Accumulation and Phytotoxicity of Two Commercial Biocides in the Lichen Evernia prunastri and the Moss Brachythecium sp.
Abstract
1. Introduction
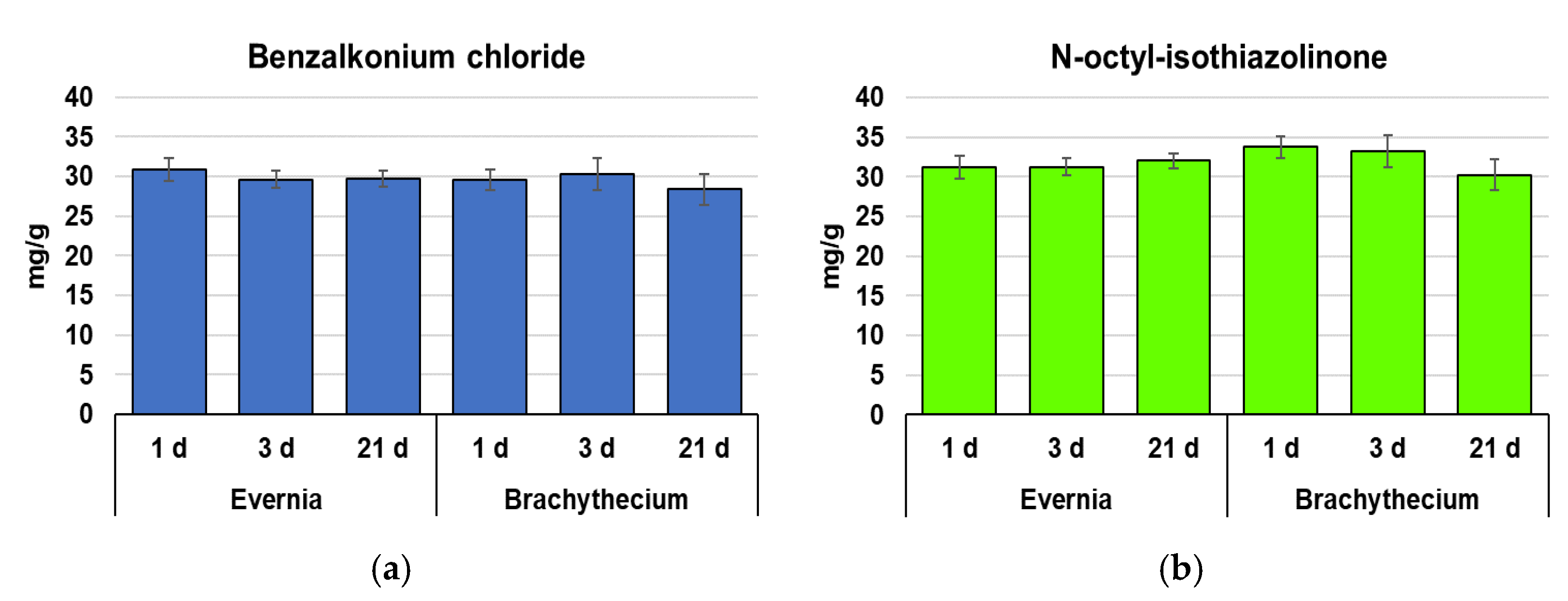
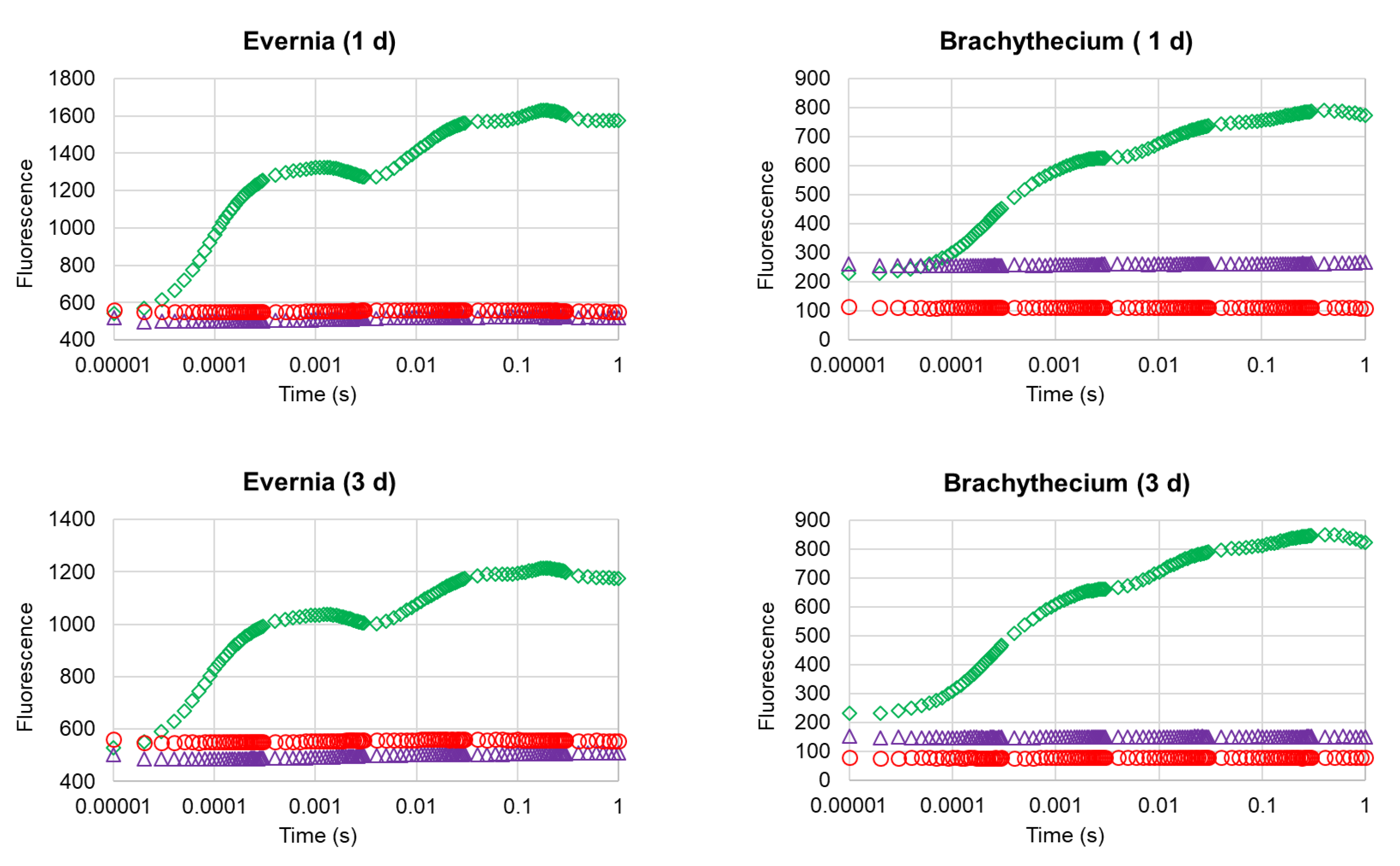
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental
4.2. Chemical Characteristics of Biotin T and Preventol RI80
4.3. Accumulation of Biocide Compounds
4.4. Photosynthetic Efficiency
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favero-Longo, S.E.; Viles, H.A. A review of the nature, role and control of lithobionts on stone cultural heritage: Weighing-up and managing biodeterioration and bioprotection. World J. Microbiol. Biotechnol. 2020, 36, 1–18. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publications: Los Angeles, CA, USA, 2008. [Google Scholar]
- Dakal, T.C.; Cameotra, S.S. Microbially induced deterioration of architectural heritages: Routes and mechanisms involved. Environ. Sci. Eur. 2012, 24, 36. [Google Scholar] [CrossRef]
- Salvadori, O.; Casanova-Municchia, A. The role of fungi and lichens in the biodeterioration of stone monuments. Open Conf. Proc. J. 2016, 7. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Garcia Rowe, J.; Rodriguez Hidalgo, J.M. Biodeterioration of polychrome Roman mosaics. Int. Biodeterior. Biodegrad. 1991, 28, 65–79. [Google Scholar] [CrossRef][Green Version]
- Altieri, A.; Ricci, S. Calcium uptake in mosses and its role in stone biodeterioration. Int. Biodeterior. Biodegrad. 1997, 40, 201–204. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.V. Biodeterioration of Stone in Tropical Environments: An Overview; Getty Publications: Los Angeles, CA, USA, 1999. [Google Scholar]
- Ricci, S.; Altieri, A. Il ruolo delle briofite nel deterioramento dei Beni Culturali. In Biologia ed Ecologia Delle Briofite; Aleffi, M., Ed.; Antonio Delfino: Roma, Italia, 2008; pp. 417–434. [Google Scholar]
- Caneva, G.; Terscari, M. Stone biodeterioration: Treatments and preventive conservation. In Proceedings of the 2017 International Symposium of Stone Conservation, Conservation Technologies for Stone Cultural Heritages: Status and Future Prospects, Seoul, Korea, 21 September 2017; National Research Institute of Cultural Heritage of Korea: Daejeon, Korea, 2017; pp. 95–114. [Google Scholar]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and future perspectives for biocides and antifouling products for stone-built cultural heritage: Ionic liquids as a challenging alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Monte, M.; Nichi, D. Effects of two biocides in the elimination of lichens from stone monuments: Preliminary findings. Sci. Technol. Cult. Herit. 1997, 6, 209–216. [Google Scholar]
- Tretiach, M.; Bertuzzi, S.; Salvadori, O. Chlorophyll a fluorescence as a practical tool for checking the effects of biocide treatments on endolithic lichens. Int. Biodeterior. Biodegrad. 2010, 64, 452–460. [Google Scholar] [CrossRef]
- De los Ríos, A.; Pérez-Ortega, S.; Wierzchos, J.; Ascaso, C. Differential effects of biocide treatments on saxicolous communities: Case study of the Segovia cathedral cloister (Spain). Int. Biodeterior. Biodegrad. 2012, 67, 64–72. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Benesperi, R.; Bertuzzi, S.; Bianchi, E.; Buffa, G.; Giordani, P.; Loppi, S.; Malaspina, P.; Matteucci, E.; Paoli, L.; et al. Species- and site-specific efficacy of commercial biocides and application solvents against Lichens. Int. Biodeterior. Biodegrad. 2017, 123, 127–137. [Google Scholar] [CrossRef]
- Vannini, A.; Contardo, T.; Paoli, L.; Scattoni, M.; Favero-Longo, S.E.; Loppi, S. Application of commercial biocides to lichens: Does a physiological recovery occur over time? Int. Biodeterior. Biodegrad. 2018, 129, 189–194. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Vannini, A.; Benesperi, R.; Bianchi, E.; Fačkovcová, Z.; Giordani, P.; Malaspina, P.; Martire, L.; Matteucci, E.; Paoli, L.; et al. The application protocol impacts the effectiveness of biocides against lichens. Int. Biodeterior. Biodegrad. 2020, 155, 105105. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Candotto Carniel, F.; Pipan, G.; Tretiach, M. Devitalization of poikilohydric lithobionts of open-air monuments by heat shock treatments: A new case study centred on bryophytes. Int. Biodeterior. Biodegrad. 2013, 84, 44–53. [Google Scholar] [CrossRef]
- Zykubek, K.; Proudfoot, T.; Lithgow, K.; Carpenter, D. Research on the selection of biocides for the ‘disinfection’ of statues and masonry at the National Trust (UK). J. Inst. Conserv. 2020, 43, 225–241. [Google Scholar] [CrossRef]
- Seaward, M.R.D. Lichens as Agents of Biodeterioration. In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 1. [Google Scholar] [CrossRef]
- Richardson, B.A. Control of microbial growth on stone and concrete. In Biodeterioration; Houghton, D.R., Smith, R.N., Eggins, H.O.W., Eds.; Elsevier Applied Science: London, UK; New York, NY, USA, 1988; Volume 7, pp. 101–106. [Google Scholar]
- Williams, T.M. The Mechanism of Action of Isothiazolone Biocide; CORROSION: San Diego, CA, USA, 2006. [Google Scholar]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. COBIOT 2015, 33, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, L.; Lamenti, G.; Tiano, P. Chlorophyll fluorescence for evaluating biocide treatments against phototrophic biodeteriogens. Anal. Microbial. 2002, 52, 197–206. [Google Scholar]
- Khan, A.H.; Libby, M.; Winnick, D.; Palmer, J.; Sumarah, M.; Ray, M.B.; Macfie, S.M. Uptake and phytotoxic effect of benzalkonium chlorides in Lepidium Sativum and Lactuca Sativa. J. Environ. Manag. 2018, 206, 490–497. [Google Scholar] [CrossRef]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone biocides: Chemistry, biological, and toxicity profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef]
- Collier, P.J.; Ramsey, A.J.; Austin, P.; Gilbert, P. Growth inhibitory and biocidal activity of some isothiazolone biocides. J. Appl. Bacteriol. 1990, 69, 569–577. [Google Scholar] [CrossRef]
- Diehl, M.A.; Chapman, J.S. Association of the biocide 5-chloro-2-methyl-isothiazol-3-one with Pseudomonas Aeruginosa and Pseudomonas fluorescens. Int. Biodeterior. Biodegrad. 1999, 44, 191–199. [Google Scholar] [CrossRef]
- García, M.R.; Cabo, M.L. Optimization of E. coli inactivation by benzalkonium chloride reveals the importance of quantifying the inoculum effect on chemical disinfection. Front. Microbiol. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Jovović, M.; Kostić, N.; Jančić-Stojanović, B.; Malenović, A. Investigation of tropicamide and benzalkonium chloride stability using liquid chromatography. J. Liq. Chromatogr. R. T. 2012, 35, 231–239. [Google Scholar] [CrossRef]
- Patrauchan, M.A.; Oriel, P.J. Degradation of benzyldimethylalkylammonium chloride by Aeromonas hydrophila sp. K. J. Appl. Microbiol. 2003, 94, 266–272. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-W.; Liu, W.-Z.; Tang, C.-C.; Liang, B.; Guo, Z.-C.; Wang, L.; Ren, Y.-X.; Wang, A.-J. Performance and microbial community responses of anaerobic digestion of waste activated sludge to residual benzalkonium chlorides. Energy Convers. Manag. 2019, 202, 112211. [Google Scholar] [CrossRef]
- Pereira, B.P.M.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Fernández-Calviño, D.; Brandt, K.K.; Storgaard, M.S.; Sanderson, H.; Bester, K. Biocide runoff from building facades: Degradation kinetics in soil. Environ. Sci. Technol. 2017, 51, 3694–3702. [Google Scholar] [CrossRef]
- Jing, G.; Zhou, Z.; Zhuo, J. Quantitative structure–activity relationship (QSAR) study of toxicity of quaternary ammonium compounds on Chlorella pyrenoidosa and Scenedesmus quadricauda. Chemosphere 2012, 86, 76–82. [Google Scholar] [CrossRef]
- DeLeo, P.C.; Huynh, C.; Pattanayek, M.; Schmid, K.C.; Pechacek, N. Assessment of ecological hazards and environmental fate of disinfectant quaternary ammonium compounds. Ecotox. Environ. Saf. 2020, 206, 111116. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Kosiróg, A.; Otlewska, A.; Rajkowska, K.; Piotrowska, M. Multistep approach to control microbial fouling of historic building materials by aerial phototrophs. Biofouling 2019, 35, 284–298. [Google Scholar] [CrossRef]
- McDonnel, G.; Russel, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microb. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Salton, M.R.J. Lytic agents, cell permeability, and monolayer penetrability. J. Gen. Physiol. 1968, 52, 227–252. [Google Scholar] [CrossRef] [PubMed]
- EPA. Reregistration Eligibility Decision for 2-Octyl-3 (2H)-isothiazolone (OIT). 2007. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/octhilinone-red.pdf (accessed on 4 January 2021).
- Küpper, H.; Benedikty, Z.; Morina, F.; Andresen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP chlorophyll fluorescence kinetics and Q A reoxidation kinetics by direct fast imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, M.J.; Gohlke, A.; Mahler, C.; Schmiedinger, A.; Beierkuhnlein, C. Quantification of wall surface heterogeneity and its influence on species diversity at medieval castles–implications for the environmentally friendly preservation of cultural heritage. J. Cult. Herit. 2013, 14, 219–228. [Google Scholar] [CrossRef]
- Elharech, M.; Benharbit, M.; Magri, N.; Benharbit, O.; Zidane, L.; Douira, A.; Dahmani, J. Study of the bryological flora at the archaeological site of Chellah, Morocco. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238834. [Google Scholar] [CrossRef]
- Rojsitthisak, P.; Wichitnithad, W.; Pipitharome, O.; Sanphanya, K.; Thanawattanawanich, P. Simple HPLC determination of benzalkonium chloride in ophthalmic formulations containing antazoline and tetrahydrozoline. PDA J. Pharm. Sci. Technol. 2005, 59, 332–337. [Google Scholar] [PubMed]
- Rosero-Moreano, M.; Canellas, E.; Nerín, C. Three-phase hollow-fiber liquid-phase microextraction combined with HPLC–UV for the determination of isothiazolinone biocides in adhesives used for food packaging materials. J. Sep. Sci. 2014, 37, 272–280. [Google Scholar] [CrossRef]
- Vannini, A.; Canali, G.; Pica, M.; Nali, C.; Loppi, S. The water content drives the susceptibility of the lichen Evernia prunastri and the moss Brachythecium sp. to high ozone concentrations. Biology 2020, 9, 90. [Google Scholar] [CrossRef]
- Castro, K.L.; Sanchez-Azofeifa, G.A. Changes in spectral properties, chlorophyll content and internal mesophyll structure of senescing Populus balsamifera and Populus tremuloides leaves. Sensors 2008, 8, 51–69. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 27 December 2020).

| Evernia | Brachythecium | ||||
|---|---|---|---|---|---|
| Days | Biotin T | Preventol | Biotin T | Preventol | |
| Chlorophyll content | 1 | 0.56 ± 0.01 * a | 0.59 ± 0.01 * a | 0.18 ± 0.00 * a | 0.34 ± 0.01 * a |
| 3 | 0.05 ± 0.00 * b | 0.29 ± 0.01 * b | 0.10 ± 0.01 * b | 0.35 ± 0.01 * a | |
| 21 | 0.00 ± 0.00 * c | 0.00 ± 0.00 * c | 0.00 ± 0.00 * c | 0.00 ± 0.00 * b | |
| Evernia | Brachythecium | ||||
| days | Biotin T | Preventol RI80 | Biotin T | Preventol RI80 | |
| NDVI | 1 | 0.70 ± 0.02 * a | 0.73 ± 0.02 * a | 0.55 ± 0.01 * a | 0.63 ± 0.01 * a |
| 3 | 0.76 ± 0.02 * a | 0.69 ± 0.01 * a | 0.46 ± 0.01 * b | 0.63 ± 0.01 * a | |
| 21 | 0.49 ± 0.01 * b | 0.46 ± 0.01 * b | 0.22 ± 0.00 * c | 0.28 ± 0.00 * b | |
| Evernia | Brachythecium | ||||
| days | Biotin T | Preventol RI80 | Biotin T | Preventol RI80 | |
| FV/FM | 1 | 0.05 ± 0.00 * | 0.04 ± 0.00 * | 0.07 ± 0.00 * | 0.05 ± 0.00 * |
| 3 | 0.07 ± 0.00 * | 0.05 ± 0.00 * | 0.05 ± 0.00 * | 0.05 ± 0.00 * | |
| 21 | 0.05 ± 0.00 * | 0.10 ± 0.00 * | 0.06 ± 0.00 * | 0.06 ± 0.00 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannini, A.; Canali, G.; Favero-Longo, S.E.; Loppi, S. Accumulation and Phytotoxicity of Two Commercial Biocides in the Lichen Evernia prunastri and the Moss Brachythecium sp. Stresses 2021, 1, 69-77. https://doi.org/10.3390/stresses1020006
Vannini A, Canali G, Favero-Longo SE, Loppi S. Accumulation and Phytotoxicity of Two Commercial Biocides in the Lichen Evernia prunastri and the Moss Brachythecium sp. Stresses. 2021; 1(2):69-77. https://doi.org/10.3390/stresses1020006
Chicago/Turabian StyleVannini, Andrea, Giulia Canali, Sergio Enrico Favero-Longo, and Stefano Loppi. 2021. "Accumulation and Phytotoxicity of Two Commercial Biocides in the Lichen Evernia prunastri and the Moss Brachythecium sp." Stresses 1, no. 2: 69-77. https://doi.org/10.3390/stresses1020006
APA StyleVannini, A., Canali, G., Favero-Longo, S. E., & Loppi, S. (2021). Accumulation and Phytotoxicity of Two Commercial Biocides in the Lichen Evernia prunastri and the Moss Brachythecium sp. Stresses, 1(2), 69-77. https://doi.org/10.3390/stresses1020006








