Abstract
The aim of this study was to investigate the morpho-physiological responses of tomato and bell pepper plants when specific nutrients were restricted. The study was conducted in a greenhouse under controlled environmental conditions and used hydroponic solution as the growth medium, with the nutrient solution being replaced as needed. Treatments consisted of a control treatment that included all nutrients at optimal concentrations and the suppression of magnesium (Mg), boron (B), zinc (Zn), and iron (Fe) for both tomato and bell pepper. The experimental design followed a completely randomized design, with a 2 (crops) × 5 (treatments) factorial scheme replicated four times. The results of this study showed that suppression of Fe had the most pronounced negative effect on the morphology and physiology of tomatoes and bell peppers and caused a reduction in parameters associated with gas exchange, leading to the development of interveinal chlorosis in the leaves. The suppression of Mg had the second most notable negative effects, with similar deficiency symptoms observed in the plant leaves as observed for the absence of Fe. While the suppression of B and Zn were less prominent compared to Fe and Mg, they still resulted in tissue malformation in the shoot apices and reductions in gas exchange and negatively impacted the morphological parameters evaluated. Therefore, our study provided important insights on how Mg, B, Zn, and Fe depletion affects tomato and bell pepper physiology and its impacts on tomato and bell pepper morphology.
1. Introduction
Bell pepper (Capsicum annuum L.) and Italian tomato (Solanum lycopersicum L.) belong to the Solanaceae family, and they are widely used in world cuisine. These vegetables hold a prominent position among the top ten economically important vegetables produced globally [1]. The cultivation of bell pepper and tomato has been steadily increasing due to the growing public demand. Pepper and tomato fruits are a rich source of minerals, vitamins, and antioxidants, including carotenoids (β-carotenoids and lycopene) and vitamins (A, C, and E) [2,3]. These compounds have been reported to help in preventing not only cancer but also cardiovascular diseases [4].
Horticultural crops in general have high requirements for external inputs so that plant yield can be maximized to its optimum. In this sense, one of the principal strategies to increase yield of agricultural produce is the use of an adequate plant nutrition plan [5,6]. For example, tomatoes’ nutrient requirement (N-P-K) in order to maximize fruit yield is around 120–150 kg N, 60–80 kg P2O5, and 80 kg K2O ha−1 [7,8]. Therefore, it is crucial to understand the morphological and physiological responses of these crops under the suppression of specific nutrients. Among all of the nutrients required for maximum plant growth, magnesium (Mg), boron (B), zinc (Zn), and iron (Fe) play significant roles in plant biochemical and developmental processes. Magnesium is a macronutrient that is present in the soil as the cation Mg2+. Under limited availability of Mg in the soil, plants can become Mg-depleted, which can result in interveinal chlorosis in older leaves [9,10]. Within plants, the majority of metabolically active Mg is bound or incorporated into cellular compartments, with the highest concentrations found in chloroplasts. [9]. A significant portion of Mg in leaves is bound as the central atom in the tetrapyrrole ring of chlorophyll a and b molecules, which are the major pigments responsible for photosynthetic light absorption. [10,11]. The significance of magnesium in phloem loading lies in its interaction with ATP fuelling, specifically with the H+-ATPase enzyme [12]. This enzyme provides energy for the phloem loading process and sustains the transport of sucrose into phloem cells [13]. In fruits, Mg deficiency can lead to reduced fruit size and alterations in acidity and vitamin C content. Moreover, Mg plays important roles in plant metabolism, including the activation of enzymes and the composition of pigments involved in photosynthesis. In addition, Mg is necessary for proper RNA polymerase function, an enzyme essential for RNA synthesis and gene expression [9,10]. Although Mg plays an important role in the growth and development of plants, limited research is available about Mg deficiency and possible effects on crop yield and this nutrient is often considered “the forgotten element” [11,12,13].
Boron is present in soils as boric acid (H3BO3) or in some cases in the form of borate anions [B(OH)4]−. This element primarily participates in cell wall biosynthesis, working in conjunction with other physiological processes. It is also involved in the translocation of organic metabolites and plays a role in the biosynthesis of proteins [14]. The symptoms of B deficiency can vary among different plant species. However, common signs include reduced growth and deformation of organs, particularly in the growing zones, such as the cauline apexes. Leaves affected by B deficiency may become brittle and exhibit a more intense green coloration [15]. Also, B promotes the absorption of Ca and increases the content of vitamin C in the fruit by improving membrane integrity, slowing biosynthesis and reducing respiration in different horticultural crops [16].
Zinc is typically present in soil as the cation Zn2+, and its movement within the soil occurs through diffusion, following a concentration gradient. Zinc deficiency in plants can occur due to some factors related to leaching: changes in pH, low organic matter, nutrient imbalance (notably P), and low soil fertility. Symptoms of Zn deficiency primarily appear in the youngest plant parts and may include shortened internodes, chlorosis of the leaves, reduced leaf size, and irregularities in leaf shape. It is estimated that approximately 8 to 10% of all eukaryotic proteins contain at least one Zn atom. This is particularly notable in enzymes belonging to various classes, including oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. As a result, Zn is recognized as one of the most widely utilized trace elements in nature, alongside Fe [17].
Plants can absorb Fe in the form of either ferrous ions (Fe2+) or ferric ions (Fe3+). When Fe deficiency occurs, plants excrete amino acids into the rhizosphere, particularly in the peripheral region of the root. This excretion leads to the formation of stable complexes, which facilitate the protected transport of iron to the plant tissues. Due to these mechanisms, plants vary in their efficiency of iron absorption and uptake [18]. In addition, Fe plays an important role in the formation of cytochrome molecules, which are involved in the transfer of electrons during photosynthesis and cellular respiration. These processes take place in the chloroplasts and mitochondria within the plant cells. One notable molecule is ferredoxin, which acts as an electron carrier in the pathway for NADPH generation during the light-dependent reactions of photosynthesis. Ferredoxin is essential for the efficient transfer of electrons in the photosynthetic electron transport chain [19].
Although numerous studies regarding nutrient application to horticultural crops have been performed, the effect of nutrient depletion on morphophysiological parameters of tomato and bell pepper are poorly understood. Therefore, the objectives of this study were to investigate the potential effects of Mg, B, Zn, and Fe suppression on tomato and bell pepper physiology and the impacts on plant morphology. Such research should help to determine how to maximize tomato and bell pepper nutrition, improving overall biomass productivity and nutrient replenishment in these important horticultural crops.
2. Results
2.1. Summary of Statistical Analysis
Leaf net photosynthetic rate (A), transpiration (E), water use efficiency (WUE), xylem diameter (XD), adaxial epidermis thickness (AET), and stomatal density (SD) were found to respond significantly to nutrient restriction (Table 1). Stomatal conductance (Gs), internal CO2 concentration in the substomatal chamber (Ci), phloem diameter (PD), and shoot growth (SG) were significantly affected by the main effects of vegetable crops and nutrient suppression (Table 1). Palisade parenchyma thickness (PPT), number of leaves (NL), shoot dry mass (SDM), and root dry mass (RDM) were affected by the interaction between vegetable crop and nutrient suppression (Table 1).

Table 1.
Summary of statistical analysis (p-values) for tomato and bell pepper leaf net photosynthetic rate (A), transpiration (E), stomatal conductance (Gs), internal CO2 concentration in the substomatal chamber (Ci), water use efficiency (WUE), phloem diameter (PD), xylem diameter (XD), palisade parenchyma thickness (PPT), adaxial epidermis thickness (AET), stomatal density (SD), stomatal functionality (SF), shoot growth (SG), number of leaves (NL), shoot dry mass (SDM), and root dry mass (RDM) affected by vegetable crop, nutrient suppression, and their interactions.
2.2. Morpho-Physiological Responses of Tomatoes and Bell Peppers to Nutrient Suppression
Tomato plants showed greater Gs, Ci, and AET compared to bell pepper plants (Table 2). However, PD was greater in bell pepper than tomato plants (Table 2). Regarding nutrient suppression, Mg and Fe absence caused lower A, E, Gs, PD, and SD compared to the control (all nutrients applied) and B and Zn absence (Table 2). Iron suppression lead to a decrease in WUE and XD compared to the other nutrients suppressed and the control treatment (Table 2). In addition, AET was lower with Fe, Zn, and B suppression compared to Mg suppression and the control treatment (Table 2). In contrast, Ci was found to be lower in the control and B suppression treatments (Table 2).

Table 2.
Physiological (leaf net photosynthetic rate (A), transpiration (E), stomatal conductance (Gs), internal CO2 concentration in the substomatal chamber (Ci), water use efficiency (WUE)), and morphological parameters (phloem diameter (PD), xylem diameter (XM), palisade parenchyma thickness (PPT), adaxial epidermis thickness (AET), stomatal density (SD), and stomatal functionality (SF)) as a function of vegetable crop and nutrient suppression.
The interaction between vegetable crop and nutrient suppression was found to be significant for PPT (Table 3). For bell pepper, PPT was greatest for the control treatment and decreased according to Mg > Zn ≥ B > Fe (Table 3). For tomato plants, PPT was greatest in the control treatment and did not differ among the limiting nutrients (Table 3). In most cases, PPT was greater in pepper plants than in tomato plants; the only exception was for the Fe-absent treatment (Table 3).

Table 3.
Interaction between vegetable crop and nutrient suppression in palisade parenchyma thickness (PPT). Average for All is the average over all treatments.
2.3. Changes in Biometric and Production Components as a Function of Nutrients Suppression in Tomatoes and Bell Peppers
Tomato plants showed greater SG and NL compared to bell pepper plants (Table 4). Regarding nutrient suppression, Fe absence caused lower SG and NL compared to the control and Mg, B, and Zn absent treatments (Table 4).

Table 4.
Shoot growth (SG) and number of leaves (NL) as a function of vegetable crops and nutrient suppression.
The parameters NL, SDM, and RDM were found to be significantly affected by the interaction between vegetable crops and nutrient suppression (Table 5). Tomato plants showed greater NL than bell pepper in the control and in the Mg, Zn, and B absent treatments (Table 5). Similarly, tomato plants showed greater SDM compared to bell pepper with Mg suppression (Table 5). In contrast, bell pepper plants showed greater RDM than tomato with Zn and Fe suppression (Table 5). Iron suppression decreased NL, SDM, and RDM compared to Mg, Zn, and B suppression and control treatments in tomato plants, in addition to RDM in bell pepper plants (Table 5). Similarly, Mg and Fe suppression decreased NL and SDM compared to Zn and B suppression and the control treatments in bell pepper plants (Table 5).

Table 5.
Interaction between vegetable species and nutrient suppression in number of leaves (NL), shoot (SDM), and root dry matter (RDM). Average for All is the average over all treatments.
2.4. Nutritional Deficiency Symptoms, Pearson’s Correlation (Heatmap) and Principal Component Analysis (PCA) as a Function of Plant Species and Nutrient Suppression
Figure 1 displays the symptoms of nutritional deficiency resulting from nutrient suppression in tomato and bell pepper plants. These visual representations provide valuable insights into the observable effects of nutrient restriction on plant growth and development. Figure 2 showcases Pearson’s linear correlations in the form of a heatmap, depicting the relationships between the evaluated treatments. This visualization allows for a comprehensive understanding of the interplay between different nutrient suppressions and their impacts on the parameters measured. Figure 3 presents the loadings and biplot graphics of principal component analysis (PCA) for tomato and bell pepper plants in relation to nutrient suppression. These analytical tools help in identifying the most influential variables and their contributions to the overall variance observed in the dataset. By examining the loadings and biplot, researchers can gain insights into the patterns and associations between the different treatments and their effects on the plant responses. The discussion section of this article provides a detailed analysis and interpretation of the most relevant data associated with the obtained results in the abovementioned figures.
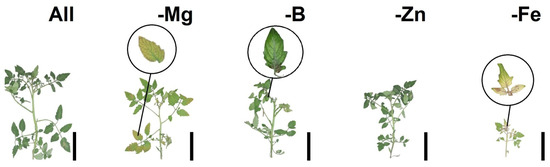
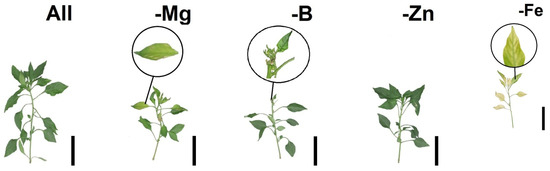
Figure 1.
Tomato and pepper seedlings after nutrient suppression. Top row: tomato Mariana and bottom row: pepper Magali R. Black bar scale: 10 cm.
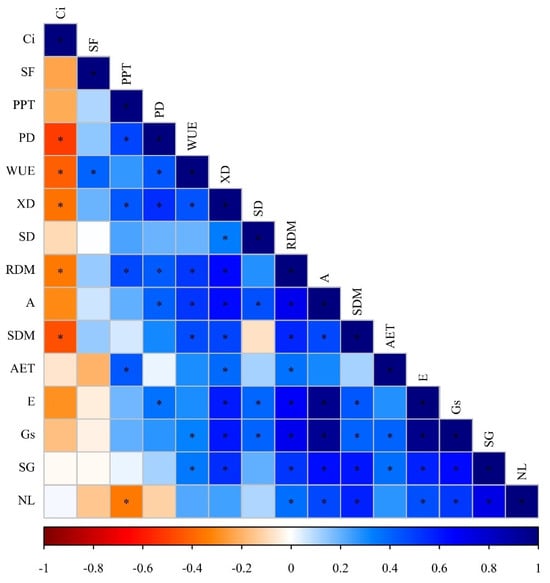
Figure 2.
Heatmap of the Pearson’s correlation coefficients obtained from variables analysed in tomato and bell pepper crops. * Indicates significant correlation (p < 0.05); leaf net photosynthetic rate = A, transpiration = E, stomatal conductance = Gs, internal CO2 concentration in the substomatal chamber = Ci, water use efficiency = WUE, phloem diameter = PD, xylem diameter = XM, palisade parenchyma thickness (PPT), adaxial epidermis thickness (AET), stomatal density (SD), stomatal functionality (SF), shoot growth (SG), number of leaves (NF), shoot dry mass (SDM), and root dry mass (RDM).
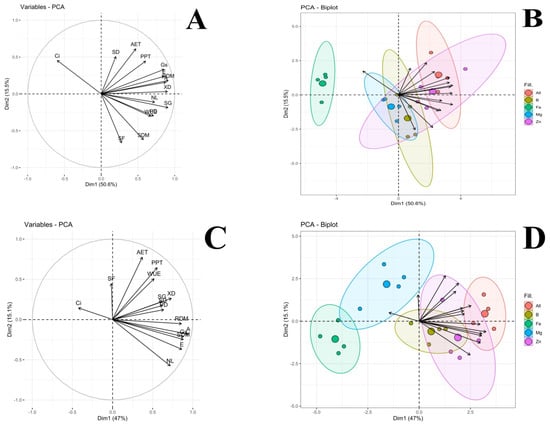
Figure 3.
Loadings and biplot graphics of principal component analysis among the relationship between leaf net photosynthetic rate (A), transpiration (E), stomatal conductance (Gs), internal CO2 concentration in the substomatal chamber (Ci), water use efficiency (WUE), phloem diameter (PD), xylem diameter (XM), palisade parenchyma thickness (PPT), adaxial epidermis thickness (AET), stomatal density (SD), stomatal functionality (SF), shoot growth (SG), number of leaves (NF), shoot dry mass (SDM), and root dry mass (RDM) evaluated in tomato (A,B) and bell pepper crops (C,D) as a function of nutrient suppression. (A,B): Eigenvalues = 7.58 (PC1), 2.31 (PC2), 1.71 (PC3) and 1.08 (PC4); Variance (%) = 50.57 (PC1), 15.45 (PC2), 11.38 (PC3) and 7.21 (PC4); Cumulative variance (%) = 50.57 (PC1), 66.03 (PC2), 77.41 (PC3) and 84.63 (PC4). (C,D): Eigenvalues = 7.06 (PC1), 2.26 (PC2), 1.66 (PC3) and 1.19 (PC4); Variance (%) = 47.01 (PC1), 15.10 (PC2), 11.10 (PC3) and 7.98 (PC4); Cumulative variance (%) = 47.01 (PC1), 62.11 (PC2), 73.21 (PC3) and 81.21 (PC4).
3. Discussion
Our results showed a significant difference in leaf net photosynthetic rate (A) with the suppression of Mg and Fe resulting in the lowest averages. Notably, the suppression of Fe had a particularly severe impact, causing a reduction of approximately 77.1% in A compared to the plants that received all the nutrients, as indicated in Table 2. This result highlights the importance of Mg and Fe for proper plant growth and development; Mg is a vital component of the chlorophyll molecule, while Fe is involved in the structure of ferredoxin within the photosynthetic system in chloroplasts [20]. The suppression of Mg and Fe also lead to a reduction in E and Gs, indicating a disruption in leaf metabolism. When plants experience decreased transpiration, it leads to a reduced replacement of water as well as a reduction in water uptake from the soil, which in turn restricts the uptake of dissolved nutrients from the soil solution, affecting nutrient assimilation in the plants [21]. Therefore, the inadequate uptake of these two nutrients adversely affects the overall water and nutrient uptake processes in tomato and bell pepper plants.
The mechanism of stomatal opening and closing is not yet fully understood, although it is known that Cl and K play active roles in this process. Recent studies have suggested that Mg may also be involved in regulating salt concentrations between the cytoplasm and vacuoles, contributing to stomatal regulation [22,23]. Additionally, Fe actively participates in the photosynthetic system and plays a crucial role in activating enzymes involved in C assimilation metabolism. The negative effect of nutrient restriction on stomatal conductance can further accentuate these effects [24]. Among the gas exchange parameters evaluated, A and Gs can be considered the most important parameters regarding the properly growth and development of tomato and bell pepper. These results could be related to the positive Pearson’s correlation between A and SDM, RDM, SG, SF, Gs, and E (Figure 2). Similarly, we verified positive Pearson’s correlation between Gs and SDM, RDM, SG, SF, E, and SD (Figure 2).
In addition to the disruption in stomatal conductance and carbon assimilation, we also observed a statistical difference in the internal concentration of CO2 in Ci. Interestingly, plants subjected to Fe suppression exhibited a higher mean concentration of Ci, as indicated in Table 2. This suggests that the photosynthesis process was not efficient, resulting in the accumulation of CO2 in the internal leaf tissues. These findings align with previous studies [24,25], which highlight the negative impact of Fe suppression on the photosynthetic efficiency and internal CO2 concentrations in leaves. The negative Pearson’s correlation between Ci and PD, XD, WUE, SDM and RDM support the hypothesis that the photosynthesis was not efficient, causing decreased plant growth and morphological development of tomato and bell pepper (Figure 2).
The positive Pearson’s correlation between WUE and XD, SDM, RDM, A, and Gs reinforces the importance of WUE in tomato and pepper cultures, directly related to productive components and photosynthetic and morphological parameters (Figure 2). Interestingly, no statistical difference was observed in stomatal functionality, indicating that the ability of stomata to open and close remained unaffected by the nutrient suppressions studied. However, a significant difference was observed in stomatal density (as shown in Table 2), highlighting the importance of both Mg and Fe in the differentiation and formation of cells within the stomatal complex. Our findings suggest that while the functionality of stomata may not be directly impacted by nutrient restrictions, the development and arrangement of stomata can be influenced by the availability of Mg and Fe [22,23].
Regarding the morphological analysis, statistical differences were found among plant species, with bell pepper exhibiting the highest average PD. The higher PD observed for bell pepper represented an increase of approximately 27.9% compared to tomato plants. On the other hand, the deficiency of Mg and Fe resulted in the lowest PD averages. Among the nutrient suppressions, the suppression of Fe had the most severe impact on reducing PD, resulting in a reduction of approximately 44.4% compared to the plants that received all of the nutrients, as indicated in Table 2. These findings highlight the importance of Mg and Fe in maintaining adequate phloem diameter in the plants, with Fe deficiency having the most significant impact on PD. Indeed, Mg has a direct impact on the photosynthetic process, as it is an integral component of the chlorophyll molecule. Its restriction can compromise the photolysis of water, resulting in a decreased availability of H+ ions required for the formation of NADPH+. This effect can be further accentuated by the inefficiency of ferredoxin due to Fe deficiency. Consequently, the reduction in NADPH+ availability leads to a decrease in sugar production, which directly affects the formation of phloem. This information aligns with studies conducted by [26] and highlights the vital role of magnesium in maintaining an efficient photosynthetic process and subsequent phloem formation. Furthermore, the xylem, being derived from the same embryonic origin as the phloem, exhibited a similar response to nutrient restriction, displaying reduced development in its diameter. Consequently, the organic components that form the xylem cells may have been compromised, potentially leading to the accumulation of lignin [20]. The positive Pearson’s correlation observed between the PD and SDM, RDM, A, and Gs, as well as between the XD and SDM, RDM, A, and Gs, further supports this hypothesis (Figure 2). Similarly to PD and XD, the PPT and AET were negatively affected by nutrient suppression. However, unlike most of the results observed for the evaluations of morphological components, the suppression of B and Zn had a more pronounced impact on PPT and AET, as indicated in Table 2 and Table 3. The restriction of these elements can compromise the metabolism of biomolecules, resulting in a reduced thickness of the epidermis. Moreover, significant changes in temperature, light intensity, and water availability can further contribute to these alterations [19].
Tomatoes exhibited greater growth compared to bell peppers, and the suppression of Fe resulted in a lower average plant growth, as indicated in Table 4. It is worth noting that the absence of Fe in plant metabolism may lead to a decline in the synthesis of lipoxygenase, an enzyme responsible for the oxidation of linoleic acids. This could potentially compromise the formation of cell biomembranes [27]. The findings reinforce the importance of Fe for optimal plant growth and underscore the impact of its deficiency on various metabolic processes. Plant growth was closely related to SDM and RDM, as verified by the positive Pearson’s correlation between SG and SDM and RDM (Figure 2).
The absence of Fe in the nutrient solution was found to compromise overall morpho-physiological parameters and shoot and root development in this study. Different plant species and cultivars exhibit varied responses in such conditions, highlighting the need for further comprehensive studies to better understand plant responses when cultivated under inappropriate conditions [28]. In response to Fe deficiency, plants undergo physiological adaptations in the mechanisms of acquiring Fe from the soil [29]. This includes enhanced Fe uptake by roots, its transport from roots to aerial parts, and its storage within cells. Iron movement within plants typically occurs through chelation with phytohydrophores, citrate, nicotianamine, or as free Fe ions [30]. Understanding the complex mechanisms involved in Fe acquisition and transport can provide insights into developing strategies to mitigate Fe deficiency-related issues in plant cultivation.
Among the treatments, bell pepper plants subjected to Mg suppression had the lowest number of leaves, as depicted in Table 4. This can be attributed to the key role of Mg in the chlorophyll molecule, as the deficiency of this nutrient leads to reduced leaf size, lower number of leaves, and the appearance of yellow spots between the veins, as seen in Figure 1. These changes in leaf pigmentation resulted in a decreased photosynthetic rate, ultimately affecting the shoot and root dry matter, as observed in Table 5. Furthermore, a significant interaction was observed between the factors for shoot dry matter. Both tomatoes and bell peppers exhibited lower averages when subjected to Fe restriction, as indicated in Table 5. The reduction in shoot dry mass reflects the disruption in metabolic processes, particularly in the photosynthetic process [29]. This deficiency also affects shoot length, as illustrated in Figure 1. These findings highlight the importance of Mg and Fe in plant growth and development, underscoring their impact on various physiological processes. Nonetheless, our results clearly indicate that the Fe and Mg suppression were the deficiencies that most affected the gas exchange parameters in tomato and bell pepper plants, negatively impairing morphologic development, reflected in lower shoot and root growth. Analysing the grouped PCA biplot graphs (PC1 and PC2) in tomato and bell pepper plants, we observed that the group formed by the treatment without nutrient suppression followed by Zn and B suppression better comprised most of the analysed parameters (Figure 3). In contrast, the group characterised by Fe and Mg suppression was found to comprise the opposed quadrants from morphophysiological and plant growth parameters (Figure 3).
Although the suppression of B and Zn had less noticeable effects compared to the suppression of Fe and Mg, it still had a negative impact on gas exchange parameters and the morphology of tomatoes and bell peppers. For instance, B suppression resulted in reduced values of A, Gs, SG, RDM, XD, PPT, and AET compared with the fully fertilized control. Similarly, Zn suppression led to reduced values of E, SG, XD, PPT, and AET compared with the fully fertilized control. These results align with the known roles of B and Zn in various important physiological processes in plants. Considering these effects, the proper replenishment of B and Zn in tomatoes and bell peppers is essential for better nutritional management of these crops. Therefore, understanding the effects of B and Zn suppression in tomatoes and bell peppers may improve nutrient management practices, ensuring that these crops receive the necessary elements for their proper development and productivity.
4. Materials and Methods
4.1. Experimental Design and Treatments
The study was carried out in a greenhouse under controlled conditions (the temperature in the greenhouse during plant growth ranged between 25.1 °C (minimum) and 34.7 °C (maximum) and averaged 29.9 °C. The average air relative humidity was 60% ± 5%, and the maximum photosynthetic photon flux density (sunlight) was approximately 2000 μmol photons m−2 s−1 at the leaf level, delivered by 18-W LED lamps. The experimental design used in this study was a completely randomized design with a 2 × 5 factorial scheme. The first factor consisted of two vegetable crops: (i) Mariana variety (Sakata®), a tomato variety with Italian-type fruit and determinate growth habits, having an average size of 11.95 cm and approximately 7 ± 1 leaflets per seedling; and (ii) Magali R variety (Sakata®), a pepper variety with an average size of 8.52 cm and approximately 5 ± 1 leaves per seedling. The second factor comprised the availability of nutrients, with the following treatments: (1) control group with the supply of all nutrients, (2) magnesium (Mg) suppression, (3) boron (B) suppression, (4) zinc (Zn) suppression, and (5) iron (Fe) suppression, totalizing 10 treatments. Each treatment was replicated four times, resulting in a total of 40 experimental pots, with one seedling per pot. The seedlings used in the study were obtained from a commercial nursery located in the same municipality as the experimental site.
The seedlings were cultivated in 5 L pots filled with a nutrient solution following the recommended concentrations provided by [31]. The nutrient solution consisted of the following components: 0.75 g L−1 of Ca(NO3)2; 0.53 g L−1 of KCl; 0.15 g L−1 of NH4H2PO4; 0.4 g L−1 of MgSO4; 1.5 × 10−2 g L−1 of CuSO4; 2.0 × 10−2 g L−1 of ZnSO4; 1.5 × 10−1 g L−1 of MnSO4; 1.5 × 10−1 g L−1 of H3BO3; 1.5 × 10−2 g L−1 of Na2MoO4; 3.0 g L−1 of EDTA + Fe (6%). The electrical conductivity of the nutrient solution was adjusted daily to 2.000 ± 100 µS, and the pH was maintained at 6.4 ± 0.2. It is important to note that the treatments involving the suppression of Mg, B, Zn, and Fe did not receive these specific nutrients in the nutrient solution, while the other treatments received all the nutrients as per the specified concentrations.
4.2. Growth Parameters—Biometric Components
Thirty days after the start of the experiment, the following variables were measured: shoot growth (SG): determined by calculating the difference between the final length of the shoot and the initial length, expressed in centimetres; number of fully expanded leaves (NL): determined by subtracting the initial number of leaves from the final number of fully expanded leaves; shoot dry matter (SDM): obtained by drying the plant samples in a circulation oven at a constant temperature of 65 °C until a constant weight was achieved; root dry matter (RDM): similarly, determined by drying the root samples in a circulation oven at a constant temperature of 65 °C until a constant weight was attained.
4.3. Physiological Parameters—Gas Exchange
At the same time as the growth parameter measurements (30 days after the start of the experiment), gas exchange measurements were conducted using an Infra-Red Gas Analyser (IRGA) device (ADC BioScientific Ltd., model LC-Pro Hoddesdon, United Kingdom). The plants were exposed to a photosynthetically active radiation (PAR) of 1200 μmol m−2 s−1, provided by LED lamps, until the gas exchange parameters stabilized. The following parameters were determined: leaf net photosynthetic rate (A): this parameter quantifies the rate of CO2 assimilation by plant leaves (μmol CO2 m−2 s−1); transpiration (E): transpiration represents the rate of water loss from the leaves (mmol H2O m−2 s−1); stomatal conductance (GS): stomatal conductance measures the ease with which water vapour moves through the stomata (mol H2O m−2 s−1); internal CO2 concentration in the substomatal chamber (Ci): this parameter represents the concentration of CO2 inside the substomatal chamber (μmol CO2 mol−1 air). The measurements were taken with a CO2 concentration of 380 ppm and with the chamber temperature of 28 °C. Water use efficiency (WUE—μmol CO2 mmol H2O−1) was determined using a formula that calculates the ratio between CO2 assimilation rate and transpiration rate (Equation (1)):
4.4. Morphological Parameters
At the same time as the physiological evaluations, a fragment of the first fully expanded leaf was collected from the apex of each plant for further analysis. The following measurements were collected from leaf tissues: phloem diameter (PD): the diameter of the phloem tissue in the leaf; xylem diameter (XD): the diameter of the xylem tissue in the leaf; palisade parenchyma thickness (PPT): the thickness of the palisade parenchyma layer in the leaf; adaxial epidermis thickness (AET): the thickness of the adaxial (upper) epidermis in the leaf. In addition, an impression of the inferior or abaxial epidermis was made on the same leaf using cyanoacrylate ester technique [32]. This impression was used to determine stomatal density (SD) and stomatal functionality (SF) following the methodology described by [33]. Ten measurements were performed per slide, resulting in a total of 40 measurements per treatment.
4.5. Statistical Analysis
The Levene’s homoscedasticity test (p > 0.05) and Shapiro–Wilk normality test (p > 0.05) were performed on the data collected. Then, a variance analysis was performed using the F test (p ≤ 0.05). Significant results were submitted to means comparison by using the Scott–Knott test (p ≤ 0.05). To identify dependent variables directly related to nutrient suppression in tomato and bell pepper crops, a Pearson’s correlation analysis (p ≤ 0.05) was performed and presented as a coloured heatmap. In addition, a principal component analysis (PCA) was performed following [34] procedures. All statistical analyses were performed using the software program R [35].
5. Conclusions
Our findings indicate that the suppression of Fe followed by Mg had the most pronounced impact on the morphology and physiology of tomatoes and bell peppers. These suppressions resulted in significant reductions in parameters associated with gas exchange, and both crops displayed deficiency symptoms such as interveinal chlorosis in the leaves. Although the effects were less pronounced compared to Fe and Mg suppressions, restrictions in B and Zn also caused malformation in the shoot apex tissues. Furthermore, these restrictions led to reductions in parameters related to gas exchange and alterations in the overall morphology of tomatoes and bell peppers. To date, this study provided important insights on how nutrient suppression changes tomato and bell paper morphology, resulting in lower plant growth mainly due to decreased photosynthetic metabolism.
Author Contributions
Conceptualization, L.A.M.L. and P.A.M.d.F.; methodology, L.A.M.L., P.A.M.d.F., F.S.G. and P.H.P.; software, L.A.M.L. and F.S.G.; validation, L.A.M.L., P.A.M.d.F., F.S.G. and P.H.P.; formal analysis, L.A.M.L.; investigation, L.A.M.L., J.I.U.P.G., M.H.O. and M.L.O.C.; resources, L.A.M.L. and P.A.M.d.F.; data curation, L.A.M.L., P.A.M.d.F., F.S.G. and P.H.P.; writing—original draft preparation, L.A.M.L. and F.S.G.; writing—review and editing, L.A.M.L., P.A.M.d.F., F.S.G. and P.H.P.; visualization, L.A.M.L. and F.S.G..; supervision, L.A.M.L. and P.A.M.d.F.; project administration, L.A.M.L. and P.A.M.d.F.; funding acquisition, L.A.M.L. and P.A.M.d.F.; All authors have read and agreed to the published version of the manuscript..
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets generated during this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brandão Filho, J.U.T.; Freitas, P.S.L.; Berian, L.O.S.; Goto, R. Hortaliças-Fruto; EDUEM: Maringá, Brazil, 2018; 535p. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Franczuk, J.; Tartanus, M.; Rosa, R.; Zaniewicz-Bajkowska, A.; Dębski, H.; Andrejiová, A.; Dydiv, A. The Effect of Mycorrhiza Fungi and Various Mineral Fertilizer Levels on the Growth, Yield, and Nutritional Value of Bell Pepper (Capsicum annuum L.). Agriculture 2023, 13, 857. [Google Scholar] [CrossRef]
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental Composition and Some Nutritional Parameters of Bell Pepper from Organic and Conventional Agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, M.; Motesharezadeh, B.; Alikhani, H.A.; Mousavi, S.M. Optimal management of plant nutrition in tomato (Lycopersicon esculent Mill) by using biologic, organic and inorganic fertilizers. J. Plant Nutr. 2023, 46, 1560–1579. [Google Scholar] [CrossRef]
- Cheraghi, M.; Motesharezadeh, B.; Alikhani, H.A. Nutritional and morpho-physiological responses of tomato plant (Lycopersicon esculentum Mill) affected by biological and chemical fertilizers. Iran. J. Soil Water Res. 2020, 51, 59–74. (In Persian) [Google Scholar] [CrossRef]
- Singh, P.; Singh, D.; Singh, A.K.; Singh, B.; Singh, T. Growth and yield of tomato grown under organic and inorganic nutrient management. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 365–375. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, L.A.M.; Cavichioli, J.C.; Vitorino, R.; Figueiredo, P.A.M.; da Silva Viana, R. Nutrient suppression in passion fruit species: An approach to leaf development and morphology. Colloq. Agrar. 2021, 17, 89–102. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhong, Y.; Wang, Y.; Li, X. Magnesium Limitation Leads to Transcriptional Down-Tuning of Auxin Synthesis, Transport, and Signaling in the Tomato Root. Front. Plant Sci 2021, 12, 802399. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J.; Konvalina, P.; Kopecký, M. Impacts of Environmental Factors and Nutrients Management on Tomato Grown under Controlled and Open Field Conditions. Agronomy 2023, 13, 916. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, A.; Yashvika; Sidhu, S.; Upadhyay, S.K. A glimpse of boron transport in plants. In Cation Transporters in Plants; Academic Press: Cambridge, MA, USA, 2022; pp. 281–306. [Google Scholar] [CrossRef]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2022, 97, 267–282. [Google Scholar] [CrossRef]
- Xu, W.; Wang, P.; Yuan, L.; Chen, X.; Hu, X. Effects of Application Methods of Boron on Tomato Growth, Fruit Quality and Flavor. Horticulturae 2021, 7, 223. [Google Scholar] [CrossRef]
- Clemens, S. The cell biology of zinc. J. Exp. Bot. 2021, 73, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Ohnishi, M.; Maekawa, S.; Wada, S.; Ifuku, K.; Miyake, C. Evaluating the Oxidation Rate of Reduced Ferredoxin in Arabidopsis thaliana Independent of Photosynthetic Linear Electron Flow: Plausible Activity of Ferredoxin-Dependent Cyclic Electron Flow around Photosystem I. Int. J. Mol. Sci. 2023, 24, 12145. [Google Scholar] [CrossRef] [PubMed]
- Stutz, S.S.; Hanson, D.T. What is the fate of xylem-transported CO2 in Kranz-type C4 plants? New Phytol. 2019, 223, 1241–1252. [Google Scholar] [CrossRef]
- Guo, B.; Liu, J.; Liu, C.; Lin, Y.; Li, H.; Zhu, D.; Zhang, Q.; Chen, X.; Qiu, G.; Fu, Q. Shade and iron plaque of Sesbania affect cadmium accumulation in rice: A new strategy for safe production in contaminated soil. Environ. Technol. Innov. 2023, 29, 102964. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Moroni, F.J.; Baby, T. Potassium and Magnesium Mediate the Light and CO2 Photosynthetic Responses of Grapevines. Biology 2020, 9, 144. [Google Scholar] [CrossRef]
- Inoue, S.; Hayashi, M.; Huang, S.; Yokosho, K.; Gotoh, E.; Ikematsu, S.; Okumura, M.; Suzuki, T.; Kamura, T.; Kinoshita, T. A tonoplast localized magnesium transporter is crucial for stomatal opening in Arabidopsis under high Mg2+ conditions. New Phytol. 2022, 236, 864–877. [Google Scholar] [CrossRef]
- Santos, M.S.; Sanglard, L.M.P.V.; Martins, S.C.V.; Barbosa, M.L.; Melo, D.C.; Gonzaga, W.F.; Damatta, F.M. Silicon alleviates the impairments of iron toxicity on the rice photosynthetic performance via alterations in leaf diffusive conductance with minimal impacts on carbon metabolism. Plant Physiol. Biochem. 2019, 143, 275–285. [Google Scholar] [CrossRef]
- Kouas, S.; Slatni, T.; Chihaoui, S.; Abdelly, C.; Mhadhbi, H. Differential behavior of Medicago truncatula to calcareous soil is explained by modulation of stomatal responses, antioxidant activity, and iron use efficiency. Arab. J. Geosci. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Alrashidi, A.A.; Alhaithloul, H.A.S.; Soliman, M.H.; Attia, M.S.; Elsayed, S.M.; Sadek, A.M.; Fakhr, M.A. Role of calcium and magnesium on dramatic physiological and anatomical responses in tomato plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12614. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Assefa, T.; Zhang, J.; Chowda-Reddy, R.V.; Lauter, A.N.M.; Singh, A.; O’rourke, J.A.; Graham, M.A.; Singh, A.K. Deconstructing the genetic architecture of iron deficiency chlorosis in soybean using genome-wide approaches. Bmc Plant Biol. 2020, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Bacaicoa, E.; Rivero, M.; Zamarreño, Á.M.; García-Mina, J.M. Complementary Evaluation of Iron Deficiency Root Responses to Assess the Effectiveness of Different Iron Foliar Applications for Chlorosis Remediation. Front. Plant Sci. 2018, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [PubMed]
- Furlani, P.R. Instruções Para o Cultivo de Hortaliças de Folhas Pela Técnica de Hidroponia—NFT; Instituto Agronômico de Campinas: Campinas, Brazil, 1997; p. 30. [Google Scholar]
- Segatto, F.B.; Bisognin, D.A.; Benedetti, M.; Costa, L.C.; Rampelotto, M.V.; Nicoloso, F.T. A technique for the anatomical study of potato leaf epidermis. Ciência Rural 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Castro, E.M.; Pereira, F.J.; Paiva, R. Histologia Vegetal: Estrutura e Função de Órgãos Vegetativos; UFLA: Lavras, Brazil, 2009; p. 234. [Google Scholar]
- Galindo, F.S.; Rodrigues, W.L.; Fernandes, G.C.; Boleta, E.H.M.; Jalal, A.; Rosa, P.A.L.; Buzetti, S.; Lavres, J.; Teixeira Filho, M.C.M. Enhancing agronomic efficiency and maize grain yield with Azospirillum brasilense inoculation under Brazilian savannah conditions. Eur. J. Agron. 2022, 134, 126471. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 3 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).