Predicting the Geographical Distribution Shift of Medicinal Plants in South Africa Due to Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Medicinal Plant Species
| Species Name | Common Name | Uses and Properties | IUCN Status | References |
|---|---|---|---|---|
| A. ferox Miller | Cape aloe | Anti-inflammatory, analgesic, wound healing, used as a laxative; relief of arthritis pain, antioxidant, anticancer, antimalarial activities | Least Concern | [35,36,37,38] |
| B. volubilis Harv | Climbing onion | Purgatives, skin disorders, pains and inflammation, antimicrobial, anti-inflammatory | Vulnerable | [8,31,39,40,41] |
| D. elephantipes Engl | Elephant’s foot | Cortisone and contraceptives | Declining | [8,42] |
2.2. Climatic Variables for Current and Future Scenarios
2.3. Predictive Modelling for Plant Distribution
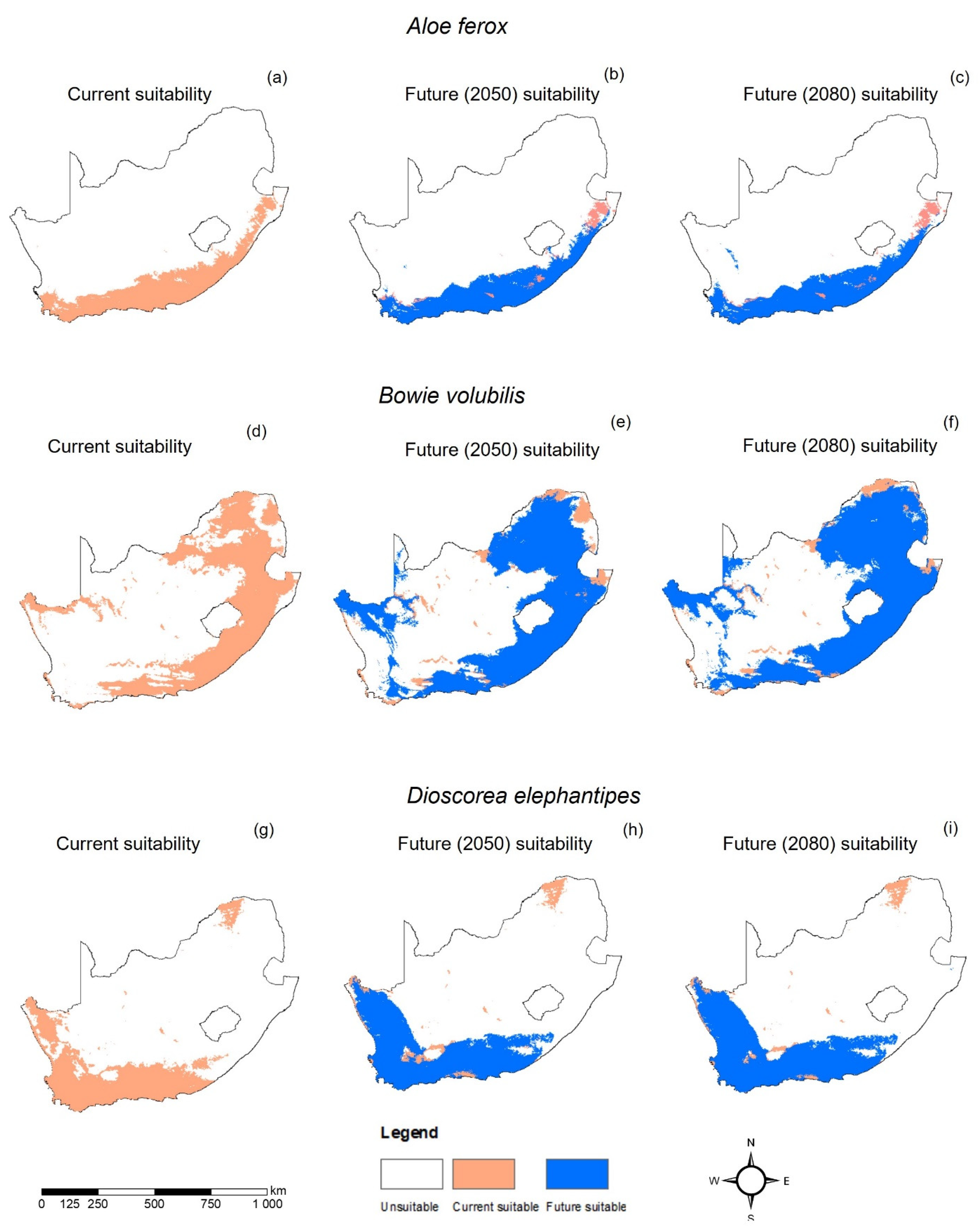
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, M.M.; Zhang, X. The World Medicines Situation 2011, Traditional Medicines: Global Situation, Issues and Challenges; World Health Organization: Geneva, Switzerland, 2011; pp. 1–2. [Google Scholar]
- Mander, M.; McKenzie, M. Southern African Trade Directory of Indigenous Natural Products; Commercial Products from the Wild Group Stellenbosch: Stellenbosch, South Africa, 2005. [Google Scholar]
- Mahmood, K.T.; Mugal, T.; Haq, I.U. Moringa oleifera: A natural gift-A review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- Mander, M. An overview of the medicinal plant market in South Africa. In Indigenous Forests and Woodlands in South Africa: Policy, People and Practice; University of KwaZulu-Natal Press: Scottsville, South Africa, 2004; pp. 440–445. [Google Scholar]
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. South Africa’s Fifth National Report to the Convention On Biological Diversity. Available online: https://www.cbd.int/doc/world/za/za-nr-05-en.pdf (accessed on 20 February 2022).
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Williams, V.; Victor, J.; Crouch, N. Red Listed medicinal plants of South Africa: Status, trends, and assessment challenges. S. Afr. J. Bot. 2013, 86, 23–35. [Google Scholar] [CrossRef]
- Wiersum, K.F.; Dold, A.P.; Husselman, M.; Cocks, M. Cultivation of medicinal plants as a tool for biodiversity conservation and poverty alleviation in the Amatola region, South Africa. Frontis 2006, 17, 43–57. [Google Scholar]
- Department of Environmental Affairs. South Africa’s 2nd Annual Climate Change Report; Department of Environmental Affairs: Pretoria, South Africa, 2017. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.; Engelbrecht, F. Chapter 3: Impacts of 1.5 °C of Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; 2018; Volume 1, pp. 177–311. Available online: https://research-repository.uwa.edu.au/en/publications/impacts-of-15%C2%BAc-global-warming-on-natural-and-human-systems (accessed on 20 February 2022).
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E.; et al. RCP4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77–94. [Google Scholar] [CrossRef]
- McSweeney, R.; Timperley, J. The Carbon Brief Profile: South Africa. Available online: https://www.carbonbrief.org/the-carbon-brief-profile-south-africa/ (accessed on 13 October 2022).
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Chang. 2011, 109, 33–57. [Google Scholar] [CrossRef]
- Bystriakova, N.; Peregrym, M.; Dragićević, S. Effect of environment on distributions of rock ferns in the Mediterranean climate: The case of the genus Asplenium in Montenegro. Flora—Morphol. Distrib. Funct. Ecol. Plants 2015, 215, 84–91. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, H.; Lu, C.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of Schisandra sphenanthera under climate change. PeerJ 2016, 4, e2554. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Predicting the distributions of Egypt’s medicinal plants and their potential shifts under future climate change. PLoS ONE 2017, 12, e0187714. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Wang, Y.; Li, R.; Song, Z.; Zhang, F.; Xu, M.; Li, D. Vulnerability of 208 endemic or endangered species in China to the effects of climate change. Reg. Environ. Chang. 2012, 13, 843–852. [Google Scholar] [CrossRef]
- Alkemade, R.; Bakkenes, M.; Eickhout, B. Towards a general relationship between climate change and biodiversity: An example for plant species in Europe. Reg. Environ. Chang. 2010, 11, 143–150. [Google Scholar] [CrossRef]
- Kudo, G.; Ida, T.Y. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 2013, 94, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Schmidhuber, J.; Tubiello, F.N. Global food security under climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19703–19708. [Google Scholar] [CrossRef]
- Komen, K.; Olwoch, J.; Rautenbach, H.; Botai, J.; Adebayo, A. Long-Run Relative Importance of Temperature as the Main Driver to Malaria Transmission in Limpopo Province, South Africa: A Simple Econometric Approach. EcoHealth 2014, 12, 131–143. [Google Scholar] [CrossRef]
- Musengimana, G.; Mukinda, F.K.; Machekano, R.; Mahomed, H. Temperature Variability and Occurrence of Diarrhoea in Children under Five-Years-Old in Cape Town Metropolitan Sub-Districts. Int. J. Environ. Res. Public Heal. 2016, 13, 859. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2020, 35, 213–226. [Google Scholar] [CrossRef]
- Khanum, R.; Mumtaz, A.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecologica 2013, 49, 23–31. [Google Scholar] [CrossRef]
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W.; et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Qian, J.; Zhuang, H.; Yang, W.; Chen, Y.; Chen, S.; Qu, Y.; Zhang, Y.; Yang, Y.; Wang, Y. Selecting flagship species to solve a biodiversity conservation conundrum. Plant Divers. 2020, 42, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W. Patterns and uncertainties of species’ range shifts under climate change. Glob. Chang. Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Hoffman, M. The pollination ecology of Aloe ferox Mill. S. Afr. J. Bot. 1988, 54, 345–350. [Google Scholar] [CrossRef]
- Aremu, A.O.; Moyo, M.; Amoo, S.O.; Van Staden, J. Ethnobotany, therapeutic value, phytochemistry and conservation status of Bowiea volubilis: A widely used bulbous plant in southern Africa. J. Ethnopharmacol. 2015, 174, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Maurin, O.; Muasya, A.M.; Catalan, P.; Shongwe, E.Z.; Viruel, J.; Wilkin, P.; van der Bank, M. Diversification into novel habitats in the Africa clade of Dioscorea (Dioscoreaceae): Erect habit and elephant’s foot tubers. BMC Evol. Biol. 2016, 16, 1–17. [Google Scholar] [CrossRef]
- SANBI. South African National Biodiversity Institute, Botanical Database of Southern Africa (BODATSA). Available online: http://posa.sanbi.org (accessed on 7 April 2022).
- GBIF. Global Biodiversity Information Facility, GBIF.org GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.qdz6k8 (accessed on 7 April 2022).
- Agyare, C.; Obiri, D.D.; Boakye, Y.D.; Osafo, N. Anti-Inflammatory and Analgesic Activities of African Medicinal Plants. Med. Plant Res. Afr. 2013, 725–752. [Google Scholar] [CrossRef]
- Chen, W.; Van Wyk, B.-E.; Vermaak, I.; Viljoen, A.M. Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem. Lett. 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar] [CrossRef]
- Raimondo, D.; Vlok, J.H.; van Wyk, B.E.; van Jaarsveld, E.J.; Mtshali, H. Aloe ferox Mill. National Assessment: Red List of South African Plants Version 2020.1. Available online: http://redlist.sanbi.org/species.php?species=2206-93 (accessed on 14 April 2022).
- Bircher, C.; Prentice, C.; Crouch, N.; Symmonds, R. Conservation concerns for Bowiea volubilis, an unusually succulent member of the Hyacinthaceae. Herbertia 1998, 53, 81–88. [Google Scholar]
- van Jaarsveld, E.J.; Potter, L. Bowiea volubilis Harv. ex Hook.f. subsp. gariepensis (Van Jaarsv.) Bruyns. National Assessment: Red List of South African Plants version 2020.1. Available online: http://redlist.sanbi.org/species.php?species=3811-4 (accessed on 14 April 2022).
- Raimondo, D.; Victor, J.E.; Scott-Shaw, C.R.; Lötter, M.; Dold, A.P.; Pfab, M.F.; Burrows, J.E. Bowiea volubilis Harv. ex Hook.f. subsp. volubilis. National Assessment: Red List of South African Plants Version 2020.1. Available online: http://redlist.sanbi.org/species.php?species=3811-3 (accessed on 14 April 2022).
- Victor, J.E.; Dold, A.P. Dioscorea elephantipes (L’Hér.) Engl. National Assessment: Red List of South African Plants Version 2020.1. Available online: http://redlist.sanbi.org/species.php?species=1777-12 (accessed on 14 April 2022).
- WorldClim. Global Climate Data, WorldClim 2.0 Beta Version 1. Available online: http://www.worldclim.org/node/1 (accessed on 18 February 2022).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Guo, D.; Arnolds, J.L.; Midgley, G.F.; Foden, W.B. Conservation of quiver trees in Namibia and South Africa under a changing climate. J. Environ. Prot. Sci. 2016, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Cahyaningsih, R.; Phillips, J.; Brehm, J.M.; Gaisberger, H.; Maxted, N. Climate change impact on medicinal plants in Indonesia. Glob. Ecol. Conserv. 2021, 30, e01752. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Navarro-Racines, C.E.; Tarapues, J.; Thornton, P.; Jarvis, A.; Ramirez-Villegas, J. High-resolution and bias-corrected CMIP5 projections for climate change impact assessments. Sci. Data 2020, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Singels, A.; Ruane, A. Simulated impacts of climate change on water use and yield of irrigated sugarcane in South Africa. Agric. Syst. 2015, 139, 260–270. [Google Scholar] [CrossRef]
- Singh, P.; Nedumaran, S.; Ntare, B.R.; Boote, K.; Singh, N.P.; Srinivas, K.; Bantilan, M.C.S. Potential benefits of drought and heat tolerance in groundnut for adaptation to climate change in India and West Africa. Mitig. Adapt. Strat. Glob. Chang. 2013, 19, 509–529. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef]
- Flato, G.; Marotzke, J.; Abiodun, B.; Braconnot, P.; Chou, S.C.; Collins, W.; Cox, P.; Driouech, F.; Emori, S.; Eyring, V. Evaluation of climate models. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014; pp. 741–866. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 8 March 2022).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pearson, R.G.; Thuiller, W.; Araújo, M.B.; Martínez-Meyer, E.; Brotons, L.; McClean, C.; Miles, L.; Segurado, P.; Dawson, T.; Lees, D.C. Model-based uncertainty in species range prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Fan, J.; Upadhye, S.; Worster, A. Understanding receiver operating characteristic (ROC) curves. Can. J. Emerg. Med. 2006, 8, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting Multicollinearity in Ecological Multiple Regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Zeng, Y.; Low, B.W.; Yeo, D.C. Novel methods to select environmental variables in MaxEnt: A case study using invasive crayfish. Ecol. Model. 2016, 341, 5–13. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Iverson, L.R.; McKenzie, D. Tree-species range shifts in a changing climate: Detecting, modeling, assisting. Landsc. Ecol. 2013, 28, 879–889. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol. Model. 2000, 128, 127–147. [Google Scholar] [CrossRef]
- Stoklosa, J.; Daly, C.; Foster, S.D.; Ashcroft, M.B.; Warton, D.I. A climate of uncertainty: Accounting for error in climate variables for species distribution models. Methods Ecol. Evol. 2015, 6, 412–423. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Barry, S.; Elith, J. Error and uncertainty in habitat models. J. Appl. Ecol. 2006, 43, 413–423. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Grenouillet, G.; Buisson, L.; Casajus, N.; Lek, S. Ensemble modelling of species distribution: The effects of geographical and environmental ranges. Ecography 2011, 34, 9–17. [Google Scholar] [CrossRef]
- Crimmins, S.M.; Dobrowski, S.Z.; Mynsberge, A.R. Evaluating ensemble forecasts of plant species distributions under climate change. Ecol. Model. 2013, 266, 126–130. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.R. Climate Change 2014–Impacts, Adaptation and Vulnerability: Regional Aspects; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Haile, G.G.; Tang, Q.; Hosseini-Moghari, S.; Liu, X.; Gebremicael, T.G.; Leng, G.; Kebede, A.; Xu, X.; Yun, X. Projected Impacts of Climate Change on Drought Patterns Over East Africa. Earth’s Future 2020, 8, e2020EF001502. [Google Scholar] [CrossRef]
- Hussein, A.; Workeneh, S. Modeling the impacts of climate changes on the distribution of Aloe vera species in Ethiopia. J. Earth Sci. Clim. Chang. 2021. [Google Scholar] [CrossRef]
- As, S.V.; van der Linden, S.; Phillips, D.P.; Rous, K.G.; Duker, R.; Beyers, A.; Calitz, W.; Cowling, R.M.; Potts, A.J. Impending local extinction of Aloe ferox Mill. populations in the absence of elephants and black rhinos? Afr. J. Ecol. 2016, 54, 504–506. [Google Scholar]
- Parker, D.; Bernard, R. Lessons from aloes in the Thicket Biome: Reconstructing past elephant browsing to understand the present: Commentary. S. Afr. J. Sci. 2008, 104, 163–164. [Google Scholar]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Kapwata, T.; Gebreslasie, M.T.; Mathee, A.; Wright, C.Y. Current and Potential Future Seasonal Trends of Indoor Dwelling Temperature and Likely Health Risks in Rural Southern Africa. Int. J. Environ. Res. Public Health 2018, 15, 952. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.C.; Gove, A.D.; Sanders, N.J.; Dunn, R.R. Climate change, plant migration, and range collapse in a global biodiversity hotspot: The Banksia (Proteaceae) of Western Australia. Glob. Chang. Biol. 2008, 14, 1337–1352. [Google Scholar] [CrossRef]
- Mohammady, M.; Pourghasemi, H.R.; Yousefi, S.; Dastres, E.; Edalat, M.; Pouyan, S.; Eskandari, S. Modeling and Prediction of Habitat Suitability for Ferula gummosa Medicinal Plant in a Mountainous Area. Nat. Resour. Res. 2021, 30, 4861–4884. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.; Park, S.-R.; Yi, T.; Kim, C.; Lee, S.-W.; An, K. Identifying key environmental factors for Paulownia coreana habitats: Implementing national on-site survey and machine learning algorithms. Land 2022, 11, 578. [Google Scholar] [CrossRef]
- Namitha, L.; Achu, A.; Reddy, C.S.; Beevy, S.S. Ecological modelling for the conservation of Gluta travancorica Bedd. An endemic tree species of southern Western Ghats, India. Ecol. Informat. 2022, 71, 101823. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for Climate Change: Forestry and Assisted Migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Cunningham, A.B. Return of the pepper-bark. Med. Plant Conserv. 2001, 7, 21–22. [Google Scholar]
- Kirk, H.; Vrieling, K.; Van Der Meijden, E.; Klinkhamer, P.G.L. Species by Environment Interactions Affect Pyrrolizidine Alkaloid Expression in Senecio jacobaea, Senecio aquaticus, and Their Hybrids. J. Chem. Ecol. 2010, 36, 378–387. [Google Scholar] [CrossRef]
- Gairola, S.; Shariff, N.M.; Bhatt, A. Influence of climate change on production of secondary chemicals in high altitude medicinal plants: Issues needs immediate attention. J. Med. Plant Res. 2010, 4, 1825–1829. [Google Scholar]

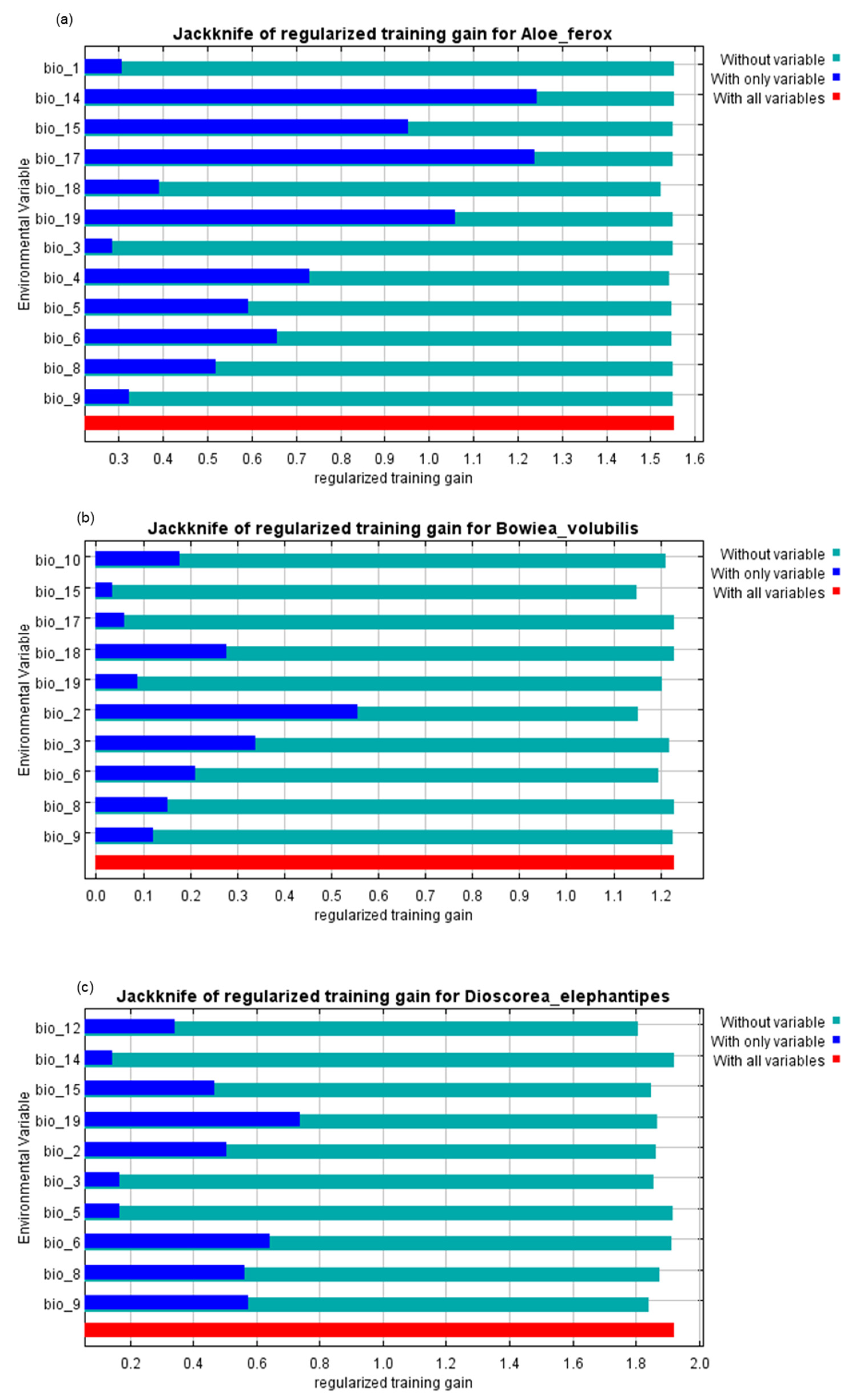
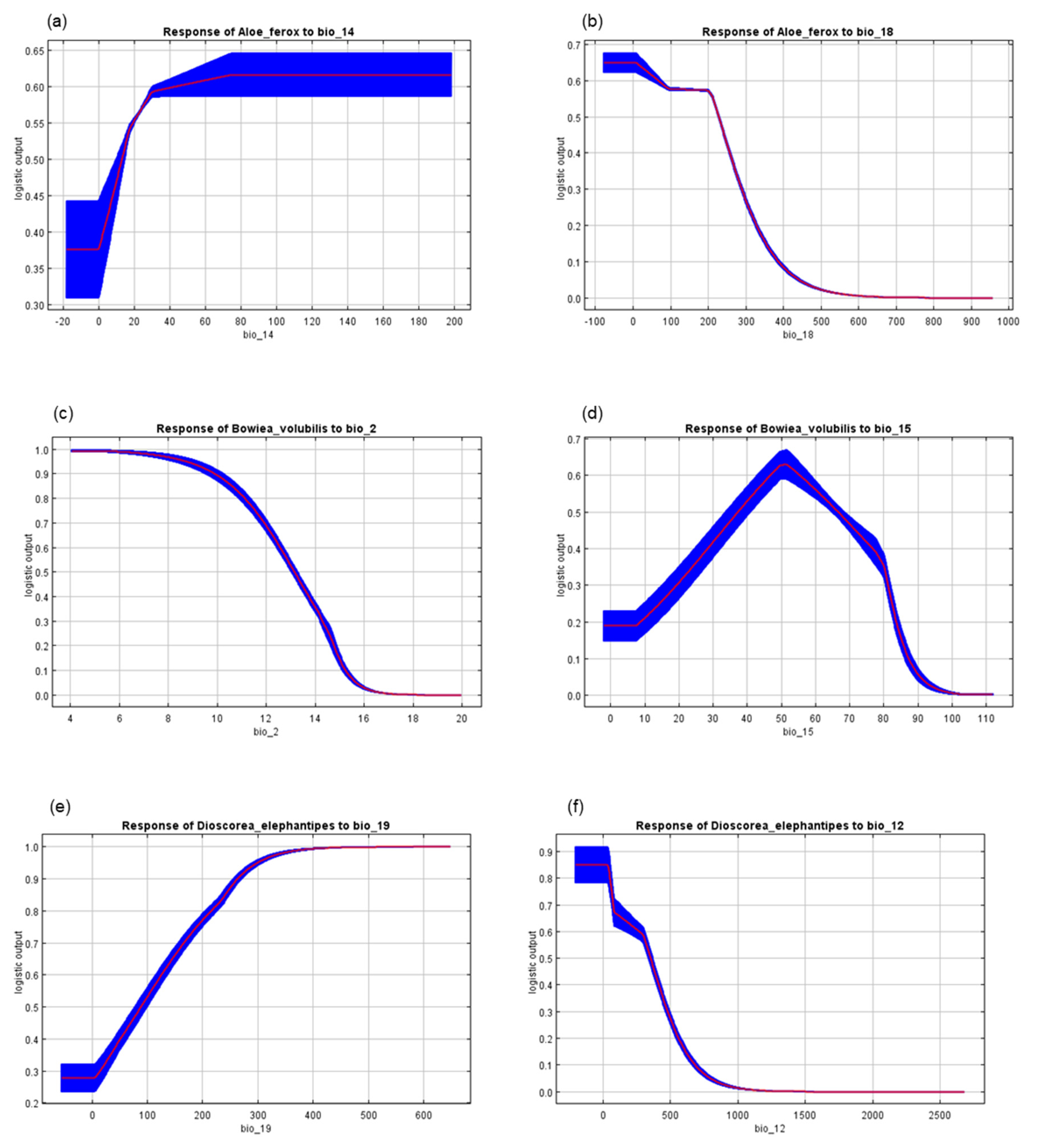
| Bioclimatic Variable | Code | Contribution | ||
|---|---|---|---|---|
| A. ferox | B. volubilis | D. elephantipes | ||
| Annual Mean Temperature | Bio1 | 1.2 | 0 | 0 |
| Mean diurnal range (Mean of monthly (max temp–min temp)) | Bio2 | 0 | 56.1 | 19.4 |
| Isothermality (Bio2/Bio7) (×100) | Bio3 | 2 | 2.2 | 6.2 |
| Temperature seasonality (standard deviation, ×100) | Bio4 | 1.6 | 0 | 0 |
| Max temperature of the warmest month | Bio5 | 0.6 | 0 | 1.0 |
| Min temperature of the coldest month | Bio6 | 7.0 | 5.1 | 6.3 |
| Temperature annual range (Bio5-Bio6) | Bio7 | 0 | 0 | 0 |
| Mean temperature of wettest quarter | Bio8 | 1.0 | 1.9 | 4.9 |
| Mean temperature of driest quarter | Bio9 | 0.5 | 9.8 | 12.2 |
| Mean temperature of warmest quarter | Bio10 | 0 | 2 | 0 |
| Mean temperature of coldest quarter | Bio11 | 0 | 0 | 0 |
| Annual precipitation | Bio12 | 0 | 0 | 17.4 |
| Precipitation of wettest month | Bio13 | 0 | 0 | 0 |
| Precipitation of driest month | Bio14 | 7.4 | 0 | 0.3 |
| Precipitation seasonality (Coefficient of Variation) | Bio15 | 5.2 | 9.8 | 4.8 |
| Precipitation of wettest quarter | Bio16 | 0 | 0 | 0 |
| Precipitation of driest quarter | Bio17 | 66.3 | 1.5 | 0 |
| Precipitation of warmest quarter | Bio18 | 6.9 | 1.4 | 0 |
| Precipitation of coldest quarter | Bio19 | 0.2 | 10.1 | 27.6 |
| Model | A. ferox | B. volubilis | D. elephantipes |
|---|---|---|---|
| Present Coverage | 194,102.52 | 460,788.04 | 211,866.66 |
| RCP2.6 2050 | 197,377.92 | 579,786.05 | 284,663.52 |
| RCP2.6 2080 | 200,958.07 | 594,234.49 | 286,920.43 |
| RCP8.5 2050 | 199,716.27 | 599,473.74 | 266,784.58 |
| RCP8.5 2080 | 203,727.99 | 550,154.24 | 278,999.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshabalala, T.; Mutanga, O.; Abdel-Rahman, E.M. Predicting the Geographical Distribution Shift of Medicinal Plants in South Africa Due to Climate Change. Conservation 2022, 2, 694-708. https://doi.org/10.3390/conservation2040045
Tshabalala T, Mutanga O, Abdel-Rahman EM. Predicting the Geographical Distribution Shift of Medicinal Plants in South Africa Due to Climate Change. Conservation. 2022; 2(4):694-708. https://doi.org/10.3390/conservation2040045
Chicago/Turabian StyleTshabalala, Thulani, Onisimo Mutanga, and Elfatih M. Abdel-Rahman. 2022. "Predicting the Geographical Distribution Shift of Medicinal Plants in South Africa Due to Climate Change" Conservation 2, no. 4: 694-708. https://doi.org/10.3390/conservation2040045
APA StyleTshabalala, T., Mutanga, O., & Abdel-Rahman, E. M. (2022). Predicting the Geographical Distribution Shift of Medicinal Plants in South Africa Due to Climate Change. Conservation, 2(4), 694-708. https://doi.org/10.3390/conservation2040045








