https://link.springer.com/article/10.1007/BF00033411 (accessed on 4 December 2022). [CrossRef]
- Kimura, E.; Islam, M.A. Seed Scarification Methods and their Use in Forage Legumes. Res. J. Seed Sci. 2012, 5, 38–50. Available online: https://scialert.net/abstract/?doi=rjss.2012.38.50 (accessed on 4 December 2022).
- Vari, A.K.; Jethani, I.; Sharma, S.P.; Khanna, M.; Barnwal, S. Seed coat-imposed dormancy in Sesbania spp. and treatments to improve germination. Seed. Sci. Technol. 2007, 35, 318–325. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of Biodiversity and Ecosystem Services by Ecological Restoration: A Meta-Analysis. Science 1979, 325, 1121–1124. [Google Scholar] [CrossRef]
- Benítez, G.; Equihua, M. Diagnóstico de la situación de los viveros oficiales de Veracruz y su papel para apoyar programas de reforestación y restauración. Rev. Chapingo 2002, 8, 5–12. Available online: https://www.redalyc.org/articulo.oa?id=62980101 (accessed on 7 February 2023).
- Rodríguez-Larramendi, L.A.; Sánchez-Cortés, M.S.; Gordillo-Ruiz, M.C. Árboles útiles del bosque tropical caducifolio secundario en la Reserva Forestal Villa Allende, Chiapas, México. Acta Bot. Mex. 2018, 125, 189–214. [Google Scholar] [CrossRef]
- Rico-Arce, M.L.; Gale, S.L.; Maxted, N. A taxonomic study of Albizia (Leguminosae: Mimosoideae: Ingeae) in Mexico and Central America. An. Jardín Bot. Madr. 2008, 65, 255–305. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=2786975 (accessed on 29 January 2023). [CrossRef]
- Allen, O.; Allen, E. The Leguminosae—A Source Book of Characteristics, Uses, and Nodulation; The University of Wisconsin Press: Madison, WI, USA, 1981; pp. 30–32. [Google Scholar]
- Pérez, J.I.J. Funciones E Importancia Económica Del Recurso Chapulixtle (Dodonaea viscosa) En Un Ejido Del Subtrópico Mexicano. Obs. Iberoam. Desarro. Local 2013, 7. Available online: https://ideas.repec.org/a/erv/oidles/y2013i141.html (accessed on 3 February 2023).
- FAO. Trees and Shrubs of the Maldives, 1st ed.; FAO: Bangkok, Thailand, 2007; pp. 83–84. [Google Scholar]
- Cansino, J.V. Botánica Económica de Cuatro Especies de San Juan Ixcaquxstla, Mixteca Poblana. Bachelor’s Thesis, Universidad Autónoma de Chapingo, Texcoco, Mexico, 2001. Available online: https://es.slideshare.net/CharlieSC4/botnica-econmica-de-cuatro-especies-de-san-juan-ixcaquixtla (accessed on 4 December 2022).
- Peralta-Juárez, I.; Gómez-Campos, A.; Romero-Castillo, P.A.; Reyes-Dorantes, A. Uso antropocéntrico Del Guaje Leucaena esculenta (Moc. y Sessé Ex. DC.) Benth, en dos comunidades de la mixteca baja oaxaqueña. Polibotánica 2017, 14, 349–364. [Google Scholar] [CrossRef]
- Andrade, M.; Greter, R.; Hernandez, H.; Medina-Lemos, R.; Rico, L.; Sousa, M. Flora del Valle de Tehuacán-Cuicatlan, 1st ed.; UNAM: Mexico City, Mexico, 2012; pp. 38–43. [Google Scholar]
- De Stefano, R.D.; Tun, C.T.; Contreras, J.E.L.; Fernández-Concha, G.C.; Leopardi-Verde, C.L.; Ramírez-Prado, J.H.; Can-Itza, L.L.; Cen, I.T. Filogenia de Lysiloma (Fabaceae), un género restringido a Megaméxico con especies atípicas en las Antillas y Florida. Acta Bot. Mex. 2021, 128, e1782. [Google Scholar] [CrossRef]
- Rodríguez-Sauceda, E.N. Análisis técnico del árbol de mezquite (Prosopis laevigata Humb. & Bonpl. ex Willd.) en Mexico. Ra Ximhai 2014, 10, 173–193. Available online: https://www.redalyc.org/articulo.oa?id=46131111013 (accessed on 4 December 2022).
- Palacios-Romero, A.; Jiménez-Muñoz, E.; Rodríguez-Laguna, R.; Razo-Zárate, R. Distribución potencial de Prosopis laevigata (Humb. et Bonpl. ex Willd.) M.C. Johnst. en el estado de Hidalgo, México. Rev. Mex. Cienc. For. 2020, 12, 71–87. [Google Scholar] [CrossRef]
- García-López, J.C.; Duran-Garcia, H.M.; De-Nova, J.A.; Álvarez-Fuentes, G.; Pinos-Rodriguez, J.; Lee-Rangel, H.A.; Lopez Aguirre, S.; Ruiz-Tavares, D.; Rendon-Huerta, J.; Vicente-Martínez, J.; et al. Producción y contenido nutrimental de vainas de tres variantes de mezquite (Prosopis laevigata) en el altiplano potosino, México. Agrociencia 2020, 53, 821–832. Available online: https://agrociencia-colpos.org/index.php/agrociencia/article/view/1846 (accessed on 3 February 2023).
- Lewis, G.P.; Irwin, H.S.; Barneby, R.C. The American Cassiinae: A Synoptical Revision of Leguminosae Tribe Cassieae Subtribe Cassiinae in the New World. Kew Bull. 1984, 39, 664–666. [Google Scholar] [CrossRef]
- Rzedowski, J.; Rzedowski, G.C. Flora del Bajío y de Regiones Adyacentes. Familia Leguminosae: Subfamilia Caesalpinioideae; Instituto de Ecología A.C.: Michoacan, Mexico, 1997; pp. 98–101. [Google Scholar]
- García-Martínez, M.A.; Rodríguez, A. Vegetation and phanerogamic flora of Piedras Bola protected area in Jalisco, Mexico. Polibotánica 2018, 48, 71–90. [Google Scholar] [CrossRef]
- Arana-Argáez, V.E.; Domínguez, F.; Moreno, D.A.; Isiordia-Espinoza, M.A.; Lara-Riegos, J.C.; Ceballos-Góngora, E.; Zapata-Morales, J.R.; Franco-de la Torre, L.; Sánchez-Enríquez, S.; Alonso-Castro, A.J. Anti-inflammatory and antinociceptive effects of an ethanol extract from Senna septemtrionalis. Inflammopharmacology 2020, 28, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Mimosaceae = Leguminosae en parte Acacia farnesiana (L.) Willd. Huizache. Malezas de México. Available online: http://www.conabio.gob.mx/malezasdemexico/mimosaceae/acacia-farnesiana/fichas/ficha.htm (accessed on 3 February 2023).
- Erkovan, H.İ.; Clarke, P.J.; Whalley, R.D.B. A review on General Description of Vachellia farnesiana (L.) Wight &Arn. Atatürk Univ. J. Agric. Fac. 2016, 47, 71–76. Available online: https://dergipark.org.tr/en/pub/ataunizfd/issue/27764/293472 (accessed on 3 February 2023).
- López-Merlín, D.; Soto-Pinto, L.; Jiménez-Ferrer, G.; Hernández-Daumás, S. Relaciones alométricas para la predicción de biomasa forrajera y leña de Acacia pennatula y Guazuma ulmifolia en dos comunidades del norte de Chiapas, México. Interciencia 2003, 28, 334–339. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442003000600005&lng=es&tlng=es (accessed on 9 March 2023).
- Niembro-Rocas, A.; Vázquez-Torres, M.; Sánchez-Sánchez, O. Árboles de Veracruz: 100 Especies para la Reforestación Estratégica, 1st ed.; Secretaría de Educación del Estado de Veracruz: Xalapa-Enríquez, Mexico, 2010; pp. 26–27. Available online: https://libros.uv.mx/index.php/UV/catalog/book/FC138 (accessed on 2 February 2023).
- Ribeiro-Oliveira, J.P.; Ranal, M.A.; Garcia de Santana, D.; Pereira, L.A. Sufficient sample size to study seed germination. Aust. J. Bot. 2016, 64, 295–301. [Google Scholar] [CrossRef]
- Ribeiro-Oliveira, J.P.; Ranal, M.A. Sample size in studies on the germination process. Botany 2016, 94, 103–115. [Google Scholar] [CrossRef]
- Bradow, J.M.; Bauer, P.J. Germination and Seedling Development. In Physiology of Cotton, 1st ed.; Stewart, J.M., Oosterhuis, D.M., Heitholt, J.J., Mauney, J.R., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 48–56. [Google Scholar] [CrossRef]
- Moares, F.G.D.; de Matos, V.P.; Silva, M.; da Ferreira, E.G.B.S.; Santos, H.H.D.; Rodrigues, I.A.S.; Bittar, S.M.B. Tratamentos pré-germinativos em sementes de Vachellia farnesiana (L.) Wight & Arn.—Leguminosae-Mimosoidae. Sci. Plena 2013, 8, 04732. Available online: https://www.scientiaplena.org.br/sp/article/view/1430 (accessed on 9 March 2023).
- Gómez-Ruiz, P.A.; Sáenz-Romero, C.; Lindig-Cisneros, R. Early performance of two tropical dry forest species after assisted migration to pine–oak forests at different altitudes: Strategic response to climate change. J. For. Res. 2020, 31, 1215–1223. [Google Scholar] [CrossRef]
- Campbell, R.K.; Sorensen, F.C. Genetic implications of nursery practices. In Forestry Nursery Manual: Production of Bareroot Seedlings, 1st ed.; Duryea, M.L., Landis, T.D., Eds.; Forestry Sciences: Dordrecht, The Netherlands, 1984; Volume 11, pp. 183–191. [Google Scholar] [CrossRef]
- Figueroa-Torres, I. Efecto en el Crecimiento de Albizia occidentalis en Inoculación Dual con Hongos Micorrícicos y Nanoparticulas como Factor de Tolerancia al Estrés Hídrico. Master’s Thesis, Universidad Michoacana de San Nicolas de Hidalgo, Michoacan, Mexico, 2021. Available online: http://bibliotecavirtual.dgb.umich.mx:8083/xmlui/handle/DGB_UMICH/6396 (accessed on 3 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).