Rutin Facilitates Dioxin Elimination and Attenuates Systemic Toxicity in a Wistar Rat Model
Abstract
1. Introduction
2. Results
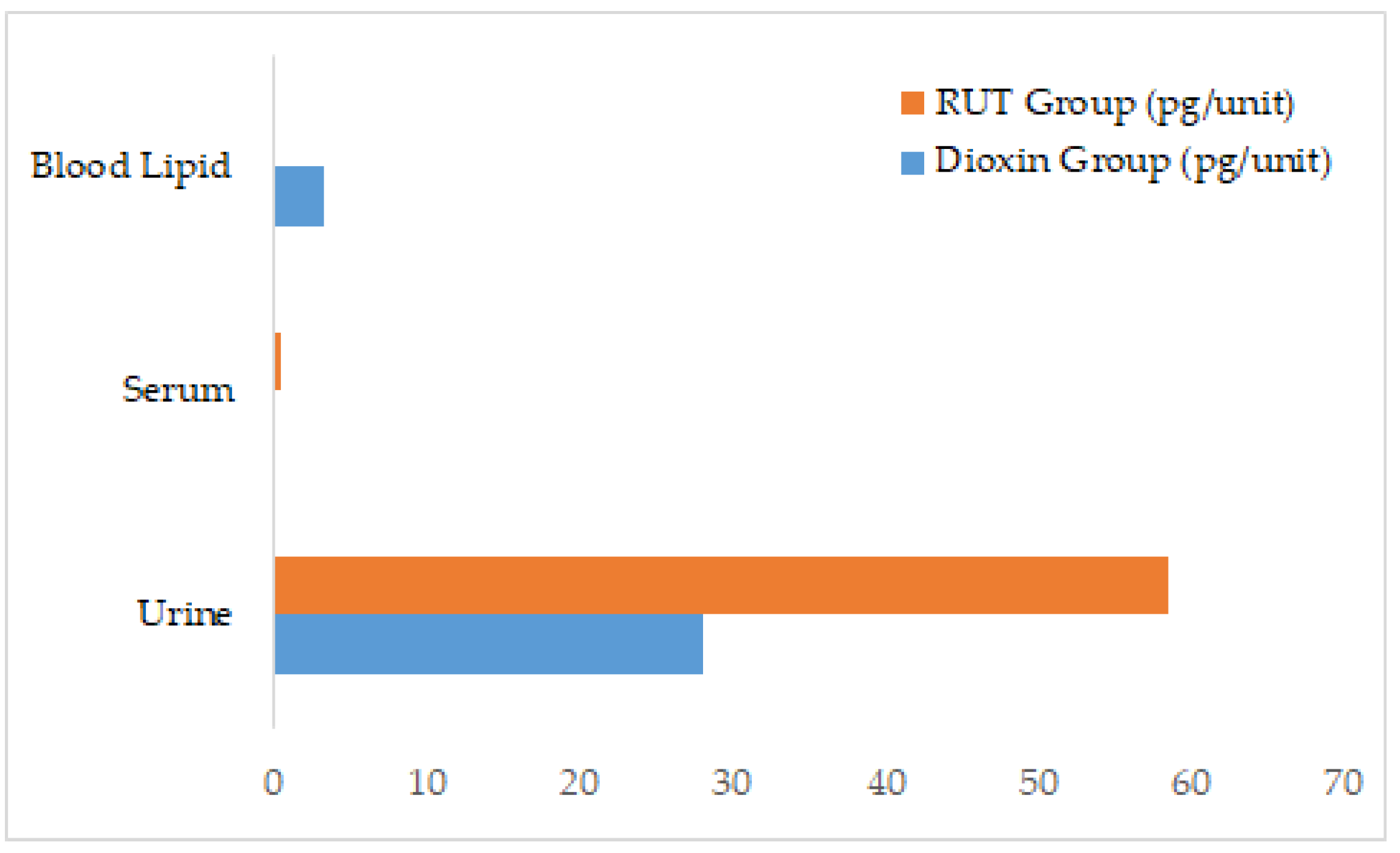
2.1. Effects of RUT on Dioxin Excretion
2.2. Quantification of Dioxin Excretion and Residual Levels
3. Discussion
4. Materials and Methods
4.1. Dioxin Mixture and Dosing
4.2. Evaluation of Dioxin and Congener Excretion by RUT in Experimental Animals
- -
- Group 1 (Dioxin group): Rats were administered a single intraperitoneal injection of a solution containing dioxin and its congeners at a dose of 10 µg/kg body weight (BW).
- -
- Group 2 (RUT group): Rats received the same dioxin and congener injection as Group 1 (10 µg/kg BW) and were additionally administered RUT orally at a dose of 0.02 g/kg BW/day.
- -
- Group 3 (Control group): Rats were given 10 mL/kg BW/day of distilled water.
4.3. Quantitative Analysis of Dioxin in Study Samples
4.3.1. Fecal Samples
4.3.2. Urine Samples
4.3.3. Blood Samples
4.3.4. Hematology and Clinical Chemistry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RUT | Rutin |
| OCDD | Octachlorodibenzo-p-dioxin |
| HRGC/ | High-Resolution Gas Chromatography/ |
| HRMS | High-Resolution Mass Spectrometry |
| GST | Glutathione S-Transferase |
| CYP1A1 | Cytochrome P450 1A1 |
| AhR | Aryl Hydrocarbon Receptor |
| PCDD | Polychlorinated Dibenzo-p-dioxins |
| PCDF | Polychlorinated Dibenzofurans |
| MQL | Method Quantification Limit |
References
- Hites, R.A. Dioxins: An overview and history. Environ. Sci. Technol. 2011, 45, 16–20. [Google Scholar] [CrossRef]
- De Oliveira Cardo, M.J.S. Study of OCDD, 1,2,3,4,6,7,8-HxCDD, 1,2,3,7,8-PeCDD and HxCDD and their bioaccumulation. Environ. Sci. Pollut. Res. 2011, 18, 678–684. [Google Scholar]
- Thornton, J. Dioxin from Cradle to Grave; Greenpeace: Washington, DC, USA, 1997. [Google Scholar]
- Malone, A. The Legacy of War: A Holistic Analysis of Socio-Economic and Healthcare Resources for People Affected by Agent Orange in Việt Nam; Routledge: London, UK, 2023. [Google Scholar]
- Nguyen, H.X.; Nguyen, X.T.; Mai, H.T.H.; Nguyen, H.T.; Vu, N.D.; Pham, T.T.P.; Tran, T.T.; Bui, M.Q. A Comprehensive Evaluation of Dioxins and Furans Occurrence in River Sediments from a Secondary Steel Recycling Craft Village in Northern Vietnam. Molecules 2024, 29, 1788. [Google Scholar] [CrossRef]
- Bartalini, A. Persistent Organic Pollutants (POPs) in Marine Species. Ph.D. Thesis, Università degli Studi di Siena, Siena, Italy, 2024. [Google Scholar]
- Eljarrat, E.; Barceló, D. Toxicity potency assessment of persistent organic pollutants in sediments and sludges. In Series Anthropogenic Compounds: Emerging Organic Pollution in Waste Waters and Sludge; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Richards, G.; Agranovski, I.E. Dioxin-like PCB emissions from cement kilns during the use of alternative fuels. J. Hazard. Mater. 2017, 323, 698–709. [Google Scholar] [CrossRef]
- Dopico, M.; Gómez, A. Review of the current state and main sources of dioxins around the world. J. Air Waste Manag. Assoc. 2015, 65, 1033–1049. [Google Scholar] [CrossRef]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Reiner, E.J. Analysis of dioxin and dioxin-like compounds. Dioxin Relat. Compd. 2016, 49, 51–94. [Google Scholar] [CrossRef]
- Schröder Barraza, S.M.; Ortiz Uribe, I.; San Román San Emeterio, M.F. Electrochemical Degradation of Key Drugs to Treat COVID-19: Experimental Analysis of the Toxic By-Products Formation (PCDD/Fs). Sci. Total Environ. 2024, 906, 167660. [Google Scholar] [CrossRef]
- Gorbunova, T.I.; Egorova, D.O.; Saloutin, V.I.; Chupakhin, O.N. Aerobic Bacterial Degradation of Polychlorinated Biphenyls and Their Hydroxy and Methoxy Derivatives. Russ. Chem. Rev. 2024, 93, 11. [Google Scholar] [CrossRef]
- Mathew, N.; Somanathan, A.; Tirpude, A.; Pillai, A.M.; Mondal, P.; Arfin, T. Dioxins and Their Impact: A Review of Toxicity, Persistence, and Novel Remediation Strategies. Anal. Methods 2025, 17, 1698–1748. [Google Scholar] [CrossRef] [PubMed]
- Scippo, M.L.; Eppe, G.; Saegerman, C.; Scholl, G.; De Pauw, E.; Maghuin-Rogister, G.; Focant, J.F. Persistent organochlorine pollutants, dioxins and polychlorinated biphenyls. Compr. Anal. Chem. 2008, 51, 457–506. [Google Scholar]
- Schiavon, M.; Torretta, V.; Rada, E.C.; Ragazzi, M. State of the art and advances in the impact assessment of dioxins and dioxin-like compounds. Environ. Monit. Assess. 2016, 188, 57. [Google Scholar] [CrossRef] [PubMed]
- Srogi, K. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: A review. Environ. Chem. Lett. 2008, 6, 1–28. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F.; Diliberto, J.J. Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs). Environ. Int. 2003, 29, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, J.; Vartiainen, T.; Tuomisto, J.T. Synopsis on Dioxins and PCBs; National Institute for Health and Welfare (THL): Helsinki, Finland, 2011. [Google Scholar]
- Borgå, K.; Fisk, A.T.; Hoekstra, P.F.; Muir, D.C. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in arctic marine food webs. Environ. Toxicol. Chem. 2004, 23, 2367–2385. [Google Scholar] [CrossRef]
- De Solla, S.R. Exposure, bioaccumulation, metabolism and monitoring of persistent organic pollutants in terrestrial wildlife. Dioxin Relat. Compd. 2016, 49, 203–252. [Google Scholar] [CrossRef]
- Byard, J.L.; Paulsen, S.C.; Tjeerdema, R.S.; Chiavelli, D. DDT, chlordane, toxaphene and PCB residues in Newport Bay and watershed: Assessment of hazard to wildlife and human health. Rev. Environ. Contam. Toxicol. 2014, 235, 49–168. [Google Scholar] [CrossRef]
- Jantunen, P. The Role of Sorption in the Ecological Risk Assessment of Xenobiotics. Ph.D. Thesis, Itä-Suomen Yliopisto, Kuopio, Finland, 2010. [Google Scholar]
- Schecter, A. (Ed.) Dioxins and Health, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Stejskalova, L.; Pavek, P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr. Pharm. Biotechnol. 2011, 12, 715–730. [Google Scholar] [CrossRef]
- Zhang, W.; Sargis, R.M.; Volden, P.A.; Carmean, C.M.; Sun, X.J.; Brady, M.J. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS ONE 2012, 7, e37103. [Google Scholar] [CrossRef]
- Jackson, E.; Shoemaker, R.; Larian, N.; Cassis, L. Adipose tissue as a site of toxin accumulation. Compr. Physiol. 2017, 7, 1085. [Google Scholar] [CrossRef]
- Bruce-Vanderpuije, P.N.A. Investigations on the State of POPs in Ghana, and Human Biomonitoring of Dioxin-like Polychlorinated Biphenyls (DlPCBs), Polychlorinated, Polybrominated and Mixed Halogenated Dibenzo-p-Dioxins and Furans (PCDD/Fs, PBDD/Fs, and PXDD/Fs) in Vulnerable Ghanaian Populations. Ph.D. Thesis, State University of New York at Buffalo, Buffalo, NY, USA, 2019. [Google Scholar]
- Olson, J.R. Pharmacokinetics of dioxins and related chemicals. In Dioxins and Health, 2nd ed.; Schecter, A., Ed.; Springer Science & Business Media: New York, NY, USA, 1994; pp. 163–197. [Google Scholar]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of xenobiotic pollutants: An environmentally sustainable approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef]
- Rice, C.P.; O’Keefe, P.; Kubiak, T. Sources, pathways, and effects of PCBs, dioxins, and dibenzofurans. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 525–598. [Google Scholar]
- Zimniak, P. Detoxification reactions: Relevance to aging. Ageing Res. Rev. 2008, 7, 281–300. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Mench, M.; Schwitzguébel, J.P.; Schroeder, P.; Bert, V.; Gawronski, S.; Gupta, S. Assessment of successful experiments and limitations of phytotechnologies: Contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ. Sci. Pollut. Res. 2009, 16, 876–900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Tian, F.; Zhang, S.Q.; Jin, H. Advances in pharmacokinetic mechanisms of transporter-mediated herb-drug interactions. Pharmaceuticals 2022, 15, 1126. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, S.M.; Nanakali, N.M. Ameliorating potential of quercetin on liver function, genotoxicity and oxidative damage induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in liver of male rats. Pak. J. Zool. 2020, 52, 535–547. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Zhai, J.; Zhong, C.; Zeng, H.; Feng, L.; Deng, J. Effect of Oxidative Stress Induced by 2,3,7,8-Tetrachlorodibenzo-p-dioxin on DNA Damage. J. Hazard. Mater. 2024, 472, 134485. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Paithankar, J.G.; Stopper, H.; Pandey, A.; Sharma, A. Environmental Chemical-Induced Reactive Oxygen Species Generation and Immunotoxicity: A Comprehensive Review. Antioxid. Redox Signal. 2024, 40, 691–714. [Google Scholar] [CrossRef]
- Beigh, S. A Phytochemicals Approach towards the Role of Dioxins in Disease Progression Targeting Various Pathways: Insights. Indian J. Pharm. Educ. Res. 2024, 58 (Suppl. S3), s732–s756. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 2010, 49, 1328–1341. [Google Scholar] [CrossRef]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: A review. Adv. Pharm. Bull. 2020, 10, 184. [Google Scholar] [CrossRef]
- Boušová, I.; Skálová, L. Inhibition and induction of glutathione S-transferases by flavonoids: Possible pharmacological and toxicological consequences. Drug Metab. Rev. 2012, 44, 267–286. [Google Scholar] [CrossRef]
- Korobkova, E.A. Effect of natural polyphenols on CYP metabolism: Implications for diseases. Chem. Res. Toxicol. 2015, 28, 1359–1390. [Google Scholar] [CrossRef]
- Ciftci, O.; Vardi, N.; Ozdemir, I. Effects of Quercetin and Chrysin on 2,3,7,8-Tetrachlorodibenzo-p-dioxin Induced Hepatotoxicity in Rats. Environ. Toxicol. 2013, 28, 146–154. [Google Scholar] [CrossRef]
- Morita, K.; Tobiishi, K. Increasing Effect of Nori on the Fecal Excretion of Dioxin by Rats. Biosci. Biotechnol. Biochem. 2002, 66, 2306–2313. [Google Scholar] [CrossRef][Green Version]
- Pohjanvirta, R.; Tuomisto, J.; Vartiainen, T.; Rozman, K. Han/Wistar Rats Are Exceptionally Resistant to TCDD. Pharmacol. Toxicol. 1987, 60, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lindén, J.; Lensu, S.; Pohjanvirta, R. Effect of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) on Hormones of Energy Balance in a TCDD-Sensitive and a TCDD-Resistant Rat Strain. Int. J. Mol. Sci. 2014, 15, 13938–13966. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An up-to-date review on citrus flavonoids: Chemistry and benefits in health and diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Fernández-González, R.; Yebra-Pimentel, I.; Martinez-Carballo, E.; Simal-Gandara, J. A critical review about human exposure to polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) through foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 1590–1617. [Google Scholar] [CrossRef]
- Jeno, J.G.A.; Rathna, R.; Nakkeeran, E. Biological implications of dioxins/furans bioaccumulation in ecosystems. Environ. Pollut. Remediat. 2021, 395–420. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Casas, M. Interactions between environmental pollutants and dietary nutrients: Current evidence and implications in epidemiological research. J. Epidemiol. Community Health 2021, 75, 108–113. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Anticancer antioxidant regulatory functions of phytochemicals. Curr. Med. Chem. 2011, 18, 2315–2338. [Google Scholar] [CrossRef]
- Breinholt, V.; Lauridsen, S.T.; Dragsted, L.O. Differential effects of dietary flavonoids on drug metabolizing and antioxidant enzymes in female rats. Xenobiotica 1999, 29, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Tsutsumi, T.; Sasaki, K.; Nakamura, M.; Yoshida, T.; Maitani, T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry 2008, 69, 3117–3130. [Google Scholar] [CrossRef]
- Nakai, R.; Fukuda, S.; Kawase, M.; Yamashita, Y.; Ashida, H. Curcumin and its derivatives inhibit 2,3,7,8-tetrachloro-dibenzo-p-dioxin-induced expression of drug metabolizing enzymes through aryl hydrocarbon receptor-mediated pathway. Biosci. Biotechnol. Biochem. 2018, 82, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Goya-Jorge, E.; Jorge Rodríguez, M.E.; Veitía, M.S.I.; Giner, R.M. Plant occurring flavonoids as modulators of the aryl hydrocarbon receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef]
- Go, R.E.; Hwang, K.A.; Choi, K.C. Cytochrome P450 1 family and cancers. J. Steroid Biochem. Mol. Biol. 2015, 147, 24–30. [Google Scholar] [CrossRef]
- Jandacek, R.J.; Tso, P. Factors affecting the storage and excretion of toxic lipophilic xenobiotics. Lipids 2001, 36, 1289–1305. [Google Scholar] [CrossRef]
- Burdová, K. Selected Chemopreventive Compounds as Cytochrome P450 Inducers. Master’s Thesis, Charles University, Prague, Czech Republic, 2009. [Google Scholar]
- Kohda, N.; Inoue, S.; Noda, T.; Saito, T. Effects of a chitosan intake on the fecal excretion of dioxins and fat in rats. Biosci. Biotechnol. Biochem. 2012, 76, 1544–1548. [Google Scholar] [CrossRef][Green Version]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Health risks associated with organic pollutants in soils. In Soil Components and Human Health; Elsevier: Amsterdam, The Netherlands, 2018; pp. 575–657. [Google Scholar][Green Version]
- LaKind, J.S. Recent global trends and physiologic origins of dioxins and furans in human milk. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 510–524. [Google Scholar] [CrossRef]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Wang, Z.; Li, Y.; Yang, H. Toxicity assessment of dioxins and their transformation by-products from inferred degradation pathways. Sci. Total Environ. 2024, 937, 173416. [Google Scholar] [CrossRef]
- Van den Berg, M.; De Jongh, J.; Poiger, H.; Olson, J.R. The toxicokinetics and metabolism of polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) and their relevance for toxicity. Crit. Rev. Toxicol. 1994, 24, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, J.A. Measurement of Polychlorinated Biphenyls (PCBs) in Fish Tissue and Prediction of Toxicity. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2005. [Google Scholar]
- Kovacic, P.; Jacintho, J.D. Reproductive toxin’s pervasive theme of oxidative stress and electron transfer. Curr. Med. Chem. 2001, 8, 863–892. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic impact of flavonoid consumption in obesity: From central to peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Cooperation and Development (OECD). Guideline for Testing of Chemicals No. 417: Toxicokinetics; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). Guidance Document No. 194 on the Validation of (Quantitative) Analytical Methods; OECD: Paris, France, 2014. [Google Scholar]
- Ministry of Health. Guidance on Pre-Clinical and Clinical Trials of Oriental Medicines and Herbal Medicines; Issued under Decision No. 141/QĐ-K2ĐT dated 27 October 2015; Medical Publishing House: Hanoi, Vietnam, 2015; pp. 13–17. (In Vietnamese) [Google Scholar]
- ISO 18073:2004; Water Quality-Determination of Tetra- to Octa-Chlorinated Dioxins and Furans-Method Using Isotope Dilution HRGC/HRMS. International Organization for Standardization: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/31687.html (accessed on 16 September 2025).

| Rat Group (n = 10) | Body Weight (g) | pt-s (Paired t-Test) | |
|---|---|---|---|
| Before Experiment | After Experiment | ||
| Dioxin Group | 163.8 ± 14.34 | 224.2 ± 15.48 | 0.005 |
| RUT Group | 164.8 ± 11.75 | 218.9 ± 12.80 | 0.005 |
| Control Group | 167.4 ± 10.51 | 218.0 ± 12.95 | 0.005 |
| Parameter | Group (n = 10) | Time | pt-s (Paired t-Test) | |
|---|---|---|---|---|
| Before Study | After Study | |||
| Red Blood Cells (T/L) | Dioxin Group | 7.99 ± 0.61 | 6.76 ± 1.04 * | 0.017 |
| RUT Group | 7.61 ± 0.48 | 7.56 ± 1.48 | >0.05 | |
| Control Group | 8.14 ± 0.52 | 7.85 ± 0.54 | >0.05 | |
| Hemoglobin (g/L) | Dioxin Group | 148.7 ± 10.60 | 121.3 ± 12.54 *** | 0.005 |
| RUT Group | 156.5 ± 12.26 | 126.2 ± 31.72 * | 0.024 | |
| Control Group | 146.4 ± 10.94 | 154.33 ± 10.2 | >0.05 | |
| Platelets (G/L) | Dioxin Group | 661.6 ± 106.28 | 525.6 ± 138.94 ** | 0.028 |
| RUT Group | 729.2 ± 104.03 | 557.8 ± 195.98 * | >0.05 | |
| Control Group | 721.7 ± 112.57 | 739.2 ± 107.62 | >0.05 | |
| White Blood Cells (G/L) | Dioxin Group | 15.33 ± 4.73 | 10.08 ± 4.08 | >0.05 |
| RUT Group | 13.47 ± 4.30 | 7.76 ± 2.98 | 0.028 | |
| Control Group | 15.21 ± 4.73 | 10.97 ± 4.45 | >0.05 | |
| Parameter | Group (n = 10) | Time | pt-s (Paired t-Test) | |
|---|---|---|---|---|
| Before Study | After Study | |||
| AST (u/L) | Dioxin Group | 122.21 ± 26.81 | 169.86 ± 95.34 | >0.05 |
| RUT Group | 118.86 ± 15.32 | 139.56 ± 60.93 | >0.05 | |
| Control Group | 122.02 ± 26.74 | 120.67 ± 19.43 | >0.05 | |
| ALT (u/L) | Dioxin Group | 43.89 ± 9.48 | 60.96 ± 8.24 * | 0.009 |
| RUT Group | 47.38 ± 12.11 | 59.7 ± 7.38 * | 0.009 | |
| Control Group | 47.64 ± 9.74 | 47.59 ± 10.74 | >0.05 | |
| GGT (u/L) | Dioxin Group | 3.1 ± 2.08 | 3.2 ± 1.81 | >0.05 |
| RUT Group | 3.0 ± 1.70 | 5.5 ± 3.75 | >0.05 | |
| Control Group | 3.8 ± 1.40 | 3.4 ± 2.32 | >0.05 | |
| Parameter | Group (n = 10) | Time | pt-s (Paired t-Test) | |
|---|---|---|---|---|
| Before Study | After Study | |||
| Total Protein (g/L) | Dioxin Group | 72.48 ± 5.94 | 62.87 ± 2.81 ** | 0.009 |
| RUT Group | 68.87 ± 7.49 | 62.64 ± 4.01 * | 0.037 | |
| Control Group | 70.3 ± 6.34 | 67.03 ± 5.08 | >0.05 | |
| Total Bilirubin (µmol/L) | Dioxin Group | 3.08 ± 1.01 | 1.31 ± 0.67 ** | 0.007 |
| RUT Group | 3.20 ± 0.99 | 1.45 ± 0.62 ** | 0.005 | |
| Control Group | 3.33 ± 1.26 | 3.65 ± 1.00 | >0.05 | |
| Glucose (mmol/L) | Dioxin Group | 6.03 ± 0.94 | 9.23 ± 1.51 *** | 0.005 |
| RUT Group | 5.37 ± 0.96 | 9.55 ± 2.78 *** | 0.009 | |
| Control Group | 5.53 ± 0.95 | 5.56 ± 1.02 | >0.05 | |
| Parameter | Group (n = 10) | Time | pt-s (Paired t-Test) | |
|---|---|---|---|---|
| Before Study | After Study | |||
| Cholesterol (µmol/L) | Dioxin Group | 1.40 ± 0.36 | 1.14 ± 0.16 | >0.05 |
| RUT Group | 1.29 ± 0.39 | 1.19 ± 0.07 | >0.05 | |
| Control Group | 1.42 ± 0.33 | 1.39 ± 0.46 | >0.05 | |
| Triglycerides (µmol/L) | Dioxin Group | 1.69 ± 0.37 | 0.88 ± 0.23 * | 0.005 |
| RUT Group | 1.55 ± 0.67 | 1.39 ± 0.32 | >0.05 | |
| Control Group | 1.64 ± 0.66 | 1.39 ± 0.53 | >0.05 | |
| Parameter | Group (n = 10) | Time | pt-s (Paired t-Test) | |
|---|---|---|---|---|
| Before Study | After Study | |||
| Urea (mmol/L) | Dioxin Group | 3.88 ± 0.88 | 6.25 ± 1.18 *** | 0.012 |
| RUT Group | 3.41 ± 1.01 | 7.04 ± 0.80 *** | 0.005 | |
| Control Group | 3.40 ± 1.02 | 3.78 ± 0.94 | >0.05 | |
| Creatinine (µmol/L) | Dioxin Group | 36.29 ± 11.66 | 45.48 ± 3.17 * | 0.037 |
| RUT Group | 35.08 ± 14.53 | 43.80 ± 4.23 * | >0.05 | |
| Control Group | 32.27 ± 15.24 | 30.23 ± 13.73 | >0.05 | |
| Compound | MQL (pg/g) | Dioxin Group | RUT Group | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| 2378-TCDD | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 12378-PeCDD | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 123478-HxCDD | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 123678-HxCDD | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 123789-HxCDD | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 1234678-HpCDD | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| OCDD | 1 | <1 | <1 | <1 | <1 |
| 2378-TCDF | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 12378-PeCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 23478-PeCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 123478-HxCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 123678-HxCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 123789-HxCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 234678-HxCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 1234678-HpCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| 1234789-HpCDF | 0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Octachlorodibenzofuran (OCDF) | 1 | <1 | <1 | <1 | <1 |
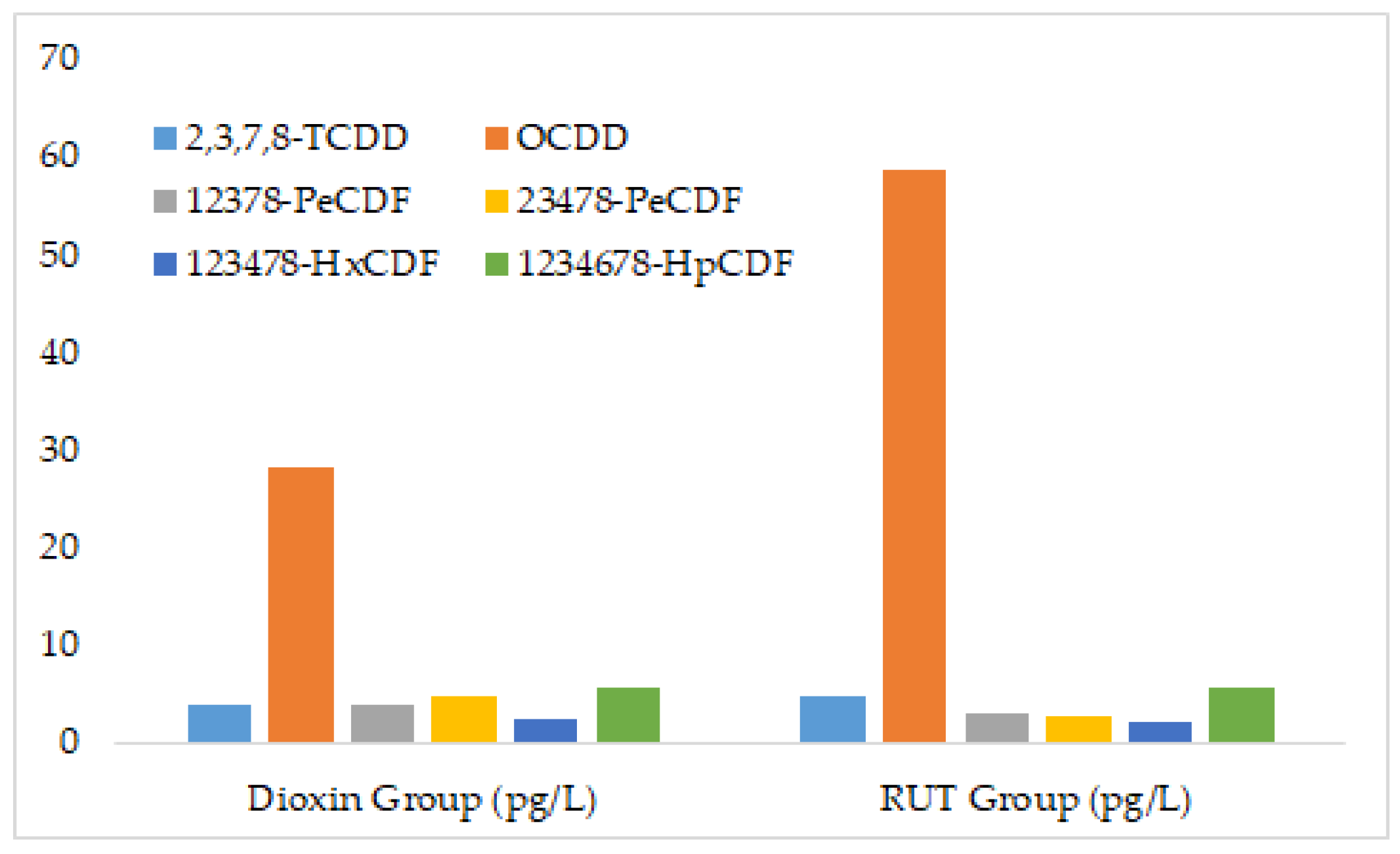
| Compound | Dioxin Group | RUT Group |
|---|---|---|
| 2378-TCDD | 3.79 | 4.68 |
| 12378-PeCDD | ND | ND |
| 123478-HxCDD | ND | ND |
| 123678-HxCDD | ND | ND |
| 123789-HxCDD | ND | ND |
| 1234678-HpCDD | ND | ND |
| OCDD | 28.1 | 58.6 |
| 2378-TCDF | 29 | 17.4 |
| 12378-PeCDF | 3.74 | 3.13 |
| 23478-PeCDF | 4.88 | 2.57 |
| 123478-HxCDF | 2.27 | 2.05 |
| 123678-HxCDF | ND | ND |
| 123789-HxCDF | ND | ND |
| 234678-HxCDF | 2.55 | 2.09 |
| 1234678-HpCDF | 5.73 | 5.61 |
| 1234789-HpCDF | ND | ND |
| OCDF | ND | ND |
| Compound | MQL (pg/L) | Dioxin Group | RUT Group |
|---|---|---|---|
| 2378-TCDD | 0.012 | <0.012 | <0.012 |
| 12378-PeCDD | 0.009 | <0.009 | <0.009 |
| 123478-HxCDD | 0.019 | <0.019 | <0.019 |
| 123678-HxCDD | 0.019 | <0.019 | 0.042 |
| 123789-HxCDD | 0.018 | <0.018 | <0.018 |
| 1234678-HpCDD | 0.016 | <0.016 | 0.396 |
| OCDD | 0.034 | <0.034 | 0.599 |
| 2378-TCDF | 0.013 | 0.053 | 0.04 |
| 12378-PeCDF | 0.023 | <0.023 | 0.072 |
| 23478-PeCDF | 0.028 | <0.028 | <0.028 |
| 123478-HxCDF | 0.017 | <0.017 | 0.078 |
| 123678-HxCDF | 0.027 | <0.027 | 0.063 |
| 123789-HxCDF | 0.017 | <0.017 | <0.017 |
| 234678-HxCDF | 0.007 | <0.007 | <0.008 |
| 1234678-HpCDF | 0.015 | <0.015 | 0.257 |
| 1234789-HpCDF | 0.026 | <0.026 | <0.026 |
| OCDF | 0.03 | <0.030 | <0.030 |
| Compound | MQL (pg/g) | Dioxin Group | RUT Group |
|---|---|---|---|
| 2378-TCDD | 1.24 | <1.24 | <1.24 |
| 12378-PeCDD | 0.91 | <0.91 | <0.91 |
| 123478-HxCDD | 1.93 | <1.93 | <1.93 |
| 123678-HxCDD | 1.89 | <1.89 | 92.7 |
| 123789-HxCDD | 1.81 | <1.81 | <1.81 |
| 1234678-HpCDD | 1.63 | <1.63 | 883 |
| OCDD | 3.42 | <3.42 | 1335 |
| 2378-TCDF | 1.31 | 124 | 88.6 |
| 12378-PeCDF | 2.3 | <2.30 | 161 |
| 23478-PeCDF | 2.79 | <2.79 | <2.79 |
| 123478-HxCDF | 1.74 | <1.74 | 174 |
| 123678-HxCDF | 2.72 | <2.72 | 140 |
| 123789-HxCDF | 0.74 | <0.74 | <0.74 |
| 234678-HxCDF | 1.69 | <1.69 | <1.69 |
| 1234678-HpCDF | 1.53 | <1.53 | 573 |
| 1234789-HpCDF | 2.59 | <2.59 | <2.59 |
| OCDF | 2.98 | <2.98 | <2.98 |
| Congener | Chlorine Atoms | Log Kow | Relative Stability | Lipid Solubility | Notable Feature |
|---|---|---|---|---|---|
| 2,3,7,8-TCDD | 4 | ~6.8 | High | Moderate | Prototype congener, most toxic |
| PeCDD | 5 | ~7.2 | Very high | High | Strong lipid affinity |
| HxCDD | 6 | ~7.6 | Very high | High | Increased persistence |
| OCDD | 8 | ~8.2 | Extremely high | Very high | Strong lipid accumulation, long half-life |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dat, N.T.; Nam, V.D.; Anh, H.L.T.; Giang, D.H.; Luyen, N.T.; Thang, H.D.; Ha, N.M.; Minh, T.N. Rutin Facilitates Dioxin Elimination and Attenuates Systemic Toxicity in a Wistar Rat Model. Stresses 2025, 5, 59. https://doi.org/10.3390/stresses5030059
Dat NT, Nam VD, Anh HLT, Giang DH, Luyen NT, Thang HD, Ha NM, Minh TN. Rutin Facilitates Dioxin Elimination and Attenuates Systemic Toxicity in a Wistar Rat Model. Stresses. 2025; 5(3):59. https://doi.org/10.3390/stresses5030059
Chicago/Turabian StyleDat, Nguyen Tien, Vu Duc Nam, Hoang Le Tuan Anh, Do Hoang Giang, Nguyen Thi Luyen, Hoang Dac Thang, Nguyen Minh Ha, and Truong Ngoc Minh. 2025. "Rutin Facilitates Dioxin Elimination and Attenuates Systemic Toxicity in a Wistar Rat Model" Stresses 5, no. 3: 59. https://doi.org/10.3390/stresses5030059
APA StyleDat, N. T., Nam, V. D., Anh, H. L. T., Giang, D. H., Luyen, N. T., Thang, H. D., Ha, N. M., & Minh, T. N. (2025). Rutin Facilitates Dioxin Elimination and Attenuates Systemic Toxicity in a Wistar Rat Model. Stresses, 5(3), 59. https://doi.org/10.3390/stresses5030059








