The Invisible Influence: Can Endocrine Disruptors Reshape Behaviors Across Generations?
Abstract
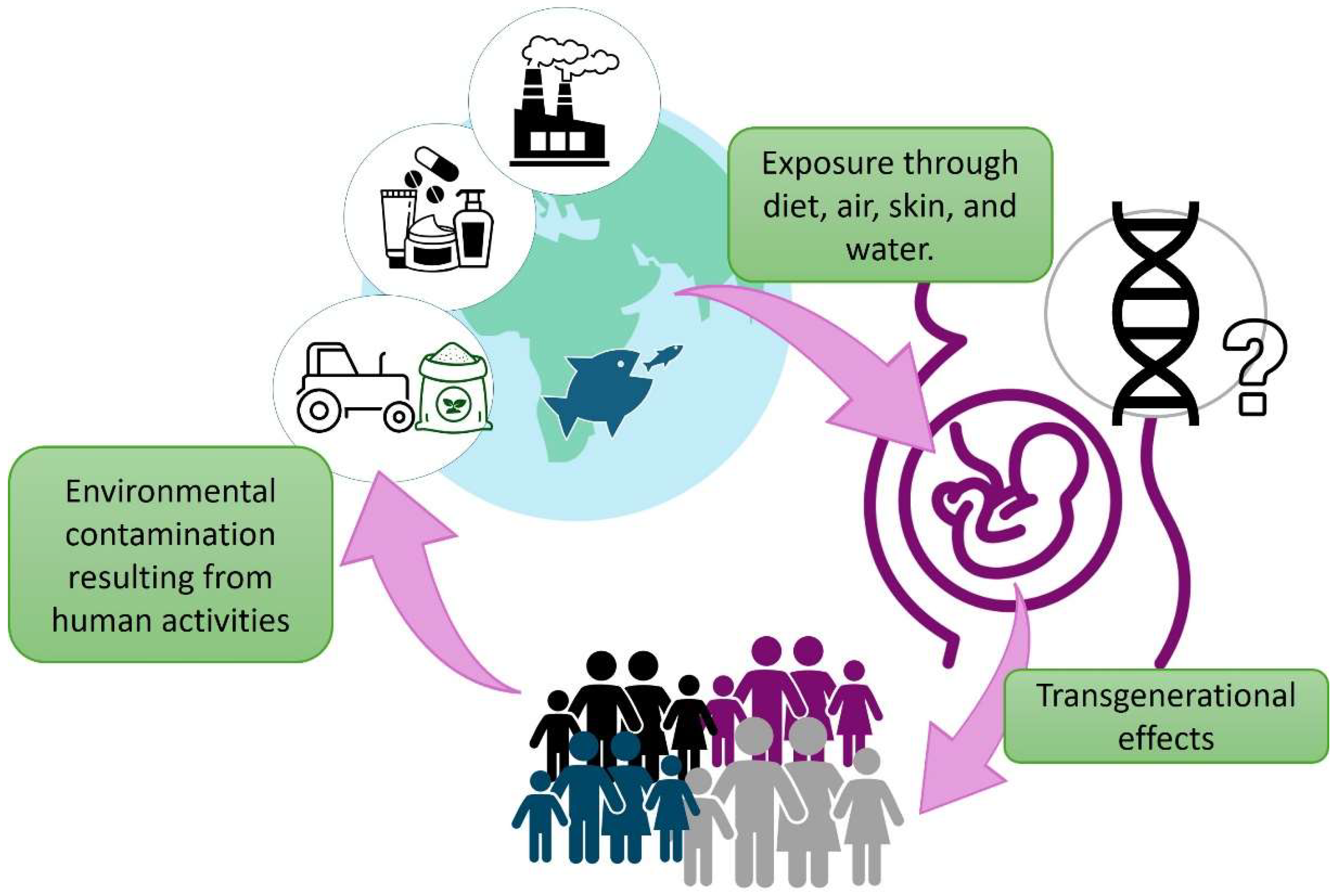
1. Introduction
2. Targets of Endocrine-Disrupting Chemicals in Neurodevelopmental Processes
3. The Epigenetics of Endocrine Disruptors in Nervous and Neuroendocrine Systems
3.1. Mechanistic Insights into Endocrine Disruptors’ Epigenetic Perspective
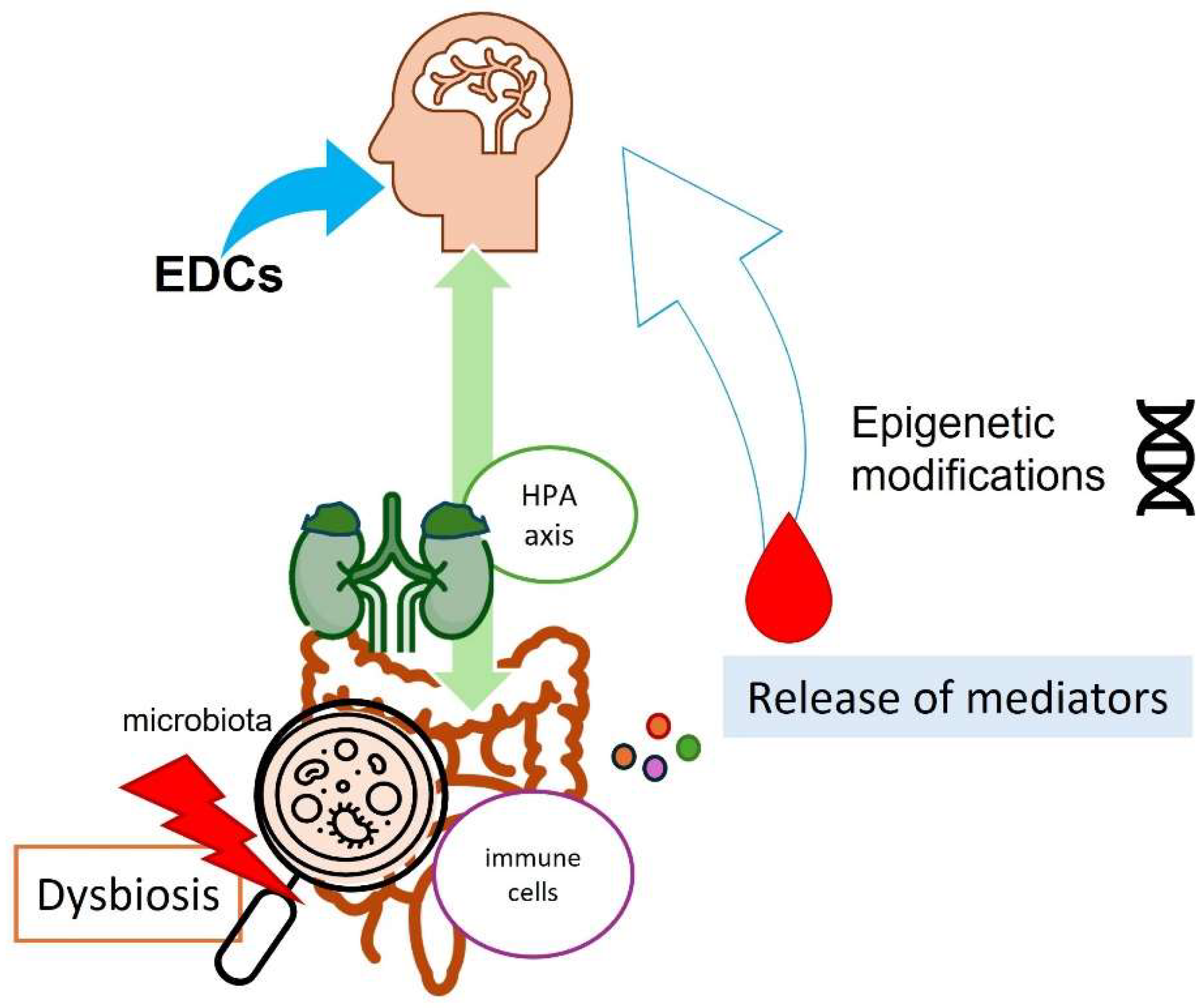
3.2. Endocrine Disruptors, Microbiota, and Epigenetic Changes: A Warning Triangle for Neuromodulation
4. From Individual Behavior to Population Dynamics: New Challenges for Behavioral Endocrinology
5. Methodological Challenges and Translational Barriers in Endocrine Disruptor Research
Future Directions and Research Priorities
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chormare, R.; Kumar, M.A. Environmental Health and Risk Assessment Metrics with Special Mention to Biotransfer, Bioaccumulation and Biomagnification of Environmental Pollutants. Chemosphere 2022, 302, 134836. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Fortenberry, G.Z.; Meeker, J.D.; Sánchez, B.N.; Barr, D.B.; Panuwet, P.; Bellinger, D.; Schnaas, L.; Solano-González, M.; Ettinger, A.S.; Hernandez-Avila, M.; et al. Urinary 3, 5, 6-Trichloro-2-Pyridinol (TCPY) in Pregnant Women from Mexico City: Distribution, Temporal Variability, and Relationship with Child Attention and Hyperactivity. Int. J. Hyg. Environ. Health 2014, 217, 405–412. [Google Scholar] [CrossRef]
- Maitre, L.; Robinson, O.; Martinez, D.; Toledano, M.B.; Ibarluzea, J.; Marina, L.S.; Sunyer, J.; Villanueva, C.M.; Keun, H.C.; Vrijheid, M.; et al. Urine Metabolic Signatures of Multiple Environmental Pollutants in Pregnant Women: An Exposome Approach. Environ. Sci. Technol. 2018, 52, 13469–13480. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.K.; Sobolewski, M.; Susiarjo, M. Exposure to Endocrine Disrupting Chemicals Impacts Immunological and Metabolic Status of Women during Pregnancy. Mol. Cell. Endocrinol. 2023, 577, 112031. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.J.; Urbanetz, A.A. Environmental Toxins and the Impact of Other Endocrine Disrupting Chemicals in Women’s Reproductive Health. JBRA Assist. Reprod. 2019, 23, 154–164. [Google Scholar] [CrossRef]
- Rizzo, R.; Bortolotti, D.; Rizzo, S.; Schiuma, G. Endocrine Disruptors, Epigenetic Changes, and Transgenerational Transmission. In Environment Impact on Reproductive Health: A Translational Approach; Marci, R., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 49–74. ISBN 978-3-031-36494-5. [Google Scholar]
- WHO/IPCS. Global Assessment on the State of the Science of Endocrine Disruptors; WHO: Geneva, Switzerland, 2002; p. 180. [Google Scholar]
- Demirtaş, İ.; Mızık, E.T.; Can-Güven, E.; Gedik, K. A Data-Driven Analysis of Global Research Trends on Dirty-Dozen Persistent Organic Pollutants. Environ. Monit. Assess. 2023, 195, 1115. [Google Scholar] [CrossRef]
- Guillotin, S.; Delcourt, N. Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14271. [Google Scholar] [CrossRef]
- Grasselli, E.; Dvorakova, M.; Graceli, J.B. Editorial: Presence and Daily Exposure to Endocrine Disruptors: How Can Human Life Change? Front. Endocrinol. 2021, 12, 790853. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A Review on Endocrine Disruptors and Their Possible Impacts on Human Health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, M.; Zhuang, D. Wastewater Treatment and Emerging Contaminants: Bibliometric Analysis. Chemosphere 2022, 297, 133932. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.K.; Padmanabhan, V. Early Pregnancy Exposure to Endocrine Disrupting Chemical Mixtures Are Associated with Inflammatory Changes in Maternal and Neonatal Circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal–Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of Phenolic Endocrine Disrupting Chemicals (EDCs) from Maternal Blood Plasma and Amniotic Fluid in Indian Population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef]
- Svensson, K.; Gennings, C.; Lindh, C.; Kiviranta, H.; Rantakokko, P.; Wikström, S.; Bornehag, C.-G. Prenatal Exposures to Mixtures of Endocrine Disrupting Chemicals and Sex-Specific Associations with Children’s BMI and Overweight at 5.5 Years of Age in the SELMA Study. Environ. Int. 2023, 179, 108176. [Google Scholar] [CrossRef]
- Maddalon, A.; Cari, L.; Iulini, M.; Alhosseini, M.N.; Galbiati, V.; Marinovich, M.; Nocentini, G.; Corsini, E. Impact of Endocrine Disruptors on Peripheral Blood Mononuclear Cells in Vitro: Role of Gender. Arch. Toxicol. 2023, 97, 3129–3150. [Google Scholar] [CrossRef]
- Maddalon, A.; Masi, M.; Iulini, M.; Linciano, P.; Galbiati, V.; Marinovich, M.; Racchi, M.; Buoso, E.; Corsini, E. Effects of Endocrine Active Contaminating Pesticides on RACK1 Expression and Immunological Consequences in THP-1 Cells. Environ. Toxicol. Pharmacol. 2022, 95, 103971. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Masi, M.; Long, A.; Chiappini, C.; Travelli, C.; Govoni, S.; Racchi, M. Ribosomes as a Nexus between Translation and Cancer Progression: Focus on Ribosomal Receptor for Activated C Kinase 1 (RACK1) in Breast Cancer. Br. J. Pharmacol. 2022, 179, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An Emerging Threat to Female Fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of Bisphenol A and Its Analogs on Reproductive Health: A Mini Review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef]
- Wang, W.; Hafner, K.S.; Flaws, J.A. In Utero Bisphenol A Exposure Disrupts Germ Cell Nest Breakdown and Reduces Fertility with Age in the Mouse. Toxicol. Appl. Pharmacol. 2014, 276, 157–164. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.L.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult Exposure to Bisphenol A (BPA) in Wistar Rats Reduces Sperm Quality with Disruption of the Hypothalamic–Pituitary–Testicular Axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism Disrupting Chemicals and Metabolic Disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Aronica, L.; Ordovas, J.M.; Volkov, A.; Lamb, J.J.; Stone, P.M.; Minich, D.; Leary, M.; Class, M.; Metti, D.; Larson, I.A.; et al. Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine. Nutrients 2022, 14, 768. [Google Scholar] [CrossRef]
- Bornman, M.; Delport, R.; Farías, P.; Aneck-Hahn, N.; Patrick, S.; Millar, R.P.; de Jager, C. Alterations in Male Reproductive Hormones in Relation to Environmental DDT Exposure. Environ. Int. 2018, 113, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Jaz, K.; Ochmańska, A.; Zgorzalewicz-Stachowiak, M.; Czarnocka, B.; Sawicka-Gutaj, N.; Ziółkowska, P.; Krela-Kaźmierczak, I.; Gut, P.; Florek, E.; et al. The Effect of Endocrine Disruptors on the Reproductive System—Current Knowledge. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4930–4940. [Google Scholar] [CrossRef] [PubMed]
- Jarman, W.M.; Ballschmiter, K. From Coal to DDT: The History of the Development of the Pesticide DDT from Synthetic Dyes till Silent Spring. Endeavour 2012, 36, 131–142. [Google Scholar] [CrossRef]
- Jeng, H.A. Exposure to Endocrine Disrupting Chemicals and Male Reproductive Health. Front. Public Health 2014, 2, 55. [Google Scholar] [CrossRef]
- McCue, K.; DeNicola, N. Environmental Exposures in Reproductive Health. Obstet. Gynecol. Clin. N. Am. 2019, 46, 455–468. [Google Scholar] [CrossRef]
- Braun, J.M. Early-Life Exposure to EDCs: Role in Childhood Obesity and Neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Rich, A.L.; Phipps, L.M.; Tiwari, S.; Rudraraju, H.; Dokpesi, P.O. The Increasing Prevalence in Intersex Variation from Toxicological Dysregulation in Fetal Reproductive Tissue Differentiation and Development by Endocrine-Disrupting Chemicals. Environ. Health Insights 2016, 10, 163–171. [Google Scholar] [CrossRef]
- Schwarzman, M. Potential Toxicity of Synthetic Chemicals: What You Should Know about Endocrine-Disrupting Chemicals. Am. Fam. Physician 2008, 78, 565–566. [Google Scholar]
- Almstrup, K.; Frederiksen, H.; Andersson, A.-M.; Juul, A. Levels of Endocrine-Disrupting Chemicals Are Associated with Changes in the Peri-Pubertal Epigenome. Endocr. Connect. 2020, 9, 845–857. [Google Scholar] [CrossRef]
- Nettore, I.C.; Franchini, F.; Palatucci, G.; Macchia, P.E.; Ungaro, P. Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Obesity. Biomedicines 2021, 9, 1716. [Google Scholar] [CrossRef]
- Panera, N.; Mandato, C.; Crudele, A.; Bertrando, S.; Vajro, P.; Alisi, A. Genetics, Epigenetics and Transgenerational Transmission of Obesity in Children. Front. Endocrinol. 2022, 13, 1006008. [Google Scholar] [CrossRef] [PubMed]
- Samtani, R.; Sharma, N.; Garg, D. Effects of Endocrine-Disrupting Chemicals and Epigenetic Modifications in Ovarian Cancer: A Review. Reprod. Sci. 2018, 25, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Cediel-Ulloa, A.; Lupu, D.L.; Johansson, Y.; Hinojosa, M.; Özel, F.; Rüegg, J. Impact of Endocrine Disrupting Chemicals on Neurodevelopment: The Need for Better Testing Strategies for Endocrine Disruption-Induced Developmental Neurotoxicity. Expert. Rev. Endocrinol. Metab. 2022, 17, 131–141. [Google Scholar] [CrossRef]
- Montero-Pedrazuela, A.; Grijota-Martínez, C.; Ausó, E.; Bárez-López, S.; Guadaño-Ferraz, A. Chapter 8—Endocrine Aspects of Development. Thyroid Hormone Actions in Neurological Processes during Brain Development. In Diagnosis, Management and Modeling of Neurodevelopmental Disorders; Martin, C.R., Preedy, V.R., Rajendram, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 85–97. ISBN 978-0-12-817988-8. [Google Scholar]
- Makris, G.; Eleftheriades, A.; Pervanidou, P. Early Life Stress, Hormones, and Neurodevelopmental Disorders. Horm. Res. Paediatr. 2023, 96, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Anifantaki, F.; Pervanidou, P.; Lambrinoudaki, I.; Panoulis, K.; Vlahos, N.; Eleftheriades, M. Maternal Prenatal Stress, Thyroid Function and Neurodevelopment of the Offspring: A Mini Review of the Literature. Front. Neurosci. 2021, 15, 692446. [Google Scholar] [CrossRef]
- Bludau, A.; Royer, M.; Meister, G.; Neumann, I.D.; Menon, R. Epigenetic Regulation of the Social Brain. Trends Neurosci. 2019, 42, 471–484. [Google Scholar] [CrossRef]
- Manley, K.; Han, W.; Zelin, G.; Lawrence, D.A. Crosstalk between the Immune, Endocrine, and Nervous Systems in Immunotoxicology. Curr. Opin. Toxicol. 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Qin, J.; Jiao, J.; Ji, F. The Roles of Immune Factors in Neurodevelopment. Front. Cell. Neurosci. 2025, 19, 1451889. [Google Scholar] [CrossRef]
- Kajta, M.; Wójtowicz, A.K. Impact of Endocrine-Disrupting Chemicals on Neural Development and the Onset of Neurological Disorders. Pharmacol. Rep. 2013, 65, 1632–1639. [Google Scholar] [CrossRef]
- Li, L.-X.; Chen, L.; Meng, X.-Z.; Chen, B.-H.; Chen, S.-Q.; Zhao, Y.; Zhao, L.-F.; Liang, Y.; Zhang, Y.-H. Exposure Levels of Environmental Endocrine Disruptors in Mother-Newborn Pairs in China and Their Placental Transfer Characteristics. PLoS ONE 2013, 8, e62526. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Albaladejo, E.; Fernandes, D.; Lacorte, S.; Porte, C. Comparative Toxicity, Oxidative Stress and Endocrine Disruption Potential of Plasticizers in JEG-3 Human Placental Cells. Toxicol. Vitr. 2017, 38, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Piscioli, F.; Pusiol, T. The Endocrine Disruptors among the Environmental Risk Factors for Stillbirth. Sci. Total Environ. 2016, 563–564, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, E.; Grimalt, J.O.; Fernández-Somoano, A.; Tardon, A. Transport of Persistent Organic Pollutants across the Human Placenta. Environ. Int. 2014, 65, 107–115. [Google Scholar] [CrossRef]
- Mao, J.; Jain, A.; Denslow, N.D.; Nouri, M.-Z.; Chen, S.; Wang, T.; Zhu, N.; Koh, J.; Sarma, S.J.; Sumner, B.W.; et al. Bisphenol A and Bisphenol S Disruptions of the Mouse Placenta and Potential Effects on the Placenta–Brain Axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4642–4652. [Google Scholar] [CrossRef]
- Forger, N.G. Cell Death and Sexual Differentiation of the Nervous System. Neuroscience 2006, 138, 929–938. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Auger, A.P.; Bale, T.L.; De Vries, G.J.; Dunn, G.A.; Forger, N.G.; Murray, E.K.; Nugent, B.M.; Schwarz, J.M.; Wilson, M.E. The Epigenetics of Sex Differences in the Brain. J. Neurosci. 2009, 29, 12815–12823. [Google Scholar] [CrossRef]
- Schug, T.T.; Blawas, A.M.; Gray, K.; Heindel, J.J.; Lawler, C.P. Elucidating the Links between Endocrine Disruptors and Neurodevelopment. Endocrinology 2015, 156, 1941–1951. [Google Scholar] [CrossRef]
- Juricek, L.; Coumoul, X. The Aryl Hydrocarbon Receptor and the Nervous System. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef]
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, High Affinity Binding of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin by Hepatic Cytosol. Evidence That the Binding Species Is Receptor for Induction of Aryl Hydrocarbon Hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the Environmental Endocrine Disruptor TCDD and Human Reproductive Dysfunction: Translating Lessons from Murine Models. Reprod. Toxicol. 2017, 68, 59–71. [Google Scholar] [CrossRef]
- Martin, N.R.; Patel, R.; Kossack, M.E.; Tian, L.; Camarillo, M.A.; Cintrón-Rivera, L.G.; Gawdzik, J.C.; Yue, M.S.; Nwagugo, F.O.; Elemans, L.M.H.; et al. Proper Modulation of AHR Signaling Is Necessary for Establishing Neural Connectivity and Oligodendrocyte Precursor Cell Development in the Embryonic Zebrafish Brain. Front. Mol. Neurosci. 2022, 15, 1032302. [Google Scholar] [CrossRef] [PubMed]
- Baraka, A.M.; Korish, A.A.; Soliman, G.A.; Kamal, H. The Possible Role of Estrogen and Selective Estrogen Receptor Modulators in a Rat Model of Parkinson’s Disease. Life Sci. 2011, 88, 879–885. [Google Scholar] [CrossRef] [PubMed]
- D’Astous, M.; Morissette, M.; Di Paolo, T. Effect of Estrogen Receptor Agonists Treatment in MPTP Mice: Evidence of Neuroprotection by an ERα Agonist. Neuropharmacology 2004, 47, 1180–1188. [Google Scholar] [CrossRef]
- Salama, R.M.; Tadros, M.G.; Schaalan, M.F.; Bahaa, N.; Abdel-tawab, A.M.; Khalifa, A.E. Potential Neuroprotective Effect of Androst-5-ene-3β, 17β-diol (ADIOL) on the Striatum, and Substantia Nigra in Parkinson’s Disease Rat Model. J. Cell. Physiol. 2018, 233, 5981–6000. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, Z.; Chen, S.; Schneider, L.S.; Brinton, R.D. Early Intervention with an Estrogen Receptor β-Selective Phytoestrogenic Formulation Prolongs Survival, Improves Spatial Recognition Memory, and Slows Progression of Amyloid Pathology in a Female Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 37, 403–419. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by Stealth—Interference of Endocrine Disrupting Chemicals on Hormonal Crosstalk with Thyroid Axis Function in Humans and Other Animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Allen, J.G.; Gale, S.; Zoeller, R.T.; Spengler, J.D.; Birnbaum, L.; McNeely, E. PBDE Flame Retardants, Thyroid Disease, and Menopausal Status in U.S. Women. Environ. Health 2016, 15, 60. [Google Scholar] [CrossRef]
- Blake, B.E.; Pinney, S.M.; Hines, E.P.; Fenton, S.E.; Ferguson, K.K. Associations between Longitudinal Serum Perfluoroalkyl Substance (PFAS) Levels and Measures of Thyroid Hormone, Kidney Function, and Body Mass Index in the Fernald Community Cohort. Environ. Pollut. 2018, 242, 894–904. [Google Scholar] [CrossRef]
- Noda, M. Chapter Thirteen—Thyroid Hormone in the CNS: Contribution of Neuron–Glia Interaction. In Vitamins and Hormones; Litwack, G., Ed.; Thyroid Hormone; Academic Press: New York, NY, USA, 2018; Volume 106, pp. 313–331. [Google Scholar]
- Gould, E.; Butcher, L.L. Developing Cholinergic Basal Forebrain Neurons Are Sensitive to Thyroid Hormone. J. Neurosci. 1989, 9, 3347–3358. [Google Scholar] [CrossRef]
- Gould, E.; Frankfurt, M.; Westlind-Danielsson, A.; McEwen, B.S. Developing Forebrain Astrocytes Are Sensitive to Thyroid Hormone. Glia 1990, 3, 283–292. [Google Scholar] [CrossRef]
- Lima, F.R.; Gervais, A.; Colin, C.; Izembart, M.; Neto, V.M.; Mallat, M. Regulation of Microglial Development: A Novel Role for Thyroid Hormone. J. Neurosci. 2001, 21, 2028–2038. [Google Scholar] [CrossRef]
- Rodriguez-Peña, A.; Ibarrola, N.; Iñiguez, M.A.; Muñoz, A.; Bernal, J. Neonatal Hypothyroidism Affects the Timely Expression of Myelin-Associated Glycoprotein in the Rat Brain. J. Clin. Investig. 1993, 91, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.M.; Leung, M.T.Y.; Man, K.K.C.; Leung, W.C.; Ip, P.; Li, G.H.Y.; Wong, I.C.K.; Kung, A.W.C.; Cheung, C.-L. Maternal Thyroid Dysfunction During Pregnancy and the Risk of Adverse Outcomes in the Offspring: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 105, 3821–3841. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Ghassabian, A.; Bongers-Schokking, J.J.; Jaddoe, V.W.V.; Hofman, A.; De Rijke, Y.B.; Verhulst, F.C.; Tiemeier, H. Association of Gestational Maternal Hypothyroxinemia and Increased Autism Risk. Ann. Neurol. 2013, 74, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-P.; Hetzel, B.S. Cretinism Revisited. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 39–50. [Google Scholar] [CrossRef]
- Meerts, I.A.T.M.; Lilienthal, H.; Hoving, S.; van den Berg, J.H.J.; Weijers, B.M.; Bergman, Å.; Koeman, J.H.; Brouwer, A. Developmental Exposure to 4-Hydroxy-2,3,3′,4′,5-Pentachlorobiphenyl (4-OH-CB107): Long-Term Effects on Brain Development, Behavior, and Brain Stem Auditory Evoked Potentials in Rats. Toxicol. Sci. 2004, 82, 207–218. [Google Scholar] [CrossRef]
- Babić Leko, M.; Pleić, N.; Lešin, M.; Gunjača, I.; Torlak, V.; Škunca Herman, J.; Vatavuk, Z.; Punda, A.; Polašek, O.; Hayward, C.; et al. Association between Thyroid Function and Ocular Parameters. Biology 2022, 11, 1847. [Google Scholar] [CrossRef]
- Pontelli, R.C.N.; Souza, M.C.O.; Fantucci, M.Z.; de Andrade, M.; Rocha, E.M. The Role of Endocrine Disruptors in Ocular Surface Diseases. Med. Hypotheses 2019, 122, 157–164. [Google Scholar] [CrossRef]
- Yang, F.; Ma, H.; Ding, X.-Q. Thyroid Hormone Signaling in Retinal Development, Survival, and Disease. Vitam. Horm. 2018, 106, 333–349. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Lin, Z.-C.; Lu, S.-Q.; Chen, X.-F.; Liao, X.-L.; Qi, Z.; Cai, Z. Azole-Induced Color Vision Deficiency Associated with Thyroid Hormone Signaling: An Integrated In Vivo, In Vitro, and In Silico Study. Environ. Sci. Technol. 2022, 56, 13264–13273. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, S.; Zhang, X.; Li, N.; Zhang, Q.; Guo, X.; Chi, X.; Tong, M. Peptidomic Analysis of Zebrafish Embryos Exposed to Polychlorinated Biphenyls and Their Impact on Eye Development. Ecotoxicol. Environ. Saf. 2019, 175, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, K.H. Visual and Neurobehavioral Impairment Associated with Polychlorinated Biphenyls. Neurotoxicology 2000, 21, 489–499. [Google Scholar] [PubMed]
- Wang, Y.-P.; Hong, Q.; Qin, D.; Kou, C.-Z.; Zhang, C.-M.; Guo, M.; Guo, X.-R.; Chi, X.; Tong, M.-L. Effects of Embryonic Exposure to Polychlorinated Biphenyls on Zebrafish (Danio Rerio) Retinal Development. J. Appl. Toxicol. 2012, 32, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Segner, H.; Ros, A.; Knapen, D.; Vergauwen, L. Thyroid Hormone Disruptors Interfere with Molecular Pathways of Eye Development and Function in Zebrafish. Int. J. Mol. Sci. 2019, 20, 1543. [Google Scholar] [CrossRef]
- Hasegawa, M.; Wada, H. Developmental Hypothyroidism Disrupts Visual Signal Detection Performance in Rats. Physiol. Behav. 2013, 112–113, 90–95. [Google Scholar] [CrossRef]
- Knudsen, E.I. Evolution of Neural Processing for Visual Perception in Vertebrates. J. Comp. Neurol. 2020, 528, 2888–2901. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Bansal, R.; Parris, C. Bisphenol-A, an Environmental Contaminant That Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology 2005, 146, 607–612. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin: A Potential Biomarker of Neurological and Mental Diseases. Front. Aging Neurosci. 2020, 12, 584743. [Google Scholar] [CrossRef]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef]
- Blanc-Legendre, M.; Sire, S.; Christophe, A.; Brion, F.; Bégout, M.-L.; Cousin, X. Embryonic Exposures to Chemicals Acting on Brain Aromatase Lead to Different Locomotor Effects in Zebrafish Larvae. Environ. Toxicol. Pharmacol. 2023, 102, 104221. [Google Scholar] [CrossRef]
- Bortolato, M.; Godar, S.C.; Davarian, S.; Chen, K.; Shih, J.C. Behavioral Disinhibition and Reduced Anxiety-like Behaviors in Monoamine Oxidase B-Deficient Mice. Neuropsychopharmacology 2009, 34, 2746–2757. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Shih, J.C. Behavioral Outcomes of Monoamine Oxidase Deficiency: Preclinical and Clinical Evidence. In International Review of Neurobiology; Youdim, M.B.H., Douce, P., Eds.; Monoamine Oxidase and their Inhibitors; Academic Press: Cambridge, MA, USA, 2011; Volume 100, pp. 13–42. [Google Scholar]
- Matsuda, S.; Matsuzawa, D.; Ishii, D.; Tomizawa, H.; Sutoh, C.; Nakazawa, K.; Amano, K.; Sajiki, J.; Shimizu, E. Effects of Perinatal Exposure to Low Dose of Bisphenol A on Anxiety like Behavior and Dopamine Metabolites in Brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 273–279. [Google Scholar] [CrossRef] [PubMed]
- McRobb, F.M.; Sahagún, V.; Kufareva, I.; Abagyan, R. In Silico Analysis of the Conservation of Human Toxicity and Endocrine Disruption Targets in Aquatic Species. Environ. Sci. Technol. 2014, 48, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, K.; Shih, J.C.; Teng, C.T. Estrogen-Related Receptors-Stimulated Monoamine Oxidase B Promoter Activity Is Down-Regulated by Estrogen Receptors. Mol. Endocrinol. 2006, 20, 1547–1561. [Google Scholar] [CrossRef]
- Dutta, H.M.; Arends, D.A. Effects of Endosulfan on Brain Acetylcholinesterase Activity in Juvenile Bluegill Sunfish. Environ. Res. 2003, 91, 157–162. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Kutsuno, Y.; Hirashima, R.; Sakamoto, M.; Ushikubo, H.; Michimae, H.; Itoh, T.; Tukey, R.H.; Fujiwara, R. Expression of UDP-Glucuronosyltransferase 1 (UGT1) and Glucuronidation Activity toward Endogenous Substances in Humanized UGT1 Mouse Brain. Drug Metab. Dispos. 2015, 43, 1071. [Google Scholar] [CrossRef]
- Ouzzine, M.; Gulberti, S.; Ramalanjaona, N.; Magdalou, J.; Fournel-Gigleux, S. The UDP-Glucuronosyltransferases of the Blood-Brain Barrier: Their Role in Drug Metabolism and Detoxication. Front. Cell. Neurosci. 2014, 8, 349. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, H.; Wu, T.; Zhu, L.; Liu, L.; Liu, X. Alterations of Cytochrome P450s and UDP-Glucuronosyltransferases in Brain Under Diseases and Their Clinical Significances. Front. Pharmacol. 2021, 12, 650027. [Google Scholar] [CrossRef]
- Arambula, S.E.; McCarthy, M.M. Neuroendocrine-Immune Crosstalk Shapes Sex-Specific Brain Development. Endocrinology 2020, 161, bqaa055. [Google Scholar] [CrossRef]
- Blalock, J.E. A Molecular Basis for Bidirectional Communication between the Immune and Neuroendocrine Systems. Physiol. Rev. 1989, 69, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Masi, M.; Limosani, R.V.; Oliviero, C.; Saeed, S.; Iulini, M.; Passoni, F.C.; Racchi, M.; Corsini, E. Endocrine Disrupting Toxicity of Bisphenol A and Its Analogs: Implications in the Neuro-Immune Milieu. J. Xenobiotics 2025, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; McCarthy, M.; Raval, A.P. Xenoestrogens Impact Brain Estrogen Receptor Signaling during the Female Lifespan: A Precursor to Neurological Disease? Neurobiol. Dis. 2022, 163, 105596. [Google Scholar] [CrossRef] [PubMed]
- He, D.-Y.; Neasta, J.; Ron, D. Epigenetic Regulation of BDNF Expression via the Scaffolding Protein RACK1. J. Biol. Chem. 2010, 285, 19043–19050. [Google Scholar] [CrossRef]
- Cirillo, F.; Lappano, R.; Bruno, L.; Rizzuti, B.; Grande, F.; Guzzi, R.; Briguori, S.; Miglietta, A.M.; Nakajima, M.; Di Martino, M.T.; et al. AHR and GPER Mediate the Stimulatory Effects Induced by 3-Methylcholanthrene in Breast Cancer Cells and Cancer-Associated Fibroblasts (CAFs). J. Exp. Clin. Cancer Res. 2019, 38, 335. [Google Scholar] [CrossRef]
- Yan, S.; Ji, J.; Zhang, Z.; Imam, M.; Chen, H.; Zhang, D.; Wang, J. Targeting the Crosstalk between Estrogen Receptors and Membrane Growth Factor Receptors in Breast Cancer Treatment: Advances and Opportunities. Biomed. Pharmacother. 2024, 175, 116615. [Google Scholar] [CrossRef]
- Masi, M.; Maddalon, A.; Iulini, M.; Linciano, P.; Galbiati, V.; Marinovich, M.; Racchi, M.; Corsini, E.; Buoso, E. Effects of Endocrine Disrupting Chemicals on the Expression of RACK1 and LPS-Induced THP-1 Cell Activation. Toxicology 2022, 480, 153321. [Google Scholar] [CrossRef]
- Żabińska, M.; Wiśniewska, K.; Węgrzyn, G.; Pierzynowska, K. Exploring the Physiological Role of the G Protein-Coupled Estrogen Receptor (GPER) and Its Associations with Human Diseases. Psychoneuroendocrinology 2024, 166, 107070. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, M.; Gao, T.; Meng, L.; Ding, X.; Meng, Y.; Jiao, Y.; Luo, P.; He, Z.; Sun, T.; et al. GPER-Deficient Rats Exhibit Lower Serum Corticosterone Level and Increased Anxiety-Like Behavior. Neural Plast. 2020, 2020, 8866187. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Oudart, M.; Avila-Gutierrez, K.; Moch, C.; Dossi, E.; Milior, G.; Boulay, A.-C.; Gaudey, M.; Moulard, J.; Lombard, B.; Loew, D.; et al. The Ribosome-Associated Protein RACK1 Represses Kir4.1 Translation in Astrocytes and Influences Neuronal Activity. Cell. Rep. 2023, 42, 112456. [Google Scholar] [CrossRef]
- Takahashi, M.; Komada, M.; Miyazawa, K.; Goto, S.; Ikeda, Y. Bisphenol A Exposure Induces Increased Microglia and Microglial Related Factors in the Murine Embryonic Dorsal Telencephalon and Hypothalamus. Toxicol. Lett. 2018, 284, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.; Reindl, M. Blood–Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, D.; Mhaouty-Kodja, S.; Grange-Messent, V. Disruption of the Blood-Brain Barrier and Its Close Environment Following Adult Exposure to Low Doses of Di(2-Ethylhexyl)Phthalate Alone or in an Environmental Phthalate Mixture in Male Mice. Chemosphere 2021, 282, 131013. [Google Scholar] [CrossRef] [PubMed]
- Alavian-Ghavanini, A.; Rüegg, J. Understanding Epigenetic Effects of Endocrine Disrupting Chemicals: From Mechanisms to Novel Test Methods. Basic Clin. Pharmacol. Toxicol. 2018, 122, 38–45. [Google Scholar] [CrossRef]
- Rattan, S.; Flaws, J.A. The Epigenetic Impacts of Endocrine Disruptors on Female Reproduction across Generations. Biol. Reprod. 2019, 101, 635–644. [Google Scholar] [CrossRef]
- Singleton, D.W.; Feng, Y.; Yang, J.; Puga, A.; Lee, A.V.; Khan, S.A. Gene Expression Profiling Reveals Novel Regulation by Bisphenol-A in Estrogen Receptor-Alpha-Positive Human Cells. Environ. Res. 2006, 100, 86–92. [Google Scholar] [CrossRef]
- Walker, D.M.; Gore, A.C. Epigenetic Impacts of Endocrine Disruptors in the Brain. Front. Neuroendocrinol. 2017, 44, 1–26. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Coussons-Read, M.E. Effects of Prenatal Stress on Pregnancy and Human Development: Mechanisms and Pathways. Obstet. Med. 2013, 6, 52–57. [Google Scholar] [CrossRef]
- Lazinski, M.J.; Shea, A.K.; Steiner, M. Effects of Maternal Prenatal Stress on Offspring Development: A Commentary. Arch. Womens Ment. Health 2008, 11, 363–375. [Google Scholar] [CrossRef]
- Lund, I.O.; Hannigan, L.J.; Ask, H.; Askelund, A.D.; Hegemann, L.; Corfield, E.C.; Wootton, R.E.; Ahmadzadeh, Y.I.; Davey Smith, G.; McAdams, T.A.; et al. Prenatal Maternal Stress: Triangulating Evidence for Intrauterine Exposure Effects on Birth and Early Childhood Outcomes across Multiple Approaches. BMC Med. 2025, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- LeWinn, K.Z.; Stroud, L.R.; Molnar, B.E.; Ware, J.H.; Koenen, K.C.; Buka, S.L. Elevated Maternal Cortisol Levels during Pregnancy Are Associated with Reduced Childhood IQ. Int. J. Epidemiol. 2009, 38, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.P.; Sandman, C.A. Prenatal Psychobiological Predictors of Anxiety Risk in Preadolescent Children. Psychoneuroendocrinology 2012, 37, 1224–1233. [Google Scholar] [CrossRef]
- Ronald, A.; Pennell, C.E.; Whitehouse, A.J.O. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in Early Childhood. Front. Psychology 2011, 1, 223. [Google Scholar] [CrossRef]
- Grundwald, N.J.; Brunton, P.J. Prenatal Stress Programs Neuroendocrine Stress Responses and Affective Behaviors in Second Generation Rats in a Sex-Dependent Manner. Psychoneuroendocrinology 2015, 62, 204–216. [Google Scholar] [CrossRef]
- Short, A.K.; Fennell, K.A.; Perreau, V.M.; Fox, A.; O’Bryan, M.K.; Kim, J.H.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Elevated Paternal Glucocorticoid Exposure Alters the Small Noncoding RNA Profile in Sperm and Modifies Anxiety and Depressive Phenotypes in the Offspring. Transl. Psychiatry 2016, 6, e837. [Google Scholar] [CrossRef]
- Yeshurun, S.; Rogers, J.; Short, A.K.; Renoir, T.; Pang, T.Y.; Hannan, A.J. Elevated Paternal Glucocorticoid Exposure Modifies Memory Retention in Female Offspring. Psychoneuroendocrinology 2017, 83, 9–18. [Google Scholar] [CrossRef]
- Di Criscio, M.; Lodahl, J.E.; Stamatakis, A.; Kitraki, E.; Bakoyiannis, I.; Repouskou, A.; Bornehag, C.-G.; Gennings, C.; Lupu, D.; Rüegg, J. A Human-Relevant Mixture of Endocrine Disrupting Chemicals Induces Changes in Hippocampal DNA Methylation Correlating with Hyperactive Behavior in Male Mice. Chemosphere 2023, 313, 137633. [Google Scholar] [CrossRef]
- Yeo, M.; Berglund, K.; Hanna, M.; Guo, J.U.; Kittur, J.; Torres, M.D.; Abramowitz, J.; Busciglio, J.; Gao, Y.; Birnbaumer, L.; et al. Bisphenol A Delays the Perinatal Chloride Shift in Cortical Neurons by Epigenetic Effects on the Kcc2 Promoter. Proc. Natl. Acad. Sci. USA 2013, 110, 4315–4320. [Google Scholar] [CrossRef]
- Yaoi, T.; Itoh, K.; Nakamura, K.; Ogi, H.; Fujiwara, Y.; Fushiki, S. Genome-Wide Analysis of Epigenomic Alterations in Fetal Mouse Forebrain after Exposure to Low Doses of Bisphenol A. Biochem. Biophys. Res. Commun. 2008, 376, 563–567. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, F.; Chang, F.; Bai, Y.; Chen, L. Persistent Overexpression of DNA Methyltransferase 1 Attenuating GABAergic Inhibition in Basolateral Amygdala Accounts for Anxiety in Rat Offspring Exposed Perinatally to Low-Dose Bisphenol A. J. Psychiatr. Res. 2013, 47, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.M.; Cunningham, S.L.; Gore, A.C. Prenatal PCBs Disrupt Early Neuroendocrine Development of the Rat Hypothalamus. Toxicol. Appl. Pharmacol. 2011, 252, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Topper, V.Y.; Walker, D.M.; Gore, A.C. Sexually Dimorphic Effects of Gestational Endocrine-Disrupting Chemicals on MicroRNA Expression in the Developing Rat Hypothalamus. Mol. Cell. Endocrinol. 2015, 414, 42. [Google Scholar] [CrossRef] [PubMed]
- Ejaredar, M.; Nyanza, E.C.; Ten Eycke, K.; Dewey, D. Phthalate Exposure and Childrens Neurodevelopment: A Systematic Review. Environ. Res. 2015, 142, 51–60. [Google Scholar] [CrossRef]
- Luu, B.E.; Green, S.R.; Childers, C.L.; Holahan, M.R.; Storey, K.B. The Roles of Hippocampal microRNAs in Response to Acute Postnatal Exposure to Di(2-Ethylhexyl) Phthalate in Female and Male Rats. NeuroToxicology 2017, 59, 98–104. [Google Scholar] [CrossRef]
- Qiu, F.; He, S.; Zhang, Z.; Dai, S.; Wang, J.; Liu, N.; Li, Z.; Hu, X.; Xiang, S.; Wei, C. MiR-93 Alleviates DEHP Plasticizer-Induced Neurotoxicity by Negatively Regulating TNFAIP1 and Inhibiting Ubiquitin-Mediated Degradation of CK2β. Food Chem. Toxicol. 2023, 178, 113888. [Google Scholar] [CrossRef]
- Hung, C.-H.; Yang, S.-N.; Kuo, P.-L.; Chu, Y.-T.; Chang, H.-W.; Wei, W.-J.; Huang, S.-K.; Jong, Y.-J. Modulation of Cytokine Expression in Human Myeloid Dendritic Cells by Environmental Endocrine-Disrupting Chemicals Involves Epigenetic Regulation. Environ. Health Perspect. 2010, 118, 67–72. [Google Scholar] [CrossRef][Green Version]
- Yeh, C.-H.; Wu, H.-C.; Kuo, T.-H.; Kuo, C.-H.; Yang, S.-N.; Wang, W.-L.; Chen, H.-N.; Wei, W.-J.; Hung, C.-H. Suppressive Effect on MDC and IP-10 Expression in Monocytes by Endocrine Disruptor Chemicals. Inflammation 2010, 33, 10–17. [Google Scholar] [CrossRef]
- O’Brien, E.; Dolinoy, D.C.; Mancuso, P. Perinatal Bisphenol A Exposures Increase Production of Pro-Inflammatory Mediators in Bone Marrow-Derived Mast Cells of Adult Mice. J. Immunotoxicol. 2014, 11, 205–212. [Google Scholar] [CrossRef]
- Calero-Medina, L.; Jimenez-Casquet, M.J.; Heras-Gonzalez, L.; Conde-Pipo, J.; Lopez-Moro, A.; Olea-Serrano, F.; Mariscal-Arcas, M. Dietary Exposure to Endocrine Disruptors in Gut Microbiota: A Systematic Review. Sci. Total Environ. 2023, 886, 163991. [Google Scholar] [CrossRef]
- Hampl, R.; Stárka, L. Endocrine Disruptors and Gut Microbiome Interactions. Physiol. Res. 2020, 69, S211–S223. [Google Scholar] [CrossRef]
- Shemtov, S.J.; Emani, R.; Bielska, O.; Covarrubias, A.J.; Verdin, E.; Andersen, J.K.; Winer, D.A. The Intestinal Immune System and Gut Barrier Function in Obesity and Ageing. FEBS J. 2023, 290, 4163–4186. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Galasso, M.; Ronfani, M.; Papale, A.; Galbiati, V.; Eberini, I.; Marinovich, M.; Racchi, M.; Corsini, E. The Scaffold Protein RACK1 Is a Target of Endocrine Disrupting Chemicals (EDCs) with Important Implication in Immunity. Toxicol. Appl. Pharmacol. 2017, 325, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Masi, M.; Galbiati, V.; Maddalon, A.; Iulini, M.; Kenda, M.; Sollner Dolenc, M.; Marinovich, M.; Racchi, M.; Corsini, E. Effect of Estrogen-Active Compounds on the Expression of RACK1 and Immunological Implications. Arch. Toxicol. 2020, 94, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Pahović, P.Š.; Iulini, M.; Maddalon, A.; Galbiati, V.; Buoso, E.; Dolenc, M.S.; Corsini, E. In Vitro Effects of Bisphenol Analogs on Immune Cells Activation and Th Differentiation. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1750–1761. [Google Scholar] [CrossRef]
- O’Brien, E.; Dolinoy, D.C.; Mancuso, P. Bisphenol A at Concentrations Relevant to Human Exposure Enhances Histamine and Cysteinyl Leukotriene Release from Bone Marrow-Derived Mast Cells. J. Immunotoxicol. 2014, 11, 84–89. [Google Scholar] [CrossRef]
- Ménard, S.; Guzylack-Piriou, L.; Lencina, C.; Leveque, M.; Naturel, M.; Sekkal, S.; Harkat, C.; Gaultier, E.; Olier, M.; Garcia-Villar, R.; et al. Perinatal Exposure to a Low Dose of Bisphenol A Impaired Systemic Cellular Immune Response and Predisposes Young Rats to Intestinal Parasitic Infection. PLoS ONE 2014, 9, e112752. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Microbiota Implications in Endocrine-Related Diseases: From Development to Novel Therapeutic Approaches. Biomedicines 2024, 12, 221. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Kumar, A.; Pramanik, J.; Goyal, N.; Chauhan, D.; Sivamaruthi, B.S.; Prajapati, B.G.; Chaiyasut, C. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals 2023, 16, 565. [Google Scholar] [CrossRef]
- Caspani, G.; Swann, J. Small Talk: Microbial Metabolites Involved in the Signaling from Microbiota to Brain. Curr. Opin. Pharmacol. 2019, 48, 99–106. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Anuar, F.; Zadjali, F.; Rafter, J.; Pettersson, S. Gut Microbial Communities Modulating Brain Development and Function. Gut Microbes 2012, 3, 366–373. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota Derived Short Chain Fatty Acids Promote Histone Crotonylation in the Colon through Histone Deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef]
- Li, L.; Zhao, S.; Xiang, T.; Feng, H.; Ma, L.; Fu, P. Epigenetic Connection between Gut Microbiota-Derived Short-Chain Fatty Acids and Chromatin Histone Modification in Kidney Diseases. Chin. Med. J. 2022, 135, 1692–1694. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A.; Riber, L. Epigenetic Effects of Short-Chain Fatty Acids from the Large Intestine on Host Cells. Microlife 2023, 4, uqad032. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Ensink, E.; Li, P.; Gordevičius, J.; Marshall, L.L.; George, S.; Pospisilik, J.A.; Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov. Disord. 2022, 37, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Reva, K.; Laranjinha, J.; Rocha, B.S. Epigenetic Modifications Induced by the Gut Microbiota May Result from What We Eat: Should We Talk about Precision Diet in Health and Disease? Metabolites 2023, 13, 375. [Google Scholar] [CrossRef]
- Romano, K.A.; Martinez-Del Campo, A.; Kasahara, K.; Chittim, C.L.; Vivas, E.I.; Amador-Noguez, D.; Balskus, E.P.; Rey, F.E. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 2017, 22, 279–290.e7. [Google Scholar] [CrossRef]
- Mellott, T.J.; Kowall, N.W.; Lopez-Coviella, I.; Blusztajn, J.K. Prenatal Choline Deficiency Increases Choline Transporter Expression in the Septum and Hippocampus during Postnatal Development and in Adulthood in Rats. Brain Res. 2007, 1151, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Gene Response Elements, Genetic Polymorphisms and Epigenetics Influence the Human Dietary Requirement for Choline. IUBMB Life 2007, 59, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The Role of the Hippocampus in Flexible Cognition and Social Behavior. Front. Hum. Neurosci. 2014, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- López-Moreno, A.; Torres-Sánchez, A.; Acuña, I.; Suárez, A.; Aguilera, M. Representative Bacillus Sp. AM1 from Gut Microbiota Harbor Versatile Molecular Pathways for Bisphenol A Biodegradation. Int. J. Mol. Sci. 2021, 22, 4952. [Google Scholar] [CrossRef]
- Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients 2019, 11, 22. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily Intake of Lactobacillus Alleviates Autistic-like Behaviors by Ameliorating the 5-Hydroxytryptamine Metabolic Disorder in VPA-Treated Rats during Weaning and Sexual Maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of Exposure to Bisphenol A and Ethinyl Estradiol on the Gut Microbiota of Parents and Their Offspring in a Rodent Model. Gut Microbes 2016, 7, 471–485. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Ohshima, M.; Ohno, S.; Nakajin, S. Inhibitory Effects of Some Possible Endocrine-Disrupting Chemicals on the Isozymes of Human 11beta-Hydroxysteroid Dehydrogenase and Expression of Their mRNA in Gonads and Adrenal Glands. Environ. Sci. 2005, 12, 219–230. [Google Scholar]
- Kermath, B.A.; Thompson, L.M.; Jefferson, J.R.; Ward, M.H.B.; Gore, A.C. Transgenerational Effects of Prenatal Endocrine Disruption on Reproductive and Sociosexual Behaviors in Sprague Dawley Male and Female Rats. Toxics 2022, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Feldman, T.B.; Chitnis, T. Interplay Between Endocrine Disruptors and Immunity: Implications for Diseases of Autoreactive Etiology. Front. Pharmacol. 2021, 12, 626107. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine Disrupting Chemicals and Disease Susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Anway, M.D.; Savenkova, M.I.; Gore, A.C.; Crews, D. Transgenerational Epigenetic Programming of the Brain Transcriptome and Anxiety Behavior. PLoS ONE 2008, 3, e3745. [Google Scholar] [CrossRef]
- Tran, M.T.M.T.; Kuo, F.-C.; Low, J.-T.; Chuang, Y.-M.; Sultana, S.; Huang, W.-L.; Lin, Z.-Y.; Lin, G.-L.; Wu, C.-F.; Li, S.-S.; et al. Prenatal DEHP Exposure Predicts Neurological Disorders via Transgenerational Epigenetics. Sci. Rep. 2023, 13, 7399. [Google Scholar] [CrossRef]
- Van Cauwenbergh, O.; Di Serafino, A.; Tytgat, J.; Soubry, A. Transgenerational Epigenetic Effects from Male Exposure to Endocrine-Disrupting Compounds: A Systematic Review on Research in Mammals. Clin. Epigenet. 2020, 12, 65. [Google Scholar] [CrossRef]
- Crews, D.; Gillette, R.; Scarpino, S.V.; Manikkam, M.; Savenkova, M.I.; Skinner, M.K. Epigenetic Transgenerational Inheritance of Altered Stress Responses. Proc. Natl. Acad. Sci. USA 2012, 109, 9143–9148. [Google Scholar] [CrossRef]
- Krishnan, K.; Rahman, S.; Hasbum, A.; Morales, D.; Thompson, L.M.; Crews, D.; Gore, A.C. Maternal Care Modulates Transgenerational Effects of Endocrine-Disrupting Chemicals on Offspring Pup Vocalizations and Adult Behaviors. Horm. Behav. 2019, 107, 96–109. [Google Scholar] [CrossRef]
- Gillette, R.; Miller-Crews, I.; Skinner, M.K.; Crews, D. Distinct Actions of Ancestral Vinclozolin and Juvenile Stress on Neural Gene Expression in the Male Rat. Front. Genet. 2015, 6, 56. [Google Scholar] [CrossRef]
- Gillette, R.; Miller-Crews, I.; Nilsson, E.E.; Skinner, M.K.; Gore, A.C.; Crews, D. Sexually Dimorphic Effects of Ancestral Exposure to Vinclozolin on Stress Reactivity in Rats. Endocrinology 2014, 155, 3853–3866. [Google Scholar] [CrossRef]
- Hatcher, K.M.; Willing, J.; Chiang, C.; Rattan, S.; Flaws, J.A.; Mahoney, M.M. Exposure to Di-(2-Ethylhexyl) Phthalate Transgenerationally Alters Anxiety-like Behavior and Amygdala Gene Expression in Adult Male and Female Mice. Physiol. Behav. 2019, 207, 7–14. [Google Scholar] [CrossRef]
- Martini, M.; Corces, V.G.; Rissman, E.F. Mini-Review: Epigenetic Mechanisms That Promote Transgenerational Actions of Endocrine Disrupting Chemicals: Applications to Behavioral Neuroendocrinology. Horm. Behav. 2020, 119, 104677. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Ponzo, O.J.; Gobetto, N.; Samaniego, Y.A.; Reynoso, R.; Moguilevsky, J.A.; Cutrera, R.A. Effect of Di(2-Ethylhexyl) Phthalate on the Neuroendocrine Regulation of Reproduction in Adult Male Rats and Its Relationship to Anxiogenic Behavior: Participation of GABAergic System. Hum. Exp. Toxicol. 2019, 38, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yu, M.L.; Rogan, W.J.; Gladen, B.C.; Hsu, C.C. A 6-Year Follow-up of Behavior and Activity Disorders in the Taiwan Yu-Cheng Children. Am. J. Public Health 1994, 84, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Amaya, E.; Gil, F.; Fernández, M.F.; Murcia, M.; Llop, S.; Andiarena, A.; Aurrekoetxea, J.; Bustamante, M.; Guxens, M.; et al. Prenatal Co-Exposure to Neurotoxic Metals and Neurodevelopment in Preschool Children: The Environment and Childhood (INMA) Project. Sci. Total Environ. 2018, 621, 340–351. [Google Scholar] [CrossRef]
- Kim, S.; Eom, S.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.; Kim, Y.D.; et al. Association between Maternal Exposure to Major Phthalates, Heavy Metals, and Persistent Organic Pollutants, and the Neurodevelopmental Performances of Their Children at 1 to 2 Years of Age- CHECK Cohort Study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef]
- Vuong, A.M.; Yolton, K.; Xie, C.; Webster, G.M.; Sjödin, A.; Braun, J.M.; Dietrich, K.N.; Lanphear, B.P.; Chen, A. Childhood Polybrominated Diphenyl Ether (PBDE) Exposure and Neurobehavior in Children at 8 Years. Environ. Res. 2017, 158, 677–684. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.-Z.; Huang, X.; Wang, M.; Wu, J. The Association between Prenatal Exposure to Phthalates and Cognition and Neurobehavior of Children-Evidence from Birth Cohorts. NeuroToxicology 2019, 73, 199–212. [Google Scholar] [CrossRef]
- León-Olea, M.; Martyniuk, C.J.; Orlando, E.F.; Ottinger, M.A.; Rosenfeld, C.S.; Wolstenholme, J.T.; Trudeau, V.L. Current Concepts in Neuroendocrine Disruption. Gen. Comp. Endocrinol. 2014, 203, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Mendola, P.; Buck, G.M.; Sever, L.E.; Zielezny, M.; Vena, J.E. Consumption of PCB-Contaminated Freshwater Fish and Shortened Menstrual Cycle Length. Am. J. Epidemiol. 1997, 146, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, R.; Dewailly, É.; Ayotte, P.; Forget-Dubois, N.; Jacobson, S.W.; Jacobson, J.L.; Muckle, G. Exposure to Organochlorines and Mercury through Fish and Marine Mammal Consumption: Associations with Growth and Duration of Gestation among Inuit Newborns. Environ. Int. 2013, 54, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M. Persistent Environmental Pollutants and Couple Fecundity: An Overview. Reproduction 2014, 147, R97–R104. [Google Scholar] [CrossRef]
- Fourrier, J.; Deschamps, M.; Droin, L.; Alaux, C.; Fortini, D.; Beslay, D.; Conte, Y.L.; Devillers, J.; Aupinel, P.; Decourtye, A. Larval Exposure to the Juvenile Hormone Analog Pyriproxyfen Disrupts Acceptance of and Social Behavior Performance in Adult Honeybees. PLoS ONE 2015, 10, e0132985. [Google Scholar] [CrossRef]
- Nilsen, E.; Smalling, K.L.; Ahrens, L.; Gros, M.; Miglioranza, K.S.B.; Picó, Y.; Schoenfuss, H.L. Critical Review: Grand Challenges in Assessing the Adverse Effects of Contaminants of Emerging Concern on Aquatic Food Webs. Environ. Toxicol. Chem. 2019, 38, 46–60. [Google Scholar] [CrossRef]
- Ward, J.L.; Korn, V.; Auxier, A.N.; Schoenfuss, H.L. Temperature and Estrogen Alter Predator–Prey Interactions between Fish Species. Integr. Org. Biol. 2020, 2, obaa008. [Google Scholar] [CrossRef]
- Eikenaar, C.; Müller, F.; Leutgeb, C.; Hessler, S.; Lebus, K.; Taylor, P.D.; Schmaljohann, H. Corticosterone and Timing of Migratory Departure in a Songbird. Proc. Biol. Sci. 2017, 284, 20162300. [Google Scholar] [CrossRef]
- Deem, S.L.; Holliday, D.K. Chapter 21—Impacts from Endocrine Disrupting Chemicals on Wildlife Health—A One Health Challenge. In Fowler’ s Zoo and Wild Animal Medicine Current Therapy, Volume 10; Miller, E., Lamberski, N., Calle, P., Eds.; W.B. Saunders: New Delhi, India, 2023; pp. 131–136. ISBN 978-0-323-82852-9. [Google Scholar]
- Godfray, H.C.J.; Stephens, A.E.A.; Jepson, P.D.; Jobling, S.; Johnson, A.C.; Matthiessen, P.; Sumpter, J.P.; Tyler, C.R.; McLean, A.R. A Restatement of the Natural Science Evidence Base on the Effects of Endocrine Disrupting Chemicals on Wildlife. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182416. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, Z.-H.; Zhu, X.-S. Are Endocrine Disruptors among the Causes of the Deterioration of Aquatic Biodiversity? Integr. Environ. Assess. Manag. 2010, 6, 492–498. [Google Scholar] [CrossRef]
- Moosa, A.; Shu, H.; Sarachana, T.; Hu, V.W. Are Endocrine Disrupting Compounds Environmental Risk Factors for Autism Spectrum Disorder? Horm. Behav. 2018, 101, 13–21. [Google Scholar] [CrossRef]
- Jankowska, A.; Polańska, K.; Koch, H.M.; Pälmke, C.; Waszkowska, M.; Stańczak, A.; Wesołowska, E.; Hanke, W.; Bose-O’Reilly, S.; Calamandrei, G.; et al. Phthalate Exposure and Neurodevelopmental Outcomes in Early School Age Children from Poland. Environ. Res. 2019, 179, 108829. [Google Scholar] [CrossRef]
- Chevrier, C.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Dardier, V.; Le Sourn-Bissaoui, S.; Rouget, F.; Monfort, C.; Gaudreau, E.; Mercier, F.; et al. Childhood Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment at Six Years of Age. NeuroToxicology 2016, 54, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cunha, Y.G.d.O.; Amaral, G.C.B.d.; Felix, A.A.; Blumberg, B.; Amato, A.A. Early-Life Exposure to Endocrine-Disrupting Chemicals and Autistic Traits in Childhood and Adolescence: A Systematic Review of Epidemiological Studies. Front. Endocrinol. 2023, 14, 1184546. [Google Scholar] [CrossRef] [PubMed]
- Coady, K.K.; Biever, R.C.; Denslow, N.D.; Gross, M.; Guiney, P.D.; Holbech, H.; Karouna-Renier, N.K.; Katsiadaki, I.; Krueger, H.; Levine, S.L.; et al. Current Limitations and Recommendations to Improve Testing for the Environmental Assessment of Endocrine Active Substances. Integr. Environ. Assess. Manag. 2017, 13, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudłak, B.; Williams, M.J.; Jönsson, J.; Schiöth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Non-Monotonic Dose Responses in Studies of Endocrine Disrupting Chemicals: Bisphenol A as a Case Study. Dose Response 2013, 12, 259–276. [Google Scholar] [CrossRef]
- Burton, N.O.; Greer, E.L. Multigenerational Epigenetic Inheritance: Transmitting Information across Generations. Semin. Cell Dev. Biol. 2022, 127, 121–132. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Jarosz, D.F. More than Just a Phase: Prions at the Crossroads of Epigenetic Inheritance and Evolutionary Change. J. Mol. Biol. 2018, 430, 4607–4618. [Google Scholar] [CrossRef]
- Peng, D.; Wang, C.; Li, K.-L.; Gan, Z.-X.; Li, Y.-H.; Wang, H.-W.; Li, Q.-Y.; Liu, X.-W.; Sun, H.-Y.; Jing, Y.-Y.; et al. The Establishment of Transgenerational Epigenetic Inheritance in the C. elegans Germline Is Mediated by Lipid Metabolism. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kumar, V.; Deepika, D.; Sharma, R.P. Integrated Translation Framework for Endocrine Disruptors in the Area of Computational Toxicology. In Challenges in Endocrine Disruptor Toxicology and Risk Assessment; The Royal Society of Chemistry: Cambridge, UK, 2020. [Google Scholar]
- Bornehag, C.-G.; Engdahl, E.; Unenge Hallerbäck, M.; Wikström, S.; Lindh, C.; Rüegg, J.; Tanner, E.; Gennings, C. Prenatal Exposure to Bisphenols and Cognitive Function in Children at 7 Years of Age in the Swedish SELMA Study. Environ. Int. 2021, 150, 106433. [Google Scholar] [CrossRef]
- Özel, F.; Stratmann, M.; Lindh, C.; Gennings, C.; Bornehag, C.-G.; Rüegg, J. Prenatal Exposure to Phthalates and Gender-Specific Play Behavior at Seven Years of Age in the SELMA Study. Environ. Int. 2023, 178, 108029. [Google Scholar] [CrossRef]
- Hyland, C.; Mora, A.M.; Kogut, K.; Calafat, A.M.; Harley, K.; Deardorff, J.; Holland, N.; Eskenazi, B.; Sagiv, S.K. Prenatal Exposure to Phthalates and Neurodevelopment in the CHAMACOS Cohort. Environ. Health Perspect. 2019, 127, 107010. [Google Scholar] [CrossRef]

| Effect Level | Main Mechanism Involved | Observed Outcomes | Type of Study/Model System Investigated |
|---|---|---|---|
| Epigenetic level | DNA methylation, histone modifications, and altered noncoding RNAs | Altered gene expression patterns, transgenerational inheritance of phenotypes [135,181,182,183,184,185,186,187] | Mechanistic studies on rodent models |
| Neurodevelopmental level | Disruption of neural circuit formation, altered neurotransmitter systems, and hormone receptor modulation | Cognitive deficits, anxiety, and social behavior alterations [135,181,188,190,192,193,194,195,208,209,210] | Mechanistic studies on rodent models; Observational studies on human cohorts |
| Behavioral level (individual) | Neuroendocrine axis disruption (hypothalamic–pituitary–gonadal axis, hypothalamic–pituitary–adrenal axis), synaptic plasticity impairment | Anxiety, hyperactivity, altered sociability, attention deficits [135,181,188,190,192,193,194,195,208,209,210] | Observational studies on human cohorts; mechanistic studies on rodent models |
| Behavioral level (population/ ecosystem) | Disrupted mating, social structures, predator-prey dynamics | Reduced fertility, altered colony stability, increased predation risk [197,198,199,200,201,202,204,205,206] | Human observational (epidemiological); observational/speculative on various wildlife species |
| Ecosystem functions | Cumulative behavioral alterations in key species | Loss of ecosystem services (pollination, seed dispersal), and biodiversity decline [200,201,202,203,204,205,206] | Observational/speculative/mechanistic studies on wildlife models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiano, A.; Caioni, G.; D’Addario, C.; Merola, C.; Francioso, A.; Amorena, M. The Invisible Influence: Can Endocrine Disruptors Reshape Behaviors Across Generations? Stresses 2025, 5, 46. https://doi.org/10.3390/stresses5030046
Damiano A, Caioni G, D’Addario C, Merola C, Francioso A, Amorena M. The Invisible Influence: Can Endocrine Disruptors Reshape Behaviors Across Generations? Stresses. 2025; 5(3):46. https://doi.org/10.3390/stresses5030046
Chicago/Turabian StyleDamiano, Antonella, Giulia Caioni, Claudio D’Addario, Carmine Merola, Antonio Francioso, and Michele Amorena. 2025. "The Invisible Influence: Can Endocrine Disruptors Reshape Behaviors Across Generations?" Stresses 5, no. 3: 46. https://doi.org/10.3390/stresses5030046
APA StyleDamiano, A., Caioni, G., D’Addario, C., Merola, C., Francioso, A., & Amorena, M. (2025). The Invisible Influence: Can Endocrine Disruptors Reshape Behaviors Across Generations? Stresses, 5(3), 46. https://doi.org/10.3390/stresses5030046








