Comparison of the Effects of Gradual and Acute Treatment with Mn on Physiological Responses of Rumex hydrolapathum Plants
Abstract
1. Introduction
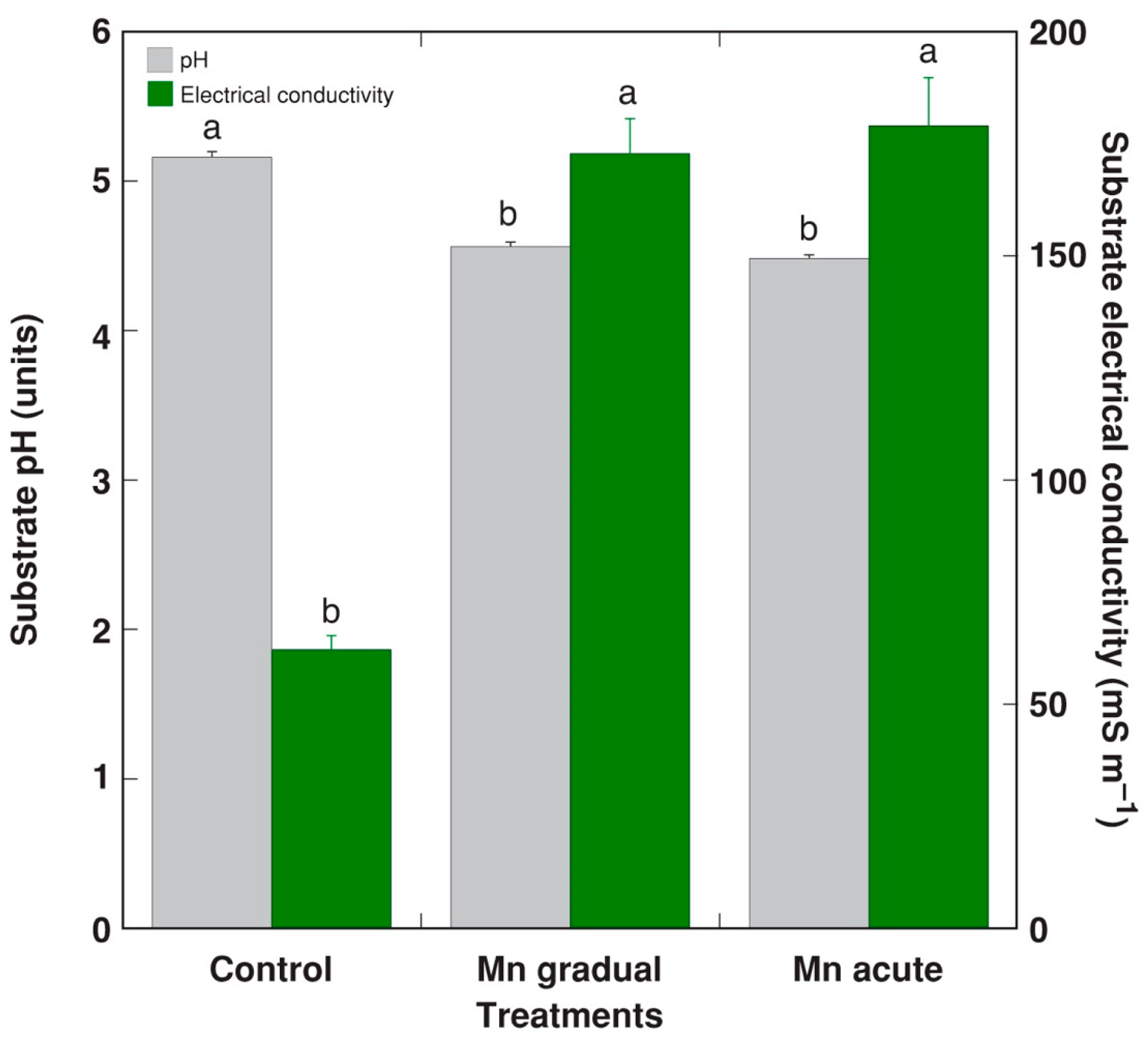
2. Results
3. Discussion
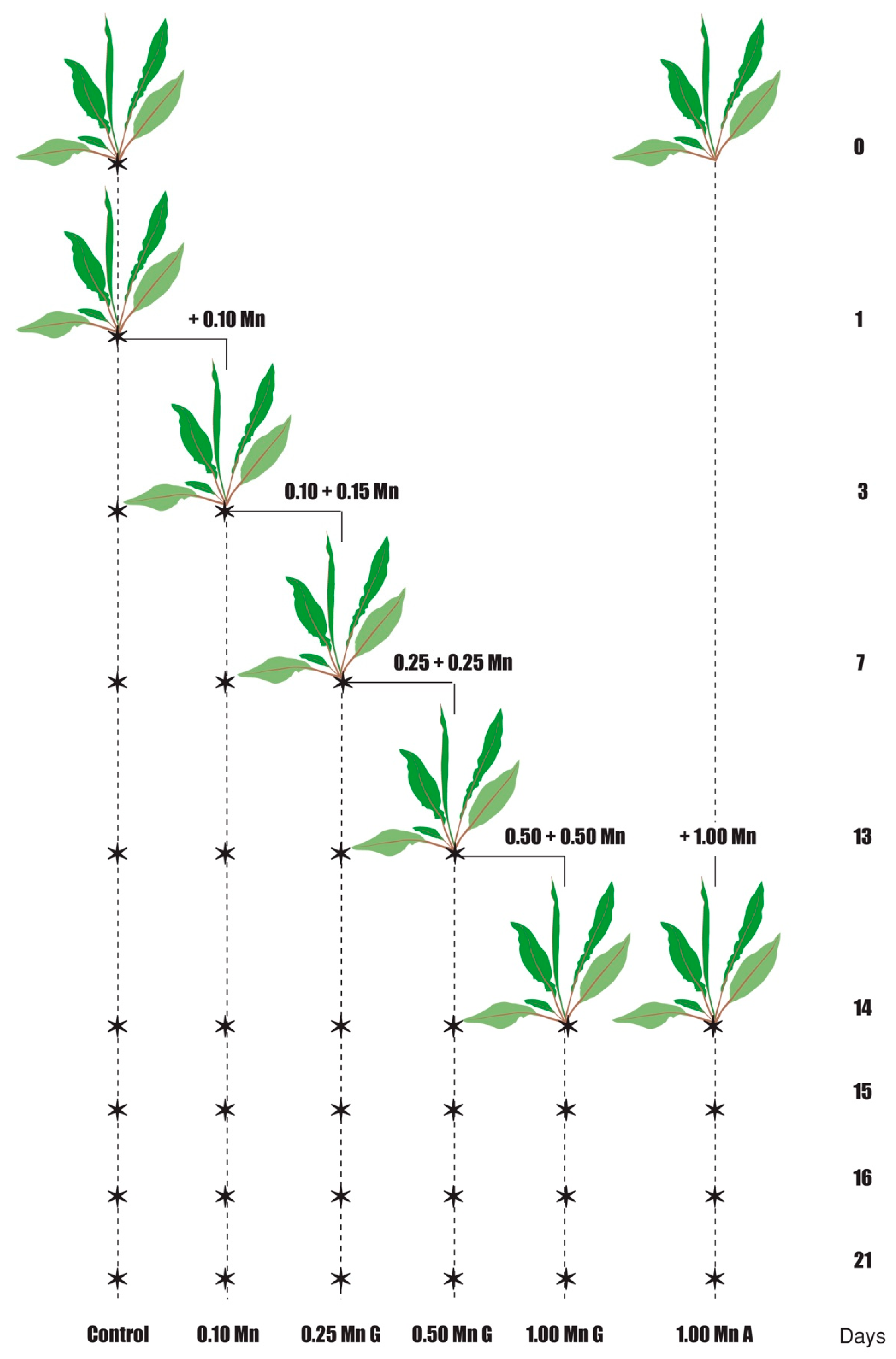
4. Materials and Methods
4.1. Plant Material and Establishment of Seedlings
4.2. Plant Cultivation and Treatment Conditions
4.3. Measurements and Experiment Termination
4.4. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegedus, C.; Pașcalău, S.-N.; Andronie, L.; Rotaru, A.-S.; Cucu, A.-A.; Dexmirean, D.S. The journey of 1000 leagues towards the decontamination of the soil from heavy metals and the impact on the soil–plant–animal–human chain begins with the first step: Phytostabilization/phytoextraction. Agriculture 2023, 13, 735. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy metals in agricultural soils: Sources, influencing factors, and remediation strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z. Metal accumulation and tolerance in wetland plants. Front. Biol. China 2009, 4, 282–288. [Google Scholar] [CrossRef]
- Teuchies, J.; Jacobs, S.; Oosterlee, L.; Bervoets, L.; Meire, P. Role of plants in metal cycling in a tidal wetland: Implications for phytoremediation. Sci. Total Environ. 2013, 445–446, 146–154. [Google Scholar] [CrossRef]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- Chandra, R.; Yadav, S.; Yadav, S. Phytoextraction potential of heavy metals by native wetland plants growing on chlorolignin containing sludge of pulp and paper industry. Ecol. Eng. 2017, 98, 134–145. [Google Scholar] [CrossRef]
- Bonanno, G.; Vymazal, J.; Cirelli, G.L. Translocation, accumulation and bioindication of trace elements in wetland plants. Sci. Total Environ. 2018, 631–632, 252–261. [Google Scholar] [CrossRef]
- Ievinsh, G.; Andersone-Ozola, U.; Landorfa-Svalbe, Z.; Karlsons, A.; Osvalde, A. Wild plants from coastal habitats as a potential resource for soil remediation. In Soil Health; Giri, B., Varma, A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 121–144. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Shavrukov, Y. Salt stress or salt shock: Which genes are we studying? J. Exp. Bot. 2013, 64, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2008, 14, 43–50. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; El Moneim, D.A.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Kartagelis, S.; Moustakas, M.; Symeonidis, L. Effects of heavy metals on isoperoxidases of wheat. Biol. Plant. 1991, 33, 3–9. [Google Scholar] [CrossRef]
- Klotz, K.L.; Liu, T.T.; Liu, L.; Lagrimini, L.M. Expression of the tobacco anionic peroxidase gene is tissue-specific and developmentally regulated. Plant Mol. Biol. 1998, 36, 509–520. [Google Scholar] [CrossRef]
- Hiraga, S.; Yamamoto, K.; Ito, H.; Sasaki, K.; Matsui, H.; Honma, M.; Nagamura, Y.; Sasaki, T.; Ohashi, Y. Diverse expression profiles of 21 rice peroxidase genes. FEBS Lett. 2000, 471, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Novo-Uzal, E.; Fernández-Pérez, F.; Herrero, J.; Gutiérrez, J.; Gómez-Ros, L.V.; Bernal, M.Á.; Díaz, J.; Cuello, J.; Pomar, F.; Pedreño, M.Á. From Zinnia to Arabidopsis: Approaching the involvement of peroxidases in lignification. J. Exp. Bot. 2013, 64, 3499–3518. [Google Scholar] [CrossRef]
- Reichman, S.M. The Responses of Plants to Metal Toxicity: A Review Focusing on Copper, Manganese and Zinc; AMEEF Paper 14; Australian Minerals and Energy Environment Foundation: Melbourne, VIC, Australia, 2002. [Google Scholar]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Husted, S. The biochemical properties of manganese in plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef]
- Singer, C.E.; Havil, D.C. Manganese as an ecological factor in salt marshes. Vegetatio 1985, 62, 287–292. [Google Scholar] [CrossRef]
- Otero, X.L.; Ferreira, T.O.; Huerta-Diaz, M.A.; Partiti, C.S.M.; Souza, V., Jr.; Vidal-Torrado, P.; Macias, F. Geochemistry of iron and manganese in soils and sediments of a mangrove system Island of Pai Matos (Cananeia—SP, Brazil). Geoderma 2009, 148, 318–335. [Google Scholar] [CrossRef]
- Cooper, A. A comparative study of the tolerance of salt marsh plants to manganese. Plant Soil 1984, 81, 47–59. [Google Scholar] [CrossRef]
- Singer, C.E.; Havil, D.C. Resistance to divalent manganese of salt marsh plants. J. Ecol. 1993, 81, 797–806. [Google Scholar] [CrossRef]
- Fernando, D.R.; Moroni, S.J.; Scott, B.J.; Conyers, M.K.; Lynch, J.P.; Marshall, A.T. Temperature and light drive manganese accumulation and stress in crops across three major families. Environ. Exp. Bot. 2016, 132, 66–79. [Google Scholar] [CrossRef]
- Santos, E.F.; Santini, J.M.K.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Mojović, M.; Maksimović, V.; Römheld, V.; Nikolic, M. Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J. Exp. Bot. 2012, 63, 2411–2420. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative stress and antioxidant responses of mulberry (Morus alba) plants subjected to deficiency and excess of manganese. Acta Physiol. Plant. 2013, 35, 3345–3356. [Google Scholar] [CrossRef]
- González, A.; Steffen, K.L.; Lynch, J.P. Light and excess manganese. Implications for oxidative stress in common bean. Plant Physiol. 1998, 118, 493–504. [Google Scholar]
- Ribera-Fonseca, A.; Inostroza-Blancheteau, C.; Cartes, P.; Rengel, Z.; Mora, M.L. Early induction of Fe-SOD gene expression is involved in tolerance to Mn toxicity in perennial ryegrass. Plant Physiol. Biochem. 2013, 73, 77–82. [Google Scholar] [CrossRef]
- Liu, P.D.; Huang, R.; Hu, X.; Jia, Y.D.; Ki, J.F.; Luo, J.J.; Liu, Q.; Luo, L.J.; Liu, G.D.; Chen, Z.J. Physiological responses and proteomic changes reveal insights into Stylosanthes response to manganese toxicity. BMC Plant Biol. 2019, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, K.; Tian, X.; Korpelainen, H.; Li, C. Effect of Mn toxicity on morphological and physiological changes in two Populus cathayana populations originating from different habitats. Trees 2007, 21, 569–580. [Google Scholar] [CrossRef]
- Younis, M.E.; Tourky, S.M.N.; Elsharkawy, S.E.A. Symptomatic parameters of oxidative stress and antioxidant defense system in Phaseolus vulgaris L. in response to copper or cadmium stress. S. Afr. J. Bot. 2018, 117, 207–214. [Google Scholar] [CrossRef]
- Lizieri, C.; Kuki, K.N.; Agular, R. The morphophysiological responses of free-floating aquatic macrophytes to a supra-optimal supply of manganese. Water Air Soil Pollut. 2012, 223, 2807–2820. [Google Scholar] [CrossRef]
- Yang, X.-J.; Deng, D.-M.; Liu, K.-H.; Yu, F.-M. Response of enzymatic and non-enzymatic antioxidant defense systems of Polygonum hydropiper to Mn stress. J. Cent. South Univ. 2016, 23, 793–797. [Google Scholar] [CrossRef]
- Tang, S.; Fang, Y. Copper accumulation by Polygonum microcephalum D. Don and Rumex hastatus D. Don from copper mine spoils in Yunnan Province, P.R. China. Environ. Geol. 2001, 40, 902–907. [Google Scholar] [CrossRef]
- Barrutia, O.; Epelde, L.; García-Plazaola, J.I.; Garbisu, C.; Becerril, J.M. Phytoextraction potential of two Rumex acetosa L. accessions collected from metalliferous and non-metalliferous sites: Effect of fertilization. Chemosphere 2009, 74, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liao, B.; Li, J.T.; Mengoni, A.; Hu, M.; Luo, W.C.; Shu, W.S. Contrasting patterns of genetic divergence in two sympatric pseudo-metallophytes: Rumex acetosa L. and Commelina communis L. BMC Evol. Biol. 2012, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, G. Resistance and distribution to heavy metals of Zoysia sinica Hance and Rumex crispus. Adv. Mater. Res. 2014, 1010–1012, 117–120. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, H.; Ma, J.; Li, Z.; Li, H.; Zhu, X.; Shao, J.; Yang, Z.; Yang, Y.; He, F.; et al. Effects of mutual intercropping on Pb and Zn accumulation of accumulator plants Rumex nepalensis, Lolium perenne and Trifolium repens. Chem. Ecol. 2018, 34, 259–271. [Google Scholar] [CrossRef]
- Sager, L.; Clerc, C. Factors influencing the distribution of Hydrocharis morsus-ranae L. and Rumex hydrolapathum Huds. in a mowed low-lying marshland, Réserve de Cheyres, lac de Neuchâtel, Switzerland. In Macrophytes in Aquatic Ecosystems: From Biology to Management; Caffrey, J.M., Dutartre, A., Haury, J., Murphy, K.J., Wade, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 223–229. [Google Scholar]
- Ievinsh, G.; Dišlere, E.; Karlsons, A.; Osvalde, A.; Vikmane, M. Physiological responses of wetland species Rumex hydrolapathum to increased concentration of biogenous heavy metals Zn and Mn in substrate. Proc. Latv. Acad. Sci. B 2020, 74, 35–47. [Google Scholar] [CrossRef]
- Ieviņa, S.; Karlsons, A.; Osvalde, A.; Andersone-Ozola, U.; Ievinsh, G. Coastal wetland species Rumex hydrolapathum: Tolerance against flooding, salinity, and heavy metals for its potential use in phytoremediation and environmental restoration technologies. Life 2023, 13, 1604. [Google Scholar] [CrossRef]
- Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Ievinsh, G. Type of anion largely determines salinity tolerance in four Rumex species. Plants 2023, 12, 92. [Google Scholar] [CrossRef]
- Hayat, K.; Khan, A.; Bibi, F.; Salahuddin; Murad, W.; Fu, Y.; Batiha, G.E.-S.; Alqarni, M.; Khan, A.; Al-Harrasi, A. Effect of cadmium and copper exposure on growth, physio-chemicals and medicinal properties of Cajanus cajan L. (pigeon pea). Metabolites 2021, 11, 769. [Google Scholar] [CrossRef]
- Hassan, M.J.; Raza, M.A.; Rehman, S.U.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Shah, G.A.; Peng, Y.; et al. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Chen, M.; He, X.; Li, J.; An, R. Defense mechanisms of two pioneer submerged plants during their optimal performance period in the bioaccumulation of lead: A comparative study. Int. J. Environ. Res. Public Health 2018, 15, 2844. [Google Scholar] [CrossRef] [PubMed]
- Alsherif, E.A.; Al-Shaikh, T.M.; Almaghrabi, O.; AbdElgawad, H. High redox status as the basis for heavy metal tolerance of Sesuvium portulacastrum L. inhabiting contaminated soil in Jeddah, Saudi Arabia. Antioxidants 2022, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Xie, T.; Yao, H.; Chen, Y.; Wang, H.; Dai, X.; Wang, Y.; Shi, L.; Luo, Y. Lead responses and tolerance mechanisms of Koelreuteria paniculata: A newly potential plant for sustainable phytoremediation of Pb-contaminated soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef]
- Ding, X.; Wu, Y.; Wang, J. Effects of waste cement on extractability of Cd, soil enzyme activities, cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in lettuce. Appl. Sci. 2023, 13, 8254. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Li, J.; Chen, J.; Yang, S.; Zhao, M.; Xue, Y. Transcriptome sequencing analysis of root in soybean responding to Mn poisoning. Int. J. Mol. Sci. 2023, 24, 12727. [Google Scholar] [CrossRef]
- Tahir, N.A.-R.; Lateef, D.D.; Mustafa, K.M.; Rasul, K.S.; Khurshid, F.F. Determination of physicochemical characteristics associated with various degrees of cadmium toerance in barley accessions. Agronomy 2023, 13, 1502. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Brreusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Brestič, M.; Deshmukh, R.; Vaculik, M. Priming mediated abiotic stress management in plants: Recent avenues and future directions. Plant Stress 2022, 5, 100097. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress management in plants: Examining provisional and unique dose-dependent responses. Int. J. Mol. Sci. 2023, 23, 5105. [Google Scholar] [CrossRef]
- Wiszniewska, A. Priming strategies for benefiting plant performance under toxic trace metall exposure. Plants 2021, 10, 623. [Google Scholar] [CrossRef]
- Chaoui, A.; El Ferjani, E. Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedlings. Comptes Rendus Biol. 2005, 328, 23–31. [Google Scholar] [CrossRef]
- Führs, H.; Götze, S.; Specht, A.; Erban, A.; Gallien, S.; Heintz, D.; Van Dorsselaer, A.; Kopka, J.; Braun, H.-P.; Horst, W.J. Characterization of leaf apoplastic peroxidases and metabolites in Vigna unguiculata in response to toxic manganese supply and silicon. J. Exp. Bot. 2009, 60, 1663–1678. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Pandey, A.K.; Zorić, L.; Sun, T.; Karanović, D.; Fang, P.; Borišev, M.; Wu, X.; Luković, J.; Xu, P. The anatomical basis of heavy metal responses in legumes and their impact on plant-rhizosphere interaction. Plants 2022, 11, 2554. [Google Scholar] [CrossRef]
- ShangGuan, X.; Qi, Y.; Wang, A.; Ren, Y.; Wang, Y.; Xiao, T.; Shen, Z.; Wang, Q.; Xia, Y. OsGLP participates in the regulation of lignin synthesis and deposition in rice against copper and cadmium toxicity. Front. Plant Sci. 2023, 13, 1078113. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Luo, Q.; Yu, B.; Liu, Y. Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J. Plant Physiol. 2005, 162, 1003–1012. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsone, I.; Ievinsh, G. Comparison of the Effects of Gradual and Acute Treatment with Mn on Physiological Responses of Rumex hydrolapathum Plants. Stresses 2024, 4, 225-237. https://doi.org/10.3390/stresses4020013
Samsone I, Ievinsh G. Comparison of the Effects of Gradual and Acute Treatment with Mn on Physiological Responses of Rumex hydrolapathum Plants. Stresses. 2024; 4(2):225-237. https://doi.org/10.3390/stresses4020013
Chicago/Turabian StyleSamsone, Ineta, and Gederts Ievinsh. 2024. "Comparison of the Effects of Gradual and Acute Treatment with Mn on Physiological Responses of Rumex hydrolapathum Plants" Stresses 4, no. 2: 225-237. https://doi.org/10.3390/stresses4020013
APA StyleSamsone, I., & Ievinsh, G. (2024). Comparison of the Effects of Gradual and Acute Treatment with Mn on Physiological Responses of Rumex hydrolapathum Plants. Stresses, 4(2), 225-237. https://doi.org/10.3390/stresses4020013






