The Effect of Biochar on the Properties of Alkali-Activated Slag Pastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Paste Mix Design
2.2. Methods
2.2.1. Absorption Test
2.2.2. Setting Time Test
2.2.3. Autogenous Shrinkage Test
2.2.4. Isothermal Calorimetry Test
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. X-ray Micro-Computed Tomography (Micro-CT) Analysis
2.2.7. Compressive Strength Test
2.2.8. Electrical Resistivity Test
3. Results
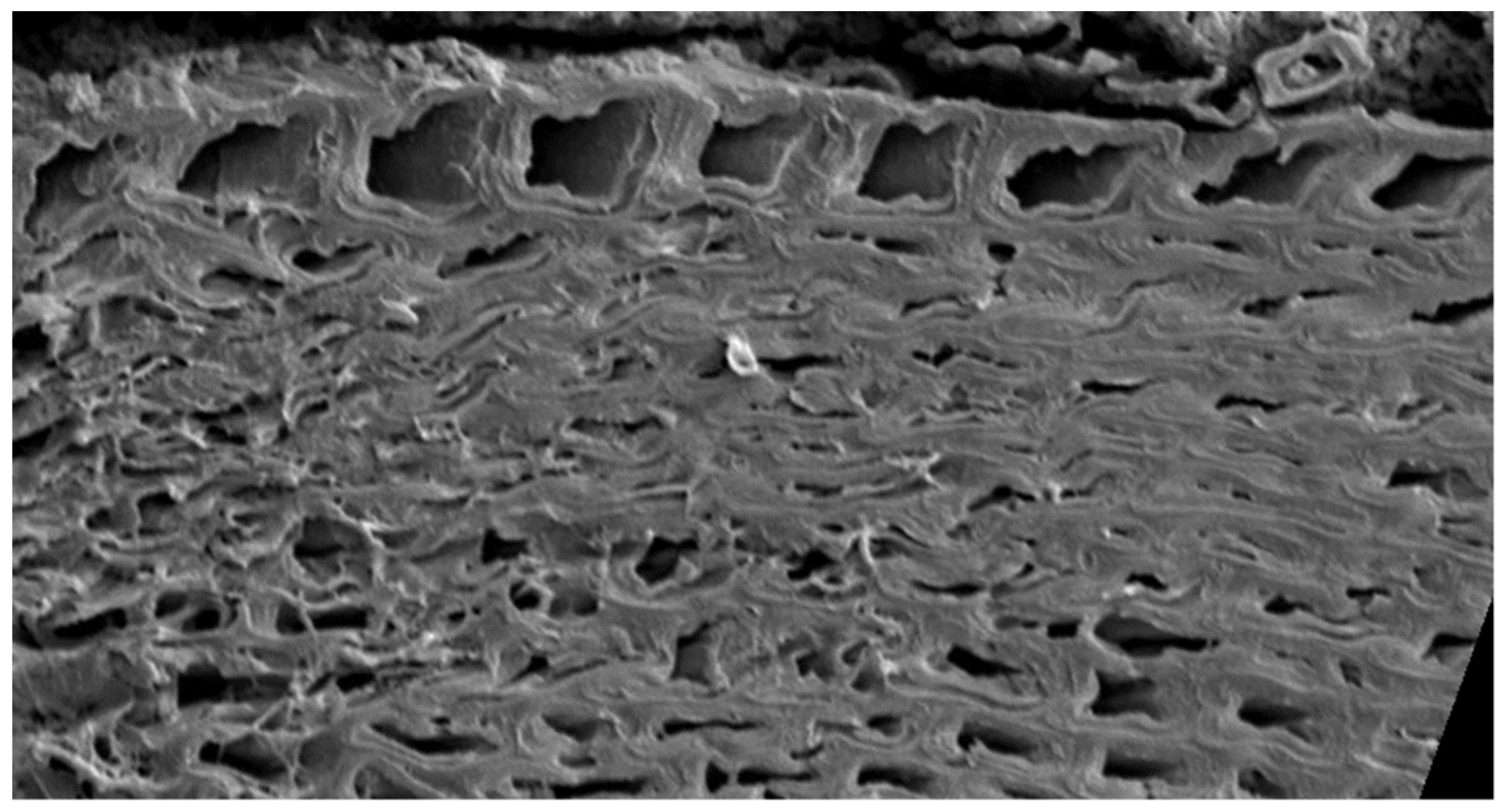
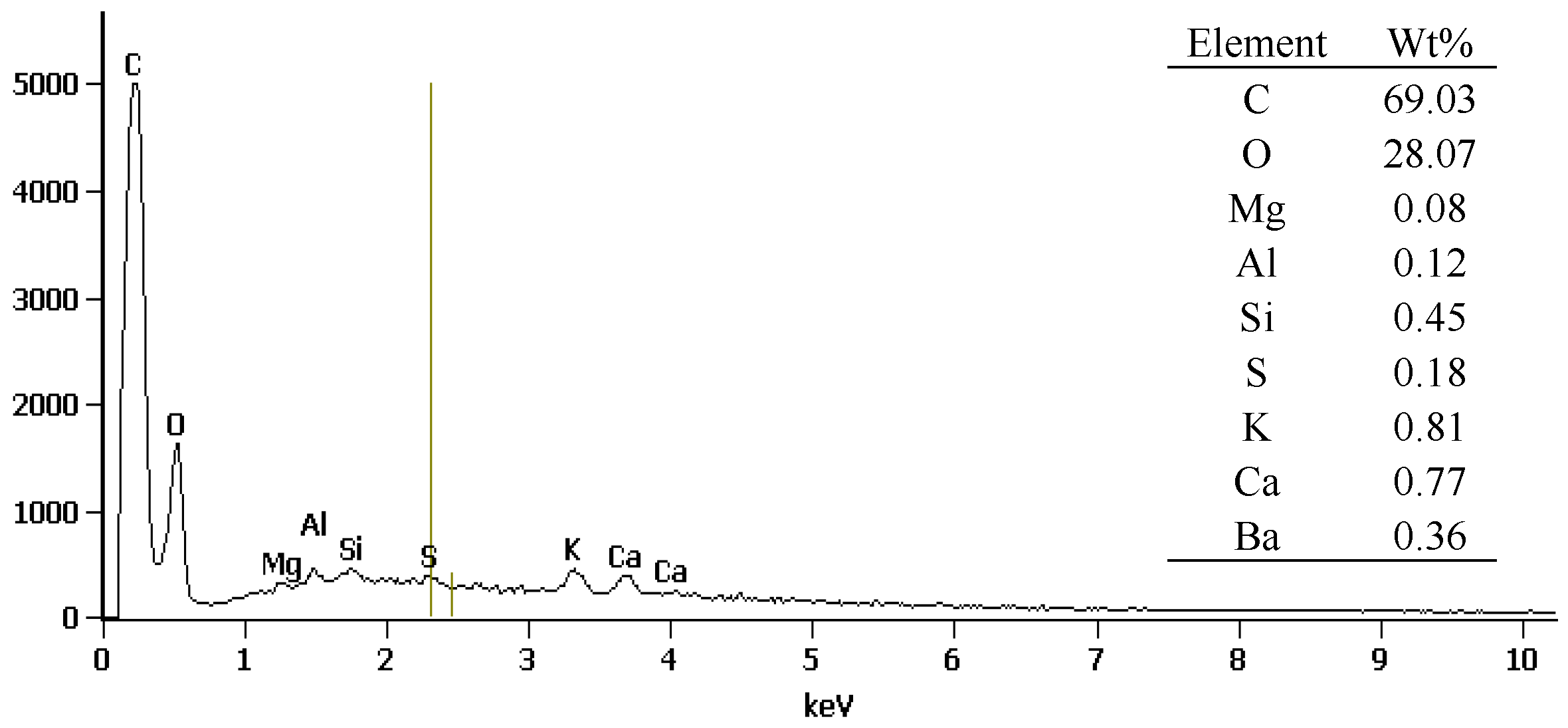
3.1. SEM, EDS, and FTIR of Biochar
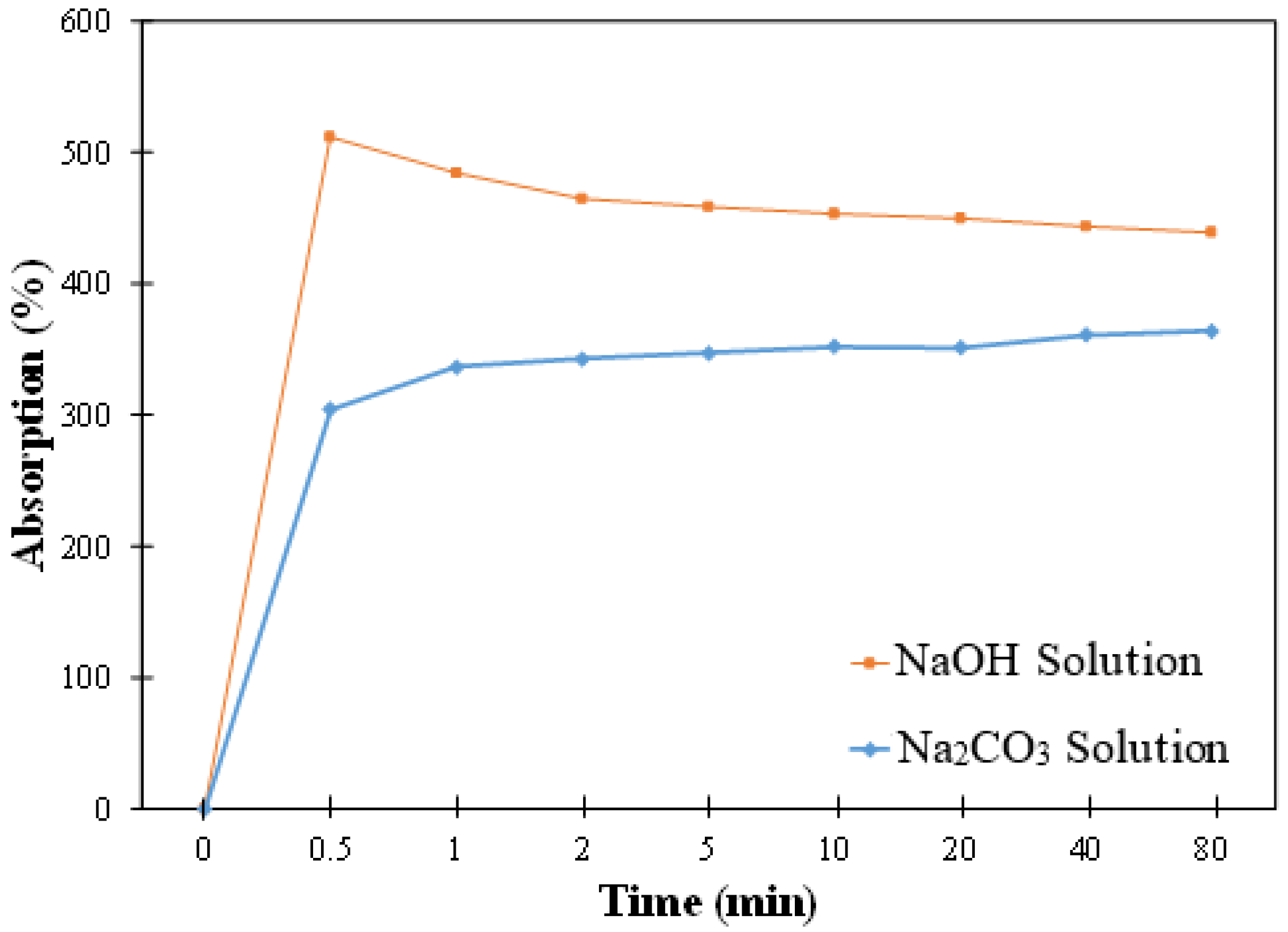
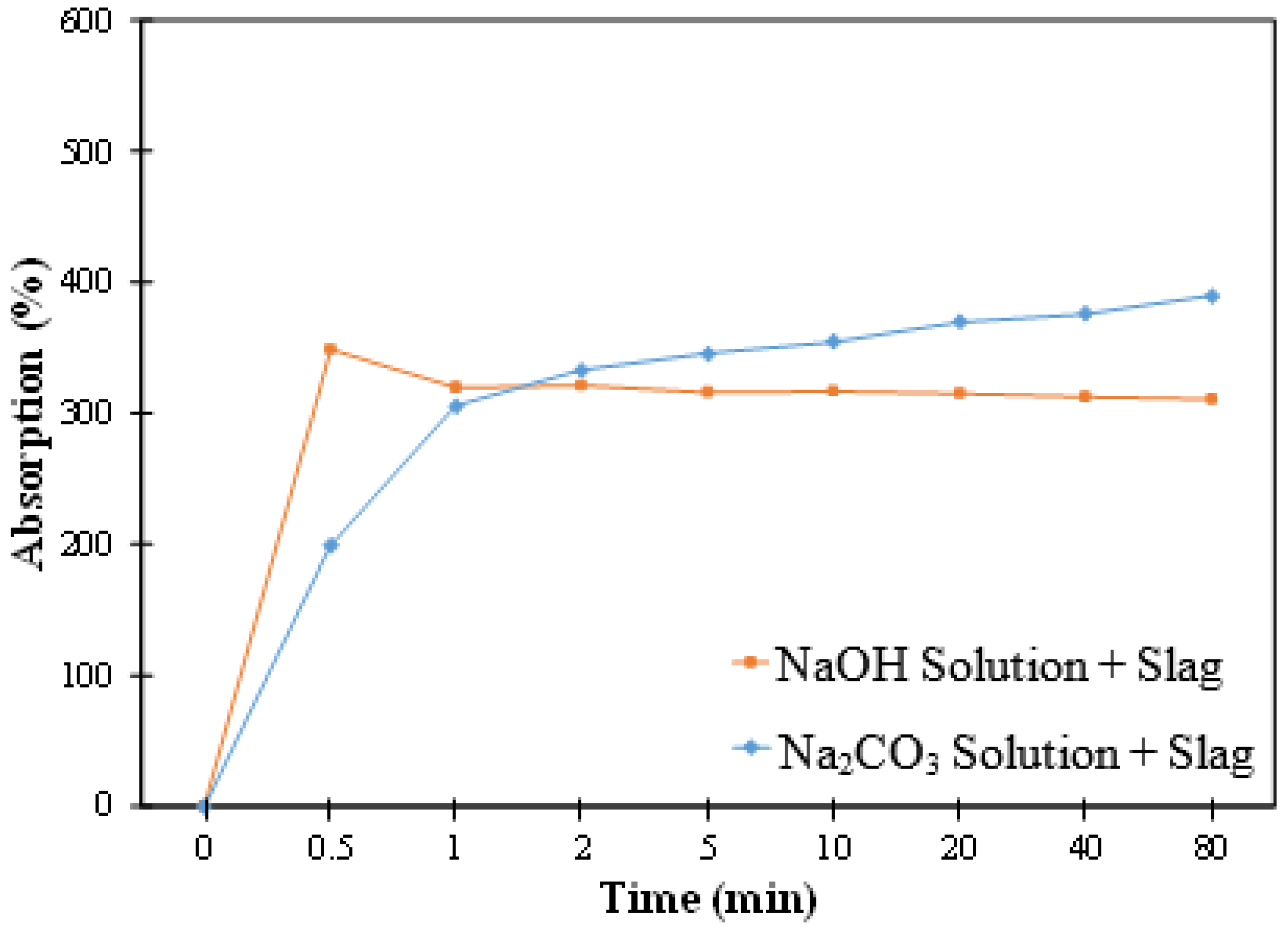
3.2. Absorption
3.2.1. Absorption in Solution
3.2.2. Absorption in the Activator Solution + Slag Mixtures
3.3. Isothermal Calorimetry
3.4. Setting Time
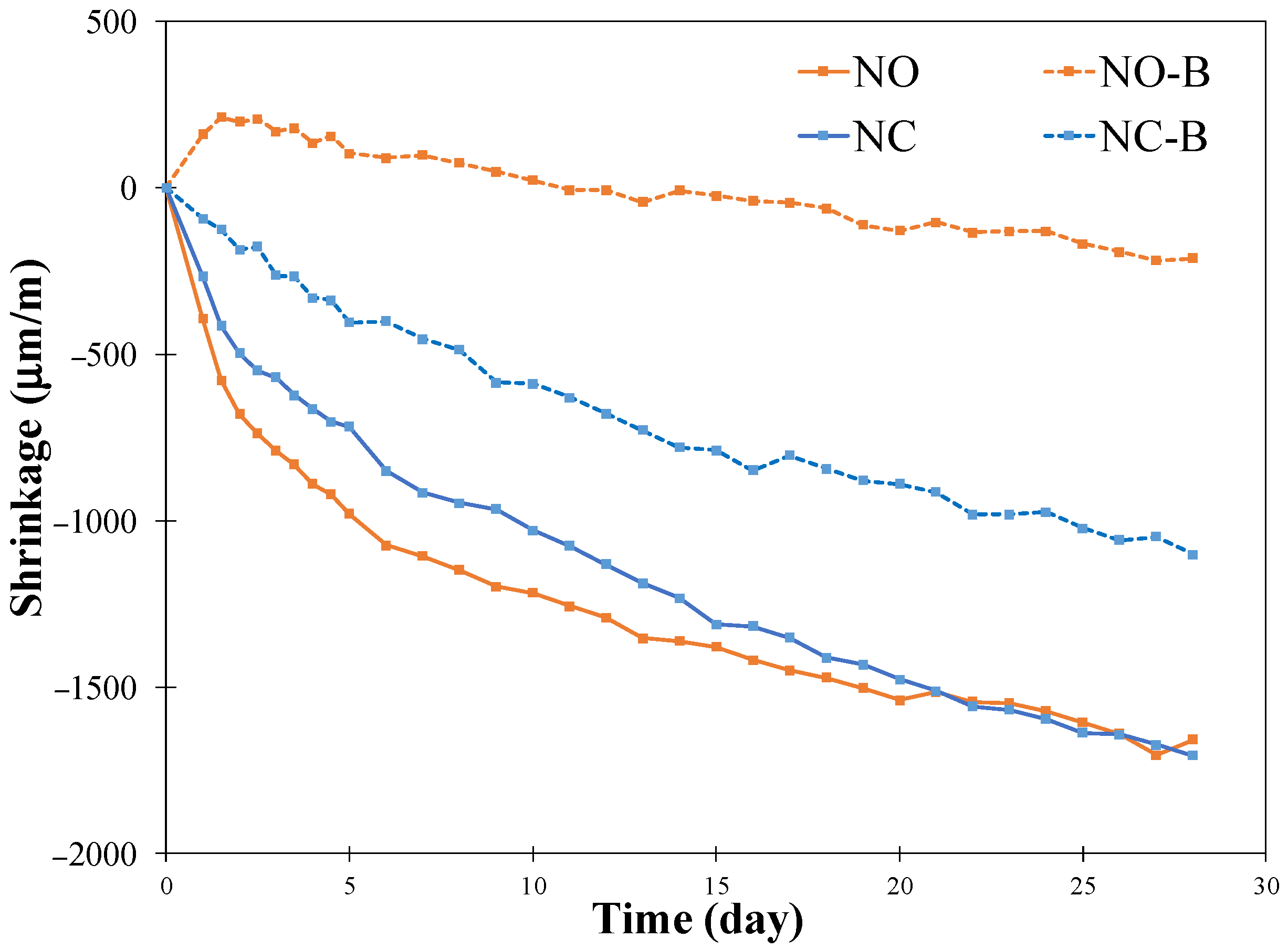
3.5. Autogenous Shrinkage
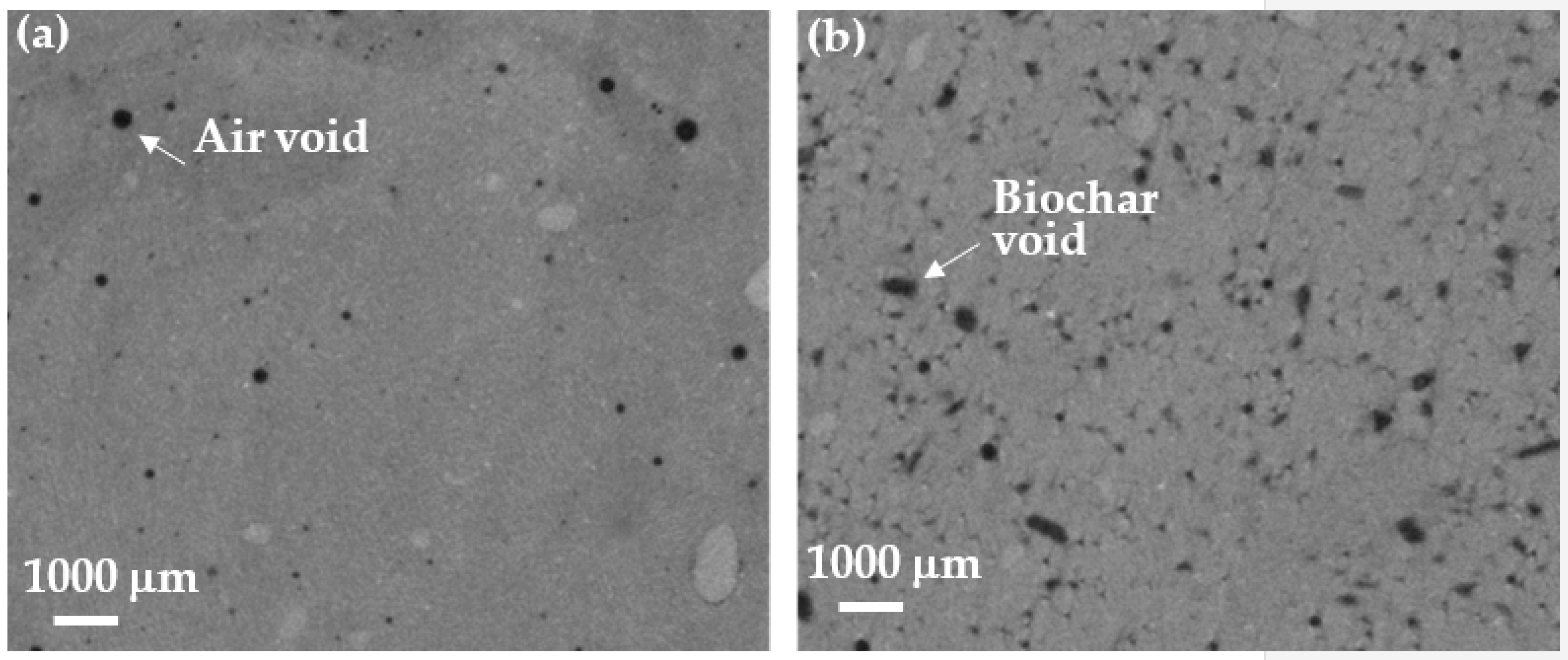
3.6. Void Structure Analysis
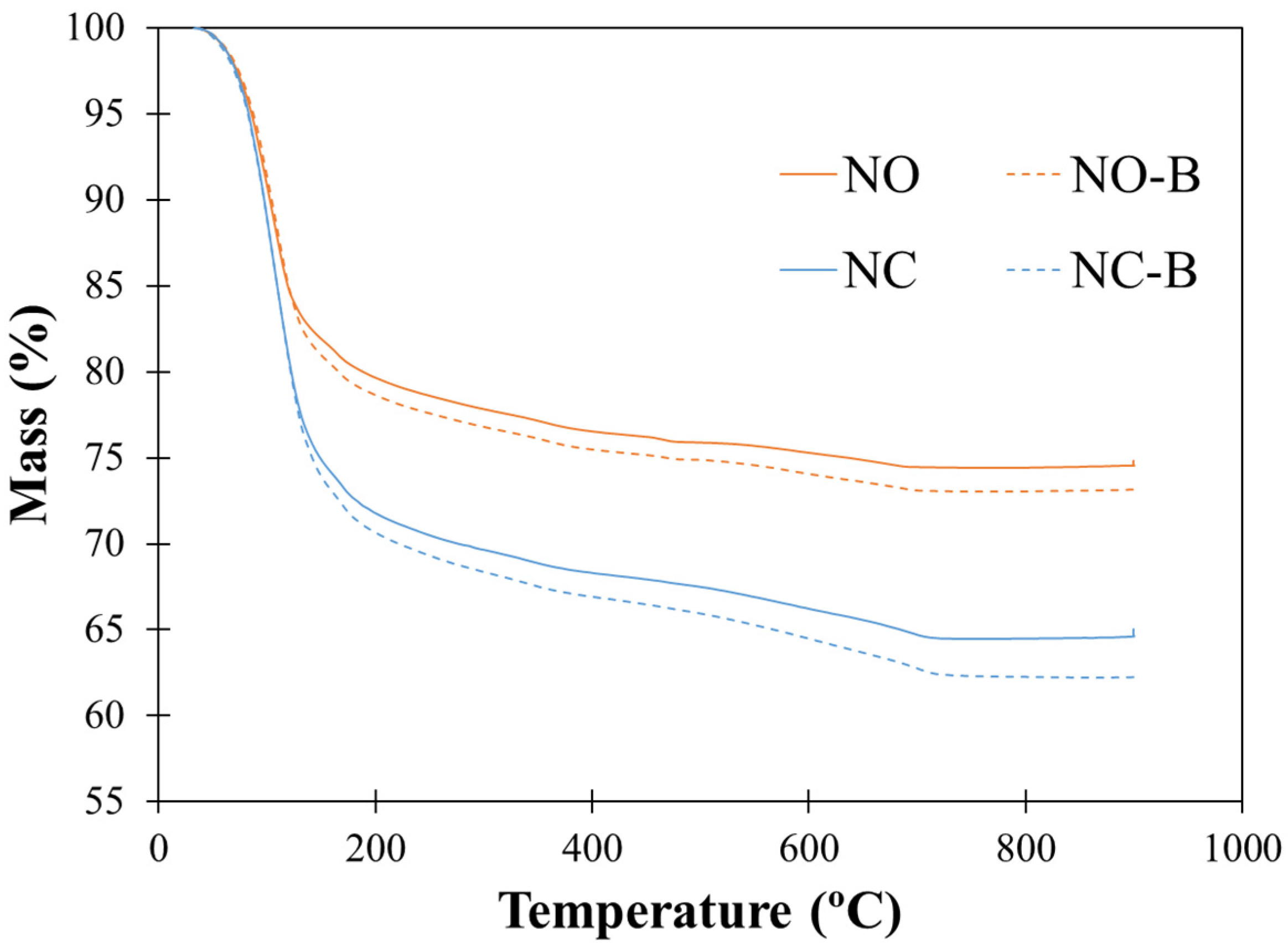
3.7. TGA
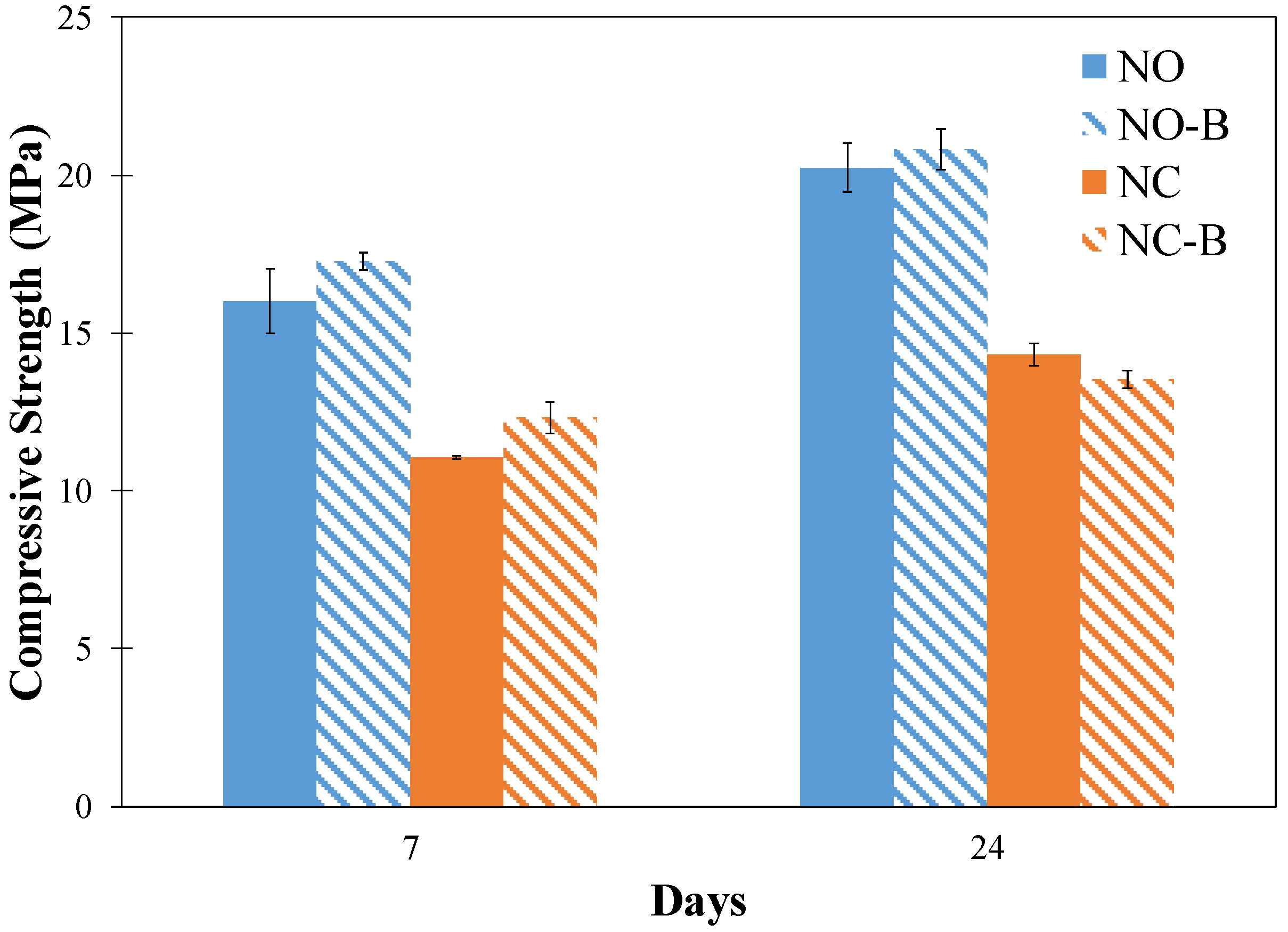
3.8. Compressive Strength
3.9. Electrical Resistivity
4. Conclusions
- The difference in the absorption values of biochar in the mixture of slag and the two activator solutions was small. However, the kinetics of absorption seemed to differ; in slag and NaOH solution, the biochar showed a rapid initial increase followed by a small reduction and then reached a plateau; on the other hand, in slag and Na2CO3 solution, the biochar showed a rapid initial increase followed by a slower increase in absorption.
- The heat flow peak was delayed in the pastes with biochar, compared to the pastes without biochar, but the cumulative heat release was higher at later hours in the pastes with biochar, compared to the pastes without biochar.
- The pastes with biochar showed a reduced autogenous shrinkage, compared to the pastes without biochar, and this effect was more pronounced in the pastes activated with NaOH solution than the paste activated with Na2CO3 solution.
- In the pastes with biochar, micro-CT analysis indicated a slightly higher increase in the number of and the porosity of the voids larger than 30 μm in the paste activated with the NaOH solution, compared to the Na2CO3 solution.
- It was found that the compressive strength of most pastes was slightly increased when biochar was added. The pore structure refinement due to reduced solution/slag is believed to compensate for the negative effect of biochar voids on compressive strength in the pastes.
- The pastes with biochar demonstrated a lower electrical resistivity, compared to the pastes without biochar. This unexpected behavior could be due to the conductive nature of biochar decreasing the overall resistivity of the pastes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Wyrzykowski, M.; Dong, H.; Granja, J.; Azenha, M.; Lura, P.; Ye, G. Internal curing by superabsorbent polymers in alkali-activated slag. Cem. Concr. Res. 2020, 135, 106123. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Anderson, E.; Schauer, C.; Barsoum, M.W. Mechanical and microstructural characterization of an alkali-activated slag/limestone fine aggregate concrete. Constr. Build. Mater. 2009, 23, 2951–2957. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Hi, C.; Krivenko, P.; Roy, D. Alkali-Activated Cements and Concrete; Taylor and Francis: New York, NY, USA, 2006. [Google Scholar]
- Cartwright, C.; Rajabipour, F.; Radlińska, A. Shrinkage Characteristics of Alkali-Activated Slag Cements. J. Mater. Civ. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Ye, H.; Cartwright, C.; Rajabipour, F.; Radlińska, A. Understanding the drying shrinkage performance of alkali-activated slag mortars. Cem. Concr. Compos. 2017, 76, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Sakulich, A.R.; Bentz, D.P. Mitigation of autogenous shrinkage in alkali activated slag mortars by internal curing. Mater. Struct. 2012, 46, 1355–1367. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of shrinkage-reducing admixtures on the properties of alkali-activated slag mortars and pastes. Cem. Concr. Res. 2007, 37, 691–702. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Neto, A.M.; Cincotto, M.A.; Repette, W. Drying and autogenous shrinkage of pastes and mortars with activated slag cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Neto, A.A.M.; Cincotto, M.A.; Repette, W. Mechanical properties, drying and autogenous shrinkage of blast furnace slag activated with hydrated lime and gypsum. Cem. Concr. Compos. 2010, 32, 312–318. [Google Scholar] [CrossRef]
- Atiş, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Effect of elevated temperature curing on properties of alkali-activated slag concrete. Cem. Concr. Res. 1999, 29, 1619–1625. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Choi, Y.C. Superabsorbent polymers as internal curing agents in alkali activated slag mortars. Constr. Build. Mater. 2018, 159, 1–8. [Google Scholar] [CrossRef]
- Vafaei, B.; Farzanian, K.; Ghahremaninezhad, A. The influence of superabsorbent polymer on the properties of alkali-activated slag pastes. Constr. Build. Mater. 2020, 236, 117525. [Google Scholar] [CrossRef]
- Song, C.; Choi, Y.C.; Choi, S. Effect of internal curing by superabsorbent polymers—Internal relative humidity and autogenous shrinkage of alkali-activated slag mortars. Constr. Build. Mater. 2016, 123, 198–206. [Google Scholar] [CrossRef]
- Krafcik, M.J.; Erk, K.A. Characterization of superabsorbent poly(sodium-acrylate acrylamide) hydrogels and influence of chemical structure on internally cured mortar. Mater. Struct. 2016, 49, 4765–4778. [Google Scholar] [CrossRef]
- Bentur, A.; Igarashi, S.-I.; Kovler, K. Prevention of autogenous shrinkage in high-strength concrete by internal curing using wet lightweight aggregates. Cem. Concr. Res. 2001, 31, 1587–1591. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success? Materials 2017, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Mechtcherine, V.; Reinhardt, H.-W. Application of Super Absorbent Polymers (SAP) in Concrete Construction; Springer: Berlin, Germany, 2012. [Google Scholar]
- Igarashi, S.; Watanabe, A. Experimental study on prevention of autogenous deformation by internal curing using super-absorbent polymer particles. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, RILEM Proceedings PRO 052; RILEM Publications S.A.R.L.: Bagneux, France, 2006; pp. 77–86. [Google Scholar]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- Justs, J.; Wyrzykowski, M.; Bajare, D.; Lura, P. Internal curing by superabsorbent polymers in ultra-high performance concrete. Cem. Concr. Res. 2015, 76, 82–90. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Dixit, A.; Gupta, S.; Pang, S.D.; Kua, H.W. Waste Valorisation using biochar for cement replacement and internal curing in ultra-high performance concrete. J. Clean. Prod. 2019, 238, 117876. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Effect of water entrainment by pre-soaked biochar particles on strength and permeability of cement mortar. Constr. Build. Mater. 2018, 159, 107–125. [Google Scholar] [CrossRef]
- Choi, W.C.; Yun, H.D.; Lee, J.Y. Mechanical Properties of Mortar Containing Bio-Char From Pyrolysis. J. Korea Inst. Struct. Maint. Insp. 2012, 16, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Kua, H.W.; Pang, S.D. Biochar-mortar composite: Manufacturing, evaluation of physical properties and economic viability. Constr. Build. Mater. 2018, 167, 874–889. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life Cycle Assessment of Biochar Systems: Estimating the Energetic, Economic, and Climate Change Potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef]
- Zhu, Q.; Barney, C.W.; Erk, K.A. Effect of ionic crosslinking on the swelling and mechanical response of model superabsorbent polymer hydrogels for internally cured concrete. Mater. Struct. 2014, 48, 2261–2276. [Google Scholar] [CrossRef]
- Siriwatwechakul, W.; Siramanont, J.; Vichit-Vadakan, W. Behavior of Superabsorbent Polymers in Calcium- and Sodium-Rich Solutions. J. Mater. Civ. Eng. 2012, 24, 976–980. [Google Scholar] [CrossRef]
- Jain, J.A.; Neithalath, N. Chloride transport in fly ash and glass powder modified concretes—Influence of test methods on microstructure. Cem. Concr. Compos. 2010, 32, 148–156. [Google Scholar] [CrossRef]
- Neithalath, N.; Weiss, W.; Olek, J. Characterizing Enhanced Porosity Concrete using electrical impedance to predict acoustic and hydraulic performance. Cem. Concr. Res. 2006, 36, 2074–2085. [Google Scholar] [CrossRef]
- Praneeth, S.; Saavedra, L.; Zeng, M.; Dubey, B.K.; Sarmah, A.K. Biochar admixtured lightweight, porous and tougher cement mortars: Mechanical, durability and micro computed tomography analysis. Sci. Total Environ. 2021, 750, 142327. [Google Scholar] [CrossRef]
- Zhong, P.; Wang, J.; Wang, X.; Liu, J.; Li, Z.; Zhou, Y. Comparison of Different Approaches for Testing Sorption by a Superabsorbent Polymer to Be Used in Cement-Based Materials. Materials 2020, 13, 5015. [Google Scholar] [CrossRef] [PubMed]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars—Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Shi, C. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J. Microcracking and strength development of alkali activated slag concrete. Cem. Concr. Compos. 2001, 23, 345–352. [Google Scholar] [CrossRef]
- Sakulich, A.; Miller, S.; Barsoum, M.W. Chemical and Microstructural Characterization of 20-Month-Old Alkali-Activated Slag Cements. J. Am. Ceram. Soc. 2010, 93, 1741–1748. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef]



| Composition | (%) |
|---|---|
| SiO2 | 31.62 |
| Al2O3 | 11.01 |
| FexOy | 0.92 |
| CaO | 44.57 |
| MgO | 6.45 |
| Na2O | 0.17 |
| K2O | 0.41 |
| SO3 | 3.13 |
| LOI | 1.14 |
| Designation | Water/Slag | Water-Reducing Admixture (% per Slag Mass) | Biochar (% per Slag Mass) | Water (g) | NaOH (g) | Na2CO3 (g) |
|---|---|---|---|---|---|---|
| NO | 0.44 | 0.17 | - | 1760 | 124.8 | - |
| NO-B | 0.44 | 0.17 | 1.54 | 1760 | 124.8 | - |
| NC | 0.68 | 0.17 | - | 1760 | - | 534.2 |
| NC-B | 0.68 | 0.17 | 1.63 | 1760 | - | 534.2 |
| Paste Designation | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|
| NO | 223 | 445 |
| NO-B | 341 | 651 |
| NC | 306 | 474 |
| NC-B | 248 | 405 |
| Paste Designation | Porosity (%) | Number of Voids |
|---|---|---|
| NO | 0.95 | 8684 |
| NO-B | 5.7 | 52,197 |
| NC | 0.58 | 10,084 |
| NC-B | 4.15 | 35,819 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabahar, J.; Vafaei, B.; Baffoe, E.; Ghahremaninezhad, A. The Effect of Biochar on the Properties of Alkali-Activated Slag Pastes. Constr. Mater. 2022, 2, 1-14. https://doi.org/10.3390/constrmater2010001
Prabahar J, Vafaei B, Baffoe E, Ghahremaninezhad A. The Effect of Biochar on the Properties of Alkali-Activated Slag Pastes. Construction Materials. 2022; 2(1):1-14. https://doi.org/10.3390/constrmater2010001
Chicago/Turabian StylePrabahar, Joshua, Babak Vafaei, Elvis Baffoe, and Ali Ghahremaninezhad. 2022. "The Effect of Biochar on the Properties of Alkali-Activated Slag Pastes" Construction Materials 2, no. 1: 1-14. https://doi.org/10.3390/constrmater2010001





