Abstract
Bird populations associated with agricultural ecosystems have declined markedly in Europe during the last quarter of the 20th century due to land-use intensification. This has meant that some very common species, in some cases even species considered as pests, are now threatened or subject to management programs to ensure their conservation. Considered pests of crops and predators of small game species, corvids are among the most persecuted common farmland birds. The consideration that these birds are pests lacks any scientific evaluation and is justified by the subjective impression that they are abundant. Here, using estimates of absolute and relative abundances of both the total and the breeding population, we show how jackdaws Corvus monedula have shown a marked negative population trend in central Spain during the last 40 years. Decline involves the loss of multiple colonies, the apparent absence of the species as a breeder in riverside forests, and an overall numerical decrease of about 75% (from 35,000 to 9000 individuals) according to counts in communal roosts. The population decline seems to be more pronounced in areas where land use has been intensified, probably in response to the reduction in the availability of once-abundant food (i.e., invertebrates and weed seeds) but also due to more direct effects such as intoxication and medium to long-term accumulation of agricultural pollutants which may have also affected reproduction and survival. Intensive hunting over decades has undoubtedly contributed to this decline and should therefore be made forbidden urgently. Generally, it seems that high-intensity agricultural management more drastically affects smaller and less adaptable common species, which are expected to decline before and at a higher extent and magnitude than jackdaws. Given that global population estimates based on direct counts of individuals are readily achievable through simultaneous counts in communal roosts, the jackdaw can serve as a model for assessing temporal trends potentially linked to large-scale anthropogenic modifications of open and agricultural environments.
1. Introduction
Birds are suffering unprecedented declines worldwide, and common farmland species are among the most affected groups [1,2]. These species represent key elements in the dynamics and functioning of open and extensive agricultural ecosystems and, as such, have been considered bio-indicators of environmental health [3,4]. Farmland bird species provide multiple ecosystem services in key processes for environmental integrity and resilience [5,6,7], and due to their high abundance, they can reflect the biomass that these landscapes can sustain associated to nutrient transfer processes and the functioning of ecological cycles, with cascading effects on food webs [5,8,9,10]. Changes in agricultural practices and other environmental alterations have been so intense and rapid in recent decades that many of these species show declines that can reach catastrophic levels [11,12,13,14], which represent one of the most worrying conservation crises today [15,16]. The current strong decline of keystone species in agro-systems poses a paradoxical situation, as some of these species have gone from being so abundant to be considered as crop pests to being threatened to the point of requiring costly conservation management programs [2,16]. Understanding the main drivers of decline on a case-by-case and global basis is therefore essential and constitutes one of the major challenges for biodiversity conservation worldwide. A direct link between agricultural practices and demographic trends of these species has been repeatedly highlighted [11,12], driven by increasingly globalized economic policies [17,18]. Therefore, coordinated and global action is imperative to try to halt this decline and propose measures to reverse it. Detailed knowledge of the abundances, population sizes, and trends of each farmland species is paramount to achieving this ambitious goal.
Estimates of abundance and population trends allow identifying priority bird species for conservation and management programs at regional, national, and continental scales [19,20]. To date, most of the information available on population trends of farmland bird species refers to changes in distribution and relative abundance, obtained on a large scale through citizen science programs [21,22]. These monitoring schemes represent a milestone in biodiversity conservation due to the massive amount of valuable information obtained at large scales in recent decades [23]. This approach is generally based on the accumulation of qualitative data that allows for semi-quantitative analytical assessments at variable spatiotemporal scales [23,24,25]. However, it generally lacks the necessary detail on abundance, population size, and long-term temporal trends for particular species and regions [26,27,28,29,30]. Intensive monitoring to obtain targeted quantitative information is especially indicated for species for which the usual surveys methods may not be the most adequate [27,31,32,33], or when conservation programs need to be implemented for species of special concern at local or regional scales [19,34]. This information can be obtained through different field protocols including sampling stations, walking or road-side surveys that quantify abundance per unit area or distance in different habitats, as well as through specific counts of the absolute abundance of breeding and wintering populations [31,35]. Extensive approaches monitoring multiple species and intensive approaches focused on particular species have pros and cons depending on the size, habitat, abundance, and ecology of the focus species [27,36,37]. Absolute abundance surveys are especially informative for species where the number of breeding pairs can be adequately accounted for or individuals gather at communal roosts, provided that counts can be done over a large enough area to be representative. However, although a combination of sampling methods may be the most appropriate for assessing population trends, simultaneous information on the relative and absolute abundance of a species is rarely available [31,35,38].
Crows, ravens, and magpies (Corvidae) are among the biggest and most persecuted common birds. Several species have historically been considered as crop pests and predators of small game and have been subjected to hunting regulations [39]. This consideration generally lacks a scientifically based empirical assessment [40] and is often justified by the subjective impression that they are abundant. This unfounded perception, derived from the high sociability and mobility of noisy feeding and roosting flocks [39], can lead to negative attitudes towards these species, especially when these flocks are associated with agricultural and urbanized areas [41]. Indeed, several species of corvids have been intensively persecuted across the Mediterranean and other regions in the Western Palearctic during the last century [42,43,44], being still subjected to regular hunting and categorized as Non-threatened or of Least Concern by the IUCN [2]. However, the transformation of agricultural landscapes due to the increasingly intensive practices over the last century, combined with intense persecution, could be driving the rapid decline of several corvid species in European agro-ecosystems [45,46,47]. It is worth mentioning that due to their generalist diet, large size, and long lifespan compared to other common birds, corvids can respond better than smaller species to the effects of agricultural intensification on individual health status and population dynamics [48,49,50,51], so the decline of these smart species could be considered as a definite warning signal of drastic changes in agrosystems.
Here we evaluated long-term population trends of the Western jackdaw (Corvus monedula) in open and agricultural landscapes of central Spain during the past five decades. Data included specific censuses of breeding pairs in a variety of nesting substrates, the estimation of occurrence and abundance in riverside forests during the breeding season, and the global population size estimated throughout winter communal roost counts. Given the methodological ease of obtaining realistic estimates of their population sizes, we discuss the value of the Western jackdaw as a bioindicator species whose population trends may inform on the degree of degradation of open and agricultural environments that may affect more drastically other smaller and less adaptable common species.
2. Materials and Methods
2.1. Study Species and Study Area
The Western jackdaw (hereafter jackdaw) is a medium-sized (c. 220 g), social corvid widely distributed throughout the Western Palearctic [52]. Breeding pairs nest colonially on cliffs, trees, and constructions, forming foraging flocks throughout the year. Trees are used as temporal or permanent communal roosts, both by breeding and non-breeding individuals [52]. Jackdaws are closely linked to open environments that have been transformed into farmed areas for centuries, where the species reaches its highest population densities [52]. Jackdaws are categorized as a species of Least Concern by the IUCN, although several European countries have alerted on population declines detected during the last decades [53]. In Spain, the jackdaw was widely distributed and considered very abundant in the past [42,52], although the actual size of the Spanish population is unknown and has been only estimated after extrapolating densities by habitat. However, huge differences have been found between these estimates and data obtained by direct counting of individuals in communal roosts during winter in areas such as Madrid [54], suggesting that estimates may also be substantially inflated for other regions and species [54,55,56]. Indeed, there has been a clear reduction of its distribution range during the last decades, and jackdaws have disappeared as breeders from large areas of the Castilian Plateaus, Ebro Valley, Central System, Sierra Morena, and the Betic Systems, while their presence is very scarce and fragmented in other regions [55].
The study was conducted in the province of Madrid, which was divided into three main zones (i.e., river meadows, high Tajuña river, and foothills of the Sierra de Guadarrama (Figure 1) according to their habitat characteristics. In the river meadows (Zone 1), jackdaws nest in gypsum and clay cliffs along the rivers Jarama, Manzanares, Tajo, and Henares, as well as in quarries, constructions, and riverside forests in the south-eastern quadrant of the province. This lowland area (about 600 m a.s.l), nowadays dedicated to irrigated cereal, maize, vegetables, and gravel extraction [57,58], has been increasingly degraded and occupied by urbanized areas due to the growth of the human population around the city of Madrid, the largest human concentration in Spain. In this zone, jackdaws mainly forage in dry cereal fields, scrubland on gypsum soils, and olive groves (Olea europaea), also relying on three large landfills. The high Tajuña River (Zone 2) is a small area within the Alcarria landscape located in the east of the province (700 m a.s.l) and characterized by clay soils and relatively well-preserved Mediterranean scrub vegetation dominated by holm oak (Quercus rotundifolia) [59]. In this area, jackdaws nest on limestone cliffs and buildings and feed in areas of scrubland, dry cereal crops, and olive groves. Finally, the foothills of the Sierra de Guadarrama (Zone 3), located in the northern part of the province, include the highest mountain ramp (about 700–1000 m a.s.l) of the Sistema Central Range occupied by extensive open grasslands, oak (Quercus pyrenaica), holm oak, and ash (Fraxinus angustifolia) forests and, to a lesser extent, arable land dedicated to dry cereal crops [59]. In this area, jackdaws forage in grassland and dry cereal crops, nesting in buildings, small cliffs, ash, holm oak, and riverside forests.
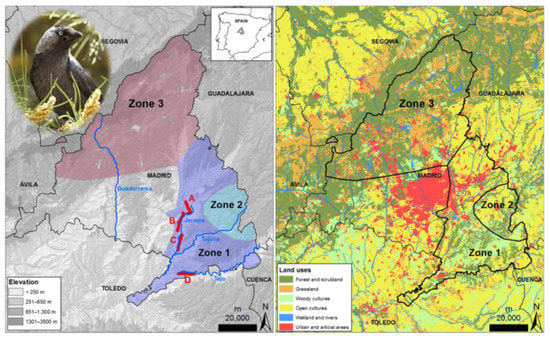
Figure 1.
Left panel: map of the study area showing the location of the cliff sectors (A–D letters and associated areas, in red), riverside forests across rivers (blue lines) surveyed for jackdaws during the breeding season in each zone (1–3) in Madrid province, Central Spain. Right panel: habitats characterizing each zone according to Corine land cover (https://land.copernicus.eu/pan-european/corine-land-cover/clc2018?tab=metadata; accessed on 24 May 2021).
2.2. Breeding Abundance in Gypsum Cliffs
We conducted walking surveys to estimate the abundance of jackdaws per km of linear gypsum cliffs located along the Manzanares, Jarama, and Tajo rivers. The cliff sectors (A, B, C, and D, in Zone 1; Figure 1) were delimited as independent survey units taking into account their continuity and orientation along the river courses [60]. Surveys were conducted along paths parallel to cliff faces so that jackdaws were counted when observed perched or in flight more or less perpendicular to the observer, attempting to not repeat individuals that might move between different areas of the cliffs. Observations were made with binoculars, and counts refer to the number of individuals present on the cliffs where jackdaws nest. The number of individuals observed is therefore a surrogate of the total abundance during the breeding season, including breeding pairs and individuals from the floating population. Transects were carried out in April–June from 1991 to 1997, although not every year in all the cliff sectors considered (from 2009 to 2021: sector A, B, and C; from 2011 onwards, except 2020: all sectors).
In the four cliff sectors where walking surveys were carried out, we also estimated the number of breeding pairs during the period 2009–2021 (except 2020). For this purpose, we visited each cliff sector for 3–5 days during the breeding season (from laying in March–April to fledging in June) to locate all breeding pairs (i.e., individuals showing reproductive activities such as nest construction, incubation, or nestling rearing). We used telescopes from distances ranging from 50–100 m to avoid disturbance. The number of pairs recorded was compared with that found in the same cliff sectors during 1984–1985 [61]. For one of these cliff sectors, there is also published information on the number of breeding pairs estimated in 1975 [62].
2.3. Colony Size
We selected a sample of 58 colonies of known size occupied in 1984–1985 [61] to determine whether they were occupied, and to determine the number of breeding pairs present in 2009–2011 using the methods described above. Colonies were grouped into three main zones (Figure 1) and were located on a variety of substrates that we classified as natural cliffs (including gypsum, limestone, and granitic cliffs, n = 18), artificial cliffs (quarry walls, n = 7), buildings (churches, castle, bridges, walls, reservoir dam walls, and ruined houses, n = 26), other artificial structures (electric pylons, and radar towers, n = 2), and other natural substrates (trees, and rabbit warrens, n = 5).
2.4. Breeding Abundance in Riverine Forest
We estimated the abundance of jackdaws in riverine forests in April–June of 2010 using standard circular plots (radius = 50 m) lasting 10 min. This methodology was previously applied in 1996 for the same purpose [63], allowing us a comparison between years (Supplementary Material). In both cases, sampling points were randomly selected but always at a distance of more than 75 m from the nearest point, in riverine forests of the Jarama, Tajo, Tajuña, and Guadarrama rivers (Figure 1). Jackdaws that flew to the point once the counting period had begun or those that flew over it were excluded. We determined the presence and number of individuals detected within each sampling point to estimate the proportion of positive points over the total sampled, and the density of jackdaws expressed as individuals/10 ha in both years, respectively. These data were compared between years for each river sampled.
2.5. Winter Communal Roosts
We located communal roosts by monitoring the movements of jackdaws from foraging areas at the afternoon to pre-roost gathering sites (i.e., places of aggregation regularly used just before entering the roost) from vantage points and car journeys. In addition, we visited during the afternoon all previously known communal roosting sites [54,61] and other suitable places with potential to be used as communal roosts (i.e., areas of dense woodland with large trees and wetlands). Once located, we conducted simultaneous counts in all communal roosts in December 2017 and 2021 to quantify the size of the winter population, including roosts located in the province of Madrid which were used by jackdaws partially foraging in neighboring provinces, and vice versa (see [54] for details). This information was compared with wintering counts of communal roosts obtained in 1984–1985 [61] and 2009–2011 [54] to assess potential changes in the distribution, total abundance, number of roosts, and roost size during the last decades. Counts from 1984–1985 did not cover all the communal roosts present in the province of Madrid, so the counts partially reflect the population size during winter. As counts in this period were not simultaneous due to logistic limitations regarding available time and observers, estimates of population numbers can be subject to some variation due to movements between roosts in the interval between counts in the different roosts.
2.6. Statistical Analysis
We compared the annual number of jackdaws observed in gypsum cliffs during the breeding season using generalized linear mixed models (GLMM; negative binomial error distribution, log link function; year as a random term). Due to survey imbalances, we first compared the number of jackdaws in 1994–1997 and 2009–2021 in sectors A and B (both included as factors in the models, as well as their interaction). The length of each survey (in km) was incorporated as a covariate to control for sampling effort. Then, we used generalized linear models (GLM) to assess annual variation in the abundance of jackdaws from 2009 to 2021 (negative binomial error distribution, log link function), when we have annual surveys (except for 2020) for each cliff sector. In this case, we included year as a covariate, the sector as a fixed factor (four levels, A–D), their interaction (yearXsector), and the length of each survey as a covariate to control for sampling effort. Similarly, we evaluated the temporal trends in the number of breeding pairs of jackdaws nesting in each cliff sector (A–D), including year as a covariate, and sector as a factor, as well as its interaction in a GLM (negative binomial error distribution, log link function). We also used GLMM to assess changes in the number of breeding pairs per colony (negative binomial error distribution, log link function; colony as a random term) from 1984–1985 to 2009–2011. The substrate (i.e., natural cliffs, buildings, other artificial structures, and other natural substrates), the period (1994–1997 and 2009–2011), the geographic area (zone) where the colony was located (Figure 1), as well as their interactions, were included as factors. Statistical analyses and checking of model assumptions were performed using the glmmTMB package in R statistical platform [64]. Interactions were further explored using post hoc comparisons (emeans and emtrends functions in the emmeans package [65], using the Benjamini-Hochberg correction to reduce the probability of obtaining false positive results). The fit of the models was evaluated using the package DHARMa [66]. Temporal trends in the number of jackdaws in winter communal roosts, and the number of communal roosts were evaluated by Spearman rank correlation coefficients. The trend of roost size (response variable) with years (factor) was evaluated by Kruskal-Wallis test.
3. Results
The number of jackdaws observed during the breeding season in gypsum cliffs (Zone 1, river meadows; Figure 1) sharply declined from 1994–1997 to 2009–2021, much more markedly in sector A than in sector B (Table 1; Figure 2A). When we assessed the annual variation in the number of jackdaws during the second period (2009–2021), all sectors showed a general decline, which was more pronounced in sector B than in the others (Table 1; Figure 2B). The number of breeding pairs nesting in each cliff sector also decreased drastically in the last decades (Table 1, Figure 3). In all sectors, the number of pairs counted in the 1980s was much higher than in later decades. In sectors A (for which a census was also taken in 1975) and B, the decline was more marked than in other ones. From 2009 onwards, when censuses were annual in all sectors, the negative trend continued until the present day (Figure 3).

Table 1.
Models obtained to assess changes in the total number and number of breeding pairs of jackdaws during the breeding season in gypsum cliffs and post hoc tests. (A) Comparison between sectors 1 and 2 between 1994–1997 and 2009–2021 and (B) among all sectors (1–4) from 2009 to 2021. mean: estimated marginal means; trend: estimated marginal means of trends; SE: standard errors; CI: confidence intervals. Model fits are shown in Figures S1 and S2.
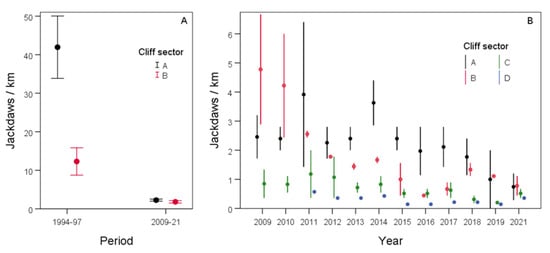
Figure 2.
Number of jackdaws (mean ± SE) during the breeding seasons in gypsum cliffs (A) in the two study periods (1994–1997 and 2009–2021) in sectors (A,B), and (B) from 2009 to 2021 in all monitored sectors (see Figure 1 for location of the cliff sectors).
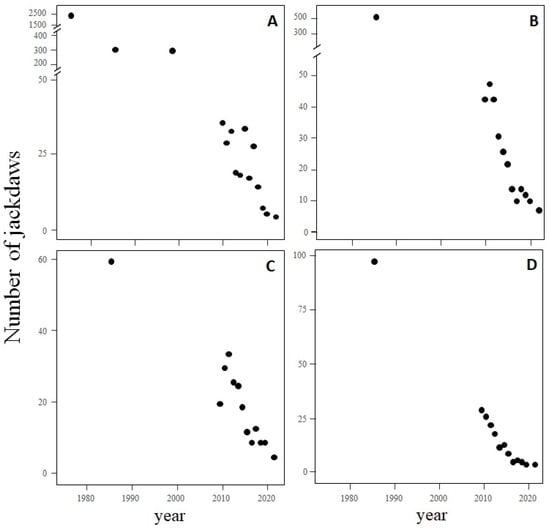
Figure 3.
Trends in the number of breeding pairs of jackdaws nesting in each cliff sector (A–D) in Madrid province, Central Spain (see Figure 1 for location of the cliffs).
From 1984–1985 to 2009–2011, a proportion of the sampled colonies in zones 1 and 3 were lost, while the total number of pairs also declined, more markedly in zone 1 than in zones 2 and 3 (Table 2; Figure 4). The same negative trend observed for the population as a whole was detected when focusing on the number of breeding jackdaws in each colony or cliff sector in this period (Figure 4). Although the decline in colony size was generalized, the drop was more marked in some areas (Zone 1) and particular nesting substrates (natural cliffs and buildings; Table 3; Figure 4 and Figure 5) than in the others.

Table 2.
Trends in the number of colonies and breeding pairs of jackdaws in Madrid, central Spain. Data refers to changes considering the same sample of colonies censused in 1984–1985 [61] and re-sampled in 2009–2011 (this study). Zone refers to the geographical areas specified in Figure 1.
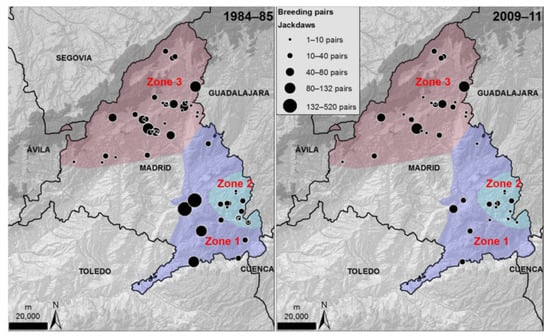
Figure 4.
Location of colonies and number of breeding pairs of jackdaws censused in 1984–1985 (left panel) according to Domínguez [61] and re-censused in 2009–2011 (right panel, this study). Zone refers to the geographical areas specified in Figure 1.

Table 3.
Model and post hoc tests (contrasts) obtained to assess changes in the number of breeding jackdaws from 1994–1997 to 2009–2011 (period) in the three areas (area) within the study region and in different substrates (NC: natural cliffs, AC: artificial cliffs, BU: buildings, OA: other artificial structures, ON: other natural substrates). Mean: estimated marginal means; SE: standard errors; CI: confidence intervals. Model fits are shown in Figures S1 and S2.
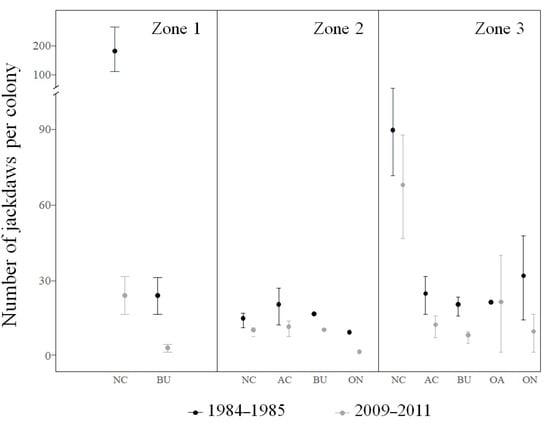
Figure 5.
Number of breeding jackdaws per colony (mean ± SE) in 1984–1985 [61] and 2009–2011 (this study) in the three zones in which we subdivided the study region (see Figure 1). The substrate in which each colony was located (NC: natural cliffs, AC: artificial cliffs, BU: buildings, OA: other artificial structures, ON: other natural substrates) is included.
Surveys in the riverside forests of four of the main rivers crossing the study area showed the presence of jackdaws at a variable proportion of the sampling points during the breeding season in 1996 (range: 3.2–16.7%), both at the riverine forest from the meadows area (Zone 1), the Guadarrama river running across the foothills of the Central Range (Zone 3), and downstream (Table 4, Figure 1). However, all surveys conducted in 2010 on the same riverside forests showed negative results (Table 4).

Table 4.
Percentage of sampling points with presence (% positive) and density (individuals/10 ha) of jackdaws during the breeding season in riverside forests of the Tajo, Tajuña, Jarama and Manzanares Rivers, Madrid, central Spain (Zone 1, see Figure 1). Data refer to sampling points selected at random in 1996 [63] and 2010 (this study).
Winter roost surveys (Figure 6) showed a negative trend from the 1980s to the present day (Spearman rank correlation coefficient rs =−0.90, p = 0.037, n = 5, Figure 7A). The number of individuals gathered in each roost (roost size) also showed a decreasing trend in the last five decades (Kruskal-Wallis test, H4,90 = 27.03, p < 0.0001, Figure 7B), while the number of communal roosts did not show a clear trend (rs =−0.30, p = 0.62, n = 5, Figure 7C).
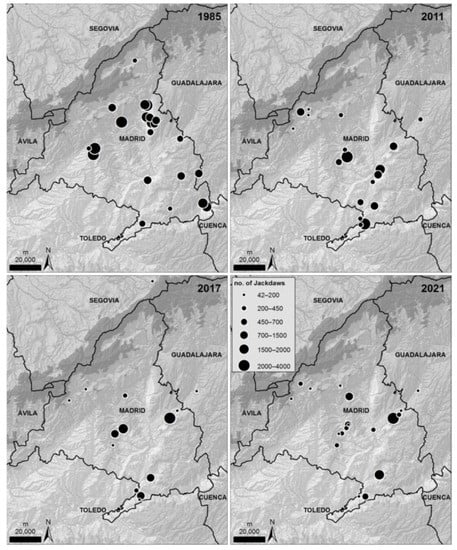
Figure 6.
Distribution and size of communal roosts of jackdaws during the non-breeding season in Madrid province, Central Spain. Data refer to simultaneous counts in mid-December 2011 [54], 2017 and 2021, including individuals foraging in neighbour provinces (Segovia, Toledo, and Guadalajara) but roosting in Madrid, or vice versa. Data from 1985 [61] refer to non-simultaneous counts partially covering the study area in autumn and winter.
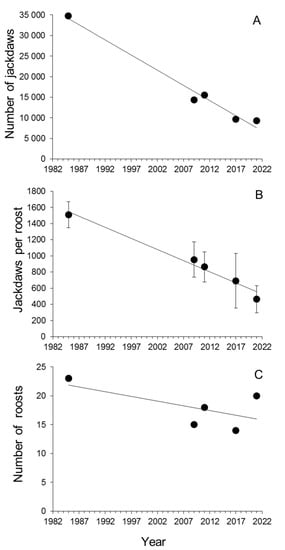
Figure 7.
Relationships between year and (A) number of jackdaws in winter communal roosts, (B) mean ± SE roost size, and (C) number of communal roosts in the study area in Central Spain (see Figure 6 for details). Least squares regression lines of the correlations are shown for graphical representation of trends.
4. Discussion
Our results show a sharp decline of the jackdaw in central Spain during the last decades. This trend is evident according to all available sources of information for relative and absolute abundance, to the point that this species has gone from being very widespread, numerous, and reaching high population densities to being relatively rare in all the habitats and geographical areas considered. These strong negative trends have also been detected in other Spanish regions [52,54,55], so the species has been proposed to enter the national catalog of threatened species in the category “Endangered” [47].
The decline of the species in Madrid, despite being general, seems to be more marked in the fertile meadows along the main rivers (Zone 1), where agricultural practices have been progressively intensified during the last decades of the 20th century. In this area, jackdaws nested in huge numbers in the apparently not limiting cavities available in the gypsum and clay cliffs along the main rivers [67], numbering thousands of pairs at least since the 1970s [62,68]. In subsequent decades, their decline was very marked, and most of the individuals breeding in these cliffs were progressively lost. At present, the number of nesting pairs on these cliffs has been quantified in a few dozens. This decline has been generalized in all the cliff sectors sampled, with some differences in magnitude when considering the total abundance surveys. These differences may be due to variable environmental conditions between cliff sectors and surrounding foraging areas, unknown local threats, or differential use of local resources by the non-breeding population. The latter possibility seems likely given the similar trends between cliff sectors when considering only breeding pairs. The collapse of these breeding nuclei could be associated with changes in agricultural practices in lowland meadows during the study period. In particular, crops have shifted from irrigated cereals riverside to cornfields and intensive vegetable crops, while a large part of the meadows and areas of dry cereal and olive groves have been completely eliminated due to quarries for the extraction of gravel and sand [57,58]. Besides, the introduction and increase of new intensive irrigated crops have been associated with the increasing application of agrochemicals such as pesticides, herbicides, and fungicides that reduce invertebrate populations and weed seeds [69,70], which dominate the diet of the species during the breeding season, resulting in a limitation of adequate food available for nestlings that potentially reduce reproductive success and productivity [52]. This side effect, in conjunction with more direct ones such as intoxication and medium to long-term accumulation of agricultural pollutants, could also have negative consequences on reproduction and survival [49,50,51]. In addition, the water used to irrigate crops in the meadows is heavily contaminated with multiple pharmaceuticals from human populations [71], with unknown impacts on the populations of jackdaws and the invertebrates that make up part of their food. Much of the gypsophilous scrubland surrounding the cliffs has been also urbanized due to the demographic pressure from Madrid city. Both the rivers and the agricultural areas of this zone are among the most polluted in Spain, with industrial developments, waste incineration, and agricultural intensification being the main sources of contamination with multiple compounds [72,73,74]. Jackdaws in this area could be seen by thousands feeding in urban waste dumps, especially outside the breeding season, until the beginning of the 21st century [68,75]. Since then, the number of individuals using these feeding sources began to decrease drastically in parallel with the decline of the breeding population, to the extreme that the presence of jackdaws is nowadays irregular and limited to a few individuals that still inhabit the surroundings. The availability of food at these sites cannot be considered as a limiting factor for the jackdaw population as the same dumps have continued to be used by thousands of white stork (Ciconia ciconia), gulls of several species, and Milvus kites [73,75].
Population trends were found to be very negative when we analyse the evolution of particular colonies over a period of about 25 years. Some of these colonies were lost in the last period, while the colonies which remained active showed a significantly reduced number of pairs. These negative trends, which continued until today, were much more pronounced in the lowland meadows than in the other zones, and the decline was more pronounced in natural cliffs and buildings than in other nesting substrates. Although the loss of some colonies may be a consequence of the destruction or the restoration of some buildings, cliffs remain nearly unchanged and have a similar availability of nesting sites than in previous decades, when thousands of pairs were recorded [61,62]. Moreover, the same trend was observed for the total number of individuals, suggesting that the main factor explaining this general decline may be shared between breeding areas and colonies. In the foothills of the mountains (Zone 3), the foraging habitats of jackdaws have been severely fragmented and degraded by intense urbanization, and by the intensification of livestock farming practices in the remaining grasslands. In particular, livestock farming has been greatly reduced, with the almost total disappearance of extensive sheep and goat farming [76], (http://www.magrama.gob.es/es/, accessed on 10 December 2021). Cattle farming has also been spatially reduced but intensified in management, which has led to the introduction and subsequent expansion of the use of ivermectin and other veterinary treatments. This antiparasitic drug has been linked to the disappearance of coprophagous insects and other invertebrates [77] that form the basis of the jackdaw′s diet during the breeding season [52]. In the high Tajuña river (zone 2), the loss of breeding colonies was not evident during the study period, although there was a decrease of about half of the breeding pairs from the 1980s to the 2010s. In this area, jackdaws use limestone cliffs and quarries that provide permanent and semi-permanent nesting sites, respectively [61]. Foraging habitats have apparently maintained their structure over the last decades, mainly due to the lower managing intensity of dry cereal crops and woody cultures (mainly olive groves) compared to irrigated cultures in the meadows. In addition, the natural shrub vegetation has been subjected to less intensive anthropogenic pressures compared to other areas. However, these apparently favorable conditions in this reduced area were not reflected in a higher abundance or density of jackdaws, probably limited by the metapopulation dynamics structured in source and sink nuclei, and by the threats to which jackdaws are subjected (especially shooting) due to long-range movements during the non-breeding season to gather in communal roosts [44,54]. Finally, abundance estimates in the riverside forests show the virtual loss of the species as a breeder. The species was very abundant nesting in these forests at least until the early 1990s, occupying tree cavities in old white poplars (Populus alba) and other typical riverside tree species [61]. In the mid-1990s, surveys still found the species during the breeding season in riverine forests, but by 2010 the species was not detected more in the rivers sampled. Given that these forests are adjacent to the agricultural meadows, the causes for the apparent lack of breeders in this habitat may be shared with those on riverine cliffs. The same negative trend was observed in the riverside forests along the Guadarrama River, despite this river running through less intensive agricultural areas and even non-agricultural areas without cliffs. The conservation status of these forests is very precarious due to their progressive elimination from long stretches of meadows as a consequence of agricultural activities [57,58]. However, the availability of nesting sites does not seem to be a limiting factor in this area, given the great availability of cavities on the cliffs and in the remaining riverside woodland.
The causes of the jackdaw decline in Central Spain seem, therefore, to be diverse and shared with those reported for other common birds of open and agricultural environments [11,78]. In particular, agricultural intensification seems to greatly limit the availability of protein food in the form of invertebrates necessary for the reproduction of many species [79]. Indeed, the global decline of birds has been linked to the global crisis of insects and other invertebrates, because of possible common causes of decline but also due to cascading effects (bottom-up regulation; [79,80,81,82]). Moreover, agricultural intensification also constitutes a direct threat through intoxication, as well as through exposure to and accumulation of agrochemicals and pharmaceuticals with sublethal effects [50,51,81,83]. Due to its omnivorous nature, the availability of food outside the breeding season does not seem to be a limiting factor, given that flocks of thousands of jackdaws foraged at urban rubbish dumps in the past, but they have been progressively scarcer in these sites. However, a presumably low nutritional quality of garbage and the acquisition of toxicants and pathogens derived from the exploitation of rubbish as food may have had long-term negative consequences on health, reproduction, and survival contributing to population decline [84,85]. Besides, beyond the general intensification of the landscape, hunting pressure on jackdaws has been very intensive in Madrid for decades, with a recent reduction in the number of individuals hunted due to their scarcity [44]. Indiscriminate hunting is expected to play a major role in the decline of the jackdaws due to the impact of increased adult mortality on the population dynamics of long-lived species [44,52,86]. In this sense, the ban on hunting of this species of no culinary interest is absolutely necessary to try to halt its collapse in the coming years, as well as to avoid hunting similar highly threatened species like the Red-billed chough, Pyrrhocorax pyrrhocorax [44]. Destruction of nesting sites may become a major problem locally, although the species′ great nesting versatility [46,52,61] may buffer this threat on a global scale. These threats need to be specifically assessed depending on the circumstances associated with human activities in each region through research and management programs that include specific agri-environmental measures to favor this and other common species.
Monitoring the trend of species and populations across space and time is essential to assess the human impact on nature [87] and evaluate the effectiveness of policy interventions [88]. As robust population monitoring is constrained by the availability of biodiversity data, governments, non-governmental organizations, and researchers increasingly rely on data collected by large numbers of volunteers with varying levels of skill [89]. However, these programs do not allow assessment of trends beyond the last three decades due to the absence of comparable past data, which may underestimate the magnitude of negative trends. Moreover, the sampling methods for multiple common species used in citizen science monitoring programs are not the most suitable for large, elusive or rare species such as corvids and raptors [27,32,35,36]. For instance, jackdaws are rarely detected in the circular census plots used in the surveys (of 25 m radius in the SACRE monitoring program in Spain [90]) due to their social habits and elusive behavior implying long flushing distance [52,91], which make this narrow radius problematic for survey corvids. As a consequence, the resulting data may be inappropriate to estimate population size. In fact, attempts to estimate population sizes based on citizen science surveys can greatly overestimate actual population sizes, due to the aforementioned sampling biases as well as analytical inconsistencies associated with habitat extrapolation and other deficiencies and difficulties [27,54,56,92,93,94]. Overestimating population sizes in the case of species with such a marked decline can be perniciously considered in hunting management plans by the competent administrations, with dramatic consequences for target and non-target species and populations [44,95,96]. Conversely, quantification of the total population size of jackdaws is feasible by communal roost counts, as the entire population is concentrated during autumn and winter [54]. The population trend assessed by these global counts in central Spain showed a decline of about 75% between the mid-1980s and today, from about 35,000 to 9000 individuals. The censuses of the 1980s were not simultaneous and did not cover the whole study area [61], so they could represent an underestimate of the actual population that would imply an even greater decline than shown in this study. Moreover, estimates made subsequently included individuals using communal roosts in the study area but foraging in neighboring provinces and vice versa [54]. This negative trend was also very pronounced in the average number of jackdaws per roost, despite the improved coverage of the most recent censuses due to a better knowledge of the population, which allowed finding and monitoring even the smallest roosts. In this sense, it is possible that the small roosts of less than 50 individuals detected in the last counts were not present when the population was much larger and have appeared as a consequence of its fragmentation into progressively smaller roosts. In any case, taking into account the most recent simultaneous censuses, the population trend is still very marked in the last decade.
As global population estimates based on direct counts of individuals are readily achievable through simultaneous counts in communal roosts, the jackdaw can serve as a model for assessing temporal trends potentially linked to large-scale anthropogenic modifications of open and agricultural environments. Given their relatively large size among common birds, omnivorous diet, long lifespan, and adaptability to environmental changes, declining population trends of the jackdaw can be especially useful as a warning signal of extreme environmental degradation and introduction of novel harmful changes for wildlife due to agricultural practices. High-intensity agricultural management generally affects more drastically smaller and less adaptable common species [79,97], which are expected to decline before and at a higher extent and magnitude than jackdaws. Therefore, large declines of jackdaws are expected when agricultural practices become completely unsustainable for the maintenance of minimum levels of biodiversity, including viable populations of smaller common birds and other organisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/conservation2010007/s1, Figure S1. qq-plot and standard residuals plots for the model obtained to describe changes in the number of jackdaws from 1994–1997 to 2009–2021 in gypsum cliffs in sectors 1 and 2 (No significant problems were detected), Figure S2. qq-plot and standard residuals plots for the model obtained to describe changes in the number of jackdaws from 2009 to 2021 in gypsum cliffs in all monitored sectors (No significant problems were detected), Figure S3. qq-plot and standard residuals plots for the model obtained to describe changes in the number of breeding jackdaws (colony size) from 1994–1997 to 2009–2011; the model included a zero-inflation term (No significant problems were detected).
Author Contributions
Conceptualization: G.B., L.D., L.F. and F.M.; methodology, G.B., L.D., L.F. F.M.; and M.C.; fieldwork: G.B., L.D., L.F., F.M., J.L.G.d.B., Ó.F. and J.A.C.; software, J.A.C.; M.C.; formal analysis, G.B. and M.C.; writing—original draft preparation, G.B. and M.C.; writing—review and editing, L.D., L.F., F.M, J.L.G.d.B., Ó.F. and J.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included within the article.
Acknowledgments
We thank C. Palacín, J.A. López Septiem, J. Lavado, J.L. Hidalgo, A. Nuñez, F. Roviralta, J. Prieto, P. Alcazar, M. Garcés, J. Osorio, S. Rebollo, J.A. Martín, J.A. Fargallo, D. Gil, A. Aparicio, Ó. Llama, A. Rodríguez, S. del Pozuelo, J.C. Quintana, R. Moreno-Opo, A. Ortega, A. Victoria, B.J. Condori, I. Farias, J.M. Rodríguez, E. García, J.A. Matesanz, C. Serrano, R. Bocca, M. Vicente, C. Ferrero, P.J. Sanz, E. Ramírez, F. Álamo and M.A. Letón for their help in the counts in communal roosts in 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- BirdLife International. State of the World’s Birds: Taking the Pulse of the Planet; BirdLife International: Cambridge, UK, 2018. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species; Version 2021-1; IUCN: London, UK, 2021. [Google Scholar]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.; Almond, R.E.; Watson, R. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Gamero, A.; Brotons, L.; Brunner, A.; Foppen, R.; Fornasari, L.; Gregory, R.D.; Herrando, S.; Hořák, D.; Jiguet, F.; Kmecl, P.; et al. Tracking progress toward EU biodiversity strategy targets: EU policy effects in preserving its common farmland birds. Conserv. Lett. 2017, 10, 395–402. [Google Scholar] [CrossRef]
- Sekercioglu, C.H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J. Valuing common species. Science 2010, 327, 154–155. [Google Scholar] [CrossRef]
- Green, A.J.; Elmberg, J.; Lovas-Kiss, Á. Beyond scatter-hoarding and frugivory: European corvids as overlooked vectors for a broad range of plants. Front. Ecol. Evol. 2019, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.J.; Fuller, R.A. Commonness, population depletion and conservation biology. Trends Ecol. Evol. 2007, 23, 14–19. [Google Scholar] [CrossRef]
- Vorisek, P.; Jiguet, F.; van Strien, A.; Škorpilová, J.; Klvanová, A.; Gregory, R.D. Trends in Abundance and Biomass of Widespread European Farmland Birds: How Much Have We Lost? BOU Proceedings–Lowland Farmland Birds III. 2010. Available online: https://www.bou.org.uk/bouproc-net/lfb3/vorisek-etal.pdf (accessed on 11 December 2021).
- Pejchar, L.; Clough, Y.; Ekroos, J.; Nicholas, K.A.; Olsson, O.; Ram, D.; Tschumi, M.; Smith, H.G. Net effects of birds in agroecosystems. BioScience 2018, 68, 896–904. [Google Scholar] [CrossRef]
- Donald, P.F.; Sanderson, F.J.; Burfield, I.J.; van Bommel, F.P.J. Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agric. Ecosyst. Environ. 2006, 116, 189–196. [Google Scholar] [CrossRef]
- Butler, S.J.; Boccaccio, L.; Gregory, R.D.; Vorisek, P.; Norris, K. Quantifying the impact of land-use change to European farmland bird populations. Agric. Ecosyst. Environ. 2010, 137, 348–357. [Google Scholar] [CrossRef]
- Guerrero, I.; Morales, M.B.; Oñate, J.J.; Geiger, F.; Berendse, F.; de Snoo, G.; Sönke, E.; Tomas, P.; Jan, B.; Lars, W.C.; et al. Response of ground-nesting farmland birds to agricultural intensification across Europe: Landscape and field level management factors. Biol. Conserv. 2012, 152, 74–80. [Google Scholar] [CrossRef]
- Emmerson, M.; Morales, M.B.; Oñate, J.J.; Batary, P.; Berendse, F.; Liira, J.; Aavik, T.; Guerrero, I.; Bommarco, R.; Eggers, S.; et al. How agricultural intensification affects biodiversity and ecosystem services. Adv. Ecol. Res. 2016, 55, 43–97. [Google Scholar]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, K.J.; Fuller, R.A. Biodiversity and extinction: Losing the common and the widespread. Prog. Phys. Geogr. 2007, 31, 213–225. [Google Scholar] [CrossRef]
- Pe’er, G.; Dicks, L.V.; Visconti, P.; Arlettaz, R.; Báldi, A.; Benton, T.G.; Scott, A.V. EU agricultural reform fails on biodiversity. Science 2014, 344, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Reif, J.; Vermouzek, Z. Collapse of farmland bird populations in an Eastern European country following its EU accession. Conserv. Lett. 2019, 12, e12585. [Google Scholar] [CrossRef]
- Nichols, J.D.; Williams, B.K. Monitoring for conservation. Trends Ecol. Evol. 2006, 21, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.C.; Blancher, P.; Rosenberg, K.V.; Panjabi, A.O.; Thogmartin, W.E. Estimating uncertainty of North American landbird population sizes. Avian Conserv. Ecol. 2019, 14, 4. [Google Scholar] [CrossRef]
- Marsh, D.M.; Trenham, P.C. Current trends in plant and animal population monitoring. Conserv. Biol. 2008, 22, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, P.; Firbank, L.; Butler, S.; Norris, K.; Jones, L.M.; Petit, S. Modelling the European farmland bird indicator in response to forecast land-use change in Europe. Ecol. Indic. 2011, 11, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.D.; Williams, B.K. The potential for citizen science to produce reliable and useful information in ecology. Conserv. Biol. 2019, 33, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Pollock, K.H.; Nichols, J.D.; Simonst, T.R.; Farnsworth, G.L.; Bailey, L.; Sauer, J.R. Large scale wildlife monitoring studies: Statistical methods for design and analysis. Environmetrics 2002, 13, 105–119. [Google Scholar] [CrossRef]
- Elphick, C.S. How you count counts: The importance of methods research in applied ecology. J. Appl. Ecol. 2008, 45, 1313–1320. [Google Scholar] [CrossRef]
- Snäll, T.; Kindvall, O.; Nilsson, J.; Pärt, T. Evaluating citizen-based presence data for bird monitoring. Biol. Conserv. 2011, 144, 804–810. [Google Scholar] [CrossRef]
- Blanco, G.; Sergio, F.; Sánchez-Zapata, J.A.; Pérez-García, J.M.; Botella, F.; Martínez, F.; Zuberogoitia, I.; Frías, Ó.; Roviralta, F.; Martínez, J.E. Safety in numbers? Supplanting data quality with fanciful models in wildlife monitoring and conservation. Biodiver. Conserv. 2012, 21, 3269–3276. [Google Scholar] [CrossRef] [Green Version]
- Overmars, K.P.; Schulp, C.J.; Alkemade, R.; Verburg, P.H.; Temme, A.J.; Omtzigt, N.; Schaminée, J.H. Developing a methodology for a species-based and spatially explicit indicator for biodiversity on agricultural land in the EU. Ecol. Indic. 2014, 37, 186–198. [Google Scholar] [CrossRef]
- Lewandowski, E.; Specht, H. Influence of volunteer and project characteristics on data quality of biological surveys. Conserv. Biol. 2015, 29, 713–723. [Google Scholar] [CrossRef]
- Kamp, J.; Oppel, S.; Heldbjerg, H.; Nyegaard, T.; Donald, P.F. 2016. Unstructured citizen science data fail to detect long-term population declines of common birds in Denmark. Divers. Distrib. 2016, 22, 1024–1035. [Google Scholar] [CrossRef]
- Sutherland, W.J. (Ed.) Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Hardey, J.; Crick, H.; Wernham, C.; Riley, H.; Etheridge, B.; Thompson, D. Raptors: A Field Guide to Surveys and Monitoring; The Stationery Office: Edinburgh, UK, 2009. [Google Scholar]
- Norman, D.; Harris, R.J.; Newson, S.E. Producing regional estimates of population size for common and widespread breeding birds from national monitoring data. Bird Study 2012, 59, 10–21. [Google Scholar] [CrossRef]
- Reynolds, J.H.; Thompson, W.L.; Russell, B. Planning for success: Identifying effective and efficient survey designs for monitoring. Biol. Conserv. 2011, 144, 1278–1284. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques, 2nd ed.; Academic Press: London, UK, 2000. [Google Scholar]
- Thompson, W.L. Sampling Rare and Elusive Species; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Tella, J.L.; Romero-Vidal, P.; Dénes, F.V.; Hiraldo, F.; Toledo, B.; Rossetto, F. Roadside car surveys: Methodological constraints and solutions for estimating parrot abundances across the world. Diversity 2021, 13, 300. [Google Scholar] [CrossRef]
- Hertzog, L.R.; Frank, C.; Klimek, S.; Röder, N.; Böhner, H.G.; Kamp, J. Model-based integration of citizen science data from disparate sources increases the precision of bird population trends. Divers. Distrib. 2021, 27, 1106–1119. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Angell, T. In the Company of Crows and Ravens; Yale University Press: New Haven, CT, USA, 2005. [Google Scholar]
- Madden, C.F.; Arroyo, B.; Amar, A. A review of the impacts of corvids on bird productivity and abundance. IBIS 2015, 157, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Benmazouz, I.; Jokimäki, J.; Lengyel, S.; Juhász, L.; Kaisanlahti-Jokimäki, M.L.; Kardos, G.; Paládi, P.; Kövér, L. Corvids in urban environments: A systematic global literature review. Animals 2021, 11, 3226. [Google Scholar] [CrossRef] [PubMed]
- Marina, G.; Bezares, E. Información sobre los cuervos en España. Inst. For. Investig. Y Exp. 1933, 12, 1–47. [Google Scholar]
- Brochet, A.L.; Van Den Bossche, W.; Jones, V.R.; Arnardottir, H.; Damoc, D.; Demko, M.; Driessens, G.; Flensted, K.; Gerber, M.; Ghasabyan, M.; et al. Illegal killing and taking of birds in Europe outside the Mediterranean: Assessing the scope and scale of a complex issue. Bird Conserv. Int. 2019, 29, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Blanco, G.; Cuevas, J.A.; Frías, Ó.; del Barrio, J.L.G. A shot in the dark: Sport hunting of declining corvids promotes the inadvertent shooting of threatened red-billed choughs. J. Nat. Conserv. 2019, 52, 125739. [Google Scholar] [CrossRef]
- BirdLife International. European Birds of Conservation Concern: Populations, Trends and National Responsibilities; BirdLife International: Cambridge, UK, 2017. [Google Scholar]
- Božič, L. Numbers, distribution and nest site characteristics of Jackdaw Corvus monedula in Slovenia and its conservation status. Acrocephalus 2016, 37, 123–150. [Google Scholar] [CrossRef] [Green Version]
- López-Jiménez, N. (Ed.) Libro Rojo de las Aves de España; SEO/BirdLife: Madrid, Spain, 2021. [Google Scholar]
- Vandewalle, M.; De Bello, F.; Berg, M.P.; Bolger, T.; Doledec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef] [Green Version]
- Mineau, P.; Whiteside, M. Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS ONE 2013, 8, e57457. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, A.J. Ecotoxicology, avian. Encycl. Toxicol. 2014, 2, 289–294. [Google Scholar]
- Goulson, D. Pesticides linked to bird declines. Nature 2014, 511, 295–296. [Google Scholar] [CrossRef]
- Soler, M. Grajilla—Corvus monedula. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Morales, M.B., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2012; Available online: http://www.vertebradosibericos.org/ (accessed on 13 November 2021).
- BirdLife International. Corvus monedula (Eurasian Jackdaw). In European Red List of Birds; Office for Official Publications of the European Communities: Luxembourg, 2015. [Google Scholar]
- Blanco, G.; Frías, Ó.; Cuevas, J.A.; González, J.L.; Martínez, F. Commonness of not-so-common birds: The need for baseline knowledge of actual population size for the validation of population size predictions. Bird Study 2014, 61, 351–360. [Google Scholar] [CrossRef]
- Frías, Ó.; González del Barrio, J.L.; Martínez, F.; Blanco, G. Grajilla Occidental, Corvus monedula. In SEO/BirdLife: III Atlas de las Aves en Época de Reproducción en ESPAÑA; SEO/BirdLife: Madrid, Spain, 2021; in press. [Google Scholar]
- Frías, Ó.; Bautista, L.M.; Dénes, F.V.; Cuevas, J.A.; Martínez, F.; Blanco, G. Influence of habitat suitability and sex-related detectability on density and population size estimates of habitat-specialist warblers. PLoS ONE 2018, 13, e0201482. [Google Scholar]
- Cuevas, J.A.; Acha, A.; Blanco, G.; Ruiz, P.; Velasco, T.; Delgado, J.A.; De Miguel, J.A. Biodiversidad en ecosistemas fluviales: Las aves acuáticas en la cuenca media del Tajo. Ser. Doc. 2000, 31, 114. [Google Scholar]
- Molina-Holgado, P.M.; Jendrzyczkowski Rieth, L.; Berrocal Menarguez, A.B.; Allende Álvarez, F. The analysis of urban fluvial landscapes in the centre of Spain, their characterization, values and interventions. Sustainability 2020, 12, 4661. [Google Scholar] [CrossRef]
- Gómez Mendoza, J.; Mata Olmo, R.; Sanz Herráiz, C.; Galiana Martín, L.; Manuel Valdés, C.M.; Molina Holgado, P. Los Paisajes de Madrid: Naturaleza y Medio Rural; Alianza Editorial-Fundación Caja Madrid: Madrid, Spain, 1999; 301p. [Google Scholar]
- Blanco, G.; Cuevas, J.A.; Fargallo, J.A. La población de Chova Piquirroja Pyrrhocorax pyrrhocorax en el Sureste de Madrid (Centro de España). Ardeola 1991, 38, 91–99. [Google Scholar]
- Domínguez, L. Ecología de la Grajilla (Corvus monedula Linnaeus 1758) en la Provincial de Madrid. Ph.D Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1999. [Google Scholar]
- Arroyo, B. La avifauna de un cantil estepárico. Ardeola 1976, 23, 41–49. [Google Scholar]
- Velasco, T.; Blanco, G. Avifauna nidificante en los sotos fluviales de la Comunidad de Madrid. Anu. Ornitológico Madr. 2001, 2000, 56–67. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means; R Package, Version 1.6.2-1. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 15 December 2021).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; R Package, Version 0.2.0. Available online: https://cran.r-project.org/package=DHARMa (accessed on 15 December 2018).
- Blanco, G.; Cuevas, J.A.; Fargallo, J.A. Breeding density and distribution of choughs Pyrrhocorax pyrrhocorax nesting in river cliffs: The role of nest-site availability. Ardea 1998, 86, 237–244. [Google Scholar]
- Ceballos, P.; Purroy, F.J. Pájaros de Nuestros Campos y Bosques; Mundi-Prensa: Madrid, Spain, 2005. [Google Scholar]
- Fagúndez, J.; Olea, P.P.; Tejedo, P.; Mateo-Tomás, P.; Gómez, D. Irrigation and maize cultivation erode plant diversity within crops in Mediterranean dry cereal agro-ecosystems. Environ. Manag. 2016, 58, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Cabodevilla, X.; Wright, A.D.; Villanua, D.; Arroyo, B.; Zipkin, E.F. The implementation of irrigation leads to declines in farmland birds. Agric. Ecosyst. Environ. 2022, 323, 107701. [Google Scholar] [CrossRef]
- Valcárcel, Y.; Alonso, S.G.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef]
- Fernández, M.; Cuesta, S.; Jiménez, O.; Garcıa, M.A.; Hernández, L.M.; Marina, M.L.; González, M.J. Organochlorine and heavy metal residues in the water/sediment system of the southeast regional park in Madrid, Spain. Chemosphere 2000, 41, 801–812. [Google Scholar] [CrossRef]
- Blanco, G.; Sergio, F.; Frías, Ó.; Salinas, P.; Tanferna, A.; Hiraldo, F.; Barceló, D.; Eljarrat, E. Integrating population connectivity into pollution assessment: Overwintering mixing reveals flame retardant contamination in breeding areas in a migratory raptor. Environ. Res. 2018, 166, 553–561. [Google Scholar] [CrossRef]
- Eljarrat, E.; Aznar-Alemany, Ò.; Sala, B.; Frías, Ó.; Blanco, G. Decreasing but still high levels of halogenated flame retardants in wetland birds in central Spain. Chemosphere 2019, 228, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Marchamalo, J. Post-breeding inland movements and use of refuse dumps by Audouin’s Gulls in Spain. Waterbirds 1999, 22, 307–309. [Google Scholar] [CrossRef]
- Blanco, G. Can livestock carrion availability influence diet of wintering red kites? Implications of sanitary policies in ecosystem services and conservation. Popul. Ecol. 2014, 56, 593–604. [Google Scholar] [CrossRef]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.P. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Traba, J.; Morales, M.B. The decline of farmland birds in Spain is strongly associated to the loss of fallowland. Sci. Rep. 2019, 9, 9473. [Google Scholar] [CrossRef] [PubMed]
- Bowler, D.E.; Heldbjerg, H.; Fox, A.D.; de Jong, M.; Böhning-Gaese, K. Long-term declines of European insectivorous bird populations and potential causes. Conserv. Biol. 2019, 33, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Camarero, P.R.; Mateo, R. Birds feeding on tebuconazole treated seeds have reduced breeding output. Environ. Pollut. 2021, 271, 116292. [Google Scholar] [CrossRef] [PubMed]
- Meyrier, E.; Jenni, L.; Bötsch, Y.; Strebel, S.; Erne, B.; Tablado, Z. Happy to breed in the city? Urban food resources limit reproductive output in Western Jackdaws. Ecol. Evol. 2017, 7, 1363–1374. [Google Scholar] [CrossRef]
- Plaza, P.I.; Lambertucci, S.A. How are garbage dumps impacting vertebrate demography, health, and conservation? Glob. Ecol. Conserv. 2017, 12, 9–20. [Google Scholar] [CrossRef]
- Bird, J.P.; Martin, R.; Akçakaya, H.R.; Gilroy, J.; Burfield, I.J.; Garnett, S.T.; Symes, A.; Taylor, J.; Şekercioğlu, Ç.H.; Butchart, S.H.M. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 2020, 34, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Balmford, A.; Green, R.E.; Jenkins, M. Measuring the changing state of nature. Trends Ecol. Evol. 2003, 18, 326–330. [Google Scholar] [CrossRef]
- Donald, P.F.; Sanderson, F.J.; Burfield, I.J.; Bierman, S.M.; Gregory, R.D.; Waliczky, Z. International conservation policy delivers benefits for birds in Europe. Science 2007, 317, 810–813. [Google Scholar] [CrossRef]
- Amano, T.; Lamming, J.D.L.; Sutherland, W.J. Spatial gaps in global biodiversity information and the role of citizen science. BioScience 2016, 66, 393–400. [Google Scholar] [CrossRef] [Green Version]
- SEO/BirdLife. SEO/BirdLife 2011 Birds Monitoring Programmes; SEO/BirdLife: Madrid, Spain, 2012. [Google Scholar]
- Møller, A.P. Flight distance and population trends in European breeding birds. Behav. Ecol. 2008, 19, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Guthery, F.S. Statistical ritual versus knowledge accrual in wildlife science. J. Wildl. Manag. 2008, 72, 1872–1875. [Google Scholar] [CrossRef]
- Murgi, E. How many common breeding birds are there in Spain? A comparison of census methods and national population size estimates. Ardeola 2011, 58, 343–364. [Google Scholar]
- Bird, T.J.; Bates, A.E.; Lefcheck, J.S.; Hill, N.A.; Thomson, R.J.; Edgar, G.J.; Stuart-Smith, R.D.; Wotherspoon, S.; Krkosek, M.; Stuart-Smith, J.F.; et al. Statistical solutions for error and bias in global citizen science datasets. Biol. Conserv. 2014, 173, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Pellikka, J.; Kuikka, S.; Lindén, H.; Varis, O. The role of game management in wildlife populations: Uncertainty analysis of expert knowledge. Eur. J. Wildl. Res. 2005, 51, 48–59. [Google Scholar] [CrossRef]
- Mustin, K.; Arroyo, B.; Beja, P.; Newey, S.; Irivine, R.J.; Kestler, J.; Redpath, S.M. Consequences of game bird management for non-game species in Europe. J. Appl. Ecol. 2018, 55, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Inger, R.; Gregory, R.; Duffy, J.P.; Stott, I.; Voříšek, P.; Gaston, K.J. Common European birds are declining rapidly while less abundant species’ numbers are rising. Ecol. Lett. 2015, 18, 28–36. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).