Benthic Fauna Assessment along the Navigation Channel from the Mouth of the Casamance Estuary to Ziguinchor City
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling Stations and Equipment
2.3. Collection of Samples
2.4. Description of Sediments and Sample Sorting in the Laboratory
2.5. Counting and Species Identification
2.6. Evaluation of Dry Biomass, Real Biovolumes, and Densities
2.7. Species Richness
2.8. Shannon and Fairness Indexes
2.9. Trophic Group
- Carnivores, which include predators that capture their prey, some of which are vagile (wandering polychaetes, gastropods, sea stars, and decapods) and others sessile (actinids and hydraires). The necrophagous are consumers of the flesh of dead animals deposited on the bottom. They are mainly gastropods and decapods.
- Herbivores, which are consumers of algae or grazers, including sea urchins and gastropods.
- Detritus feeders, which are vagile organisms, such as amphipods, isopods, tanaids, decapods, and some nereids, which consume detritus, mainly of plant origin.
- Suspension feeders, which feed by filtering organic particles suspended in the water above the sediment (polychaetes Sabellidae, Serpulidae, and some bivalves).
- Selective deposit feeders, which are composed of organisms (sedentary polychaetes, some bivalve mollusks and crustaceans) that use the surface sedimentary layer to feed. They feed on organic particles, supporting bacteria, and single-celled algae, which are deposited on the sediment.
- Non-selective deposit feeders, which include organisms that live at depth and ingest sediment from reduced layers to collect organic matter. These are mainly sedentary polychaetes.
- This classification is completed and adapted to a more recent trophic group classification proposed by Gaudêncio and Cabral [31].
2.10. Correspondence Factorial Analysis, Factor Analysis for Mixed Data, and Spearman Rank Correlations
3. Results
3.1. Structure of Benthic Fauna
3.2. Spatial Distribution
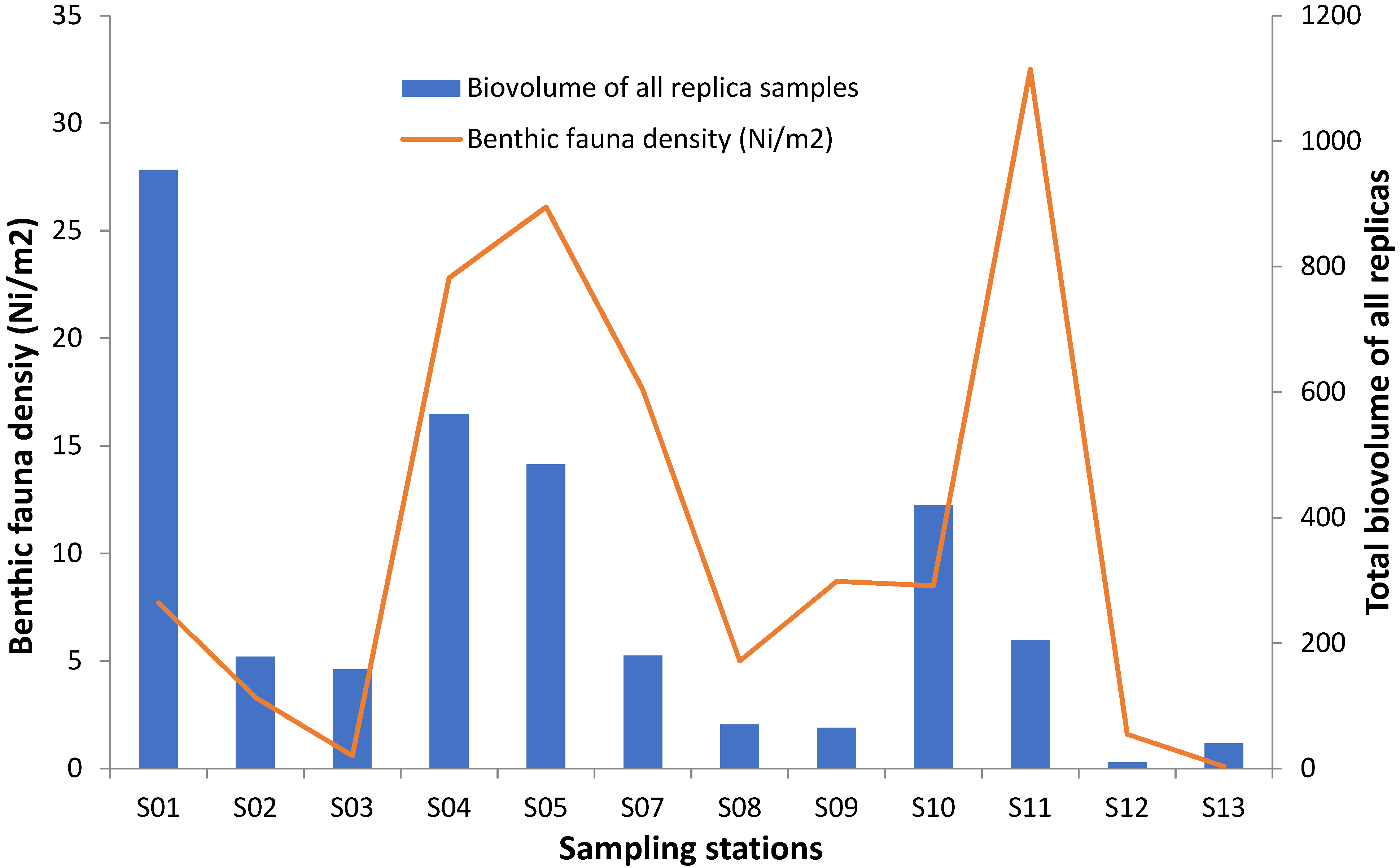
3.3. Evaluation of Dry Biomass, Living Biovolumes, and Densities
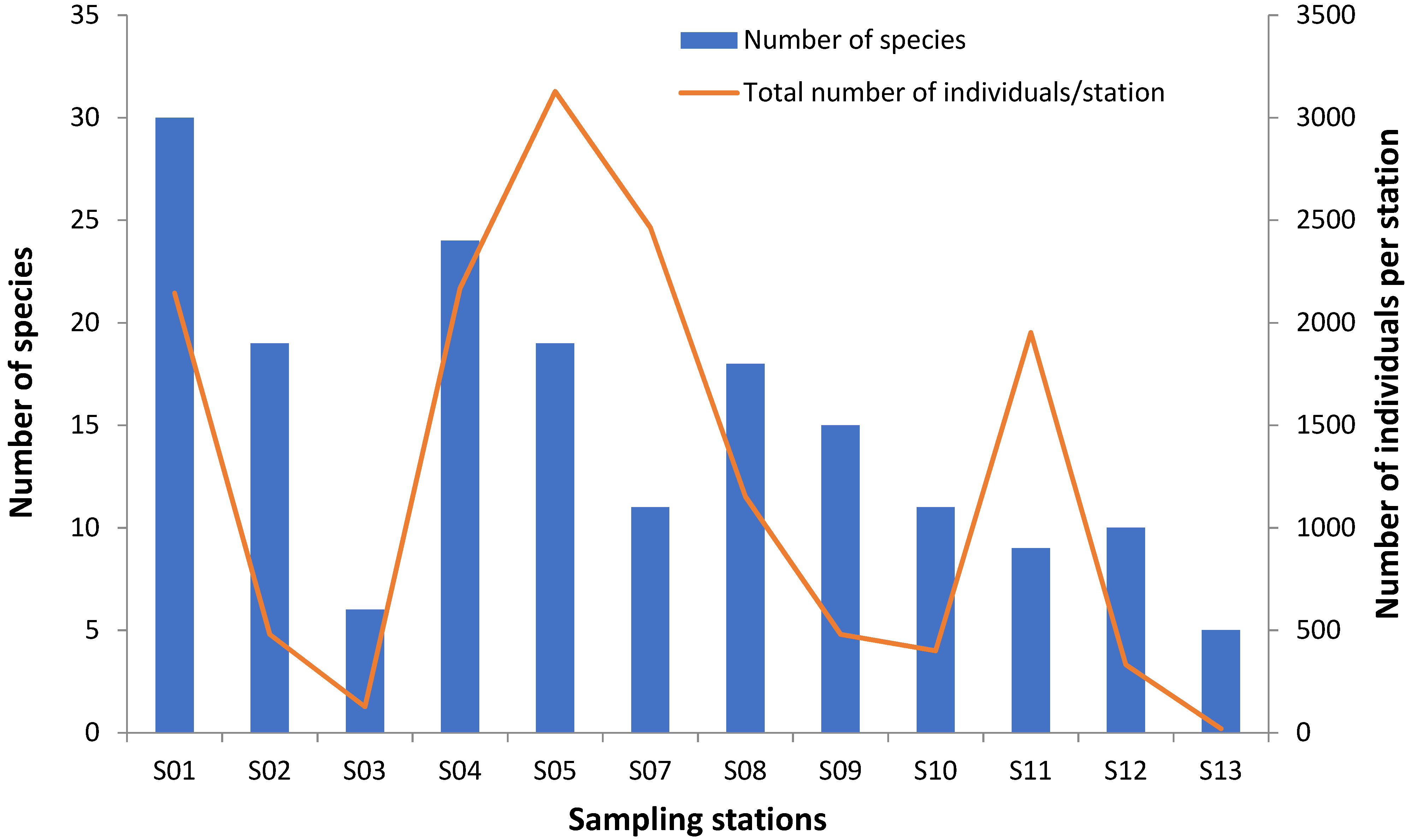
3.4. Species Abundance
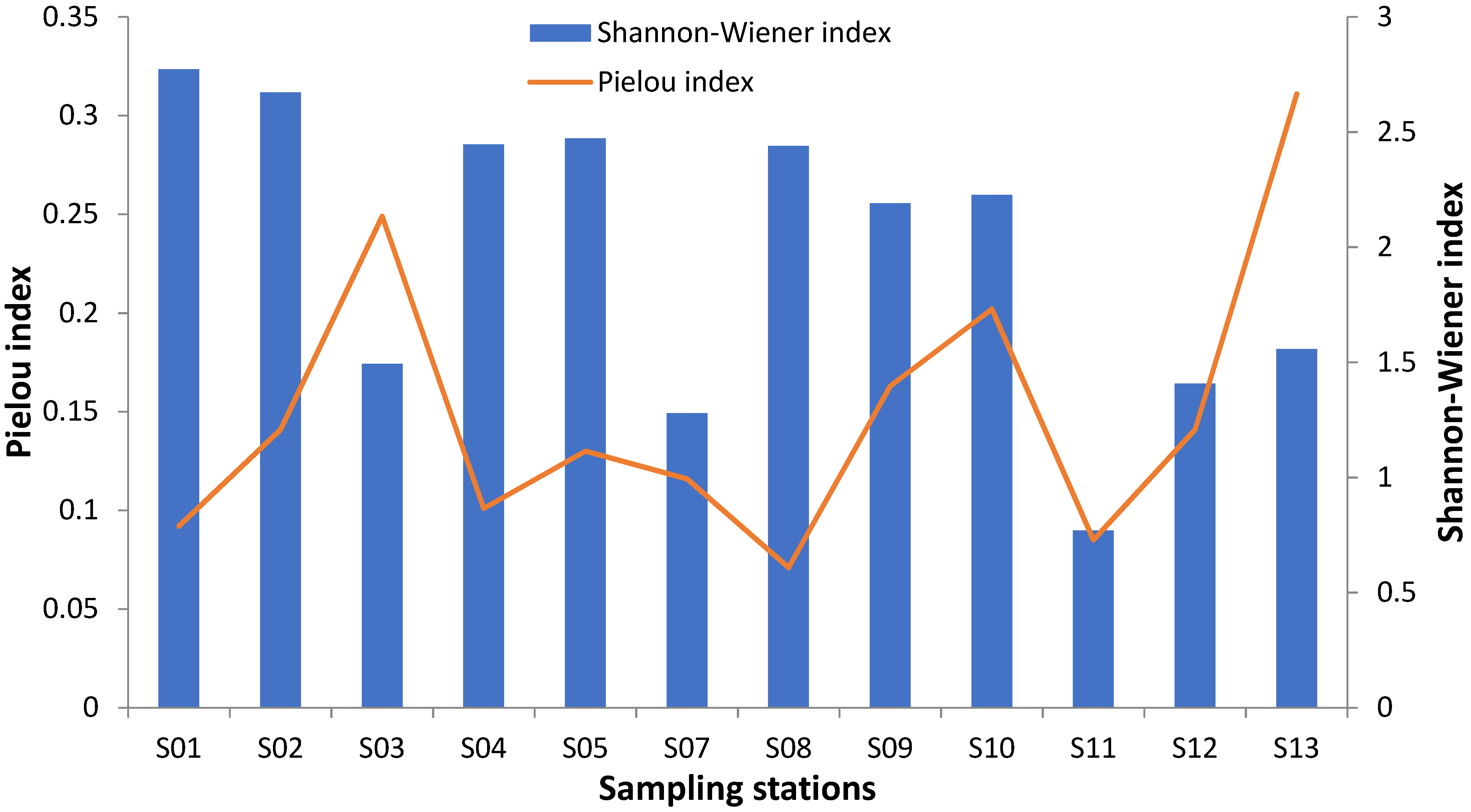
3.5. Shannon-Weaver and Pielou Indexes
3.6. Trophic Groups and Spatial Distribution
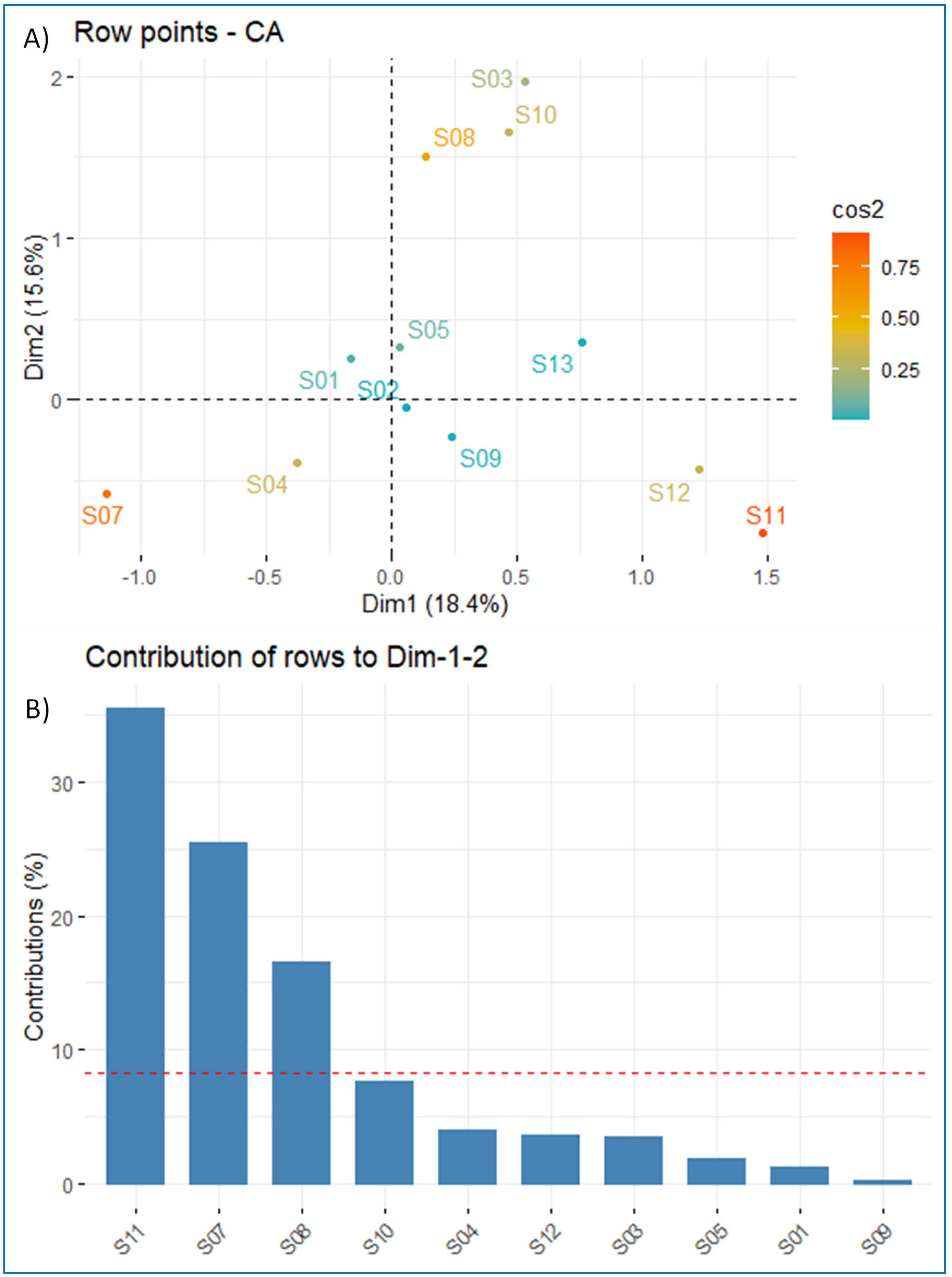
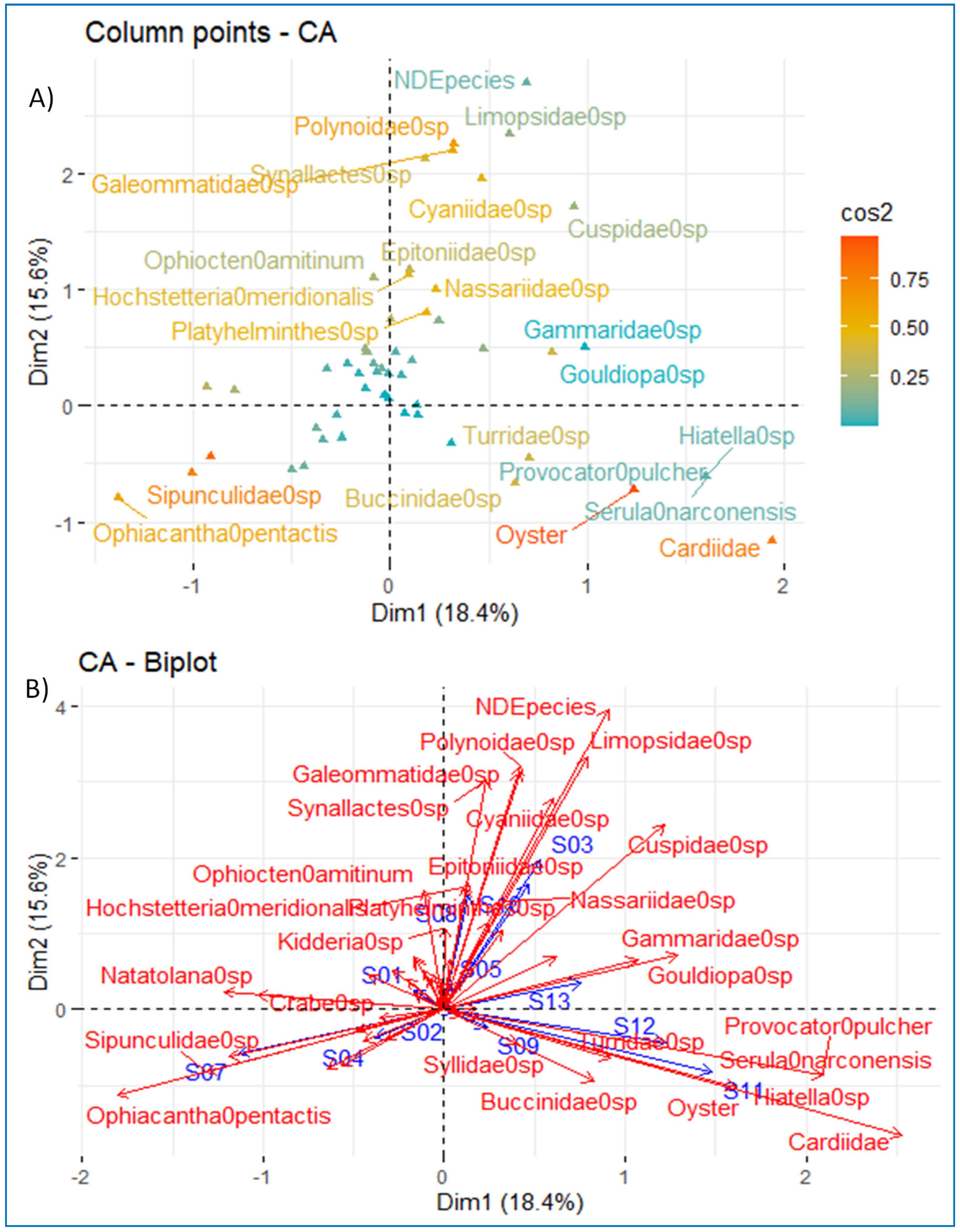
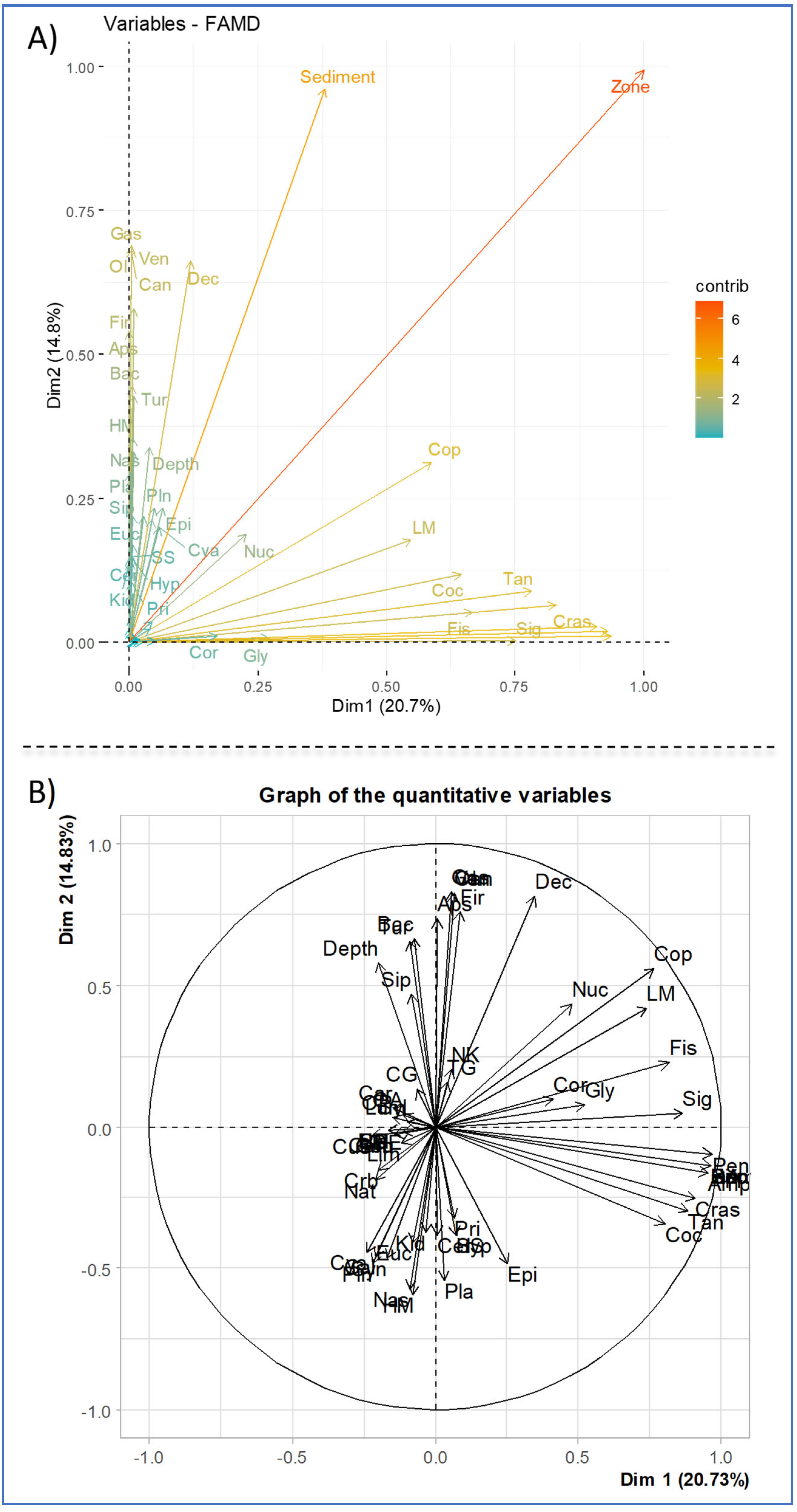
3.7. Correspondence Factorial Analysis (CFA) and Factor Analysis for Mixed Data (FAMD)
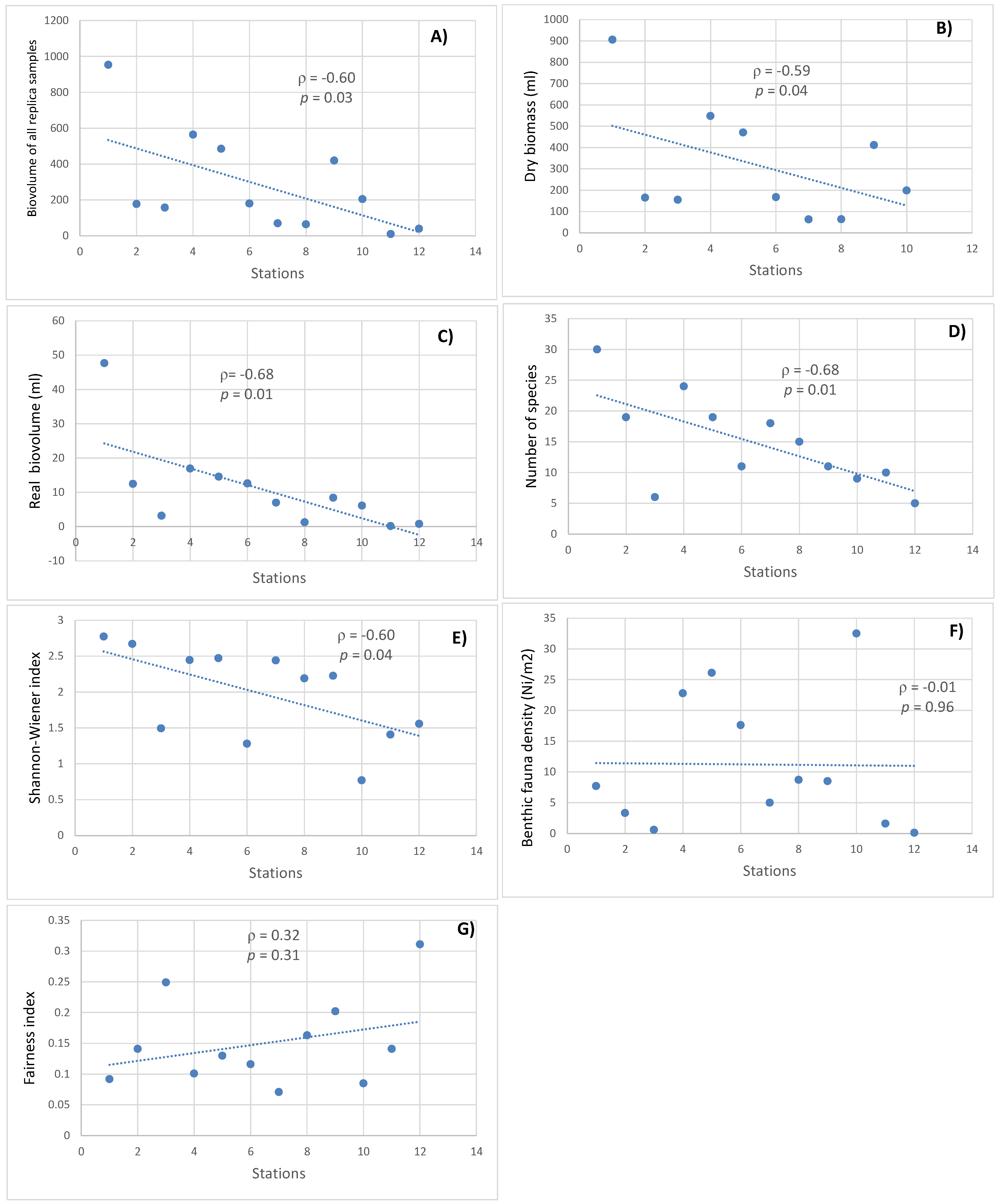
3.8. Correlation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
International Review Board Statement:
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, E. Biodiversity of Estuarine Fish Faunas in West Africa; Naga, The ICLARM Quarterly: Metro Manila, Philippines, 2000; Volume 23. [Google Scholar]
- Cloern, J.; Jassby, A. Drivers of change in estuarine-coastal ecosystems: Discoveries from four decades of study in San Francisco Bay. Rev. Geophys. 2012, 50, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Dolbeth, M.; Cardoso, P.G.; Grilo, T.F.; Bordalo, M.D.; Raffaelli, D.; Pardal, M.A. Long-Term Changes in the Production by Estuarine Macrobenthos Affected by Multiple Stressors. Estuar. Coast. Shelf Sci. 2011, 92, 10–18. [Google Scholar] [CrossRef]
- Villanueva, M.C. Contrasting tropical estuarine ecosystem functioning and stability: A comparative study. Estuar. Coast. Shelf Sci. 2015, 155, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.; Whitfield, A.K. Challenging paradigms in estuarine ecology and management. Estuar. Coast. Shelf Sci. 2011, 94, 306–314. [Google Scholar] [CrossRef]
- McLusky, D.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats, and Management. In Elliott Threats and Management; McLusky, M.D.S., Ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Edgar, G.J.; Barrett, N.S.; Graddon, D.J.; Last, P.R. The conservation significance of estuaries: A classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biol. Conserv. 2000, 92, 383–397. [Google Scholar] [CrossRef]
- Edgar, G.J.; Barrett, N.S.; Last, P.R. The Distribution of Macroinvertebrates and Fishes in Tasmanian Estuaries. J. Biogeogr. 1999, 26, 1169–1189. [Google Scholar] [CrossRef]
- Grizzetti, B.; Passy, P.; Billen, G.; Bouraoui, F.; Garnier, J.; Lassaletta, L. The role of water nitrogen retention in integrated nutrient management: Assessment in a large basin using different modelling approaches. Environ. Res. Lett. 2015, 2015, 10. [Google Scholar] [CrossRef]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Chatzinikolaou, E.; Mandalakis, M.; Damianidis, P.; Dailianis, T.; Gambineri, S.; Rossano, C.; Scapini, F.; Carucci, A.; Arvanitidis, C. Spatio-temporal benthic biodiversity patterns and pollution pressure in three Mediterranean touristic ports. Sci. Total Environ. 2017, 624, 648–660. [Google Scholar] [CrossRef]
- Blanchard, A.L.; Feder, H.M. Adjustment of benthic fauna following sediment disposal at a site with multiple stressors in Port Valdez, Alaska. Mar. Pollut. Bull. 2003, 46, 1590–1599. [Google Scholar] [CrossRef]
- Boyd, W.A.; Brewer, S.K.; Williams, P.L. Altered behaviour of invertebrates living in polluted environments. In Behavioural Ecotoxicology; Dell’Omo, G., Ed.; John Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- Kravitz, M.J.; Lamberson, J.O.; Ferraro, S.P.; Swartz, R.C.; Boese, B.L.; Specht, D.T. Avoidance response of the estuarine amphipod Eohaustorius estuarius to polycyclic aromatic hydrocarbon-contaminated, field-collected sediments. Environ. Toxicol. Chem. 1999, 18, 1232–1235. [Google Scholar] [CrossRef]
- Scarlett, A.; Canty, M.N.; Smith, E.L.; Rowland, S.J.; Galloway, T.S. Can Amphipod Behavior Help to Predict Chronic Toxicity of Sediments? Hum. Ecol. Risk Assess. Int. J. 2007, 13, 506–518. [Google Scholar] [CrossRef]
- Reddy, C.M.; Eglinton, T.I.; Hounshell, A.; White, H.K.; Xu, L.; Gaines, R.B.; Frysinger, G.S. The West Falmouth oil spill after thirty years: The persistence of petroleum hydrocarbons in marsh sediments. Environ. Sci. Technol. 2002, 36, 4754–4760. [Google Scholar] [CrossRef]
- Reimer, J.D.; Yang, S.Y.; White, K.N.; Asami, R.; Fujita, K.; Hongo, C.; Ito, S.; Kawamura, I.; Maeda, I.; Mizuyama, M.; et al. Effects of causeway construction on environment and biota of subtropical tidal flats in Okinawa, Japan. Mar. Pollut. Bull. 2015, 94, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Baudin, F.; Disnar, J.R.; Martinez, P.; Dennielou, B. Distribution of the organic matter in the channel-levees systems of the Congo mud-rich deep-sea fan (West Africa). Implication for deep offshore petroleum source rocks and global carbon cycle. Mar. Pet. Geol. 2010, 27, 995–1010. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, A.M.; Ballesteros, E.; Fay, M.; Sala, E. Marine Communities on Oil Platforms in Gabon, West Africa: High Biodiversity Oases in a Low Biodiversity Environment. PLoS ONE 2014, 9, e103709. [Google Scholar] [CrossRef]
- Ober, H.K. Effects of Oil Spills on Marine and Coastal Wildlife; UF/IFAS Extension WEC 285; Wildlife Ecology and Conservation Department: Gainesville, FL, USA; North Florida Research and Education Center (NFREC): Quincy, FL, USA, 2010. [Google Scholar]
- Pradella, N.; Fowler, A.M.; Booth, D.J.; Macreadie, P.I. Fish assemblages associated with oil industry structures on the continental shelf of northwestern Australia. J. Fish Biol. 2013, 84, 247–255. [Google Scholar] [CrossRef]
- DPZ. Groundbreaking at Ziguinchor Port Marks Important Economic Milestone; Degla, R., Ed.; Project Manager: Dakar, Senegal, 2010. [Google Scholar]
- Thiam, E.; Singh, V. Space-time-frequency analysis of rainfall, runoff and temperature in the Casamance River basin, southern Senegal, West Africa. Water SA 2002, 28, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Saos, J.-L.; Bouteiller, C.; Diop, S. Aspects Géologiques et Géomorphologiques de la Casamance: Étude de la Sédimentation Actuelle. Rev. D’hydrobiologie Trop. 1987, 20 (3–4), 219–232. [Google Scholar]
- FAO. The State of the World’s Biodiversity for Food and Agriculture; Bélanger, J., Pilling, D., Eds.; FAO Commission on Genetic Resources for Food and Agriculture Assessments: Rome, Italy, 2019; 572p, Licence: CC BY-NC-SA 3.0 IGO; Available online: https://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed on 12 May 2022).
- Mikhailov, V.; Isupova, M. Hypersalinization of river estuaries in West Africa. Water Resour. 2008, 35, 367–385. [Google Scholar] [CrossRef]
- Savenije, H.; Pagès, J. Hypersalinity: A dramatic change in the hydrolog of Sahelian estuaries. J. Hydrol. 1992, 135, 157–174. [Google Scholar] [CrossRef]
- Pages, J.; Citeau, J.E.; Demarcq, H. Bathymétrie par imagerie spot sur la Casamance (Sénégal). Résultats préliminaires. In Proceedings of the International Colloqium on Spectral Signatures of Objects in Remote Sensing Held at Aussois, Modane, France, 18–22 January 1988; pp. 387–392. [Google Scholar]
- Hibberd, T.; Moore, K. Field Identification Guide to Heard Island and McDonald Islands Benthic Invertebrates: A Guide for Scientific Observers Aboard Fishing Vessels; Fisheries Research and Development Corporation: Canberra, Australia, 2019; 159p, Available online: https://www.ccamlr.org/en/document/publications/field-identification-guide-heard-island-and-mcdonald-islands-benthic (accessed on 12 May 2022).
- Grall, J.; Hilly, C. Tratement des Données Stationnelles (Faune); 2003; 2010p. Available online: http://www.rebent.org//medias/documents/www/contenu/pdf/document/Fiches_techniques/FT10-2003-01.pdf (accessed on 12 May 2022).
- Gaudêncio, M.J.; Cabral, H.N. Cabral Trophic structure of macrobenthos in the Tagus estuary and adjacent coastal shelf. Hydrobiologia 2007, 587, 241–251. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Diouf, P.S.; Diallo, A. Variations spatio-temporelles du zooplancton d’un estuaire hyperhalin: La Casamance. Rev. D’hydrobiologie Trop. 1987, 20, 257–269. [Google Scholar]
- Akaahan, T.; Manyi, M.M.; Azua, E. Variation of Benthic Fauna Composition in River Benue at Makurdi, Benue State, Nigeria. Int. J. Fauna Biol. Stud. 2016, 3, 71–76. [Google Scholar]
- Gutperlet, R.; Capperucci, R.M.; Bartholomä, A.; Kröncke, I. Benthic biodiversity changes in response to dredging activities during the construction of a deep-water port. Mar. Biodivers. 2015, 45, 819–839. [Google Scholar] [CrossRef]
- Nephin, J.; Juniper, S.K.; Archambault, P. Diversity, abundance and community structure of benthic macro- and megafauna on the Beaufort Shelf and Slope. PLoS ONE 2014, 9, e101556. [Google Scholar] [CrossRef]
- Millet, B. Caractéristiques de la marée dans l’estuaire de la Casamance: Etalonnage et Analyse harmonique d’enregistrements courantographiques de longue durée à Zigunichor. CRODT Doc. Sci. N 1991, 123, 12. [Google Scholar]
- Ceesay, A.; Konan, Y.A.; Njie, E.; Koné, T. Diversity and spatial variation of benthic macroinvertebrates in the River Gambia estuary, West Africa. Int. J. Fish. Aquat. Stud. 2019, 7, 83–88. [Google Scholar]
- Graca, B.; Burska, D.; Matuszewska, K. The impact of dredging deep pits on organic matter decomposition in sediments(Article). Water Air Soil Pollut. 2004, 158, 237–259. [Google Scholar] [CrossRef]
- Kumar, P.S.; Khan, A. The distribution and diversity of benthic macroinvertebrate fauna in Pondicherry mangroves, India. Aquat. Biosyst. 2013, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, P.; Buatois, L.; Mangano, M. Tidal Flats and Subtidal Sand Bodies. Dev. Sedimentol. 2012, 10, 529–561. [Google Scholar] [CrossRef]
- Herman, P.; Middelburg, J.; Heip, C. Benthic community structure and sediment processes on an intertidal flat: Results from the ECOFLAT project. Cont. Shelf Res. 2001, 21, 2055–2071. [Google Scholar] [CrossRef]
- Debenay, J.P.; Pagès, J.; Diouf, P.S. Ecological zonation of the hyperhaline estuary of the Casamance river (Senegal): Foraminifera, zooplanktoon and abiotic variables. Hydrobiologia 1989, 174, 161–176. [Google Scholar] [CrossRef]
- Bernardino, A.F.; Gomes, L.E.O.; Hadlich, H.L.; Andrades, R.; Correa, L.B. Mangrove clearing impacts on macrofaunal assemblages and benthic food webs in a tropical estuary. Mar. Pollut. Bull. 2018, 126, 228–235. [Google Scholar] [CrossRef]
- Bissoli, L.; Bernardino, A. Benthic macrofaunal structure and secondary production in tropical estuaries on the Eastern Marine Ecoregion of Brazil. PeerJ 2018, 6, e4441. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Covich, A.; Palmer, M.; Crowl, T. The Role of Benthic Invertebrate Species in Freshwater Ecosystems. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.; Elliott, M. Ecology of Marine Sediments: Science to Management; OUP: Oxford, UK, 2009; 260p. [Google Scholar]
- Olsgard, F.; Gray, J.S. A comprehensive analysis of the effects of offshore oil and gas exploration and production on the benthic communities of the Norwegian continental shelf. Mar. Ecol. Prog. Ser. 1995, 122, 277–306. [Google Scholar] [CrossRef]


| Station | Location | Location Codes | Depth (m) | Type of Sediment | Codes of Sediment Type | Number of Replicates for Sampling of Benthic Fauna |
|---|---|---|---|---|---|---|
| S01 | Dredge footprint (Zone C) | ZNC | 2 | mud | MD | 3 |
| S02 | Upstream port | UP | 4 | mud | MD | 3 |
| S03 | Refueling pontoon | RP | 4 | mud | MD | 3 |
| S04 | Approach berth | AB | 10 | shell debris mixed with sandy mud | SD1 | 3 |
| S05 | River bed upstream | RB1 | 7 | coarse particles in muddy sand | CP | 5 |
| S06 | Boudody pontoon | 4 | silty mud | no benthic fauna at this location | ||
| S07 | River bed downstream | RB2 | 9 | sandy mud | SM1 | 3 |
| S08 | Eastern channel—river influence | EC | 1 | solid dark grey slightly sandy mud | SD2 | 4 |
| S09 | North bank opposite Ile Carabanes | NO | 8 | sandy mud mixed with shell debris | SM2 | 3 |
| S10 | West dredge footprint (Zone B) | ZNB | 2 | muddy sand mixed with great number of shell debris | MG | 4 |
| S11 | East dredge footprint (Zone B) | ZNB | 4 | muddy sand mixed with shell debris | MS | 5 |
| S12 | River bed toward Pt St Georges | RB3 | 5 | grey mud | GM1 | 3 |
| S13 | Navigation channel o warddiogue | NC | 4 | grey muddy sand | GM2 | 3 |
| Phylum | Species | Phylum | Species |
|---|---|---|---|
| Neantheskerguelensis | Cerithiopsidae sp. | ||
| Aphroditidae sp. | Inuncula sp. | ||
| Serulanarconensis | Buccinidae sp. | ||
| Glyceridae sp. | Turridae sp. | ||
| Annelids | Sigalionidae sp. | Gastropod mollusks | Cancellariidae sp. |
| 9 | Polynoidae sp. | Nassariidae sp. | |
| Lumbrineridae sp. | 11 | Epitoniidae sp. | |
| Syllidae sp. Sipunculidae sp. | |||
| Gasteropoda sp. | |||
| Ophiacantha imago | Provocatorpulcher | ||
| Ophiacanthapentactis | Epoitonidae | ||
| Echinoderms | Ophioctenamitinum | Fissurellidae sp. | |
| 4 | Synallactes sp. | Crassatellidae sp. | |
| Lithodesmurrayi | Nuculana sp. | ||
| Euphausicés | Bivalvia sp. | ||
| Decapod sp. | Crassostreagasar | ||
| Crustaceans | Copepod sp. | Cuspidae sp. | |
| 6 | Coryceaus | Kidderia sp. | |
| Cumacea sp. | Bivalve mollusks | Euciroa sp. | |
| Veneroida sp. | |||
| Hyperiidea sp. | 15 | Hochstetteriameridionalis | |
| Amphipods | Thermistogaudicaudii | Cyaniidae sp. | |
| 4 | Amphipodae sp. | Galeommatidae sp. | |
| Gammaridae sp. | Limopsidae sp. | ||
| Serilis sp. | Cardiidae | ||
| Arthropods | Dliahiscusaft | Hiatella sp. | |
| 4 | Isopa sp. | Gouldiopa sp. | |
| Natatolana sp. | Priapulidae | Priapulidae sp. | |
| Tanaids | Tanaidacea sp. | Cnidaria | Pennatulacae sp. |
| 2 | Apseudomorpha sp. | Plathelminthes | Platyhelminthes sp. |
| Broken eggs | Fish | ||
| Corals | Undetermined | ||
| Chordata | Polycarpa (Sea squirt) | ||
| Unidentified species | Undetermined |
| Stations | Biovolume of All Samples (mL) | Percentage (%) of Debris | Dry Biomass (mL) | Real Biovolume (mL) | Densities (Nind/m2) |
|---|---|---|---|---|---|
| S01 | 953.85 | 95 | 906.16 | 47.69 | 7.70 |
| S02 | 178.0 | 93 | 165.5 | 12.46 | 3.31 |
| S03 | 158.0 | 98 | 154.84 | 3.16 | 0.60 |
| S04 | 565.0 | 97 | 548.05 | 16.95 | 22.80 |
| S05 | 485.0 | 97 | 470.45 | 14.55 | 26.10 |
| S06 | 0 | ||||
| S07 | 180.0 | 93 | 167.4 | 12.6 | 17.60 |
| S08 | 70.0 | 95 | 63.0 | 7.0 | 5.00 |
| S09 | 65.0 | 98 | 63.7 | 1.3 | 8.70 |
| S10 | 420.0 | 98 | 411.6 | 8.4 | 8.50 |
| S11 | 205.0 | 97 | 198.85 | 6.15 | 32.50 |
| S12 | 10.0 | 98 | 9.8 | 0.2 | 1.60 |
| S13 | 40.0 | 98 | 39.2 | 0.8 | 0.10 |
| Stations | Number of Species | Total Number of Individuals/Station | Shannon-Wiener Index | Pielou Index |
|---|---|---|---|---|
| S01 | 30 | 2144 | 2.772 | 0.092 |
| S02 | 19 | 480 | 2.671 | 0.141 |
| S03 | 6 | 127 | 1.494 | 0.249 |
| S04 | 24 | 2168 | 2.446 | 0.101 |
| S05 | 19 | 3128 | 2.473 | 0.13 |
| S06 | ||||
| S07 | 11 | 2464 | 1.279 | 0.116 |
| S08 | 18 | 1152 | 2.44 | 0.071 |
| S09 | 15 | 480 | 2.19 | 0.163 |
| S10 | 11 | 400 | 2.227 | 0.202 |
| S11 | 9 | 1952 | 0.77 | 0.085 |
| S12 | 10 | 332 | 1.407 | 0.141 |
| S13 | 5 | 20 | 1.557 | 0.311 |
| Trophic Groups | S01 | S02 | S03 | S04 | S05 | S07 | S08 | S09 | S10 | S11 | S12 | S13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Omnivores | 240 | 520 | 416 | 1344 | ||||||||
| Filter feeders | 240 | 32 | 288 | 312 | 68 | 16 | 1632 | 212 | 12 | |||
| Detritus feeders | 56 | 8 | 80 | 16 | 4 | 16 | ||||||
| Filter feeder & Detritivores | 32 | 288 | ||||||||||
| Carnivores | 96 | 64 | 8 | 304 | 48 | 56 | 48 | |||||
| Necrophagous | 176 | 136 | 64 | 88 | 2 | |||||||
| Carnivores & Detritivores | 240 | 8 | 16 | 32 | 16 | |||||||
| Bottom deposit feeders | 16 | |||||||||||
| Detritivores & Herbivores | 16 | |||||||||||
| Detritivores & Omnivores | 16 | |||||||||||
| Suspension feeders | 480 | 72 | 232 | 32 | 64 | 48 | 12 | |||||
| Zooplankton feeders | 16 | 32 | 480 | 768 | 48 | 16 | 6 | |||||
| Plankton & Nutriment feeder | 32 | 8 | 384 | 32 | 48 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tine, M.; Diop, P.; Diadhiou, H.D. Benthic Fauna Assessment along the Navigation Channel from the Mouth of the Casamance Estuary to Ziguinchor City. Conservation 2022, 2, 367-387. https://doi.org/10.3390/conservation2020025
Tine M, Diop P, Diadhiou HD. Benthic Fauna Assessment along the Navigation Channel from the Mouth of the Casamance Estuary to Ziguinchor City. Conservation. 2022; 2(2):367-387. https://doi.org/10.3390/conservation2020025
Chicago/Turabian StyleTine, Mbaye, Penda Diop, and Hamet Diaw Diadhiou. 2022. "Benthic Fauna Assessment along the Navigation Channel from the Mouth of the Casamance Estuary to Ziguinchor City" Conservation 2, no. 2: 367-387. https://doi.org/10.3390/conservation2020025
APA StyleTine, M., Diop, P., & Diadhiou, H. D. (2022). Benthic Fauna Assessment along the Navigation Channel from the Mouth of the Casamance Estuary to Ziguinchor City. Conservation, 2(2), 367-387. https://doi.org/10.3390/conservation2020025






