The Influence of Molarity Activity on the Green and Mechanical Properties of Geopolymer Concrete
Abstract
1. Introduction
2. Materials and the Experimental Procedure
2.1. Materials
2.2. Mixture Proportions
2.3. Mixing Process and Specimen Preparation
2.4. Testing Procedure
3. Result and Discussion
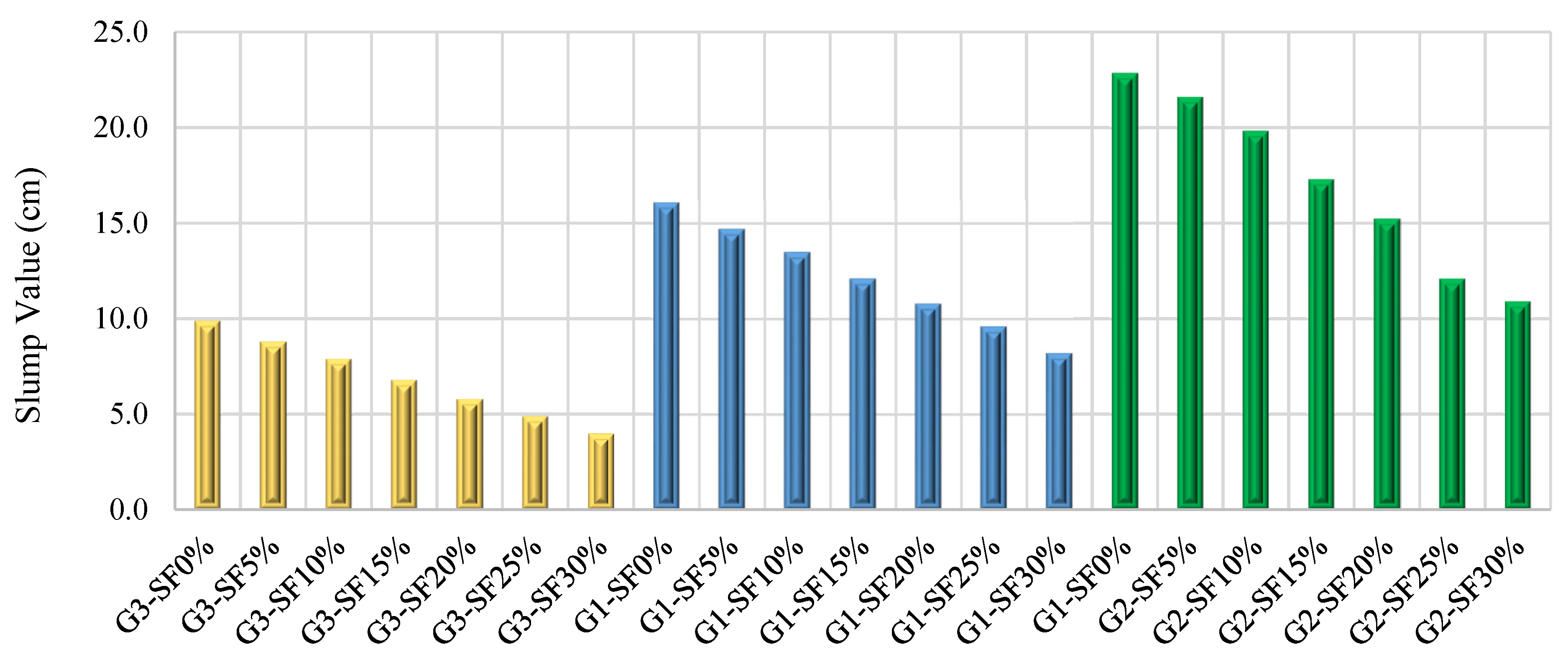
3.1. Workability
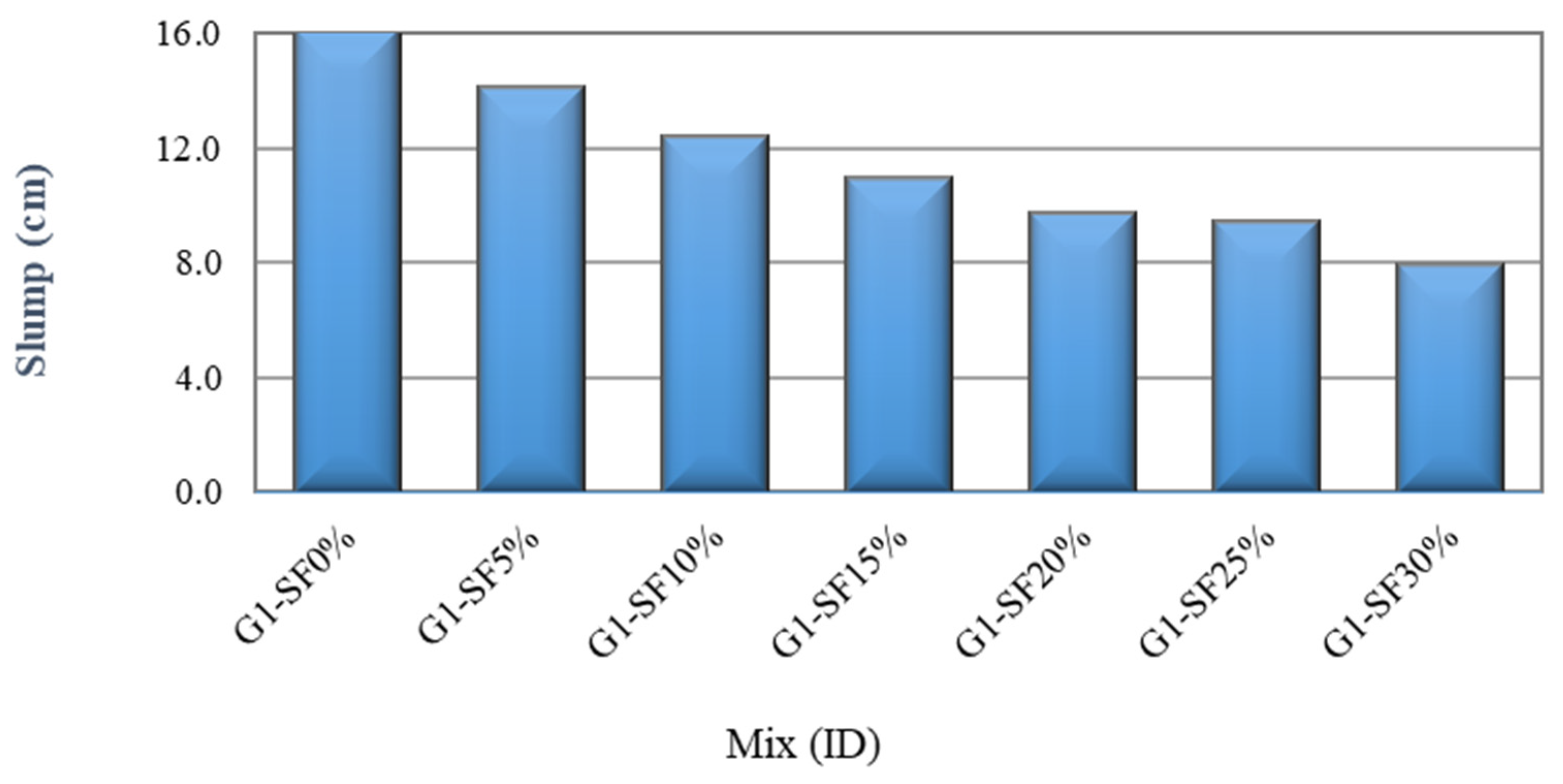
3.1.1. The Effect of Silica Fume Content
3.1.2. Effect of Alkaline Activator Solution Content
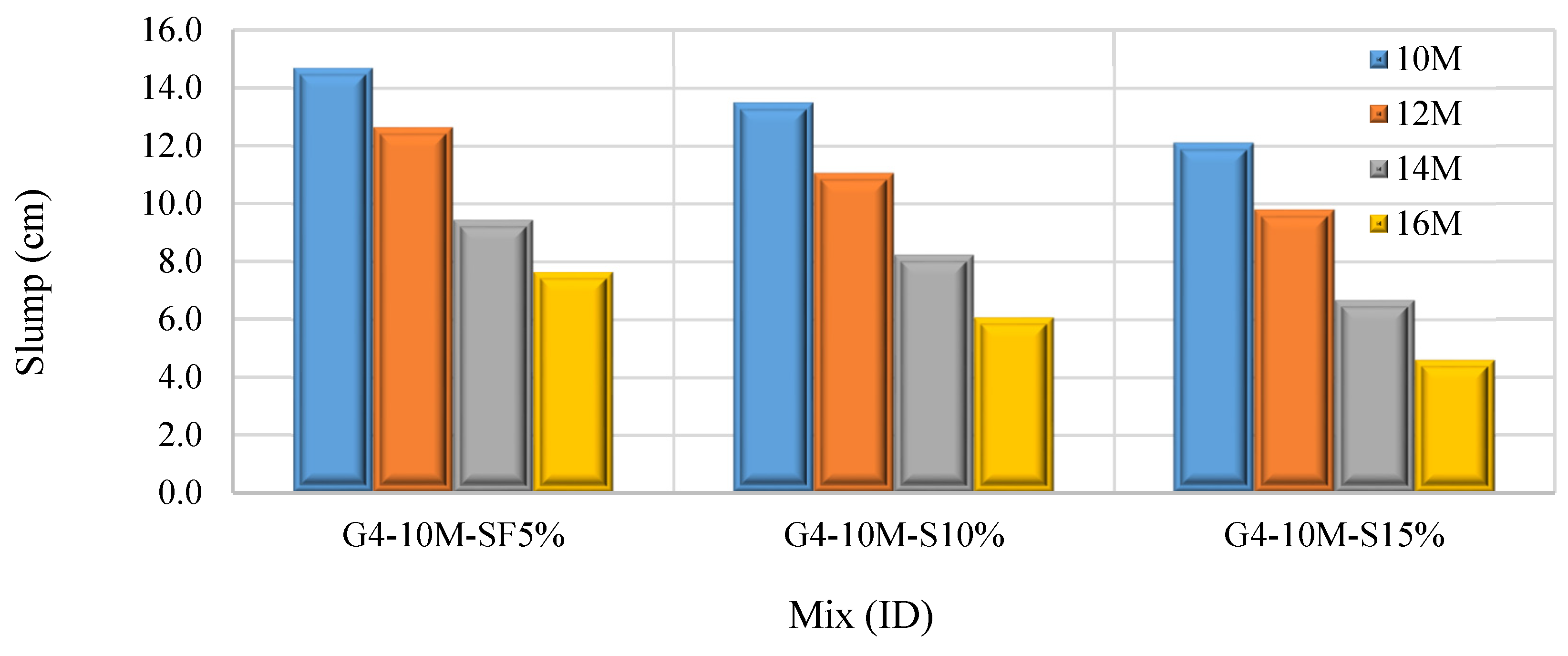
3.1.3. Effect of Sodium Hydroxide Molarity
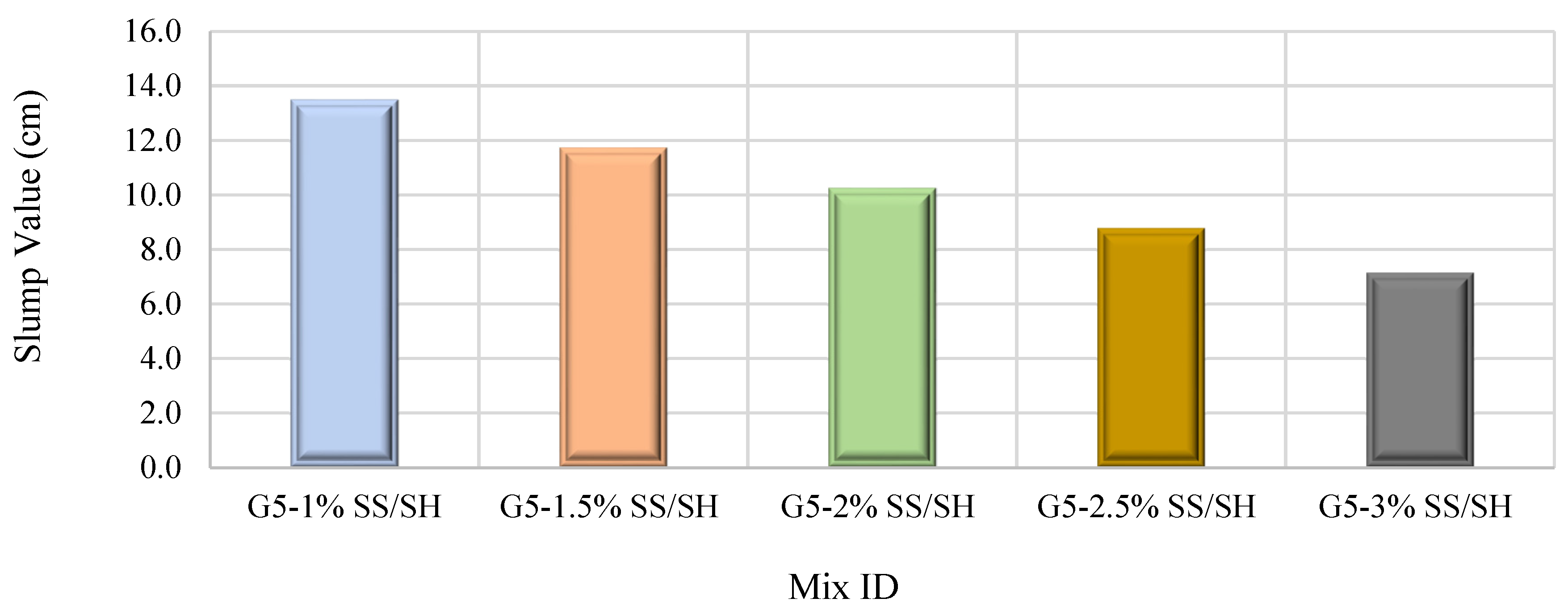
3.1.4. Impact of Sodium Silicate-to-Sodium Hydroxide Ratio
3.1.5. Effect of Additional Water
3.2. Compressive Strength
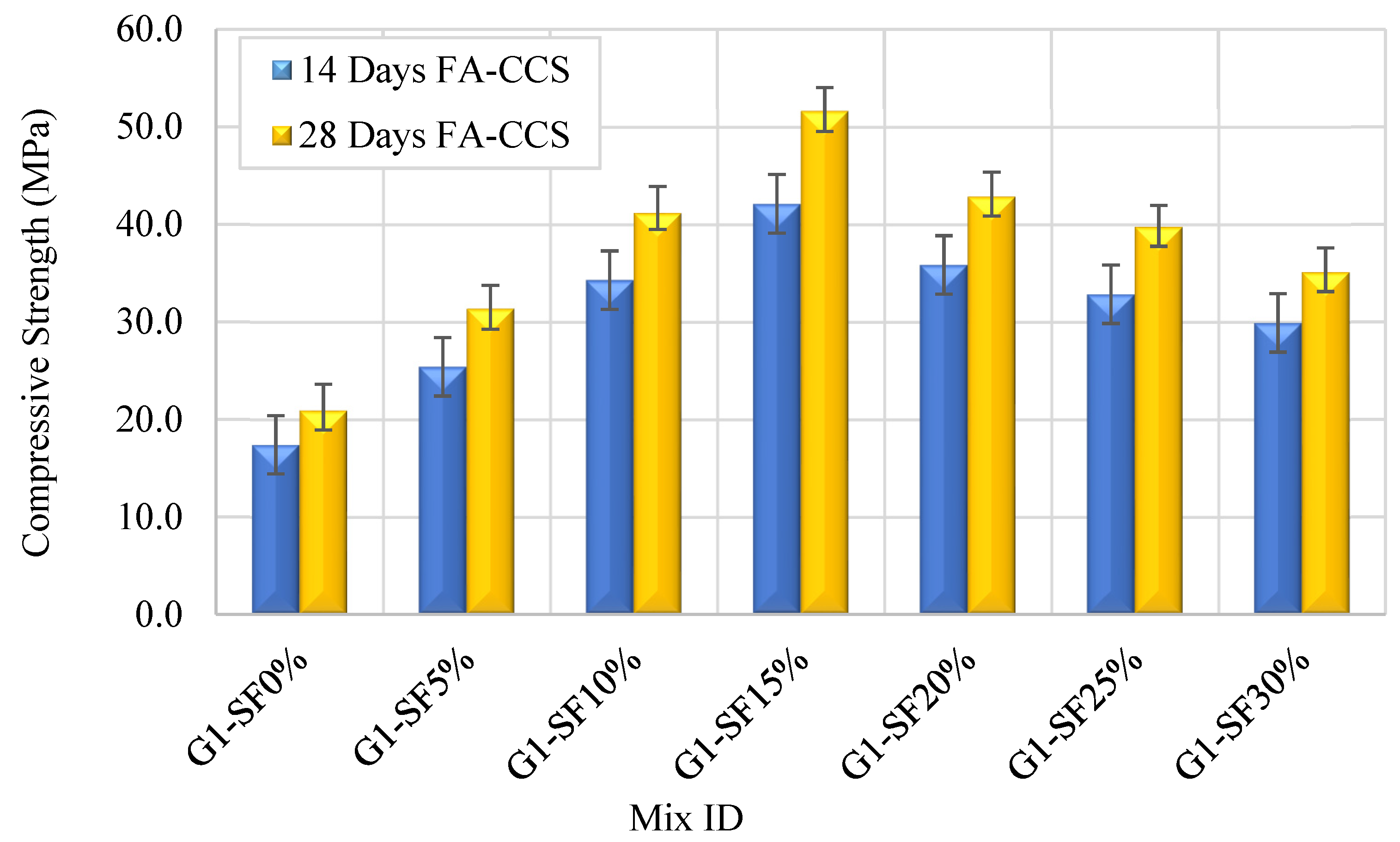
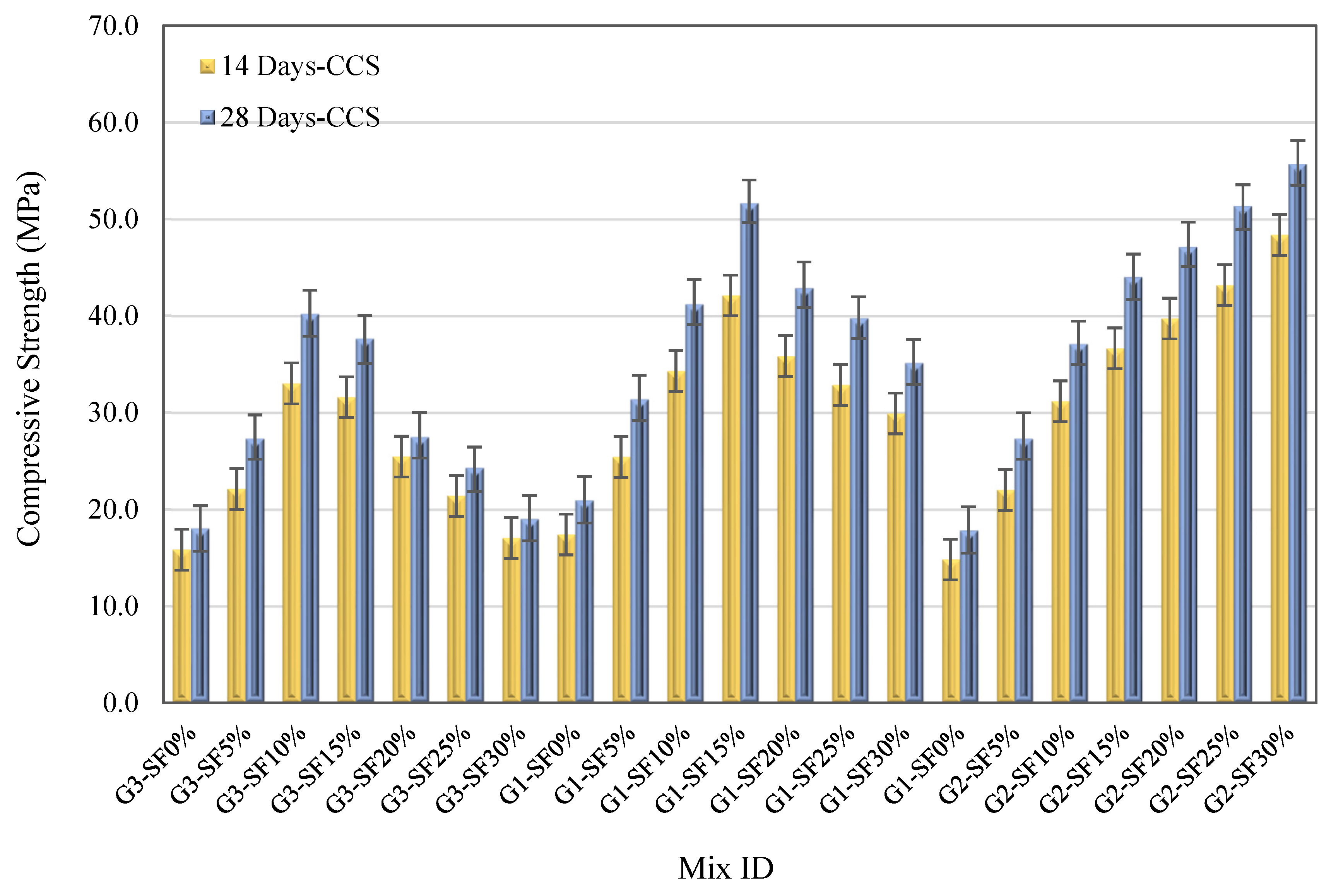
3.2.1. Impact of Silica Fume Content
3.2.2. Effect of Alkaline Activator Solution Content
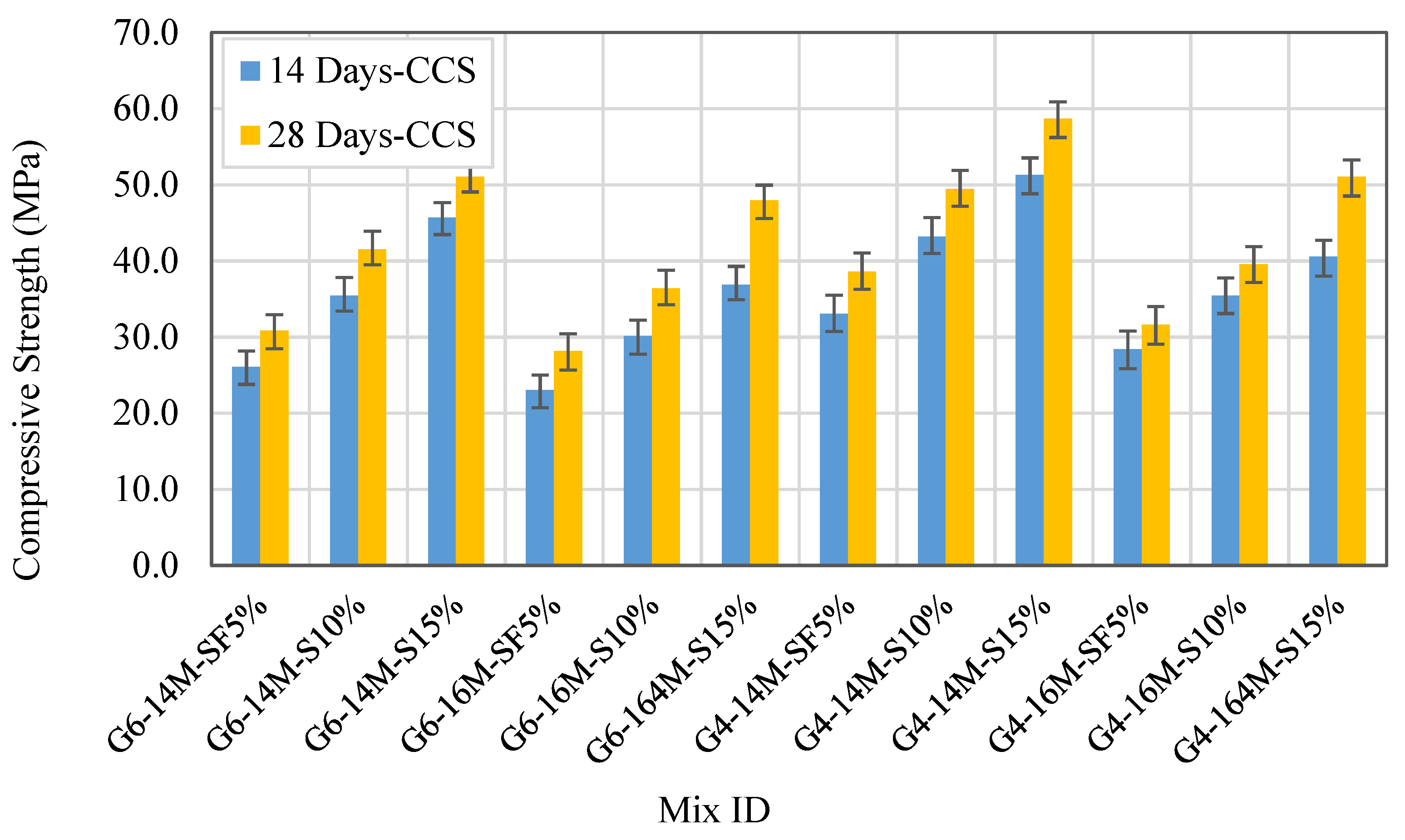
3.2.3. Effect of NaOH Molarity
3.2.4. Influence of Sodium Silicate-to-Sodium Hydroxide Ratio
3.2.5. Impact of Additional Water
4. Conclusions
- Increasing the SF content (5% to 30%), SS/SH ratio (1% to 3%), and NaOH molarity (10 M to 16 M) declines the workability of FA–GPCMs. On the other hand, reducing alkaline activator percentages to 35% results in a decrease in the FA–GPCM’s workability. The impact of low AAS content (35%) and high NaOH molarity (14 M and 16 M) on the FA–GPCM’s workability is more obvious in FA–GPCMs prepared with higher SF percentages.
- Increasing the SF replacement percentages from 5% to 15% in FA–GPCMs leads to significant 28- and 14-day compressive strength enhancements compared to FA–GPCM produced with 0% SF.
- The FA–GPC compressive strength development depends on the AAS percentages and SF content. The highest 28-day FA–CCS results can be realized from GPC samples produced with mixes SF30% and 45%AAS, SF15% and 40%AAS, as well as SF10% and 35%AAS.
- NaOH concentration involves a significant impact on the FA-GPC compressive strength. Increasing NaOH molarity from 10 to 14 M improves the FA–CCS. On the other hand, increasing NaOH concentration up to 16 M results in an adverse impact on the FA–GPC compressive strengths.
- Increasing the SS/SH (Na2SiO3/NaOH) ratio from 1% to 3% results in improving the 14- and 28-day FA–GPS concrete compressive strength due to modification of the FA-GPC matrix microstructure owing to improving silica content (SS) in a proportion that is compatible with the NaOH content to ensure an effective dissolution process.
- The 14- and 28-day compressive strengths of FA–GPC decline with additional water added to FA–GPCMs. The impact of extra water is less noticeable on FA–GPCMs produced with high SF content and NaOH molarity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Roche, M.Y. Built for net-zero: Analysis of long-term greenhouse gas emission pathways for the Nigerian cement sector. J. Clean. Prod. 2023, 383, 135446. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A.-L. Microencapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Hunger, M.; Entrop, A.; Mandilaras, I.; Brouwers, H.; Founti, M. The behavior of self-compacting concrete containing micro-encapsulated Phase Change Materials. Cem. Concr. Compos. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Wembe, J.T.; Ngueyep, L.L.M.; Moukete, E.E.A.; Eslami, J.; Pliya, P.; Ndjaka, J.-M.B.; Noumowe, A. Physical, mechanical properties and microstructure of concretes made with natural and crushed aggregates: Application in building construction. Clean. Mater. 2023, 7, 100173. [Google Scholar] [CrossRef]
- Hamada, H.M.; Abed, F.; Katman, H.Y.B.; Humada, A.M.; Al Jawahery, M.S.; Majdi, A.; Yousif, S.T.; Thomas, B.S. Effect of silica fume on the properties of sustainable cement concrete. J. Mater. Res. Technol. 2023, 24, 8887–8908. [Google Scholar] [CrossRef]
- Singaram, K.K.; Khan, M.A.; Talakokula, V.; Gurnani, C. Expansion in low calcium fly ash-based geopolymer concrete: Chemical factors influenced by silica fume and NaOH concentration. J. Sustain. Cem. Mater. 2024, 14, 74–88. [Google Scholar] [CrossRef]
- Mossie, A.T.; Khatiwada, D.; Palm, B.; Bekele, G. Investigating energy saving and climate mitigation potentials in cement production—A case study in Ethiopia. Energy Convers. Manag. 2023, 287, 117111. [Google Scholar] [CrossRef]
- Karadumpa, C.S.; Pancharathi, R.K. Study on energy use and carbon emission from manufacturing of OPC and blended cements in India. Environ. Sci. Pollut. Res. 2024, 31, 5364–5383. [Google Scholar] [CrossRef]
- Ghayeb, H.H.; Razak, H.A.; Sulong, N.R. Evaluation of the CO2 emissions of an innovative composite precast concrete structure building frame. J. Clean. Prod. 2019, 242, 118567. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Z.; Xie, Z.; Wei, L.; Hua, J.; Huang, L.; Yap, P.-S. Recent developments on natural fiber concrete: A review of properties, sustainability, applications, barriers, and opportunities. Dev. Built Environ. 2023, 16, 100255. [Google Scholar] [CrossRef]
- Waqas, R.M.; Butt, F.; Zhu, X.; Jiang, T.; Tufail, R.F. A Comprehensive Study on the Factors Affecting the Workability and Mechanical Properties of Ambient Cured Fly Ash and Slag Based Geopolymer Concrete. Appl. Sci. 2021, 11, 8722. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Kasehchi, E.; Arjomand, M.A.; Elizei, M.H.A. Experimental investigation of the feasibility of stabilizing inshore silty sand soil using geopolymer based on ceramic waste powder: An approach to upcycling waste material for sustainable construction. Case Stud. Constr. Mater. 2024, 20, e02979. [Google Scholar] [CrossRef]
- Al-Qutaifi, S.; Nazari, A.; Bagheri, A. Mechanical properties of layered geopolymer structures applicable in concrete 3D-printing. Constr. Build. Mater. 2018, 176, 690–699. [Google Scholar] [CrossRef]
- Divvala, S.; Rani, M.S. Early strength properties of geopolymer concrete composites: An experimental study. Mater. Today Proc. 2021, 47, 3770–3777. [Google Scholar] [CrossRef]
- Okoye, F.; Durgaprasad, J.; Singh, N. Effect of silica fume on the mechanical properties of fly ash based-geopolymer concrete. Ceram. Int. 2016, 42, 3000–3006. [Google Scholar] [CrossRef]
- Alghannam, M.; Albidah, A.; Abbas, H.; Al-Salloum, Y. Influence of Critical Parameters of Mix Proportions on Properties of MK-Based Geopolymer Concrete. Arab. J. Sci. Eng. 2021, 46, 4399–4408. [Google Scholar] [CrossRef]
- Ndahirwa, D.; Zmamou, H.; Lenormand, H.; Leblanc, N. The role of supplementary cementitious materials in hydration, durability and shrinkage of cement-based materials, their environmental and economic benefits: A review. Clean. Mater. 2022, 5, 100123. [Google Scholar] [CrossRef]
- Anitha, M.; Garg, A.; Babu, T.R. Experimental study of geopolymer concrete with recycled fine aggregates and alkali activators. Case Stud. Chem. Environ. Eng. 2023, 8, 100501. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Rangan, B.V. Fly ash-based geopolymer concrete. In Proceedings of the International Workshop on Geopolymer Cement and Concrete, Mumbai, India, 23 February 2008. [Google Scholar]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of elevated temperatures on mechanical properties of lightweight geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Al-Qutaifi, S.; Ethaib, S.; Awei, Y.R. Evaluating the Impact of Inclusion Metakaolin and Silica Fume on the Green and Mechanical Properties of Low Calcium Fly Ash Concrete. Ann. De Chim. Sci. Des Mater. 2022, 46, 323–331. [Google Scholar] [CrossRef]
- Komnitsas, K.A. Potential of geopolymer technology towards green buildings and sustainable cities. Procedia Eng. 2011, 21, 1023–1032. [Google Scholar] [CrossRef]
- Hamada, H.M.; Tayeh, B.A.; Al-Attar, A.; Yahaya, F.M.; Muthusamy, K.; Humada, A.M. The present state of the use of eggshell powder in concrete: A review. J. Build. Eng. 2020, 32, 101583. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Obla, K.H.; Hill, R.L.; Thomas, M.D.A.; Shashiprakash, S.G.; Perebatova, O. Properties of concrete containing ultra-fine fly ash. ACI Mater. J. 2003, 100, 426–433. [Google Scholar]
- Koushkbaghi, M.; Alipour, P.; Tahmouresi, B.; Mohseni, E.; Saradar, A.; Sarker, P.K. Influence of different monomer ratios and recycled concrete aggregate on mechanical properties and durability of geopolymer concretes. Constr. Build. Mater. 2019, 205, 519–528. [Google Scholar] [CrossRef]
- Biswal, P.; Patel, N.; Samal, A.K.; Rao, M. Investigation on Mechanical Properties of Pondash/Natural Fiber Based Geopoly-Meric Products. Int. J. Res. Anal. Rev. 2019, 6, 324–332. [Google Scholar]
- Graytee, A.; Sanjayan, J.G.; Nazari, A. Development of a high strength fly ash-based geopolymer in short time by using microwave curing. Ceram. Int. 2018, 44, 8216–8222. [Google Scholar] [CrossRef]
- Bayrak, B.; Alcan, H.G.; Tanyildizi, M.; Kaplan, G.; İpek, S.; Aydin, A.C.; Güneyisi, E. Effects of silica fume and rice husk ash contents on engineering properties and high-temperature resistance of slag-based prepacked geopolymers. J. Build. Eng. 2024, 92, 10974. [Google Scholar] [CrossRef]
- İpek, S. Macro and micro characteristics of eco-friendly fly ash-based geopolymer composites made of different types of recycled sand. J. Build. Eng. 2022, 52, 104431. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Jiang, S.; Xia, Z.; Zhang, Q. Effect of silica fume with various fineness levels on the strength degradation of concrete after steam curing. Constr. Build. Mater. 2025, 464, 140169. [Google Scholar] [CrossRef]
- Uddin, A.; Uddin, A.; Bashir, M.T.; Bashir, M.T.; Khan, A.M.; Khan, A.M.; Alsharari, F.; Alsharari, F.; Farid, F.; Farid, F.; et al. Effect of Silica Fume on Compressive Strength and Water Absorption of the Portland Cement–Silica Fume Blended Mortar. Arab. J. Sci. Eng. 2024, 49, 4803–4811. [Google Scholar] [CrossRef]
- Mermerdaş, K.; İpek, S.; Mahmood, Z. Visual inspection and mechanical testing of fly ash-based fibrous geopolymer composites under freeze-thaw cycles. Constr. Build. Mater. 2021, 283, 122756. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shariq, M.; Mahdi, F. Multioptimization of FA-Based Geopolymer Concrete Mixes: A Synergistic Approach Using Gray Relational Analysis and Principal Component Analysis. J. Struct. Des. Constr. Pract. 2025, 30, 04024101. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Andrushia, D.; Cashell, K.A. Post-Fire Performance of Binary-Blended Geopolymer Concrete Structural Members. Fire Technol. 2025, 1–32. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N. Mechanical properties of silica fume based concrete: A review. Mater. Today Proc. 2024, in press. [Google Scholar] [CrossRef]
- Dutta, D.; Thokchom, S.; Ghosh, P.; Ghosh, S. Effect of silica fume additions on porosity of fly ash geopolymers. J. Eng. Appl. Sci. 2010, 5, 74–79. [Google Scholar]
- Al-Qutaifi, S.; Bagheri, A. Evaluating Fresh and Hardened Properties of High-Strength Concrete Including Closed Steel Fibres. Open Civ. Eng. J. 2021, 15, 104–114. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Li, Q.; Zhang, J. Relationship between Microstructure and Thermal Conductivity in Coal Slags with Variable Silica and Alumina. Energy Fuels 2019, 33, 6226–6233. [Google Scholar] [CrossRef]
- Nurruddin, M.F.; Sani, H.; Mohammed, B.S.; Shaaban, I. Methods of curing geopolymer concrete: A review. Int. J. Adv. Appl. Sci. 2018, 5, 31–36. [Google Scholar] [CrossRef]
- ASTM C143/C143M-15; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C39/C39M-14; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2014.
- Arunachalam, S.K.; Kadarkarai, A.; Thankaswamy, J.; Karuppasamy, M.; Vagestan, P.K.; Pradeep, D.; Sakhamuri, S.S.K. Effect of silica fume on rheological, mechanical and durability properties of ground granulated blast furnace slag based geopolymer concrete. In Proceedings of the International Conference on Recent Innovations in Science and Technology (RIST2022), Malappuram, India, 8–9 July 2022. [Google Scholar]
- Pan, Z.; Tan, M.; Zheng, G.; Wei, L.; Tao, Z.; Hao, Y. Effect of silica fume type on rheology and compressive strength of geopolymer mortar. Constr. Build. Mater. 2024, 430, 136488. [Google Scholar] [CrossRef]
- Zhang, R.; He, H.; Song, Y.; Zhi, X.; Fan, F. Influence of mix proportioning parameters and curing regimes on the properties of ultra-high strength alkali-activated concrete Quality of English Language. Constr. Build. Mater. 2023, 393, 132139. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Ebailila, M. Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution. Materials 2023, 16, 2400. [Google Scholar] [CrossRef]
- Venkatesan, G.; Alengaram, U.J.; Ibrahim, S.; Ibrahim, M.S. Effect of Fly Ash characteristics, sodium-based alkaline activators, and process variables on the compressive strength of siliceous Fly Ash geopolymers with microstructural properties: A comprehensive review. Constr. Build. Mater. 2024, 437, 136808. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix design and mechanical properties of geopolymer and alkali activated concrete: Review of the state-of-the-art and the development of a new unified approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Fan, L.; Chen, D.; Zhong, W. Effects of slag and alkaline solution contents on bonding strength of geopolymer-concrete composites. Constr. Build. Mater. 2023, 406, 133391. [Google Scholar] [CrossRef]
- Huseien, G.F.; Hussein, Z.J.; Kubba, Z.; Nikolaevich, B.M.; Mirza, J. Improved Bond Strength Performance of Geopolymer Mortars: Role of High Volume Ground Blast Furnace Slag, Fly Ash, and Palm Oil Fuel Ash Incorporation. Minerals 2023, 13, 1096. [Google Scholar] [CrossRef]
- Xu, F.; Chen, G.; Li, K.; Zhang, Z.; Luo, Z.; Li, S.; Yang, D.; Li, X.; Zhang, X. Interfacial bond behavior between normal OPC concrete and self-compacting geopolymer concrete enhanced by nano-SiO. Constr. Build. Mater. 2024, 411, 134617. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Singh, P.K.; Rajhans, P. Comparative Analysis of Regression and ANN Algorithm for Predicting Compressive Strength of Sustainable Geopolymer Concrete at Varying NaOH Concentration and Curing Temperature. Iran. J. Sci. Technol. Trans. Civ. Eng. 2024, 48, 1273–1298. [Google Scholar] [CrossRef]

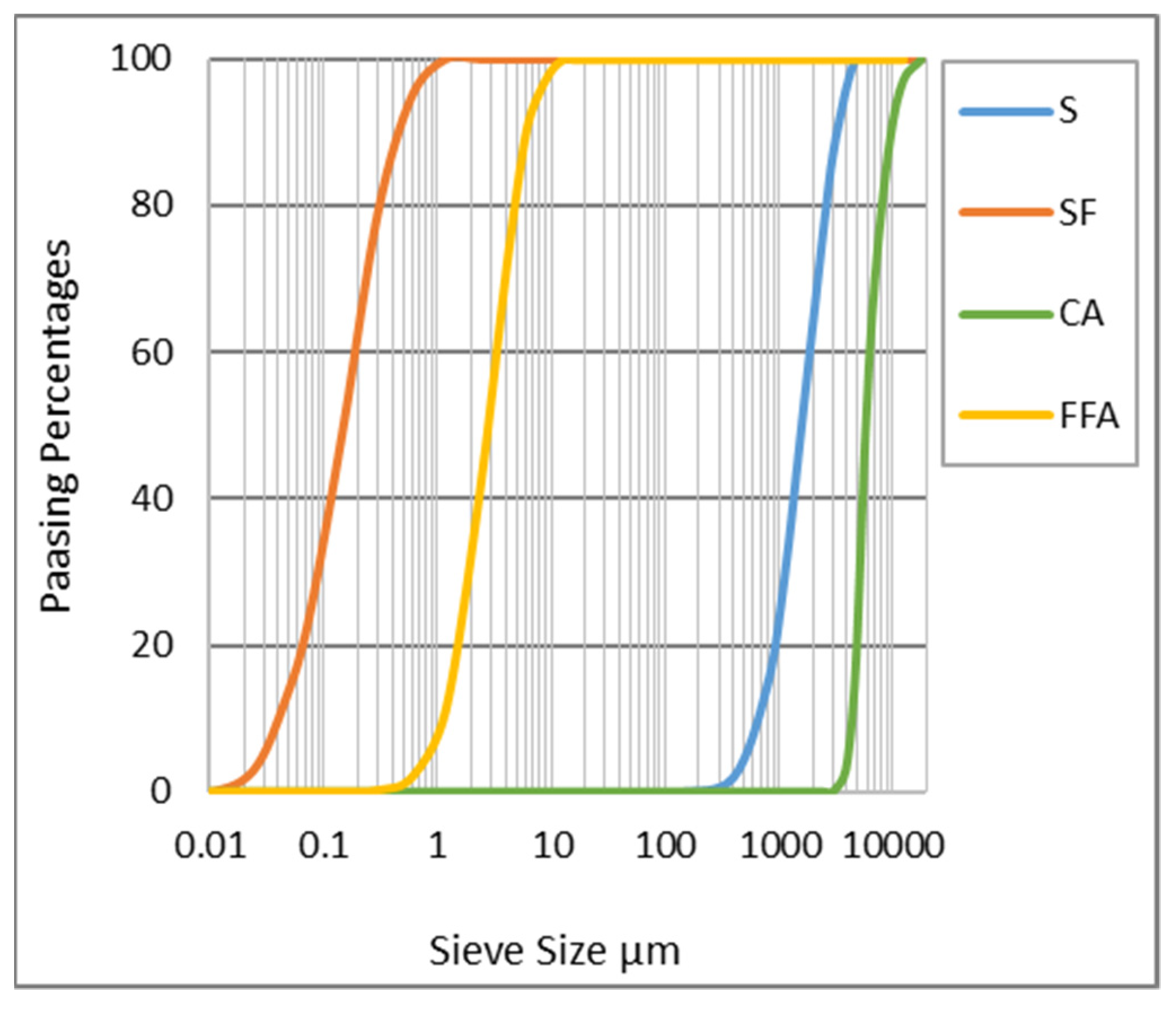





| Chemicals | FFA% | SF% |
|---|---|---|
| SiO2 | 51.23 | 91.9 |
| Al2O3 | 25.65 | 0.67 |
| CaO | 4.36 | 0.42 |
| Fe2O3 | 12.7 | 1.23 |
| MgO | 1.48 | 1.69 |
| Na2O | 0.81 | 0.43 |
| SO3 | 0.24 | — |
| K2O | 0.73 | 1.19 |
| LOI | 0.58 | 1.18 |
| P2O5 | 0.87 | — |
| Color | Gray | Dark gray |
| Group No. | Mix No. | Mix ID | FA | S | CA | SF% | AAS% | SS/SH% | M | AW% | SP% | The Studied Factor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit Content (kg/m3) | ||||||||||||
| 1 | 1 | G1–SF0% | 500 | 580 | 1110 | 0 | 40 | 1 | 10 | - | 4 | Effect of SF Content Effect of AAS Content Effects of NaOH Molarity Impact of SS/SH Ratio Impact of additional water |
| 2 | G1–SF5% | 475 | 580 | 1110 | 5 | 40 | 1 | 10 | - | 4 | ||
| 3 | G1–SF10% | 450 | 580 | 1110 | 10 | 40 | 1 | 10 | - | 4 | ||
| 4 | G1–SF15% | 425 | 580 | 1110 | 15 | 40 | 1 | 10 | - | 4 | ||
| 5 | G1–SF20% | 400 | 580 | 1110 | 20 | 40 | 1 | 10 | - | 4 | ||
| 6 | G1–SF25% | 375 | 580 | 1110 | 25 | 40 | 1 | 10 | - | 4 | ||
| 7 | G1–SF30% | 350 | 580 | 1110 | 25 | 40 | 1 | 10 | - | 4 | ||
| 2 | 8 | G2–SF0% | 500 | 580 | 1110 | 0 | 45 | 1 | 10 | - | 4 | |
| 9 | G2–SF5% | 475 | 580 | 1110 | 5 | 45 | 1 | 10 | - | 4 | ||
| 10 | G2–SF10% | 450 | 580 | 1110 | 10 | 45 | 1 | 10 | - | 4 | ||
| 11 | G2–SF15% | 425 | 580 | 1110 | 15 | 45 | 1 | 10 | - | 4 | ||
| 12 | G2–SF20% | 400 | 580 | 1110 | 20 | 45 | 1 | 10 | - | 4 | ||
| 13 | G2–SF25% | 375 | 580 | 1110 | 20 | 45 | 1 | 10 | - | 4 | ||
| 14 | G2–SF30% | 350 | 580 | 1110 | 25 | 45 | 1 | 10 | - | 4 | ||
| 3 | 15 | G3–SF0% | 500 | 580 | 1110 | 0 | 35 | 1 | 10 | - | 4 | |
| 16 | G3–SF5% | 475 | 580 | 1110 | 5 | 35 | 1 | 10 | - | 4 | ||
| 17 | G3–SF10% | 450 | 580 | 1110 | 10 | 35 | 1 | 10 | - | 4 | ||
| 18 | G3–SF15% | 425 | 580 | 1110 | 15 | 35 | 1 | 10 | - | 4 | ||
| 19 | G3–SF20% | 400 | 580 | 1110 | 15 | 35 | 1 | 10 | - | 4 | ||
| 20 | G3–SF25% | 375 | 580 | 1110 | 20 | 35 | 1 | 10 | - | 4 | ||
| 21 | G3–SF30% | 350 | 580 | 1110 | 25 | 35 | 1 | 10 | - | 4 | ||
| 4 | 22 | G4–10M–SF5% | 475 | 580 | 1110 | 5 | 40 | 1 | 10 | - | 4 | |
| 23 | G4–12M–SF5% | 475 | 580 | 1110 | 5 | 40 | 1 | 12 | - | 4 | ||
| 24 | G4–14M–SF5% | 475 | 580 | 1110 | 5 | 40 | 1 | 14 | - | 4 | ||
| 25 | G4–16M–SF5% | 475 | 580 | 1110 | 5 | 40 | 1 | 16 | - | 4 | ||
| 26 | G4–10M–S10% | 450 | 580 | 1110 | 10 | 40 | 1 | 10 | - | 4 | ||
| 27 | G4–12M–S10% | 450 | 580 | 1110 | 10 | 40 | 1 | 12 | - | 4 | ||
| 28 | G4–14M–S10% | 450 | 580 | 1110 | 10 | 40 | 1 | 14 | - | 4 | ||
| 29 | G4–16M–S10% | 450 | 580 | 1110 | 10 | 40 | 1 | 16 | - | 4 | ||
| 30 | G4–10M–S15% | 425 | 580 | 1110 | 15 | 40 | 1 | 10 | - | 4 | ||
| 31 | G4–12M–S15% | 425 | 580 | 1110 | 15 | 40 | 1 | 12 | - | 4 | ||
| 32 | G4–14M–S15% | 425 | 580 | 1110 | 15 | 40 | 1 | 14 | - | 4 | ||
| 33 | G4–16M–S15% | 425 | 580 | 1110 | 15 | 40 | 1 | 16 | - | 4 | ||
| 5 | 34 | G5–SS/SH1% | 450 | 580 | 1110 | 10 | 40 | 1 | 10 | - | 4 | |
| 35 | G5–SS/SH1.5% | 450 | 580 | 1110 | 10 | 40 | 1.5 | 10 | - | 4 | ||
| 36 | G5–SS/SH2% | 450 | 580 | 1110 | 10 | 40 | 2 | 10 | - | 4 | ||
| 37 | G5–SS/SH2.5% | 450 | 580 | 1110 | 10 | 40 | 2.5 | 10 | - | 4 | ||
| 38 | G5–SS/SH3% | 450 | 580 | 1110 | 10 | 40 | 3 | 10 | - | 4 | ||
| 6 | 39 | G6–SF0% | 500 | 580 | 1110 | 0 | 35 | 1 | 10 | 14.5 | 4 | |
| 40 | G6–SF5% | 475 | 580 | 1110 | 5 | 35 | 1 | 10 | 14.5 | 4 | ||
| 41 | G6–SF10% | 450 | 580 | 1110 | 10 | 35 | 1 | 10 | 14.5 | 4 | ||
| 42 | G6–SF15% | 425 | 580 | 1110 | 15 | 35 | 1 | 10 | 14.5 | 4 | ||
| 43 | G6–SF20% | 400 | 580 | 1110 | 15 | 35 | 1 | 10 | 14.5 | 4 | ||
| 44 | G6–SF25% | 375 | 580 | 1110 | 20 | 35 | 1 | 10 | 14.5 | 4 | ||
| 45 | G6–SF30% | 350 | 580 | 1110 | 25 | 35 | 1 | 10 | 14.5 | 4 | ||
| 46 | G6–16M–SF5% | 475 | 580 | 1110 | 5 | 40 | 1 | 16 | 14.5 | 4 | ||
| 47 | G6–16M–S10% | 450 | 580 | 1110 | 10 | 40 | 1 | 16 | 14.5 | 4 | ||
| 48 | G6–16M–S15% | 425 | 580 | 1110 | 15 | 40 | 1 | 16 | 14.5 | 4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qutaifi, S.; K. Hanan, A.; Hamza, A.J. The Influence of Molarity Activity on the Green and Mechanical Properties of Geopolymer Concrete. Constr. Mater. 2025, 5, 16. https://doi.org/10.3390/constrmater5010016
Al-Qutaifi S, K. Hanan A, Hamza AJ. The Influence of Molarity Activity on the Green and Mechanical Properties of Geopolymer Concrete. Construction Materials. 2025; 5(1):16. https://doi.org/10.3390/constrmater5010016
Chicago/Turabian StyleAl-Qutaifi, Sarah, Aliaa K. Hanan, and Ahmed Jabbar Hamza. 2025. "The Influence of Molarity Activity on the Green and Mechanical Properties of Geopolymer Concrete" Construction Materials 5, no. 1: 16. https://doi.org/10.3390/constrmater5010016
APA StyleAl-Qutaifi, S., K. Hanan, A., & Hamza, A. J. (2025). The Influence of Molarity Activity on the Green and Mechanical Properties of Geopolymer Concrete. Construction Materials, 5(1), 16. https://doi.org/10.3390/constrmater5010016




