Group Size Buffers against Energetic Stress in Honeybee Workers (Apis mellifera)
Abstract
1. Introduction
2. Results
2.1. Starvation Response
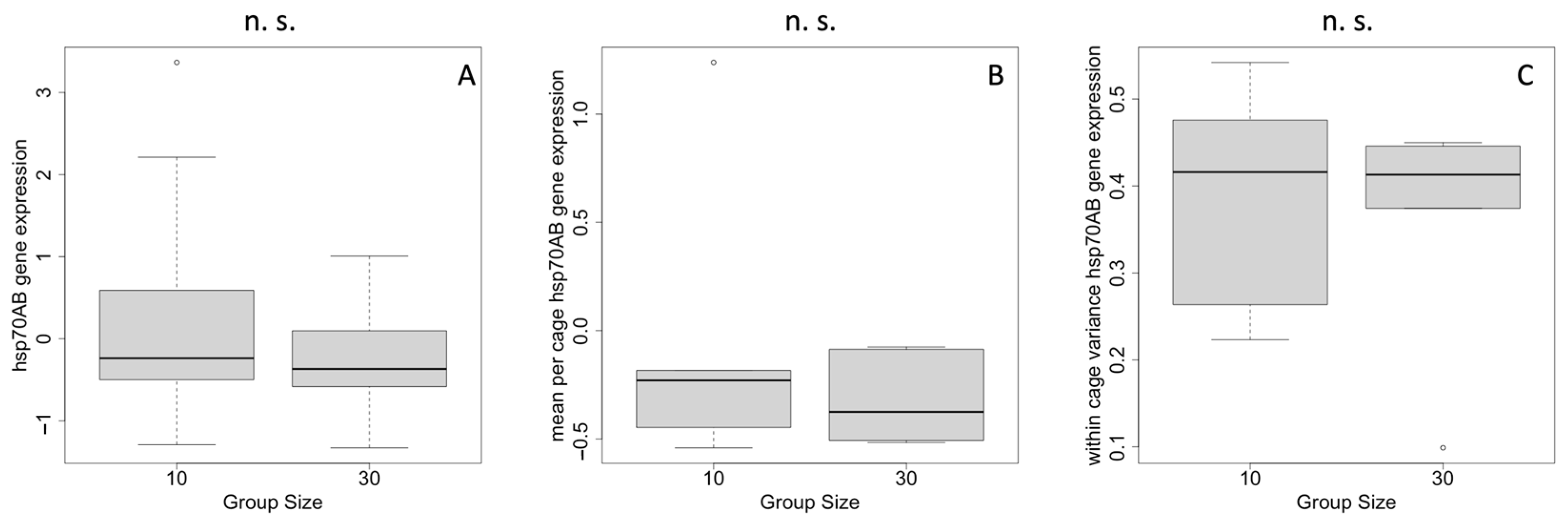
2.2. Heat-Shock Response
3. Discussion
4. Materials and Methods
4.1. Experimental Set Up
4.2. Hemolymph Collection
4.3. Glucose/Trehalose Assay
4.4. RNA Extraction, cDNA Synthesis, and qRT-PCR
4.5. Normalization of Gene Expression
4.6. Statistical Data Analysis
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szathmáry, E.; Maynard-Smith, J. The major evolutionary transitions. Nature 1995, 374, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D. The genetical evolution of social behaviour I. J. Theor. Biol. 1964, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D. The genetical evolution of social behaviour II. J. Theor. Biol. 1964, 7, 17–52. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.A.; Tarnita, C.E.; Wilson, E.O. The evolution of eusociality. Nature 2010, 466, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Abbot, P.; Abe, J.; Alcock, J.; Alizon, S.; Alpedrinha, J.A.C.; Andersson, M.; Andre, J.-B.; van Baalen, M.; Balloux, F.; Balshine, S.; et al. Inclusive fitness theory and eusociality. Nature 2011, 471, E1–E4. [Google Scholar] [CrossRef]
- Beshers, S.N.; Fewell, J.H. Models of Division of labor in social insects. Annu. Rev. Entomol. 2001, 46, 413–440. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social Immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef]
- Abbot, P. Defense in social insects: Diversity, division of labor, and evolution. Annu. Rev. Entomol. 2022, 67, 407–436. [Google Scholar] [CrossRef]
- Schultner, E.; Oettler, J.; Helanterä, H. The role of brood in eusocial Hymenoptera. Q. Rev. Biol. 2017, 92, 39–78. [Google Scholar] [CrossRef]
- Visscher, P.K. Group decision making in nest-site selection among social insects. Annu. Rev. Entomol. 2007, 52, 255–275. [Google Scholar] [CrossRef]
- Even, N.; Devaud, J.M.; Barron, A.B. General Stress Responses in the Honey Bee. Insects 2012, 3, 1271–1298. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Loeschcke, V. Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J. Insect Physiol. 2001, 47, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Koto, A.; Mersch, D.; Hollis, B.; Keller, L. Social isolation causes mortality by disrupting energy homeostasis in ants. Behav. Ecol. Sociobiol. 2015, 69, 583–591. [Google Scholar] [CrossRef]
- Wang, Z.Y.; McKenzie-Smith, G.C.; Liu, W.; Cho, H.J.; Pereira, T.; Dhanerawala, Z.; Shaevitz, J.W.; Kocher, S.D. Isolation disrupts social interactions and destabilizes brain development in bumblebees. Curr. Biol. 2022, 32, 2754–2764. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Dyer, F. The biology of the dance language. Annu. Rev. Entomol. 2002, 47, 917–949. [Google Scholar] [CrossRef] [PubMed]
- Lattorff, H.M.G. Increased stress levels in caged honeybee (Apis mellifera) (Hymenoptera: Apidae) workers. Stresses 2022, 2, 373–384. [Google Scholar] [CrossRef]
- Bosua, H.J.; Nicolson, S.W.; Archer, C.R.; Pirk, C.W.W. Effects of cage volume and bee density on survival and nutrient intake of honeybees (Apis mellifera L.) under laboratory conditions. Apidologie 2018, 49, 734–746. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Baxter, J.R. Honey Bees: The Effect of Group Size on Longevity and Hoarding in Laboratory Cages. Ann. Entomol. Soc. Am. 1978, 71, 732. [Google Scholar] [CrossRef]
- Lattorff, H.M.G.; Moritz, R.F.A. Genetic underpinnings of division of labor in the honeybee. Trends Genet. 2013, 29, 641–648. [Google Scholar] [CrossRef]
- Blatt, J.; Roces, F. Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): Dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J. Exp. Biol. 2001, 204, 2709–2716. [Google Scholar] [CrossRef]
- Bartlett, L.J.; Rozins, C.; Brosi, B.J.; Delaplane, K.S.; de Roode, J.C.; White, A.; Wilfert, L.; Boots, M. Industrial bees: The impact of apicultural intensification on local disease prevalence. J. Appl. Ecol. 2019, 56, 2195–2205. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Kryger, P.; Le Conte, Y.; Lattorff, H.M.G.; Kraus, F.B.; Moritz, R.F.A. Four quantitative trait loci associated with low Nosema ceranae (Microsporidia) spore load in the honeybee Apis mellifera. Apidologie 2014, 45, 248–256. [Google Scholar] [CrossRef]
- Kurze, C.; Le Conte, Y.; Dussaubat, C.; Erler, S.; Kryger, P.; Lewkowski, O.; Müller, T.; Widder, M.; Moritz, R.F.A. Nosema Tolerant Honeybees (Apis mellifera) Escape Parasitic Manipulation of Apoptosis. PLoS ONE 2015, 10, e0140174. [Google Scholar] [CrossRef]
- Kurze, C.; Mayack, C.; Hirche, F.; Stangl, G.I.; Le Conte, Y.; Kryger, P.; Moritz, R.F.A. Nosema spp. infections cause no energetic stress in tolerant honeybees. Parasitol. Res. 2016, 115, 2381–2388. [Google Scholar] [CrossRef]
- Ruiz-González, M.X.; Moret, Y.; Brown, M.J.F. Rapid induction of immune density-dependent prophylaxis in adult social insects. Biol. Lett. 2009, 5, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Helbing, S.; Erler, S.; Lattorff, H.M.G. Social context dependent immune gene expression in bumblebees (Bombus terrestris). Behav. Ecol. Sociobiol. 2012, 66, 791–796. [Google Scholar] [CrossRef]
- Lattorff, H.M.G. Tissue specificity in social context-dependent lysozyme expression in bumblebees. Antibiotics 2020, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Rehan, S.M.; Toth, A.L. Climbing the social ladder: The molecular evolution of sociality. Trends Ecol. Evol. 2015, 30, 426–433. [Google Scholar] [CrossRef]
- Stow, A.; Briscoe, D.; Gillings, M.; Holley, M.; Smith, S.; Leys, R.; Silberbauer, T.; Turnbull, C.; Beattie, A. Antimicrobial defences increase with sociality in bees. Biol. Lett. 2007, 3, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, C.; Hoggard, S.; Gillings, M.; Palmer, C.; Stow, A.; Beattie, D.; Briscoe, D.; Smith, S.; Wilson, P.; Beattie, A. Antimicrobial strength increases with group size: Implications for social evolution. Biol. Lett. 2011, 7, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Hartfelder, K.; Bitondi, M.M.G.; Brent, C.S.; Guidugli-Lazzarini, K.R.; Simões, Z.L.P.; Stabentheiner, A.; Tanaka, E.D.; Wang, Y. Standard methods for physiology and biochemistry research in Apis mellifera. J. Apic. Res. 2013, 52, 1–48. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.P.; Mackert, A.; Cristino, A.S.; Simões, Z.L.P. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 2008, 39, 372–385. [Google Scholar] [CrossRef]
- Andersen, C.L.; Ledet-Jensen, J.; Ørntoft, T. Normalization of real-time quantitative RT-PCR data: A model based variance estimation approach to identify genes suited for normalization—Applied to bladder- and colon-cancer data-sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Erler, S.; Popp, M.; Lattorff, H.M.G. Dynamics of Immune System Gene Expression upon Bacterial Challenge and Wounding in a Social Insect (Bombus terrestris). PLoS ONE 2011, 6, e18126. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattorff, H.M.G. Group Size Buffers against Energetic Stress in Honeybee Workers (Apis mellifera). Stresses 2023, 3, 397-403. https://doi.org/10.3390/stresses3020029
Lattorff HMG. Group Size Buffers against Energetic Stress in Honeybee Workers (Apis mellifera). Stresses. 2023; 3(2):397-403. https://doi.org/10.3390/stresses3020029
Chicago/Turabian StyleLattorff, H. Michael G. 2023. "Group Size Buffers against Energetic Stress in Honeybee Workers (Apis mellifera)" Stresses 3, no. 2: 397-403. https://doi.org/10.3390/stresses3020029
APA StyleLattorff, H. M. G. (2023). Group Size Buffers against Energetic Stress in Honeybee Workers (Apis mellifera). Stresses, 3(2), 397-403. https://doi.org/10.3390/stresses3020029




