Abstract
Masting, or the synchronous and intermittent production of seeds, can have profound consequences for Tropical Montane Cloud Forest (TMCF) tree populations and the trophic webs that depend on their mass flowering and seeds. Over the past 80 years, the importance of Fagus mexicana Martínez (Mexican beech) masting has become apparent in terms of conservation and management, promoting regeneration, and conserving endangered tree species, as well as the conscientious development of edible beechnuts as a non-timber forest product. The establishment of the relict-endemic Mexican beech is unknown, and several microenvironmental factors could influence natural regeneration. Thus, this study was conducted in two well-preserved Mexican beech forests to assess the influence of light incidence and soil moisture on the natural germination and seedling establishment of beeches. During two masting years (2017 and 2024), we assessed in situ beechnut germination and establishment. We tested the effect of the microenvironment of the oldest beeches on beechnut germination and seedling establishment. Our study highlights the complexity of the microenvironment of old beeches influencing the early stages of establishment and provides insights into possible conservation actions aimed at mitigating the impact of environmental change and humans.
1. Introduction
Tropical Montane Cloud Forests (TMCFs) are sensitive to climatic variation because they are characterized by species adapted to high-moisture environments [1]. Nevertheless, drought events have a considerable influence on montane ecosystems [2], and their impact on phenological processes will undoubtedly affect the recruitment of relict-endemic tree species. Mexican beech (Fagus mexicana Martínez) is a long-lived (>200 years old) Oligo-Miocene relict-endemic tree inhabiting fragmented TMCFs (2–42.5 ha) in the Sierra Madre Oriental, eastern Mexico. Older Mexican beeches (≥50 years old [3]) have a significant effect on seed production, germination, and seedling establishment [4,5]. During masting years (2- to 8-year intervals [6]), residents collect seeds (henceforth beechnuts) as a food source or to sell locally, decreasing the natural regeneration [7].
Beechnuts develop and ripen by late summer (June–July), and they disperse and germinate immediately in early fall (late August–early September). Certain aspects of beechnut biology, such as rapid viability loss and low germination rates, could affect the conservation of natural populations [4]. Older trees significantly contribute to both seed quantity and viability during masting events. As tree age increases (≥50 years old), beechnuts are dispersed randomly near each tree [3]. Mexican beech forests are closely associated with fog, light incidence, soil moisture, pH, and soil temperature [8]. Even slight variations in temperature and moisture can disrupt the timing of germination, influencing seedling survival and, consequently, the continuity of this relict-endangered TMCF tree in Mexico [6].
Although the ecology of beechnuts at the local level has been scarcely studied, there are a few notable exceptions: American beech (Fagus grandifolia Ehrh.) in the USA [9], Japanese beech (Fagus crenata Blume) in Japan [10], oriental beech (Fagus orientalis Lipsky) in Turkey [11], and common beech (Fagus sylvatica L.) in Sweden [12]; likewise, older beeches’ sheltering effect during germination and seedling establishment stages are unknown. In this study, we assess for the first time how older beeches’ microenvironmental shelter affects beechnut germination, and seedling establishment. Thus, this study aimed at (1) assessing the beechnut variability of regeneration performance of Mexican beech (Fagus mexicana) between two masting years (2017 and 2020); and (2) assessing the beechnut ecological influence of older Mexican beeches’ microenvironmental light incidence and soil moisture. Specifically, we address the following questions: (i) What are the strongest microenvironmental drivers of beechnut germination and seedling establishment in Mexican beech species? (ii) Is there an influence of the older beech tree microenvironmental factors on germination and establishment? (iii) What are the ecological implications of these findings for Mexican beech conservation and management?
2. Results
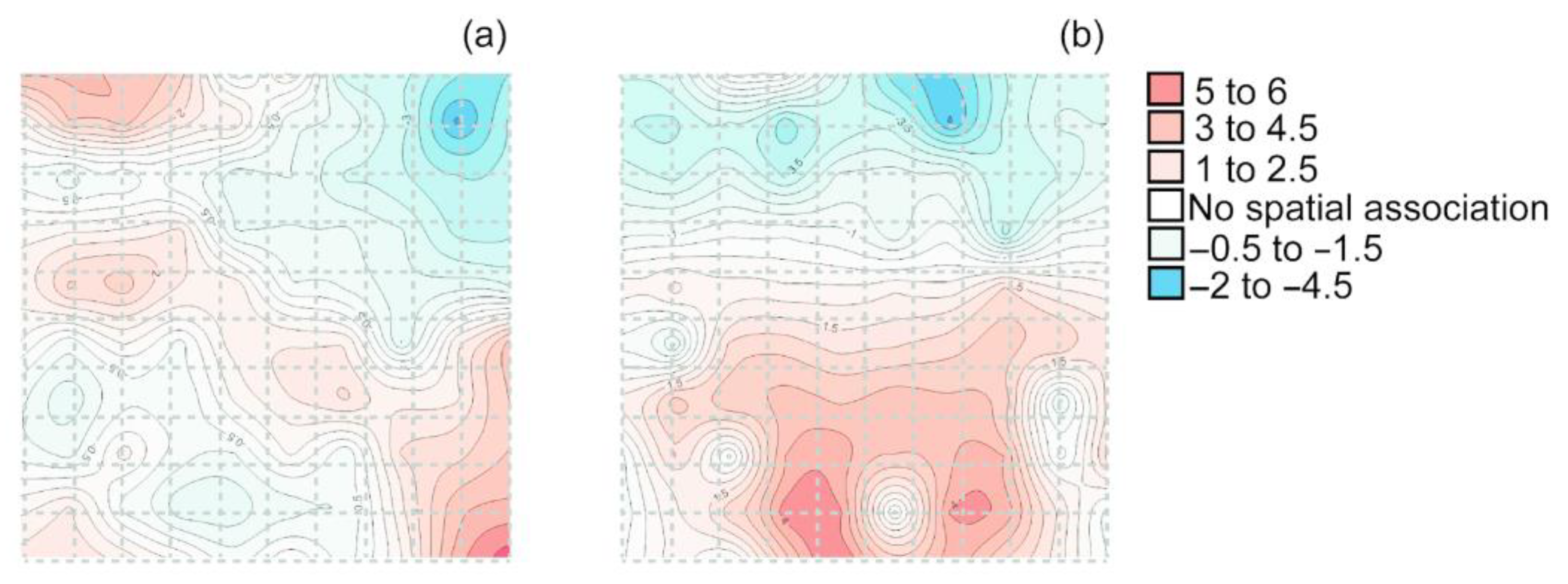
Contour maps of the index of clustering visually displayed a spatial older beech (≥50 years old) distribution of the main gaps (blue color) and hotspots (red color) within each permanent plot (Figure 1). In the two study forests, older beeches exhibited significant spatial aggregation (Figure 1a, Tutotepec: Ia > 1; and Figure 1b, La Cantera: Ia > 2.54).
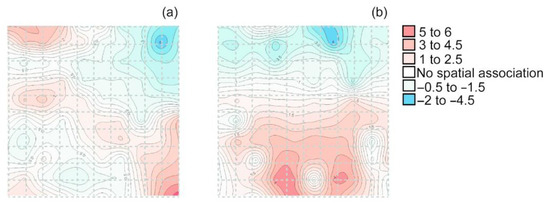
Figure 1.
Interpolated map of clustering of older beeches in two Mexican beech forests (a) Tutotepec and (b) La Cantera. Blue color = gaps, and red color = hotspots.
The older beech trees exhibited considerable age variation, ranging from 102 to 265 years (Table 1). The growth rings of Mexican beech also displayed a small number of false rings (less than 0.2%), which contributed to a higher mean sensitivity (MS) in the species chronology. The independent chronologies extended up to 120 years for Tutotepec and 265 years for La Cantera (Table 1). The R-bar values for Tutotepec (0.48) and La Cantera (0.40) indicate a strong common signal among trees at each site.

Table 1.
Tree-ring characteristics for two Mexican beech forests.
During 2017, in both study forests, beechnut germination was high from July to September. A total of 400 beechnuts were placed in the boxes; 189 seedlings emerged and survived in Tutotepec (94% survival), and 268 seedlings were detected in La Cantera (89% survival). Mortality was low in both forests (approximately 5%). Nevertheless, in 2020, beechnut production was absent.
Survival analysis indicated that the emergence date is a key determinant of seedling survival (Table 2). The parameters β0 and β1 were positive and statistically significant in both study forests, indicating greater survival among seedlings that germinated before September. In contrast, β4 was associated with lower seedling survival. Beechnuts that germinated between July_1 and July_2, as well as August_1 and August_2, exhibited significantly higher survival rates compared to those that emerged in September. During the August_2 period, seedling mortality was low, with β1 + β2 = −0.124 in Tutotepec and β1 + β2 = −0.512 in La Cantera. This was followed by September_1 and September_2, which had higher seedling mortality values of −0.554 and −0.785 for Tutotepec, and −0.702 and −0.666, respectively, for La Cantera (Table 2).

Table 2.
The goodness of fit and estimated parameters for the survival of Mexican beech seedlings by date in two Mexican beech forests (Tutotepec and La Cantera).
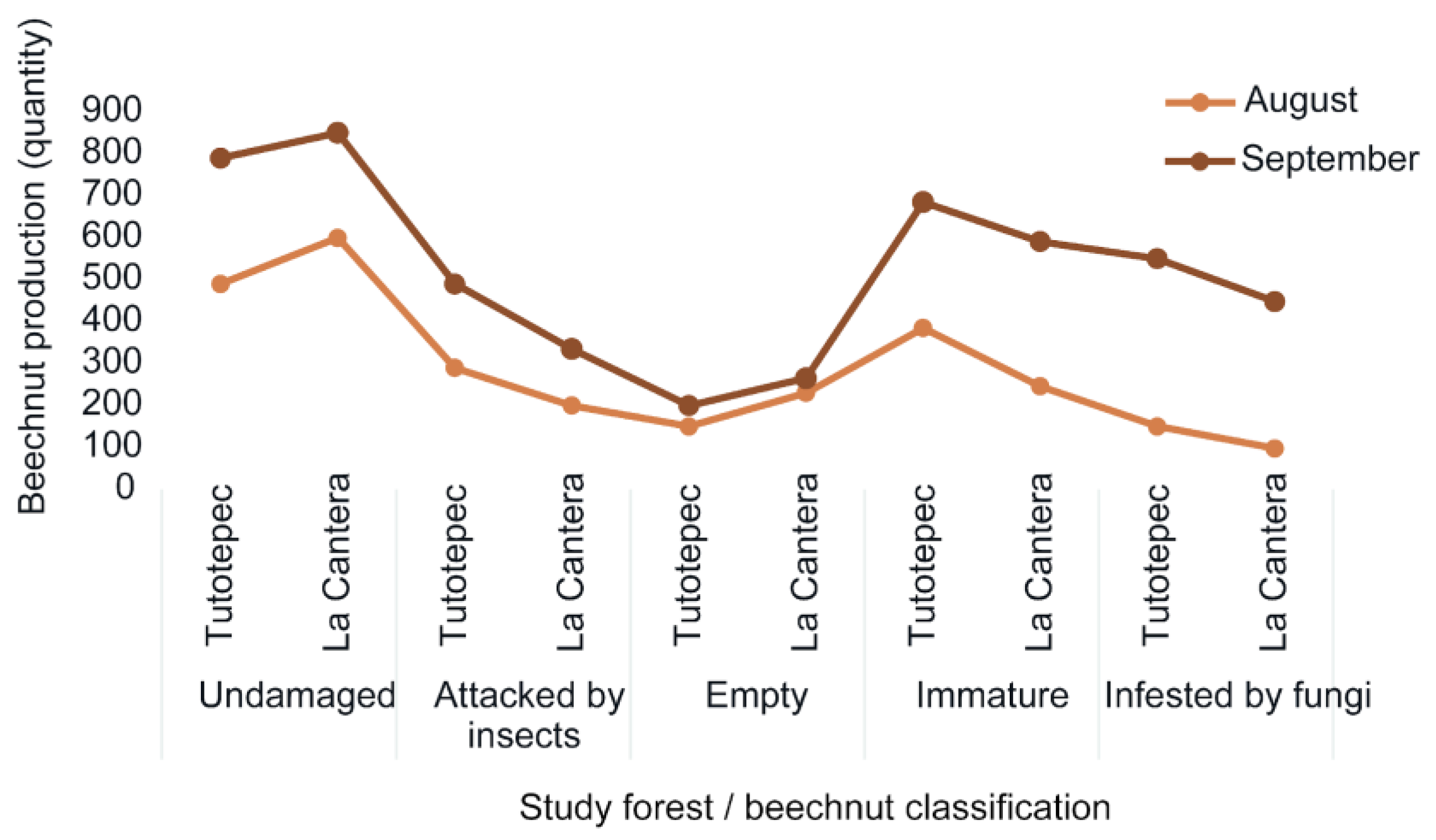
Overall, between 650 and 825 undamaged beechnuts were observed in the two study forests (Figure 2). During the masting year, both forests exhibited synchronous seed production, with most seeds being dispersed in August and September 2017 (Figure 2).
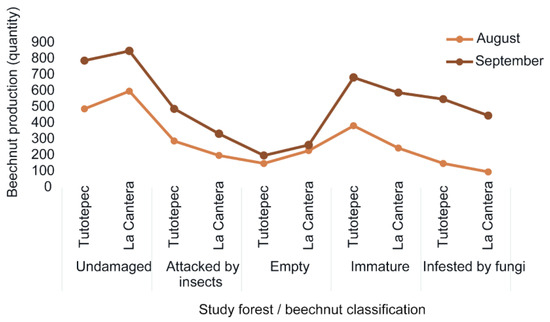
Figure 2.
Values of beechnut production are expressed as total number of seeds counted for the entire sampling period, and by beechnut classification in each study forests (Tutotepec and La Cantera).
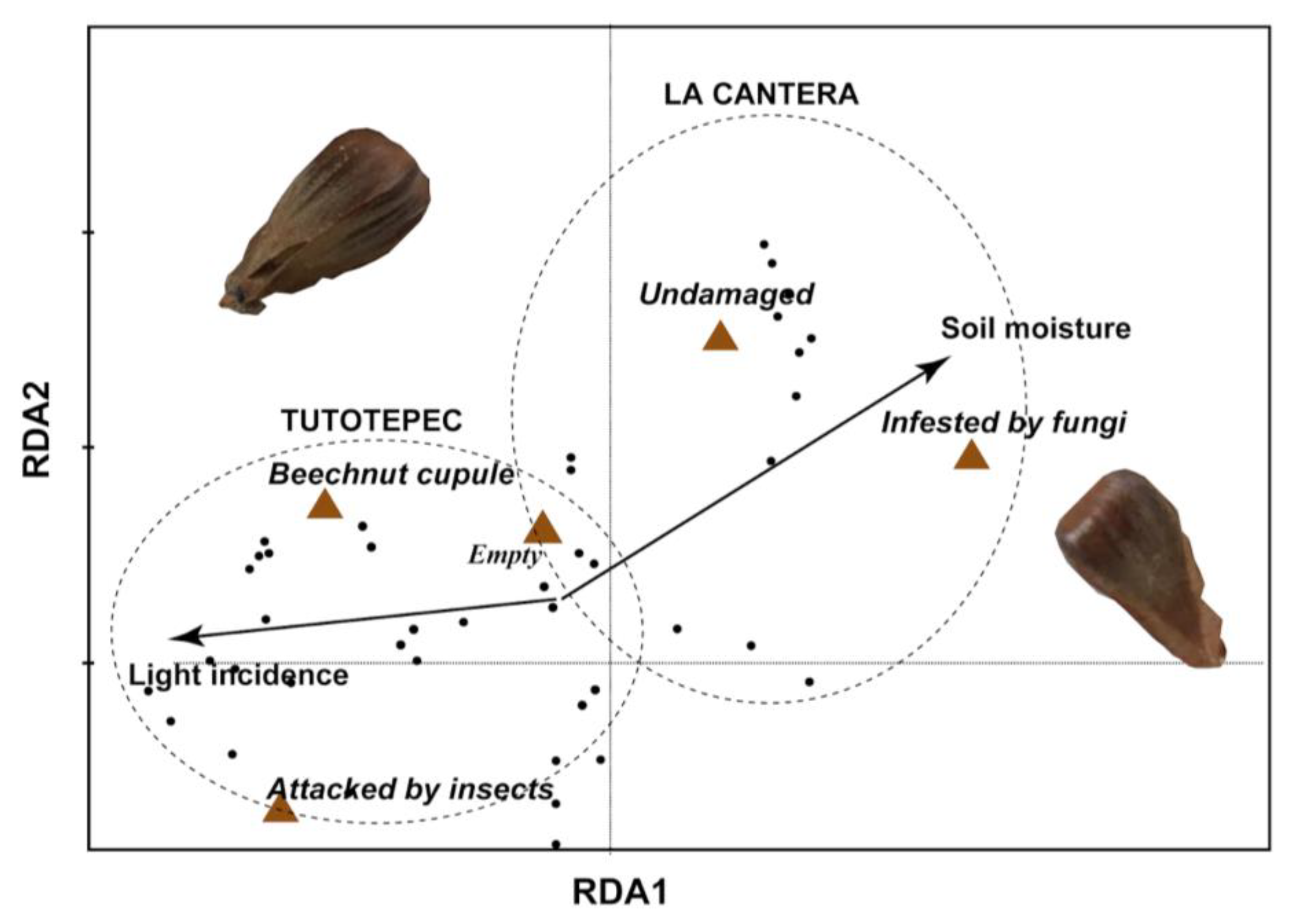
We found that specific older beech’s microenvironment factors influenced on beechnut quality. The RDA exhibited that the relationship between beechnut quality and microenvironmental variables (light incidence and soil moisture) were significant (p < 0.01). The first two axes explained 95% of the variance in beechnut quality and between beechnut quality and microenvironmental relationship. Soil moisture in La Cantera is associated with undamaged beechnuts as well as increased fungal infestation. In contrast, in Tutotepec, light incidence on beechnuts is linked to higher rates of insect attack, a greater proportion of empty cupules, and an increased total number of cupules (Figure 3).
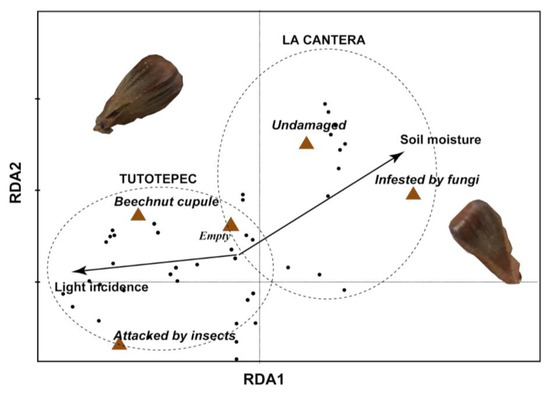
Figure 3.
Projection of older beeches’ microenvironmental factors, study forests (Tutotepec and La Cantera) and beechnut quality in the ordination space constructed with RDA. The length and direction of the arrows show the relative degree and direction of association.
3. Discussion
Spatial pattern sequences demonstrated important hotspots related to the natural older beech distribution in Mexican beech forests. Specific phenological processes (such as masting) have a wide impact on forest ecosystem dynamics (i.e., mass flowering, seed production, dispersal, or establishment success [15]). We detected that beechnuts harvested under older beeches displayed a high undamaged quantity and germinated successfully. Similar results concerning productivity and survival were found for Fagus orientalis in the Middle East [11], F. sylvatica in Europe [16], F. grandifolia in the United States [17], F. crenata and F. japonica in Japan [18], and F. hayatae, F. chienii, F. lucida, F. longipetiolata, and F. engleriana in China [19]. Furthermore, we found a significant interaction between seedling establishment, light incidence, and soil moisture, in which the older mother beeches directly influence on seed germination [4,20]. Notwithstanding, the differences detected in this study according to the age of beeches could also be because the fact that there are performing reproductive alternatives during the tree’s lifetime [6,21].
Moreover, this study shows that the beechnut from older mother beeches have a clear advantage over those produced by young beeches concerning germination. If this is correct, it could explain why older mother beeches develop beechnuts with higher germination rates since they would express specific microenvironment factors that gave them an adaptive advantage in their establishment. Likewise, older beech tree shelter effect on seed germination and seedling establishment has received limited attention despite its potential key role in forest regeneration and resilience to biotic and abiotic stresses [22].
Several reports have been published on the older beech tree shelter effects of microenvironmental factors on the subsequent seed germination [4,6]. This study suggests that older beech shelter is crucial for seedling establishment, providing specific abiotic factors (i.e., soil moisture and light incidence). Unfortunately, no previous studies about older beech effect on beechnut viability identify seed productivity on a fine scale. The scarcity of published studies in Mexican beech forests along a broad range of habitat conditions makes it difficult to assess whether this potential relationship between age beech and quality beechnut production fulfills this prediction for a wider spatial scale.
Overall, older beech spatial distribution can trigger beechnut germination and seedling aggregation [5]. Beechnut quality (undamaged), and (infested by fungi) strongly varied from forest to forest, and it was linked to high moisture rates during each masting year. During 2017, Tutotepec displayed a high amount beechnut attacked by insects, whereas La Cantera showed high beechnuts undamaged. Beechnut quality may be strongly influenced by local climatic fluctuations [23,24]; nevertheless, in Fagus orientales Lipsky from Japan, beechnut quality is influenced by the litterfall amount and summer temperature [25]. The present study has shown how older beeches’ microenvironmental factors are linked to beechnut germination and seedling establishment. This shelter association explains the spatial variability of beechnut production at local scale.
Beech trees exhibit masting behavior, characterized by highly variable and synchronized seed production across years [6,10]. Nonetheless, even during pronounced masting events—years typically marked by very low seed output—beechnut production is rarely [4], if ever, truly zero (as 2024 year). Several ecological and physiological factors explain this phenomenon. Microenvironmental differences may allow some individual trees to produce a small number of seeds even in years when most do not [24,25]. For example, beechnut production is influenced by summer weather conditions in the two years preceding a mast event, but these conditions may not be uniform across all individuals or sites [22]. Likewise, tree-specific factors, including age, height, crown size, and resource availability, contribute to seed production. Some trees may have accumulated more resources or may be less affected by stressors, enabling limited seed production even when most of the population is in a low-output phase [3]. This individual variation ensures that a small amount of seeds are developed nearly every year.
Although masting is driven by climatic cues—such as mild, wet summers two years before, and hot, dry summers one year before the mast year—the timing and intensity of these cues can differ regionally and locally [3,4]. As a result, not all trees respond identically, leading to some seed production even in years classified as “off” or low-mast years [4]. The internal resource dynamics of beech trees are complex. While most resources may be diverted away from reproduction in non-mast years, some trees may still allocate enough to produce a limited number of beechnuts, particularly if their resource reserves are higher because of favorable conditions in previous years [12,15,17].
Beechnut production is rarely zero during masting events because of the interplay of microenvironmental variability, individual tree differences, incomplete synchronization of climatic cues, internal resource allocation, and evolutionary strategies [3,12]. These factors together ensure that some level of seed output persists, even in years of otherwise minimal production. Recent studies in Croatia and other European regions (Fagus sylvatica) have reported years with total absence of beechnut production at the population level. For example, long-term monitoring between 2015 and 2022 revealed that 2015, 2017, 2019, and 2020 were years with no beechnut crops at all in multiple forest sites, despite consistent sampling methods and coverage of tree crowns [3]. This phenomenon is not limited to a single region and has been observed in Mexican beech population during 2024 as well. Years with zero beechnut production have significant ecological consequences, affecting wildlife that depend on beechnuts as a food resource and influencing forest regeneration patterns [10,17,18]. These events highlight the sensitivity of masting cycles to climate variability and underscore the importance of long-term monitoring for understanding and managing beech forests under changing environmental conditions [3,4].
According to [23], a beech forest has specific microenvironmental conditions (i.e., moisture, pH, essential carbon, water, and nutrients to germinate); nevertheless, a complete understanding of the beech seedlings and saplings ecological requirements are rarely included in forest conservation and management strategies of the endangered TMCF tree species [5,26]. Most studies of beechnut germination in situ show that seedlings (i.e., Fagus sylvatica [27,28]; F. grandifolia [17]; F. orientalis [29]; F. crenata [30], and F. japonica [31]) can survive under specific microenvironmental factors such as light incidence (3–5%) and soil moisture (90%). Today, the greatest challenge is to link behavior patterns of animal dispersers [32,33]. The consequences of seed dispersal patterns should be assessed more explicitly, including test predictions based on disperser behavior against field data. In this way, we might hope to develop an ecological understanding of seed dispersal by animals.
4. Materials and Methods
4.1. Study Area
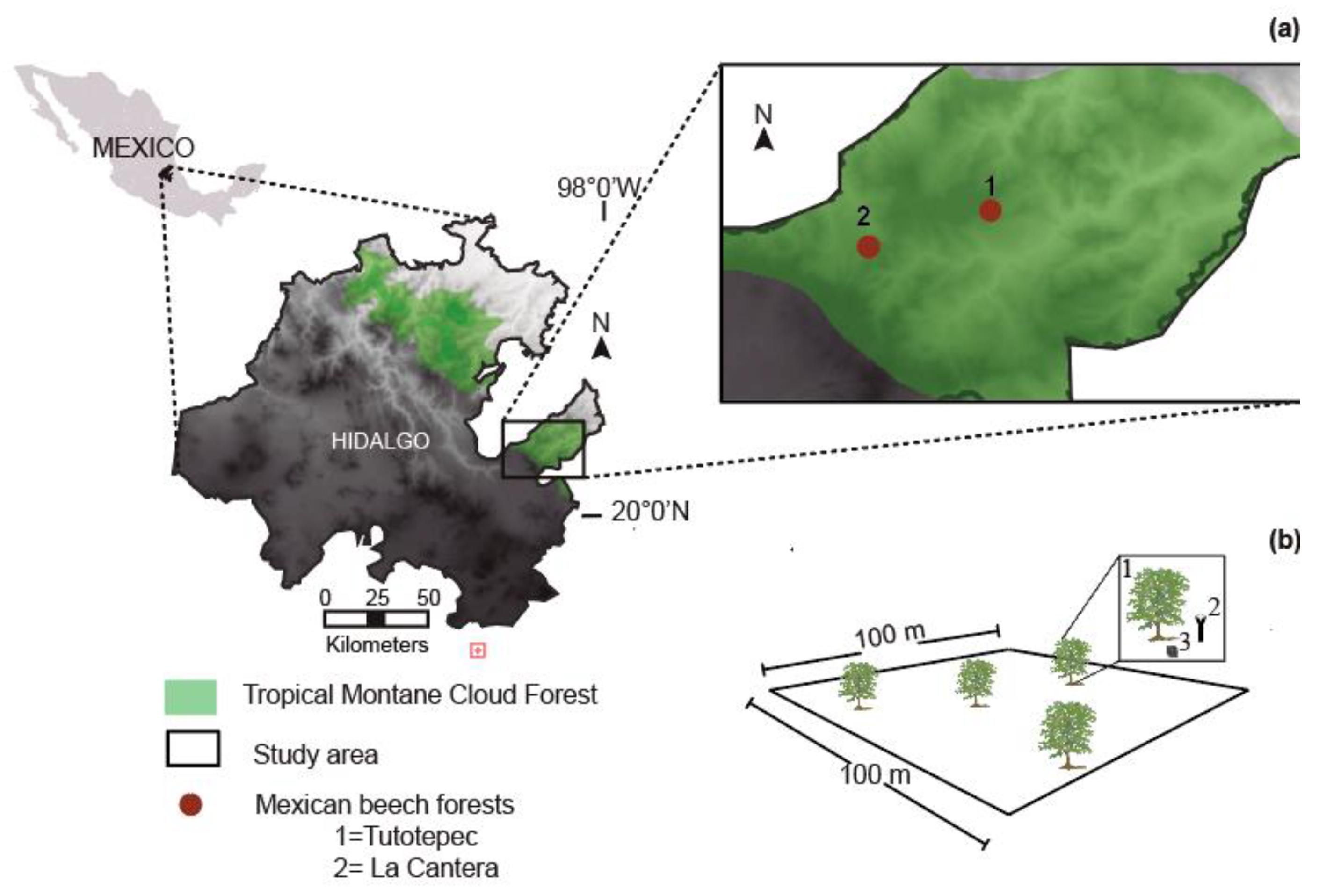
The study was conducted in two Mexican beech forests, San Bartolo Tutotepec, Hidalgo state, central-eastern Mexico: (1) Tutotepec, 20.409158, −98.250946; 1800 m asl; and (2) La Cantera, 20.412155, −98.281866; 1944 m asl; Figure 4a. The climate is temperate (Cwb [34]), with temperatures of 14.5 to 24.4 °C and 824 to 2458 mm annual precipitation [20]. The soil type is Humic (Th) [35] with a light sandy-clay loam texture and pH values between 4 and 6 [3].
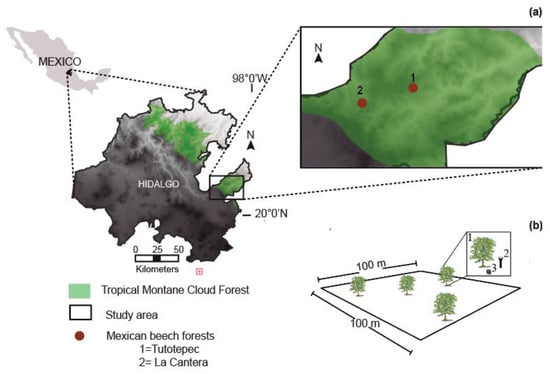
Figure 4.
(a) Geographical location of the Mexican beech forest in the Tropical montane cloud forest of the Sierra Madre Oriental, Hidalgo state, Mexico. (b) Schematic representation of the 100 × 100 m2 permanent plot; (1) mother Mexican beech selected; (2) circular seed trap; and (3) box of 20 × 20 cm2.
The vegetation is dominated by relict-endemic species, including Fagus mexicana, Magnolia schiedeana Schltdl., Quercus trinitatis Trel., Q. meavei S. Valencia, Sabas & O.J. Soto, and Q. delgadoana S. Valencia, Nixon & L.M. Kelly [8]. The study forest is surrounded by pine or pine–oak forests.
4.2. Sampling Protocol and Mother Mexican Beech Selection
A permanent plot of 100 × 100 m2 [8] was set up in two representative Mexican beech forests during two masting year (2017 and 2024), as far as possible from human settlements and roads. In each permanent plot, we randomly selected 20 dominant old beeches (Figure 4b) with a diameter of ≥100 cm at breast height (DBH ≥ 15 m), and ≥50 years old. To determine the ages of the beeches (geographically referenced), we assessed the tree ring chronologies according to standard dendrochronological procedures [36].
We described the spatial pattern of older beech distribution in each permanent plot (we set 10 transects, 100 m in length and 10 m apart) using the software SADIE v1.22 (Spatial Analysis by Distance IndicEs [37,38]). SADIE incorporates spatial information of the counted data from geographically referenced points [38], allowing us to identify the exact mother beech hotspots. To assess distance regularity (D), which is considered by a high proportion of zeros and non-normal distribution, SADIE uses the transportation algorithm from the linear programming literature [38].
In the present case, the mother beech distribution across subplots was related to 999 randomly created distributions. We assessed the spatial distribution using an aggregation index (Ia) of the degree of mother beech clustering, which was achieved for each data set to determine overall 99,999 randomizations where Ia = 1 shows a random arrangement of counts of no significant spatial pattern; Ia > 1 shows aggregated clusters; and Ia < 1 shows a regular arrangement of counts [39].
Finally, we visualized the spatial mother beech aggregation with the red-blue method [37,39], using the software Surfer® v. 14 (Golden Software, Denver, CO, USA).
4.3. Beechnut Sample Collection
From late July to September, we placed a circular seed trap (1 m2 in surface area) below each older beech selected (Figure 4b(1)). Each trap consisted of a plastic funnel attached to a PVC tube with a diameter of 5.08 cm and 1 m in height (Figure 4b(2)) to avoid beechnut removal by animals (the main seed predator) after a beechnut drops. The seed trap establishment (Figure 4b(3)) was carried out in early June before the beechnuts are released. Hence, beechnuts were collected and counted from July to September from each trap.
We classified the beechnuts into five morphological categories: (1) undamaged; (2) attacked by insects (by the larvae presence); (3) empty; (4) immature, and (5) infested by fungi (Figure 5 [6]). Therefore, the sum of beechnuts in all five categories estimates the total seed crop.

Figure 5.
Beechnut classification. (a) Undamaged; (b) attacked by insects; (c) empty; (d) beechnut cupule; and (e) infested by fungi.
Beechnut Germination
Next to each mature beech tree selected, we placed a box (20 × 20 cm) containing 10 beechnuts that were randomly collected. These boxes were positioned in situ, directly adjacent to each older beech, to allow for natural germination conditions. Each box was sealed with a protective mesh to prevent predation, while the mesh holes permitted sufficient light penetration necessary for germination. The boxes were examined every 15 days over a two-month period during the masting event (July to September). Beechnut survival was assessed using a General Linear Model (GLM) with a binomial distribution [40] using Gaussian (for count data) distributions [41]. GLM analyses were achieved using R software v.3.4.3 (R Development Core Team) through the glm function in stats package [42].
The adjustment of total beechnut survival for each box was identified using a complementary log-log link structure. The linear model used begins with the following formula:
where n is the linear model of the complementary log-log function, β1 is the model parameters, and t is the time in days in which the change in the proportion of seedling survival and death occurs. In this way, mortality is a function of time in days from the date of emergence. Beechnut survival is given by β1 depending on the moment the seedlings have emerged, and the mortality rate fluctuates with the date of emergence. The linear model was expanded to the following expression to consider both effects:
The value reference of the model was the date (i.e., 1 August) that makes up all the observations recorded after June (10 July, 24 July, 1 August, 15 August, 1 September, and 15 September).
4.4. Relationship Among Beechnut Classification and Microenvironmental Factors
We measured soil moisture and light incidence next to the 20 selected older beeches. These microenvironment variables were assessed for three months (24 July, 1 August, 15 August, 1 September, and 15 September) between 8:00 am and 12:00 pm to standardize environmental daily fluctuations [8].
Soil moisture was measured to a depth of 2 cm using a hygrometer (Lincoln Soil Moisture Meters) located below each mother beech. The soil moisture value varied from 1 to 10, where 1 = dry and 10 = saturated. We obtained four soil moisture measurements at the four main cardinal points (i.e., N, S, E, and W) of the 20 selected mother beeches. We performed a statistical analysis of these microenvironmental data using the average values to represent soil moisture.
Light incidence was measured for each mother beech at the four cardinal points using a canopy densitometer Model A [43]. These data were averaged and verified for normality using the Shapiro–Wilk test for statistical analyses.
Relationships among beechnut classification were performed for each study forest with stepwise Redundancy Analysis (RDA [44]), a canonical multivariate method to analyze the relationship between mother beech microenvironmental factors and beechnut amount according to its classification between the study forests. We intend for the technique to display main trends in variations in a multi-dimensional data set in a reduced space of selected, linearly independent dimensions [45]. We achieved the analyses with the R–software and the vegan package [46].
5. Conclusions
The present study shows the complexity of the relationships between the age of older beech and microenvironment factors influencing beechnut germination and seedling establishment. Not all the patterns and sources of variation in seed dispersion that we discuss here will prove important to understanding beechnut germination and seedling establishment. To determine which microenvironmental factors are essential, we need longer term studies at a larger scale among masting events. Spatial indices obtained with SADIE exhibited that the older beech tree distribution enhances beechnut germination and seedling establishment. We suggest that older beeches provide a shelter-effect to seedlings and saplings, as suggested by [23]. Seed germination is favored by specific light incidence and soil moisture under mother beech; nevertheless, the suitable micro-habitat for beech seedlings appears to be influenced by the degree of canopy openness and the presence of Magnolia schiedeana and several oaks [5]. The present study advances our understanding of the older beeches’ microenvironmental processes involved in the natural establishment of this endangered-relict TMCF tree species; notwithstanding, the differences in the vegetation composition between forests regulate moisture and light incidence in the understory [5,47].
The beechnut ecology understanding shown in this paper may help evolve strategies for conservation and reforestation of disturbed TMCFs in eastern Mexico. The natural regeneration during each masting event of Mexican beech through seedlings is relatively poor in this region because of the high density of seed and seedling predators (i.e., birds, mammals, insects, and fungi), and unfavorable fragments of Mexican beech forest anthropic pressure in mountainous areas. Nonetheless, beechnut germination on most hotspots has implications for endangered-relict TMCF management and conservation.
Spatial ecology, along with the mathematical techniques developed to investigate the microenvironmental effects of older beeches on beechnuts, provides both a framework and tools for deepening our understanding of seed ecological processes. These approaches are also valuable for applying ecological insights to the conservation and management of Mexican beech forests. Such advances will depend on the use of models to integrate information from field studies (e.g., [48,49,50,51,52]), enabling researchers to explore large-scale consequences that are difficult to assess directly in the field.
Author Contributions
E.C.R.-R. and B.A.-M.: Conceptualization; B.A.-M. and E.C.R.-R.: investigation, methodology; E.C.R.-R.: project administration and supervision; E.C.R.-R.: writing—original draft; B.A.-M. and E.C.R.-R.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
I. Luna-Vega received financial support through grant IN223118 project from PAPIIT, DGAPA-UNAM. The first author acknowledges the financial support granted by the postdoctoral fellowship CONACYT 2019–2020.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thankful to Susana Guzmán Gómez (Laboratorio de Microscopía y Fotografía de la Biodiversidad II, Instituto de Biología, UNAM) for technical assistance with the digital beechnut images. The second author acknowledges the funding provided by Isolda Luna-Vega.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TMCF | Tropical Montane Cloud Forest |
References
- Williams-Linera, G. El Bosque de Niebla Del Centro de Veracruz: Ecologia, Historia y Destino En Tiempos de Fragmentacion y Cambio Climatico; Instituto de Ecología A.C.: Xalapa, Mexico, 2012; ISBN 9707091010. [Google Scholar]
- Rahbek, C.; Borregaard, M.K.; Antonelli, A.; Colwell, R.K.; Holt, B.G.; Nogues-Bravo, D.; Rasmussen, C.M.Ø.; Richardson, K.; Rosing, M.T.; Whittaker, R.J.; et al. Building Mountain Biodiversity: Geological and Evolutionary Processes. Science 2019, 365, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Architecture and Development of Mexican Beech Forest. In Vegetation Science in Forestry; Box, E.O., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1995; pp. 325–343. [Google Scholar]
- Alvarez-Aquino, C.; Williams-Linera, G. Seedling Bank Dynamics of Fagus grandifolia var. mexicana before and after a Mast Year in a Mexican Cloud Forest. J. Veg. Sci. 2002, 13, 179–184. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Martínez-Falcón, A.P.; Luna-Vega, I. Spatial Patterns of Mexican Beech Seedlings (Fagus grandifolia subsp. mexicana (Martínez) A.E. Murray): Influence of Canopy Openness and Conspecific Trees on Recruitment Mechanisms. Ann. For. Sci. 2018, 75, 27. [Google Scholar] [CrossRef]
- Godínez-Ibarra, O.; Ángeles-Pérez, G.; López-Mata, L.; García-Moya, E.; Valdez-Hernández, J.I.; de Los Santos-Posadas, H.; Trinidad-Santos, A. Seed Rain and Seedling Emergence of Fagus grandifolia subsp. mexicana at La Mojonera, Hidalgo, Mexico. Rev. Mex. Biodivers. 2007, 78, 117–128. [Google Scholar]
- Rodríguez-Ramírez, E.C.; Sánchez-González, A.; Ángeles-Pérez, G. Current Distribution and Coverage of Mexican Beech Forests Fagus grandifolia subsp. mexicana. Mexico. Endanger. Species Res. 2013, 20, 205–216. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Sánchez-González, A.; Ángeles-Pérez, G. Relationship between Vegetation Structure and Microenvironment in Fagus grandifolia subsp. mexicana Forest Relicts in Mexico. J. Plant Ecol. 2016, 11, 237–247. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Fairbairn, M.; Fahey, T.J. Growth and Survivorship of American Beech (Fagus grandifolia Ehrh.) Seedlings in a Northern Hardwood Forest Following a Mast Event. J. Torrey Bot. Soc. 2008, 135, 328–345. [Google Scholar] [CrossRef]
- Hiroki, S.; Matsubara, T. Fluctuation of Nut Production and Seedling Appearance of a Japanese Beech (Fagus crenata Blume). Ecol. Res. 1995, 10, 161–169. [Google Scholar] [CrossRef]
- Nasiri, N.; Marvie Mohadjer, M.R.; Etemad, V.; Sefidi, K.; Mohammadi, L.; Gharehaghaji, M. Natural Regeneration of Oriental Beech (Fagus orientalis Lipsky) Trees in Canopy Gaps and under Closed Canopy in a Forest in Northern Iran. J. For. Res. 2018, 29, 1075–1081. [Google Scholar] [CrossRef]
- Vacchiano, G.; Hacket-Pain, A.; Turco, M.; Motta, R.; Maringer, J.; Conedera, M.; Drobyshev, I.; Ascoli, D. Spatial Patterns and Broad-Scale Weather Cues of Beech Mast Seeding in Europe. New Phytol. 2017, 215, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Guide for Computer Program ARSTAN. In The International Tree-Ring Data Bank Program Library Version 2.0 User’s Manual; Grissino-Mayer, H.D., Holmes, R.L., Fritts, H.C., Eds.; Laboratory of Tree-Ring Research: Tucson, AZ, USA, 1996; pp. 75–87. [Google Scholar]
- Burns, K.C. Masting in a Temperate Tree: Evidence for Environmental Prediction? Austral Ecol. 2012, 37, 175–182. [Google Scholar] [CrossRef]
- Peltier, A.; Touzet, M.-C.; Armengaud, C.; Ponge, J.-F. Establishment of Fagus Sylvatica and Fraxinus Excelsior in an Old-Growth Beech Forest. J. Veg. Sci. 1997, 8, 13–20. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Fahey, T.J. Seed Production of Sugar Maple and American Beech in Northern Hardwood Forests, New Hampshire, USA. Can. J. For. Res. 2017, 47, 985–990. [Google Scholar] [CrossRef]
- Shibata, M.; Tanaka, H.; Iida, S.; Abe, S.; Masaki, T.; Niiyama, K.; Nakashizuka, T. Synchronized Annual Seed Production by 16 Principal Tree Species in a Temperate Deciduous Forest, Japan. Ecology 2002, 83, 1727–1742. [Google Scholar] [CrossRef]
- Cao, K.-F.; Peters, R.; Oldeman, R.A.A. Climatic Ranges and Distribution of Chinese Fagus Species. J. Veg. Sci. 1995, 6, 317–324. [Google Scholar] [CrossRef]
- Peters, R. Ecology of Beech Forests in the Northern Hemisphere. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1992. [Google Scholar]
- Alonso-Crespo, I.M.; Silla, F.; Jiménez del Nogal, P.; Fernández, M.J.; Martínez-Ruiz, C.; Fernández-Santos, B. Effect of the Mother Tree Age and Acorn Weight in the Regenerative Characteristics of Quercus faginea. Eur. J. For. Res. 2020, 139, 513–523. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Bagniewska-Zadworna, A.; Suszka, J.; Pukacka, S. Dehydration Sensitivity at the Early Seedling Establishment Stages of the European Beech (Fagus sylvatica L.). Forests 2019, 10, 900. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-Plant Communication through Mycorrhizal Networks Mediates Complex Adaptive Behaviour in Plant Communities. AoB Plants 2015, 7, plv050. [Google Scholar] [CrossRef] [PubMed]
- Hilton, G.M.; Packham, J.R. Variation in the Masting of Common Beech (Fagus sylvatica L.) in Northern Europe over Two Centuries (1800–2001). Forestry 2003, 76, 319–328. [Google Scholar] [CrossRef]
- Etemad, V.; Sefidi, K. Seed Production and Masting Behaviour in Oriental Beech (Fagus orientalis Lipsky) Forests of Northern Iran. For. Ideas 2017, 23, 65–76. [Google Scholar]
- Bruijnzeel, L.A.; Scatena, F.N.; Hamilton, L.S. Tropical Montane Cloud Forests: Science for Conservation and Management; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Collet, C.; Chenost, C. Using Competition and Light Estimates to Predict Diameter and Height Growth of Naturally Regenerated Beech Seedlings Growing under Changing Canopy Conditions. Forestry 2006, 79, 489–502. [Google Scholar] [CrossRef]
- Collet, C.; Lanter, O.; Pardos, M. Effects of Canopy Opening on Height and Diameter Growth in Naturally Regenerated Beech Seedlings. Ann. For. Sci. 2001, 58, 127–134. [Google Scholar] [CrossRef]
- Tavankar, F.; Picchio, R.; Nikooy, M.; Jourgholami, M.; Naghdi, R.; Latterini, F.; Venanzi, R. Soil Natural Recovery Process and Fagus orientalis Lipsky Seedling Growth after Timber Extraction by Wheeled Skidder. Land 2021, 10, 113. [Google Scholar] [CrossRef]
- Abe, M.; Izaki, J.; Miguchi, H.; Masaki, T.; Makita, A.; Nakashizuka, T. The Effects of Sasa and Canopy Gap Formation on Tree Regeneration in an Old Beech Forest. J. Veg. Sci. 2002, 13, 565–574. [Google Scholar] [CrossRef]
- Ohkubo, T. Structure and Dynamics of Japanese Beech (Fagus japonica Maxim.) Stools and Sprouts in the Regeneration of the Natural Forests. Vegetaio 1992, 101, 65–80. [Google Scholar] [CrossRef]
- Awan, S.; Footitt, S.; Finch-Savage, W.E. Interaction of Maternal Environment and Allelic Differences in Seed Vigour Genes Determines Seed Performance in Brassica oleracea. Plant J. 2018, 94, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, E.C.; Camacho-Islas, L.; Martínez-Falcón, A.P.; Luna-Vega, I.; Carbó-Ramírez, P. Masting Effect on Alpha and Beta Avian Diversity in Fragmented Forests of Relict-Endangered Mexican Beech (Fagus grandifolia subsp. mexicana). Avian Res. 2021, 12, 49. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- FAO. FAO-UNESCO Soil Map of the World; Revised Legend; FAO: Rome, Italy, 1988. [Google Scholar]
- Rodríguez-Ramírez, E.C.; Luna-Vega, I.; Rozas, V. Tree-Ring Research of Mexican Beech (Fagus grandifolia subsp. mexicana) A Relict Tree Endemic to Eastern Mexico. Tree Ring Res. 2018, 74, 94–107. [Google Scholar] [CrossRef]
- Perry, J.N.; Bell, E.D.; Smith, R.H.; Woiwod, I.P. SADIE: Software to Measure and Model Spatial Pattern. Asp. Appl. Biol. 1996, 46, 95–102. [Google Scholar]
- Perry, J.N.; Winder, L.; Holland, J.M.; Alston, R.D. Red-Blue Plots for Detecting Clusters in Count Data. Ecol. Lett. 1999, 2, 106–113. [Google Scholar] [CrossRef]
- Perry, J.N.; Dixon, P.M. A New Method to Measure Spatial Association for Ecological Count Data. Ecoscience 2002, 9, 133–141. [Google Scholar] [CrossRef]
- Balzano, S.; Porzio, G.C.; Salvatore, R.; Vistocco, D. Statistical Learning and Modeling in Data Analysis; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 9783030699437. [Google Scholar]
- Zeileis, A.; Meyer, D.; Hornik, K. Residual-Based Shadings for Visualizing (Conditional) Independence. J. Comput. Graph. Stat. 2007, 16, 507–525. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R. Spatstat: An R Package for Analyzing Spatial Point Patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Fiala, A.C.S.; Garman, S.L.; Gray, A.N. Comparison of Five Canopy Cover Estimation Techniques in the Western Oregon Cascades. For. Ecol. Manag. 2006, 232, 188–197. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1998; ISBN 0444892508. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-1; CRAN: Durham, NC, USA, 2016; Available online: https://CRAN.R-project.org/package=vegan (accessed on 24 May 2025).
- Peña, L.; Amezaga, I.; Onaindia, M. At Which Spatial Scale Are Plant Species Composition and Diversity Affected in Beech Forests? Ann. For. Sci. 2011, 68, 1351–1362. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Terrazas, T.; Luna-Vega, I. The Influence of Climate on the Masting Behavior of Mexican Beech: Growth Rings and Xylem Anatomy. Trees 2018, 33, 23–35. [Google Scholar] [CrossRef]
- Ames-Martínez, F.N.; Luna-Vega, I.; Dieringer, G.; Rodríguez-Ramírez, E.C. The Effect of Climate Change on Arcto-Tertiary Mexican Beech Forests: Exploring Their Past, Present, and Future Distribution. Ecol. Evol. 2022, 12, e9228. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, E.C.; Williams-Linera, G.; Díaz-Ávalos, C.; Luna-Vega, I. Masting Effect on Canopy Greenness and Climate Response on Seed Production of Fagus grandifolia subsp. mexicana across the Sierra Madre Oriental, Mexico. Clim. Chang. Ecol. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Gavranović Markić, A.; Vujnović, Z.; Kičić, M.; Ivanković, M.; Škvorc, Ž.; Bogdan, S.; Oršanić, M. Seed Quantity and Quality Variation in European Beech (Fagus sylvatica L.): A Comparative Analysis of Different Crop Years. South-East Eur. For. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Nagel, T.A.; Kutsch, W.L.; Oßwald, R.; Skomarkova, M.V.; Sippell, L.; Rennenberg, H. Extreme summer heat and drought lead to early fruit abortion in European beech. Tree Physiol. 2019, 39, 1283–1298. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).