Characterization of Sugarcane Germplasm for Physiological and Agronomic Traits Associated with Drought Tolerance Across Various Soil Types
Abstract
1. Introduction
2. Results
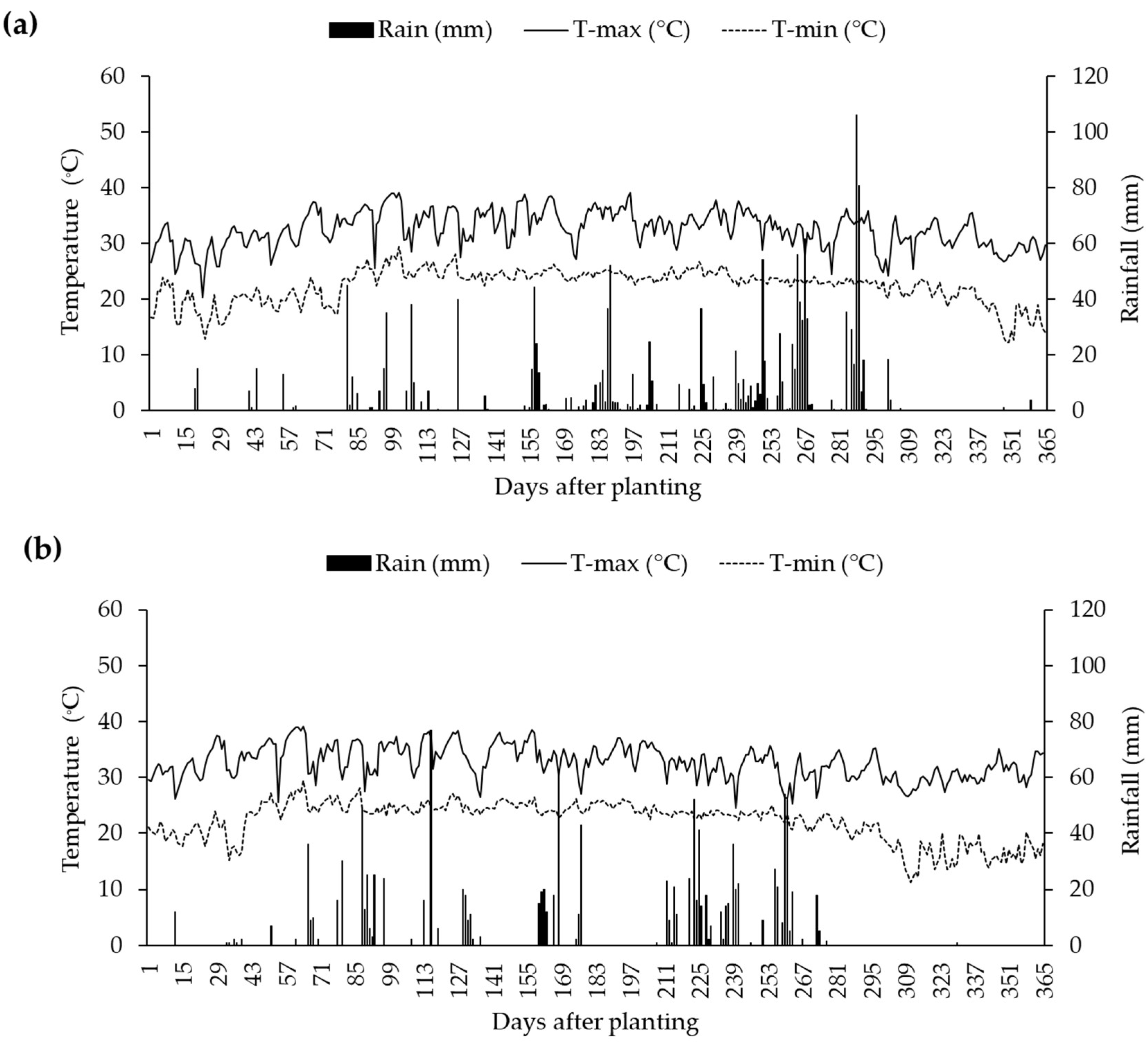
2.1. Soil Moisture Content and Meteorological Conditions
2.2. Physiological Variations in Progressive Drought Stress and Recovery
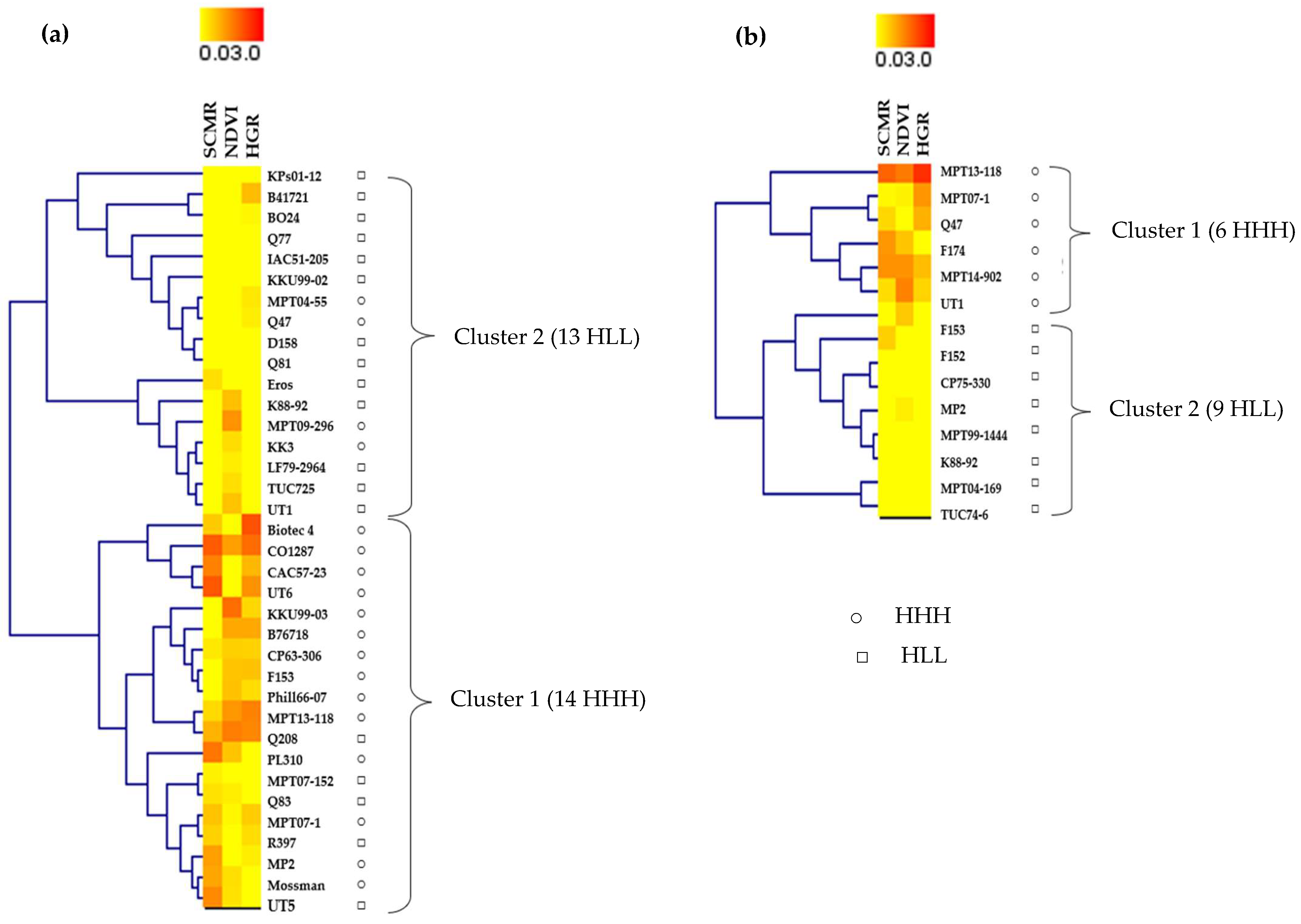
2.3. Hierarchical Clustering of Sugarcane Genotypes Based on Physiological Traits
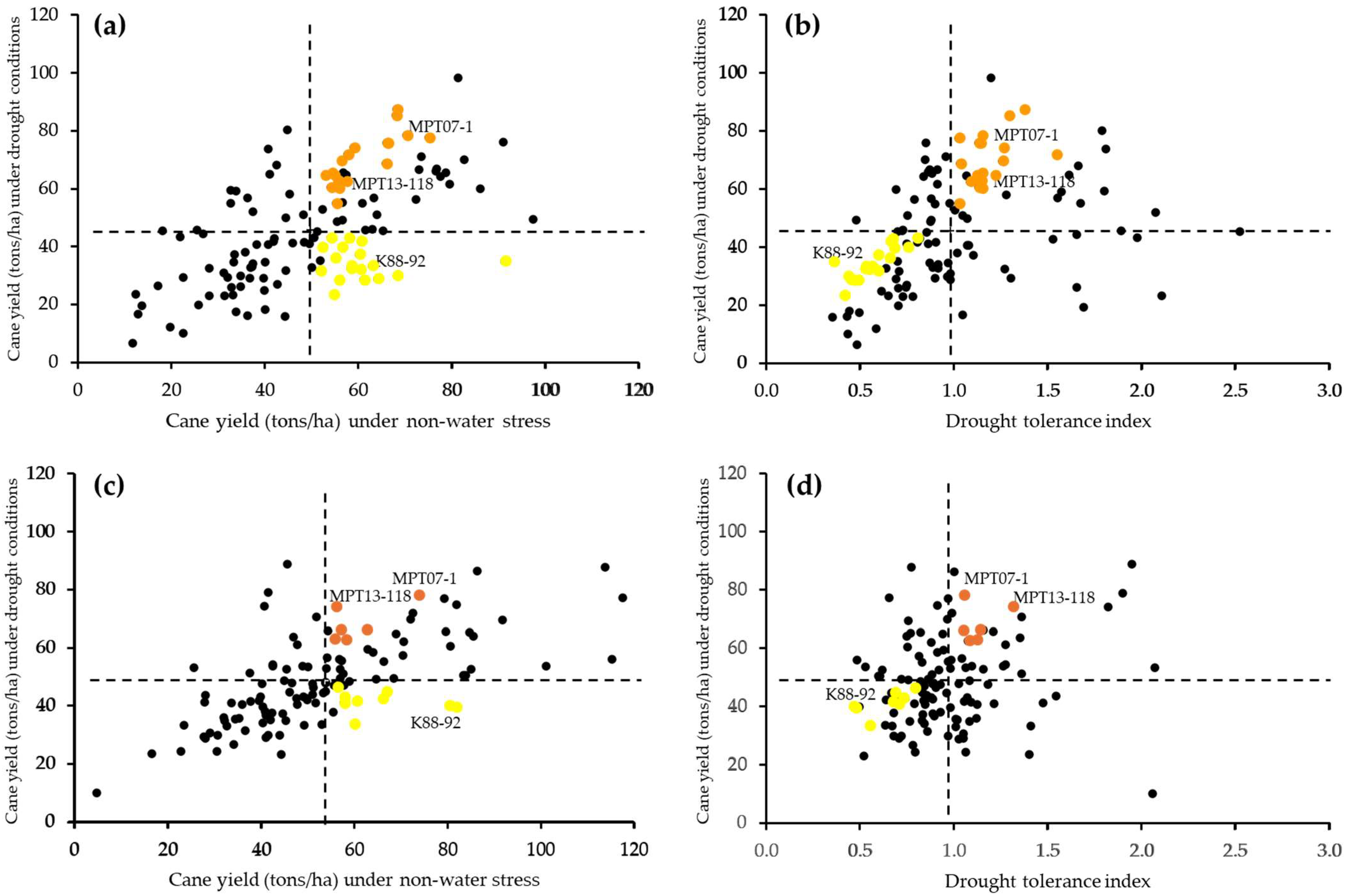
2.4. Relationships Among Cane Yield and Physiological Traits
3. Discussion
3.1. Physiological Variations in Progressive Drought Stress and Recovery
3.2. Hierarchical Clustering of Sugarcane Genotypes Based on Physiological Traits
3.3. Relationships Among Cane Yield and Physiological Traits
4. Materials and Methods
4.1. Plant Materials and Stress Treatment
4.2. Meteorological Condition Soil Moisture Measurements
4.3. Morpho-Physiological Measurements
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| % | Percentage |
| ΔH | Difference in plant height between the two measurements |
| ΔT | Time interval between the two measurements |
| a.m. | Ante meridiem |
| °C | Degree celsius |
| cm | Centimeter |
| CT | Control condition |
| CY | Cane yield |
| DS | Drought condition |
| DTI | Drought tolerance index |
| Fv/Fm | Maximum quantum efficiency of photosystem II photochemistry or chlorophyll fluorescence |
| HGR | Height growth rate |
| HHH | High cane yield under irrigated conditions, high cane yield under rainfed conditions, and a high drought tolerance index |
| HLL | High cane yield under irrigated conditions, low cane yield under rainfed conditions, and a low drought tolerance index |
| km | Kilo metters |
| L1 | Sandy loam soil |
| L2 | Loam soil |
| LR | Leaf rolling |
| LSD | Least significant difference test |
| MAP | Months after planting |
| NDVI | Normalized difference vegetation index |
| mm | Millimeters |
| p.m. | Post-meridiem |
| RHG | Relative height growth rate |
| SCMR | SPAD chlorophyll meter reading |
| SMC | Soil moisture contents |
| ton/ha | Tons per hectare |
| TVD | Top visible dewlap |
References
- Khonghintaisong, J.; Onkaeo, A.; Songsri, P.; Jongrungklang, N. Water use efficiency characteristics and their contributions to yield in diverse sugarcane genotypes with varying drought resistance levels under different field irrigation conditions. Agriculture 2024, 14, 1952. [Google Scholar] [CrossRef]
- Singh, S.K.; Katiyar, A. The economic importance of sugarcane: An imperative grass of Indian sub-continent. J. Exp. Zool. India 2016, 19, 401–406. [Google Scholar]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Growth and physiological patterns of sugarcane cultivars to mimic drought conditions in late rainy season system. Naresuan Univ. J. Sci. Technol. 2017, 2, 102–112. [Google Scholar]
- Kyei-Mensah, C.; Kyerematen, R.; Adu-Acheampong, S. Impact of rainfall variability on crop production within the Worobong ecological area of Fanteakwa district, Ghana. Adv. Agric. 2019, 7930127. [Google Scholar] [CrossRef]
- Wu, Q.; Li, A.; Zhao, P.; Xia, H.; Zhang, Y.; Que, Y. Theory to practice: A success in breeding sugarcane variety YZ08–1609 known as the King of Sugar. Front. Plant Sci. 2024, 15, 1413108. [Google Scholar] [CrossRef]
- Tippayawat, A.; Jogloy, S.; Vorasoot, N.; Jongrungklang, N.; Kimbeng, C.A.; Jifon, J.L.; Khonghintaisong, J.; Songsri, P. Timing and duration of drought differentially affect growth and yield components among sugarcane genotypes. Plants 2025, 14, 796. [Google Scholar] [CrossRef]
- Manivong, P.; Bourgois, E. White Paper: Thai Sugarcane Sector and Sustainability. Available online: https://bonsucro.com/white-paper-thai-sugarcane-industry-sustainability-released/ (accessed on 25 August 2025).
- Reyes, J.A.O.; Carpentero, A.S.; Santos, P.J.A.; Delfin, E.F. Effects of water regime, genotype, and formative stages on the agro-physiological response of sugarcane (Saccharum officinarum L.) to drought. Plants 2020, 9, 661. [Google Scholar] [CrossRef]
- Jangpromma, N.; Thammasirirak, S.; Jaisil, P.; Songsri, P. Effects of drought and recovery from drought stress on above ground and root, root growth, and water use efficiency in sugarcane (Saccharum officinarum L.). Aust. J. Crop Sci. 2012, 6, 1298–1304. [Google Scholar]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araujo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.J.B.; dos Santos, D.M.M.; Ferraudo, A.S.; Carlin, S.D.; Silva, M.D.A. Biochemical and physiological responses of sugarcane cultivars to soil water deficiencies. Sci. Agric. 2011, 68, 469–476. [Google Scholar] [CrossRef]
- Bunce, J. Leaf gas exchange and photosystem II fluorescence responses to CO2 cycling. Plants 2023, 12, 1620. [Google Scholar] [CrossRef]
- Wirojsirasak, W.; Songsri, P.; Jongrungklang, N.; Tangphatsornruang, S.; Klomsa-ard, P.; Ukoskit, K. Determination of morpho-physiological traits for assessing drought tolerance in sugarcane. Plants 2024, 13, 1072. [Google Scholar] [CrossRef]
- da Graca, J.; Rodrigues, F.; Farias, J.; Oliveira, M.; Hoffmann-Campo, C.; Zingaretti, S. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar] [CrossRef]
- Supavetch, S. In-season yield prediction and monitoring of sugarcane using cumulative growth of normalized difference vegetation index. In Proceedings of the 2022 IEEE Mediterranean and Middle-East Geoscience and Remote Sensing Symposium (M2GARSS), Istanbul, Turkey, 7–9 March 2022; pp. 173–176. [Google Scholar]
- Gunnula, W.; Kosittrakun, M.; Righetti, T.L.; Weerathaworn, P. Relationship between MODIS NDVI and rainfall patterns for sugarcane farmers’ fields in northeastern Thailand. Aust. J. Crop Sci. 2011, 5, 1845–1851. [Google Scholar]
- Janpuk, T.; Pinata, P.; Klomsa-Ard, P.; Jongrungklang, N.; Nanta, N.; Bootprom, N.; Songsri, P. Evaluation of Genetic Diversity in Sugarcane Germplasm Based on Physiological and Agronomic Traits. Kaen Kaset=Khon Kaen Agric. J. 2019, 47, 761–772. [Google Scholar]
- Zhou, L.; Wang, S.; Chi, Y.; Li, Q.; Huang, K.; Yu, Q. Responses of photosynthetic parameters to drought in subtropical forest ecosystem of China. Sci. Rep. 2015, 5, 18254. [Google Scholar] [CrossRef]
- Kumar, R.A.; Vasantha, S.; Tayade, A.S.; Anusha, S.; Geetha, P.; Hemaprabha, G. Physiological efficiency of sugarcane clones under water-limited conditions. Trans. ASABE 2020, 63, 133–140. [Google Scholar] [CrossRef]
- Zhao, D.; Irey, M.; LaBorde, C.; Hu, C.-J. Physiological and yield characteristics of 18 sugarcane genotypes grown on sand soil. Crop Sci. 2019, 59, 2741–2751. [Google Scholar] [CrossRef]
- Mubeen, M.; Ahmed, M.; Ijaz, M.; Mehmood, K.; Javed, H.U.; Ali, Q.; Batool, A. Comparative analysis of growth and physiological responses of sugarcane elite genotypes to water stress and sandy loam soils. Plants 2023, 12, 2759. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A review of environmental droughts: Increased risk under global warming. Earth Sci. Rev. 2020, 200, 102953. [Google Scholar] [CrossRef]
- Faranda, D.; Vrac, M.; Yiou, P. ClimaMeter: Contextualizing extreme weather in a changing climate. Weather Clim. Dyn. 2024, 5, 959–978. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Contiliani, D.F.; Nebo, J.F.C.d.O.; Ribeiro, R.V.; Landell, M.G.d.A.; Pereira, T.C.; Ming, R.; Figueira, A.; Creste, S. Drought-triggered leaf transcriptional responses disclose key molecular pathways underlying leaf water use efficiency in sugarcane (Saccharum spp.). Front. Plant Sci. 2023, 14, 1182461. [Google Scholar] [CrossRef]
- Khuynh, B.T.; Thang, V.N.; Chinh, V.D.; Thom, P.T. Growth and physiological responses of sugarcane to drought stress at an early growth stage. Vietnam J. Agric. Sci. 2019, 2, 451–460. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Kaura, V.; Malhotra, P.K.; Mittal, A.; Sanghera, G.S.; Kaur, N.; Bhardwaj, R.D.; Cheema, R.S.; Kaur, G. Physiological, biochemical, and gene expression responses of sugarcane under cold, drought and salt stresses. J. Plant Growth Regul. 2023, 42, 6367–6376. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Jifon, J.L.; Da Silva, J.A.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef]
- Abbas, S.R.; Ahmad, S.D.; Sabir, S.M.; Shah, A.H. Detection of drought tolerant sugarcane genotypes (Saccharum officinarum) using lipid peroxidation, antioxidant activity, glycine-betaine and proline contents. J. Soil Sci. Plant Nutr. 2014, 14, 233–243. [Google Scholar] [CrossRef]
- Dinh, H.T.; Watanable, K.; Takaragawa, H.; Kawamitsu, Y. Effects of drought stress at early growth stage on response of sugarcane to different nitrogen application. Sugar Tech 2018, 20, 420–430. [Google Scholar] [CrossRef]
- Rao, M.; Rao, P.; Charumathi, C.; Adilakshmi, A.; Devi, C.; Bharathalakshmi, B. Sugarcane Clones for soil moisture stress/drought conditions of Andhra Pradesh. Theor. Biol. Forum 2023, 12, 400–407. [Google Scholar]
- Khuimphukhieo, I.; Bhandari, M.; Enciso, J.; Da Silva, J.A. Assessing drought stress of sugarcane cultivars using Unmanned Vehicle System (UAS)-Based vegetation indices and physiological parameters. Remote Sens. 2024, 16, 1433. [Google Scholar] [CrossRef]
- Masoabi, M.; Snyman, S.; Van Der Vyver, C. Characterisation of an ethyl methanesulfonate-derived drought-tolerant sugarcane mutant line. Ann. Appl. Biol. 2023, 182, 343–360. [Google Scholar] [CrossRef]
- Devi, K.; Gomathi, R.; Kumar, R.A.; Manimekalai, R.; Selvi, A. Field tolerance and recovery potential of sugarcane varieties subjected to drought. Indian J. Plant Physiol. 2018, 23, 271–282. [Google Scholar] [CrossRef]
- De Almeida Silva, M.; Jifon, J.L.; Sharma, V.; Da Silva, J.a.G.; Caputo, M.M.; Damaj, M.B.; Guimarães, E.R.; Ferro, M.I.T. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Tech 2011, 13, 191–197. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yang, S.L.; Dao, J.M.; Deng, J.; Shahzad, A.N.; Fan, X.; Li, R.D.; Quan, Y.J.; Bukhari, S.A.H.; Zeng, Z.H. Drought-induced alterations in photosynthetic, ultrastructural and biochemical traits of contrasting sugarcane genotypes. PLoS ONE 2020, 15, e0235845. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Understanding growth rate patterns among different drought resistant sugarcane cultivars during plant and ratoon crops encountered water deficit at early growth stage under natural field conditions. Agronomy 2021, 11, 2083. [Google Scholar] [CrossRef]
- De Almeida Silva, M.; Jifon, J.L.; Santos, C.M.D.; Jadoski, C.J.; Da Silva, J.a.G. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz. Arch. Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Li, D.M.; Guo, D.J.; Rajput, V.D.; Chen, G.L.; Barakhov, A.; Minkina, T.M.; Li, Y.R. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- Sultana, M.S.; Gazi, M.Y.; Mia, M.B. Multiple indices based agricultural drought assessment in the northwestern part of Bangladesh using geospatial techniques. Environ. Chall. 2021, 4, 100120. [Google Scholar] [CrossRef]
- Balbaa, M.G.; Osman, H.T.; Kandil, E.E.; Javed, T.; Lamlom, S.F.; Ali, H.M.; Kalaji, H.M.; Wróbel, J.; Telesinski, A.; Brysiewicz, A.; et al. Determination of morpho-physiological and yield traits of maize inbred lines (Zea mays L.) under optimal and drought stress conditions. Front. Plant Sci. 2022, 13, 959203, Erratum in Front. Plant Sci. 2022, 13, 1069938. [Google Scholar] [CrossRef]
- Endres, L.; Santos, C.M.D.; Souza, G.V.D.; Menossi, M.; Santos, J.C.M.D. Morphological changes recorded in different phenophases of sugarcane plants subjected to water stress in tropical field conditions. Aust. J. Crop Sci. 2018, 12, 1041–1050. [Google Scholar] [CrossRef]
- Harakotr, P.; Sornpha, W.; Khonghintaisong, J.; Gonkhamdee, S.; Songsri, P.; Jongrungklang, N. Correlation Between Rapid Measurement and Leaf Chlorophyll Content of Various Sugarcane Genotypes at Different Growth Phases. Asian J. Plant Sci. 2024, 23, 367–376. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Lean, F.L.; Ismail, M.; Berahim, Z.; Rahman, M.; Islam, M. Physiological and molecular characterization of drought responses and screening of drought tolerant rice varieties. Biosci. J. 2015, 31, 709–718. [Google Scholar] [CrossRef]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Muhammad, N.; Farooq Ahmad, K.; Farasat, S. Divergence analysis and association of some economical characters of sugarcane (Saccharum officinarum L.). J. Plant Breed. Genet. 2013, 1, 1–6. [Google Scholar]
- Carretillo Moctezuma, C.D.; Guzmán Martínez, M.; Godínez-Jaimes, F.; García-Preciado, J.C.; Reyes Carreto, R.; Terrones Salgado, J.; Pérez Arriaga, E. Growth Curve Models and Clustering Techniques for Studying New Sugarcane Hybrids. AgriEngineering 2025, 7, 114. [Google Scholar] [CrossRef]

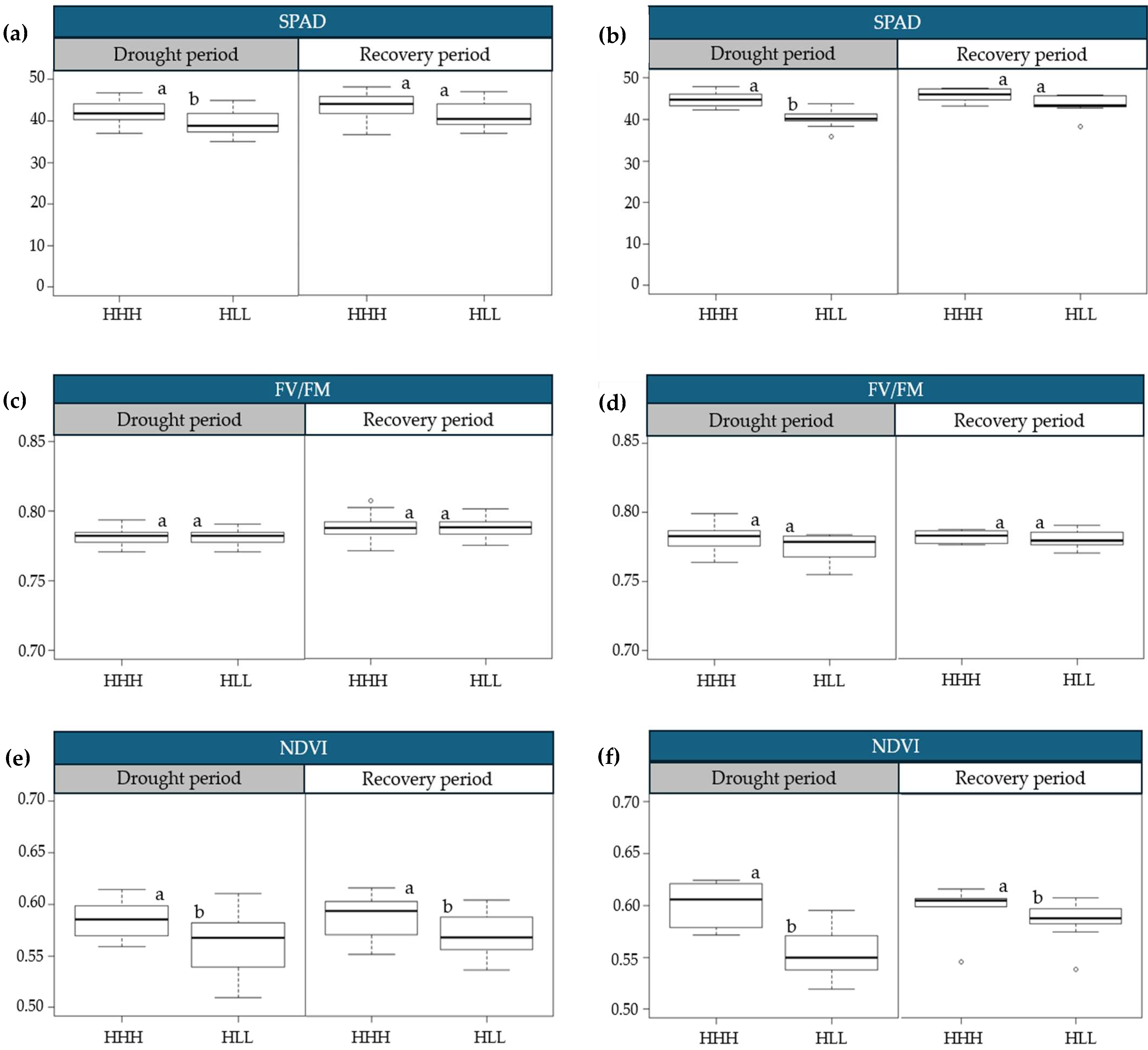
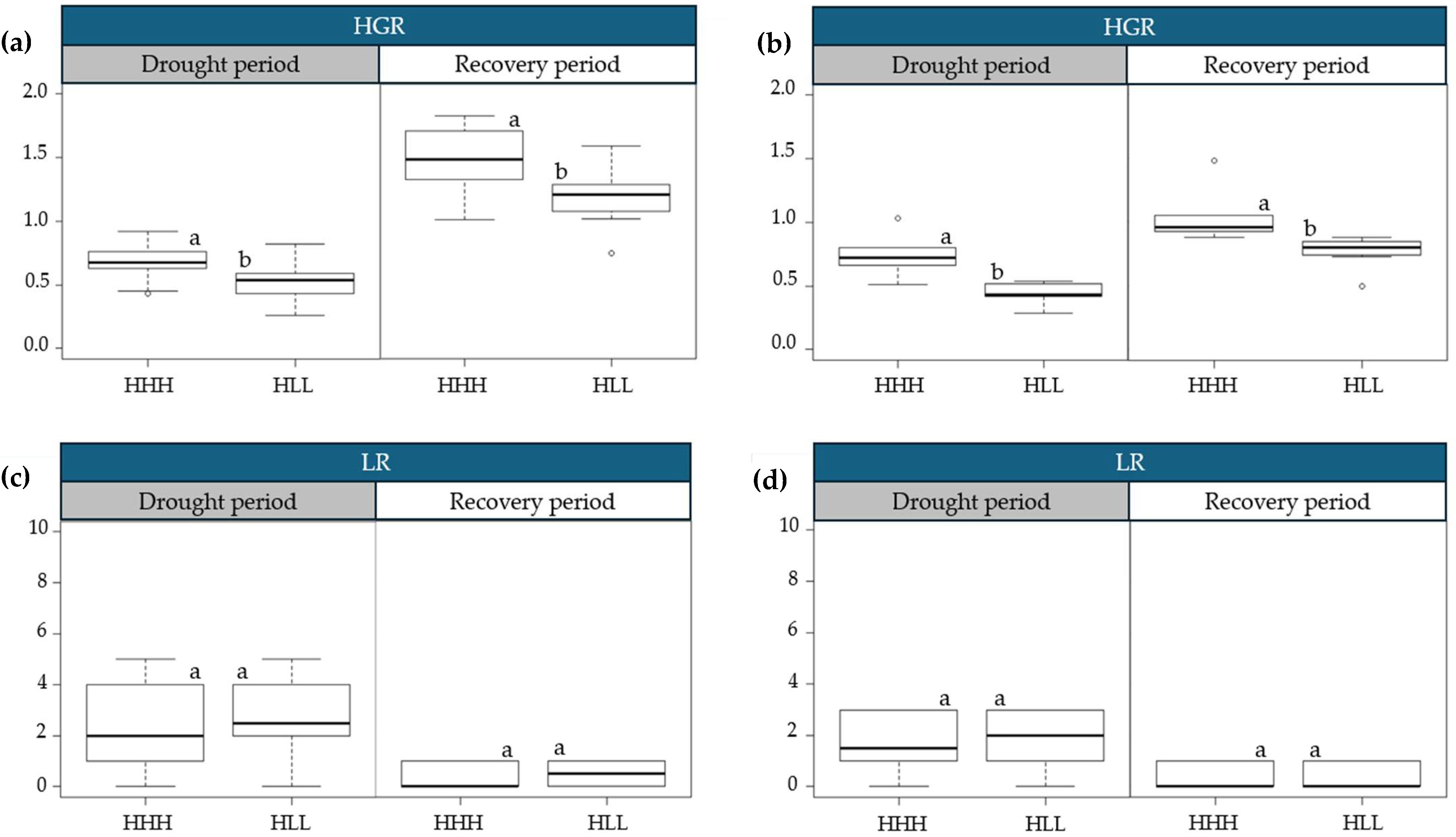

| Cluster | Drought Period | Recovery Period | Yield (ton/ha) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCMR | NDVI | HGR | SCMR | NDVI | HGR | |||||||||
| L1 | L2 | L1 | L2 | L1 | L2 | L1 | L2 | L1 | L2 | L1 | L2 | L1 | L2 | |
| 1 | 42.09 a | 44.83 a | 0.5849 a | 0.6013 a | 0.68 a | 0.75 a | 43.61 a | 45.71 a | 0.5904 a | 0.6115 a | 1.49 a | 1.01 a | 70.11 a | 68.34 a |
| 2 | 39.46 b | 40.19 b | 0.5631 b | 0.5565 b | 0.52 b | 0.45 b | 41.57 a | 43.53 a | 0.5705 b | 0.5849 b | 1.21 b | 0.77 b | 34.35 b | 39.27 b |
| Difference (%) | (−) 6.25 | (−) 10.35 | (−) 3.71 | (−) 7.45 | (−) 23.41 | (−) 40.36 | (−) 4.67 | (−) 4.77 | (−) 3.37 | (−) 4.34 | (−) 18.79 | (−) 23.51 | (−) 51.01 | (−) 42.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laotongkam, P.; Jongrungklang, N.; Banterng, P.; Klomsa-ard, P.; Wirojsirasak, W.; Songsri, P. Characterization of Sugarcane Germplasm for Physiological and Agronomic Traits Associated with Drought Tolerance Across Various Soil Types. Stresses 2025, 5, 57. https://doi.org/10.3390/stresses5030057
Laotongkam P, Jongrungklang N, Banterng P, Klomsa-ard P, Wirojsirasak W, Songsri P. Characterization of Sugarcane Germplasm for Physiological and Agronomic Traits Associated with Drought Tolerance Across Various Soil Types. Stresses. 2025; 5(3):57. https://doi.org/10.3390/stresses5030057
Chicago/Turabian StyleLaotongkam, Phunsuk, Nakorn Jongrungklang, Poramate Banterng, Peeraya Klomsa-ard, Warodom Wirojsirasak, and Patcharin Songsri. 2025. "Characterization of Sugarcane Germplasm for Physiological and Agronomic Traits Associated with Drought Tolerance Across Various Soil Types" Stresses 5, no. 3: 57. https://doi.org/10.3390/stresses5030057
APA StyleLaotongkam, P., Jongrungklang, N., Banterng, P., Klomsa-ard, P., Wirojsirasak, W., & Songsri, P. (2025). Characterization of Sugarcane Germplasm for Physiological and Agronomic Traits Associated with Drought Tolerance Across Various Soil Types. Stresses, 5(3), 57. https://doi.org/10.3390/stresses5030057









