Abstract
Oxidative stress is caused by an imbalance between the production and subsequent accumulation of reactive oxygen species (ROS) in cells and tissues and the capacity of a biological system to eliminate these reactive substances. Systemic oxidative stress biomarkers in plasma, serum, urine, or red blood cells have been found to be elevated in many diseases, including skin cancer. UV radiation (UVR) induces damage to biomolecules that enter the bloodstream, reinforcing systemic oxidative stress. On the other hand, pre-existing systemic oxidative stress does not supply the skin with the adequate micronutrients and antioxidant resources to ameliorate the skin’s antioxidant defense against UVR. In both scenarios, skin cancer patients are exposed to oxidative conditions. In the case of warts, oxidation is linked to chronic inflammation, while impaired cutaneous antioxidant defense could ineffectively deal with possible oxidative stimuli from viral agents, such as HPV. Therefore, the aim of our study is to evaluate the existing data on systemic oxidative stress in skin diseases such as non-melanoma skin cancer (NMSC), basal-cell carcinoma (BCC), squamous-cell carcinoma (SCC), and melanoma as well as benign lesions such as actinic keratosis (AK), sebaceous keratosis (SK), and warts. Previous studies have demonstrated that patients with NMSC, melanoma, AK, and warts (both genital and non-genital) are subjected to severe oxidative stress, indicated by disturbed antioxidant enzyme levels, accumulated oxidized proteins and lipid products, and, to a lesser extent, lower concentrations of micronutrients. Interestingly, medical history of NMSC or melanoma as well as stage of skin cancer and treatment approach were found to affect systemic oxidative stress parameters. In the case of warts (both genital and non-genital), high oxidative stress levels were also detected, and they were found to be aligned with their recalcitrant character.
1. Introduction
1.1. The Concept of Oxidative Stress
Oxidative stress is a term used to describe a disturbance of equilibrium between the generation of reactive oxygen species (ROS) within cells and tissues and the ability of a biological system to eliminate these reactive substances. ROS include radical and non-radical oxygen derivatives formed by the partial reduction of oxygen such as superoxide anions (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (HO•) [1]. External stressors, like UV radiation, ionizing radiation, pollutants, and heavy metals, along with xenobiotic substances like anticancer drugs substantially raise ROS production. Excessive levels of ROS cause harmful outcomes and, if not mitigated adequately by the enzymatic and non-enzymatic antioxidant mechanisms of the targeted cell or tissue, they induce modifications of significant biomolecules, processes implicated in the pathophysiology of diseases [1]. It is worth mentioning that ROS serve a dual role in living systems, contributing to important cellular functions in low or moderate levels. More specifically, they act beneficially as mediators of immunity [2] and intracellular signaling pathways [3]. They are also involved in cellular proliferation, differentiation, and programmed cell death [4].
In order to determine oxidative stress levels, most studies evaluate the enzymatic and non-enzymatic mechanisms activated by a given cell, tissue, or organism to deal with the oxidative changes mediated by the contributing stressor. Usually, the findings are compared with the respective results in the control group or individuals that were not exposed to the oxidative factor. In the case of disease, in the majority of cases, patients with a specific disease and occasionally with certain eligible criteria (a certain disease severity or patients without any intervention or medical treatment, etc.) are compared to disease-free individuals in terms of oxidative stress parameters. These parameters can be evaluated in erythrocytes, biological fluids (plasma, serum, or urine), or a specific tissue (for example a skin biopsy), reflecting the redox status of the specific system [5].
The most important enzymes that cells are armed with are superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione reductase (GR), and catalase (CAT). Firstly, SOD catalyzes the dismutation of superoxide anion free radicals (O2•−) into molecular oxygen and hydrogen peroxide (H2O2). Secondly, hydrogen peroxide is subsequently reduced to water by the enzymatic actions of GPX and CAT [1]. GPx catalyzes this reaction via the oxidation of reduced GSH into its disulfide form (GSSH), while GR replenishes cellular GSH levels by converting GSSG into its reduced form using NADPH as a cofactor [6]. Studies usually determine the activity of those enzymes to assess oxidative stress. For example, reduced GPx-1 activity can increase vulnerability to oxidative stress by permitting the buildup of ROS, while excess GPx-1 might foster reductive stress, marked by an insufficient presence of necessary ROS required for cellular signaling functions [6].
Non-enzymatic molecules can also have antioxidant capacities, inactivating radicals and oxidants. Minerals exert their antioxidant action through involvement in certain enzymatic reactions. For example, in the case of Zn, the SOD1 enzyme comprises an eight-stranded β-barrel with one Cu and Zn ion bound in each monomer. Their presence is crucial for the catalytic activity of the enzyme. Besides this, zinc competes with iron (Fe) and copper (Cu) ions for binding to cell membranes and proteins, displacing these redox-active metals, which catalyze the production of ⋅OH from H2O2 [7]. Generally, the most important antioxidant micronutrients are vitamins A, C, and E, copper, zinc, and selenium [8].
Besides the focus on innate protection against oxidative stress, it is common for studies to assess the impact of oxidative stress on cellular components like DNA, lipids, and proteins. Oxidative modifications can lead to the production of 8-oxoguanine (also called 8-hydroxyguanine), a tautomer of guanine in nucleic acids that is formed when DNA is exposed to excessive ROS. As a result, 8-oxoguanine has gained significant recognition as a biomarker of oxidative damage [9]. As an index for lipid peroxidation, thiobarbituric acid reactive substance (TBARS) assay is a frequently used method. This assay measures malondialdehyde (MDA), a breakdown product originating from the oxidation of lipid substrates, specifically from an endoperoxide of unsaturated fatty acids [10]. 15-F2t-isoprostane is also a lipid peroxidation product that is a frequently used oxidative stress marker [11].
As for the impact of oxidative stress on proteins, protein carbonylation, which is the most common form of protein oxidation, is an irreversible process that promotes protein degradation. Advanced byproducts of lipid peroxidation such as 4-Hydroxy-2-nonenal (4-HNE) and MDA, regarded as reactive carbonyl species, have been correlated with protein modifications [12]. Another relevant mechanism involves the oxidation of sulfur-containing amino acids, present in thiols [13]. These intracellular compounds are especially susceptible to direct oxidation by ROS due to their strong nucleophilic properties. The oxidation of these thiols leads to changes in the structure and function of proteins [12,13].
Regarding antioxidant micronutrients, vitamin A, or retinol, and carotenoids exhibit their antioxidant properties through a hydrophobic chain composed of polyene units. This chain has the capability to extinguish singlet oxygen and to counteract thiol radicals, as well as to enhance the stability of peroxyl radicals. Secondly, vitamin C is chemically capable of reacting with most of the physiologically important radicals and oxidants and acts as a proven hydrosoluble antioxidant, while vitamin E is a fat-soluble antioxidant that terminates the production of ROS that forms when fat undergoes oxidation. Therefore, the recognition of a reduced quantity of serum macronutrients may be indicative of oxidative stress [14].
It is important to outline that each study may use a different technique or different protocol to assess the same oxidative stress parameter, rendering the exclusion of definite or additive conclusions challenging. Also, it is worth mentioning that oxidative stress markers can differ between several samples of the same organism (tissue or type of cell). For example, in the case of psoriasis, in research conducted by Yldirim and colleagues, serum MDA levels in individuals with psoriasis were not notably elevated compared to those in the control group. Nonetheless, higher lipid peroxidation levels were observed in samples obtained by lesional skin biopsies, indicating different oxidative stress parameters between cutaneous and systemic oxidative stress [15].
1.2. Oxidative Stress in Dermatology—The Interaction between Cutaneous and Systemic Oxidative Stress
Oxidative stress has been widely investigated in dermatology and skin diseases. Reviews focusing on common dermatoses such as acne [16], psoriasis [17], and atopic dermatitis [18] have been published recently, indicating it as a contributor factor in the pathogenesis of the focus disease. Oxidative stress is considered part of the internal exposome and, along with other contributors such as genetic variants and internal organism characteristics like the microbiota and metabolics, predisposes an individual to disease. External contributors, including diet and exercise, in turn affect systemic oxidative stress [19]. However, a question occurs on how a skin disease, or a skin stressor, can affect systemic oxidative stress and, on the contrary, how the latter is associated with cutaneous oxidative stress.
As mentioned previously, exposure to ultraviolet radiation (UVR) serves as the primary trigger for ROS production in the skin and the main etiology of skin cancer. The spectrum of wavelengths responsible for this effect predominantly falls within the UVA range (320–400 nm), although there is some overlap with the UVB region (280–315 nm). The process of ROS generation following UVA and UVB irradiation is based on the absorption of photons by intrinsic photosensitizer molecules like cytochromes, riboflavin, heme, and porphyrin. Following exposure to sunlight, damaged biomolecules and signaling molecules resulting from UV exposure can permeate into the bloodstream, thereby inducing systemic oxidative stress. Also, skin cancer cells produce excessive ROS by themselves [20]. This is the reason why skin cancer patients tend to have high levels of systemic oxidative stress [21]. Also, patients with certain gene polymorphisms have misfunctioning antioxidant enzymes [22]. In this case, the default found in red blood cells (RBCs) would be present in every cell of the same organism, including skin keratinocytes, fibroblasts, and melanocytes, forming a generalized flawed antioxidant defense [23]. As for inflammatory dermatoses, systemic inflammation corresponds to systemic oxidative stress [24].
The reverse relationship has been also observed, indicating that systemic oxidative stress can affect skin integrity. Notably, the consumption of certain antioxidants can ameliorate systemic oxidative stress and subsequently reduce skin disorder severity. For example, flavonoids can act beneficially, as they can repair damaged biomolecules and enhance the activities of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase respectively. In the case of skin cancer patients, it has been proven that dietary flavonoid-rich polyphenols exert skin-protective effects against the potential hazards of UV-induced skin cancers by reducing cutaneous inflammation and oxidative stress [25].
1.3. Oxidative Stress and Skin Cancer
Skin cancer encompasses melanoma and non-melanoma skin cancer (NMSC) and represents the most prevalent form of cancer among individuals of Caucasian ethnicity. Non-melanoma skin cancers predominantly comprise basal-cell carcinoma (BCC) and cutaneous squamous-cell carcinoma (SCC), alongside some less common skin tumors. BCC originates from the basal layer of the epidermis and its associated structures, whereas SCC emerges from the unregulated growth of atypical epidermal keratinocytes. Melanoma, a malignant tumor arising from melanocytes, is the deadliest form of skin cancer, being capable of metastasizing to both regional and distant sites [26].
1.3.1. Oxidative Stress and NMSC
NMSC initiation is influenced by a combination of environmental triggers, phenotypic characteristics (including lighter skin tones with less natural protection), and genetic factors that make the individual more prone to oxidative stress in the skin microenvironment. Among environmental factors, exposure to UVR stands out as the most significant risk factor, due to the induction of DNA damage, particularly in the UVB range of 290–320 nm, which produces two major types of lesions: cyclobutene pyrimidine dimers (CPDs) and 6–4 photoproducts (6-4PPs). If this damage is not repaired by nucleotide excision repair mechanisms, its products can disrupt proper base-pairing and impede vital cellular processes such as transcription and replication [5,27]. These harmful modifications may lead to progressive alterations in genes, including tumor suppressor genes and proto-oncogenes, eventually resulting in the formation of tumors. In the case of BCC, for example, exposure to UVR and oxidative stress promote mutations in the PTCH (patched-1) gene located on the cell membrane, resulting in an abnormal activation of the hedgehog signaling pathway. This, in turn, plays a significant role in the development of BCC [28]. Newer studies make efforts to relate oxidative stress and skin cancer, especially NMSC, with a third parameter, more frequently a third exposome variant such as the skin microbiome [29] and vitamin D adequacy [5].
1.3.2. Oxidative Stress and Melanoma
Considering cases of NMSC, melanoma is related to exceptionally high oxidative stress levels. Melanocytes, due to their physical location, are directly exposed to environmental stressors, such as UV radiation, that induce oxidative stress. Also, melanocytes are particularly susceptible to oxidative changes due to the pro-oxidant state generated during the synthesis of melanin and the intrinsic antioxidant defenses that may be disrupted in pathologic conditions. Damaged cellular components formed by elevated ROS disturb the structural integrity and functionality of cells. Ion channels can be stimulated or blocked depending on the intensity of oxidative stress, determining melanoma progression. As a consequence, ion channels and oxidative stress may serve as possible therapy targets [30,31].
1.4. Oxidative Stress and Benign Skin Lesions
Data regarding benign skin lesions and oxidative stress seem to be less abundant compared to those on skin cancer, probably due to the benign nature of the lesions. Actinic keratosis results from UV-provoked dysplastic proliferations of keratinocytes with the potential for malignant transformation, considered pre-malignant lesions. Actinic keratosis tends to follow, just as skin cancer, the general terms of UV-induced oxidative stress discussed above [5]. Secondly, seborrheic keratoses (SKs) are very common benign epithelial skin tumors due to skin aging, chronic UV exposure, and possibly the involvement of HPV [32]. The above-mentioned etiological factors are closely related to oxidative stress.
1.5. Oxidative Stress and Warts
Warts are mucocutaneous growths caused by the human papillomavirus (HPV). To date, over 200 different types of HPV have been identified, with warts commonly associated with HPV types 1, 2, 4, and 7. In immunosuppressed patients, HPV types 75, 76, and 77 have been observed. HPV infects host cells without integrating viral DNA into the host genome. In the case of HPV infection, viral infection does not trigger a state of prolonged inflammation. This is primarily due to the fact that the virus initially infects basal epithelial cells, which are protected from circulating immune cells during the early phases of infection. However, it is worth noting that ROS and reactive nitrogen species (RNS) could potentially play a role in the progression of viral-induced wart formation and, rarely, HPV-related carcinogenesis. Oxidative stress can significantly impact both processes, ultimately establishing favorable conditions for effective viral integration. Then, HPV-transformed cells may avoid apoptosis by the expression of the viral E6 protein, which promotes the ubiquitination and subsequent proteolytic degradation of the cellular protein p53. Furthermore, oxidative-stress-mediated regulation of viral oncogenes at the level of transcriptional activation may lead to HPV carcinogenesis [33]. Beyond the skin, oxidative stress has been found to be present in patients with HPV-related CIN [34] and medical history of multiple HPV infections [33].
Both skin cancer and lesions with benign and pre-malignant capacities are related to oxidative stress. In studies evaluating oxidative stress parameters of patients with skin diseases, authors tend to give a more holistic approach by measuring systemic oxidative stress in patients’ blood samples [5]. However, this assessment might reflect oxidative stress caused by other systemic diseases such as hypertension, diabetes, and a medical history of heart attacks. Systemic oxidative stress is a general but important term which includes processes ranging from damage at the cellular level to aging and traces of immune dysfunction in antioxidant mechanisms that can result in disease development. Therefore, our review aims to collect studies focused on systemic oxidative stress in NMSC and melanoma as well as patients with benign lesions (AK, SK, and warts), provide a biological-system-specific assessment of skin disease patients, and further investigate the vicious circle between systemic and cutaneous oxidative stress.
2. Result and Methods
In order to perform our narrative review, we searched PubMed articles published until the end of April 2023 based on terms such as “melanoma” OR “Non-melanoma skin cancer” OR “BCC” OR “cutaneous SCC” OR “actinic keratosis” OR “seborrheic keratosis “ OR “ warts” AND “oxidative stress” OR “glutathione” OR “catalase” OR “ TBARS” OR “ carbonyls” OR “ vitamin A” OR “ vitamin C” OR “zinc” OR “selenium” OR “ vitamin E”. Where no results were found, we expanded our research beyond PubMed. Eligible criteria were references to systemic oxidative stress parameters in skin cancer patients or patients with benign lesions such as actinic keratosis and seborrheic keratosis and their comparison with the respective parameters in a control group. As systemic oxidative stress markers, we considered oxidative stress parameters assessed either in the bloodstream, plasma, serum, RBCs, or urine. Excluded studies were those that assessed only cutaneous and not systemic oxidative stress and those that determined alterations in oxidative stress following antioxidant supplementation. Comparisons based on child populations were excluded.
Following our research, we found fourteen studies on NMSC, fifteen studies on melanoma, four studies on benign lesions (we expanded our research on SK, as no results were found on PubMed, and we included one study on SK), and seven studies on warts. In the following tables, the selected studies are presented, including references, the biological system based on which oxidative stress parameters were assessed, redox biomarkers, the method used, and the outcome of the comparison. Statistical significance was determined by the original studies.
BCC patients were detected in ten studies, of which three reported enzymatic mechanisms, five included oxidative stress byproducts, and seven reported antioxidant vitamin concentrations. The studies revealed 50 comparisons between BCC patients and other groups, such as healthy individuals, patients with another type of NMSC, patients with medical history of NMSC excision, or patients with AK. Comparison of BCC patients with patients with AK or SCC is indicative of a comparison with sun-exposed patients.Table 1, Table 2, Table 3, Table 4 and Table 5 detail studies on NMSC patients, Table 6, Table 7 and Table 8 on melanoma patients, Table 9 on benign lesion patients, and Table 10 and Table 11 on wart patients.

Table 1.
Comparisons of enzymatic antioxidants (catalase, glutathione peroxidase (GPx), superoxide dismutase (SOD), and NAD(P)H:quinone oxidoreductase 1 (NQO1)) detected in BCC patients with a comparison study group. The method used is described in each case. The results refer only to statistically significant differences found by the authors of the respective studies.
Table 1.
Comparisons of enzymatic antioxidants (catalase, glutathione peroxidase (GPx), superoxide dismutase (SOD), and NAD(P)H:quinone oxidoreductase 1 (NQO1)) detected in BCC patients with a comparison study group. The method used is described in each case. The results refer only to statistically significant differences found by the authors of the respective studies.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [5] | BCC vs. control | Erythrocytes | [35] | Catalase (U/mg Hb) | No significant difference detected |
| [5] | BCC vs. AK | Erythrocytes | [35] | Catalase (U/mg Hb) | No significant difference detected |
| [5] | BCC vs. SCC | Erythrocytes | [35] | Catalase (U/mg Hb) | No significant difference detected |
| [36] | BCC vs. control | Plasma | Kit protocol from Cayman Chemical (Ann Arbor, MI, USA) | Catalase activity (unit/mg protein) | Lower in BCC patients than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | Kit protocol from Cayman Chemical (Ann Arbor, MI) | Catalase activity (unit/mg protein) | Lower in BCC patients than NMSC-excised patients |
| [36] | BCC vs. control | Plasma | [37] | GPx (unit/mg protein) | Lower in BCC patients than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | [37] | GPx (unit/mg protein) | Lower in BCC patients than NMSC-excised patients |
| [36] | BCC vs. control | Plasma | [38] and kit protocol from Cayman Chemical (Ann Arbor, MI) | SOD (unit/mg protein) | Higher in BCC than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | [38] and kit protocol from Cayman Chemical (Ann Arbor, MI) | SOD (unit/mg protein) | No significant difference detected |
| [36] | BCC vs. control | Plasma | [39] | NQO1 (µmol 2,6-dichloroindophenol reduced/min/mg protein) | Lower in BCC patients than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | [39] | NQO1 (µmol 2,6-dichloroindophenol reduced/min/mg protein) | Lower in BCC patients than NMSC-excised patients |
Interestingly, three studies [5,36,40] included eleven comparisons concerning antioxidant enzymes, four of which showed no statistically significant differences (Table 1). Worth mentioning is NAD(P)H:quinone oxidoreductase 1 (NQO1), which is a crucial cellular defense enzyme against oxidative stress.

Table 2.
Comparisons of non-enzymatic antioxidants, including metabolic antioxidants and dietary micronutrients, detected in BCC patients compared with a study group.
Table 2.
Comparisons of non-enzymatic antioxidants, including metabolic antioxidants and dietary micronutrients, detected in BCC patients compared with a study group.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [5] | BCC vs. control | Erythrocytes | [35] | GSH (μmol/g Hb) | Lower in BCC patients than control |
| [5] | BCC vs. AK | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [5] | BCC vs. SCC | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [36] | BCC vs. control | Plasma | DTNB enzymatic recycling method following kit protocol from Sigma-Aldrich (St. Louis, MO, USA) | GSH (µmol/mg protein) | Higher in BCC than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | DTNB enzymatic recycling method following kit protocol from Sigma-Aldrich (MO, USA) | GSH (µmol/mg protein) | No significant difference detected |
| [40] | BCC vs. control | Erythrocytes | [41] | GSH (mg/dL) | Lower in BCC patients compared to control |
| [40] | BCC vs. AK | Erythrocytes | [41] | GSH (mg/dL) | Lower in BCC patients compared to AK |
| [5] | BCC vs. control | Plasma | [42] | TAC (mmol DPPH/L) | Lower in BCC patients than control |
| [5] | BCC vs. AK | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [5] | BCC vs. SCC | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [40] | BCC vs. control | Plasma | [43] | Ascorbic acid (mg/dL) | Lower in BCC patients compared to control |
| [40] | BCC vs. AK | Plasma | [43] | Ascorbic acid (mg/dL) | No significant difference detected |
| [40] | BCC vs. control | Plasma | [44] | a-tocopherol (mg/L) | Lower in BCC patients compared to control |
| [40] | BCC vs. AK | Plasma | [44] | a-tocopherol (mg/L) | Lower in BCC patients compared to AK |
| [45] | NMSC (BCC included) | Serum | [46] | Carotenoids (μmol/L) | No significant difference detected |
| [45] | NMSC (BCC included) | Serum | [46] | Selenium (μmol/L) | Lower in patients with NMSC |
| [45] | NMSC (BCC included) | Serum | [47] | a-tocopherol (μmol/L) | No significant difference detected |
| [48] | BCC vs. controls | Serum | [46] | Carotenoids (μmol/L) | No significant difference detected |
| [48] | BCC vs. controls | Serum | [46] | a-tocopherol (μmol/L) | No significant difference detected |
| [49] | BCC vs. controls | Serum | HPLC analysis (described in [50]) | a-tocopherol (μg/mL) | No significant difference detected |
| [49] | BCC vs. controls | Serum | HPLC analysis (described in [50]) | Retinol (μg/mL) | Lower in BCC patients than control |
| [51] | BCC vs. controls | Serum | [52] | Selenium (μg/dL) | No significant difference detected |
| [51] | BCC vs. controls | Serum | [53] | b-carotenoid (μg/dL) | No significant difference detected |
| [51] | BCC vs. controls | Serum | [53] | a-tocopherol (mg/dl) | No significant difference detected |
| [51] | BCC vs. controls | Serum | [53] | Retinol (μg/dL) | Higher in BCC patients compared to control |
| [54] | BCC vs. controls | Serum | Atomic absorption spectroscopy (AAS) | Zinc (μg/dL) | Lower in BCC than control |
| [40] | BCC vs. control | Plasma | [55] | Total thiol groups (mmol/L) | Lower in BCC patients compared to control |
| [40] | BCC vs. AK | Plasma | [55] | Total thiol groups (mmol/L) | No significant difference detected |
The micronutrient concentrations under comparison included ascorbic acid, selenium, carotenoids, vitamin E (a-tocopherol), vitamin A (retinol), and zinc. Ten of these indicated no statistical significance (Table 2). TAC assay is also included, as it refers to the cumulative action of several antioxidant components [35]. Other molecules with antioxidant capacities detected were glutathione (GSH) and total thiol groups, as plasma total sulfhydryl groups have also been suggested to contribute significantly to the antioxidant capacity of plasma [55]. Different results were observed concerning the same micronutrient marker, such as serum a-tocopherol in comparisons of BCC patients vs. controls. In total, no significant differences were found in 15 out of 28 comparisons.

Table 3.
Comparisons of markers of oxidative damage to proteins, lipids, or DNA detected in BCC patients compared with a study group.
Table 3.
Comparisons of markers of oxidative damage to proteins, lipids, or DNA detected in BCC patients compared with a study group.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [5] | BCC vs. control | Plasma | [56] | TBARS (μmol/L) | Higher in BCC patients than control |
| [5] | BCC vs. control | Plasma | [57] | CARBS (nmol/mg protein) | No significant difference detected |
| [5] | BCC vs. AK | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | BCC vs. AK | Plasma | [57] | CARBS (nmol/mg protein) | No significant difference detected |
| [5] | BCC vs. SCC | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | BCC vs. SCC | Plasma | [57] | CARBS (nmol/mg protein) | Higher in SCC patients than BCC |
| [36] | BCC vs. controls | Urine | Competitive enzyme immunoassay (STA-320, Cell Biolabs, San Diego, CA, USA) | 8-oxo-dGuo levels (ng/mg creatinine) | Higher in BCC patients than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Urine | Competitive enzyme immunoassay (STA-320, Cell Biolabs, San Diego, CA, USA) | 8-oxo-dGuo levels (ng/mg creatinine) | No significant difference detected |
| [58] | NMSC (BCC and SCC) vs. controls | Peripheral blood | [59] | H2O2-induced DNA damage (mean tail length after H2O2)—(basal mean tail length) | H2O2-induced DNA damage was significantly higher in NMSC (BCC and SCC) than in control |
| [54] | BCC vs. controls | Serum | Colorimetric assay, protocol kit by Sigma-Aldrich Company, catalog number MAK085 | MDA (nmol/mL) | Higher in BCC than control |
The impact of oxidative stress on DNA, lipids, and proteins in BCC patients was observed in four studies including 10 comparisons. Lipid byproducts in the studies were assessed in terms of TBARS and MDA and included four comparisons. DNA byproducts were expressed in urine 8-oxo-dGuo levels [36] and H2O2-induced DNA [58] damage, while protein oxidation was defined by CARBS (protein carbonyls) [5] (Table 3). No significant difference was detected in four out of ten comparisons.

Table 4.
Comparisons of enzymatic and non-enzymatic antioxidants as well as oxidative damage products present in SCC patients compared with a study group.
Table 4.
Comparisons of enzymatic and non-enzymatic antioxidants as well as oxidative damage products present in SCC patients compared with a study group.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [5] | SCC vs. AK | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [5] | SCC vs. AK | Erythrocytes | [35] | Catalase activity (U/mg Hb) | Lower in SCC patients than AK patients |
| [5] | SCC vs. AK | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [5] | SCC vs. AK | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | SCC vs. AK | Plasma | [57] | CARBS (nmol/mg protein) | Higher in SCC patients than AK patients |
| [5] | BCC vs. SCC | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [5] | BCC vs. SCC | Erythrocytes | [35] | Catalase activity (U/mg Hb) | No significant difference detected |
| [5] | BCC vs. SCC | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [5] | BCC vs. SCC | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | BCC vs. SCC | Plasma | [57] | CARBS (nmol/mg protein) | Higher in SCC patients than BCC |
| [5] | SCC vs. control | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [5] | SCC vs. control | Erythrocytes | [35] | Catalase (U/mg Hb) | Lower in SCC patients than control |
| [5] | SCC vs. control | Plasma | [42] | TAC (mmol DPPH/L) | Lower in SCC patients than control |
| [5] | SCC vs. control | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | SCC vs. control | Plasma | [57] | CARBS (nmol/mg protein) | Higher in SCC patients than control |
| [58] | NMSC (BCC and SCC) vs. controls | Peripheral blood | [59] | H2O2-induced DNA damage (mean tail length after H2O2)—(basal mean tail length) | H2O2-induced DNA damage was significantly higher in NMSC (BCC and SCC) than in controls |
| [45] | NMSC (SCC included) | Serum | [46] | Carotenoids (μmol/L) | No significant difference detected |
| [45] | NMSC (SCC included) | Serum | [47] | Selenium (μmol/L) | Lower in patients with NMSC |
| [45] | NMSC (SCC included) | Serum | [46] | a-tocopherol (μmol/L) | No significant difference detected |
| [48] | SCC vs. controls | Serum | [46] | Carotenoids (μmol/L) | No significant difference detected |
| [48] | SCC vs. controls | Serum | [46] | a-tocopherol (μmol/L) | No significant difference detected |
| [51] | SCC vs. controls | Serum | [53] | Retinol (μg/dL) | No significant difference detected |
| [51] | SCC vs. controls | Serum | [53] | b-carotenoid (μg/dL) | No significant difference detected |
| [51] | SCC vs. controls | Serum | [53] | a-tocopherol (mg/dL) | No significant difference detected |
| [51] | SCC vs. controls | Serum | [52] | Selenium (μg/dL) | No significant difference detected |
| [60] | SCC vs. controls | Plasma | [61] | b-carotene (ng/mL) | No significant difference detected |
| [60] | SCC vs. controls | Plasma | [62] | a-tocopherol (μg/mL) | No significant difference detected |
| [60] | SCC vs. controls | Plasma | [62] | Retinol (ng/mL) | No significant difference detected |
| [60] | SCC vs. controls | Plasma | [63] | Selenium (ppm) | No significant difference detected |
SCC patients were examined in six studies [5,45,48,51,58,60] totaling 30 comparisons. SCC vs. controls were the most studied groups, featured in 14 comparisons [5]. Other comparisons involved SCC vs. BCC, SCC vs. AK, and SCC vs. medical history of NMSC. Among those comparisons, six reported enzymatic antioxidant mechanisms, seven discussed oxidative damage (oxidized products of lipids, proteins, and DNA), and seventeen evaluated antioxidant micronutrients. The oxidative biomarkers calculated were similar to those reported in BCC patients. Specifically, 20 of them showed no statistically significant differences; of these, most focused on micronutrients (Table 4) [47,49,59].

Table 5.
Comparisons of enzymatic and non-enzymatic antioxidants as well as oxidative damage products present in patients with medical history of NMSC.
Table 5.
Comparisons of enzymatic and non-enzymatic antioxidants as well as oxidative damage products present in patients with medical history of NMSC.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [64] | Medical history of NMSC (BCC and SCC) vs. control | Plasma | [65] | TBARS (nmol/L) | No significant difference detected |
| [64] | Medical history of NMSC (BCC and SCC) vs. control | Plasma | Enzyme-linked immunosorbent assay-(Isoprostane Express EIA Kit; Cayman, USA) | 15-F2t-isoprostane levels (pg/mL) | Higher in NMSC-excised patients compared to control |
| [64] | Medical history of NMSC (BCC and SCC) vs. control | Plasma | [66] | Nitrate (mmol/L × 10−1) | No significant difference detected |
| [64] | Medical history of NMSC (BCC and SCC) vs. control | Plasma | Antioxidant Assay Kit protocol from Cayman, USA). | TAC (mmol × 10−2) | No significant difference detected |
| [36] | Medical history of NMSC (BCC) vs. control | Urine | Competitive enzyme immunoassay (STA-320, Cell Biolabs, San Diego, CA, USA) | 8-oxo-dGuo levels (ng/mg creatinine) | Higher in NMSC-excised patients than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Urine | Competitive enzyme immunoassay (STA-320, Cell Biolabs, San Diego, CA, USA) | 8-oxo-dGuo levels (ng/mg creatinine) | No significant difference detected |
| [36] | Medical history of NMSC (BCC) vs. control | Plasma | Kit protocol from Cayman Chemical (Ann Arbor, MI, USA) | Catalase Activity (unit/mg protein) | No significant difference detected |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | Kit protocol from Cayman Chemical (Ann Arbor, MI, USA) | Catalase Activity (unit/mg protein) | Lower in BCC patients than NMSC-excised patients |
| [36] | Medical history of NMSC (BCC) vs. control | Plasma | [37] | GPx (unit/mg protein) | No significant difference detected |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | [37] | GPx (unit/mg protein) | Lower in BCC patients than NMSC-excised patients |
| [36] | Medical history of NMSC (BCC) vs. control | Plasma | [39] | NQO1 (µmol 2,6-dichloroindophenol reduced/min/mg protein) | No significant difference detected |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | [39] | NQO1 (µmol 2,6-dichloroindophenol reduced/min/mg protein) | Lower in BCC patients than NMSC-excised patients |
| [36] | Medical history of NMSC (BCC) vs. control | Plasma | DTNB enzymatic recycling method following kit protocol from Sigma-Aldrich (St louis, MO, USA) | GSH (µmol/mg protein) | Higher in NMSC-excised than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | DTNB enzymatic recycling method following kit protocol from Sigma-Aldrich (St louis, MO, USA) | GSH (µmol/mg protein) | No significant difference detected |
| [36] | Medical history of NMSC (BCC) vs. control | Plasma | [38] and kit protocol from Cayman Chemical (Ann Arbor, MI, USA) | SOD (unit/mg protein) | Higher in NMSC-excised than control |
| [36] | BCC vs. medical history of NMSC (BCC) | Plasma | [38] and kit protocol from Cayman Chemical (Ann Arbor, MI, USA) | SOD (unit/mg protein) | No significant difference detected |
| [67] | Medical history of NMSC (BCC and SCC) vs. control | Plasma | Protocol by Antioxidant Assay Kit (Cayman, USA). | TAC (nmol/L) | No significant difference detected |
| [68] | Medical history of NMSC (BCC) vs. control | Serum | [53] | Carotenoids | No significant difference detected |
| [68] | Medical history of NMSC (BCC) vs. control | Serum | [53] | a-tocopherol | No significant difference detected |
| [68] | Medical history of NMSC (BCC) vs. control | Serum | [52] | Selenium | No significant difference detected |
Patients with medical history of NMSC were also included in our review, as their exposure to extensive sunlight can lead to skin cancer development. However, they do not present with oxidative stress produced by cancer cells, since in these patients, the tumors are excised or treated with destructive methods. Regarding the outcomes, twenty-one comparisons were detected, of which nine focused on antioxidant enzymes, five on oxidized byproducts, and seven on antioxidant molecules (GSH and micronutrients) (Table 5). Interestingly, 13 of the 21 showed no differences, including 10 NMSC history–control comparisons, revealing that systemic oxidative stress parameters tend to resemble those of controls after skin cancer removal.
Concerning NMSC (BCC and SCC patients), most studies relied on their comparison with healthy controls, while only two of the studies compared NMSC with actinic keratosis patients [5,40]. Considering that those two groups have received the same external stressor, UV, the comparison of oxidative stress in these patients can be regarded as more trustworthy if the impact of oxidative stress on skin carcinogenesis is in question (see Table 1, Table 2 and Table 9). In view of the comparison of BCC vs. AK [5,40], one study indicated lower erythrocyte GSH levels in BCC patients, while another did not detect any differences regarding this biomarker. However, no changes were observed in any of the other examined parameters related to enzymatic mechanisms (catalase activity, etc.) or micronutrients (ascorbic acid, etc.). In the case of comparisons of BCC and controls, previous studies have examined eleven oxidative stress parameters in plasma, ten parameters in serum, and three in RBCs, compared to controls. Worth mentioning is that BCC patients presented significant alterations in redox biomarkers in plasma (10/11, 90.9%), whereas there were few differences in serum (4/10, 40%) and RBCs (2/3, 66%). This difference may be attributed to the fact that most studies on the serum of BCC patients were focused on micronutrients.
Regarding antioxidant enzyme activities in NMSC patients, the results seem scattered. Also, studies assessing postoperative oxidative stress modifications reveal a stress reduction that depends on the time of assessment as well as the therapeutic procedure, as chemotherapy is connected with a period of oxidative stress. Moreover, when assessing the differences between patients with BCC and patients with medical history of NMSC, the former displayed lower antioxidant enzyme activity regarding catalase, GPX, and NQO1, and no difference in SOD activity [Table 5].
In the case of comparisons between SCC patients and controls, seven oxidative stress markers were evaluated in plasma, six in serum, and two in RBCs. Interestingly, no serum antioxidant marker indicated any significant differences when compared to healthy individuals (Table 4).

Table 6.
Comparisons of enzymatic antioxidants retrieved from melanoma patients compared with a study group.
Table 6.
Comparisons of enzymatic antioxidants retrieved from melanoma patients compared with a study group.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [69] | Melanoma patients vs. control | Serum | [70] | SOD (total superoxide dismutase activity) (U/mL) | Higher in melanoma (especially stage III) patients compared to control |
| [69] | Melanoma patients vs. control | Serum | [70] | Mn-SOD (U/mL) | Higher in melanoma (especially stage IV) patients compared to control |
| [69] | Melanoma patients vs. control | Serum | [71] | CAT (kU/L) | Higher in melanoma (especially stages I, II, and III) patients compared to control |
| [72] | Melanoma patients vs. control | Serum | [73] | Mn-SOD (ng/mL) | Higher in melanoma (all stages) patients compared to control |
| [74] | Melanoma patients vs. control | Erythrocytes | [75] | SOD (U/g Hb) | No significant difference detected |
| [74] | Melanoma patients vs. control | Erythrocytes | [71] | CAT (absorption/min/g Hb × 103) | No significant difference detected |
| [76] | Melanoma patients vs. control | Erythrocytes | [70] | SOD (U/g Hb) | Lower in melanoma patients compared to control |
| [76] | Melanoma patients vs. patients with excised melanoma | Erythrocytes | [70] | SOD (U/g Hb) | No significant difference detected |
| [76] | Melanoma patients vs. control | Erythrocytes | [77] | CAT (U/g Hb) | Higher in melanoma patients compared to control |
| [76] | Melanoma patients vs. patients with excised melanoma | Erythrocytes | [77] | CAT (U/g Hb) | No significant difference detected |
| [78] | Melanoma patients vs. control | Erythrocytes | [75] | CAT ((V abs/min) Hb−1) | Lower in melanoma patients compared to control |
| [78] | Melanoma patients vs. melanoma patients with metastasis | Erythrocytes | [75] | CAT ((V abs/min) Hb−1) | No significant difference detected |
| [78] | Melanoma patients with metastasis vs. controls | Erythrocytes | [75] | CAT ((V abs/min) Hb−1) | No significant difference detected |
| [78] | Melanoma patients vs. control | Erythrocytes | [75] | SOD (U/g Hb) | No significant difference detected |
| [78] | Melanoma patients vs. melanoma patients with metastasis | Erythrocytes | [75] | SOD (U/g Hb) | No significant difference detected |
| [78] | Melanoma patients with metastasis vs. control | Erythrocytes | [75] | SOD (U/g Hb) | No significant difference detected |
Melanoma patients were the subjects of sixteen comparisons, including with healthy individuals, between patients at different stages of the disease, and with patients with metastasis. Notably, oxidative stress parameters were influenced by the different stages of the disease. For example, serum total superoxide dismutase activity was higher in melanoma stage III patients compared to controls [69]. However, nine of those comparisons revealed no differences (Table 6).

Table 7.
Comparisons of non-enzymatic antioxidants, including metabolic antioxidants and dietary micronutrients, detected in melanoma patients.
Table 7.
Comparisons of non-enzymatic antioxidants, including metabolic antioxidants and dietary micronutrients, detected in melanoma patients.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [74] | Melanoma patients vs. control | Erythrocytes | [79] | GSH (μM/g Hb−1) | Lower in melanoma patients compared to control |
| [72] | Melanoma patients vs. control | Erythrocytes | [79] | GSH (μΜ/g Hb) | Lower in melanoma patients compared to control |
| [72] | Melanoma patients vs. melanoma patients with metastasis | Erythrocytes | [79] | GSH (μΜ/g Hb) | No significant difference detected |
| [72] | Melanoma patients with metastasis vs. control | Erythrocytes | [79] | GSH (μΜ/g Hb) | No significant difference detected |
| [69] | Melanoma patients vs. control | Serum | [80] | Superoxide anion radical (mmol red nitroblue-tetrazolium/min/L) | Higher in all clinical stage melanoma patients compared to control |
| [81] | Melanoma patients vs. patients with excised melanoma | Serum | [82] | Albumin thiols (μmol/100 mL) | No significant difference detected |
| [74] | Melanoma patients vs. control | Plasma | [83] | Total thiols (μΜ) | Higher total thiols in melanoma patients compared to control |
| [79] | Melanoma patients vs. control | Plasma | [83] | Total thiols (μΜ) | No significant difference detected |
| [78] | Melanoma patients vs. melanoma patients with metastasis | Plasma | [83] | Total thiols (μΜ) | No significant difference detected |
| [78] | Melanoma patients with metastasis vs. control | Plasma | [83] | Total thiols (μΜ) | Higher in patients with melanoma metastasis compared to control |
| [78] | Melanoma patients vs. control | Plasma | [84] | TRAP (total radical-trapping antioxidant parameter) (μΜ Trolox) | No significant difference detected |
| [78] | Melanoma patients vs. melanoma patients with metastasis | Plasma | [84] | TRAP (total radical-trapping antioxidant parameter) (μΜ Trolox) | No significant difference detected |
| [78] | Melanoma patients with metastasis vs. control | Plasma | [84] | TRAP (total radical-trapping antioxidant parameter) (μΜ Trolox) | Higher in patients with melanoma metastasis compared to control |
| [81] | Melanoma patients vs. patients with excised melanoma | Serum | [85,86] | Serum antioxidants (μg/L) | No significant difference detected |
| [74] | Melanoma patients vs. control | Plasma | [87] | TRAP (total radical-trapping antioxidant parameter) (μΜ Trolox) | No significant difference detected |
| [88] | Melanoma patients | Serum | Mass spectrometry (ICP-MS NexION 350D, Perkin Elmer) | Selenium (µg/L) | A low selenium level might contribute to worse survival for patients with melanoma |
| [89] | Melanoma patients vs. control | Serum | Spectrometry | Selenium (μg/L) | All clinical melanoma stages (especially stage III) had lower selenium levels than the controls |
| [90] | Melanoma patients | Serum | Spectrometry | Selenium (μg/L) | Lower selenium correlates with worse disease severity |
| [90] | Melanoma patients | Serum | Spectrometry | Selenium (μg/L) | Selenium concentration was significantly lower for stage I and II melanomas with recurrence compared to those without recurrence |
| [51] | Melanoma patients vs. control | Serum | [53] | Retinol (μg/dL) | No significant difference detected |
| [51] | Melanoma patients vs. control | Serum | [53] | b-carotenoid (μg/dL) | No significant difference detected |
| [51] | Melanoma patients vs. control | Serum | [53] | a-tocopherol (mg/dl) | No significant difference detected |
| [51] | Melanoma patients vs. controls | Serum | [52] | Selenium (μg/dL) | No significant difference detected |
| [91] | Melanoma patients vs. control | Serum | Atomic absorption spectroscopy | Zinc (μg/100 mL) | No significant difference detected |
| [91] | Melanoma patients with metastasis vs. patients | Serum | Atomic absorption spectroscopy | Zinc (μg/100 mL) | No significant difference detected |
| [92] | Melanoma patients vs. control | Serum | [93] | Zinc (μg/100 mL) | Lower in melanoma patients compared to control |
| [94] | Melanoma patients vs. control | Serum | Atomic absorption spectroscopy | Zinc (μg/dL) | Higher in melanoma patients compared to control |

Table 8.
Comparisons of oxidative damage products present in melanoma patients compared to a study group.
Table 8.
Comparisons of oxidative damage products present in melanoma patients compared to a study group.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [69] | Melanoma patients vs. control | Serum | [95] | mmol MDA/L | Higher in melanoma (especially stage IV) patients compared to control |
| [81] | Melanoma patients vs. patients with excised melanoma | Serum | [96] | Serum lipid peroxides (μmol/100 mL) | No significant difference detected |
| [74] | Melanoma patients vs. control | Plasma | [87] | MDA (nM) | Higher in melanoma patients compared to control |
| [76] | Melanoma patients vs. control | Plasma | [97] | MDA (μΜ) | Higher in melanoma patients compared to control |
| [76] | Melanoma patients vs. patients with excised melanoma | Plasma | [97] | MDA (μΜ) | Higher in melanoma patients compared to patients with melanoma history |
| [76] | Patients with excised melanoma vs. control | Plasma | [97] | MDA (μΜ) | No significant difference detected |
| [78] | Melanoma patients vs. control | Plasma | [98] | MDA (nM) | Higher in melanoma patients compared to control |
| [78] | Melanoma patients vs. melanoma patients with metastasis | Plasma | [98] | MDA (nM) | No significant difference detected |
| [78] | Melanoma patients with metastasis vs. control | Plasma | [98] | MDA (nM) | Higher in patients with melanoma history compared to control |
| [78] | Melanoma patients vs. control | Plasma | [99] | AOPPs (advanced oxidation protein products) (μΜ × mg protein) | No significant difference detected |
| [78] | Melanoma patients vs. melanoma patients with metastasis | Plasma | [99] | AOPPs (advanced oxidation protein products) (μΜ × mg protein) | No significant difference detected |
| [78] | Melanoma patients with metastasis vs. control | Plasma | [99] | AOPPs (advanced oxidation protein products) (μΜ × mg protein) | Higher in patients with melanoma metastasis compared to control |
The previous tables present comparisons of oxidative stress markers (oxidized protein and lipid products and vitamins). Superoxide anion radicals were considered an index of antioxidant defense, since their evaluation is based on a nitroblue–tetrazolium reduction [69]. Another significant antioxidant marker was the total radical-trapping antioxidant parameter (TRAP), based on the cumulative action of individual serum antioxidants such as uric acid, protein thiols, ascorbate, α-tocopherol, and bilirubin [78]. Interestingly, cancer stage and history of previous melanoma played a crucial role in the outcome of oxidative stress comparisons. No association was found in five of the twelve comparisons of oxidative damage markers, in ten of the seventeen comparisons of micronutrients, and in fifteen out of the twenty-eight comparisons of antioxidant molecules examined (Table 7 and Table 8).
Considering benign lesions, in our review, we included patients with AK and SK lesions. We found 25 comparisons of AK patients. By extending our research to SK, we also found two comparisons that reported no significant differences.

Table 9.
Comparisons of oxidative stress parameters present in patients with benign lesions (AK and SK).
Table 9.
Comparisons of oxidative stress parameters present in patients with benign lesions (AK and SK).
| Study | Patients | Tested | Method | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [5] | AK vs. control | Erythrocytes | [35] | GSH (μmol/g Hb) | Lower in AK patients than control |
| [5] | AK vs. control | Erythrocytes | [35] | Catalase (U/mg Hb) | No significant difference detected |
| [5] | AK vs. control | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [5] | AK vs. control | Plasma | [56] | TBARS (μmol/L) | Higher in AK patients than control |
| [5] | AK vs. control | Plasma | [57] | CARBS (nmol/mg protein) | No significant difference detected |
| [5] | SCC vs. AK | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [5] | SCC vs. AK | Erythrocytes | [35] | Catalase activity (U/mg Hb) | Lower in SCC patients than AK patients |
| [5] | SCC vs. AK | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [5] | SCC vs. AK | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | SCC vs. AK | Plasma | [57] | CARBS (nmol/mg protein) | Higher in SCC patients than AK patients |
| [5] | BCC vs. AK | Erythrocytes | [35] | GSH (μmol/g Hb) | No significant difference detected |
| [5] | BCC vs. AK | Erythrocytes | [35] | Catalase (U/mg Hb) | No significant difference detected |
| [5] | BCC vs. AK | Plasma | [42] | TAC (mmol DPPH/L) | No significant difference detected |
| [5] | BCC vs. AK | Plasma | [56] | TBARS (μmol/L) | No significant difference detected |
| [5] | BCC vs. AK | Plasma | [57] | CARBS (nmol/mg protein) | No significant difference detected |
| [40] | BCC vs. AK | Plasma | [43] | Ascorbic acid (mg/dL) | No significant difference detected |
| [40] | BCC vs. AK | Plasma | [43] | a-tocopherol (mg/L) | Lower in BCC patients compared to AK |
| [40] | BCC vs. AK | Plasma | [57] | Total thiol groups (mmol/L) | No significant difference detected |
| [40] | BCC vs. AK | Erythrocytes | [41] | GSH (mg/dl) | Lower in BCC patients compared to AK |
| [40] | AK vs. control | Plasma | [49] | a-tocopherol (mg/L) | Lower in AK patients compared to control |
| [40] | AK vs. control | Plasma | [57] | Total thiol groups (mmol/L) | Lower in AK patients compared to control |
| [40] | AK vs. control | Plasma | [43] | Ascorbic acid (mg/dL) | Lower in AK patients compared to control |
| [40] | AK vs. control | Erythrocytes | [41] | GSH (mg/dL) | Lower in AK patients compared to control |
| [99] | SK vs. control | Plasma | TBARS, method not explained | MDA (mmol/L) | No significant difference detected |
| [99] | SK vs. control | Plasma | ELISA[100] | SOD (U/L) | No significant difference detected |
The following tables refer to the redox status of patients with common and recalcitrant warts (Table 10 and Table 11).

Table 10.
Comparisons of enzymatic and non-enzymatic antioxidants, as well as oxidative damage products, detected in patients with warts, including the type of wart (genital, etc.) and the number of lesions. The above-mentioned wart types are not recalcitrant (>36 months).
Table 10.
Comparisons of enzymatic and non-enzymatic antioxidants, as well as oxidative damage products, detected in patients with warts, including the type of wart (genital, etc.) and the number of lesions. The above-mentioned wart types are not recalcitrant (>36 months).
| Study | Patients | Tested | Number/Chronicity of the Lesions | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|---|
| [101] | Patients with non-genital warts vs. control | Serum | NM/Most of the lesions occurred over 1 year (19.6 ± 3.8 months) | [102] | Disulfide (μm/L) | Higher in wart patients compared to control |
| [101] | Patients with non-genital warts vs. control | Serum | NM/Most of the lesions occurred over 1 year (19.6 ± 3.8 months) | [102] | Total serum thiol (μm/L) | Higher in wart patients compared to control |
| [101] | Patients with non-genital warts vs. control | Serum | NM/Most of the lesions occurred over 1 year (19.6 ± 3.8 months) | [102] | Disulfide/native thiol ratio | Higher in wart patients compared to control |
| [101] | Patients with non-genital warts vs. control | Serum | NM/Most of the lesions occurred over 1 year (19.6 ± 3.8 months) | [102] | Native thiol (µm/L) | No significant difference detected |
| [101] | Patients with non-genital warts vs. control | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | [102] | Disulfide/total thiol | No significant difference detected |
| [101] | Patients with non-genital warts vs. control | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | [102] | Native thiol/total thiol | No significant difference detected |
| [103] | Patients with genital and non-genital warts vs. controls | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | Enzyme-linked immunosorbent assay kit (Human CoQ10-ELISA kit/Shanghai Sunred Biological Technology Co, Ltd., Shanghai, China) | Coenzyme Q10 levels (ng/mL) | No significant difference detected |
| [103] | Patients with genital and non-genital warts vs. controls | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | Double heating method of Draper and Hadley [103] | MDA (µmol/L) | Higher in wart patients compared to control |
| [103] | Patients with genital and non-genital warts vs. controls | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | Perkin Elmer AAnalyst 800 atomic absorption spectrometer (USA) with a deuterium background correction [104] | Zinc (µg/dL) | Lower in wart patients compared to control |
| [103] | Patients with genital vs. patients with non-genital warts | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | Enzyme-linked immunosorbent assay kit (Human CoQ10-ELISA kit/Shanghai Sunred Biological Technology Co, Ltd., Shanghai, China) | Coenzyme Q10 levels (ng/mL) | No significant difference detected |
| [103] | Patients with genital vs. patients with non-genital warts | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | Double heating method of Draper and Hadley [103] | MDA (µmol/L) | No significant difference detected |
| [103] | Patients with genital vs. patients with non-genital warts | Serum | Genital (10 lesions) Non-genital (4 lesions)/Most of the lesions occurred over 1 year (19.35 ± 28.82 months) | Perkin Elmer AAnalyst 800 atomic absorption spectrometer (USA) with a deuterium background correction [105] | Zinc (µg/dL) | No significant difference detected |
| [106] | Patients with non-genital warts vs. controls | Serum | From <5 to >10 lesions/Lesions occurred from <1 to >6 months | Spectrophotometric method (Randox reagents, HumaStar 300 analyzer) | Total oxidant status (µmol Trolox Eq/L) | Higher in wart patients compared to control |
| [106] | Patients with non-genital warts vs. controls | Serum | From <5 to >10 lesions/Lesions occurred from <1 to >6 months | Spectrophotometric method (Randox reagents, HumaStar 300 analyzer) | Total antioxidant status (µmol H2O2 Eq/L) | Lower in wart patients compared to control |
| [106] | Patients with non-genital warts vs. controls | Serum | From <5 to >10 lesions/Lesions occurred from <1 to >6 months | Spectrophotometric method (Randox reagents, HumaStar 300 analyzer) | Oxidative stress index (arbitrary units) | Higher in wart patients compared to control |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non- recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | Enzyme-linked immunosorbent assay (ELISA) kit (Cayman, Canada, USA). | 8-hydroxy-2-deoxyguanosine (ng/mL) | No significant difference detected |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [107] | Total oxidant status (µmol Trolox Eq/L) | No significant difference detected |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [108] | Total antioxidant status (µmol H2O2 Eq/L) | No significant difference detected |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [109] | Oxidative stress index (arbitrary units) | No significant difference detected |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [102] | Total thiol (μmol/L) | Higher in wart patients compared to controls |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [102] | Native thiol (μmol/L) | Higher in wart patients compared to controls |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [102] | Disulphide (μmol/L) | Higher in wart patients compared to control |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [102] | Native thiol/total thiol | Higher in wart patients compared to control |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | Non-recalcitrant warts (mean number of 5.5 lesions)/(Mean duration of 4.5 months) | [102] | Disulphide/total thiol | Lower in wart patients compared to control |
| [107] | Patients with genital or non-genital warts vs. controls | Serum | NM/Most of the warts lasted less than 1 year | [102] | Disulphide/native thiol | Lower in wart patients compared to control |
| [110] | Patients with genital warts vs. controls | Serum | NM/Most of the warts lasted less than 1 year | [111] | Paraoxonase (ng/mL) | No significant difference detected |
| [110] | Patients with genital warts vs. controls | Erythrocytes | NM/Most of the warts lasted less than 1 year | [111] | GPx (IU/gHb) | Higher in wart patients compared to control |
| [110] | Patients with genital warts vs. controls | Serum | NM/Most of the warts lasted less than 1 year | High-pressure liquid chromatography via Chromsystems (Chromsystems®, Mannheim, Germany) kits and an Agilent 1200 series autoanalyzer (Agilent Technologies®, CA, USA). | MDA (mmol/L) | Higher in wart patients compared to control |
| [110] | Patients with genital warts vs. controls | Serum | NM/Most of the warts lasted less than 1 year | [111] | CAT (kU/L) | Higher in wart patients compared to control |
| [112] | Patients with non-genital warts vs. controls | Erythrocytes | 19 patients with less than 10 lesions and 12 patients with more than 10 lesions/Most of the warts lasted less than 1 year | [40] | CAT (U/g Hb) | Higher in wart patients compared to control |
| [112] | Patients with non-genital warts vs. controls | Erythrocytes | 19 patients with less than 10 lesions and 12 patients with more than 10 lesions/Most of the warts lasted less than 1 year | [40] | G6PD (U/g Hb) | Higher in wart patients compared to control |
| [112] | Patients with non-genital warts vs. controls | Erythrocytes | 19 patients with less than 10 lesions and 12 patients with more than 10 lesions/Most of the warts lasted less than 1 year | [113] | SOD (U/g Hb) | Higher in wart patients compared to control |
| [112] | Patients with non-genital warts vs. controls | Plasma | 19 patients with less than 10 lesions and 12 patients with more than 10 lesions/Most of the warts lasted less than 1 year | [114] | MDA (nmol/mL) | Higher in wart patients compared to control |
NM: not mentioned.

Table 11.
Comparisons of oxidative stress parameters in recalcitrant (>36 months) wart patients.
Table 11.
Comparisons of oxidative stress parameters in recalcitrant (>36 months) wart patients.
| Study | Patients | Tested | Method Used | Redox Biomarker | Result |
|---|---|---|---|---|---|
| [107] | Recalcitrant wart patients vs. control | Serum | Enzyme-linked immunosorbent assay (ELISA) kit (Cayman, Canada, USA). | 8-hydroxy-2-deoxyguanosine (ng/mL) | Higher in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [107] | Total oxidant status (µmol Trolox Eq/L) | No significant difference detected |
| [107] | Recalcitrant wart patients vs. control | Serum | [108] | Total antioxidant status (µmol H2O2 Eq/L) | Higher in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [109] | Oxidative stress index (arbitrary units) | Higher in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [102] | Total thiol (μmol/L) | Higher in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [102] | Native thiol (μmol/L) | Higher in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [102] | Disulphide (μmol/L) | No significant difference detected |
| [107] | Recalcitrant wart patients vs. control | Serum | [102] | Native thiol/total thiol | Higher in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [102] | Disulphide/total thiol | Lower in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. control | Serum | [102] | Disulphide/native thiol | Lower in recalcitrant patients compared to control |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | Enzyme-linked immunosorbent assay (ELISA) kit (Cayman, Canada, USA). | 8-hydroxy-2-deoxyguanosine (ng/mL) | No significant difference detected |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [107] | Total oxidant status (µmol Trolox Eq/L) | No significant difference detected |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [108] | Total antioxidant status (µmol H2O2 Eq/L) | No significant difference detected |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [109] | Oxidative stress index (arbitrary units) | No significant difference detected |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [102] | Total thiol (μmol/L) | Lower in recalcitrant wart patients compared with wart patients |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [102] | Native thiol (μmol/L) | Lower in recalcitrant wart patients compared with wart patients |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [102] | Disulphide (μmol/L) | Lower in recalcitrant wart patients compared with wart patients |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [102] | Native thiol/total thiol | No significant difference detected |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [102] | Disulphide/total thiol | No significant difference detected |
| [107] | Recalcitrant wart patients vs. wart patients | Serum | [102] | Disulphide/native thiol | No significant difference detected |
The measurement of dynamic thiol/disulfide (T/DS) homeostasis was used as a redox status biomarker. Thiols are proteins with organic sulfur compounds possessing antioxidant properties that operate through various mechanisms and fluctuations in dynamic disulfide bonds and are likely to be associated with oxidative stress levels [107]. Moreover, the oxidative stress index was estimated from the ratio of total antioxidant status to total oxidant status [107].
Differences between wart patients were observed to mainly depend on wart location (genital and non-genital) and their recalcitrant and non-recalcitrant state. Systemic oxidative stress parameters of recalcitrant wart patients were mainly evaluated in [107] (Table 11). Regarding the association of the number of warts with oxidative stress markers, the results are confusing, since some studies reported no association [110], while others displayed the opposite [103,107]. Regarding the comparisons of redox biomarkers, 19 differences were found, influenced mainly by the chronic nature of the disease and its recalcitrant nature. As for the comparison between recalcitrant and non-recalcitrant warts, no association was found in seven out of ten studies.
Given that, in general, the main interests of researchers lie in the comparison between control groups and skin diseases, we found 26 comparisons with BCC, 16 with SCC, 9 with AK, 30 with melanoma, 31 with warts, and 10 for recalcitrant warts, respectively. Among these comparisons, we excluded total oxidant and antioxidant status as well as the oxidation stress index as those markers belong to a specific redox biomarker category. The percentages of statistically significant and non-significant comparisons between patients and controls are indicated in Figure 1, with significant results proving that there is a difference in systemic oxidative stress parameters. Firstly, concerning antioxidant enzyme level comparisons, significant differences were found in 80% of BCC patients, 100% of SCC patients, 0% of AK patients, 70% of melanoma patients, 83.3% of non-recalcitrant wart patients, and 0% in recalcitrant wart patients when compared to controls. Secondly, when comparing non-enzymatic antioxidants between skin disease patients and controls, notable differences were observed in 62.5% of cases of BCC, 8.3% of SCC, 83.3% of AK, 46.7% of melanoma, 66.7% of non-recalcitrant warts, and 83.3% of recalcitrant warts. Finally, the respective percentages regarding differences in oxidative damage molecules are 80% for BCC, 66.7% for SCC, 50% for AK, 80% for melanoma, 50% for non-recalcitrant warts, and 100% for recalcitrant warts. Table 12 also summarizes statistically significant differences between controls and patients with malignant or benign lesions.
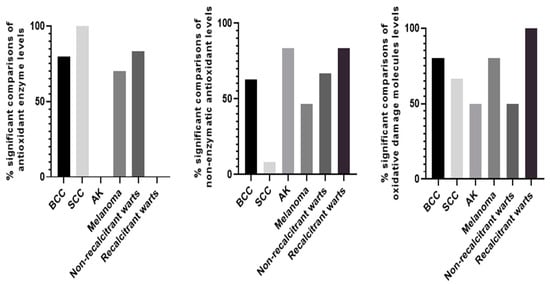
Figure 1.
Bar graphs illustrating % of statistically significant comparisons between disease and control groups in terms of antioxidant enzyme levels, non-enzymatic antioxidants, and oxidative damage molecules.

Table 12.
Summary of the results of statistically significant comparisons between controls and cases of malignant or benign lesions.
Table 12.
Summary of the results of statistically significant comparisons between controls and cases of malignant or benign lesions.
| Malignant | Benign | ||||||
|---|---|---|---|---|---|---|---|
| BCC | Redox Biomarker Reported | Reference | Results | AK | Redox Biomarker Report | Reference | Results |
| Antioxidant enzyme levels | CAT | [36] | Lower | Non-enzymatic antioxidants | GSH | [5] | Lower |
| GPx | [36] | Lower | a-tocopherol | [40] | Lower | ||
| SOD | [36] | Higher | Total thiol groups | [40] | Lower | ||
| NQO1 | [36] | Lower | Ascorbic acid | [40] | Lower | ||
| Non-enzymatic antioxidants | GSH | [5] | Lower | GSH | [40] | Lower | |
| GSH | [36] | Higher | Oxidative damage molecules | TBARS | [5] | Higher | |
| GSH | [40] | Lower | Warts (non-recalcitrant) | ||||
| TAC | [5] | Lower | Antioxidant enzyme levels | GPx | [110] | Higher | |
| Ascorbic acid | [40] | Lower | CAT | [109] | Higher | ||
| a-tocopherol | [40] | Lower | CAT | [112] | Higher | ||
| Retinol | [48] | Lower | G6PD | [112] | Higher | ||
| Retinol | [50] | Higher | SOD | [112] | Higher | ||
| Total thiol groups | [40] | Lower | Non-enzymatic antioxidants | Disulfide | [101] | Higher | |
| Oxidative damage molecules | TBARS | [5] | Higher | Total serum thiol | [101] | Higher | |
| 8-oxo-dGuo levels | [36] | Higher | Disulfide/native thiol ratio | [101] | Higher | ||
| MDA | [53] | Higher | Zinc | [103] | Lower | ||
| SCC | Total thiol | [107] | Higher | ||||
| Antioxidant enzyme levels | CAT | [5] | Lower | Native thiol | [107] | Higher | |
| Non-enzymatic antioxidants | TAC | [5] | Lower | Disulphide | [107] | Higher | |
| Oxidative damage molecules | CARBS | [5] | Higher | Disulphide/total thiol | [107] | Higher | |
| H2O2-induced DNA damage | [58] | Higher | Disulphide/native thiol | [107] | Lower | ||
| Melanoma | Oxidative damage molecules | MDA | [103] | Higher | |||
| Antioxidant enzyme levels | SOD | [69] | Higher | MDA | [110] | Higher | |
| Mn-SOD | [69] | Higher | MDA | [72] | Higher | ||
| CAT | [69] | Higher | Recalcitrant warts | ||||
| Mn-SOD | [70] | Higher | Non-enzymatic antioxidants | Total thiol | [107] | Higher | |
| SOD | [77] | Lower | Native thiol | [107] | Higher | ||
| CAT | [77] | Higher | Native thiol/total thiol | [107] | Higher | ||
| CAT | [72] | Lower | Disulphide/total thiol | [107] | Higher | ||
| Non-enzymatic antioxidants | GSH | [74] | Lower | Disulphide/native thiol | [107] | Lower | |
| GSH | [72] | Lower | Oxidative damage molecules | 8-hydroxy-2-deoxyguanosine | [107] | Higher | |
| Total thiols | [74] | Higher | |||||
| Selenium | [89] | Lower | |||||
| Zinc | [92] | Lower | |||||
| Zinc | [94] | Higher | |||||
| Oxidative damage molecules | MDA | [69] | Higher | ||||
| MDA | [74] | Higher | |||||
| MDA | [75] | Higher | |||||
| MDA | [72] | Higher |
3. Discussion
In our review, we highlighted elevated levels of oxidative stress markers in patients with skin diseases, particularly NMSC, melanoma, and wart patients as well as patients with pre-malignant lesions such as AK, compared to healthy individuals. In the case of UV-related lesions, UV radiation, particularly UVA, can penetrate deep into the dermis and has the capacity to directly influence blood and lymph vessels [5,27]. Conversely, UVB radiation induces numerous direct photochemical alterations that lead skin cells to release cytokines and other signaling molecules. However, both UVA and UVB radiation induce an excess production of ROS within skin cells, inducing oxidative stress and its impact on DNA, proteins, and lipids.
The disruption of cellular metabolism within the skin, particularly through extracellular signaling, extend its effects to other tissues. Therefore, UVR plays a major role in systemic oxidative stress [27]. As a result, individuals with diseases related to UVR exposure such as skin cancer present altered systemic oxidative stress markers compared to controls [5]. The differences in redox status between BCC and SCC patients might be attributed to different exposure types. SCC is mostly associated with cumulative lifetime sun exposure, while intermittent and intense sun exposure is more related to the risk of BCC; in terms of oxidative stress, intense sun exposure can boost oxidative stress more than frequent stimuli [115]. Also, biomarkers of systemic oxidative stress correlate with human skin lightness levels, further complicating the correlation between UVR exposure and systemic oxidative stress [116]. The incidence of skin cancer in darker skin is greater, while systemic oxidative stress, even in individuals with skin of color, can contribute to the development of skin cancer when combined with other risk factors [20,117]. On the contrary, in pre-existing oxidative stress, a deficiency of antioxidant micronutrients is associated with compromised antioxidant capacity, enhancing to the vulnerability of the skin to oxidative stimuli like UV and subsequently to skin damage [118].
Systemic oxidative stress is a complex phenomenon, mediated by endogenous and/or exogenous triggers. Examined individuals vary in their susceptibility to oxidative damage due to genetic factors or lifestyle choices. For example, in [5], patients with vitamin D deficiency and NMSC revealed higher systemic oxidative stress parameters than controls, indicating the influence of environmental factor such as UV. Moreover, the involvement of oxidative stress in several pathological processes and diseases other than UVR-related disorders complicates the evaluation of systemic oxidative stress and its association with skin cancer even more. Therefore, increased redox biomarkers might reflect the outcome of co-morbidities. Indeed, high lipid peroxidation and ROS levels were detected in diabetic nephropathy patients. Increased levels of lipid oxidation have also been observed in obese patients with obstructive sleep apnea [119,120].
The complexity of oxidative stress can be further increased by the evaluation of redox biomarkers in various biological specimens like plasma and serum, which may affect the tested activity. Generally, plasma is considered to provide a more comprehensive view of the body’s antioxidant status compared to serum, as it reflects the antioxidant levels of all blood components. Nevertheless, the differences in antioxidant properties between plasma and serum are still ambiguous. Of note, a recent study revealed that plasma samples demonstrated greater resistance to oxidative stress, while serum exhibited a stronger ABTS cation radical-scavenging effect, probably attributed to serum proteins, including albumin. However, a previous investigation showed that TBARS levels in camels could be evaluated either in plasma or in serum, with no significant difference. The visible variations may result from methodological differences in the approaches used or the different organisms’ responses [121,122].
Concerning the comparisons demonstrated in this review, BCC and SCC patients presented increased levels of oxidative damage markers, accompanied with decreased antioxidant defenses, which is evidence of redox equilibrium disturbance. As for micronutrients, except for the minerals selenium and zinc, we also focused on a-tocopherol, ascorbic acid, and carotenoids, which are commonly found in plant-based diets, and on retinol and vitamin D, which are frequently present in animal-based diets. However, findings on micronutrients seem to be less clear in the aforementioned patients, which is probably affected by the different diet plans of the tested individuals. A previous study showed that vegan and vegetarian diets might lead to inadequate intake of critical micronutrients such as iron, zinc, vitamin D and A, due to the poor bioavailability of some of these from plant-derived foods or their poor concentration within those diets [123,124].
The main difference between UVR-exposed patients and UVR-related skin cancer patients is obviously the existence of skin cancer, which increases oxidative stress [36]. Cancer cells in BCC or SCC display an aberrant redox state. More particularly, the rapid energy metabolism and proliferation that characterize tumor cells result in elevated ROS production. ROS generation is also stimulated by UV radiation, which plays a key role in its carcinogenic properties. However, cells are able to adapt to this oxidative pressure by enhancing their antioxidant defense to optimize ROS-induced proliferation, along with avoiding ROS thresholds that would activate senescence, apoptosis, or ferroptosis [78]. Interestingly, NMSC patients subjected to surgical removal improved their redox status. Chaisiriwong et al. [36] reported that elevated urinary 8-oxo-dGuo levels remained high within 1 month of BCC excision, whereas normal levels were restored after a 6-month period. Nevertheless, this pattern was not followed by 1-month postoperative total SOD activities and GSH levels [36].
Oxidative stress caused by UVR can simultaneously cause systemic oxidative stress and promote cutaneous carcinogenesis. The contribution of oxidative stress to melanoma generation is considered the result of an intricate interplay. Melanocytes irradiated by UV can generate ROS, and especially H2O2, in a dose-dependent fashion. Excessive ROS production disrupts redox homeostasis, leading to oxidative stress and subsequently to melanoma genesis. As for the progression of the disease, oxidative stress affects the metastatic ability of melanoma cells [78]. Patients with metastatic melanoma did not exhibit differences in redox biomarkers (enzyme activity) in comparison to the control group [78]. Noteworthily, oxidative stress is associated with tumor stage [69]. One study showed that after surgical removal, patients with thicker tumors maintained elevated MDA levels compared to both healthy individuals and patients with thinner tumors. Patients with tumors exhibited notably elevated MDA levels even up to 5 years post surgical removal despite no evidence of recurrence. This was possibly caused by the chronic inflammation resulting from this medical condition [76]. Notably, plasma MDA levels were raised in melanoma patients with either primary or metastatic tumors. Elevated levels of total radical-trapping antioxidant parameter (TRAP), thiol, and advanced oxidation protein products (AOPPs) were identified only in patients with metastatic melanoma [78]. As for total serum SOD activity, it was found to be elevated significantly in stage III melanoma, while SOD2 activity was only elevated in stage IV melanoma. On the other hand, catalase activity was elevated in stage I, II, and III melanoma and not in stage IV melanoma patients. The above finding indicated that certain parameters are elevated at specific stages [69]. In addition, treatment procedure[124] and time of recovery play crucial roles as well [81]. However, further research needs to elucidate the melanoma-stage-specific relationship with oxidative stress.
In the case of wart patients, oxidative stress is evident in all types of warts, including genital and non-genital warts. In particular, an imbalance between oxidants and antioxidants, as well as prolonged HPV infection as a result of chronic inflammation, play a critical role in the development and progression of genital warts. Lesion chronicity is an important aspect and is associated with systemic oxidative stress in both skin cancer and wart patients [107]. Oxidative stress seemed to be in accordance with the recalcitrant wart state. Moreover, an association of redox biomarkers, such as catalase activity, TBARS, CARBS, and TAC, with the time of the lesion’s appearance was found by a study focused on the appearance of NMSC and systemic oxidative stress [5]. In case of benign solid lesions, no results were reported in dermatofibroma, sarcomas such as Kaposi sarcoma, and angiomas. However, these lesions are linked to skin fibrosis [125] and cutaneous angiogenesis disturbances [126] connected to oxidative stress. More studies should be performed in these types of patients to assure if systemic oxidative stress is also impaired.
The above-mentioned findings suggest that antioxidant strategies are of great importance. Previous studies revealed the beneficial role of natural bioactive compounds in oncological diseases, finding them to be a promising strategy for cancer treatment [127]. On the contrary, certain cosmetics [128] and drugs with distinct chemical characteristics [129] have the ability to absorb UV–visible radiation and undergo photosensitization. Photosensitized products lead to the formation of ROS within the cell, leading to reactions such as photoallergic and phototoxic reactions, irritant contact dermatitis, and anaphylactic reactions and contributing to skin carcinogenesis [130]. How oxidative stress contributes to skin carcinogenesis is yet to be fully elucidated. Further research needs to be performed to identify the implicated molecular mechanisms that could be targeted to develop novel therapeutic agents and improve patients’ clinical outcomes.
4. Conclusions
In general, patients with NMSC and melanoma present with disturbances of redox homeostasis, expressed in lower enzyme activities, increased ROS-modified products of lipid and protein oxidation, and, to a lesser extent, by antioxidant micronutrients. Medical history of NMSC as well as stage of skin cancer and treatment approach affect systemic oxidative stress parameters. AK patients follow the same pattern as skin cancer, while individuals with SK present no significant differences with controls. Wart patients (both genital and non-genital) are also characterized by oxidative stress levels, aligned with their resistant character.
Author Contributions
Conceptualization, E.K.; methodology, E.K., P.-M.N. and K.E.G.; writing—original draft preparation, E.K., P.-M.N., K.E.G. and G.G.; writing—review and editing, E.K., A.V.R.S., E.Z. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data described in this study are available upon request from the corresponding author.
Conflicts of Interest
We declare no conflict of interest.
References
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Bassoy, E.Y.; Walch, M.; Martinvalet, D. Reactive Oxygen Species: Do They Play a Role in Adaptive Immunity? Front. Immunol. 2021, 12, 755856. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Aloizou, A.-M.; Zafiriou, E.; Bargiota, A.; Skaperda, Z.; Kouretas, D.; Roussaki-Schulze, A.-V. Non-Melanoma Skin Cancer and Vitamin D: The “Lost Sunlight” Paradox and the Oxidative Stress Explanation. Antioxidants 2023, 12, 1107. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc Is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Adjepong, M.; Agbenorku, P.; Brown, P.; Oduro, I. The Role of Antioxidant Micronutrients in the Rate of Recovery of Burn Patients: A Systematic Review. Burns Trauma 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.H.N.; Furtado, C.; Repolês, B.M.; Ribeiro, G.A.; Mendes, I.C.; Peloso, E.F.; Gadelha, F.R.; Macedo, A.M.; Franco, G.R.; Pena, S.D.J.; et al. Oxidative Stress and DNA Lesions: The Role of 8-Oxoguanine Lesions in Trypanosoma Cruzi Cell Viability. PLoS Negl. Trop. Dis. 2013, 7, e2279. [Google Scholar] [CrossRef]
- Clemente, S.M.; Martínez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Targeting Lipid Peroxidation for Cancer Treatment. Molecules 2020, 25, 5144. [Google Scholar] [CrossRef]
- Waugh, R.J.; Morrow, J.D.; Roberts, L.J.; Murphy, R.C. Identification and Relative Quantitation of F2-Isoprostane Regioisomers Formed in Vivo in the Rat. Free Radic. Biol. Med. 1997, 23, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein Oxidation—Formation Mechanisms, Detection and Relevance as Biomarkers in Human Diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The Role of Thiols in Antioxidant Systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Farbstein, D.; Kozak-Blickstein, A.; Levy, A.P. Antioxidant Vitamins and Their Use in Preventing Cardiovascular Disease. Molecules 2010, 15, 8098–8110. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Inaloz, H.; Baysal, V.; Delibas, N. The Role of Oxidants and Antioxidants in Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.L.; Mitran, C.I.; Mitran, M.I.; Tampa, M.; Matei, C.; Popa, M.I.; Georgescu, S.R. Markers of Oxidative Stress in Patients with Acne: A Literature Review. Life 2023, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Medovic, M.V.; Jakovljevic, V.L.; Zivkovic, V.I.; Jeremic, N.S.; Jeremic, J.N.; Bolevich, S.B.; Ravic Nikolic, A.B.; Milicic, V.M.; Srejovic, I.M. Psoriasis between Autoimmunity and Oxidative Stress: Changes Induced by Different Therapeutic Approaches. Oxid. Med. Cell. Longev. 2022, 2022, 2249834. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Zhang, P.; Arora, M.; Chaleckis, R.; Isobe, T.; Jain, M.; Meister, I.; Melén, E.; Perzanowski, M.; Torta, F.; Wenk, M.R.; et al. Tackling the Complexity of the Exposome: Considerations from the Gunma University Initiative for Advanced Research (GIAR) Exposome Symposium. Metabolites 2019, 9, 106. [Google Scholar] [CrossRef]
- Shih, B.B.; Farrar, M.D.; Vail, A.; Allan, D.; Chao, M.-R.; Hu, C.-W.; Jones, G.D.D.; Cooke, M.S.; Rhodes, L.E. Influence of Skin Melanisation and Ultraviolet Radiation on Biomarkers of Systemic Oxidative Stress. Free Radic. Biol. Med. 2020, 160, 40–46. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Han, J.; Colditz, G.A.; Hunter, D.J. Manganese Superoxide Dismutase Polymorphism and Risk of Skin Cancer (United States). Cancer Causes Control. 2007, 18, 79–89. [Google Scholar] [CrossRef]
- Preissner, S.C.; Hoffmann, M.F.; Preissner, R.; Dunkel, M.; Gewiess, A.; Preissner, S. Polymorphic Cytochrome P450 Enzymes (CYPs) and Their Role in Personalized Therapy. PLoS ONE 2013, 8, e82562. [Google Scholar] [CrossRef] [PubMed]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-Inflammatory Activity of Natural Dietary Flavonoids. Food Funct. 2010, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.-P. UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Narendhirakannan, R.T.; Hannah, M.A.C. Oxidative Stress and Skin Cancer: An Overview. Indian. J. Clin. Biochem. 2013, 28, 110–115. [Google Scholar] [CrossRef]
- Azzimonti, B.; Ballacchino, C.; Zanetta, P.; Cucci, M.A.; Monge, C.; Grattarola, M.; Dianzani, C.; Barrera, G.; Pizzimenti, S. Microbiota, Oxidative Stress, and Skin Cancer: An Unexpected Triangle. Antioxidants 2023, 12, 546. [Google Scholar] [CrossRef]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative Stress in Melanogenesis and Melanoma Development. Współczesna Onkol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Marino, A.; Pusch, M.; Morabito, R.; Dossena, S. Oxidative Stress and Immune Response in Melanoma: Ion Channels as Targets of Therapy. Int. J. Mol. Sci. 2023, 24, 887. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. Seborrheic Keratoses—The Most Common Benign Skin Tumor of Humans. Clinical Presentation and an Update on Pathogenesis and Treatment Options. Open Access Maced. J. Med. Sci. 2018, 6, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.M.; Filippova, M.; Filippov, V.; Payne, K.J.; Duerksen-Hughes, P. Human Papillomavirus Type 16 E6* Induces oxidative Stress and DNA Damage. J. Virol. 2014, 88, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.; Patel, S.; Dey, T.; Pandey, U.; Mishra, S.P. A Study of Oxidative Stress in Cervical Cancer—An Institutional Study. Biochem. Biophys. Rep. 2021, 25, 100881. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.S.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric Assays for Measuring Redox Biomarkers in Blood. Biomarkers 2016, 21, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Chaisiriwong, L.; Wanitphakdeedecha, R.; Sitthinamsuwan, P.; Sampattavanich, S.; Chatsiricharoenkul, S.; Manuskiatti, W.; Panich, U. A Case-Control Study of Involvement of Oxidative DNA Damage and Alteration of Antioxidant Defense System in Patients with Basal Cell Carcinoma: Modulation by Tumor Removal. Oxid. Med. Cell. Longev. 2016, 2016, 5934024. [Google Scholar] [CrossRef] [PubMed]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic Acid and Ferulic Acid Inhibit UVA-Induced Matrix Metalloproteinase-1 through Regulation of Antioxidant Defense System in Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- Johns, E.J.; O’Shaughnessy, B.; O’Neill, S.; Lane, B.; Healy, V. Impact of Elevated Dietary Sodium Intake on NAD(P)H Oxidase and SOD in the Cortex and Medulla of the Rat Kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R234–R240. [Google Scholar] [CrossRef]
- Siegel, D.; Kepa, J.K.; Ross, D. Biochemical and Genetic Analysis of NAD(P)H:Quinone Oxidoreductase 1 (NQO1). Curr. Protoc. Toxicol. 2007, 32, 4–22. [Google Scholar] [CrossRef]
- Vural, P.; Canbaz, M.; Selçuki, D. Plasma Antioxidant Defense in Actinic Keratosis and Basal Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 1999, 13, 96–101. [Google Scholar] [CrossRef]
- BEUTLER, E.; DURON, O.; KELLY, B.M. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Janaszewska, A.; Bartosz, G. Assay of Total Antioxidant Capacity: Comparison of Four Methods as Applied to Human Blood Plasma. Scand. J. Clin. Lab. Invest. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Denson, K.W.; Bowers, E.F. The Determination of Ascorbic Acid in White Blood Cells. A Comparison of W.B.C. Ascorbic Acid. and Phenolic Acid. Excretion in Elderly Patients. Clin. Sci. 1961, 21, 157–162. [Google Scholar] [PubMed]
- Brown, M.A. Resistance of Human Erythrocytes Containing Elevated Levels of Vitamin E to Radiation-Induced Hemolysis. Radiat. Res. 1983, 95, 303–316. [Google Scholar] [CrossRef] [PubMed]
- van der Pols, J.C.; Heinen, M.M.; Hughes, M.C.; Ibiebele, T.I.; Marks, G.C.; Green, A.C. Serum Antioxidants and Skin Cancer Risk: An 8-Year Community-Based Follow-up Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1167–1173. [Google Scholar] [CrossRef]
- Sowell, A.L.; Huff, D.L.; Yeager, P.R.; Caudill, S.P.; Gunter, E.W. Retinol, Alpha-Tocopherol, Lutein/Zeaxanthin, Be-ta-Cryptoxanthin, Lycopene, Alpha-Carotene, Trans-Beta-Carotene, and Four Retinyl Esters in Serum Determined Simultaneously by Reversed-Phase HPLC with Multiwavelength Detection. Clin. Chem. 1994, 40, 411–416. [Google Scholar] [CrossRef]
- Carnrick, G.R.; Manning, D.C.; Slavin, W. Determination of Selenium in Biological Materials with Platform Furnace At-om-ic-Absorption Spectroscopy and Zeeman Background Correction. Analyst 1983, 108, 1297. [Google Scholar] [CrossRef]
- Dorgan, J.F.; Boakye, N.A.; Fears, T.R.; Schleicher, R.L.; Helsel, W.; Anderson, C.; Robinson, J.; Guin, J.D.; Lessin, S.; Ratnasinghe, L.D.; et al. Serum Carotenoids and α-Tocopherol and Risk of Nonmelanoma Skin Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1276–1282. [Google Scholar] [CrossRef]
- Ghaedi, E.; Rahrovani, F.; Javanbakht, M.H.; Ehsani, A.-H.; Esrafili, A.; Mohammadi, H.; Zarei, M.; Djalali, M. Retinol and α-Tocopherol Levels in the Serum and Subcutaneous Adipose Tissue of Newly Diagnosed Basal Cell Carcinoma Patients. Iran. J. Public. Health 2019, 48, 1838–1846. [Google Scholar] [CrossRef]
- Katrangi, N.; Kaplan, L.A.; Stein, E.A. Separation and Quantitation of Serum Beta-Carotene and Other Carotenoids by High Performance Liquid Chromatography. J. Lipid Res. 1984, 25, 400–406. [Google Scholar] [CrossRef]
- Breslow, R.A.; Alberg, A.J.; Helzlsouer, K.J.; Bush, T.L.; Norkus, E.P.; Morris, J.S.; Spate, V.E.; Comstock, G.W. Serological Precursors of Cancer: Malignant Melanoma, Basal and Squamous Cell Skin Cancer, and Prediagnostic Levels of Retinol, Beta- Carotene, Ly-copene, Alpha-Tocopherol, and Selenium. Cancer Epidemiol. Biomarkers Prev. 1995, 4, 837–842. [Google Scholar]
- Lavi, N.; Mantel, M.; Alfassi, Z.B. Determination of Selenium in Biological Materials by Neutron Activation Analysis. Analyst 1988, 113, 1855. [Google Scholar] [CrossRef] [PubMed]
- Driskell, W.J.; Bashor, M.M.; Neese, J.W. Beta-Carotene Determined in Serum by Liquid Chromatography with an Internal Standard. Clin. Chem. 1983, 29, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Majidi, Z.; Djalali, M.; Javanbakht, M.H.; Fathi, M.; Zarei, M.; Foladsaz, K. Evaluation of the Level of Zinc and Malondialdehyde in Basal Cell Carcinoma. Iran. J. Public. Health 2017, 46, 1104–1109. [Google Scholar] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol Redox State (TRS) and Oxidative Stress in the Mouse Hippocampus after Pentylenetetrazol-Induced Epileptic Seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Bendesky, A.; Michel, A.; Sordo, M.; Calderón-Aranda, E.S.; Acosta-Saavedra, L.C.; Salazar, A.M.; Podoswa, N.; Ostrosky-Wegman, P. DNA Damage, Oxidative Mutagen Sensitivity, and Repair of Oxidative DNA Damage in Nonmelanoma Skin Cancer Patients. Environ. Mol. Mutagen. 2006, 47, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Sordo, M.; Herrera, L.A.; Ostrosky-Wegman, P.; Rojas, E. Cytotoxic and Genotoxic Effects of As, MMA, and DMA on Leukocytes and Stimulated Human Lymphocytes. Teratog. Carcinog. Mutagen. 2001, 21, 249–260. [Google Scholar] [CrossRef]
- Karagas, M.R.; Greenberg, E.R.; Nierenberg, D.; Stukel, T.A.; Morris, J.S.; Stevens, M.M.; Baron, J.A. Risk of Squamous Cell Carcinoma of the Skin in Relation to Plasma Selenium, Alpha-Tocopherol, Beta-Carotene, and Retinol: A Nested Case-Control Study. Cancer Epidemiol. Biomark. Prev. 1997, 6, 25–29. [Google Scholar]
- Nierenberg, D.W. Serum and Plasma β-Carotene Levels Measured with an Improved Method of High-Performance Liquid Chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1985, 339, 273–284. [Google Scholar] [CrossRef]
- Nierenberg, D.W.; Lester, D.C. Determination of Vitamins a and e in Serum and Plasma Using a Simplified Clarification Method and High-Performance Liquid Chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1985, 345, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Stampfer, M.; Underwood, B.; Speizer, F.; Rosner, B.; Hennekens, C. Validation of a Dietary Questionnaire with Plasma Carotenoid and α-Tocopherol Levels. Am. J. Clin. Nutr. 1983, 38, 631–639. [Google Scholar] [CrossRef]
- e Silva de Almendra Freitas, B.; Lloret, G.R.; Visacri, M.B.; Tuan, B.T.; Amaral, L.S.; Baldini, D.; de Sousa, V.M.; de Castro, L.L.; Aguiar, J.R.S.; Pincinato, E.D.C.; et al. High 15-F2t-Isoprostane Levels in Patients with a Previous History of Nonmelanoma Skin Cancer: The Effects of Supplementary Antioxidant Therapy. Biomed. Res. Int. 2015, 2015, 963569. [Google Scholar] [CrossRef]
- Attia, D.M.; Goldschmeding, R.; Attia, M.A.; Boer, P.; Koomans, H.A.; Joles, J.A. Male Gender Increases Sensitivity to Renal Injury in Response to Cholesterol Loading. Am. J. Physiol. Ren. Physiol. 2003, 284, F718–F726. [Google Scholar] [CrossRef] [PubMed]
- Tatsch, E.; Bochi, G.V.; Pereira, R.D.S.; Kober, H.; Oliveira, J.R.D.; Moresco, R.N. Influência Dos Anticoagulantes e Da Temperatura de Armazenamento Sobre Os Níveis Sanguíneos de Nitrito. J. Bras. Patol. Med. Lab. 2011, 47, 147–150. [Google Scholar] [CrossRef][Green Version]
- e Silva de Almendra Freitas, B.; de Castro, L.L.; Aguiar, J.R.S.; de Araújo, C.G.B.; Visacri, M.B.; Tuan, B.T.; de Carvalho Pincinato, E.; Moriel, P. Antioxidant Capacity Total in Non-Melanoma Skin Cancer and Its Relationship with Food Consumption of Antioxidant Nutrients. Nutr. Hosp. 2015, 31, 1682–1688. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Marks, G.C.; Gaffney, P.; Williams, G.; Green, A.C. Antioxidants and Basal Cell Carcinoma of the Skin: A Nested Case–Control Study. Cancer Causes Control 2005, 16, 609–618. [Google Scholar] [CrossRef]
- Bisevac, J.P.; Djukic, M.; Stanojevic, I.; Stevanovic, I.; Mijuskovic, Z.; Djuric, A.; Gobeljic, B.; Banovic, T.; Vojvodic, D. Association Between Oxidative Stress and Melanoma Progression. J. Med. Biochem. 2018, 37, 12–20. [Google Scholar] [CrossRef]
- Sun, M.; Zigman, S. An Improved Spectrophotometric Assay for Superoxide Dismutase Based on Epinephrine Autoxidation. Anal. Biochem. 1978, 90, 81–89. [Google Scholar] [CrossRef]
- Góth, L. A Simple Method for Determination of Serum Catalase Activity and Revision of Reference Range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Zuberbier, T.; Diehl, S.; Schadendorf, C.; Czarnetzki, B.M. Serum Manganese Superoxide Dismutase Is a New Tumour Marker for Malignant Melanoma. Melanoma Res. 1995, 5, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Akai, F.; Iwasaki, H.; Hosokawa, K.; Kusunoki, T.; Suzuki, K.; Taniguchl, N.; Hashimoto, S.; Tamura, T.T. Manganese Superoxide Dismutase Content and Localization in Human Thyroid Tumours. J. Pathol. 1993, 169, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, S.S.; de Souza-Neto, F.P.; Ramalho, L.N.Z.; Derossi, D.R.; Guarnier, F.A.; da Silva, C.F.N.; Melo, G.P.; Simão, A.N.C.; Cecchini, R.; Cecchini, A.L. Systemic Oxidative Profile after Tumor Removal and the Tumor Microenvironment in Melanoma Patients. Cancer Lett. 2015, 361, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Herrera, A.C.S.A.; Victorino, V.J.; Campos, F.C.; Freitas, L.F.; De Rossi, T.; Colado Simão, A.N.; Cecchini, A.L.; Cecchini, R. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res. Treat. 2012, 133, 89–97. [Google Scholar] [CrossRef]
- Gadjeva, V.; Dimov, A.; Georgieva, N. Influence of Therapy on the Antioxidant Status in Patients with Melanoma. J. Clin. Pharm. Ther. 2008, 33, 179–185. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymoly 105; Sies, H., Kaplan, N., Colowick, N., Eds.; Academic Press: San Diego, CA, USA, 1984; pp. 121–1266. [Google Scholar]
- Santos Bernardes, S.; de Souza-Neto, F.P.; Pasqual Melo, G.; Guarnier, F.A.; Marinello, P.C.; Cecchini, R.; Cecchini, A.L. Correlation of TGF-Β1 and Oxidative Stress in the Blood of Patients with Melanoma: A Clue to Understanding Melanoma Progression? Tumor Biol. 2016, 37, 10753–10761. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Auclair, C.; Voisin, E. Nitroblue Tetrazolium Reduction. In Handbook of Methods for Oxygen Radical Research, 3rd ed.; Green Wald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Păsărică, M.A.; Curcă, P.F.; Burcea, M.; Schmitzer, S.; Dragosloveanu, C.D.M.; Grigorescu, A.C. The Effects of Oncological Treatment on Redox Balance in Patients with Uveal Melanoma. Diagnostics 2023, 13, 1907. [Google Scholar] [CrossRef]
- Albini, A. Standardization of Protein Free SH Groups in Blood Plasma. Boll. Soc. Ital. Sperim. 1990, 18, 1829–1898. [Google Scholar]
- Hu, M.-L. Measurement of Protein Thiol Groups and Glutathione in Plasma. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1994; pp. 380–385. [Google Scholar] [CrossRef]
- Repetto, M.; Reides, C.; Gomez Carretero, M.L.; Costa, M.; Griemberg, G.; Llesuy, S. Oxidative Stress in Blood of HIV Infected Patients. Clin. Chim. Acta 1996, 255, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhatt, M.L.B.; Misra, M.K. Lipid Peroxidation and Antioxidant Status in Head and Neck Squamous Cell Carcinoma Patients. Oxid. Med. Cell. Longev. 2009, 2, 68–72. [Google Scholar] [CrossRef]
- Rogoża-Janiszewska, E.; Malińska, K.; Baszuk, P.; Marciniak, W.; Derkacz, R.; Lener, M.; Jakubowska, A.; Cybulski, C.; Huzarski, T.; Masojć, B.; et al. Serum Selenium Level and 10-Year Survival after Melanoma. Biomedicines 2021, 9, 991. [Google Scholar] [CrossRef]
- Reinhold, U.; Biltz, H.; Bayer, W.; Schmidt, K.H. Serum Selenium Levels in Patients with Malignant Melanoma. Acta Derm. Venereol. 1989, 69, 132–136. [Google Scholar]
- Deffuant, C.; Celerier, P.; Boiteau, H.L.; Litoux, P.; Dreno, B. Serum Selenium in Melanoma and Epidermotropic Cutaneous T-Cell Lymphoma. Acta Derm. Venereol. 1994, 74, 90–92. [Google Scholar] [CrossRef]
- Fisher, G.L.; McNeill, K.L.; Spitler, L.E.; Rosenblatt, L.S. Serum Copper and Zinc Levels in Melanoma Patients. Cancer 1981, 47, 1838–1844. [Google Scholar] [CrossRef]
- Horčičko, J.; Pantůček, M. Hypozincemia in Patients with Malignant Melanoma. Clin. Chim. Acta 1983, 130, 279–282. [Google Scholar] [CrossRef]
- Dawson, J.B.; Walker, B.E. Direct Determination of Zinc in Whole Blood, Plasma and Urine by Atomic Absorption Spectroscopy. Clin. Chim. Acta 1969, 26, 465–475. [Google Scholar] [CrossRef]
- Ros-Bullón, M.R.; Sánchez-Pedreño, P.; Martínez-Liarte, J.H. Serum Zinc Levels Are Increased in Melanoma Patients. Melanoma Res. 1998, 8, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.J.; Khan, N.; Mclellan, B.A. Early Measurement of Systemic Lipid Peroxidation Products in the Plasma of Major Blunt Trauma Patients. J. Trauma Inj. Infect. Crit. Care 1991, 31, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Carbonneau, M.A.; Peuchant, E.; Sess, D.; Canioni, P.; Clerc, M. Free and Bound Malondialdehyde Measured as Thiobarbituric Acid Adduct by HPLC in Serum and Plasma. Clin. Chem. 1991, 37, 1423–1429. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of Product of Lipid Peroxidation (Malonyl Dialdehyde) in Biochemical Systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Victorino, V.J.; Panis, C.; Campos, F.C.; Cayres, R.C.; Colado-Simão, A.N.; Oliveira, S.R.; Herrera, A.C.S.A.; Cecchini, A.L.; Cecchini, R. Decreased Oxidant Profile and Increased Antioxidant Capacity in Naturally Postmenopausal Women. Age 2013, 35, 1411–1421. [Google Scholar] [CrossRef][Green Version]
- Elvira, Y.; Putra, I.B.; Jusuf, N.K. Correlation between Superoxide Dismutase (SOD) with Malondialdehyde (MDA) Level in Blood Plasma of Seborrheic Keratosis. Bali Med. J. 2023, 12, 1291–1294. [Google Scholar]
- Descamps-Latscha, B.; Witko-Sarsat, V.; Nguyen-Khoa, T.; Nguyen, A.T.; Gausson, V.; Mothu, N.; Cardoso, C.; Noël, L.-H.; Guérin, A.P.; London, G.M.; et al. Early Prediction of IgA Nephropathy Progression: Proteinuria and AOPP Are Strong Prognostic Markers. Kidney Int. 2004, 66, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Sener, S.; Kilinc, F.; Akbas, A.; Erdogan, S.; Erel, O.; Metin, A. Oxidative Stress and Thiol/Disulfide Homeostasis in Human Papillomavirus Infections. Indian J. Dermatol. 2022, 67, 228. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A Novel and Automated Assay for Thiol/Disulphide Homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Korkmaz, S.; Şirin, F.B.; Erturan, İ.; Büyükbayram, H.İ.; Yildirim, M. Coenzyme Q10, Zinc and MDA Levels in Verruca Vulgaris. Turk. J. Med. Sci. 2020, 50, 1387–1392. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1990; pp. 421–431. [Google Scholar]
- Smith, J.C.; Butrimovitz, G.P.; Purdy, W.C. Direct Measurement of Zinc in Plasma by Atomic Absorption Spectroscopy. Clin. Chem. 1979, 25, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Mitran, C.I.; Nicolae, I.; Tampa, M.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Ene, C.D.; Matei, C.; Ionescu, A.C.; Georgescu, S.R.; et al. The Relationship between the Soluble Receptor for Advanced Glycation End Products and Oxidative Stress in Patients with Palmoplantar Warts. Medicina 2019, 55, 706. [Google Scholar] [CrossRef] [PubMed]
- Erturan, İ.; Kumbul Doğuç, D.; Korkmaz, S.; Büyükbayram, H.İ.; Yıldırım, M.; Kocabey Uzun, S. Evaluation of Oxidative Stress in Patients with Recalcitrant Warts. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1952–1957. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Demirbag, R.; Gur, M.; Yilmaz, R.; Kunt, A.S.; Erel, O.; Andac, M.H. Influence of Oxidative Stress on the Development of Collateral Circulation in Total Coronary Occlusions. Int. J. Cardiol. 2007, 115, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cokluk, E.; Sekeroglu, M.R.; Aslan, M.; Balahoroglu, R.; Bilgili, S.G.; Huyut, Z. Determining Oxidant and Antioxidant Status in Patients with Genital Warts. Redox Rep. 2015, 20, 210–214. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Sasmaz, S.; Arican, O.; Kurutas, E.B. Oxidative Stress in Patients with Nongenital Warts. Mediat. Inflamm. 2005, 2005, 233–236. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide Dismutases. In Advances in Enzymology and Related Areas of Molecular Biology; Meister, A., Ed.; Wiley: Hoboken, NJ, USA, 1974; pp. 35–97. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Savoye, I.; Olsen, C.M.; Whiteman, D.C.; Bijon, A.; Wald, L.; Dartois, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Kvaskoff, M. Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: The E3N-SunExp Study. J. Epidemiol. 2018, 28, 27–33. [Google Scholar] [CrossRef]
- Tsuchida, K.; Sakiyama, N.; Ogura, Y.; Kobayashi, M. Skin Lightness Affects Ultraviolet A-induced Oxidative Stress: Evaluation Using Ultraweak Photon Emission Measurement. Exp. Dermatol. 2023, 32, 146–153. [Google Scholar] [CrossRef]
- Karampinis, E.; Lallas, A.; Lazaridou, E.; Errichetti, E.; Apalla, Z. Race-Specific and Skin of Color Dermatoscopic Characteristics of Skin Cancer: A Literature Review. Dermatol. Pract. Concept. 2023, 13, e2023311S. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S. Antioxidants in Dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef]
- Abu Youssef, H.A.; Elshazly, M.I.; Rashed, L.A.; Sabry, I.M.; Ibrahim, E.K. Thiobarbituric Acid Reactive Substance (TBARS) a Marker of Oxidative Stress in Obstructive Sleep Apnea. Egypt. J. Chest Dis. Tuberc. 2014, 63, 119–124. [Google Scholar] [CrossRef]
- Goycheva, P.; Petkova-Parlapanska, K.; Georgieva, E.; Karamalakova, Y.; Nikolova, G. Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications. Int. J. Mol. Sci. 2023, 24, 13541. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Paprotny, Ł.; Celejewska, A.; Szewczak, D.; Wianowska, D. Comparison of the Antioxidant Properties of Serum and Plasma Samples as Well as Glutathione under Environmental and Pharmacological Stress Factors Involving Different Classes of Drugs. Environ. Toxicol. Pharmacol. 2022, 94, 103936. [Google Scholar] [CrossRef] [PubMed]
- Salar-Amoli, J.; Hejazy, M.; Ali Esfahani, T. Comparison between Some Oxidative Stress Biomarkers Values in Serum and Plasma of Clinically Healthy Adult Camels (Camelus Dromedarius) in Iran. Vet. Res. Commun. 2009, 33, 849–854. [Google Scholar] [CrossRef]
- Biesalski Hans, K.; Jana, T. Micronutrients in the Life Cycle: Requirements and Sufficient Supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Conklin, K.A. Chemotherapy-Associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef]
- Shroff, A.; Mamalis, A.; Jagdeo, J. Oxidative Stress and Skin Fibrosis. Curr. Pathobiol. Rep. 2014, 2, 257–267. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Byzova, T.V. Oxidative Stress in Angiogenesis and Vascular Disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Baek, J.; Lee, M.-G. Oxidative Stress and Antioxidant Strategies in Dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.F.; Masih, A.P.; Alqasmi, I.; Alsulimani, A.; Khan, F.H.; Haque, S. Oxidative-Stress-Induced Cellular Toxicity and Glycoxidation of Biomolecules by Cosmetic Products under Sunlight Exposure. Antioxidants 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals 2021, 14, 723. [Google Scholar] [CrossRef]
- Haisma, M.S.; Greven, N.; Logendran, M.; Bos, J.; Bert, V.D.; Horváth, B.; De Vos, S.; De Bock, G.H.; Hak, E.; Rácz, E. Chronic Use of Hydrochlorothiazide and Risk of Skin Cancer in Caucasian Adults: A PharmLines Initiative Inception Cohort Study. Acta Derm. Venereol. 2023, 103, adv3933. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).