Abstract
Several studies have shown the feasibility and thermal potential of gypsum plaster with microspheres of PCM, but very few of them investigated an approach with practical and standards concern. In this work, different characterizations are performed according to European standards on a standard gypsum plaster and two different gypsum plasters formulated with 20 wt.% of PCM microspheres. A material is experimentally made by mixing conventional gypsum and PCM microspheres, whereas the other is an already prepared commercial mix. For the laboratory material, the addition of PCM increases the consistency of the fresh paste of plaster. In order to reach a consistency in agreement with the standards more water is required. This higher amount of water causes further issues on the densification and cohesion properties. In contrary, the properties of the commercial mix are closer to a common plaster. It is therefore assumed that the commercial material incorporates thinner additives. In view of these results, it is assumed that most of the drawbacks due to the addition of PCM microspheres in gypsum plasters could effectively be encountered by adequate addition of additives in order to reduce the amount of water, and binding resins in order to improve the adhesion and mechanical properties.
1. Introduction
In this current context of temperature rises due to global warming, the need of disruptive materials and technologies becomes increasingly essential in order to be able to maintain comfort standings in buildings in a sustainable way. Following this vision, active and passive solutions are developed in order to provide energy and cost-efficient buildings [1]. Among these new technologies, the use of phase changing materials (PCM) gains in interest. These materials are commonly composed of paraffins who undergo phase transition, e.g., mostly solid–liquid transformation [2,3,4].
The phase transition enables to store a large amount of latent heat in the form of phase change enthalpy and to reduce the temperature elevation. When the temperature cools down, the PCM undergo a reverse process and are regenerated. Thanks to this free physical characteristic, the PCM enable to prevent, or at least reduce, the use of the high energy-consuming air conditionings [5]. Saffari et al. [6] determined the optimum PCM temperature for different countries and highlighted that usually the temperature of transition of the PCM should be comprised between 24 °C and 26 °C to maintain comfort standings.
In addition to their advantage of storing heat at constant temperature, the latent heat storage materials are also attractive for integration in building structural components, where they can both stabilize the composite material temperature and reduce the deterioration caused by frequent thermal expansion and contraction [7].
The scientific interest about PCMs has been considerably growing over the last decade, due to the increase of the available products at reasonable costs, with promising areas of utilization in building applications [7,8].
One of the most practical way to use PCM consists in using these materials incorporated inside the building materials themselves. Macroscopic (⌀ ~1–5 cm) or microscopic spheres can be used. In the case of microscopic spheres, the PCM are a core active material microencapsulated/coated in a thin spherical shell or capsule of polymers. The manufacturers either supply liquid dispersion of tiny microscopic spheres (⌀ ~5 µm) or powders consisting of clusters of about 200 µm of these microscopic spheres coated with a polymer [9,10]. Organic polymer shells (e.g., poly methyl methacrylate, melamine-formaldehyde resin, urea formaldehyde resin, etc.) are usually employed to protect the PCM cores and improve their structural, impermeability and thermal stability properties [3]. In both cases, the advantages are that the PCM filled microcapsules can be mixed with other materials (e.g., concrete or gypsum plaster), which improves heat transfer to the surrounding through the large surface to volume ratio and improves the cycling stability since the phase separation is restricted to microscopic distances [11]. This integration has been recorded for concrete, mortars and gypsum plaster [8].
Some studies suggests that the use of PCM might be economically beneficial. In this way, Borreguero et al. [12] estimated that the addition of 15 wt.% of microcapsules of PCM to gypsum plaster boards would allow to save 4.5 kWh of energy per operating cycle in a conventional room covered with 1 m3 of this material. A study performed in 2004 by BASF company goes along the same way and estimated that the use of PCM materials instead of classic air conditioning installation could enable to save up to 0.53 EURO/m2/month and would be profitable after 5 years [13]. The average price of electricity having generally increased in all countries since then, the gains are currently even more important.
Commonly, the academic world only investigated intrinsic properties such as the heat latent storage, the thermal conductivity or the thermal mass of gypsum plaster doped with PCM [14,15,16]. In this way, the efficiency and interest of gypsum doped with PCM is no longer to prove [8,12,17,18].
Nowadays, some commercial gypsum products containing PCM are available [19], [20]. However, despite the commercial availability of such materials, a huge gap exists between the formulation of materials exhibited in the literature and the commercial products. For obvious reasons, the manufacturers do not reveal the steps of fabrication of their materials. Academic studies were essentials at the beginning for the launch of these new materials to highlight the feasibility and the thermal efficiency. However, in order to assure the spread of this materials, a next step is to gain more information about their practical use. Indeed, despite a neat screening of the literature about gypsum plaster materials containing PCM, the present authors found no references to standard practical characterizations required by the construction standards. No information was found about the determination of the consistency of the fresh paste of gypsum, the water retention, the shrinkage or the setting time. In order to popularize these materials, the industrials and the contractors need to be sure that different criteria about the practical properties and the durability of these materials are validated by established standards.
The aim of this article is therefore to highlight the gaps that exist between a gypsum plaster developed experimentally in a laboratory and a commercially available gypsum plaster. These results come from a larger study about the technical evaluation of plasters and PCM materials. For this reason, only the technical assessment of the practical performances of the materials is evaluated in this article.
2. Materials and Methods
2.1. Materials
In this study, 3 materials are investigated:
- -
- A conventional gypsum plaster composed of calcium sulfate hemihydrate CaSO4·0.5H2O. This material is referred to as “Plaster”;
- -
- An experimental mix of conventional gypsum plaster and 20 wt.% powder of microencapsulated PCM (Micronal® DS 5001 X, also known as Micronal 26®). The raw PCM microspheres were received as a powder. It consists of capsules made of microscopic PCM particles of n-heptadecane of about 5 µm trapped in tough polymer shell of polymethacrylate (PMMA) of about 200 µm [10]. The melting point indicated by the manufacturer is around 26 °C and the ΔH is 110 kJ·kg−1.
- -
- The mix of common plaster and PCM is synthetized in our laboratory according to standards procedures used in the construction. In this study this material is called “Lab PCM”;
- -
- A commercially available material composed of gypsum plaster and with PCM microspheres at the concentration of 20 wt.%. For ethical reasons and in order to remain strictly fact oriented, the commercial name of this material is not given. In this study this material is called “Commercial PCM”.
2.2. Characterizations
2.2.1. Consistency
Consistency measurements were performed with a standard flow table and using the European standard EN 13279-2 [21] specialized for premixed gypsum plasters. Demineralized water and plaster powder are added into a mixer and mixed for 1 min manually and then 1 additional minute mechanically with a rotation speed of 140 rpm. The water/plaster ratio is determined by the trial and error method until a pat of a specified diameter is formed, when a truncated cone, filled with the slurry, is removed and jolted. The required consistency is achieved, when the empirically determined diameter of the pat of plaster reaches 165 ± 5 mm.
2.2.2. Density of Fresh Paste
The density of the fresh pastes were determined according to the standard EN 1015-6 [22]. In this method the mix is set into a recipient of a known volume (1 L) and vibrated until it becomes compact.
2.2.3. Determination of Water Retention
The water retention of the fresh pastes were determined in accordance with the guideline ETAG 004 [23] and using ASTM C91 equipment. The principle of the test consists in exerting a depression of 6.7 kPa for 15 min on a mixture prepared 15 min in advance. The amount of water extracted allows the retention to be calculated.
2.2.4. Setting Time
The setting time, also known as solidification time, is defined as the time between the initial mixing of the raw materials and the hardening. It was determined by the Vicat cone method referenced in the standard EN 13279-2 [21]. This method is the standard method for all premixed gypsum plasters which incorporate additives and/or retarders. The principle of the test is to measure the penetration of a needle into the mixture after different setting times. The final setting time is defined as the time corresponding to a given penetration (distance of 22 mm between the tip of the needle and the bottom of the container).
2.2.5. Shrinkage
The shrinkages have been determined in agreement with the guideline ETAG 004 [24] and the standard EN 13872 [25]. For each composition, 2 samples of 10 × 40 × 160 mm are prepared, and markers are set on their extremities. Thereafter, the samples are set in a climate chamber with T = 23 °C and a relative humidity of 50 ± 5%. The lengths of the samples are measured over 28 days.
2.2.6. Bulk Densities
The densities of the hardened samples were determined by dimension and weight measurements.
2.2.7. Intrinsic Mechanical Properties
The mechanical properties of the samples were determined with specimens prepared according to the standard EN 13279-2 [21]. Specimens of 160 × 40 × 40 mm are made and stored at T = 23 ± 2 °C and a relative humidity of HR = 50 ± 5%. Thereafter, they are dried at T = 40 ± 2 °C until reaching a constant mass.
Measurement of dynamic modulus of elasticity, Edyn, have been performed by acquiring the fundamental resonance frequency thanks to a Grindo-sonic apparatus. The Equation (1) gives the relation between the resonance frequency and the modulus of elasticity.
Equation (1): Flexural dynamic modulus of elasticity, determined according to EN 14,146 [26].
Where Edyn is the dynamic modulus of elasticity (MPa), L is the length (mm), A is the surface of the section (mm2), I is the inertia moment (mm4), i is the square root of I divided by A (mm), FF is the flexural resonance frequency (Hz), ρ is the density (kg/m3) and C is a correction factor depending on the value I and the Poisson’s coefficient.
Three points bending tests have been performed with a mechanical press according to the standard EN 13279-2. The bending strengths have been determined according to Equation (2).
Equation (2): Bending strength formula.
Where f is the bending strength (N/mm2), b is the width (mm), d is the thickness (mm), F is the maximal flexural load (N) and l is the support span (mm).
Compressive tests have been performed with a mechanical press according to the standard EN 13279-2 [21]. The flexural strengths have been determined according to Equation (3).
Equation (3): Compressive strength formula.
Where R is the compressive strength (N/mm2), Fc is the maximal load in compression (N) and A is the surface of the section (mm2).
2.2.8. Adhesion Properties
The adhesion of a plaster to a specific substrate is measured as the maximum load supported when a metal disc fixed to the plaster is pulled perpendicular to the surface. In this study, the tests were performed according to the standard EN 13279-2 [21]. The fresh mixes are applied on dried substrates, in order to reach a thickness of 1.5 cm. The specimen are thereafter set in a climatic chamber at a temperature T = 23 ± 2 °C and relative humidity RH = 50 ± 5%. Thereafter, metal plates of 5 cm diameter are glued to the surface with an epoxy resin. The edges of the plates are cut until reaching the substrate. The adhesion value is determined by applying a traction force with a rate of 100 N/s on the metal plate. After the traction tests, visual observations of the samples determine the areas of rupture.
2.2.9. Resistance to Mold
The resistance to mold was determine according to the standard ASTM D 5590-94 [27]. As element of comparison, a plaster composed of cement and aerial lime (type C150-CL250) was also investigated. Five specimens consisting of squares of 80 × 80 × 2 mm are tested for each material. The principle of the method consists of immersing the test specimens in a solution containing mold germs and all the nutrients necessary for their development. The aim of the test is to assess the extent to which the material can inhibit this development. The duration of the test is 4 weeks, and the development is evaluated visually 1×/week. The following types of mold were used: Penicillium funicolosum, Aspergillus versicolor, Aureobasidium pullulans, Cladosporium cladosporioides, Penicillium purpurogrnum, Phoma violacae, Rhodotorula rubra, Sporobolomyces roseus, Stachybotrys chartarum, Ulocladium atrum.
2.2.10. Durability Tests
For durability tests, 5 specimens of each material underwent 250 cycles of temperature variations that provoked the phase transition of the PCM microspheres on all the volume of the material. The cycles are as follows: (1) increase of temperature from T = 15 °C to T = 30 °C during t = 60 min; (2) stage at T = 30 °C for t = 30 min; (3) decrease of temperature from T = 30 °C to T = 15 °C during t = 60 min; 4) stage at T = 15 °C for t = 30 min. The cycles were performed thanks to a Heraeus 4034 climatic chamber, and the relative humidity was set at 50%.
2.2.11. SEM Measurements
Microscopic measurements were performed with an SEM Quanta 200 FEG equipped with an EDS (EDAX). The pictures are taken with a HV of 15.0 kV and a pressure of 130.0 Pa.
2.2.12. FTIR Measurements
FTIR measurements were performed with a Spectrum Two Perkin Elmer apparatus. The measurements are performed either on the raw samples or on the evaporation residue obtained after an extraction with petroleum ether using the Soxhlet technique (for 8 h and 15 cycles per hour).
3. Results and Discussions
3.1. Preparation and Characterizations of the Fresh Mixes
In order to put the materials in conditions as close as possible to field reality, the preparations of the samples were adjusted in order to fit with construction standards. The first parameter investigated was the determination of the amount of water needed for a standardized consistency of the fresh paste of gypsum paste. Indeed, the consistency of a fresh paste of gypsum plaster is a decisive parameter for a proper application of the material and the range of consistency declared as correct according to the European standard EN 13279-2 [21] is narrow: 165 ± 5 mm. This parameter is essential for an easy application of the plaster and an aesthetic finish. The amount of water needed to determine the right consistency of materials Plaster and Lab PCM were determined experimentally and empirically. However, for the material Commercial PCM the amount of water used was the one recommended by the manufacturer.
Table 1 lists the different consistency measurements performed with a standard flow table. Figure 1 shows the apparatus used. Despite a larger amount of water used for the Lab PCM fresh paste, the consistency after 2 min of stirring was still too thick (spread of 133 mm). However, it was observed that after 15 min of mechanical mix its consistency reached the desired value of 165 mm. For the material Commercial PCM the consistency of the fresh paste did not reach the standard value of 165 ± 5 mm expected by the standard EN 13279-2 [21], but was closer to it (143 mm).

Table 1.
Compositions and properties of fresh mixes of materials.

Figure 1.
Apparatus used for fresh mixes characterizations (from the left to the right): (a) Standard slump cone used for density measurement; (b) Table flow used to determine consistency; (c) Büchner funnel for water retention determination; (d) Vicat cone for setting time evaluation.
The results presented in Table 1 highlight that the addition of microencapsulated PCM modifies the properties of the fresh gypsum paste. In order to reach a conventional consistency the fresh paste of Lab PCM needs a higher amount of water (33% more) and needs to be mixed for much longer time (15 min instead of 2 min). This observation could be explained by the fact that the average diameter of the microencapsulate Micronal 26® spheres is 5 µm and that standard grains of calcium sulphate plaster have diameters comprised between 0.5 and 2 µm. The larger microcapsules of PCM might hinder the proper repartition of the CaSO4·0.5H2O grains in the fresh mix. However, this physical interference of the PCM capsules with the plaster grains appear to be encountered by a thorough mixing. The lower density observed for the fresh mix of Lab PCM can be explained by the fact that it contains more water and more PCM, which are both lighter than calcium sulphate hemihydrate. The amount of water added to reach an acceptable value for the fresh paste of Commercial PCM was in contrary very similar to the one needed for sample Plaster that was made of raw gypsum (33 and 39 wt.%). This observation suggests that the fresh paste of Commercial PCM might contains thinner additives. Indeed, additives such as lignosulfates, polycarboxylate polyoxyethylen (PCP), polyacrylic acid (PAA), polymethacrylic acid (PMAA) or polyphosphonate polyoxyethylen (PPP)) are commonly added to gypsum plaster in order to obtain the adequate consistencies and reduce the amount of water of fresh mixes [28,29].
Trials were thereafter performed in order to observe the influence of PCM addition on the water retention of the fresh mixes. Water retention determines the extent to which a fresh paste retains water in its fresh state. The results are shown in Table 1 and are expressed as a percentage of the initial water content. It can be observed that the addition of PCM does not modify the water retention of the fresh mix neither for sample Lab PCM nor sample Commercial PCM. This can be explained by the fact that the surface of the microcapsules are made of a hydrophobic polymer (PMMA) that does not retains water.
The setting time of fresh paste is an important value for practical application and an aesthetic finish. It is defined as the time between the initial mixing of the raw materials and the hardening. According to the criterion of the standard EN 13279-1 [30], renders made of plaster must have setting times higher than 20 min for manual ceiling application and higher than 50 min for mechanically projected ceiling application. It can be observed in Table 1 that all fresh pastes respected these criteria. Nevertheless, it can be noticed that the addition of PCM microcapsules significantly reduces the setting time (t = 138 min for Lab PCM instead of t = 239 min for Plaster and t = 264 min for Commercial PCM). Similar results have been observed by Toppi et al. [18] and Collepardi [31]. The authors made the hypothesis that the presence of very small microcapsules creates Van Der Waals forces which increase the gypsum viscosity, making the mixing process more difficult; on the other side, microcapsules provide crystallization nuclei which promote the gypsum solidification. As the solidification time shortens, the number of the bubbles which remain trapped in the gypsum increases. The authors also informs that these phenomena, which result in a higher material porosity, can be limited, but not completely eliminated, by the use of additives. These interpretations could explain why the setting time of the material Commercial PCM is similar to the one made of raw gypsum Plaster (264 and 239 min) and is significantly different from the material Lab PCM (138 min). It also strengthen the hypothesis that additives are added to the gypsum plaster Commercial PCM to correct the influence of the PCM addition and to tend to properties similar to standard gypsum plaster.
3.2. Influence on the Shrinkage and on the Density
Swelling and shrinkage is a parameter to control for gypsum plasters. The occurrence of shrinkage is mainly due to the fact that the amount of water added to the gypsum plaster is usually higher than what is required for the hydration of hemihydrate to dihydrate. This larger amount of water is usually necessary to meet the required consistency and to obtain the higher workability for ease of application. Consequently, the setting expansion of gypsum plaster during the initial stages of the gypsum hydration occurs. As the time progresses the gypsum plaster undergo dimensional changes due to the loss of moisture and evaporation of excess water used for obtaining plasticity [32].
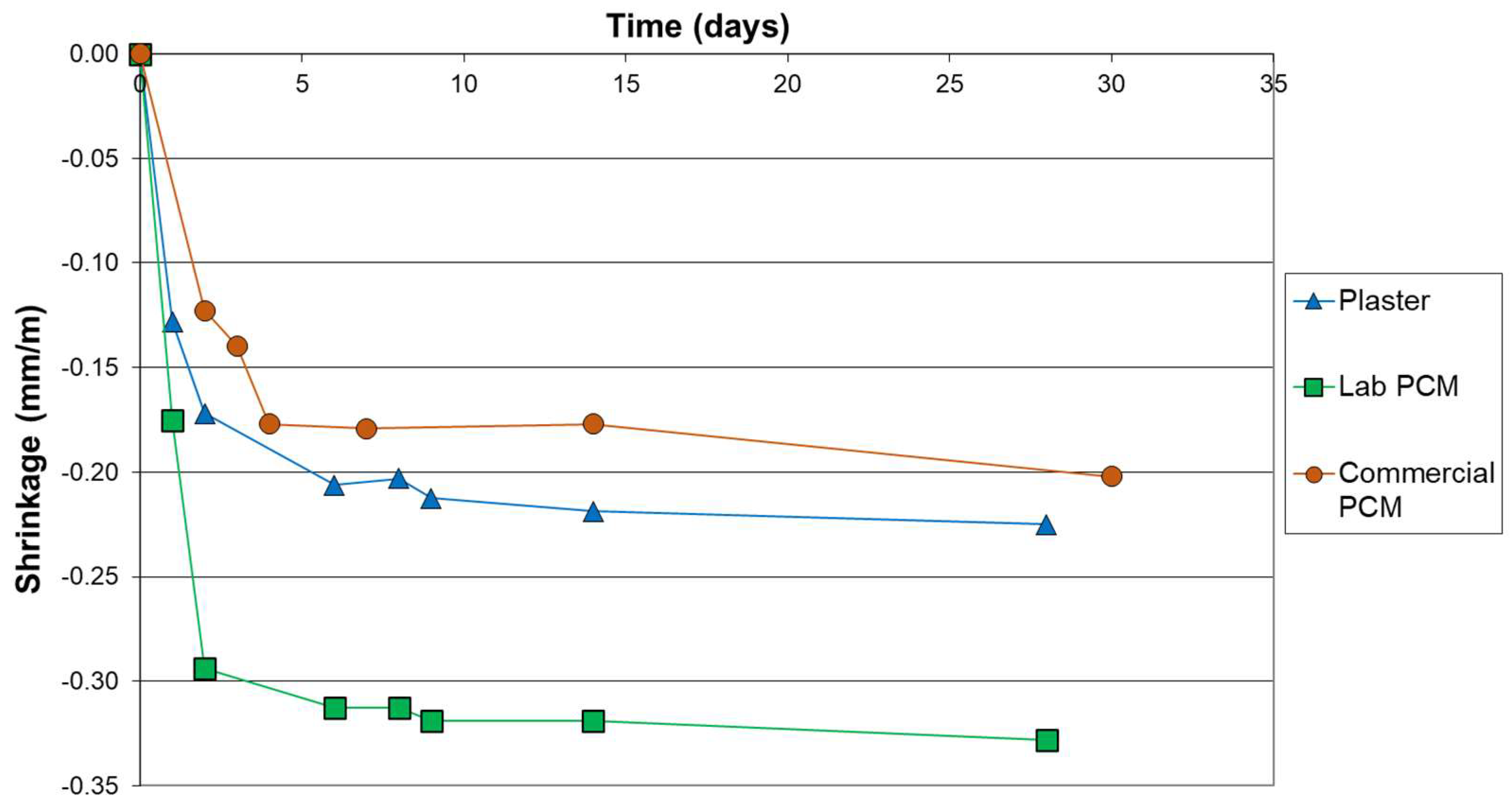
Figure 2 shows that the laboratory mix of PCM and gypsum (Lab PCM) exhibits a significantly more important shrinkage than the commercial one. Indeed, after the 28 days of curing, the material Lab PCM have shrunk of 0.33 mm/m, whereas the materials Plaster and Commercial PCM have shrunk of 0.23 and 0.20 mm/m. This more important shrinkage is certainly due to the fact that the material Lab PCM needed a higher amount of water (33 wt.%) in order to reach an acceptable consistency (see Table 1). Indeed, the shrinkages of the three materials were all related to the amount of water added. It is to notice that the samples were weighed and that the drying steps of the samples evolved in the same trends as the shrinkages. No maximal shrinkage criterion are defined for gypsum plaster, however, as a comparison, for clay plaster the maximal shrinkage is set at 20 mm/m [33] and for concrete it can goes from 0.1 to 0.8 mm/m [34]. Hence, all the samples of this study present shrinkage values that could be considered as acceptable.
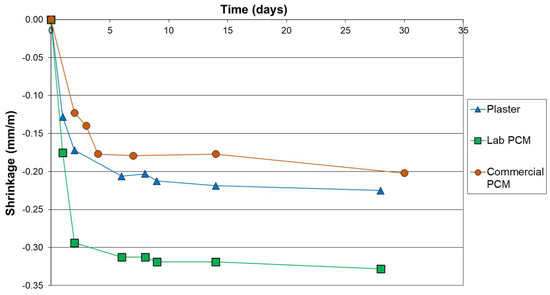
Figure 2.
Shrinkage curves of samples.
Table 2 shows the bulk densities of the dried materials. In order to get a better understanding, the table also shows the theoretical bulk density of the material Lab PCM. This value was calculated by integrating the amounts and the bulk densities of plaster and PCM microspheres (respectively 1007 kg/m3 and 300 kg/m3). It can be observed that the real density of the material Lab PCM is lower than the theoretical value expected for it (680 kg/m3 instead of 844 kg/m3). Furthermore, it is to notice that the density of Lab PCM is actually the lowest of the three samples. Lower density due to the addition of PCM microsphere has already been recorded in the literature. Fort et al. [35] observed that the densities of matrix of gypsum significantly decreased with increasing addition of PCM microspheres. The author attributed this to the fact that the PCM particles apparently filled large pores and promoted the increase of pores in the range from 0.01μm to 1 μm. However the author did not observe major changes in the total open porosity. A more conceivable answer can be found in the article of Toppi at al. [18]. The authors highlighted a clear correlation between the initial water amount and the final density. It was assumed that the decrease of the density can be explained considering that the amount of water which reacts with gypsum is constant and close to the stoichiometric value, while the excess of water evaporates, leaving pores which increase the volume of the sample, without increasing the mass. The lower density observed for material Lab PCM might therefore be more influenced by the larger initial amount of water rather than by the presence of PCM microspheres. This reasoning is strengthened by the fact that the material Commercial PCM, which necessitated a low amount of water, exhibited a high density (923 kg/m3) close to the one observed for raw Plaster (1007 kg/m3). The lower density observed for material Lab PCM could also be partially influenced by the fact that its shorter setting time might prevent the proper evacuation of the air bubbles trapped in the fresh mix [18,31].

Table 2.
Bulk densities of dried material.
3.3. Mechanical Properties
3.3.1. Intrinsic Mechanical Performances
The intrinsic mechanical properties of the material were determined by measuring the dynamic modulus (Edyn), bending strengths and compressive strengths.
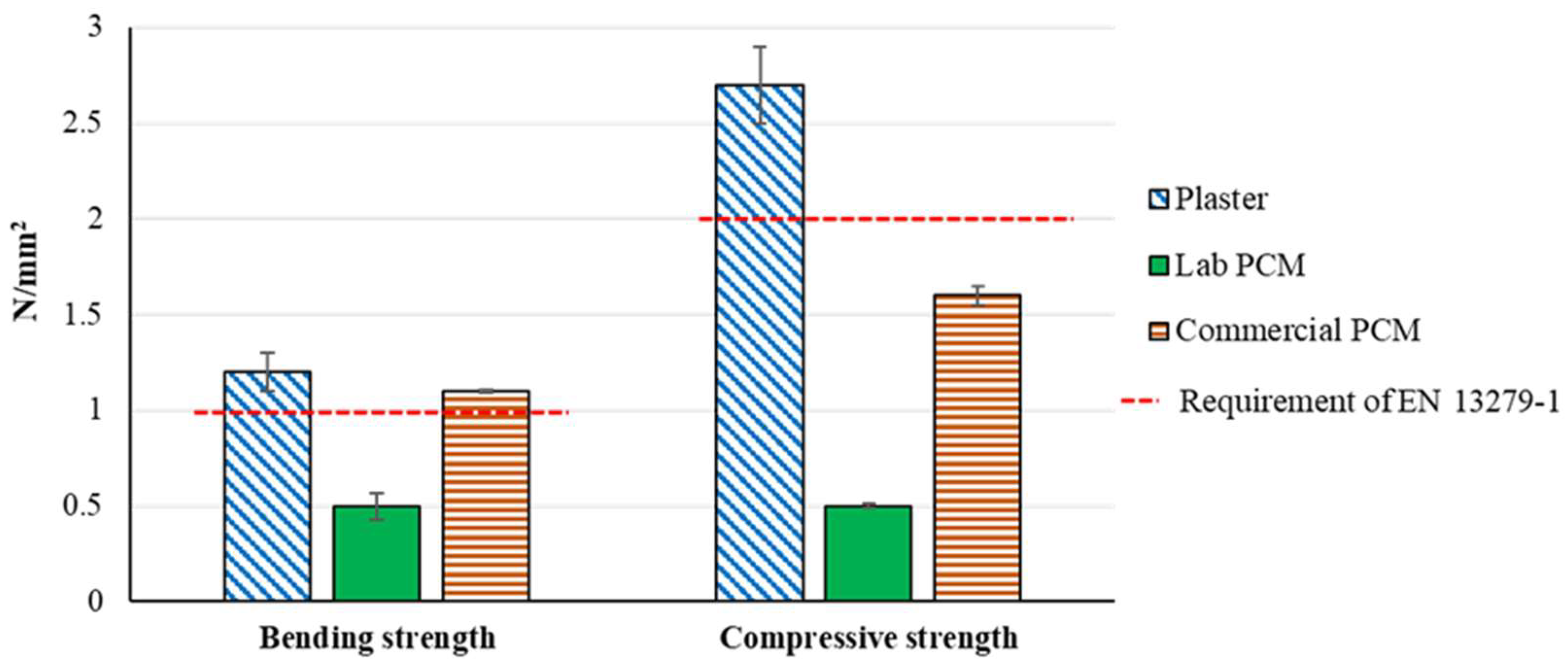
Results presented in Table 3 and in Figure 3 show that the addition of PCM causes major losses of the mechanical properties in the case of the experimental lab preparation. In this way, compare to the raw material Plaster, the material Lab PCM has a loss of dynamic modulus elasticity of about 90%, a loss of bending strength of 58% and a loss of compressive strength of 81%. Furthermore, Figure 3 shows that because of these lower performances, the material Lab PCM does not meet the mechanical resistance requirements of the EN 13279-1 [30]. This might be due to an insufficient cohesion of the gypsum’s grains and/or the presence of voids due to the initially larger amount of water. This is strengthened by the fact that the material Lab PCM shows a lower density than expected (see Table 2) and by the fact that the material Commercial PCM showed much better bending and compressive strengths.

Table 3.
Intrinsic mechanical performances of samples.
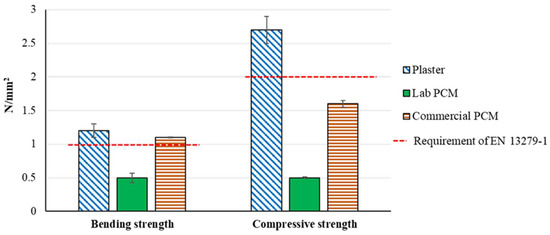
Figure 3.
Bending and compressive strengths.
In their studies, Bajare et al. [14,36] also observed losses of mechanical properties between a common gypsum plaster and laboratory samples made with two different type of commercial PCM microspheres. However, the authors observed losses that were smaller than what is observed in the present study (around 40% for flexural strengths and around 60% for compressive strengths). No information was present about the amount of water used and the consistency of the fresh mixes in their work. However, the densities of all their materials being similar to conventional plaster, it is assumed that the authors did not investigate the consistency of the fresh pastes and used the same amount of water for the preparation of all their materials.
This hypothesis is strengthened by the study of Borreguero et al. [12]. The authors designed gypsum plasters with 15 wt.% of microcapsules and used the same amount of water for both materials. Though the author observed a loss of the compressive strength of about 2.5 time, the material with PCM still had sufficient mechanical resistance (5 MPa). However, no practical aspects such as the consistency, the setting time and the shrinkage were investigated.
These comparisons between the literature and results of the present study tend to confirm that the loss of mechanical properties of material Lab PCM was more impacted by a too high initial amount of water rather than by the presence of PCM microspheres.
3.3.2. Adhesion Properties
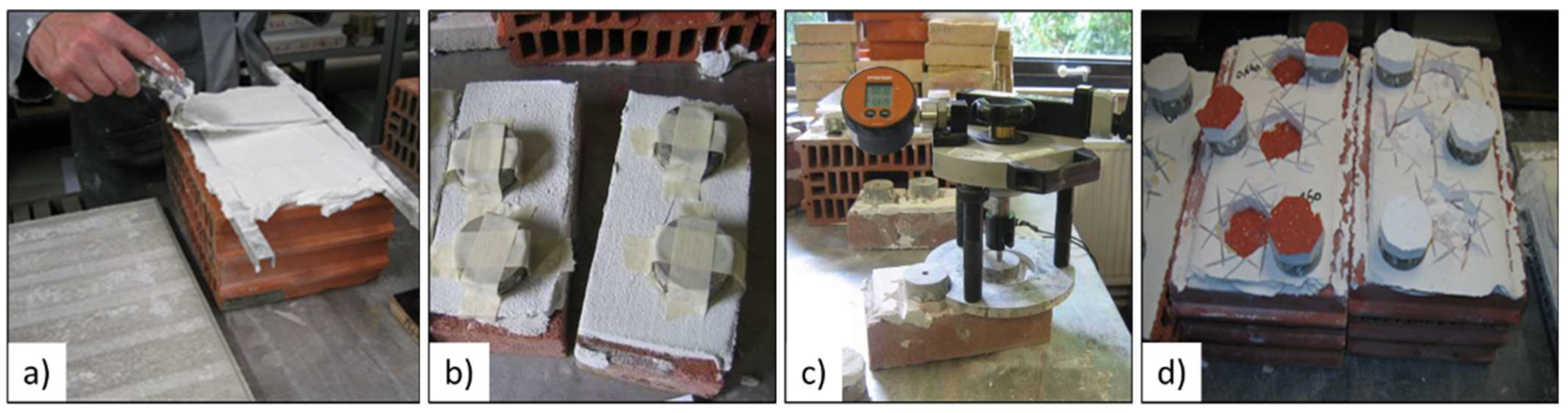
The compatibility of a plaster with a substrate is mostly determined by its adhesion. This property is one of the most important for practical applications. In this study, in order to cover several possible cases, three types of substrates were tested: a concrete slab, a standard solid brick and a hollow clay brick. The initial rates of water absorption by capillarity of these materials were determined according to the standard EN 771-1 [37]. The concrete slabs can be considered as not very absorbent (IW < 0.5 kg/m2·min), the solid bricks are very absorbent (IW > 4 kg/m2·min) and the hollow clay bricks are slightly absorbent (IW comprised between 1.5 and 4 kg/m2·min) [38]. Figure 4 shows the different steps of the trials.

Figure 4.
Stages of adhesion tests: (a) Application of the fresh mix on a brick; (b) Metal plates used for the test; (c) Traction apparatus; (d) Samples after test.
Table 4 lists the information given by the adhesion tests. In order to fulfil the requirement of the standard EN 13279-1 [30] for gypsum plaster, the adhesion value must be ≥0.1 N/mm2 when the rupture occurs at the interface. The requirement is also fulfilled if the rupture occurs strictly in the plaster or in the substrate.

Table 4.
Results from the adhesion tests after 28 days of curing at T = 23 °C and RH = 50%. Each value is the average of 3 measurements.
The material Plaster validates the standard for each case, which is in agreement with what is claimed in the technical sheet. For material Lab PCM, the rupture occurred only in the plaster for the concrete slab, which makes it conform according to the standard. In the case of hollow clay brick, it can be observed that the rupture occurs both at the interface (40%) and in the plaster (60%), and that the adhesion value is slightly below the standard requirement (0.04 N/mm2). In the case of a solid brick, the rupture occurred both at the interface and in the plaster, but the adhesion value was sufficient (0.12 N/mm2).
The highest adhesion value for the material Lab PCM is observed for the solid brick, which is the substrate that have the highest capillarity absorption. This observation strengthen the hypothesis formulated above that the lower mechanical properties of the material Lab PCM seem to be due to a too high amount of water in the initial mix (see Table 3). Despite this, it is observed that the material Lab PCM exhibited lower adhesion values for all substrates. This suggests that the higher amount of water in the initial fresh mix of Lab PCM is not the only reason for lower final mechanical properties. The loss of mechanical properties is therefore not only due to a larger amount of water, but also probably to the presence of PCM microspheres. Finding the impact of each component might be the subject of another study.
The Commercial PCM material presents sufficient adhesion value for all substrates. However, it is not possible to say if these correct performances are due to a lower presence of water or to the potential presence of additives. Indeed, it is common to use binding resins composed of dispersions of polymers to encounter low values of adhesion [39,40].
3.4. Resistance to Mold
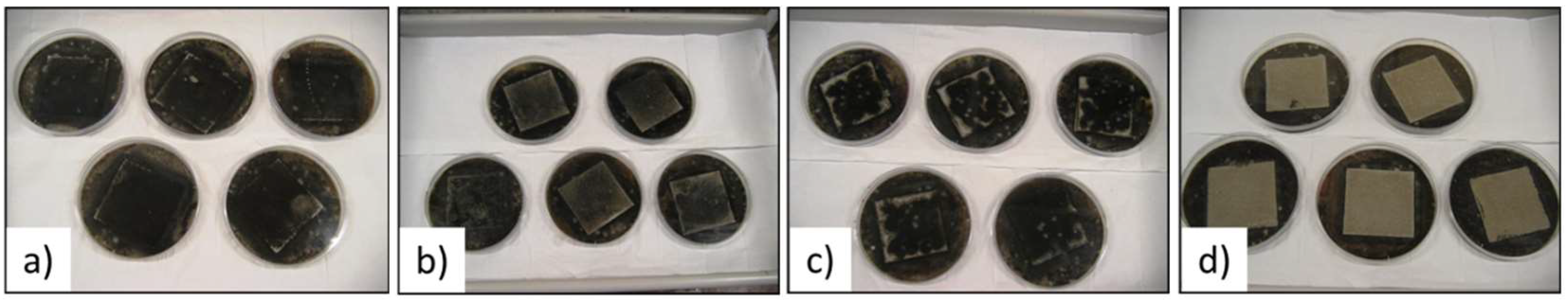
In order to see the impact of the addition of PCM microspheres on the growing of mold, the materials were tested in accordance with the standard ASTM D 5590-94 [27]. As element of comparison, a plaster composed of cement and aerial lime (type C150-CL250) was also investigated. It can be observed in Figure 5 that none of the materials exhibited a fungicide behavior. Hence, the addition of PCM microsphere does not worsen the mold development and no additives acting as anti-molding were added in the material Commercial PCM. In comparison, the plaster made of cement and aerial lime shows a good resistance against mold formation. Indeed, the presence of CaO is known to be an effective fungicide [41]. In the most usual case, the occurrence of mold is mostly caused by insufficient ventilation.

Figure 5.
Visual observation of the samples at the end of the mold resistance test. (a) sample Plaster; (b) sample Lab PCM; (c) sample Commercial PCM; (d) sample of cement + lime.
3.5. Durability
In order to evaluate the durability of the products with the time, the materials undergone 250 cycles of temperature variations between 15 and 30 °C. Visual observations performed on the samples after the cycles did not reveal any differences of aspect. In order to be sure that the cycles did not damage the structure of the materials, the samples were cut in two and the slices were observed by environmental scanning electron microscopy.
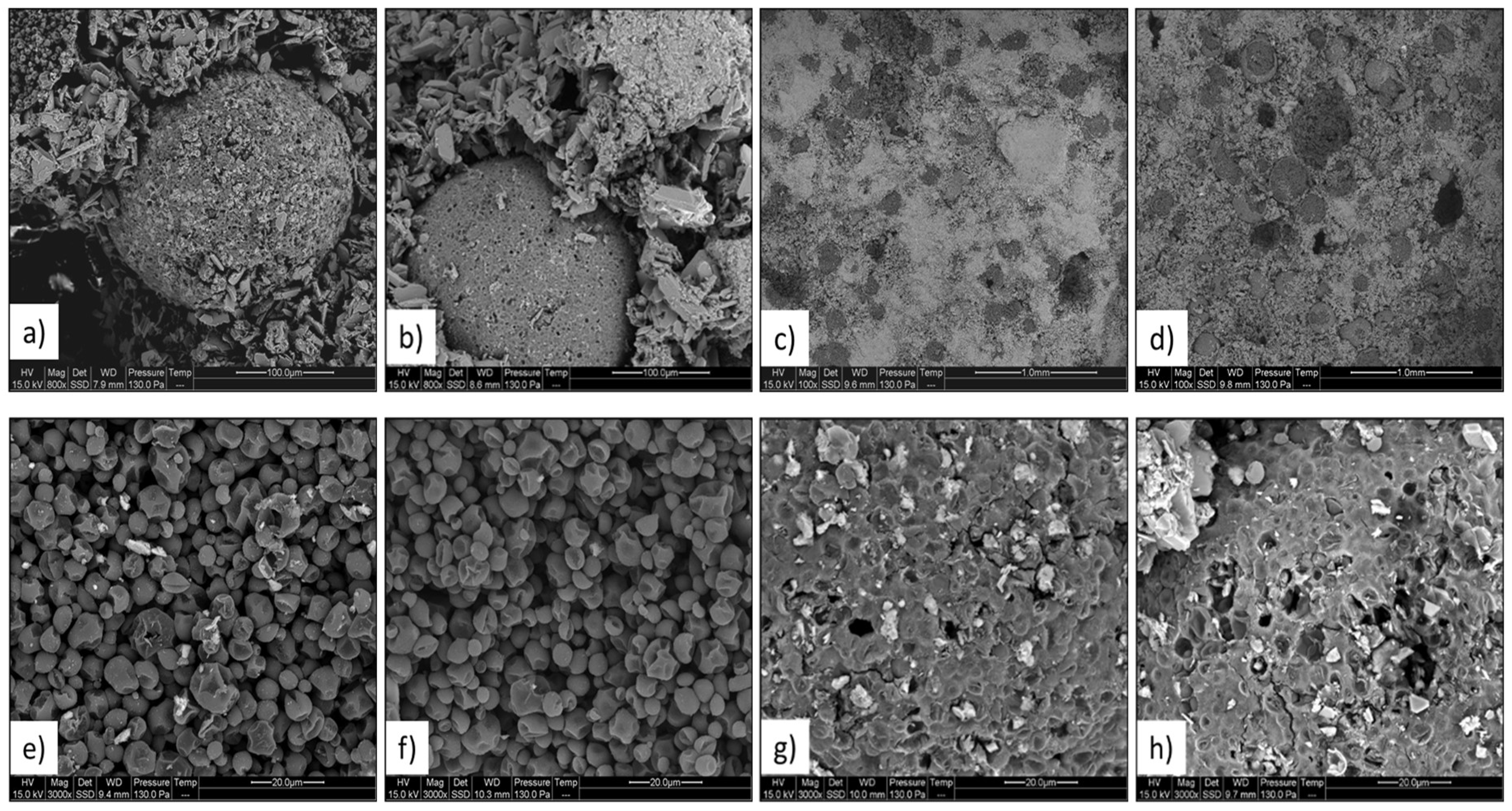
For both Commercial PCM and Lab PCM materials, the scanning electron microscopy revealed a homogeneous distribution of the capsules who contained tiny wax particles. This is in agreement with the manufacturer announcement [10]. Furthermore, no visible modification of the structure of the plaster due to the addition of PCM microsphere was visible. The multiple observations of Figure 6 revealed that the thermal cycles did not affect in either way the PCM microspheres, the clusters or created cracks in the matrix. The shapes of the PCM grains located inside the microspheres were also not affected by the thermal cycles.
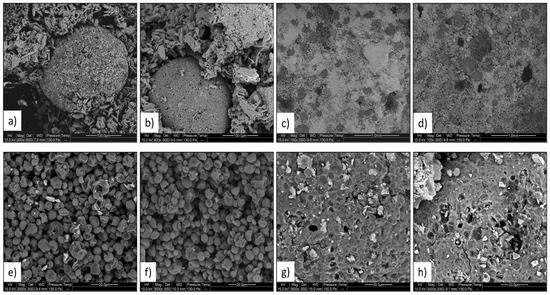
Figure 6.
Microscopic observations of the samples Commercial PCM and Lab PCM before and after 250 thermal cycles. Capsule before and after thermal cycles (a,b); matrix and capsules before and after thermal cycles (c,d); solid PCM grains inside capsule before and after thermal cycles (e,f); surface of capsule before and after thermal cycles (g,h).
3.6. FTIR Measurements
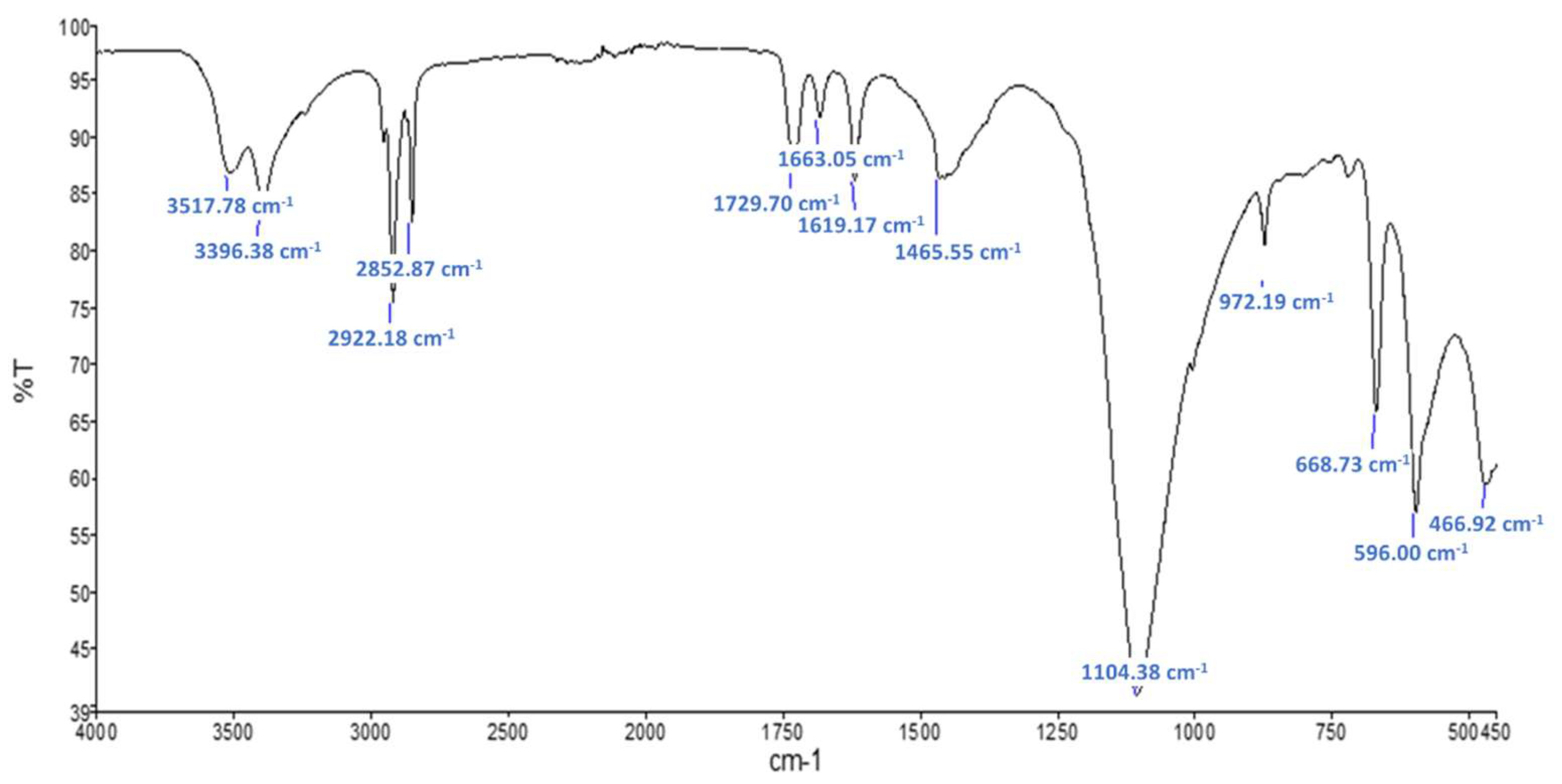
FTIR measurements were performed in order to highlight the presence and the type of additives. On the spectra of the raw material Plaster (not shown here) only gypsum (CaSO4·2H2O) was observable. The spectra obtained after Soxhlet extraction revealed the presence of peaks at 2921, 1466, 1378 and 720 cm−1 typic of a paraffin. This is assumed to be the oil which was used for mold lubrication.
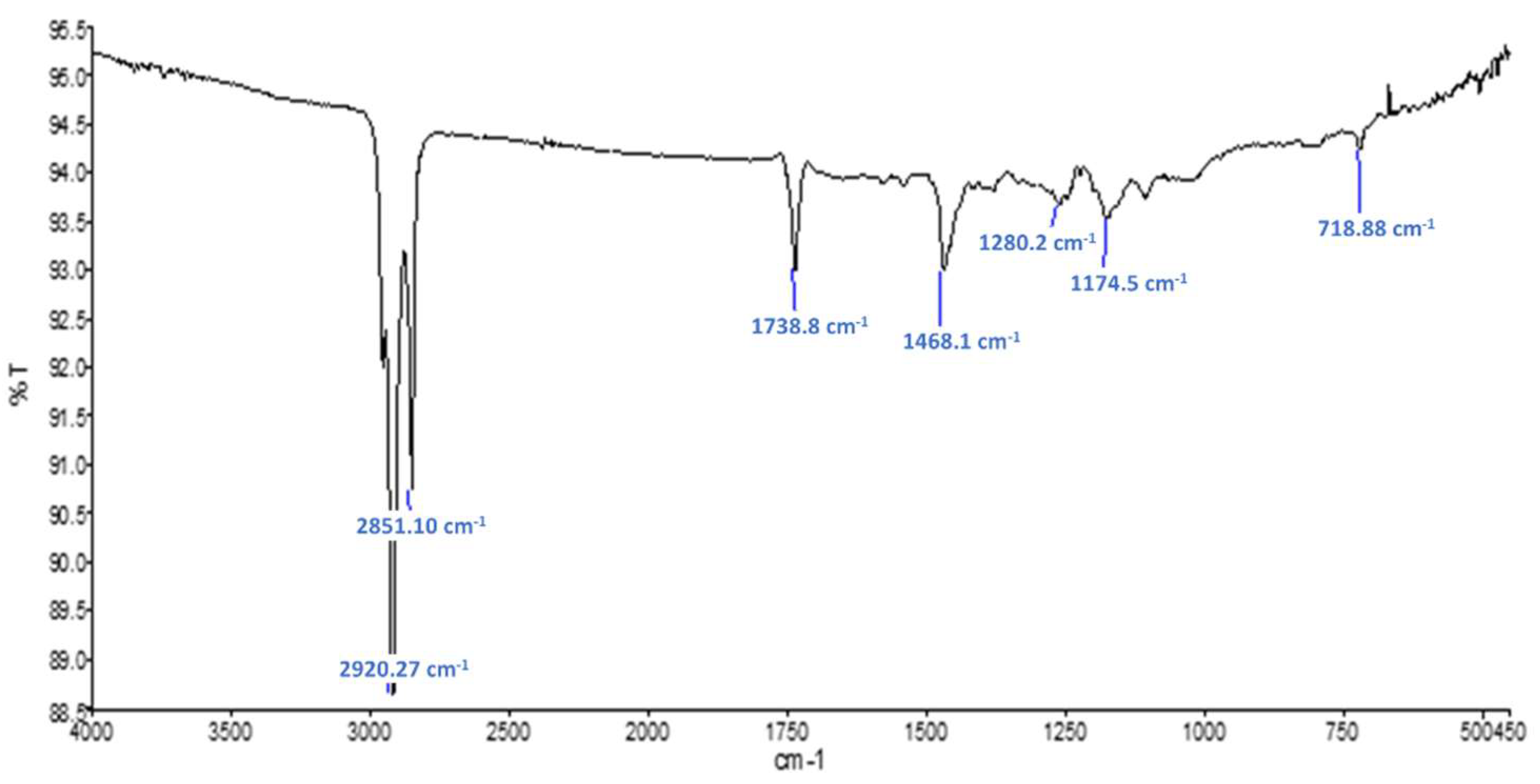
Figure 7 shows the FTIR spectra obtained on the raw sample Commercial PCM. Typical gypsum (CaSO4·2H2O) peaks are also observable. The presence of absorption bands at 2922, 2852, 1729 and 720 cm−1 suggests that a polymer is present. These peaks are certainly caused by the microcapsule shells that are known to be made of polymethacrylate (PMMA) [10]. Figure 8 shows the FTIR spectra obtained on the residues obtained after Soxhlet extraction for this material. Absorption bands at 1735, 1280 and 1174 cm−1 suggest the presence of a polymer of acrylic-type. It is difficult to say if additive such as polyacrylate acid have been used since their peaks might be too small or hidden by the other peaks of the PMMA shell. The typical peaks of paraffin observed at 2921, 1466, 1378 and 720 cm−1 are stronger in that case and might be an association of the mineral oil used as mold lubricant and the n-heptadecane used as PCM inside the microsphere.
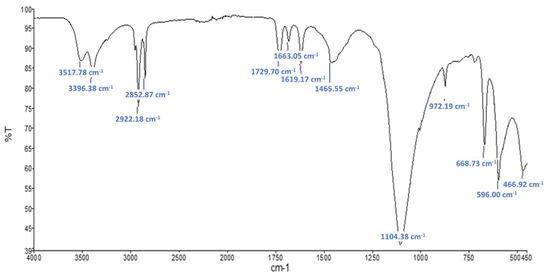
Figure 7.
FTIR spectra for raw sample Commercial PCM.
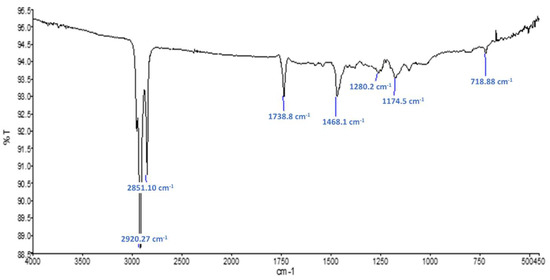
Figure 8.
FTIR spectra obtained for evaporation residue of sample Commercial PCM.
The additives being usually added with extremely low percentages (0.01–1%), other characterizations technics such as high-performance liquid chromatography (HPLC) might be more adequate to trace them. This might be the subject of another study.
4. Conclusions
The addition of PCM tends to increase the consistency of the fresh paste of plaster, and in order to reach a consistency in agreement with the construction standards the addition of more water is necessary. Because of the initial higher amount of water, the laboratory mix exhibited a higher shrinkage during its curing step, had a lower densification and a lower cohesion between the gypsum grains. As a consequence, the mechanical properties of the laboratory mix containing PCM microspheres were lower. Indeed, the bending and compressive strength did not match the standards value expected for gypsum plasters. Adhesion tests lead to more optimistic results. Though showing lower adhesion values, the material containing PCM was in agreement with the standards for two of the three types of substrates investigated. Its properties were also better than the ones of a conventional natural hydraulic lime.
In comparison, the properties of the commercial mix of plaster and PCM were closer to a common plaster. During its synthesis, this material needed less water in order to reach the requirements of practical properties such as consistency, setting time and shrinkage. It is therefore strongly assumed that the commercial material used thinner additives in order to produce a fresh paste of gypsum plaster with adequate consistency without the use of excessive amount of water that could lower the performances of the material.
Mold growing tests showed that the PCM addition did not increase nor decrease the resistance of the material on this aspect. Microscopic observations revealed that the PCM microspheres were homogeneously dispersed among the gypsum matrix and that the materials had excellent durability since 250 thermal cycles did not have incidence on the material aspect.
FTIR measurements performed on the two materials did not permit to identify with certainty a type of additive used for the commercial mix.
This study also highlighted the lack of investigation of elemental characterizations and properties of gypsum plaster such as consistency, setting time and shrinkage in the academic studies. Indeed, in order to popularize such disruptive materials, one should also focus on the standards requirements and on the practical needs that regulate their use.
In conclusion, most of the drawbacks due to the addition of PCM microspheres in gypsum plasters could effectively be encountered by adequate addition of thinner additives in order to reduce the amount of water, and binding resins composed of dispersions of polymers in order to improve the adhesion and structure of the plasters to the substrates. The lower mechanical performances that are observed in the literature could therefore easily be encountered. However, to our knowledge, for the moment no detailed information have been found about the type of additives to use to encounter the drawbacks of PCM addition. Studies with molecules such as lignosulfates, polycarboxylate polyoxyethylen (PCP), poly-acrylic acid (PAA), polymethacrylic acid (PMAA) or polyphosphonate polyoxyethylen (PPP)) could be considered.
With these solutions, a conventional execution of the plasters made with PCM might respect the standards requirements and thus assure to obtain a proper and aesthetic finish. However, more research should be done to determine which additive to use and to determine if the processing steps would be feasible in a construction site.
Author Contributions
Conceptualization, I.D., V.C. and F.d.B.; methodology, I.D. and V.C.; validation, I.D. and F.d.B.; formal analysis, V.C., I.D. and F.d.B.; investigation, I.D. and F.d.B.; resources, I.D. and F.d.B.; data curation, V.C., I.D., S.C. and F.d.B.; writing—original draft preparation, I.D. and V.C.; writing—review and editing, V.C.; supervision, F.d.B. and S.C.; project administration, F.d.B. and S.C.; funding acquisition, F.d.B. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Region Wallonne with the grant of the project RETERMAT under the convention number 716707. This research was also funded by the Region Wallonne and to the Fonds Européen de Développement Régional (FEDER) with the grant of the project S.T.O.C.C. included in the portfolio 500324-352426 and the folder 3441.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The Belgian Building Research Institute is thankful to the Region Wallonne and to the Fonds Européen de Développement Régional (FEDER).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pisello, A.L.; D’Alessandro, A.; Fabiani, C.; Fiorelli, A.P.; Ubertini, F.; Cabeza, L.F.; Materazzi, A.L.; Cotana, F. Multifunctional Analysis of Innovative PCM-filled Concretes. Energy Procedia 2017, 111, 81–90. [Google Scholar] [CrossRef]
- Alawadhi, E.M. The Design, Properties, and Performance of Concrete Masonry Blocks with Phase Change Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9781782423188. [Google Scholar]
- Drissi, S.; Ling, T.C.; Mo, K.H.; Eddhahak, A. A review of microencapsulated and composite phase change materials: Alteration of strength and thermal properties of cement-based materials. Renew. Sustain. Energy Rev. 2019, 110, 467–484. [Google Scholar] [CrossRef]
- Cellat, K.; Beyhan, B.; Güngör, C.; Konuklu, Y.; Karahan, O.; Dündar, C.; Paksoy, H. Thermal enhancement of concrete by adding bio-based fatty acids as phase change materials. Energy Build. 2015, 106, 156–163. [Google Scholar] [CrossRef]
- Sinopoli, J. Heating, Ventilating, and Air Conditioning Systems; Smart Build. Syst. Archit. Owners Build., chapter 3, 31–46; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; ISBN 978-1-85617-653-8. [Google Scholar] [CrossRef]
- Saffari, M.; De Gracia, A.; Fernández, C.; Zsembinszki, G.; Cabeza, L.F. Study on the optimum PCM melting temperature for energy savings in residential buildings worldwide. IOP Conf. Ser. Mater. Sci. Eng. 2017, 251, 012113. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Pisello, A.L.; Fabiani, C.; Ubertini, F.; Cabeza, L.F.; Cotana, F. Multifunctional smart concretes with novel phase change materials: Mechanical and thermo-energy investigation. Appl. Energy 2018, 212, 1448–1461. [Google Scholar] [CrossRef]
- Kośny, J. PCM-Enhanced Building Components: An Application of Phase Change Materials in Building Envelopes and Internal Structures; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319142852. [Google Scholar]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Biggin, I. Soaking up the heat. Phase Change Materials in Construction; BASF CPD Phase Change Material, Phase Energy: Hull, UK, 2015. [Google Scholar]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Garrido, I.; Valverde, J.L.; Rodríguez, J.F.; Carmona, M. Development of smart gypsum composites by incorporating thermoregulating microcapsules. Energy Build. 2014, 76, 631–639. [Google Scholar] [CrossRef]
- BASF. BASF Report; 2007. Available online: http://docplayer.org/25546766-Waermedaemmung-vermindert-verluste-durch-die-wand-im-winter-im-sommer-groesster-energieeintrag-durch-fenster-und-innere-lasten.html (accessed on 3 November 2021).
- Bajare, D.; Kazjonovs, J.; Korjakins, A. The thermal characteristics of gypsum boards with phase change materials (PCM). Environ. Technol. Resour. 2011, 2, 132–138. [Google Scholar] [CrossRef][Green Version]
- Oliver, A. Thermal characterization of gypsum boards with PCM included: Thermal energy storage in buildings through latent heat. Energy Build. 2012, 48, 1–7. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Carmona, M.; Sanchez, M.L.; Valverde, J.L.; Rodriguez, J.F. Improvement of the thermal behaviour of gypsum blocks by the incorporation of microcapsules containing PCMS obtained by suspension polymerization with an optimal core/coating mass ratio. Appl. Therm. Eng. 2010, 30, 1164–1169. [Google Scholar] [CrossRef]
- Toppi, T.; Mazzarella, L. Gypsum based composite materials with micro-encapsulated PCM: Experimental correlations for thermal properties estimation on the basis of the composition. Energy Build. 2013, 57, 227–236. [Google Scholar] [CrossRef]
- Micronal Website. Available online: https://www.maisonpassive.be/IMG/pdf/Micronal_EN.pdf (accessed on 3 November 2021).
- Website Alba Saint-Gobain. Available online: https://www.construction21.org/data/sources/users/3474/extracto-rigipsalbabalance.pdf (accessed on 3 November 2021).
- EN 13279-2: Gypsum Binders and Gypsum Plasters—Part 2: Test Methods; European Committee for Standardization: Brussels, Belgium, 2014.
- EN 1015-6: Methods of Test for Mortar for Masonry—Part 6: Determination of Bulk Density of Fresh Mortar; European Committee for Standardization: Brussels, Belgium, 1998.
- Guide Technique UEAtc Pour l’agrément des Systèmes d’isolation Extérieure des Façades avec Enduits Minéraux; nr 331, Cahier 2602, 23 blz; Cahiers du CSTB, CSTB: Champs-sur-Marne, France, 1992.
- ETAG 004 (2000): Guideline for European Technical Approval of External Thermal Insulation Composite Systems with Rendering; European Organisation for Technical Approvals: Brussels, Belgium, 2000.
- EN 13872-1: Method of Test for Smoothing and/or Levelling Compounds—Determination of Shrinkage; European Committee for Standardization: Brussels, Belgium, 2004.
- EN 14146 : Natural Stone Test Methods—Determination of the Dynamic Modulus of Elasticity (by Measuring the Fundamental Resonance Frequency); European Committee for Standardization: Brussels, Belgium, 2004.
- ASTM D5590-94-Standard Test Method for Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay; ASTM International: West Conshohocken, PA, USA, 1994.
- Neuville, M. Les Fluidifiants du Plâtre. Ph.D. Thesis, Université de Nice-Sophia-Antipolis, Nice, France, 2013. Available online: https://tel.archives-ouvertes.fr/tel-00913652 (accessed on 3 November 2021).
- Michon, M. Etude de l’effet D’adjuvants Chimiques sur la Conversion du Plâtre en Gypse. Master’s Thesis, University of Sherbrooke, Sherbrooke, QC, Canada, 2002. [Google Scholar]
- EN 13279-1: Gypsum Binders and Gypsum Plasters—Part 1: Definitions and Requirements; European Committee for Standardization: Brussels, Belgium, 2006.
- Collepardi, M. Scienza e Tecnologia del Calcestruzzo; Hoepli: Milan, Italy, 1992; ISBN 8820319101. [Google Scholar]
- Raja, M.A.; Sophia, M. Influence of Expansive Agent on the Dimensional Stability and Mechanical Properties of Gypsum Plaster. Int. J. Recent Technol. Eng. 2019, 8, 928–931. [Google Scholar] [CrossRef]
- DIN 18947-Earth plasters—Requirements, Test and Labelling; Deutsches Institut Fur Normung E.V. (German National Standard): Berlin, Germany, 2018.
- Les Dossiers du CSTC –N° 2/2009 –Cahier n° 3. Available online: Available: https://www.cstc.be/umbraco/Surface/PublicationItem/DownloadFile?file=31850%2Ffr%2Funprotected%2Fcstc_artonline_2009_3_no3.pdf (accessed on 3 November 2021).
- Fořt, J.; Novotný, R.; Trník, A.; Černý, R. Preparation and characterization of novel plaster with improved thermal energy storage performance. Energies 2019, 12, 3318. [Google Scholar] [CrossRef]
- Bajare, D.; Kazjonovs, J.; Korjakins, A. Development of latent heat storage phase change material containing plaster. Medziagotyra 2016, 22, 94–97. [Google Scholar] [CrossRef][Green Version]
- EN 771-1: Specification for Masonry Units—Part 1: Clay Masonry Units; European Committee for Standardization: Brussels, Belgium, 2015.
- Note D’information Technique 271; Belgium Building Research Institute: Limelette, Belgium, 2020.
- Fischer, H.B. Primers role in plastering systems on concrete surfaces. IOP Conf. Ser. Mater. Sci. Eng. 2015, 71, 012020. [Google Scholar] [CrossRef]
- Gartner, E.M. Cohesion and expansion in polycrystalline solids formed by hydration reactions—The case of gypsum plasters. Cem. Concr. Res. 2009, 39, 289–295. [Google Scholar] [CrossRef]
- Edwards, A.J. Properties of Hydraulic and Non-Hydraulic Limes for Use in Construction. Ph.D. Thesis, Napier University, Edinburgh, UK, 2005. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).