Orbital Inflammation in Thyroid Eye Disease: Stress Responses and Their Implications
Abstract
:1. Introduction
2. Epidemiology, Risk Factors and Clinical Understanding of TED
2.1. Epidemiology
2.2. Risk Factors
2.3. Signs and Symptoms
2.4. Complications
2.5. Diagnostic Criteria
2.6. Clinical Evaluation
2.7. Treatment
3. Pathophysiology of TED
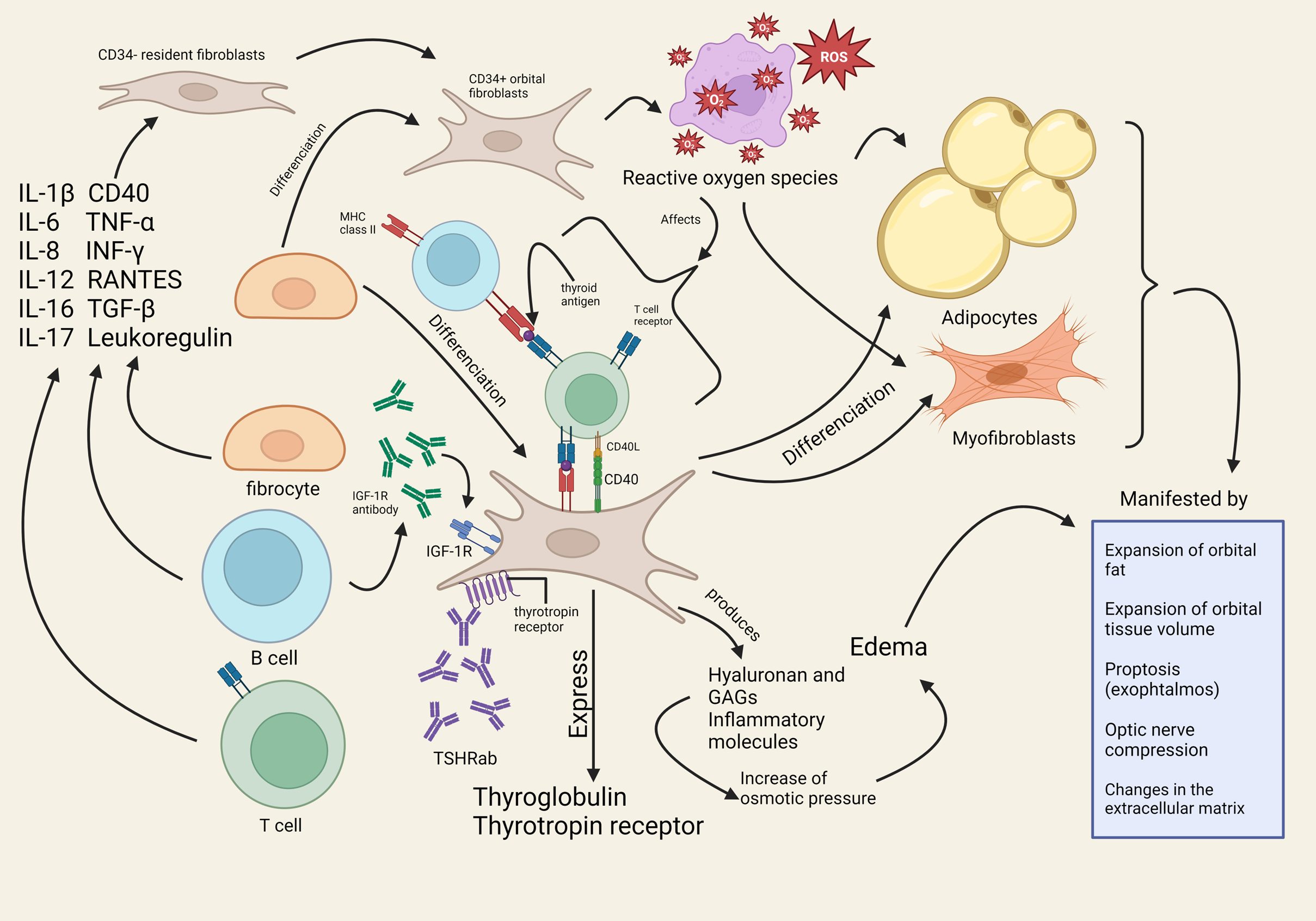
3.1. Overview of Pathophysiological Changes in TED and Its Implication
3.2. Role of IGF-1R and Potential IGF-1R Autoantibody
3.3. Implications: Understanding Mechanisms of Orbital Inflammation Can Guide Development of Novel Therapies
4. A Molecular Perspective on TED’s Pathophysiology
4.1. Cytokines and Chemokines: Role in the Inflammation Process and Current Research
4.1.1. Interleukins
4.1.2. Tumor Necrosis Factors (TNF)
4.1.3. Interferons
4.1.4. Chemokines
5. Oxidative Stress and TED’s Pathophysiology
5.1. Overview of Oxidative Stress
5.2. Current Role of Oxidative Stress in TED
| Author | Year | Conclusion |
|---|---|---|
| Heufelder et al. [145] | 1992 | H2O2 induced the expression of a heat shock protein-72 (which has a role in antigen recognition and T-cells recruitment). |
| Burch et al. [125] | 1997 | Superoxide anions trigger, in patients with TED: Retro-orbital fibroblasts proliferation, Glycosaminoglycan production, Pro-inflammatory cytokines production. |
| Lu et al. [128] | 1999 | IL-1β increases ROS production in TED patients and control-derived retro-orbital fibroblasts; IL-1β increases production of GAG in a dose-dependent manner in all retro-orbital fibroblasts; ROS were expressed in retro-orbital fibroblasts from TED patients but not in controls. |
| Bednarek et al. [123] | 2005 | Orbital inflammation contributes to increased oxidative stress parameters in hyperthyroid and euthyroid TED patients; Stabilization of oxidative stress parameters was achieved only in non-TED patients. |
| Tsai et al. [139] | 2007 | There are increased levels of urinary 8-OHdG (a marker of oxidative DNA damage) in patients with active TED. |
| Hondur et al. [137] | 2008 | Decreased activity of superoxide dismutase, GPx, and glutathione peroxidase occurs in orbital fibroblasts from TED patients. |
| Tsai et al. [138] | 2009 | Oxidative stress perpetuates oxidative damage to DNA, as seen with increased urinary levels of 8-OHdG in TED patients; Oxidative DNA damage positively correlates to clinical activity of TED; Higher levels of urinary 8-OHdG are seen in smokers compared to never-smokers. |
| Tsai et al. [113] | 2010 | Oxidative stress increases lipid peroxidation and oxidative DNA damage in TED orbital fibroblasts; TED orbital fibroblasts accumulate higher amounts of intracellular ROS (such as superoxide anions and H2O2) compared to those of normal controls. |
| Tsai et al. [126] | 2011 | H2O2 exacerbates elevation of ROS in TED orbital fibroblasts; Potential hypersensitivity of TED orbital fibroblasts to oxidative stress may be involved in the pathogenesis of TED. |
| Tsai et al. [129] | 2013 | H2O2 induces expression of intracellular pro-inflammatory cytokines TGF- and IL-1 in TED orbital fibroblasts; H2O2 (at low level) induced proliferation of orbital fibroblasts; Antioxidants (such as vitamin C) protect against these peroxide-induced effects. |
| Akarsu et al. [140] | 2011 | There are increased MDA levels and decreased GSH levels in sera of GD patients with TED compared to GD patients without TED and controls; MDA levels positively correlate with the disease’s clinical activity score; GC therapy decreases serum MDA levels, whether taken orally or IV. |
| Marique et al. [146] | 2015 | Increased oxidative stress activity was found in extraocular muscle and adipocytes from TED patients, along with upregulation of antioxidants; Serum TSHR antibody levels are related to the expression of oxidative stress. |
| Choi et al. [141] | 2018 | There are increased markers of oxidative stress (MDA and 8-OHdG levels) in tear film of TED patients; Concentration of these markers correlates with disease severity. |
| Yuksel et al. [143] | 2019 | Imbalance in thiol-disulfide homeostasis (TDH) indicates presence of oxidative stress in moderate-to-severe TED; Imbalance is significant in TED patients compared to controls, in active TED patients compared to inactive TED patients, and in smokers; TDH imbalance correlates with clinical activity score. |
| Acibucu et al. [144] | 2019 | Thiol-disulfide homeostasis (TDH) disbalance is associated with proptosis in TED patients. |
6. Implications for Treatment
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chin, Y.H.; Ng, C.H.; Lee, M.H.; Koh, J.W.H.; Kiew, J.; Yang, S.P.; Sundar, G.; Khoo, C.M. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin. Endocrinol. 2020, 93, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Mohyi, M.; Smith, T.J. IGF1 receptor and thyroid-associated ophthalmopathy. J. Mol. Endocrinol. 2018, 61, T29–T43. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Baldeschi, L.; Dickinson, A.; Eckstein, A.; Kendall-Taylor, P.; Marcocci, C.; Mourits, M.; Perros, P.; Boboridis, K.; Boschi, A.; et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur. J. Endocrinol. 2008, 158, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Bruscolini, A.; Sacchetti, M.; La Cava, M.; Nebbioso, M.; Iannitelli, A.; Quartini, A.; Lambiase, A.; Ralli, M.; de Virgilio, A.; Greco, A. Quality of life and neuropsychiatric disorders in patients with Graves’ Orbitopathy: Current concepts. Autoimmun. Rev. 2018, 17, 639–643. [Google Scholar] [CrossRef]
- Weiler, D.L. Thyroid eye disease: A review. Clin. Exp. Optom. 2017, 100, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Quality of life in Graves’ ophthalmopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Ponto, K.A.; Merkesdal, S.; Hommel, G.; Pitz, S.; Pfeiffer, N.; Kahaly, G.J. Public health relevance of Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2013, 98, 145–152. [Google Scholar] [CrossRef]
- Coulter, I.; Frewin, S.; Krassas, G.E.; Perros, P. Psychological implications of Graves’ orbitopathy. Eur. J. Endocrinol. 2007, 157, 127–131. [Google Scholar] [CrossRef]
- Taylor, P.N.; Zhang, L.; Lee, R.W.J.; Muller, I.; Ezra, D.G.; Dayan, C.M.; Kahaly, G.J.; Ludgate, M. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat. Rev. Endocrinol. 2020, 16, 104–116. [Google Scholar] [CrossRef]
- Bartalena, L.; Piantanida, E.; Gallo, D.; Lai, A.; Tanda, M.L. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Front. Endocrinol. 2020, 11, 615993. [Google Scholar] [CrossRef]
- Cyranska-Chyrek, E.; Olejarz, M.; Szczepanek-Parulska, E.; Stajgis, P.; Pioch, A.; Ruchala, M. Severe unilateral orbitopathy in a patient with Hashimoto’s thyroiditis—A case report. BMC Ophthalmol. 2019, 19, 9. [Google Scholar] [CrossRef]
- Perros, P.; Neoh, C.; Dickinson, J. Thyroid eye disease. BMJ 2009, 338, b560. [Google Scholar] [CrossRef]
- Shah, S.S.; Patel, B.C. Thyroid Eye Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hoang, T.D.; Stocker, D.J.; Chou, E.L.; Burch, H.B. 2022 Update on Clinical Management of Graves Disease and Thyroid Eye Disease. Endocrinol. Metab. Clin. N. Am. 2022, 51, 287–304. [Google Scholar] [CrossRef]
- Perros, P.; Hegedüs, L.; Bartalena, L.; Marcocci, C.; Kahaly, G.J.; Baldeschi, L.; Salvi, M.; Lazarus, J.H.; Eckstein, A.; Pitz, S.; et al. Graves’ orbitopathy as a rare disease in Europe: A European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J. Rare Dis. 2017, 12, 72. [Google Scholar] [CrossRef]
- Bartley, G.B. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans. Am. Ophthalmol. Soc. 1994, 92, 477–588. [Google Scholar]
- Abraham-Nordling, M.; Byström, K.; Törring, O.; Lantz, M.; Berg, G.; Calissendorff, J.; Nyström, H.F.; Jansson, S.; Jörneskog, G.; Karlsson, F.A.; et al. Incidence of hyperthyroidism in Sweden. Eur. J. Endocrinol. 2011, 165, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Berman, D.C.; Bülow Pedersen, I.; Andersen, S.; Carlé, A. Incidence and clinical presentation of moderate to severe graves’ orbitopathy in a Danish population before and after iodine fortification of salt. J. Clin. Endocrinol. Metab. 2012, 97, 2325–2332. [Google Scholar] [CrossRef]
- Douglas, R.S.; Gupta, S. The pathophysiology of thyroid eye disease: Implications for immunotherapy. Curr. Opin. Ophthalmol. 2011, 22, 385–390. [Google Scholar] [CrossRef]
- Şahlı, E.; Gündüz, K. Thyroid-associated Ophthalmopathy. Turk. J. Ophthalmol. 2017, 47, 94–105. [Google Scholar] [CrossRef]
- Perros, P.; Crombie, A.L.; Matthews, J.N.; Kendall-Taylor, P. Age and gender influence the severity of thyroid-associated ophthalmopathy: A study of 101 patients attending a combined thyroid-eye clinic. Clin. Endocrinol. 1993, 38, 367–372. [Google Scholar] [CrossRef]
- Manji, N.; Carr-Smith, J.D.; Boelaert, K.; Allahabadia, A.; Armitage, M.; Chatterjee, V.K.; Lazarus, J.H.; Pearce, S.H.; Vaidya, B.; Gough, S.C.; et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J. Clin. Endocrinol. Metab. 2006, 91, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.; Kelly, S.P.; Harrison, R.A.; Edwards, R. Cigarette smoking and thyroid eye disease: A systematic review. Eye 2007, 21, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Gontarz-Nowak, K.; Szychlińska, M.; Matuszewski, W.; Stefanowicz-Rutkowska, M.; Bandurska-Stankiewicz, E. Current Knowledge on Graves’ Orbitopathy. J. Clin. Med. 2020, 10, 16. [Google Scholar] [CrossRef]
- O’Dell, J.M.; Mussatto, C.C.; Chu, R.L.; Al-Sabbagh, M.Q.; Timoney, P.J.; Sokol, J.A. Effects of Smoking on Outcomes of Thyroid Eye Disease Treated with Teprotumumab: A Retrospective Cohort Study. Kans. J. Med. 2023, 16, 62–64. [Google Scholar] [CrossRef]
- Aranyosi, J.K.; Galgoczi, E.; Erdei, A.; Katko, M.; Fodor, M.; Ujhelyi, Z.; Bacskay, I.; Nagy, E.V.; Ujhelyi, B. Different Effects of Cigarette Smoke, Heated Tobacco Product and E-Cigarette Vapour on Orbital Fibroblasts in Graves’ Orbitopathy; a Study by Real Time Cell Electronic Sensing. Molecules 2022, 27, 3001. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Marcocci, C.; Bogazzi, F.; Manetti, L.; Tanda, M.L.; Dell’Unto, E.; Bruno-Bossio, G.; Nardi, M.; Bartolomei, M.P.; Lepri, A.; et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N. Engl. J. Med. 1998, 338, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, T.; Wu Li, A.; El-Kaissi, S.; Wall, J.R. Stress and thyroid autoimmunity. Thyroid 2004, 14, 1047–1055. [Google Scholar] [CrossRef]
- Cao, J.; Su, Y.; Chen, Z.; Ma, C.; Xiong, W. The risk factors for Graves’ ophthalmopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1043–1054. [Google Scholar] [CrossRef]
- Zawadzka-Starczewska, K.; Stasiak, B.; Wojciechowska-Durczyńska, K.; Lewiński, A.; Stasiak, M. Novel Insight into Non-Genetic Risk Factors of Graves’ Orbitopathy. Int. J. Environ. Res. Public Health 2022, 19, 16941. [Google Scholar] [CrossRef]
- Lee, J.; Kang, J.; Ahn, H.Y.; Lee, J.K. Sex-specific risk factors associated with graves’ orbitopathy in Korean patients with newly diagnosed graves’ disease. Eye 2023, 37, 3382–3391. [Google Scholar] [CrossRef]
- Khong, J.J.; McNab, A.A.; Ebeling, P.R.; Craig, J.E.; Selva, D. Pathogenesis of thyroid eye disease: Review and update on molecular mechanisms. Br. J. Ophthalmol. 2016, 100, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.K.; Bahn, R.S. Pathogenesis of Graves’ ophthalmopathy: The role of autoantibodies. Thyroid 2007, 17, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Ophtalmology. Thyroid Eye Disease. Available online: https://eyewiki.aao.org/Thyroid_Eye_Disease#:~:text=The%20most%20commonly%20affected%20muscle,adducted%20i.e.,%20double%20elevator%20palsy (accessed on 2 October 2023).
- Bartley, G.B.; Fatourechi, V.; Kadrmas, E.F.; Jacobsen, S.J.; Ilstrup, D.M.; Garrity, J.A.; Gorman, C.A. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am. J. Ophthalmol. 1996, 121, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Lippa, J.; Rootman, J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch. Ophthalmol. 1993, 111, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chng, C.L.; Seah, L.L.; Khoo, D.H. Ethnic differences in the clinical presentation of Graves’ ophthalmopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Marinò, M.; Ionni, I.; Lanzolla, G.; Sframeli, A.; Latrofa, F.; Rocchi, R.; Marcocci, C. Orbital diseases mimicking graves’ orbitopathy: A long-standing challenge in differential diagnosis. J. Endocrinol. Investig. 2020, 43, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Kennerdell, J.S.; Rosenbaum, A.E.; El-Hoshy, M.H. Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch. Ophthalmol. 1981, 99, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Dayan, C.M.; Dickinson, A.J.; Ezra, D.; Estcourt, S.; Foley, P.; Hickey, J.; Lazarus, J.H.; MacEwen, C.J.; McLaren, J.; et al. Management of patients with Graves’ orbitopathy: Initial assessment, management outside specialised centres and referral pathways. Clin. Med. 2015, 15, 173–178. [Google Scholar] [CrossRef]
- Mourits, M.P.; Koornneef, L.; Wiersinga, W.M.; Prummel, M.F.; Berghout, A.; van der Gaag, R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: A novel approach. Br. J. Ophthalmol. 1989, 73, 639–644. [Google Scholar] [CrossRef]
- Barrio-Barrio, J.; Sabater, A.L.; Bonet-Farriol, E.; Velázquez-Villoria, Á.; Galofré, J.C. Graves’ Ophthalmopathy: VISA versus EUGOGO Classification, Assessment, and Management. J. Ophthalmol. 2015, 2015, 249125. [Google Scholar] [CrossRef]
- Werner, S.C. Modification of the classification of the eye changes of Graves’ disease. Am. J. Ophthalmol. 1977, 83, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L. Role of teprotumumab in the treatment of active moderate-to-severe Graves’ orbitopathy. Eur. Thyroid J. 2022, 11, e220185. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Diana, T.; Ponto, K.A.; Kahaly, G.J. Thyrotropin receptor antibodies and Graves’ orbitopathy. J. Endocrinol. Investig. 2021, 44, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Gerding, M.N.; van der Meer, J.W.; Broenink, M.; Bakker, O.; Wiersinga, W.M.; Prummel, M.F. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin. Endocrinol. 2000, 52, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Sarić Matutinović, M.; Kahaly, G.J.; Žarković, M.; Ćirić, J.; Ignjatović, S.; Nedeljković Beleslin, B. The phenotype of Graves’ orbitopathy is associated with thyrotropin receptor antibody levels. J. Endocrinol. Investig. 2023, 46, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Selter, J.H.; Gire, A.I.; Sikder, S. The relationship between Graves’ ophthalmopathy and dry eye syndrome. Clin. Ophthalmol. 2015, 9, 57–62. [Google Scholar] [CrossRef]
- Philipp, S.; Horstmann, M.; Hose, M.; Daser, A.; Görtz, G.E.; Jesenek, C.; Flögel, U.; Hansen, W.; Bechrakis, N.; Banga, J.P.S.; et al. An Early Wave of Macrophage Infiltration Intertwined with Antigen-Specific Proinflammatory T Cells and Browning of Adipose Tissue Characterizes the Onset of Orbital Inflammation in a Mouse Model of Graves’ Orbitopathy. Thyroid 2022, 32, 283–293. [Google Scholar] [CrossRef]
- Dik, W.A.; Virakul, S.; van Steensel, L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp. Eye Res. 2016, 142, 83–91. [Google Scholar] [CrossRef]
- Pappa, A.; Jackson, P.; Stone, J.; Munro, P.; Fells, P.; Pennock, C.; Lightman, S. An ultrastructural and systemic analysis of glycosaminoglycans in thyroid-associated ophthalmopathy. Eye 1998, 12 Pt 2, 237–244. [Google Scholar] [CrossRef]
- Kumar, S.; Nadeem, S.; Stan, M.N.; Coenen, M.; Bahn, R.S. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves’ ophthalmopathy. J. Mol. Endocrinol. 2011, 46, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. The insulin-like growth factor-I receptor and its role in thyroid-associated ophthalmopathy. Eye 2019, 33, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.; Smith, T.J. Lessons Learned from Targeting IGF-I Receptor in Thyroid-Associated Ophthalmopathy. Cells 2021, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B.; Dehina, N.; Welsink, T.; Schwiebert, C.; Morgenthaler, N.G.; Köhrle, J.; Eckstein, A.; Schomburg, L. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2013, 98, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Weightman, D.R.; Perros, P.; Sherif, I.H.; Kendall-Taylor, P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity 1993, 16, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Krieger, C.C.; Neumann, S.; Place, R.F.; Marcus-Samuels, B.; Gershengorn, M.C. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves’ disease immunoglobins. J. Clin. Endocrinol. Metab. 2015, 100, 1071–1077. [Google Scholar] [CrossRef]
- Krieger, C.C.; Place, R.F.; Bevilacqua, C.; Marcus-Samuels, B.; Abel, B.S.; Skarulis, M.C.; Kahaly, G.J.; Neumann, S.; Gershengorn, M.C. TSH/IGF-1 Receptor Cross Talk in Graves’ Ophthalmopathy Pathogenesis. J. Clin. Endocrinol. Metab. 2016, 101, 2340–2347. [Google Scholar] [CrossRef]
- Pritchard, J.; Horst, N.; Cruikshank, W.; Smith, T.J. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J. Immunol. 2002, 168, 942–950. [Google Scholar] [CrossRef]
- Varewijck, A.J.; Boelen, A.; Lamberts, S.W.; Fliers, E.; Hofland, L.J.; Wiersinga, W.M.; Janssen, J.A. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab. 2013, 98, 769–776. [Google Scholar] [CrossRef]
- Neumann, S.; Krieger, C.C.; Gershengorn, M.C. Targeting TSH and IGF-1 Receptors to Treat Thyroid Eye Disease. Eur. Thyroid J. 2020, 9, 59–65. [Google Scholar] [CrossRef]
- Girnita, L.; Smith, T.J.; Janssen, J. It Takes Two to Tango: IGF-I and TSH Receptors in Thyroid Eye Disease. J. Clin. Endocrinol. Metab. 2022, 107, S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, D.; Cushing, G.W.; Moses, A.C.; Ingbar, S.H. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology 1986, 119, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Tsui, S.; Naik, V.; Hoa, N.; Hwang, C.J.; Afifiyan, N.F.; Sinha Hikim, A.; Gianoukakis, A.G.; Douglas, R.S.; Smith, T.J. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: A tale of two antigens implicated in Graves’ disease. J. Immunol. 2008, 181, 4397–4405. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Gianoukakis, A.G.; Kamat, S.; Smith, T.J. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J. Immunol. 2007, 178, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.; Han, R.; Horst, N.; Cruikshank, W.W.; Smith, T.J. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J. Immunol. 2003, 170, 6348–6354. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hoa, N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J. Clin. Endocrinol. Metab. 2004, 89, 5076–5080. [Google Scholar] [CrossRef]
- Smith, T.J.; Janssen, J. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr. Rev. 2019, 40, 236–267. [Google Scholar] [CrossRef]
- Matos, K.; Manso, P.G.; Marback, E.; Furlanetto, R.; Alberti, G.N.; Nosé, V. Protein expression of VEGF, IGF-1 and FGF in retroocular connective tissues and clinical correlation in Graves’ ophthalmopathy. Arq. Bras. Oftalmol. 2008, 71, 486–492. [Google Scholar] [CrossRef]
- Lanzolla, G.; Ricci, D.; Nicolì, F.; Sabini, E.; Sframeli, A.; Brancatella, A.; Mantuano, M.; Dottore, G.R.; Bucci, I.; Figus, M.; et al. Putative protective role of autoantibodies against the insulin-like growth factor-1 receptor in Graves’ Disease: Results of a pilot study. J. Endocrinol. Investig. 2020, 43, 1759–1768. [Google Scholar] [CrossRef]
- Smith, T.J. Is IGF-I receptor a target for autoantibody generation in Graves’ disease? J. Clin. Endocrinol. Metab. 2013, 98, 515–518. [Google Scholar] [CrossRef]
- Gulbins, A.; Horstmann, M.; Daser, A.; Flögel, U.; Oeverhaus, M.; Bechrakis, N.E.; Banga, J.P.; Keitsch, S.; Wilker, B.; Krause, G.; et al. Linsitinib, an IGF-1R inhibitor, attenuates disease development and progression in a model of thyroid eye disease. Front. Endocrinol. 2023, 14, 1211473. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit. Health 2019, 1, e271–e297. [Google Scholar] [CrossRef] [PubMed]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.R.; Leung, P.S.C.; Hodge, D.L.; Fenimore, J.M.; Jeon, S.M.; Thovarai, V.; Dzutsev, A.; Welcher, A.A.; Boedigheimer, M.; Damore, M.A.; et al. Multi-omics: Differential expression of IFN-γ results in distinctive mechanistic features linking chronic inflammation, gut dysbiosis, and autoimmune diseases. J. Autoimmun. 2020, 111, 102436. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.B.; Barroso, W.A.; da Silva, N.N.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflam. 2017, 2017, 3406215. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Vannucchi, G.; Currò, N.; Introna, M.; Rossi, S.; Bonara, P.; Covelli, D.; Dazzi, D.; Guastella, C.; Pignataro, L.; et al. Small dose of rituximab for graves orbitopathy: New insights into the mechanism of action. Arch. Ophthalmol. 2012, 130, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Monson, J.P.; Wood, D.F.; Besser, G.M.; Burrin, J.M. Serum cytokines in thyrotoxicosis. J. Clin. Endocrinol. Metab. 1999, 84, 435–439. [Google Scholar] [CrossRef]
- Molnár, I.; Balázs, C. High circulating IL-6 level in Graves’ ophthalmopathy. Autoimmunity 1997, 25, 91–96. [Google Scholar] [CrossRef]
- Raychaudhuri, N.; Fernando, R.; Smith, T.J. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: Clear delineation of its cAMP-independent actions. PLoS ONE 2013, 8, e75100. [Google Scholar] [CrossRef]
- Paik, J.S.; Cho, W.K.; Oh, E.H.; Lee, S.B.; Yang, S.W. Palmitate induced secretion of IL-6 and MCP-1 in orbital fibroblasts derived from patients with thyroid-associated ophthalmopathy. Mol. Vis. 2012, 18, 1467–1477. [Google Scholar] [PubMed]
- Pérez-Moreiras, J.V.; Alvarez-López, A.; Gómez, E.C. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bilbao, L.; Martínez-López, D.; Revenga, M.; López-Vázquez, Á.; Valls-Pascual, E.; Atienza-Mateo, B.; Valls-Espinosa, B.; Maiz-Alonso, O.; Blanco, A.; Torre-Salaberri, I.; et al. Anti-IL-6 Receptor Tocilizumab in Refractory Graves’ Orbitopathy: National Multicenter Observational Study of 48 Patients. J. Clin. Med. 2020, 9, 2816. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, P.; Italiani, P.; Pratesi, F.; Puxeddu, I.; Boraschi, D. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020, 19, 102617. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Rong, S.S.; Chong, K.K.; Young, A.L.; Pang, C.P.; Chen, L.J. Genetic Associations of Interleukin-related Genes with Graves’ Ophthalmopathy: A Systematic Review and Meta-analysis. Sci. Rep. 2015, 5, 16672. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Smith, T.J. Regulation of IL-1 receptor antagonist by TSH in fibrocytes and orbital fibroblasts. J. Clin. Endocrinol. Metab. 2014, 99, E625–E633. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Smith, T.J. Divergent expression of IL-1 receptor antagonists in CD34⁺ fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: Contribution of fibrocytes to orbital inflammation. J. Clin. Endocrinol. Metab. 2013, 98, 2783–2790. [Google Scholar] [CrossRef]
- Boutet, M.A.; Blanchard, F.; Le Goff, B. Response to: ‘Does IL-38 act on macrophages and/or dendritic cells in arthritis?’ by Jiang et al. Ann. Rheum. Dis. 2018, 77, e13. [Google Scholar] [CrossRef]
- Shi, L.; Ye, H.; Huang, J.; Li, Y.; Wang, X.; Xu, Z.; Chen, J.; Xiao, W.; Chen, R.; Yang, H. IL-38 Exerts Anti-Inflammatory and Antifibrotic Effects in Thyroid-Associated Ophthalmopathy. J. Clin. Endocrinol. Metab. 2021, 106, e3125–e3142. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, M.; Chen, X.; Chen, Y.; Ai, S.; Wang, M.; Su, W.; Liang, D. Elevated IL-38 inhibits IL-23R expression and IL-17A production in thyroid-associated ophthalmopathy. Int. Immunopharmacol. 2021, 91, 107300. [Google Scholar] [CrossRef]
- Gu, L.Q.; Jia, H.Y.; Zhao, Y.J.; Liu, N.; Wang, S.; Cui, B.; Ning, G. Association studies of interleukin-8 gene in Graves’ disease and Graves’ ophthalmopathy. Endocrine 2009, 36, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A.P.; Bennett, G.L.; Wong, W.L. Thyroid follicular cells produce interleukin-8. J. Clin. Endocrinol. Metab. 1992, 75, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Myśliwiec, J.; Kretowski, A.; Stepień, A.; Mirończuk, K.; Kinalska, I. Interleukin 18 and transforming growth factor beta1 in the serum of patients with Graves’ ophthalmopathy treated with corticosteroids. Int. Immunopharmacol. 2003, 3, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, B.; Gogolak, P.; Erdei, A.; Nagy, V.; Balazs, E.; Rajnavolgyi, E.; Berta, A.; Nagy, E.V. Graves’ orbitopathy results in profound changes in tear composition: A study of plasminogen activator inhibitor-1 and seven cytokines. Thyroid 2012, 22, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Xu, F.; Xu, W.; Zhu, H. Elevated expression of interleukin-27, IL-35, and decreased IL-12 in patients with thyroid-associated ophthalmopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bahn, R.S. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with graves’ ophthalmopathy. J. Clin. Endocrinol. Metab. 2003, 88, 4246–4250. [Google Scholar] [CrossRef]
- Cawood, T.J.; Moriarty, P.; O’Farrelly, C.; O’Shea, D. The effects of tumour necrosis factor-alpha and interleukin1 on an in vitro model of thyroid-associated ophthalmopathy; contrasting effects on adipogenesis. Eur. J. Endocrinol. 2006, 155, 395–403. [Google Scholar] [CrossRef]
- Heufelder, A.E.; Bahn, R.S.; Boergen, K.P.; Scriba, P.C. Detection, localization and modulation of hyaluronic acid/CD44 receptor expression in patients with endocrine orbitopathy. Med. Klin. 1993, 88, 181–184+277. [Google Scholar]
- Ayabe, R.; Rootman, D.B.; Hwang, C.J.; Ben-Artzi, A.; Goldberg, R. Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 415–419. [Google Scholar] [CrossRef]
- Durrani, O.M.; Reuser, T.Q.; Murray, P.I. Infliximab: A novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit 2005, 24, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, J.; Jankiewicz-Wika, J.; Siejka, A.; Lawnicka, H.; Kłysik, A.; Goś, R.; Majos, A.; Stefańczyk, L.; Stepień, H. Monoclonal anti-TNFalpha antibody (infliximab) in the treatment of patient with thyroid associated ophthalmopathy. Klin. Ocz. 2007, 109, 457–460. [Google Scholar]
- Hodge, D.L.; Berthet, C.; Coppola, V.; Kastenmüller, W.; Buschman, M.D.; Schaughency, P.M.; Shirota, H.; Scarzello, A.J.; Subleski, J.J.; Anver, M.R.; et al. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J. Autoimmun. 2014, 53, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Ragusa, F.; Ruffilli, I.; Elia, G.; Paparo, S.R.; Antonelli, A. Th1 Chemokines in Autoimmune Endocrine Disorders. J. Clin. Endocrinol. Metab. 2020, 105, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Camastra, S.; Miccoli, M.; Cavallini, G.; Benvenga, S.; et al. Cytokines as Targets of Novel Therapies for Graves’ Ophthalmopathy. Front. Endocrinol. 2021, 12, 654473. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Frascerra, S.; Santini, E.; Franceschini, S.S.; Ferrannini, E. Monokine induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma inducible T-cell alpha-chemoattractant (CXCL11) involvement in Graves’ disease and ophthalmopathy: Modulation by peroxisome proliferator-activated receptor-gamma agonists. J. Clin. Endocrinol. Metab. 2009, 94, 1803–1809. [Google Scholar] [CrossRef]
- Han, R.; Smith, T.J. T helper type 1 and type 2 cytokines exert divergent influence on the induction of prostaglandin E2 and hyaluronan synthesis by interleukin-1beta in orbital fibroblasts: Implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology 2006, 147, 13–19. [Google Scholar] [CrossRef]
- Chen, B.; Tsui, S.; Smith, T.J. IL-1 beta induces IL-6 expression in human orbital fibroblasts: Identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J. Immunol. 2005, 175, 1310–1319. [Google Scholar] [CrossRef]
- Antonelli, A.; Rotondi, M.; Ferrari, S.M.; Fallahi, P.; Romagnani, P.; Franceschini, S.S.; Serio, M.; Ferrannini, E. Interferon-gamma-inducible alpha-chemokine CXCL10 involvement in Graves’ ophthalmopathy: Modulation by peroxisome proliferator-activated receptor-gamma agonists. J. Clin. Endocrinol. Metab. 2006, 91, 614–620. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Franceschini, S.S.; Gelmini, S.; Ferrannini, E.; Fallahi, P. Extra-ocular muscle cells from patients with Graves’ ophthalmopathy secrete α (CXCL10) and β (CCL2) chemokines under the influence of cytokines that are modulated by PPARγ. Autoimmun. Rev. 2014, 13, 1160–1166. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Cheng, C.Y.; Kao, S.C.; Kau, H.C.; Chiou, S.H.; Hsu, W.M.; Wei, Y.H. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves’ ophthalmopathy: Evidence that oxidative stress has a role in this disorder. Eye 2010, 24, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Leo, M.; Altea, M.A. Oxidative stress in graves’ disease. Eur. Thyroid J. 2012, 1, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Ophthalmology. Basic Ophtalmology: Essentials for Medical Students, 10th ed.; American Academy of Ophthalmology: San Francisco, CA, USA, 2016; p. 275. [Google Scholar]
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Tanda, M.L.; Piantanida, E.; Lai, A. Oxidative stress and Graves’ ophthalmopathy: In vitro studies and therapeutic implications. Biofactors 2003, 19, 155–163. [Google Scholar] [CrossRef]
- Wilson, R.; Chopra, M.; Bradley, H.; McKillop, J.H.; Smith, W.E.; Thomson, J.A. Free radicals and Graves’ disease: The effects of therapy. Clin. Endocrinol. 1989, 30, 429–433. [Google Scholar] [CrossRef]
- Zarković, M. The role of oxidative stress on the pathogenesis of graves’ disease. J. Thyroid Res. 2012, 2012, 302537. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell. Mol. Life Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Bednarek, J.; Wysocki, H.; Sowiński, J. Oxidative stress peripheral parameters in Graves’ disease: The effect of methimazole treatment in patients with and without infiltrative ophthalmopathy. Clin. Biochem. 2005, 38, 13–18. [Google Scholar] [CrossRef]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.C.; et al. Roles of hydrogen peroxide in thyroid physiology and disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B.; Lahiri, S.; Bahn, R.S.; Barnes, S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves’ ophthalmopathy. Exp. Eye Res. 1997, 65, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Wu, S.B.; Cheng, C.Y.; Kao, S.C.; Kau, H.C.; Lee, S.M.; Wei, Y.H. Increased response to oxidative stress challenge in Graves’ ophthalmopathy orbital fibroblasts. Mol. Vis. 2011, 17, 2782–2788. [Google Scholar]
- Buonfiglio, F.; Böhm, E.W.; Pfeiffer, N.; Gericke, A. Oxidative Stress: A Suitable Therapeutic Target for Optic Nerve Diseases? Antioxidants 2023, 12, 1465. [Google Scholar] [CrossRef]
- Lu, R.; Wang, P.; Wartofsky, L.; Sutton, B.D.; Zweier, J.L.; Bahn, R.S.; Garrity, J.; Burman, K.D. Oxygen free radicals in interleukin-1beta-induced glycosaminoglycan production by retro-ocular fibroblasts from normal subjects and Graves’ ophthalmopathy patients. Thyroid 1999, 9, 297–303. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Kao, S.C.; Kau, H.C.; Lee, F.L.; Wei, Y.H. The protective effect of antioxidants on orbital fibroblasts from patients with Graves’ ophthalmopathy in response to oxidative stress. Mol. Vis. 2013, 19, 927–934. [Google Scholar]
- Hou, T.Y.; Wu, S.B.; Kau, H.C.; Tsai, C.C. The Role of Oxidative Stress and Therapeutic Potential of Antioxidants in Graves’ Ophthalmopathy. Biomedicines 2021, 9, 1871. [Google Scholar] [CrossRef]
- Kim, B.Y.; Jang, S.Y.; Choi, D.H.; Jung, C.H.; Mok, J.O.; Kim, C.H. Anti-inflammatory and Antioxidant Effects of Selenium on Orbital Fibroblasts of Patients with Graves Ophthalmopathy. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Rotondo Dottore, G.; Leo, M.; Casini, G.; Latrofa, F.; Cestari, L.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Antioxidant Actions of Selenium in Orbital Fibroblasts: A Basis for the Effects of Selenium in Graves’ Orbitopathy. Thyroid 2017, 27, 271–278. [Google Scholar] [CrossRef]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur. Thyroid J. 2016, 5, 9–26. [Google Scholar] [CrossRef]
- Marcocci, C.; Kahaly, G.J.; Krassas, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. Selenium and the course of mild Graves’ orbitopathy. N. Engl. J. Med. 2011, 364, 1920–1931. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, H.J.; Chae, M.K.; Byun, J.W.; Lee, E.J.; Yoon, J.S. Therapeutic Effect of Resveratrol on Oxidative Stress in Graves’ Orbitopathy Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6352–6361. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Lee, H.J.; Chae, M.K.; Lee, S.Y.; Lee, E.J. Cigarette smoke extract-induced adipogenesis in Graves’ orbital fibroblasts is inhibited by quercetin via reduction in oxidative stress. J. Endocrinol. 2013, 216, 145–156. [Google Scholar] [CrossRef]
- Hondur, A.; Konuk, O.; Dincel, A.S.; Bilgihan, A.; Unal, M.; Hasanreisoglu, B. Oxidative stress and antioxidant activity in orbital fibroadipose tissue in Graves’ ophthalmopathy. Curr. Eye Res. 2008, 33, 421–427. [Google Scholar] [CrossRef]
- Tsai, C.C.; Cheng, C.Y.; Liu, C.Y.; Kao, S.C.; Kau, H.C.; Hsu, W.M.; Wei, Y.H. Oxidative stress in patients with Graves’ ophthalmopathy: Relationship between oxidative DNA damage and clinical evolution. Eye 2009, 23, 1725–1730. [Google Scholar] [CrossRef]
- Tsai, C.C.; Kao, S.C.; Cheng, C.Y.; Kau, H.C.; Hsu, W.M.; Lee, C.F.; Wei, Y.H. Oxidative stress change by systemic corticosteroid treatment among patients having active graves ophthalmopathy. Arch. Ophthalmol. 2007, 125, 1652–1656. [Google Scholar] [CrossRef]
- Akarsu, E.; Buyukhatipoglu, H.; Aktaran, S.; Kurtul, N. Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin. Endocrinol. 2011, 74, 118–124. [Google Scholar] [CrossRef]
- Choi, W.; Li, Y.; Ji, Y.S.; Yoon, K.C. Oxidative stress markers in tears of patients with Graves’ orbitopathy and their correlation with clinical activity score. BMC Ophthalmol. 2018, 18, 303. [Google Scholar] [CrossRef]
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993, 686, 12–28. [Google Scholar] [CrossRef]
- Yuksel, N.; Tanriverdi, B.; Ipteç, B.; Erel, O. Thiol-disulfide homeostasis as an oxidative stress marker in patients with Graves’ ophthalmopathy. Orbit 2019, 38, 370–375. [Google Scholar] [CrossRef]
- Acibucu, F.; Öztürk, D.D.; Kizildag, C.; Aslan, M.Z.; Gulumsek, E.; Sumbul, M.S.; Neselioglu, S.; Erel, O.; Sen, S.; Bankir, M.; et al. Proptosis is associated with thiol-disulfide in patients with Graves’ ophthalmopathy. Arch. Endocrinol. Metab. 2022, 66, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Heufelder, A.E.; Wenzel, B.E.; Bahn, R.S. Methimazole and propylthiouracil inhibit the oxygen free radical-induced expression of a 72 kilodalton heat shock protein in Graves’ retroocular fibroblasts. J. Clin. Endocrinol. Metab. 1992, 74, 737–742. [Google Scholar] [CrossRef]
- Marique, L.; Senou, M.; Craps, J.; Delaigle, A.; Van Regemorter, E.; Wérion, A.; Van Regemorter, V.; Mourad, M.; Nyssen-Behets, C.; Lengelé, B.; et al. Oxidative Stress and Upregulation of Antioxidant Proteins, Including Adiponectin, in Extraocular Muscular Cells, Orbital Adipocytes, and Thyrocytes in Graves’ Disease Associated with Orbitopathy. Thyroid 2015, 25, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Wei, R. Interleukin-7 expression in tears and orbital tissues of patients with Graves’ ophthalmopathy. Endocrine 2013, 44, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Bajkowska, D.; Szelachowska, M.; Buczyńska, A.; Krętowski, A.J.; Siewko, K. Tears as a Source of Biomarkers in the Diagnosis of Graves’ Orbitopathy. Biomolecules 2022, 12, 1620. [Google Scholar] [CrossRef]
- Huang, D.; Luo, Q.; Yang, H.; Mao, Y. Changes of lacrimal gland and tear inflammatory cytokines in thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4935–4943. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, H.F.; Fan, X.Q. Ocular surface changes in Graves’ ophthalmopathy. Int. J. Ophthalmol. 2021, 14, 616–621. [Google Scholar] [CrossRef]
- Xu, N.; Cui, Y.; Fu, D.; Sun, F. Tear inflammatory cytokines and ocular surface changes in patients with active thyroid eye disease treated with high-dose intravenous glucocorticoids. J. Endocrinol. Investig. 2020, 43, 901–910. [Google Scholar] [CrossRef]
- Khazaei, H.; Khazaei, D.; Verma, R.; Ng, J.; Wilmarth, P.A.; David, L.L.; Rosenbaum, J.T. The potential of tear proteomics for diagnosis and management of orbital inflammatory disorders including Graves’ ophthalmopathy. Exp. Eye Res. 2021, 213, 108813. [Google Scholar] [CrossRef]

| Only parameters 1–7 are scored for initial CAS | |
|---|---|
| 1 | Spontaneous orbital pain |
| 2 | Gaze-evoked orbital pain |
| 3 | Eyelid swelling that is considered to be due to active TED |
| 4 | Eyelid erythema |
| 5 | Conjunctival redness that is considered to be due to active TED |
| 6 | Chemosis |
| 7 | Inflammation of caruncle or plica |
| For following assessments, parameters 1 to 10 can be included | |
| 8 | Increase of ˃2 mm in proptosis |
| 9 | Decrease in uniocular ocular excursion in any one direction of ˃ 8° |
| 10 | Decrease in acuity equivalent to 1 Snellen line |
| Class | Grade | Suggestions for Grading |
|---|---|---|
| 0 | - | No physical signs or symptoms |
| I | - | Only signs |
| II | 0 a b c | Soft tissue involvement Absent Minimal Moderate Marked |
| III | 0 a b c | Proptosis Absent 3–4 mm over upper normal 5–7 mm increase 8 mm increase |
| IV | 0 a b c | Extraocular muscle involvement Absent Limitation of motion at extreme gaze Evident restriction of motion Fixation of a globe or globes |
| V | 0 a b c | Corneal involvement Absent Stippling of cornea Ulceration Clouding, necrosis, and perforation |
| VI | 0 a b c | Sight loss (due to optic nerve involvement) Absent Disc pallor or choking or visual field defect, vision 20/20-20/60 Disc pallor or choking, or visual field defect, vision 20/70-20/200 Blindness, vision less than 20/200 |
| For All Patients | 1. Restore Euthyroid State 2. Urge Smoking Cessation | |
|---|---|---|
| Severity | Active | Inactive |
| Mild | Artificial tears Sunglasses Elevating head of bed Prismatic glasses | Artificial tears Prismatic glasses Surgical Müllerectomy Blepharoplasty |
| Moderate-severe | Intravenous methylprednisolone In steroid-resistant patients: cyclosporin A + oral steroid, immunosuppressive therapies, anti-cytokine/lymphocyte agents If motility dysfunction is pronounced: orbital radiotherapy | Orbital decompression Strabismus surgery Palpebral surgery |
| Threat to vision Optic neuropathy Severe corneal involvement | Intravenous methylprednisolone: 1 gr for 3 days If non-responsive: orbital decompression +/− intravenous steroid +/− radiotherapy Intravenous steroid, lubrication, tarsorrhaphy, orbital decompression | Urgent surgical decompression Lateral tarsorrhaphy, orbital decompression, amniotic membrane transplant, keratoplasty |
Effects of ROS at cellular level [117]
|
Antioxidant mechanisms in the eye include [117,118]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoun, T.; Danielova Gueorguieva, D.; Wu, K.Y. Orbital Inflammation in Thyroid Eye Disease: Stress Responses and Their Implications. Stresses 2024, 4, 54-78. https://doi.org/10.3390/stresses4010004
Aoun T, Danielova Gueorguieva D, Wu KY. Orbital Inflammation in Thyroid Eye Disease: Stress Responses and Their Implications. Stresses. 2024; 4(1):54-78. https://doi.org/10.3390/stresses4010004
Chicago/Turabian StyleAoun, Tracy, Diana Danielova Gueorguieva, and Kevin Y. Wu. 2024. "Orbital Inflammation in Thyroid Eye Disease: Stress Responses and Their Implications" Stresses 4, no. 1: 54-78. https://doi.org/10.3390/stresses4010004
APA StyleAoun, T., Danielova Gueorguieva, D., & Wu, K. Y. (2024). Orbital Inflammation in Thyroid Eye Disease: Stress Responses and Their Implications. Stresses, 4(1), 54-78. https://doi.org/10.3390/stresses4010004







