1. Introduction
Knee injuries, particularly those involving the anterior cruciate ligament (ACL), are among the most common musculoskeletal injuries in sports and physically active populations. These injuries can cause significant functional impairment, long-term instability, and an elevated risk of osteoarthritis [
1,
2,
3]. It has been reported that patients with complete ACL tears tend to develop more severe gonarthrosis, indicating a link between chronic joint instability and progressive osteoarticular degeneration [
4]. At the cellular level, an increase in inflammatory cytokines in synovial fluid and alterations in chondral metabolism after ACL injury and reconstruction are described, providing evidence for early degenerative processes that may compromise long-term functional recovery [
5].
Beyond physical consequences, they also affect psychological well-being and quality of life. Therefore, accurate evaluation and targeted rehabilitation of knee function are essential.
Inertial measurement units (IMUs) and optical motion capture systems have been used to analyze knee kinematics during movements such as loaded squats and pivot landings [
6,
7]. However, these methods are often applied under ex vivo conditions or with the patient at rest, which may not accurately reflect how the knee behaves during real functional activities [
8]. As a result, there is increasing interest in tools that can capture joint mechanics during functional tasks, offering a more comprehensive understanding of knee behavior under real-life conditions.
Recent studies have highlighted the importance of assessing knee function during movement, as demonstrated by the development of a dynamic scoring system based on knee kinematics of six degrees of freedom (6-DOF) during gait analysis in patients with ACL injury, underscoring the clinical relevance of dynamic evaluations [
9]. Similarly, the validation of three-dimensional knee kinematics using an instrumented knee brace during treadmill walking has demonstrated its practical use in clinical settings [
10]. IMU-based algorithms have been validated against optical motion capture during high-risk ACL injury tasks such as landing and cutting, showing RMS errors of less than 3° across the three planes of rotation, with particularly strong accuracy in the sagittal plane. While IMUs and optical motion capture systems have shown high accuracy in estimating joint kinematics under controlled conditions, they typically rely on indirect measurements from markers or limb segments [
11,
12]. Finally, recent work has shown that on-body calibration improves the accuracy of portable goniometry, underscoring the importance of calibrating the user directly [
13].
Advances in sensor technologies have facilitated the development of wearable devices that record motion data in various environments, providing clinicians with meaningful information on joint performance and function [
14]. Among these, electrogoniometry has emerged as a valuable approach to capture three-dimensional joint kinematics. However, many available systems are costly or lack the sensitivity required for clinical precision.
This study presents the development of a novel three-dimensional electrogoniometer designed to directly assess knee mobility and stability during gait. By providing continuous motion data, the device seeks to improve the precision of clinical evaluations and support more effective rehabilitation strategies for individuals with knee injuries. While not intended to replace gold-standard systems, the electrogoniometer offers a portable and accessible alternative for real-time functional evaluation, particularly valuable in clinical and low-resource environments. In doing so, this work addresses key limitations of existing measurement practices and contributes to the advancement of biomechanical assessment tools in clinical care.
The primary objective of this study is to develop and validate an electrogoniometer capable of accurately quantifying knee joint behavior during functional activities, particularly gait. To achieve this, the study explores several key research questions: How the device’s performance compares with traditional systems in measuring knee kinematics such as flexion, extension, tibial rotation, and translation; whether it can provide reliable real-time data that reflect joint stability throughout movement, including its responsiveness and clinical utility; what the practical implications of the measurements are for clinical practice, especially in the rehabilitation of patients with ACL injuries; and whether the device is comfortable and easy to use without interfering with natural gait, supporting its feasibility for routine clinical application. By addressing these aspects, the study aims to contribute to the development of advanced measurement technologies in biomechanics and support improved outcomes in the treatment of knee injuries.
Given the dynamic nature of knee instability and gait alterations in various injuries and arthritic conditions, there is a clear need for accessible tools that allow objective functional assessment. Although full gait analysis systems provide valuable data, they are not routinely used to inform surgical decisions due to their high cost and limited availability. As a result, clinical evaluations often rely on subjective observations or static measurements that do not reflect joint behavior during daily activities, highlighting the need for alternative technologies capable of providing dynamic, real-time assessments in clinical settings.
2. Materials and Methods
A methodology was proposed to dynamically assess knee mobility, stability, and displacement during daily activities. This includes evaluating its muscular dynamic compensatory mechanisms through the collection of test results from human subjects.
Data capture involved a descriptive observational study methodology aimed at identifying patterns of mobility and instability between healthy individuals and those with specific knee injuries. Specifically, the study focused on characterizing patients with anterior cruciate ligament (ACL) instability patterns, particularly given their increased frequency compared to other knee injuries.
2.1. Design
The study employs a descriptive observational design, focusing on the development and evaluation of a novel three-dimensional electrogoniometry device. This design comprises two primary phases: device development and performance testing. Each phase is carefully structured to ensure the reliability and validity of the device in measuring knee mobility and stability.
2.1.1. Device Development
The electrogoniometry device is designed to capture knee movements in three dimensions: flexion, extension, tibial rotation, and tibial translation. The device integrates several key components:
Angular Position Encoders: These sensors are crucial for measuring joint angles and movements accurately. With a resolution of 1°, they provide real-time data on knee position, allowing for precise tracking of mobility during gait.
Microcontroller Unit: The device uses an Arduino microcontroller to process encoder signals, sampling data at a frequency of 50 Hz. This unit is responsible for converting raw data into usable information that can be transmitted to an external application.
Wireless Communication Module: A Bluetooth HC-05 communication module enables the device to transmit data wirelessly to an Android application. The transmission speed was set to 57,600 baud, corresponding to approximately 173.611 µs per data, which ensures sufficient throughput for real-time monitoring and analysis of knee movements during dynamic activities.
User Interface: The Android application provides a user-friendly interface for visualizing real-time data. It plots knee movements graphically, facilitating immediate feedback for both clinicians and patients.
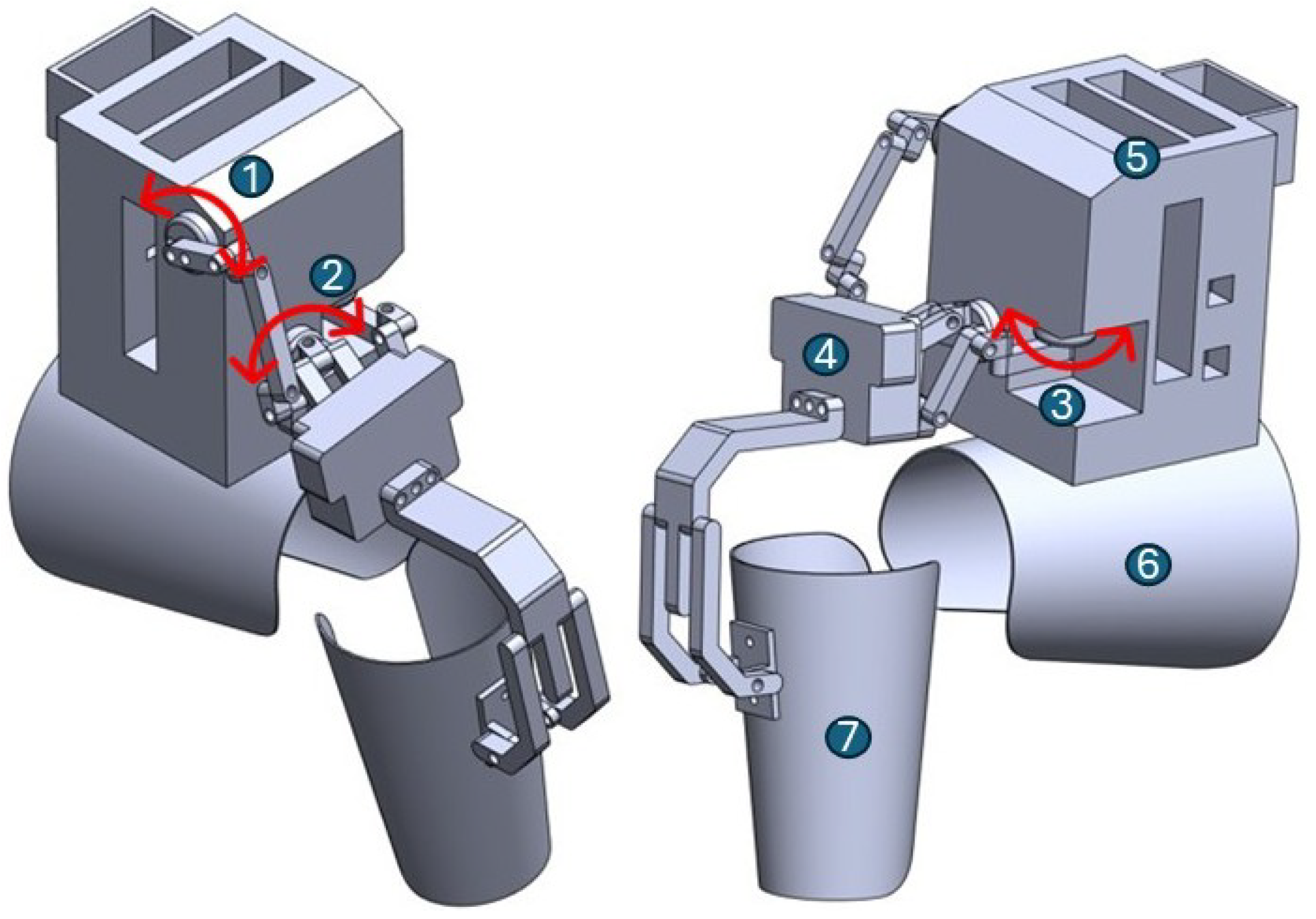
The mechanical structure of the electrogoniometry device shown in
Figure 1 was 3D-printed using PLA (polylactic acid), providing a lightweight yet rigid framework. It includes housings for three rotary encoders aligned with the anatomical axes corresponding to flexion-extension, tibial rotation, and tibial translation. Motion is transmitted through printed couplings and articulated arms. The thigh and shank attachments consist of adjustable Velcro straps to ensure stability during gait without restricting natural movement. The central module houses the electronics, including a microcontroller, power supply, and Bluetooth transmission unit.
Control system
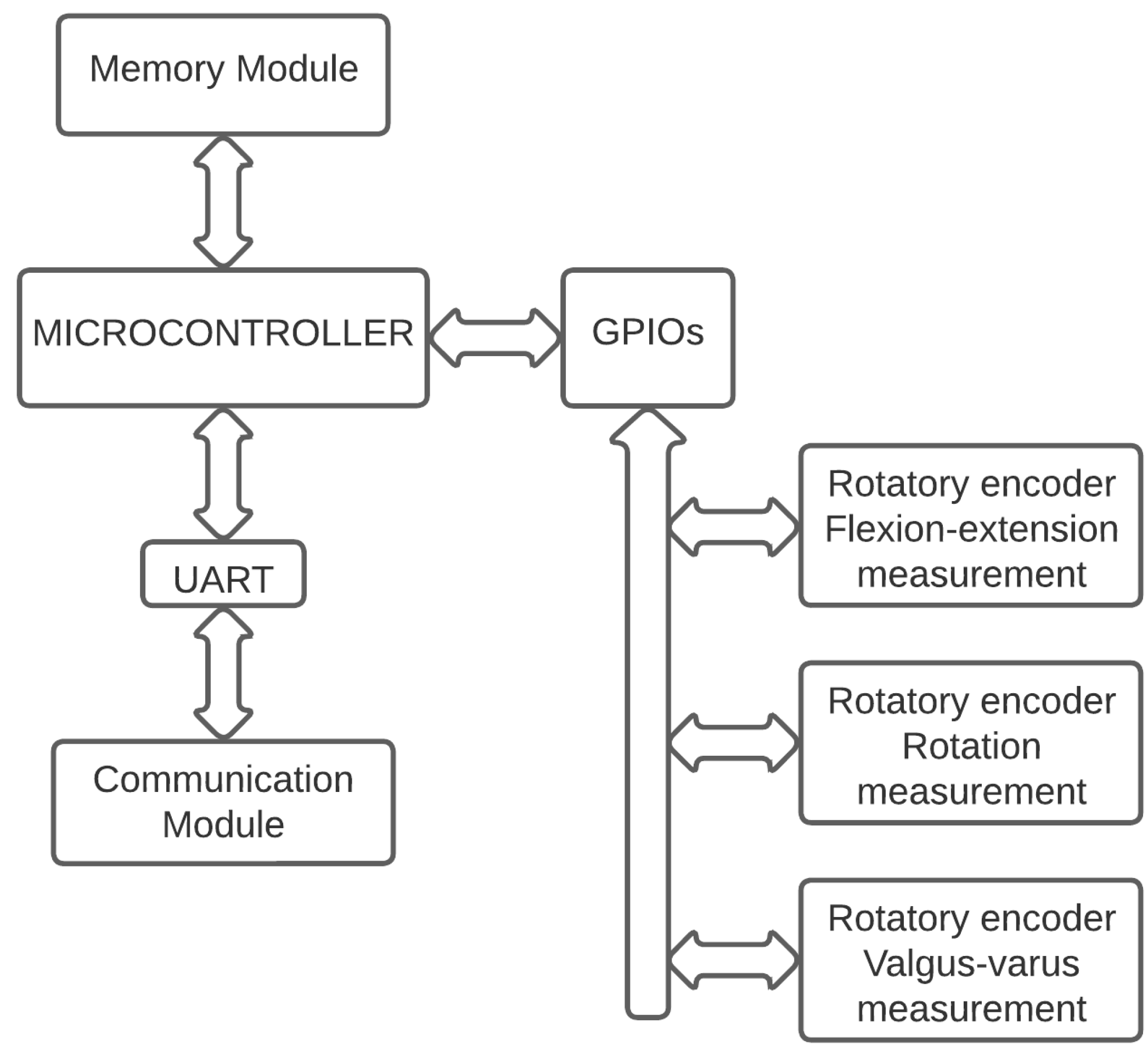
As seen in the electronic board wiring diagram of the electrogoniometry device in
Figure 2, the electronic board consists of a processing medium or microcontroller, which is the appropriate means for processing the signals captured by the angular position encoder sensors. The microcontroller is connected to a communications module via a communication protocol (UART hardware interface). The communications module is designed to perform data communication between the developed electrogoniometry device and an external user equipment (Android application) using a Bluetooth connection.
2.1.2. Performance Testing
To evaluate the effectiveness of the electrogoniometry device, a series of tests were performed involving both healthy participants and those with ACL injuries. The testing procedures include the following:
Calibration and Validation: The device was calibrated to ensure accurate measurements. Validation was performed by comparing electrogoniometry readings with those obtained from a standard 3D motion capture system (3DMA) from Motio STT Systems using full-body and lower-body protocols based on Helen Hayes, which is known for its reliability in biomechanical assessments.
Participant Testing: Participants were instructed to walk at their preferred pace while wearing the device. Data will be collected during these activities to assess the device’s performance in capturing dynamic knee movements accurately.
Data processing: To obtain the same data with the device as the 3DMA system, a cubic spline data interpolation was performed in MATLAB® R2023a (MathWorks, Natick, MA, USA) software to estimate the values at the unknown points based on the knowledge of the set of known values to be able to compare them in a statistical analysis with the 3DMA system.
Data Analysis: The collected data were analyzed to determine the device’s sensitivity and specificity in measuring knee mobility and stability. This analysis also included comparing the results of the electrogoniometry device with traditional measurement techniques to highlight improvements in data acquisition and clinical relevance.
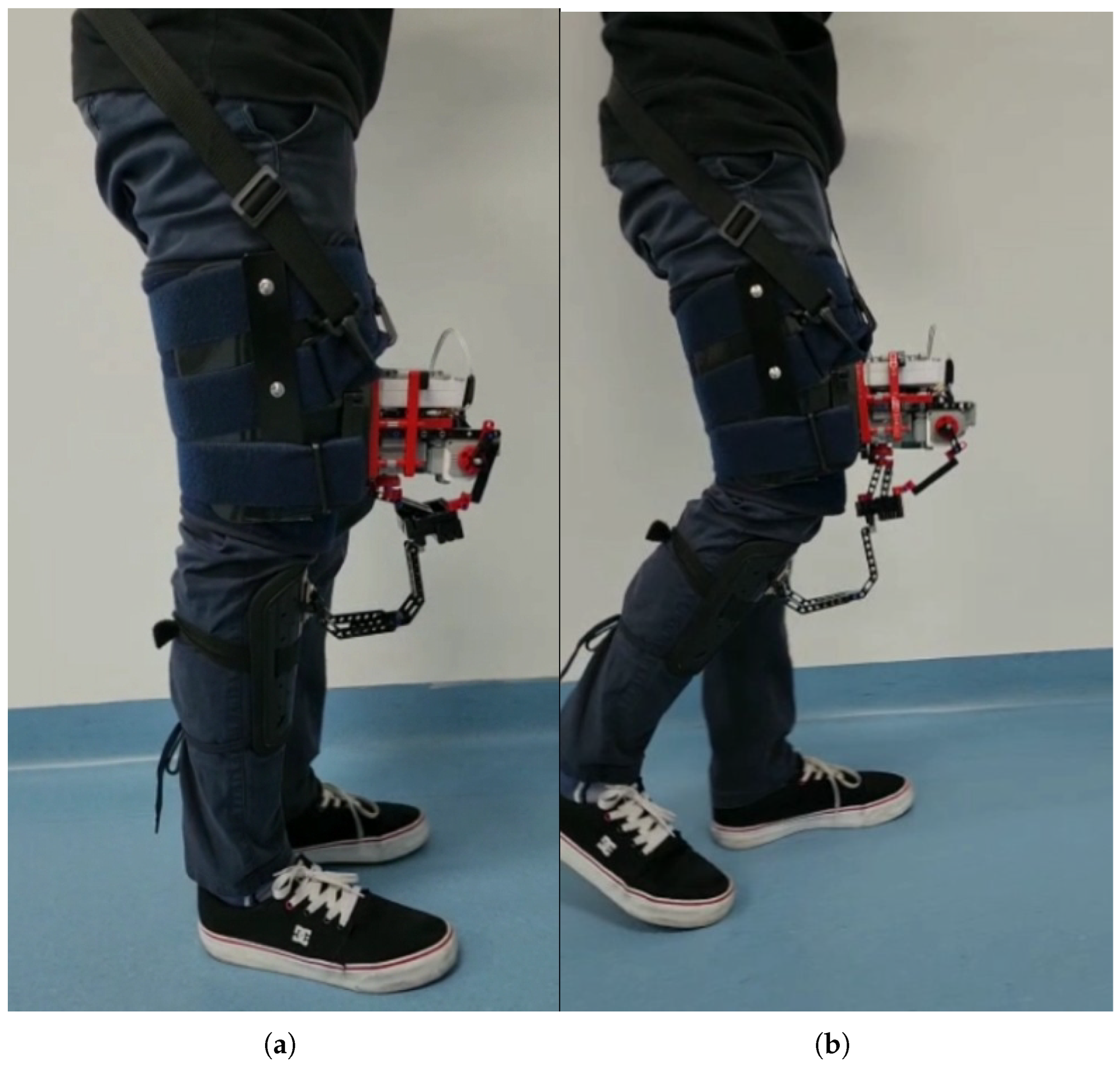
Figure 3 shows the electrogoniometry device implemented in a volunteer during knee extension and flexion, simulating gait. This illustrates that the device does not hinder normal movement, thanks to its mechanical design, which allows it to be positioned on the thigh and shank without interfering with joint motion. The total weight of the device, including all structural parts, sensors, electronics, battery, fastening straps, and wiring, is approximately 900 g (
Table 1). Its mass is distributed along the thigh and shank, and the fastening strap system improves fixation and stability, minimizing perceived load during use, and ensuring secure attachment throughout the gait cycle.
The calibration and setup process for the electrogoniometry device typically takes less than 5 min per subject. This includes positioning the device on the thigh and shank, adjusting the straps for secure fixation, and performing a brief signal check to ensure proper functionality. No complex calibration procedures are required, as the system relies on mechanical alignment with anatomical landmarks. This streamlined procedure facilitates rapid deployment in clinical settings without the need for specialized personnel or extensive preparation.
2.2. Main Measures
The study focuses on several key measures to assess knee mobility and stability using the developed electrogoniometry device. These measures are designed to provide a comprehensive evaluation of knee function during dynamic activities, particularly gait. The following main measures will be utilized:
Flexion and Extension: These movements will be measured to assess the range of motion of the knee joint. The electrogoniometry device will provide real-time data on the angles achieved during walking, allowing a detailed analysis of flexion-extension patterns.
Tibial rotation: The device will capture rotational movements of the knee, which are critical to understanding the stability and function of the joint during various activities, including sports and daily tasks.
Tibial translation: This measure refers to the anterior–posterior and medial–lateral movement of the tibia relative to the femur. Assessment of translation is essential for identifying instability, particularly in individuals with ACL injuries.
2.2.1. Data Acquisition
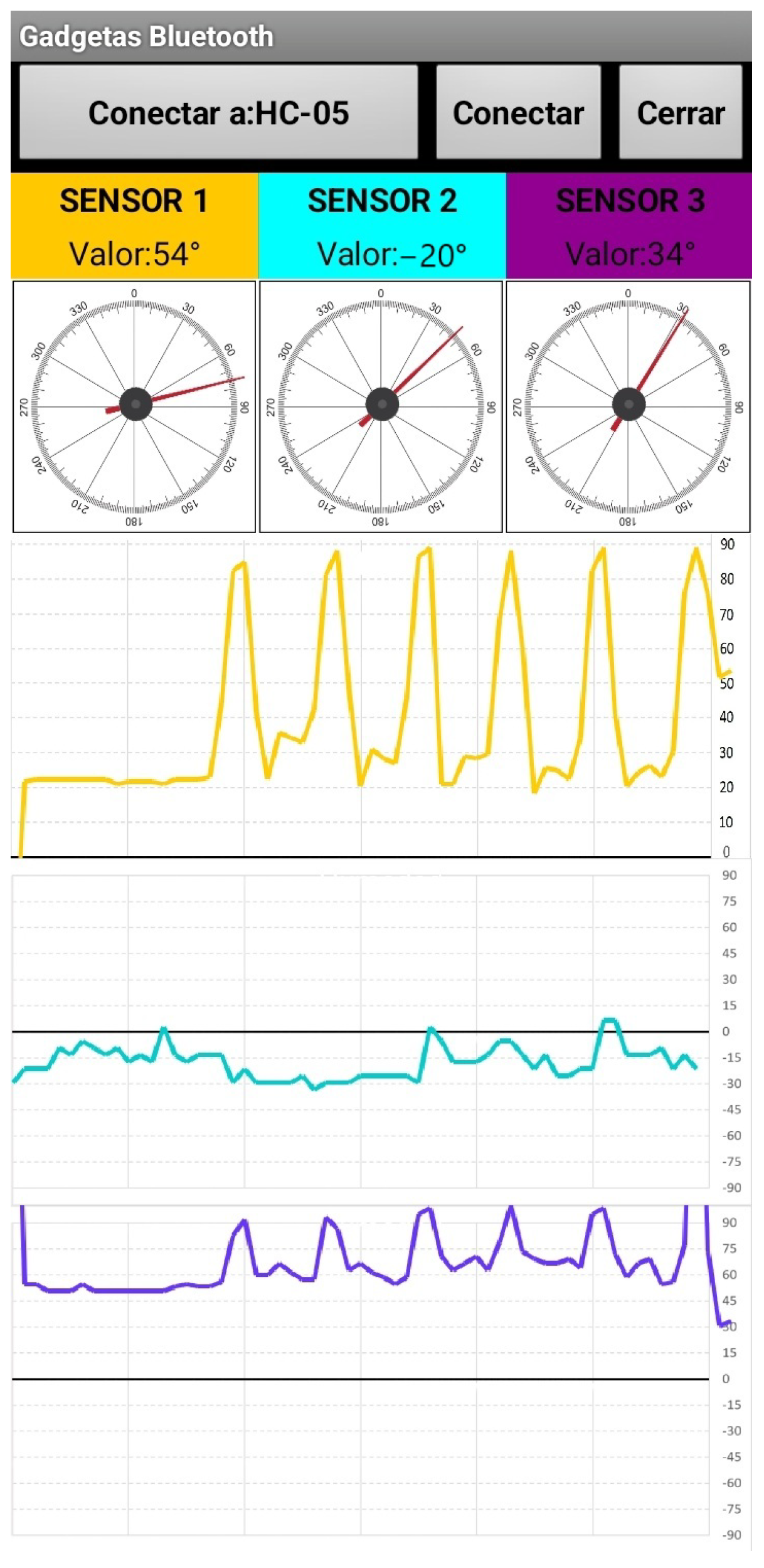
The electrogoniometry device continuously captures and records knee movement data during gait. These data are transmitted wirelessly to an Android application for visualization. The application displays real-time graphs of knee flexion, extension, tibial rotation, and tibial translation, as shown in
Figure 4.
2.2.2. Functional Assessment
Clinical Evaluation Scales: Standardized clinical evaluation tools such as the Lysholm Knee Scoring Scale and the Subjective Knee Form of the International Knee Documentation Committee (IKDC) were used to correlate the findings of the electrogoniometry device. These tools provide insight into the functional implications of knee injuries and help evaluate overall knee function.
Gait Analysis Parameters: The study also measured spatio-temporal parameters, including gait speed, cadence, and stride length. These metrics were analyzed in conjunction with knee movement data to provide a holistic view of participants’ functional mobility.
2.3. Participants
The study involved a total of 30 participants, divided equally between individuals with anterior cruciate ligament (ACL) injury and healthy controls. Participants were recruited from local clinics and sports organizations to ensure demographic diversity. The sample size was determined primarily by feasibility constraints related to participant availability and laboratory resources, in accordance with common practice for preliminary device validation studies. Furthermore, a post hoc power estimate indicated that with n = 15 per group, and power, the study can detect differences between groups corresponding to large effect sizes (Cohen’s ) or correlations of approximately . This level of sensitivity is sufficient for detecting moderate-to-large effects relevant to a proof-of-concept evaluation but not for small-effect clinical inferences. The present sample therefore supports the primary objective of assessing device performance against a gold-standard optical motion capture system, while informing the design parameters for future large-scale studies.
2.3.1. Sample Characteristics
Data were collected on demographic variables such as age, sex, height, weight, and level of physical activity. This information will help contextualize the findings and ensure that the results can be generalized across different populations. The descriptive demographic data for both groups are as follows:
Healthy Group (n = 15):
Participants aged between 18 and 50 years.
Eight men and seven women.
Mean height = 165 ± 10 cm.
Mean weight = 70 ± 10 kg.
ACL Injury Group (n = 15):
Participants aged between 18 and 50 years.
Eleven men and four women.
Mean height = 160 ± 10 cm.
Mean weight = 67 ± 8 kg.
By carefully selecting participants and adhering to ethical standards, the study aims to obtain a comprehensive understanding of knee mobility and stability, contributing to the development of effective assessment tools in clinical practice.
2.3.2. Recruitment Process
Participants were recruited through advertisements in local sports clubs, community centers, and rehabilitation clinics. Potential participants were subjected to a screening process that included the following:
Initial Questionnaire: To gather basic demographic information and medical history.
Physical Assessment: Conducted by a licensed physical therapist or physician to confirm eligibility and ensure safety during the test.
2.3.3. Inclusion Criteria
Healthy Group:
ACL Injury Group:
Clinically diagnosed with an ACL injury, confirmed by medical records and imaging (e.g., MRI).
At least 12 months post-injury to ensure stability in assessment.
Exclusion criteria (both groups):
Neurological disorders or conditions affecting motor control.
Any pain, discomfort, or inflammation that limits gait.
Use of walking aids or assistive devices.
Ongoing physical therapy or rehabilitation during data collection.
Previous surgeries in the lower extremities.
Other significant knee or lower limb injuries (e.g., meniscus tears, fractures) that could affect gait.
2.3.4. Ethical Considerations
All participants provided their informed consent in writing prior to participating in the study. They were informed about the purpose of the research, procedures, potential risks, and benefits of participation. The study adhered to ethical guidelines established by the Ethics Committee of the Central Military Hospital, ensuring confidentiality and the right to withdraw from the study at any time without consequences. The study was approved by the Ethics Committee of the Central Military Hospital (2022-077).
2.3.5. User Experience
Comfort and Usability Surveys: Participants completed surveys assessing their comfort and usability of the electrogoniometry device during testing. This feedback is crucial to understanding the practicality of the device in clinical settings.
2.4. Statistical Analysis Method
The sample size was determined for a comparative study aimed at validating the hypothesis that the proposed measurement method is at least as sensitive and accurate as an optoelectronic system. To evaluate this, three complementary metrics were applied to the time series data for each movement axis: (1) root mean square error (RMSE) to quantify point-by-point differences, (2) cross-correlation coefficients to assess phase alignment and waveform similarity, and (3) the coefficient of multiple correlation (CMC) to capture both amplitude and shape agreement across the full waveform. These analyses provide a comprehensive evaluation of the agreement between the electrogoniometry device and the optical motion capture system during gait cycles, going beyond the limitations of single-metric comparisons.
The study aims to provide a comprehensive evaluation of knee mobility and stability. This multifaceted approach will improve the understanding of knee function in both healthy individuals and those with ACL injuries, paving the way for improved clinical assessments and rehabilitation strategies.
3. Results
The evaluation of the newly developed three-dimensional electrogoniometry device yielded significant findings regarding its effectiveness in measuring knee mobility and stability. The results can be summarized as follows.
3.1. Measurement Accuracy
The results of both devices are graphed to facilitate interpretation, allowing a graphical comparison of dynamic knee motion measurement, and a cross-method visualization of the control group kinematics, amplitude, and overall precision. For demonstration purposes, four participants (subjects 1, 3, 5, and 9) were randomly selected from a total sample of 15 individuals in the control group.
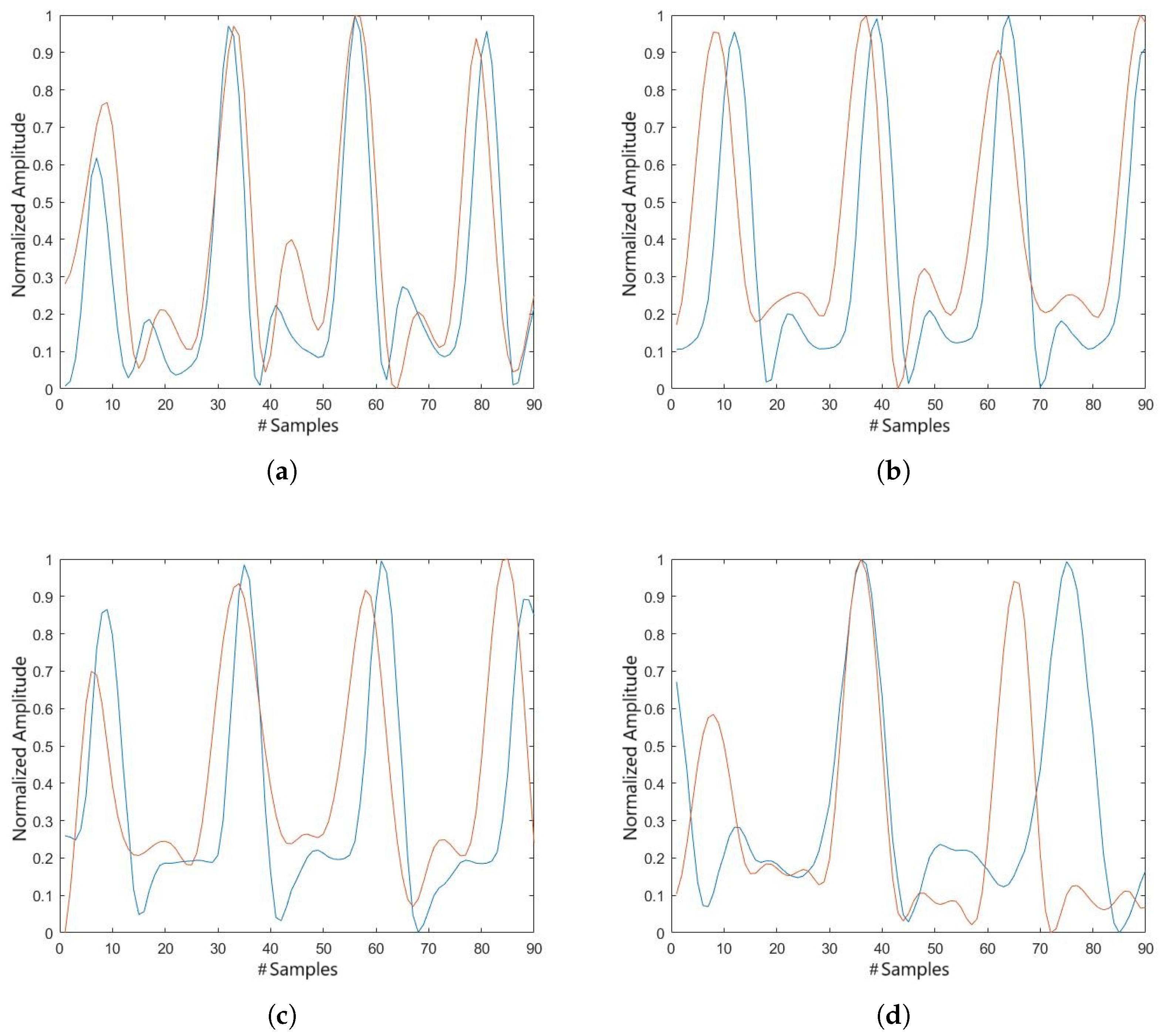
Figure 5 shows the comparison between the data obtained by optoelectronic motion analysis (3DMA System) and those from goniometric measurements.
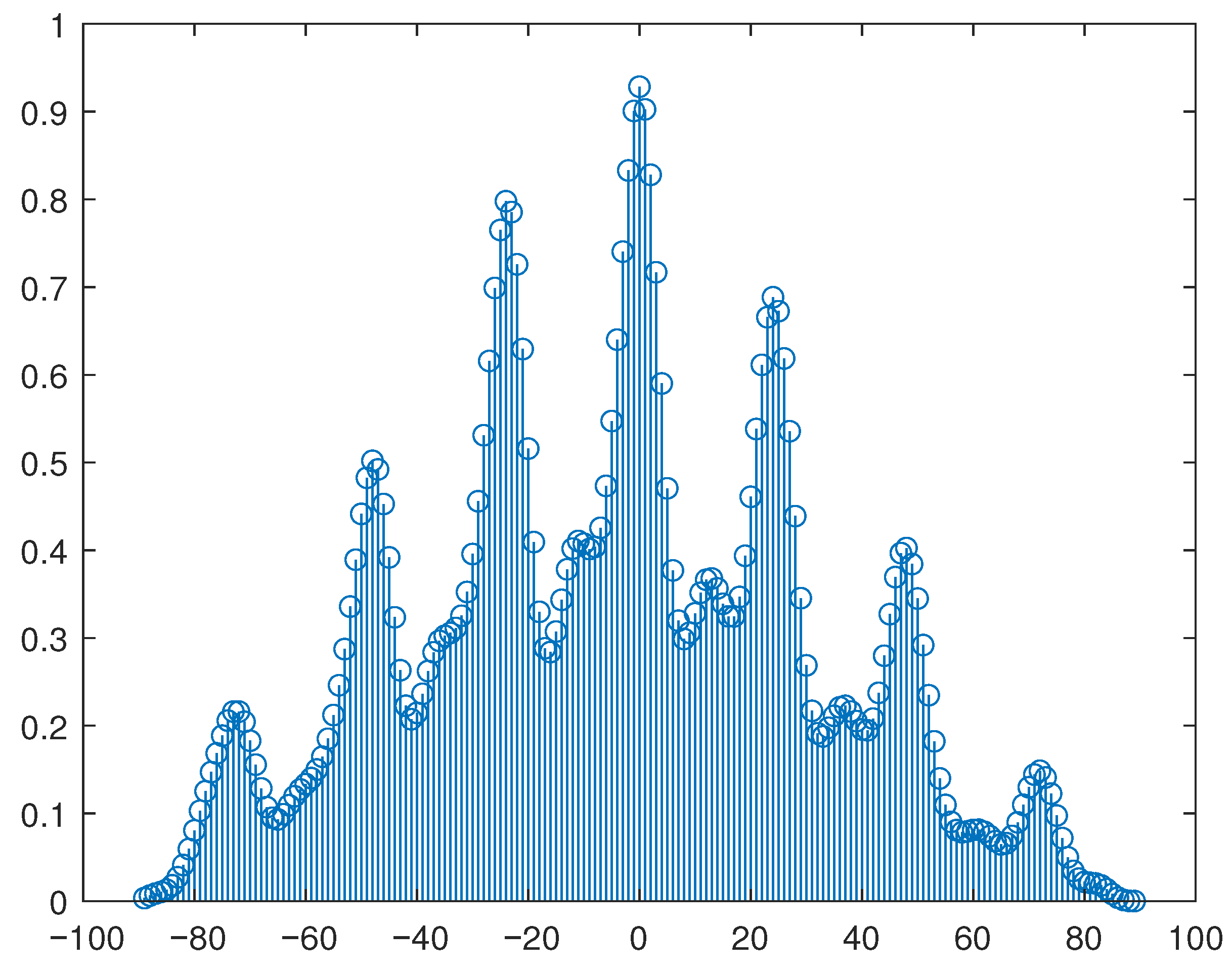
A statistical analysis of the information obtained from each system was performed using a cross-correlation function to compare the signals, which produces an estimate of the correlation between two sequences, which is a measure of the similarity between two signals.
Figure 6 illustrates the resulting cross-correlation between the electrogoniometer and the 3DMA system for the flexion-extension movement.
The device accurately measured knee movement during gait, with flexion–extension values aligned closely with those recorded by the standard 3DMA system, demonstrating a high cross-correlation coefficient shown in
Table 2. This strong correlation highlights the reliability of the electrogoniometry device in clinical evaluations [
15].
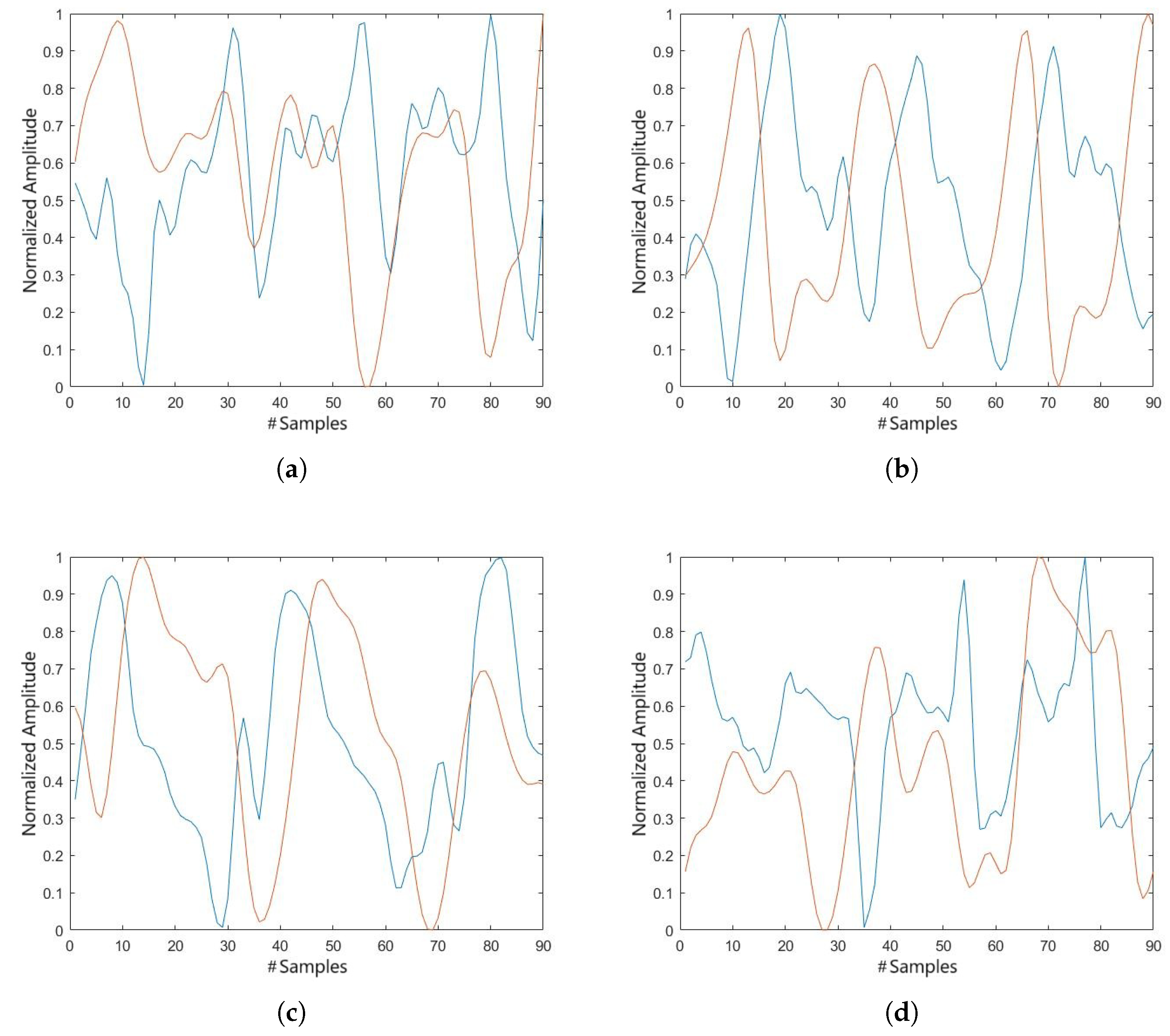
Having established a reliable comparison between flexion and extension in the healthy population, and having confirmed the precision of both systems, we proceeded to evaluate the precision of the system in other planes of motion, as well as in a population with knee instability. In this context, we observed that the tibial rotation graph is capable of providing comparable data, although the phase of movement appears to be slightly but significantly greater in the electrogoniometer, as illustrated in
Figure 7.
The cross-correlation coefficients for the tibial rotation, presented in
Table 3, show a low correlation for this movement, possibly due to the phase shift between the compared signals.
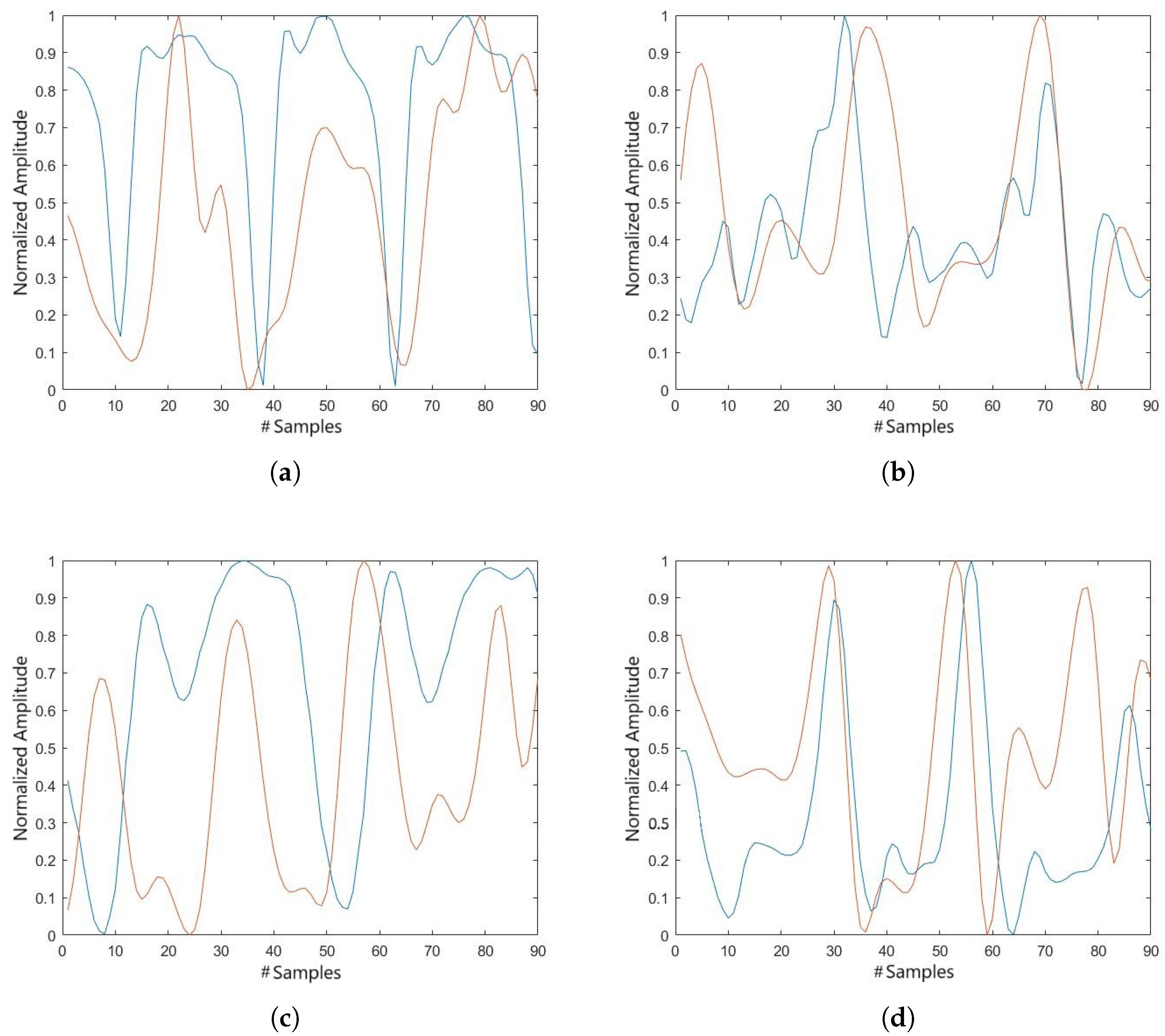
Similarly to the tibial rotation results, we obtained a graph showing the tibial translation of subjects in the ACL group, in which a comparable signal is observed between both devices, although with a difference in amplitude, as shown in
Figure 8.
The cross-correlation coefficients for the tibial translation, presented in
Table 4, are generally high, indicating good agreement between the signals. However, a few lower values were observed, which may be related to differences in amplitude between the signals rather than temporal misalignment.
3.2. Real-Time Data Transmission
Data transmission via Bluetooth to the Android application was successful, with real-time graphs of knee movements displayed during testing. The end-to-end latency of the system—from encoder signal acquisition by the microcontroller to graphical display in the Android application—was measured using software-based timing. The average latency was 51.6 ± 4.3 ms ( CI: 50.4–52.8 ms), confirming that the device provides real-time visualization with negligible delay. Participants reported high satisfaction with the ease of use and comfort of the device, strengthening its practicality in clinical settings.
3.3. Statistical Analysis and Sensitivity
All statistical analyzes were performed with MATLAB. Time series from the electrogoniometry device and the optical motion capture system were temporally aligned, resampled to 100 points per gait cycle, and amplitude normalized. The agreement between systems was assessed using three complementary metrics: (1) root mean square error (RMSE) to quantify point-by-point differences, (2) cross-correlation coefficients to evaluate phase alignment and waveform similarity, and (3) the coefficient of multiple correlation (CMC) to capture both amplitude and shape agreement across the full waveform.
The electrogoniometry device demonstrated angular sensitivity comparable to that of traditional optoelectronic motion capture systems. Although the encoders used have a resolution of
, the RMSE between the electrogoniometer and the optical system was
for flexion–extension,
for tibial rotation, and
for tibial translation. CMC values were high for flexion–extension (mean: 0.88), moderate for tibial translation (mean: 0.69), and lower for tibial rotation (mean: 0.41), reflecting the greater complexity and variability of the rotational measurements. These results support the capability of the device for dynamic knee assessment in clinical and field settings [
11,
12,
16].
3.4. Functional Assessment Correlation
For knee flexion–extension, cross-correlation coefficients ranged from 0.40 to 0.95 (mean: 0.80), with of participants () above 0.75, indicating strong agreement in waveform patterns. These findings support the reliability of the electrogoniometry device in capturing knee motion during gait and suggest its potential for use in functional assessment and clinical evaluation of joint stability.
The tibial rotation showed the lowest cross-correlation values among the three measured motions, with coefficients ranging from 0.06 to 0.53 and a mean value of approximately 0.23. Only four participants showed moderate agreement (coefficients ≥ 0.30), while six participants had values below 0.20, indicating weak correspondence between the two systems. These lower correlations may reflect the increased complexity and variability in measuring rotational motion. This suggests that rotational measurements may require further optimization to reduce signal error and improve reliability.
For the tibial translation motion, the cross-correlation coefficients revealed greater variability across participants, with values ranging from 0.04 to 0.84 and an average of approximately 0.47. Although six participants showed moderate to high agreement (coefficients ≥ 0.60), five showed low correlations below 0.30, which may be related to differences in signal amplitude or other sources of noise. Despite these variations, the results highlight the device’s ability to detect translational motion and its potential for refinement to improve consistency in this plane of movement. The observed inter-subject variability suggests opportunities for further optimization of the device’s mechanical design and signal processing algorithms to improve the stability measurement between different users.
Preliminary correlations between device-derived stability measures and Lysholm Knee Scoring Scale outcomes suggested that participants with greater measured knee stability also reported better functional outcomes (Lysholm scores > 70). These findings highlight the potential of the device to provide objective clinically relevant kinematic data to complement subjective functional assessments.
3.5. Assessment of the Impact on Normal Movement
To quantitatively assess whether the device hinders normal gait, spatiotemporal parameters were measured in a subset of 10 participants walking under two conditions: (1) without the device and (2) with the device attached. Walking speed, stride length, and cadence were recorded using the 3DMA system and compared using paired
t-tests. As shown in
Table 5, no statistically significant differences (
p > 0.05) were observed for any parameter, indicating that the device does not significantly affect normal gait patterns.
3.6. Participant Feedback
Post-test surveys revealed that of the participants rated the device as comfortable and expressed a preference for it over conventional static evaluation methods. The main reasons cited included real-time data feedback () and the absence of physical discomfort or restriction during movement ().
All participants reported that the device did not interfere with their gait pattern during the trials. This perception is consistent with the mechanical design of the system, which allows full knee flexion, extension, and tibial rotation without obstructing joint motion. While minor gait influence cannot be completely ruled out, the configuration and distribution of the 900 g device—secured with thigh and shank straps—appears to minimize perceived burden and allow natural walking.
These results support the potential of the electrogoniometry device to assist in the assessment of knee mobility and stability. While its accuracy does not exceed that of gold-standard systems, the device demonstrated an adequate level of precision consistent with values reported in the literature. Combined with its portability, ease of use, and ability to provide real-time data, this makes it a practical alternative for functional evaluation and rehabilitation monitoring in clinical and field settings.
4. Discussion
This preliminary validation study focused on evaluating the agreement between the proposed electrogoniometry device and a reference optical motion capture system (3DMA). Intra-device and inter-session repeatability assessments were not performed at this stage; however, such analyses, particularly the intraclass correlation coefficient (ICC) across repeated gait cycle are recognized as critical for clinical adoption and will be prioritized in future work to ensure measurement robustness and clinical reliability [
17,
18].
Data from the electrogoniometer were sampled at 50 Hz, while the 3DMA system recorded at 100 Hz. To allow a meaningful comparison, the electrogoniometer data were interpolated to match the 3DMA system frame count. No filtering was applied prior to interpolation, preserving the raw signal features. Visual inspection confirmed that key waveform features, such as peaks and zero-crossings, remained consistent. Temporal alignment between systems was achieved by identifying a shared gait event, specifically the peak extension angle during terminal stance. Although sufficient for this exploratory analysis, the absence of hardware synchronization and reliance on interpolation may introduce minor timing inaccuracies. Future work will address this by implementing matched sampling rates, optimized filtering, and hardware-based synchronization [
19].
Participants walked at a self-selected pace under standardized environmental conditions. Although footwear was controlled and limb dominance was recorded, variables such as body mass index (BMI), walking speed, and limb dominance were not explicitly normalized. These factors may contribute to inter-subject variability, and future studies should implement tighter controls or statistical normalization to reduce variability and enhance interpretability.
The additional spatiotemporal analysis confirmed that the device does not significantly alter normal walking patterns, as no significant differences in walking speed, stride length, or cadence were detected compared to walking without the device. This supports its suitability for functional gait assessments without introducing gait artifacts due to its weight or structure.
Although inertial measurement units (IMUs) provide valuable kinetic data, they typically lack the precision required for accurate kinematic analysis, which is critical for clinical and surgical decision-making [
20]. The proposed electrogoniometer addresses this gap by offering high-resolution, multiplanar measurements, including tibial translation and rotation, through a novel arrangement of encoders. Although not yet compared to pressure-sensitive insoles or similar wearable tools, the ability of the system to accurately capture continuous joint motion supports its potential as a valuable alternative in clinical biomechanics.
Regarding system latency, the average end-to-end delay latency was 51.6 ± 4.3 ms, which ensures sufficient temporal resolution to capture functional tibial translations and rotations during gait and supports real-time visualization of knee motion. This negligible delay confirms the suitability of the device for both experimental and potential clinical applications where timing precision is critical.
The device also shows potential for multimodal neuromuscular assessment. It has already been used experimentally alongside surface Electromyography (sEMG), though clinical validation of correlations between EMG and kinematic data remains limited [
21]. Interpretation of such data can be complex, so future development should aim to standardize and validate multimodal signal integration.
Although this study concentrated on anterior cruciate ligament (ACL) pathology, the utility of the system extends to other conditions, such as early osteoarthritis or post–total knee arthroplasty (TKA). In particular, it may help identify mid-flexion instability, a common but poorly quantified problem that contributes to patient dissatisfaction after TKA [
22,
23]. Real-time objective kinematic feedback during functional activities could enable earlier detection of joint dysfunction in a range of musculoskeletal and neuromuscular disorders.
Though this study focused on angular position data, the system also allows the derivation of angular velocity and acceleration, parameters that may be crucial in detecting dynamic instability. These higher-order variables were not analyzed here, but remain accessible through post-processing and may enhance diagnostic capabilities in future applications.
One of the key advantages of this device is its ability to provide dynamic and objective evaluations of tibial translation and rotation, parameters traditionally evaluated through subjective, passive clinical tests. Current orthopedic practice lacks standardized quantitative tools to evaluate functional joint instability, particularly during real-life activities. The electrogoniometer addresses this gap, offering continuous kinematic data that can potentially redefine clinical thresholds and improve decision making.
The analysis in this study compared full waveform profiles over the gait cycle without segmenting into discrete gait phases. While this simplification was useful for initial validation, future implementations could benefit from phase-specific analyses using event-based segmentation, dynamic time warping, or machine learning to enhance temporal interpretability and detect instability during specific phases such as mid-stance or terminal swing [
24,
25].
Although the 3DMA system used as a gold standard limited the study to level-ground walking in a controlled laboratory setting, the electrogoniometer was designed for use in real-world and high-demand environments. Preliminary data collection on uneven surfaces demonstrated the system functionality, but these results were not reported here due to the lack of a concurrent reference system. Nonetheless, the mechanical robustness and portability of the device make it suitable for future studies in field conditions, including military and sports scenarios [
26].
We are committed to translating this research into clinical practice, in order to support physicians in the objective assessment of dynamic knee function under everyday conditions. Validating the system accuracy was a necessary first step. Although the study involved a relatively small sample size (15 participants per group), this number was adequate to detect moderate-to-large differences between groups, aligning with the proof-of-concept nature of this work. The sample size, determined by feasibility constraints, limits the generalizability of the findings; however, it allowed us to confirm the technical feasibility and agreement with a gold-standard system. With this completed, our next phase involves designing a clinical protocol, obtaining ethical approval, and conducting trials in surgical populations to evaluate whether real-time kinematic data can inform or improve clinical interventions. Future work will also include larger cohorts to enable more robust statistical evaluations and strengthen the clinical evidence base.
Although the device does not match the sub-degree precision of high-end optical motion capture systems, the accuracy observed in this study is consistent with the values reported for wearable systems intended for field and clinical purposes [
11,
12,
16]. The high agreement for flexion–extension (mean CMC = 0.88, RMSE
) and moderate agreement for tibial translation (mean CMC = 0.69, RMSE
) indicate that the device captures clinically relevant changes in knee motion. The lower accuracy in tibial rotation (mean CMC = 0.41, RMSE
) reflects known challenges for wearable sensors in this plane and highlights opportunities for further optimization. Importantly, preliminary correlations with functional scores suggest sensitivity to functional knee stability, supporting clinical relevance. In this context, the goal is not to replace gold-standard laboratory systems, but to provide a portable, low-cost, and deployable alternative capable of delivering objective kinematic data where laboratory measurements are not feasible, such as in clinical screening, rehabilitation monitoring, and sports performance assessment.
While the electrogoniometry device demonstrated strong general agreement with the optical motion capture system, reflected in a mean cross-correlation coefficient of 0.80 across participants, minor discrepancies were observed during the stance phase of the gait cycle. These deviations may be attributed to soft tissue artifacts and load-induced effects on strap fixation during weight bearing. Although these variations can influence instantaneous readings, they did not significantly affect the overall waveform similarity or compromise the ability to identify key kinematic features. These findings suggest that the device, despite some phase-specific limitations, provides sufficiently accurate data for functional assessment and monitoring of knee joint behavior in dynamic tasks. In addition, user feedback confirmed that the device was well tolerated and did not hinder natural gait, supporting its potential for routine use in clinical environments.
Beyond its technical capabilities, the system offers practical advantages in terms of cost, usability, and accessibility. With an estimated per unit cost of USD 100–120—including sensors, microcontroller, Bluetooth module, mechanical structure, and power supply—it presents a highly affordable alternative to gait labs and optical motion capture systems, which typically cost between USD 20,000 and USD 100,000. This affordability, coupled with its ease of use and portability, supports its adoption in outpatient clinics, rehabilitation centers, and possibly even in sports settings.
Unlike conventional tools such as the KT-1000 or GNRB, which are limited to measuring passive translational laxity [
27], the proposed electrogoniometry system captures functional and dynamic knee instability during real-life activities. This capability improves diagnostic precision and supports more comprehensive therapeutic monitoring, especially in ACL-related conditions. Future validation in broader pathological populations, including individuals with meniscal injuries or osteoarthritis, will be essential to establish clinical sensitivity and specificity. In addition, long-term performance considerations such as sensor drift, battery duration, and ease of integration into existing workflows remain to be addressed. By combining low-cost, portability, and real-time measurement, the system holds promise not only for advancing biomechanical assessment but also for democratizing access to high-quality joint evaluation in resource-limited settings.
5. Conclusions
This study presents the successful development and preliminary validation of a three-dimensional electrogoniometer capable of accurately recording knee kinematics, including flexion–extension, tibial rotation, and tibial translation, during gait. The system demonstrated good agreement with a gold standard optical motion capture system (3DMA system), supporting its use as a portable and low-cost alternative in contexts where access to high-end laboratory equipment is limited or impractical. Rather than replacing these systems, the device offers a more accessible solution for functional knee assessment in clinical, field, or remote settings. Feedback from participants supported its comfort and ease of use, reinforcing its potential for routine application in rehabilitation and orthopedic care.
By enabling real-time, dynamic evaluation of joint behavior, this device addresses key limitations of conventional static or subjective assessment methods. Future work will focus on broader clinical validation, evaluation of various pathologies, long-term repeatability studies, and possible integration into telemedicine and multimodal monitoring platforms. Ultimately, the device aims to support improved clinical decision-making and patient outcomes in musculoskeletal health.
In general, the system represents a practical, objective, and accessible solution for dynamic knee assessment, especially in settings where conventional motion capture is not feasible.