The Effects of Aging and Cognition on Gait Coordination Analyzed Through a Network Analysis Approach
Abstract
1. Introduction
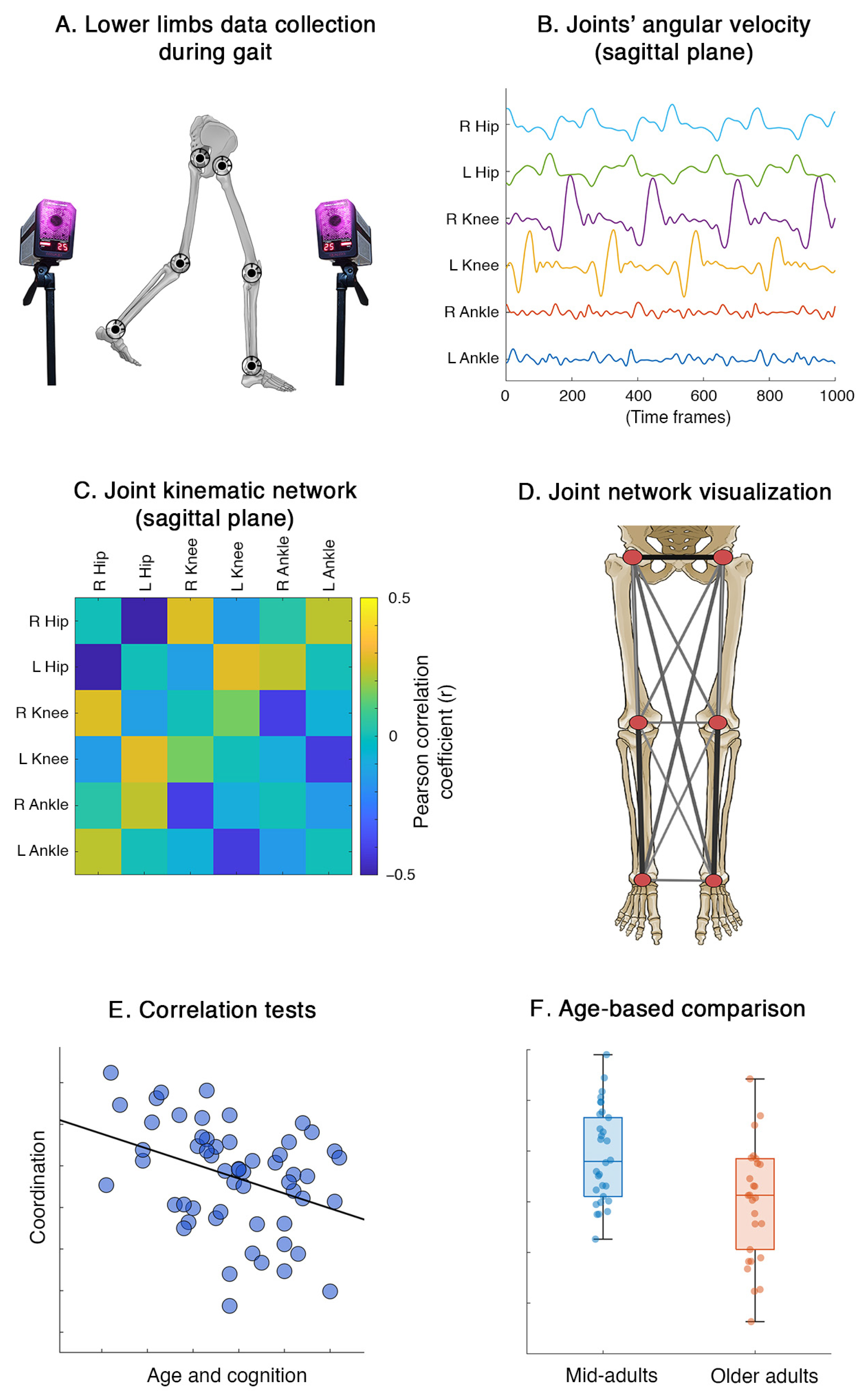
2. Materials and Methods
2.1. Participants and Data Collection
2.2. Recording System and Processing Pipeline
2.3. Lower Limbs Network
2.4. Statistics
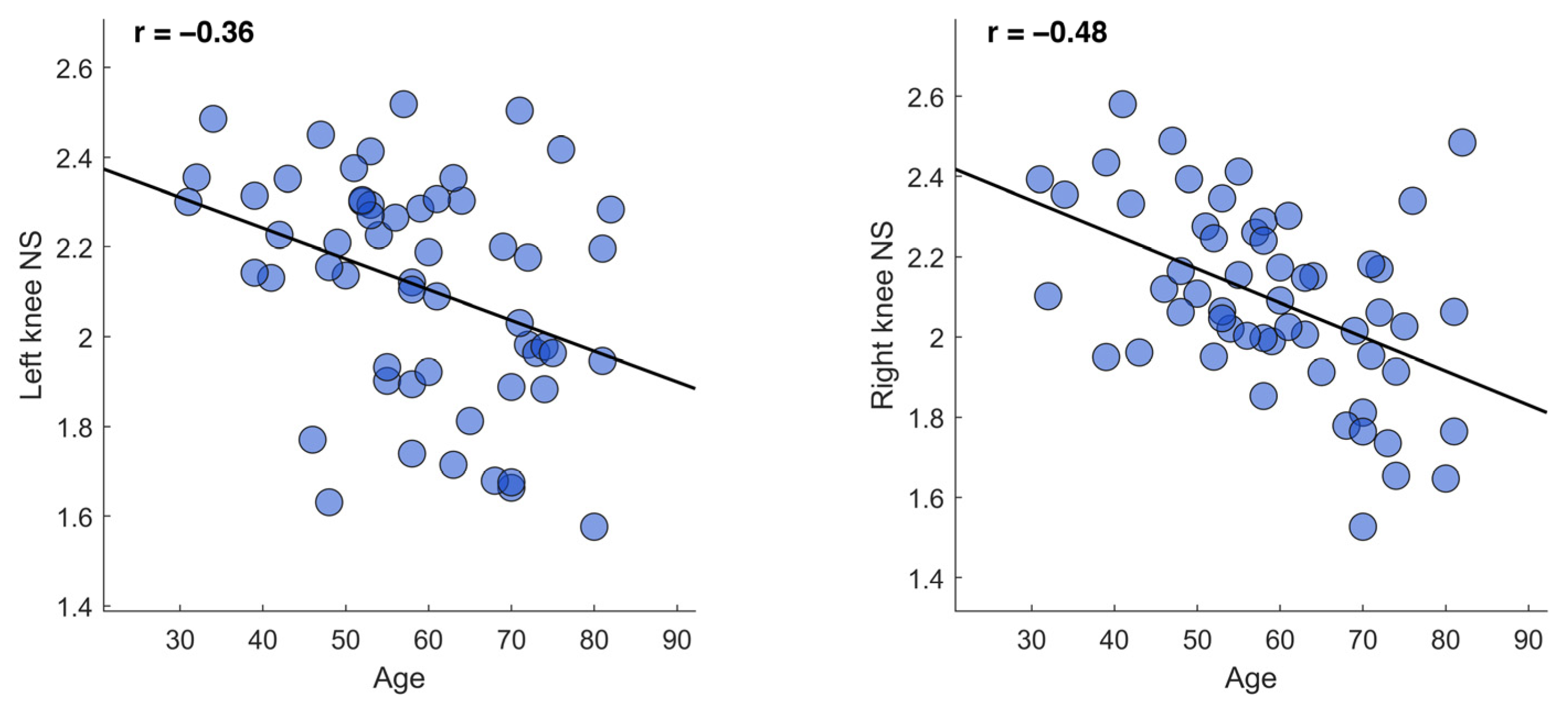
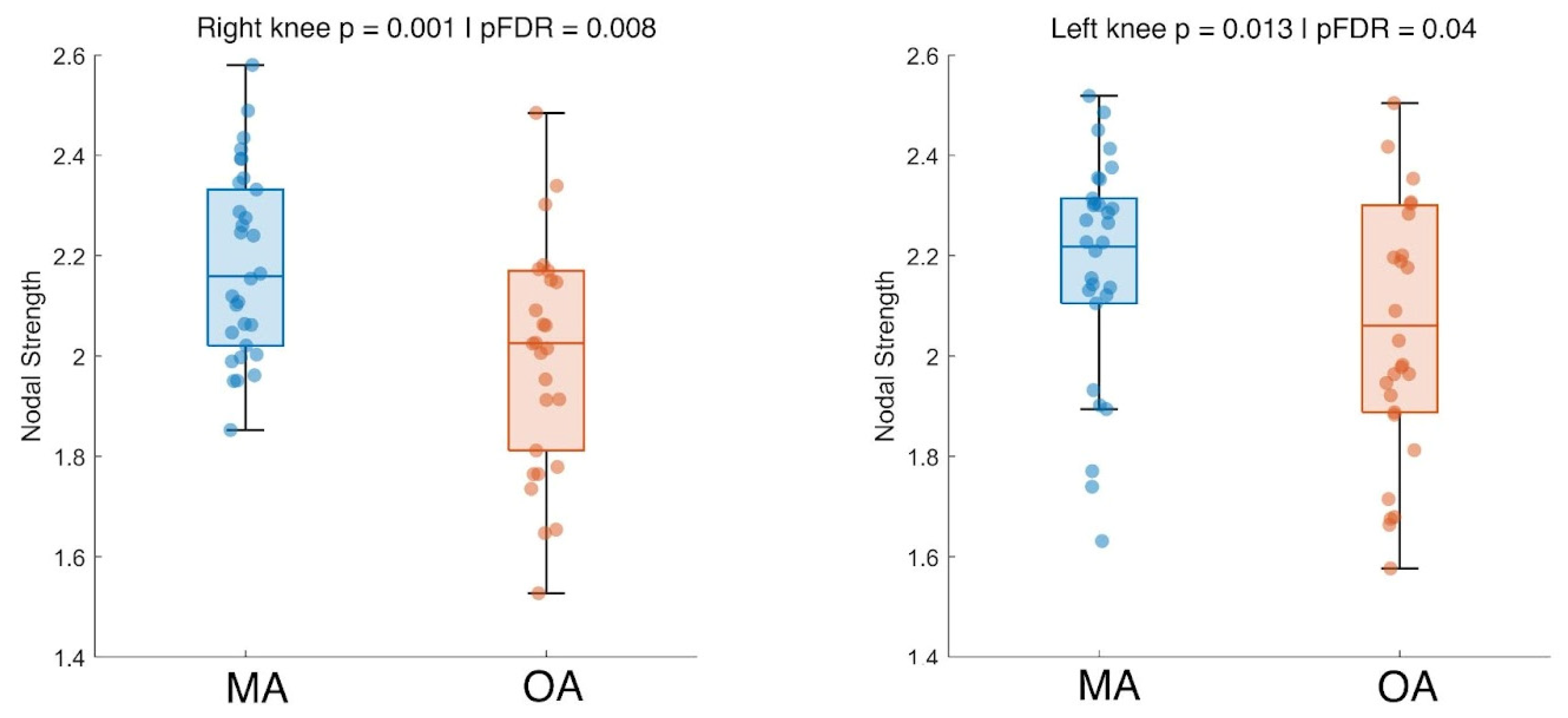
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCI | Principal Component Analysis |
| MMSE | Mini-Mental State Examination |
| FAB | Front Assessment Battery |
| BDI | Back Depression Inventory |
| MA | Mid-Adults |
| OA | Older Adults |
| FDR | False Discovery Rate |
References
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking Speed: The Functional Vital Sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.T.; Liparoti, M.; Minino, R.; Romano, A.; Polverino, A.; Carotenuto, A.; Tafuri, D.; Sorrentino, G.; Sorrentino, P. Kinematic network of joint motion provides insight on gait coordination: An observational study on Parkinson’s disease. Heliyon 2024, 10, e35751. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.T.; Minino, R.; Sorrentino, P.; Rucco, R.; Carotenuto, A.; Agosti, V.; Tafuri, D.; Manzo, V.; Liparoti, M.; Sorrentino, G. A synthetic kinematic index of trunk displacement conveying the overall motor condition in Parkinson’s disease. Sci. Rep. 2021, 11, 2736. [Google Scholar] [CrossRef]
- Macie, A.; Matson, T.; Schinkel-Ivy, A. Age affects the relationships between kinematics and postural stability during gait. Gait Posture 2023, 102, 86–92. [Google Scholar] [CrossRef]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef]
- Klimova, B.; Dostalova, R. The Impact of Physical Activities on Cognitive Performance among Healthy Older Individuals. Brain Sci. 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Kozlowska, K.; Latka, M.; West, B.J. Asymmetry of short-term control of spatio-temporal gait parameters during treadmill walking. Sci. Rep. 2017, 7, srep44349. [Google Scholar] [CrossRef]
- Scarano, S.; Tesio, L.; Rota, V.; Cerina, V.; Catino, L.; Malloggi, C. Dynamic Asymmetries Do Not Match Spatiotemporal Step Asymmetries during Split-Belt Walking. Symmetry 2021, 13, 1089. [Google Scholar] [CrossRef]
- Gimmon, Y.; Rashad, H.; Kurz, I.; Plotnik, M.; Riemer, R.; Debi, R.; Shapiro, A.; Melzer, I. Gait Coordination Deteriorates in Independent Old-Old Adults. J. Aging Phys. Act. 2018, 26, 382–389. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, C.O.; Kim, K.J.; Jeon, J.; Chang, H.; Kim, E.S.; Park, H.; Yakovenko, S. Quantitative analysis of the bilateral coordination and gait asymmetry using inertial measurement unit-based gait analysis. PLoS ONE 2019, 14, e0222913. [Google Scholar] [CrossRef]
- James, E.G.; Conatser, P.; Karabulut, M.; Leveille, S.G.; Hausdorff, J.M.; Travison, T.; Bean, J.F. Walking Speed Affects Gait Coordination and Variability Among Older Adults With and Without Mobility Limitations. Arch. Phys. Med. Rehabil. 2020, 101, 1377–1382. [Google Scholar] [CrossRef]
- Swanson, C.W.; Fling, B.W. Associations between gait coordination, variability and motor cortex inhibition in young and older adults. Exp. Gerontol. 2018, 113, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zadik, S.; Benady, A.; Gutwillig, S.; Florentine, M.M.; Solymani, R.E.; Plotnik, M. Age related changes in gait variability, asymmetry, and bilateral coordination—When does deterioration starts? Gait Posture 2022, 96, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N. The Co-ordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- Kribus-Shmiel, L.; Zeilig, G.; Sokolovski, B.; Plotnik, M.; Scholz, H. How many strides are required for a reliable estimation of temporal gait parameters? Implementation of a new algorithm on the phase coordination index. PLoS ONE 2018, 13, e0192049. [Google Scholar] [CrossRef] [PubMed]
- Turvey, M.T. Coordination. Am. Psychol. 1990, 45, 938–953. [Google Scholar] [CrossRef]
- Ippersiel, P.; Robbins, S.; Dixon, P. Lower-limb coordination and variability during gait: The effects of age and walking surface. Gait Posture 2021, 85, 251–257. [Google Scholar] [CrossRef]
- Yen, H.-C.; Chen, H.-L.; Liu, M.-W.; Liu, H.-C.; Lu, T.-W. Age effects on the inter-joint coordination during obstacle-crossing. J. Biomech. 2009, 42, 2501–2506. [Google Scholar] [CrossRef]
- Romano, A.; Liparoti, M.; Minino, R.; Polverino, A.; Cipriano, L.; Carotenuto, A.; Tafuri, D.; Sorrentino, G.; Sorrentino, P.; Lopez, E.T. The effect of dopaminergic treatment on whole body kinematics explored through network theory. Sci. Rep. 2024, 14, 1913. [Google Scholar] [CrossRef]
- Lopez, E.T.; Sorrentino, P.; Liparoti, M.; Minino, R.; Polverino, A.; Romano, A.; Carotenuto, A.; Amico, E.; Sorrentino, G. The kinectome: A comprehensive kinematic map of human motion in health and disease. Ann. N.Y. Acad. Sci. 2022, 1516, 247–261. [Google Scholar] [CrossRef]
- Roeder, L.; Breakspear, M.; Kerr, G.K.; Boonstra, T.W. Dynamics of brain-muscle networks reveal effects of age and somatosensory function on gait. iScience 2024, 27, 109162. [Google Scholar] [CrossRef]
- Minino, R.; Liparoti, M.; Romano, A.; Mazzeo, F.; Sorrentino, P.; Tafuri, D.; Lopez, E.T. The influence of auditory stimulation on whole body variability in healthy older adults during gait. J. Biomech. 2024, 172, 112222. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Minino, R.; Lopez, E.T.; Sorrentino, P.; Rucco, R.; Lardone, A.; Pesoli, M.; Tafuri, D.; Mandolesi, L.; Sorrentino, G.; Liparoti, M. The effects of different frequencies of rhythmic acoustic stimulation on gait stability in healthy elderly individuals: A pilot study. Sci. Rep. 2021, 11, 19530. [Google Scholar] [CrossRef]
- Flanders, M. Voluntary Movement. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 4371–4375. [Google Scholar] [CrossRef]
- Tessari, F.; Hermus, J.; Sugimoto-Dimitrova, R.; Hogan, N. Brownian processes in human motor control support descending neural velocity commands. Sci. Rep. 2024, 14, 8341. [Google Scholar] [CrossRef]
- Atkeson, C.; Hollerbach, J. Kinematic features of unrestrained vertical arm movements. J. Neurosci. 1985, 5, 2318–2330. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Bracey, E.F.; Faville, R.A.; Campagner, D.; Tyson, A.L.; Lenzi, S.C.; Branco, T.; Margrie, T.W. Multisensory coding of angular head velocity in the retrosplenial cortex. Neuron 2022, 110, 532–543.e9. [Google Scholar] [CrossRef]
- Zhou, Y.; Romijnders, R.; Hansen, C.; van Campen, J.; Maetzler, W.; Hortobágyi, T.; Lamoth, C.J.C. The detection of age groups by dynamic gait outcomes using machine learning approaches. Sci. Rep. 2020, 10, 4426. [Google Scholar] [CrossRef] [PubMed]
- Goodway, J.D.; Ozmun, J.C.; Gallahue, D.L. Understanding Motor Development: Infants, Children, Adolescents, Adults: Infants, Children, Adolescents, Adults; Jones & Bartlett Learning: Burlington, MA, USA, 2019. [Google Scholar]
- Campbell, M.J. Statistics at Square One; Wiley-Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sunderaraman, P.; Maidan, I.; Kozlovski, T.; Apa, Z.; Mirelman, A.; Hausdorff, J.M.; Stern, Y. Differential Associations Between Distinct Components of Cognitive Function and Mobility: Implications for Understanding Aging, Turning and Dual-Task Walking. Front. Aging Neurosci. 2019, 11, 166. [Google Scholar] [CrossRef]
- Morya, E.; Okano, A.H.; Moscaleski, L.A.; Moreira, A. Transcranial Direct Current Stimulation Effect on Locomotion and Posture. In Locomotion and Posture in Older Adults: The Role of Aging and Movement Disorders; Barbieri, F.A., Vitório, R., Santos, P.C.R.D., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 561–573. [Google Scholar] [CrossRef]
- Neptune, R.R.; Zajac, F.E.; Kautz, S.A. Muscle force redistributes segmental power for body progression during walking. Gait Posture 2004, 19, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Andriacchi, T.P.; Mündermann, A.; Smith, R.L.; Alexander, E.J.; Dyrby, C.O.; Koo, S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann. Biomed. Eng. 2004, 32, 447–457. [Google Scholar] [CrossRef]
- Mian, O.S.; Thom, J.M.; Ardigò, L.P.; Narici, M.V.; Minetti, A.E. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol. 2006, 186, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef]
- Bocksnick, J.; Sharp-Chrunik, B.; Bjerkseth, A. Changes in Range of Motion in Response to Acute Exercise in Older and Younger Adults: Implications for Activities of Daily Living. Act. Adapt. Aging 2016, 40, 20–34. [Google Scholar] [CrossRef]
- Hwang, J.; Jung, M.-C. Age and sex differences in ranges of motion and motion patterns. Int. J. Occup. Saf. Ergon. 2015, 21, 173–186. [Google Scholar] [CrossRef]
- Intolo, P.; Milosavljevic, S.; Baxter, D.G.; Carman, A.B.; Pal, P.; Munn, J. The effect of age on lumbar range of motion: A systematic review. Man. Ther. 2009, 14, 596–604. [Google Scholar] [CrossRef]
- Mundt, M.; Thomsen, W.; Bamer, F.; Makert, B. Determination of gait parameters in real-world environment using low-cost inertial sensors. PAMM 2018, 18, e201800014. [Google Scholar] [CrossRef]
- Hafer, J.F.; Boyer, K.A. Age related differences in segment coordination and its variability during gait. Gait Posture 2018, 62, 92–98. [Google Scholar] [CrossRef]
- Toda, H.; Nagano, A.; Luo, Z. Age-related differences in muscle control of the lower extremity for support and propulsion during walking. J. Phys. Ther. Sci. 2016, 28, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Gueugnon, M.; Stapley, P.J.; Gouteron, A.; Lecland, C.; Morisset, C.; Casillas, J.-M.; Ornetti, P.; Laroche, D. Age-Related Adaptations of Lower Limb Intersegmental Coordination During Walking. Front. Bioeng. Biotechnol. 2019, 7, 173. [Google Scholar] [CrossRef]
- Wingert, J.R.; Welder, C.; Foo, P. Age-Related Hip Proprioception Declines: Effects on Postural Sway and Dynamic Balance. Arch. Phys. Med. Rehabil. 2014, 95, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhao, W.; Kimura, T.; Ukawa, S.; Kadoya, K.; Kondo, K.; Tamakoshi, A. Association of gait with global cognitive function and cognitive domains detected by MoCA-J among community-dwelling older adults: A cross-sectional study. BMC Geriatr. 2021, 21, 523. [Google Scholar] [CrossRef] [PubMed]
- Jo, S. Hypothetical neural control of human bipedal walking with voluntary modulation. Med Biol. Eng. Comput. 2008, 46, 179–193. [Google Scholar] [CrossRef]
- Vernooij, C.A.; Rao, G.; Berton, E.; Retornaz, F.; Temprado, J.-J. The Effect of Aging on Muscular Dynamics Underlying Movement Patterns Changes. Front. Aging Neurosci. 2016, 8, 309. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chang, W.H.; Choi, B.-O.; Ryu, G.-H.; Kim, Y.-H. Age-related differences in muscle co-activation during locomotion and their relationship with gait speed: A pilot study. BMC Geriatr. 2017, 17, 44. [Google Scholar] [CrossRef]
- Zhai, M.; Huang, Y.; Zhou, S.; Jin, Y.; Feng, J.; Pei, C.; Wen, L. Effects of age-related changes in trunk and lower limb range of motion on gait. BMC Musculoskelet. Disord. 2023, 24, 234. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Callisaya, M.L.; De Cock, A.-M.; Helbostad, J.L.; Kressig, R.W.; Srikanth, V.; Steinmetz, J.-P.; Blumen, H.M.; Verghese, J.; et al. Poor Gait Performance and Prediction of Dementia: Results from a Meta-Analysis. J. Am. Med Dir. Assoc. 2016, 17, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Blizzard, L.; Wood, A.G.; Srikanth, V.; Thomson, R.; Sanders, L.M.; Callisaya, M.L. Cognitive Function, Gait, and Gait Variability in Older People: A Population-Based Study. J. Gerontol. Ser. A 2013, 68, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-S.; Ko, H.-J. Lower Limb Function in Elderly Korean Adults Is Related to Cognitive Function. J. Clin. Med. 2018, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Wennberg, A.M.; Hagen, C.; Edwards, K.; Roberts, R.O.; Hollman, J.H.; Knopman, D.S.; Boeve, B.F.; Machulda, M.M.; Petersen, R.C.; et al. Comparison of Gait Parameters for Predicting Cognitive Decline: The Mayo Clinic Study of Aging. J. Alzheimer’s Dis. 2017, 55, 559–567. [Google Scholar] [CrossRef]

| Full Sample (n = 56) | ||
| (mean ± standard deviation (minimum–maximum) | ||
| Age (years) | 58.88 ± 13 (31–82) | |
| Education (years) | 13.92 ± 4.08 (5–18) | |
| Sex | 36 M/20 W | |
| MMSE (maximum score = 30) | 27.57 ± 1.36 (24.85–30) | |
| FAB (maximum score = 18) | 16 ± 1.48 (12–18) | |
| BDI (maximum score = 30) | 5.87 ± 3.44 (0–13) | |
| Un 60 | Ov 60 | |
| Age (years) | 49.03 ± 8.11 | 70.23 ± 6.70 |
| Education (years) | 14.83 ± 3.18 | 12.88 ± 4.78 |
| Sex | 20 M/10 W | 16 M/10 W |
| MMSE | 27.46 ± 1.16 | 27.70 ± 1.58 |
| FAB | 15.84 ± 1.27 | 16.19 ± 1.70 |
| BDI | 5.03 ± 2.86 | 6.85 ± 3.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Minino, R.; Polverino, A.; Gallo, E.; Mandolesi, L.; Sorrentino, P.; Sorrentino, G.; Troisi Lopez, E. The Effects of Aging and Cognition on Gait Coordination Analyzed Through a Network Analysis Approach. Biomechanics 2025, 5, 43. https://doi.org/10.3390/biomechanics5030043
De Luca M, Minino R, Polverino A, Gallo E, Mandolesi L, Sorrentino P, Sorrentino G, Troisi Lopez E. The Effects of Aging and Cognition on Gait Coordination Analyzed Through a Network Analysis Approach. Biomechanics. 2025; 5(3):43. https://doi.org/10.3390/biomechanics5030043
Chicago/Turabian StyleDe Luca, Mario, Roberta Minino, Arianna Polverino, Enrica Gallo, Laura Mandolesi, Pierpaolo Sorrentino, Giuseppe Sorrentino, and Emahnuel Troisi Lopez. 2025. "The Effects of Aging and Cognition on Gait Coordination Analyzed Through a Network Analysis Approach" Biomechanics 5, no. 3: 43. https://doi.org/10.3390/biomechanics5030043
APA StyleDe Luca, M., Minino, R., Polverino, A., Gallo, E., Mandolesi, L., Sorrentino, P., Sorrentino, G., & Troisi Lopez, E. (2025). The Effects of Aging and Cognition on Gait Coordination Analyzed Through a Network Analysis Approach. Biomechanics, 5(3), 43. https://doi.org/10.3390/biomechanics5030043








