Pesticides: Environmental Stressors Implicated in the Development of Central Nervous System Disorders and Neurodegeneration
Abstract
:1. Introduction
2. Pesticides Definition and Classification
- Contact: Their action is performed upon direct contact with the target organism, disrupting essential biological processes or causing physical harm to the pests.
- Ingestion: When ingested, they are absorbed by the pest, affecting its digestive or metabolic system and leading to death.
- Systemic: These pesticides are taken up by plants and animals. For plants, once applied, they are absorbed and transported through the vascular system to the whole plant. Depending on their nature, some move to the top of the plant, others to the bottom, and some can move in both directions. These pesticides enter animals through the ingestion of plants that contain them.
- Fumigants: These are gases or vapors that, once inhaled by pests, interfere with their biological processes and cause their elimination.
- Defoliants: They eliminate plant leaves, affecting photosynthetic capacity and weakening the organism.
- Repellents: They do not cause pest death but act as a deterrent, preventing target organisms from approaching treated plants or surfaces.
3. Pesticides Mode of Action
3.1. Bipyridyls
3.2. Carbamates
3.3. Formamidines
3.4. Neonicotinoids
3.5. Organochlorines
3.6. Organophosphates
3.7. Pyrethrins and Pyrethroids
3.8. Triazines
4. Pesticide Environmental Stressor
5. Environmental Stress-Induced Neurotoxicity
5.1. Formamidines
5.2. Neonicotinoids
5.3. Organochlorine
5.4. Pyrethroids
5.5. Organophosphates
6. CNS Disorders Associated with Stress Responses Induced by Pesticide Exposure in Humans and Mice
6.1. Alzheimer’s Disease (AD)
6.1.1. Pesticides and AChE Inhibition
6.1.2. Pesticides Promote the Characteristics of AD Tauopathy and Neuroinflammation
6.2. Parkinson’s Disease (PD)
6.3. Dementia
6.4. Attention Deficit Hyperactivity Disorder (ADHD)
6.5. Neuroinflammation
6.5.1. Astrocytes
6.5.2. Microglia
6.6. Natural Products with Anti-Neuroinflammatory Effects
7. Alternatives to Chemical Pesticides
7.1. Agroecological Crop Protection (ACP)
7.2. Crop Rotation
7.3. Biological Pesticides (Biological Control)
| Organisms | Activity | Trade Name of the Product | Crops | Pest | Reference |
|---|---|---|---|---|---|
| Plants | |||||
| Jatropha curcas | Insecticide, nematicide, and molluscicide | Jatropha oil | Vegetables, fruit trees, and cereals | Moths, butterflies, aphids, bugs, beetles, flies, and cockroaches | [261] |
| Tagetes erecta | Nematicide | Nemagold | Tomato, potato, tobacco, cucumber, watermelon, melon, banana, etc. | Nematode root-knot | [262] |
| Eucalyptus globulus | Insecticide, fungicide | Eucalyptus oils | Tomatoes, cucumbers, carrots, lettuce, etc. | Flies, mosquito larvae, lice, etc. | [263] |
| Fungi | |||||
| Beauveria bassiana | Insecticide | BEA-SIN® | Pepper, bell pepper, tomato, peeled tomato, potato, and eggplant | Whiteflies | [257,264] |
| Metarhizium anisopliae | Insecticide | Met52® | Tomatoes, cucumbers, lettuce, carrots, apples, pears, citrus, corn, wheat, rice, oatmeal, etc. | Whiteflies, mosquitoes, thrips, aphids, mites, ticks, etc. | [265] |
| Metarhizium flavoviride | Insecticide | Green Muscle® | Tomatoes, cucumbers, lettuce, carrots, melons, grapes, apples, pears, citrus, corn, wheat, rice, etc. | Grasshoppers | [266] |
| Verticillium chlamydosporium | Nematicide | Ecocill® | Tomatoes, carrots, cucumbers, corn, wheat, oats, | Wireworms, rootworms, soil cockroaches, soil beetles, etc. | [267] |
| Lecanicillium longisporum | Insecticide | Eday®, Vertalec® | Tomatoes, cucumbers, lettuce, peppers, cabbage, spinach, etc. | Whitefly, blind hen, aphids, thrips, etc. | [268] |
| Bacteria | |||||
| Bacillus thuringiensis | Insecticide | Dipel® | Soybean, grapevines, and fruit trees (apple, peach, pear, and plum) | Lepidopteran larvae (Caterpillars) | [269] |
| Bacillus sphaericus | Insecticide | VectoLex® | - | Mosquito larvae | [270] |
| Pseudomonas aeruginosa | Fungicide | Gluticid® | Tomatoes | Fungicide | [271] |
| Pseudomonas fluorescens | Fertilizer and Fungicide | Bio Tak P® | Plant growth promoter | Fungicide | [272] |
| Nematodes | |||||
| Steinernema carpocapsae | Insecticide | ScanMask® | Tomatoes, apples, pears, citrus, corn, wheat, etc. | Weevils, root maggots, mosquitoes, cutworms, flea larvae, fire ants, etc. | [273] |
| Steinernema glaseri | Insecticide | Entonem® | Tomatoes, apples, pears, citrus, corn, wheat, rice, etc. | Fly larvae, beetle larvae, caterpillars, etc. | [274] |
| Steinernema feltiae | Insecticide, nematicide | Nemasys® | Tomatoes, apples, pears, citrus, corn, wheat, rice, oatmeal, etc. | Thrips, beetles, caterpillars, bedbugs, flies, etc. | [275] |
| Heterorhabditis bacteriophora | Insecticide | Grub-Guard® | Tomatoes, cucumbers, lettuce, apples, pears, citrus, corn, wheat, oats, etc. | Insect larvae, Japanese beetle larvae, etc. | [276] |
| Protozoan | |||||
| Nosema locustae | Insecticide | Nolo Bait® | Corn, wheat, oats, barley, tomatoes, carrots, lettuce, peppers, cucumbers, etc. | Grasshoppers and crickets | [277] |
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandran, C.S.; Thomas, S.; Unni, M.R. Pesticides: Classification, detection, and degradation. In Organic Farming; Chandran, C.S., Thomas, S., Unni, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 71–87. [Google Scholar] [CrossRef]
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticide use and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef] [PubMed]
- Maksymiv, I. Pesticides: Benefits and hazards. J. Vasyl Stefanyk Precarpath. Nat. Univ. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Shattuck, A.; Werner, M.; Mempel, F.; Dunivin, Z.; Galt, R. Global pesticide use and trade database (GloPUT): New estimates show pesticide use trends in low-income countries substantially underestimated. Glob. Environ. Change 2023, 81, 102693. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Pesticides Use. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 1 December 2024).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Narayanan, M.; Kandasamy, S.; He, Z.; Kumarasamy, S. Ecological impacts of pesticides on soil and water ecosystems and its natural degradation process. In Pesticides in the Natural Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–49. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Thakur, K.; Mahajan, D.; Brar, B.; Sharma, D.; Kumar, S.; Sharma, A.K. Impact of pesticides application on aquatic ecosystem and biodiversity: A review. Biol. Bull. 2023, 50, 1362–1375. [Google Scholar] [CrossRef]
- Weiss, F.T.; Ruepert, C.; Echeverría-Sáenz, S.; Eggen, R.I.; Stamm, C. Agricultural pesticides pose a continuous ecotoxicological risk to aquatic organisms in a tropical horticulture catchment. Environ. Adv. 2023, 11, 100339. [Google Scholar] [CrossRef]
- Beringue, A.; Queffelec, J.; Le Lann, C.; Sulmon, C. Sublethal pesticide exposure in non-target terrestrial ecosystems: From known effects on individuals to potential consequences on trophic interactions and network functioning. Environ. Res. 2024, 260, 119620. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of increasing pesticides and fertilizers on human health: A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2024, 11, 100410. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Coutinho, T.E.; Silva, T.L.; Andreani, T.; Silva, A.M. Neurotoxicity assessment of four different pesticides using in vitro enzymatic inhibition assays. Toxics 2022, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Honatel, K.F.; Arbo, B.D.; Leal, M.B.; da Silva Júnior, F.M.R.; Garcia, S.C.; Arbo, M.D. An update of the impact of pesticide exposure on memory and learning. Discov. Toxicol. 2024, 1, 11. [Google Scholar] [CrossRef]
- Mwaka, O.; Mwamahonje, A.; Nene, W.; Rweyemamu, E.; Maseta, Z. Pesticides use and its effects on grape production: A review. Sustain. Environ. 2024, 10, 2366555. [Google Scholar] [CrossRef]
- Saroop, S.; Tamchos, S. Impact of pesticide application: Positive and negative side. Pestic. Environ. 2024, 155–178. [Google Scholar] [CrossRef]
- Garud, A.; Pawar, S.; Patil, M.S.; Kale, S.R.; Patil, S. A Scientific Review of Pesticides: Classification, Toxicity, Health Effects, Sustainability, and Environmental Impact. Cureus 2024, 16, e67945. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Academic Press: Cambridge, MA, USA, 2020; pp. 29–42. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural (SADER); Servicio Nacional de Sanidad Inocuidad y Calidad Agroalimentaria (SENASICA). Manual para el Buen uso y Manejo de Plaguicidas en Campo. 1a Edición. 2019; pp. 1–80. Available online: https://www.gob.mx/cms/uploads/attachment/file/452645/MANUAL_PARA_EL_BUEN_USO_Y_MANEJO_DE_PLAGUICIDAS_EN_CAMPO.pdf (accessed on 5 December 2024).
- Yadav, I.C.; Devi, N.L. Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 2017, 6, 140–158. [Google Scholar]
- Libs, E.; Salim, E. Formulation of essential oil pesticides technology and their application. Agric. Res. Technol. 2017, 9, 555759. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019, 2019th ed.; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/332193/9789240005662-eng.pdf (accessed on 5 December 2024).
- Jayaprakas, C.A.; Tom, J.; Sreejith, S. Impact of Insecticides on Man and Environment. In Biomedical Applications and Toxicity of Nanomaterials; Springer Nature Singapore: Singapore, 2023; pp. 751–768. [Google Scholar] [CrossRef]
- EPA. Chemically-Related Groups of Active Ingredients. 2018. Available online: https://www.epa.gov/ingredients-used-pesticide-products/chemically-related-groups-active-ingredients (accessed on 5 December 2024).
- Rodríguez, A.; Castrejón-Godínez, M.L.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Pesticide bioremediation: OMICs technologies for understanding the processes. In Pesticides Bioremediation; Springer International Publishing: Cham, Switzerland, 2022; pp. 197–242. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Fu, X.; Nageotte, J.R.; Silverman, J.; Bretsnyder, E.C.; Chen, D.; Rydel, T.J.; Bean, G.J.; Li, K.S.; et al. Bacillus thuringiensis Cry1Da_7 and Cry1B. 868 protein interactions with novel receptors allow control of resistant fall armyworms, Spodoptera frugiperda (JE Smith). Appl. Environ. Microbiol. 2019, 85, e00579-19. [Google Scholar] [CrossRef] [PubMed]
- Baratzhanova, G.; Fournier, A.; Delannoy, M.; Baubekova, A.; Altynova, N.; Djansugurova, L.; Cakir-Kiefer, C. The mode of action of different organochlorine pesticides families in mammalians. Environ. Toxicol. Pharmacol. 2024, 110, 104514. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 141–147. [Google Scholar]
- Li, S.; Crooks, P.A.; Wei, X.; Leon, J. Toxicity of dipyridyl compounds and related compounds. Crit. Rev. Toxicol. 2004, 34, 447–460. [Google Scholar] [CrossRef]
- Agrawal, A.N.J.U.; Sharma, B. Pesticides induced oxidative stress in mammalian systems. Int. J. Biol. Med. Res. 2010, 1, 90–104. [Google Scholar]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of herbicides. In Veterinary Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 553–567. [Google Scholar] [CrossRef]
- Quinn, L.; de Vos, J.; Fernandes-Whaley, M.; Roos, C.; Bouwman, H.; Kylin, H.; Pieters, R.; van den Berg, J. Pesticide use in South Africa: One of the largest importers of pesticides in Africa. In Pesticides in the Modern World—Pesticides Use and Management; IntechOpen: London, UK, 2011; pp. 49–96. [Google Scholar]
- Rosic, N.; Bradbury, J.; Lee, M.; Baltrotsky, K.; Grace, S. The impact of pesticides on local waterways: A scoping review and method for identifying pesticides in local usage. Environ. Sci. Policy 2020, 106, 12–21. [Google Scholar] [CrossRef]
- Umar, A.M.; Aisami, A. Acetylcholinesterase enzyme (AChE) as a biosensor and biomarker for pesticides: A mini review. Bull. Environ. Sci. Sustain. Manag. 2020, 4, 7–12. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, R.; Carrageta, D.F.; Alves, M.G.; Seco-Rovira, V.; Oliveira, P.F.; de Lourdes Pereira, M. Carbamate pesticides: Shedding light on their impact on the male reproductive system. Int. J. Mol. Sci. 2022, 23, 8206. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.; Sousa, S.F. The buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Costa, L.G. Neurotoxicity of amitraz, a formamidine pesticide. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 255–276. [Google Scholar]
- Bhandarwar, A.; Burute, R.B.; Soni, N.P. A study of Clinical Profile and Management of Amitraz Poisoning: A Case Series of Not So (UN) Common Poisoning. Int. J. Med. Pharm. Res. 2023, 4, 87–90. [Google Scholar]
- Giorgini, M.; Taroncher, M.; Ruiz, M.J.; Rodríguez-Carrasco, Y.; Tolosa, J. In vitro and predictive computational toxicology methods for the neurotoxic pesticide amitraz and its metabolites. Brain Sci. 2023, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Iken, I.; Abdessadek, M.; El Attari, A.; Achour, S. Poisoning by Amitraz, uncommon pesticide revealed by high performance liquid chromatography: About two cases. Toxicol. Anal. Clin. 2020, 32, 200–204. [Google Scholar] [CrossRef]
- Reynoso, E.C.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and perspectives in immunosensors for determination of currently-used pesticides: The case of glyphosate, organophosphates, and neonicotinoids. Biosensors 2019, 9, 20. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Martínez, M.A. Neurotoxicity of neonicotinoids. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 167–207. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.F.; Liu, L.Y.; Zhu, F.J.; Ma, W.L. National-scale monitoring of historic used organochlorine pesticides (OCPs) and current used pesticides (CUPs) in Chinese surface soil: Old topic and new story. J. Hazard. Mater. 2023, 443, 130285. [Google Scholar] [CrossRef]
- Ali, U.; Syed, J.H.; Malik, R.N.; Katsoyiannis, A.; Li, J.; Zhang, G.; Jones, K.C. Organochlorine pesticides (OCPs) in South Asian region: A review. Sci. Total Environ. 2014, 476, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, H.A.; Yahaya, A.; Abdullahi, S.S.A.; Jagaba, A.H.; Birniwa, A.H. Mitigating water contamination by controlling anthropogenic activities of organochlorine pesticides (OCPs) for surface water quality assurance. Case Stud. Chem. Environ. Eng. 2023, 8, 100474. [Google Scholar] [CrossRef]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef]
- Uğurlu, P.; Satar, E.İ.; Ünlü, E. Toxic effects of commercial grade indoxacarb and endosulfan on Gammarus kischineffensis (Schellenberg, 1937) (Crustacea: Amphipoda). Chemosphere 2024, 360, 142387. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; de Araujo Furtado, M.; Pidoplichko, V.I.; Braga, M.F. Mechanisms of organophosphate toxicity and the role of acetylcholinesterase inhibition. Toxics 2023, 11, 866. [Google Scholar] [CrossRef]
- Hodoșan, C.; Gîrd, C.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Nistor, L.; Bărbuică, I.S.; Marin, S.C.; Mihalache, A.; Popa, L. Pyrethrins and pyrethroids: A comprehensive review of natural occurring compounds and their synthetic derivatives. Plants 2023, 12, 4022. [Google Scholar] [CrossRef]
- Ranatunga, M.; Kellar, C.; Pettigrove, V. Toxicological impacts of synthetic pyrethroids on non-target aquatic organisms: A review. Environ. Adv. 2023, 12, 100388. [Google Scholar] [CrossRef]
- Arsuffi-Marcon, R.; Souza, L.G.; Santos-Miranda, A.; Joviano-Santos, J.V. Neurotoxicity of Pyrethroids in exacerbating neurodegenerative diseases: From animals’ models to humans’ studies. Chem. Biol. Interact. 2024, 391, 110911. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Skouras, P.; Varvarelis, O.P.; Aloizou, A.M.; Hernández, A.F.; Liampas, I.; Rikos, D.; Dasramani, M.; Golokhvast, K.S.; Bogdanos, D.P.; et al. Pesticides and tremor: An overview of association, mechanisms and confounders. Environ. Res. 2023, 229, 115442. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.S.; Lein, P.J. Neurotoxicity. In Encyclopedia of Toxicology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 6, pp. 727–740. [Google Scholar] [CrossRef]
- Klementova, S.; Keltnerova, L. Triazine herbicides in the environment. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: Croatia, Balkan, 2015; pp. 71–96. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Ru, S.; Wang, J.; Xiong, J.; Yang, L.; Hao, L.; Zhang, J.; Zhang, X. Effects of co-exposure of the triazine herbicides atrazine, prometryn and terbutryn on Phaeodactylum tricornutum photosynthesis and nutritional value. Sci. Total Environ. 2022, 807, 150609. [Google Scholar] [CrossRef]
- LeBaron, H.M. The Triazine Herbicides. 50 Years Revolutionizing Agriculture; LeBaron, H.M., McFarland, J.E., Burnside, O.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; 600p. [Google Scholar]
- Chaudhary, N.; Choudhary, K.K.; Agrawal, S.B.; Agrawal, M. Pesticides usage, uptake and mode of action in plants with special emphasis on photosynthetic characteristics. In Pesticides in Crop Production: Physiological and Biochemical Action; Srivastava, P.K., Singh, V.P., Singh, A., Tripathi, D.K., Singh, S., Prasad, S.M., Chauhan, D.K., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 159–180. [Google Scholar] [CrossRef]
- Rao, I.S.; Madhulety, T.Y. Role of herbicides in improving crop yields. In Developments in Physiology, Biochemistry and Molecular Biology of Plants; Bose, B., Hemantaranjan, A., Eds.; New India Publishing Agency: New Delhi, India, 2005; Volume 1, pp. 203–287. [Google Scholar]
- Panigrahi, K.K.; Mohanty, A.; Padhan, S.R.; Guru, R.K.S. Genotoxicity and DNA damage induced by herbicides and toxins in plants. In Induced Genotoxicity and Oxidative Stress in Plants; Khan, Z., Ansari, M.Y.H., Shahwar, D., Eds.; Springer: Singapore, 2021; pp. 29–63. [Google Scholar] [CrossRef]
- Himani, P.U.; Mahawer, S.K.; Kumar, R.; Prakash, O. Plant protection through agrochemicals and its consequences. In Plant Protection: From Chemicals to Biologicals; Soni, R., Suyal, D.C., Goel, R., Eds.; De Gruyter: Beilin, Germany, 2022; pp. 25–44. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water-a review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Roy, D.; Ghosh, D.; Chander, S.; Saha, B.; Moulick, D. Herbicide Contamination in Ground Water and Their Probable Remediation. In Environmental Contaminants; Apple Academic Press: Boca Raton, FL, USA, 2024; pp. 111–140. [Google Scholar]
- Ahmad, S.; Chandrasekaran, M.; Ahmad, H.W. Investigation of the persistence, toxicological effects, and ecological issues of S-triazine herbicides and their biodegradation using emerging technologies: A Review. Microorganisms 2023, 11, 2558. [Google Scholar] [CrossRef]
- Yao, T.; Sun, P.; Zhao, W. Triazine herbicides risk management strategies on environmental and human health aspects using in-silico methods. Int. J. Mol. Sci. 2023, 24, 5691. [Google Scholar] [CrossRef]
- Arabi, S.; Heidari-Beni, M.; Poursafa, P.; Roshanaei, M.; Kelishadi, R. A review of the potential adverse health impacts of atrazine in humans. Rev. Environ. Health 2024. [Google Scholar] [CrossRef]
- Raj, A.; Dubey, A.; Malla, M.A.; Kumar, A. Pesticide Pestilence: Global Scenario and Recent Advances in Detection and Degradation Methods. J. Environ. Manag. 2023, 338, 117680. [Google Scholar] [CrossRef]
- Phat, C.; Ty, B.; Kuok, F.; Andrews, E.M.; Kurniawan, W.; Hinode, H. Residual Pesticides. In Water and Life in Tonle Sap Lake; Yoshimura, C., Khanal, R., Sovannara, U., Eds.; Springer: Singapore, 2022; pp. 387–397. [Google Scholar] [CrossRef]
- Bagheri, A.; Emami, N.; Damalas, C.A. Farmers’ behavior towards safe pesticide handling: An analysis with the theory of planned behavior. Sci. Total Environ. 2021, 751, 141709. [Google Scholar] [CrossRef]
- Sarker, S.; Akbor, M.A.; Nahar, A.; Hasan, M.; Islam, A.R.M.T.; Siddique, M.A.B. Level of pesticides contamination in the major river systems: A review on South Asian countries perspective. Heliyon 2021, 7, e07270. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, J.; Xu, Y.; Liu, H.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Suspecting screening “known unknown” pesticides and transformation products in soil at pesticide manufacturing sites. Sci. Total Environ. 2022, 808, 152074. [Google Scholar] [CrossRef] [PubMed]
- Khatib, I.; Rychter, P.; Falfushynska, H. Pesticide Pollution: Detrimental Outcomes and Possible Mechanisms of Fish Exposure to Common Organophosphates and Triazines. J. Xenobiot. 2022, 12, 236–265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity Analysis of Endocrine Disrupting Pesticides on Non-Target Organisms: A Critical Analysis on Toxicity Mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef]
- Wan, N.F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.Q.; Xin, F.; Goulson, D.; Woodcock, B.A.; Vanbergen, A.J.; Spurgeon, D.J.; et al. Pesticides have negative effects on non-target organisms. Nat. Commun. 2025, 16, 1360. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S. Ecotoxicological Implications of Residual Pesticides to Beneficial Soil Bacteria: A Review. Pest. Biochem. Physiol. 2022, 188, 105272. [Google Scholar] [CrossRef]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R.P. Pesticide effects on soil fauna communities—A meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-Effects of Pesticides on Non-Target Insects in Agriculture: A Mini-Review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- Goritschnig, L.; Burtscher-Schaden, H.; Durstberger, T.; Zaller, J.G. Ecotoxicity of Pesticides Approved for Use in European Conventional or Organic Agriculture for Honeybees, Birds, and Earthworms. Environments 2024, 11, 137. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and Pesticides: Their Impact on Soil Health and Environment. In Soil Health, Soil Biology; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 59. [Google Scholar] [CrossRef]
- Grung, M.; Lin, Y.; Zhang, H.; Steen, A.O.; Huang, J.; Zhang, G.; Larssen, T. Pesticide levels and environmental risk in aquatic environments in China: A review. Environ. Int. 2015, 81, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Farah, I.F.; dos Santos, C.R.; Pinto, M.C.F.; Araújo, C.R.; Amaral, M.C.S. Pesticides in aquatic environment: Occurrence, ecological implications and legal framework. J. Environ. Chem. Eng. 2024, 12, 114072. [Google Scholar] [CrossRef]
- Kadiru, S.; Patil, S.; D’Souza, R. Effect of pesticide toxicity in aquatic environments: A recent review. Int. J. Fish. Aquat. Stud. 2022, 10, 113–118. [Google Scholar] [CrossRef]
- Shahid, N.; Liess, M.; Knillmann, S. Environmental stress increases synergistic effects of pesticide mixtures on Daphnia magna. Environ. Sci. Technol. 2019, 53, 12586–12593. [Google Scholar] [CrossRef] [PubMed]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological Effects of Pesticides on Hematological Parameters and Oxidative Enzymes in Freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Katagi, T. Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2010; pp. 1–132. [Google Scholar] [CrossRef]
- Tongo, I.; Onokpasa, A.; Emerure, F.; Balogun, P.T.; Enuneku, A.A.; Erhunmwunse, N.; Asemota, O.; Ogbomida, E.; Ogbeide, O.; Ezemonye, L. Levels, bioaccumulation and biomagnification of pesticide residues in a tropical freshwater food web. Int. J. Environ. Sci. Technol. 2022, 19, 1467–1482. [Google Scholar] [CrossRef]
- Farcas, A.; Matei, A.V.; Florian, C.; Badea, M.; Coman, G. Health effects associated with acute and chronic exposure to pesticides. In Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–110. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, S.M.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic Effects of Organic Pesticides on the Aquatic Environment and Human Health: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, M.K. Soil pollution and human health. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 205–220. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Iqubal, A.; Ahmed, M.; Ahmad, S.; Sahoo, C.R.; Iqubal, M.K.; Haque, S.E. Environmental neurotoxic pollutants. Environ. Sci. Pollut. Res. 2020, 27, 41175–41198. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wakode, S.; Sharma, A.; Nair, N.; Dhobi, M.; Wani, M.A.; Pottoo, F.H. Effect of environmental toxicants on neuronal functions. Environ. Sci. Pollut. Res. 2020, 27, 44906–44921. [Google Scholar] [CrossRef]
- Costa, L.G. Interactions of neurotoxicants with neurotransmitter systems. Toxicology 1988, 49, 359–366. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Z.; Zhang, W.; Ren, Y. Investigating the neurotoxicity of environmental pollutants using zebrafish as a model organism: A review and recommendations for future work. Neurotoxicology 2023, 94, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, J.; Wang, H. Advancements in Targeting Ion Channels for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1462. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Mostafalou, S. Neurotoxicity of pesticides in the context of CNS chronic diseases. Int. J. Environ. Health Res. 2022, 32, 2718–2755. [Google Scholar] [CrossRef]
- Vellingiri, B.; Chandrasekhar, M.; Sabari, S.S.; Gopalakrishnan, A.V.; Narayanasamy, A.; Venkatesan, D.; Iyer, M.; Kesari, K.; Dey, A. Neurotoxicity of pesticides–A link to neurodegeneration. Ecotoxicol. Environ. Saf. 2022, 243, 113972. [Google Scholar] [CrossRef]
- Akinaga, J.; García-Sáinz, J.A.; Pupo, A.S. Updates in the function and regulation of α1-adrenoceptors. Br. J. Pharmacol. 2019, 176, 2343–2357. [Google Scholar] [CrossRef]
- Costa, L.G.; Wu, D.S.; Olibet, G.; Murphy, S.D. Formamidine pesticides and alpha 2-adrenoceptors: Studies with amitraz and chlordimeform in rats and development of a radioreceptor binding assay. Neurotoxicol. Teratol. 1989, 11, 405–411. [Google Scholar] [CrossRef]
- Del Pino, J.; Moyano, P.; Ruiz, M.; Anadón, M.; Díaz, M.; García, J.; Labajo González, E.; Frejo, M. Amitraz changes NE, DA and 5-HT biosynthesis and metabolism mediated by alterations in estradiol content in CNS of male rats. Chemosphere 2017, 181, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Jacome, L.F.; Gautreaux, C.; Inagaki, T.; Mohan, G.; Alves, S.; Lubbers, L.S.; Luine, V. Estradiol and ERb agonists enhance recognition memory, and DPN, an ERb agonist, alters brain monoamines. Neurobiol. Learn Mem. 2010, 94, 488–498. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Inter. 2017, 99, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, B.; Siefert, P. Acetylcholine and its receptors in honeybees: Involvement in development and impairments by neonicotinoids. Insects 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Sheets, L.P.; Li, A.A.; Minnema, D.J.; Collier, R.H.; Creek, M.R.; Peffer, R.C. A critical review of neonicotinoid insecticides for developmental neurotoxicity. Crit. Rev. Toxicol. 2016, 46, 153–190. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Yang, Y.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, X.W.; Anadón, A.; Martinez, M.A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022, 96, 1493–1520. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Goldstein, L.B.; Bullman, S.; Tu, T.; Khan, W.A.; Dechkovskaia, A.M.; Abdel-Rahman, A.A. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J. Toxicol. Environ. Health Part A 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Bal, R.; Erdogan, S.; Theophilidis, G.; Baydas, G.; Naziroglu, M. Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording. Neurotoxicology 2010, 31, 113–120. [Google Scholar] [CrossRef]
- Tanaka, T. Effects of maternal clothianidin exposure on behavioral development in F1 generation mice. Toxico. Ind. Health 2012, 28, 697–707. [Google Scholar] [CrossRef]
- Tanaka, T. Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet. Birth Defects Res. B Dev. Reprod. Toxicol. 2012, 95, 151–159. [Google Scholar] [CrossRef]
- Özdemir, H.H.; Kara, M.; Yumrutas, O.; Uckardes, F.; Eraslan, E.; Demir, C.F.; Bal, R. Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cogn. Neurodyn. 2014, 8, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ou, S.W.; Wang, Y.J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, Y.; Jiang, D.; Behnke, C.; Nomura, Y.; Zhorov, B.S.; Dong, K. The receptor site and mechanism of action of sodium channel blocker insecticides. J. Biol. Chem. 2016, 291, 20113–20124. [Google Scholar] [CrossRef]
- Zhorov, B.S.; Dong, K. Elucidation of pyrethroid and DDT receptor sites in the voltage-gated sodium channel. Neurotoxicology 2017, 60, 171–177. [Google Scholar] [CrossRef]
- Narahashi, T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996, 78, 1–14. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Faro, L.R. Systematic review of calcium channels and intracellular calcium signaling: Relevance to pesticide neurotoxicity. Int. J. Mol. Sci. 2021, 22, 13376. [Google Scholar] [CrossRef]
- Bloomquist, J.R.; Soderlund, D.M. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol. Pharmacol. 1988, 33, 543–550. [Google Scholar] [CrossRef]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-Gated Sodium Channels as Insecticide Targets. Adv. Insect Phys. 2014, 46, 389–433. [Google Scholar] [CrossRef]
- Penticoff, H.B.; Fortin, J.S. Toxic/metabolic diseases of the nervous system. In Neurobiology of Brain Disorders, 2nd ed.; Zigmond, M.J., Wiley, C.A., Chesselet, M.F., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 379–401. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Morris, R.H.; Counsell, S.J.; McGonnell, I.M.; Thornton, C. Early life exposure to air pollution impacts neuronal and glial cell function leading to impaired neurodevelopment. BioEssays 2021, 43, 2000288. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Camoni, L.; Calamandrei, G.; Chiarotti, F.; Venerosi, A. The contribution of environmental pollutants to the risk of autism and other neurodevelopmental disorders: A systematic review of case-control studies. Neurosci. Biobehav. Rev. 2024, 164, 105815. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Dong, X.; Zhang, X.; Ye, F.; Cheng, J.; Dan, G.; Zhao, Y.; Zou, Z.; Cao, J.; Sai, Y. Pesticides exposure and dopaminergic neurodegeneration. Expo. Health 2021, 13, 295–306. [Google Scholar] [CrossRef]
- Franjesevic, A.J.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase. Chemistry 2019, 25, 5337–5371. [Google Scholar] [CrossRef]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci Rep. 2021, 11, 21486. [Google Scholar] [CrossRef]
- Yadav, B.; Kaur, S.; Yadav, A.; Verma, H.; Kar, S.; Sahu, B.K.; Pati, K.R.; Sarkar, B.; Dhiman, M.; Mantha, A.K. Implications of organophosphate pesticides on brain cells and their contribution toward progression of Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2024, 38, e23660. [Google Scholar] [CrossRef]
- Go, R.C.P.; Corley, M.J.; Ross, G.W.; Petrovitch, H.; Masaki, K.H.; Maunakea, A.K.; He, Q.; Tiirikainen, M.I. Genome-wide epigenetic analyses in Japanese immigrant plantation workers with Parkinson’s disease and exposure to organochlorines reveal possible involvement of glial genes and pathways involved in neurotoxicity. BMC Neurosci. 2020, 21, 1–18. [Google Scholar] [CrossRef]
- Kalia, V.; Niedzwiecki, M.M.; Bradner, J.M.; Lau, F.K.; Anderson, F.L.; Bucher, M.L.; Manz, K.E.; Schlotter, A.P.; Fuentes, Z.C.; Pennell, K.D.; et al. Cross-species metabolomic analysis of tau- and DDT-related toxicity. PNAS Nexus 2022, 1, pgac050. [Google Scholar] [CrossRef]
- Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak, S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer’s disease: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 44724–44742. [Google Scholar] [CrossRef]
- Torres-Sánchez, E.D.; Ortiz, G.G.; Reyes-Uribe, E.; Torres-Jasso, J.H.; Salazar-Flores, J. Effect of pesticides on phosphorylation of tau protein, and its influence on Alzheimer’s disease. World J. Clin. Cases. 2023, 11, 5628–5642. [Google Scholar] [CrossRef]
- Mohammadzadeh, L.; Abnous, K.; Razavi, B.M.; Hosseinzadeh, H. Crocin-protected malathion-induced spatial memory deficits by inhibiting TAU protein hyperphosphorylation and antiapoptotic effects. Nutr. Neurosci. 2020, 23, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Credle, J.; Oaks, A.W.; Duka, V.; Lee, J.H.; Jones, J.; Sidhu, A. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS ONE 2012, 7, e30745. [Google Scholar] [CrossRef]
- Barbeau, A.; Dallaire, L.; Buu, N.T.; Poirier, J.; Rucinska, E. Comparative behavioral, biochemical and pigmentary effects of MPTP, MPP+ and paraquat in Rana pipiens. Life Sci. 1985, 37, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Liou, H.H.; Chen, R.C.; Tsai, Y.F.; Chen, W.P.; Chang, Y.C.; Tsai, M.C. Effects of paraquat on the substantia nigra of the Wistar rats: Neurochemical, histological, and behavioral studies. Toxicol. Appl. Pharmacol. 1996, 137, 34–41. [Google Scholar] [CrossRef]
- Brooks, A.I.; Chadwick, C.A.; Gelbard, H.A.; Cory-Slechta, D.A.; Federoff, H.J. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999, 823, 1–10. [Google Scholar] [CrossRef]
- Prasad, K.; Winnik, B.; Thiruchelvam, M.J.; Buckley, B.; Mirochnitchenko, O.; Richfield, E.K. Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environ. Health Perspect. 2007, 115, 1448–1453. [Google Scholar] [CrossRef]
- Vaccari, C.; El Dib, R.; Gomaa, H.; Lopes, L.C.; de Camargo, J.L. Paraquat and Parkinson’s disease: A systematic review and meta-analysis of observational studies. J. Toxicol. Environ. Health B Crit. Rev. 2019, 22, 172–202. [Google Scholar] [CrossRef]
- Tong, T.; Duan, W.; Xu, Y.; Hong, H.; Xu, J.; Fu, G.; Wang, X.; Yang, L.; Deng, P.; Zhang, J.; et al. Paraquat exposure induces Parkinsonism by altering lipid profile and evoking neuroinflammation in the midbrain. Environ. Int. 2022, 169, 107512. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Tinkov, A.A.; Skalny, A.V.; Siokas, V.; Dardiotis, E.; Tsatsakis, A.; Bowman, A.B.; da Rocha, J.B.T.; Aschner, M. Brain diseases in changing climate. Environ. Res. 2019, 177, 108637. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Adeyinka, A.; Muco, E.; Regina, A.C.; Pierre, L. Organophosphates. In StatPearls [Internet]; StatPearls: Petersburg, FL, USA, 2023. [Google Scholar]
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkung. Pflüg. Arch. Eur. J. Phy. 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Parsons, S.M.; Prior, C.; Marshall, I.G. Acetylcholine transport, storage, and release. Int Rev. Neurobiol. 1993, 35, 279–390. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.J.; Butcher, L.L. Cholinergic systems mediate action from movement to higher consciousness. Behav. Brain Res. 2011, 221, 488–498. [Google Scholar] [CrossRef]
- Boccia, M.M.; Blake, M.G.; Baratti, C.M.; McGaugh, J.L. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiol. Learn. Mem. 2009, 91, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; Delon, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef]
- Nathanson, N.M. A multiplicity of muscarinic mechanisms: Enough signaling pathways to take your breath away. Proc. Natl. Acad. Sci. USA 2000, 97, 6245–6247. [Google Scholar] [CrossRef]
- Kudo, Y.; Ogura, A.; Iijima, T. Stimulation of muscarinic receptor in hippocampal neuron induces characteristic increase in cytosolic free Ca2+ concentration. Neurosci. Lett. 1988, 85, 345–350. [Google Scholar] [CrossRef]
- Porter, A.C.; Bymaster, F.P.; DeLapp, N.W.; Yamada, M.; Wess, J.; Hamilton, S.E.; Nathanson, N.M.; Felder, C.C. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res. 2002, 944, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Segal, M. Rat hippocampal neurons in culture: Responses to electrical and chemical stimuli. J. Neurophysiol. 1983, 50, 1249–1264. [Google Scholar] [CrossRef]
- Tobin, A.B. A golden age of muscarinic acetylcholine receptor modulation in neurological diseases. Nat. Rev. Drug Discov. 2024, 23, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef]
- Kaur, S.; Chowdhary, S.; Kumar, D.; Bhattacharyya, R.; Banerjee, D. Organophosphorus and carbamate pesticides: Molecular toxicology and laboratory testing. Clin. Chim. Acta 2023, 551, 117584. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mercado, J.P.; Sierra-Santoyo, A.; Verdín-Betancourt, F.A.; Rojas-García, A.E.; Quintanilla-Vega, B. Temephos, an organophosphate larvicide for residential use: A review of its toxicity. Crit. Rev. Toxicol. 2022, 52, 113–124. [Google Scholar] [CrossRef]
- Musilek, K.; Dolezal, M.; Gunn-Moore, F.; Kuca, K. Design, evaluation and structure-Activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med. Res. Rev. 2009, 31, 548–575. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 2013, 203, 221–225. [Google Scholar] [CrossRef]
- Franco, R.; Li, S.; Rodriguez-Rocha, H.; Burns, M.; Panayiotidis, M.I. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2010, 188, 289–300. [Google Scholar] [CrossRef]
- Nagami, H.; Nishigaki, Y.; Matsushima, S.; Matsushita, T.; Asanuma, S.; Yajima, N.; Usuda, M.; Hirosawa, M. Hospital-based survey of pesticide poisoning in Japan, 1998–2002. Int. J. Occup. Environ. Health 2005, 11, 180–184. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gupta, R.D. Organophosphorus nerve agents: Types, toxicity, and treatments. J. Toxicol. 2020, 2020, 3007984. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Anwar, H.; Rasul, A.; Imran, A.; Qasim, M.; Zafar, S.; Imran, M.; Kamran, S.K.S.; Aziz, N.; Razzaq, A.; et al. Lipids as biomarkers of brain disorders. Crit. Rev. Food Sci. Nutr. 2020, 60, 351–374. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Ab: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neurophathol. 2020, 140, 417–447. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef]
- Estrada-Atehortúa, A.F.; Berrouet-Mejía, M.C.; Giraldo, J.A. Toxicidad por neonicotinoides: Revisión de tema y reporte de dos casos. Med. UPB 2016, 35, 41–46. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine pathway in Parkinson’s disease-an update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Wilson, R.; Lakshmipathy, G.; Vellingiri, B. The association between multiple risk factors, clinical correlations and molecular insights in Parkinson’s disease patients from Tamil Nadu population, India. Neurosci. Lett. 2021, 755, 135903. [Google Scholar] [CrossRef]
- Gorell, J.M.; Peterson, E.L.; Rybicki, B.A.; Johnson, C.C. Multiple risk factors for Parkinson’s disease. J. Neurol. Sci. 2004, 217, 169–174. [Google Scholar] [CrossRef]
- Dardiotis, E.; Aloizou, A.M.; Siokas, V.; Tsouris, Z.; Rikos, D.; Marogianni, C.; Aschner, M.; Kovatsi, L.; Bogdanos, D.P.; Tsatsakis, A. Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology 2019, 411, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Iteire, K.A.; Sowole, A.T.; Ogunlade, B. Exposure to pyrethroids induces behavioral impairments, neurofibrillary tangles and tau pathology in Alzheimer’s type neurodegeneration in adult Wistar rats. Drug Chem. Toxicol. 2022, 45, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Niu, C.Y.; Lei, C.L.; Cui, J.J.; Desneux, N. Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 2010, 19, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Flaskos, J. The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. Toxicol. Lett. 2012, 209, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, F.; Ozturk, M.; Akdogan, M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch. Toxicol. 2000, 74, 533–538. [Google Scholar] [CrossRef]
- Zhang, X.; Wallace, A.D.; Du, P.; Kibbe, W.A.; Jafari, N.; Xie, H.; Lin, S.; Baccarelli, A.; Soares, M.B.; Hou, L. DNA methylation alterations in response to pesticide exposure in vitro. Environ. Mol. Mutagen. 2012, 53, 542–549. [Google Scholar] [CrossRef]
- Zhang, X.; Wallace, A.D.; Du, P.; Lin, S.; Baccarelli, A.A.; Jiang, H.; Jafari, N.; Zheng, Y.; Xie, H.; Soares, M.B.; et al. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ. Toxicol. Pharmacol. 2012, 34, 959–968. [Google Scholar] [CrossRef]
- Cortese, S.; Coghill, D. Twenty years of research on attention-deficit/hyperactivity disorder (ADHD): Looking back, looking forward. BMJ Ment. Health 2018, 21, 173–176. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Tripp, G.; Wickens, J.R. Neurobiology of ADHD. Neuropharmacology 2009, 57, 579–589. [Google Scholar] [CrossRef]
- Asghar, U.; Malik, M.F.; Javed, A. Pesticide exposure and human health: A review. J. Ecosys. Ecograph S. 2016, 5. [Google Scholar] [CrossRef]
- Shah, R. Pesticides and human health. In Emerging Contaminants; Nuro, A., Ed.; InTech: Nappanee, IN, USA, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Nabi, M.; Tabassum, N. Role of environmental toxicants on neurodegenerative disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Suarez-Lopez, J.R.; Himes, J.H.; Jacobs, D.R., Jr.; Alexander, B.H.; Gunnar, M.R. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics 2013, 132, e1649–e1658. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Du, J.C.; Chiou, H.C.; Chung, M.Y.; Yang, W.; Chen, Y.S.; Fuh, M.R.; Chien, L.C.; Hwang, B.; Chen, M.L. Increased risk of attention-deficit/hyperactivity disorder associated with exposure to organophosphate pesticide in Taiwanese children. Andrology 2016, 4, 695–705. [Google Scholar] [CrossRef]
- Marks, A.R.; Harley, K.; Bradman, A.; Kogut, K.; Barr, D.B.; Johnson, C.; Calderon, N.; Eskenazi, B. Organophosphate pesticide exposure and attention in young Mexican American children: The CHAMACOS study. Environ. Health Perspect. 2010, 118, 1768–1774. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Jiménez-Ferrer, E.; González-Cortazar, M.; Zamilpa, A.; Cardoso-Taketa, A.; Arenas-Ocampo, M.L.; Jiménez-Aparicio, A.R.; Monterrosas-Brisson, N. Potential Use of Agave Genus in Neuroinflammation Management. Plants 2022, 11, 2208. [Google Scholar] [CrossRef]
- Cresto, N.; Forner-Piquer, I.; Baig, A.; Chatterjee, M.; Perroy, J.; Goracci, J.; Marchi, N. Pesticides at brain borders: Impact on the blood-brain barrier, neuroinflammation, and neurological risk trajectories. Chemosphere 2023, 324, 138251. [Google Scholar] [CrossRef] [PubMed]
- Sreekanthreddy, P.; Gromnicova, R.; Davies, H.; Phillips, J.; Romero, I.A.; Male, D. A three-dimensional model of the human blood-brain barrier to analyse the transport of nanoparticles and astrocyte/endothelial interactions. F1000Research 2015, 4, 1279. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.; Reindl, M. Blood-Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef]
- Ravid, O.; Elhaik Goldman, S.; Macheto, D.; Bresler, Y.; De Oliveira, R.I.; Liraz-Zaltsman, S.; Gosselet, F.; Dehouck, L.; Beeri, M.S.; Cooper, I. Blood-Brain Barrier Cellular Responses Toward Organophosphates: Natural Compensatory Processes and Exogenous Interventions to Rescue Barrier Properties. Front. Cell Neurosci. 2018, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.; Li, W.; Ehrich, M. Assessments of tight junction proteins occludin, claudin 5 and scaffold proteins ZO1 and ZO2 in endothelial cells of the rat blood-brain barrier: Cellular responses to neurotoxicants malathion and lead acetate. Neurotoxicology 2011, 32, 58–67. [Google Scholar] [CrossRef]
- Balbuena, P.; Li, W.; Magnin-Bissel, G.; Meldrum, J.B.; Ehrich, M. Comparison of two blood-brain barrier in vitro systems: Cytotoxicity and transfer assessments of malathion/oxon and lead acetate. Toxicol. Sci. 2010, 114, 260–271. [Google Scholar] [CrossRef]
- Hsu, S.S.; Jan, C.R.; Liang, W.Z. Uncovering malathion (an organophosphate insecticide) action on Ca2+ signal transduction and investigating the effects of BAPTA-AM (a cell-permeant Ca2+ chelator) on protective responses in glial cells. Pestic. Biochem. Physiol. 2019, 157, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.; Jan, C.R.; Liang, W.Z. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology 2019, 417, 1–14. [Google Scholar] [CrossRef]
- Mense, S.M.; Sengupta, A.; Lan, C.; Zhou, M.; Bentsman, G.; Volsky, D.J.; Whyatt, R.M.; Perera, F.P.; Zhang, L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol. Sci. 2006, 93, 125–135. [Google Scholar] [CrossRef]
- Maurya, S.K.; Rai, A.; Rai, N.K.; Deshpande, S.; Jain, R.; Mudiam, M.K.; Prabhakar, Y.S.; Bandyopadhyay, S. Cypermethrin induces astrocyte apoptosis by the disruption of the autocrine/paracrine mode of epidermal growth factor receptor signaling. Toxicol. Sci. 2012, 125, 473–487. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Weis, G.C.C.; Assmann, C.E.; Mostardeiro, V.B.; Alves, A.O.; da Rosa, J.R.; Pillat, M.M.; de Andrade, C.M.; Schetinger, M.R.C.; Morsch, V.M.M.; da Cruz, I.B.M.; et al. Chlorpyrifos pesticide promotes oxidative stress and increases inflammatory states in BV-2 microglial cells: A role in neuroinflammation. Chemosphere 2021, 278, 130417. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Liu, J.; Richardson, J.R. Pyrethroid Insecticides Directly Activate Microglia Through Interaction With Voltage-Gated Sodium Channels. Toxicol. Sci. 2017, 155, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Rajput, C.; Singh, M.P. Cypermethrin Induces the Activation of Rat Primary Microglia and Expression of Inflammatory Proteins. J. Mol. Neurosci. 2021, 71, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Xu, Y.N.; Ren, X.Y.; Zhang, X.F. Inactivation of microglia dampens blood-brain barrier permeability and loss of dopaminergic neurons in paraquat-lesioned mice. Food Chem. Toxicol. 2023, 174, 113692. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Podolak, I. Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review. Molecules 2024, 29, 866. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Granger, K.T.; Barnett, J.H. Postoperative cognitive dysfunction: An acute approach for the development of novel treatments for neuroinflammation. Drug Discov. 2021, 26, 1111–1114. [Google Scholar] [CrossRef]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer’s Disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Boyden, T.; Liras, J.; El-Kattan, A.; Brodfuehrer, J. Current advances in delivery of biotherapeutics across the blood-brain barrier. Curr. Drug Discov. Technol. 2011, 8, 87–101. [Google Scholar] [CrossRef]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-κB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Mao, Z.; Yang, S.; Wu, G.; Chen, Y.; Yin, L.; Qi, Y.; Han, L.; Xu, L. Dioscin alleviates Alzheimer’s disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed. Pharmacother. 2022, 152, 113248. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Liagre, B.; Cook-Moreau, J.; Pinon, A.; Beneytout, J.L. Cyclooxygenase-2 and 5-lipoxygenase pathways in diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer cells. Int. J. Oncol. 2010, 36, 1183–1191. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Jiménez-Ferrer, E.; Tortoriello, J.; Zamilpa, A.; Alegría-Herrera, E.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Martínez-Duncker, I.; Monterrosas-Brisson, N. Anti-neuroinflammatory effect of agaves and cantalasaponin-1 in a model of LPS-induced damage. Nat. Prod. Res. 2021, 35, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L. The Neuroprotective Effects of Moderate and Regular Caffeine Consumption in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 5568011. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7–28. [Google Scholar] [CrossRef]
- Jaturapatporn, D.; Isaac, M.G.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012, 2, CD006378. [Google Scholar] [CrossRef]
- Shukla, M.; Wongchitrat, P.; Govitrapong, P. A Synopsis of Multitarget Potential Therapeutic Effects of Huperzine A in Diverse Pathologies-Emphasis on Alzheimer’s Disease Pathogenesis. Neurochem. Res. 2022, 47, 1166–1182. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, W.; Wang, T.; Xie, J.W.; Wang, S.L.; Zhao, B.L.; Teng, W.P.; Wang, Z.Y. Huperzine A activates Wnt/β-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 2011, 36, 1073–1089. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. B-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. BioMed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [CrossRef]
- Hijam, A.C.; Tongbram, Y.C.; Nongthombam, P.D.; Meitei, H.N.; Koijam, A.S.; Rajashekar, Y.; Haobam, R. Neuroprotective Potential of Traditionally Used Medicinal Plants of Manipur against Rotenone-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. J. Ethnopharmacol. 2024, 330, 118197. [Google Scholar] [CrossRef]
- Hijam, A.C.; Tongbram, Y.C.; Nongthombam, P.D.; Meitei, H.N.; Koijam, A.S.; Rajashekar, Y.; Haobam, R. Traditionally used edible medicinal plants protect against rotenone induced toxicity in SH-SY5Y cells-a prospect for the development of herbal nutraceuticals. Neurochem. Int. 2024, 180, 105855. [Google Scholar] [CrossRef] [PubMed]
- Rhioui, W.; Al Figuigui, J.; Lahlali, R.; Laasli, S.E.; Boutagayout, A.; El Jarroudi, M.; Belmalha, S. Towards sustainable vegetable farming: Exploring agroecological alternatives to chemical products in the Fez-Meknes region of Morocco. Sustainability 2023, 15, 7412. [Google Scholar] [CrossRef]
- Singh, M. Pesticide predicament: Exploring environmental and health impacts, and possible eco-friendly solutions. Int. J. Environ. Health Sci. 2024, 6, 38–47. [Google Scholar]
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Deguine, J.P.; Aubertot, J.N.; Bellon, S.; Côte, F.; Lauri, P.E.; Lescourret, F.; Ratnadass, A.; Scopel, E.; Andrieu, N.; Bàrberi, P.; et al. Agroecological crop protection for sustainable agriculture. Adv. Agron. 2023, 178, 1–59. [Google Scholar] [CrossRef]
- Vikas; Ranjan, R. Agroecological approaches to sustainable development. Front. Sustain. Food Syst. 2024, 8, 1405409. [Google Scholar] [CrossRef]
- Jeanneret, P.; Aviron, S.; Alignier, A.; Lavigne, C.; Helfenstein, J.; Herzog, F.; Kay, S.; Petit, S. Agroecology landscapes. Landsc. Ecol. 2021, 36, 2235–2257. [Google Scholar] [CrossRef]
- Rastogi, M.; Verma, S.; Kumar, S.; Bharti, S.; Kumar, G.; Azam, K.; Singh, V. Soil health and sustainability in the age of organic amendments: A review. Int. J. Environ. Clim. Change 2023, 13, 2088–2102. [Google Scholar] [CrossRef]
- Somashekar, K.S.; Abhishek, G.J.; Kumar, V. Agroecology Principles, Practices and their Impact on Sustainable Food Systems. Eur. J. Nutr. Food Saf. 2024, 16, 249–260. [Google Scholar] [CrossRef]
- Hatt, S.; Döring, T.F. Designing pest suppressive agroecosystems: Principles for an integrative diversification science. J. Clean. Prod. 2023, 432, 139701. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Ahmad, M.A.; Karthik, S.; Raju, B.J. Organic insect pest management: A sustainable approach for the Indian Farming Community. In Agriculture Entomology; Singh, K., Ed.; Rohini: Delhi, India, 2021; Volume 2, pp. 127–150. [Google Scholar]
- Sharma, S. Cultivating sustainable solutions: Integrated Pest Management (IPM) for safer and greener agronomy. Corp. Sustain. Manag. J. 2023, 1, 103–108. [Google Scholar] [CrossRef]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. Co-Evol. Second. Metab. 2020, 505–519. [Google Scholar] [CrossRef]
- Yu, T.; Mahe, L.; Li, Y.; Wei, X.; Deng, X.; Zhang, D. Benefits of crop rotation on climate resilience and its prospects in China. Agronomy 2022, 12, 436. [Google Scholar] [CrossRef]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified crop rotation: An approach for sustainable agriculture production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Meena, R.K.; Mishra, P. Bio-pesticides for agriculture and environment sustainability. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 85–107. [Google Scholar] [CrossRef]
- Ximhai, R.; Nava-Pérez, E.; García-Gutiérrez, C.; Camacho-Báez, J.R.; Vázquez-Montoya, E.L. Bioplaguicidas: Una opción para el control biológico de plagas. Ra Ximhai 2012, 8, 17–29. [Google Scholar]
- Verma, D.K.; Guzmán, K.N.R.; Mohapatra, B.; Talukdar, D.; Chávez-González, M.L.; Kumar, V.; Srivastava, S.; Singh, V.; Yulianto, R.; Malar, S.E.; et al. Recent trends in plant-and microbe-based biopesticide for sustainable crop production and environmental security. In Recent Developments in Microbial Technologies; Prasad, R., Kumar, V., Singh, J., Upadhyaya, C.P., Eds.; Springer: Singapore, 2021; pp. 1–37. [Google Scholar] [CrossRef]
- Bel, Y.; Ferré, J.; Hernández-Martínez, P. Bacillus thuringiensis toxins: Functional characterization and mechanism of action. Toxins 2020, 12, 785. [Google Scholar] [CrossRef]
- Heckel, D.G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Physiol. 2020, 104, e21673. [Google Scholar] [CrossRef]
- Pinos, D.; Andrés-Garrido, A.; Ferré, J.; Hernández-Martínez, P. Response mechanisms of invertebrates to Bacillus thuringiensis and its pesticidal proteins. Microbiol. Mol. Biol. Rev. 2021, 85, 10–1128. [Google Scholar] [CrossRef]
- Azizoglu, U.; Salehi Jouzani, G.; Sansinenea, E.; Sanchis-Borja, V. Biotechnological advances in Bacillus thuringiensis and its toxins: Recent updates. Rev. Environ. Sci. Biotechnol. 2023, 22, 319–348. [Google Scholar] [CrossRef]
- Mwamburi, L.A. Beauveria. Benef. Microbes Agro-Ecol. 2020, 727–748. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as promising alternatives to chemical pesticides: A review of their current and future status. Online J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Cappa, F.; Baracchi, D.; Cervo, R. Biopesticides and insect pollinators: Detrimental effects, outdated guidelines, and future directions. Sci. Total Environ. 2022, 837, 155714. [Google Scholar] [CrossRef] [PubMed]
- Daraban, G.M.; Hlihor, R.M.; Suteu, D. Pesticides vs. biopesticides: From pest management to toxicity and impacts on the environment and human health. Toxics 2023, 11, 983. [Google Scholar] [CrossRef]
- Eisa, M.A.S.; Hamid, H.A.; Ishag, A.E.S. Jatropha (Jatropha curcas) and Argel (Solenostemma argel) extracts as an oviposition inhibitor to Spiny Bollworm [Earias insulana (Lepidoptera: Noctuidae)]. Int. J. Life Sci. Res. 2020, 8, 5–11. [Google Scholar]
- Bratu, E.; Şovărel, G.; Cenuşă, A.E.; Velea, M. Nemagold”–bio-product of perspective in the control of root-knot nematode (Meloidogyne spp.) At tomato crops under plastic tunnel. Int. Multidiscip. Sci. GeoConference SGEM 2017, 17, 837–844. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Swathy, K.; Parmar, M.K.; Vivekanandhan, P. Biocontrol efficacy of entomopathogenic fungi Beauveria bassiana conidia against agricultural insect pests. Environ. Qual. Manag. 2024, 34, e22174. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Huang, S.T.; Chen, J.T.; Li, N.; Wei, Y.; Nawaz, A.; Deng, S.Q. An update of a green pesticide: Metarhizium anisopliae. All Life 2022, 15, 1141–1159. [Google Scholar] [CrossRef]
- Yapa, A.T.; Thambugala, K.M.; Samarakoon, M.C.; de Silva, N. Metarhizium species as bioinsecticides: Potential, progress, applications & future perspectives. N. Z. J. Bot. 2024, 63, 439–461. [Google Scholar] [CrossRef]
- Naz, I.; Khan, R.A.A.; Masood, T.; Baig, A.; Siddique, I.; Haq, S. Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biol. Control. 2021, 152, 104429. [Google Scholar] [CrossRef]
- Kim, J.J.; Goettel, M.S.; Gillespie, D.R. Evaluation of Lecanicillium longisporum, Vertalec® for simultaneous suppression of cotton aphid, Aphis gossypii, and cucumber powdery mildew, Sphaerotheca fuliginea, on potted cucumbers. Biol. Control. 2008, 45, 404–409. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Heckel, D.G.; Ferré, J. Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef]
- Jamal, Q.M.S.; Ahmad, V. Lysinibacilli: A biological factories intended for bio-insecticidal, bio-control, and bioremediation activities. J. Fungi. 2022, 8, 1288. [Google Scholar] [CrossRef]
- Terry Alfonso, E.; Ruiz Padrón, J.; Tejeda Peraza, T. Efecto de un bioproducto a base de Pseudomona aeruginosa en el cultivo del tomate (Solanum licopersicum Mill). Rev. Colomb. Biotecnol. 2010, 12, 32–38. [Google Scholar]
- Sarkar, B.; Kumar, C.; Pasari, S.; Goswami, B. Review on Pseudomonas fluorescens: A plant growth promoting rhizobacteria. J. Posit. Sch. Psychol. 2022, 6, 2701–2709. [Google Scholar]
- Nalinci, E.; Karagoz, M.; Gulcu, B.; Ulug, D.; Gulsen, S.H.; Cimen, H.; Touray, M.; Shapiro-Ilan, D.; Hazir, S. The effect of chemical insecticides on the scavenging performance of Steinernema carpocapsae: Direct effects and exposure to insects killed by chemical insecticides. J. Invertebr. Pathol. 2021, 184, 107641. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martínez, C.I.; Rodríguez-Hernández, A.I.; López-Cuellar, M.D.R.; Chavarría-Hernández, N.; De los Santos-Romero, R. Culture of Steinernema glaseri on three solid media and their virulence against Galleria mellonella larvae. Plant Prot. Sci. 2023, 59, 278–283. [Google Scholar] [CrossRef]
- Ropek, D.R.; Gospodarek, J. Entomopathogenic Nematode Steinernema feltiae as an Indicator of Soil Pollution with Oil Derivatives in Bioremediation Process. Agriculture 2022, 12, 2033. [Google Scholar] [CrossRef]
- Kary, N.E.; Sanatipour, Z.; Mohammadi, D.; Dillon, A.B. Combination effects of entomopathogenic nematodes, Heterorhabditis bacteriophora and Steinernema feltiae, with Abamectin on developmental stages of Phthorimaea operculella (Lepidoptera, Gelechiidae). Crop. Prot. 2021, 143, 105543. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M. Nosema locustae (Protozoa, Microsporidia), a biological agent for locust and grasshopper control. Agronomy 2021, 11, 711. [Google Scholar] [CrossRef]
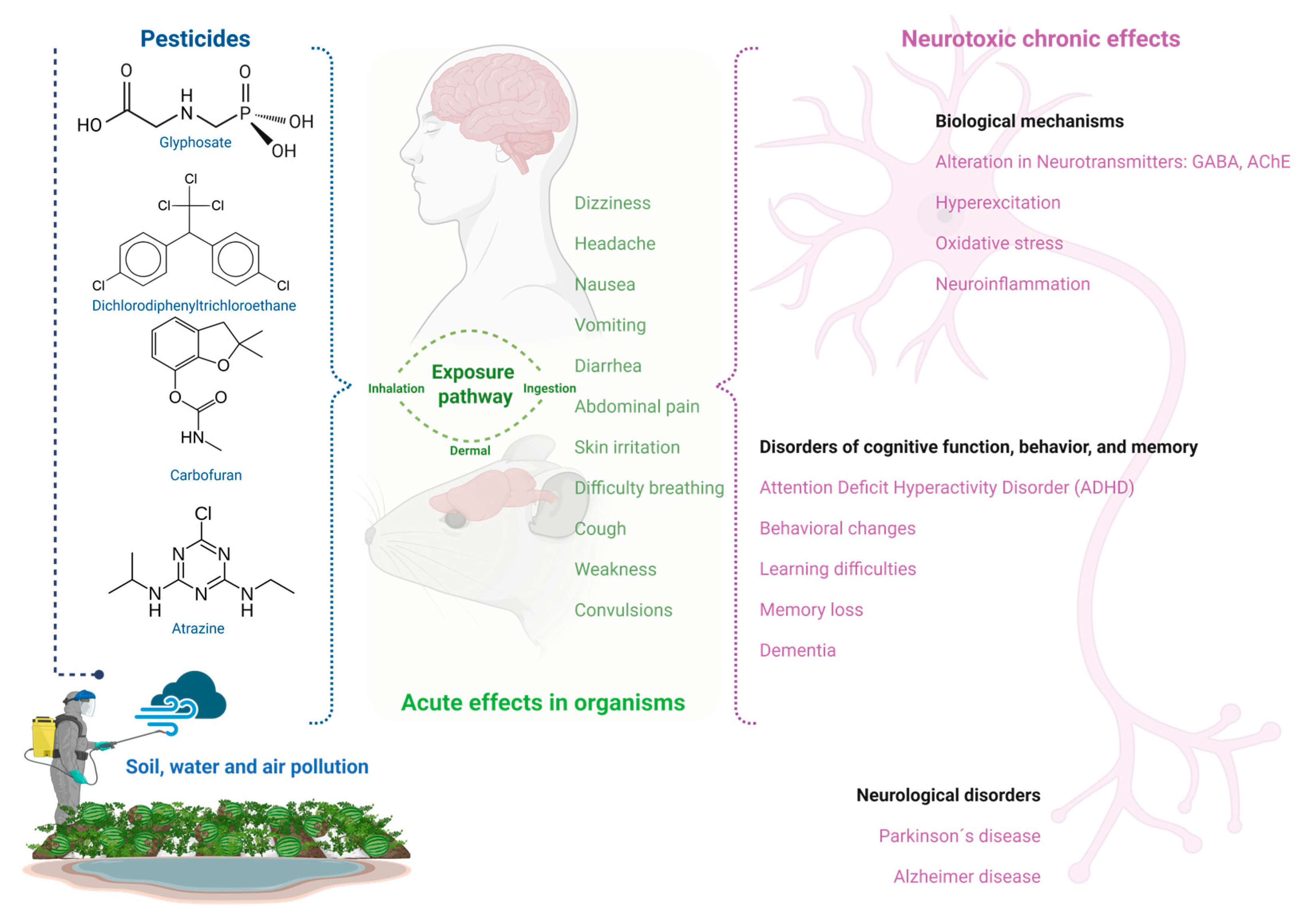
| Pesticide Class | Chemical Action and Molecular Mechanism of Action | Description | Examples |
|---|---|---|---|
| Bipyridyls | Herbicides Photosynthesis inhibition | Quaternary ammonium compounds of aromatic nature with herbicide and desiccant activities. | 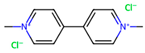 Paraquat-dichloride |
| Carbamates | Insecticides Reversible inactivation of the enzyme acetylcholinesterase (AChE) in nervous system | Derived from carbamic acid, carbamates are organic compounds that exhibit both structural and mechanistic resemblance to organophosphate insecticides. | 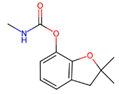 Carbofuran |
| Formamidines | Insecticides/Acaricides Inhibition of the monoamine oxidase activity and neuromuscular transmission blocking | Group or insecticidal and acaricide molecules (based on the characteristic nitrogen structure –N=CHN–) that are used to control pesticide-resistant mites, ticks, and insects in early stages (i.e., eggs and larvae). |  Amitraz |
| Neonicotinoids | Insecticides Modulation of nicotinic acetylcholine receptors (nAChRs) in the central system of insects | Neuroactive systemic insecticides are structurally related to nicotine. | 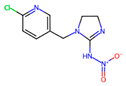 Imidacloprid |
| Organochlorines | Insecticides Blocking of inhibitory neurotransmitter GABA receptors and voltage-gated chloride channels | These are organic compounds with at least one covalently attached chlorine atom, characterized by their high persistence, nonpolar nature, and lipophilicity. |  DDT 1 |
| Organophosphates | Herbicides Competitive inhibition of the enzyme 5-enolpyruvylsikimate-3-phosphate synthase (EPSPS) | Derivatives of phosphoric or thiophosphoric acids are formed through an esterification process involving phosphoric acid and alcohol. | 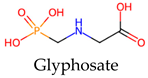 Glyphosate |
| Insecticides Inhibition of the nervous system enzyme acetylcholinesterase (AChE) |  Methyl-parathion | ||
| Pyrethrins | Insecticides Modulation/alteration of the opening of sodium channels of nerve cells | Insecticides that are derived from pyrethrum, a natural insecticide from the flowers of Chrysanthemum cinerariaefolium and Chrysanthemum cineum. |  Pyrethrin I |
| Pyrethroids | Synthetic analogs of pyrethrins with a longer duration of action, higher toxicity, and greater stability. | 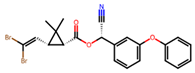 Deltamethrin | |
| Triazines | Herbicides Photosynthesis inhibition | Pesticides based on nitrogen-containing heterocycles with herbicide activity; triazines are classified as persistent organic compounds. | 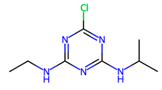 Atrazine |
| Biopesticides | Multiple activities Antagonism, competition, hyperparasitism, and activation of plant resistance | Pesticides are derived from natural origins such as animals, microorganisms, plants, and specific minerals. |  Cry toxins 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, A.; Castrejón-Godínez, M.L.; Monterrosas-Brisson, N. Pesticides: Environmental Stressors Implicated in the Development of Central Nervous System Disorders and Neurodegeneration. Stresses 2025, 5, 31. https://doi.org/10.3390/stresses5020031
Rodríguez A, Castrejón-Godínez ML, Monterrosas-Brisson N. Pesticides: Environmental Stressors Implicated in the Development of Central Nervous System Disorders and Neurodegeneration. Stresses. 2025; 5(2):31. https://doi.org/10.3390/stresses5020031
Chicago/Turabian StyleRodríguez, Alexis, María Luisa Castrejón-Godínez, and Nayeli Monterrosas-Brisson. 2025. "Pesticides: Environmental Stressors Implicated in the Development of Central Nervous System Disorders and Neurodegeneration" Stresses 5, no. 2: 31. https://doi.org/10.3390/stresses5020031
APA StyleRodríguez, A., Castrejón-Godínez, M. L., & Monterrosas-Brisson, N. (2025). Pesticides: Environmental Stressors Implicated in the Development of Central Nervous System Disorders and Neurodegeneration. Stresses, 5(2), 31. https://doi.org/10.3390/stresses5020031








