Abstract
A brain aneurysm is a structural deterioration of the arterial wall in the brain, resulting in the formation of a bulge in or ballooning of a blood vessel. Around 3–5% of the global population is affected by brain aneurysms, wherein only a small fraction results in rupture. Although an unruptured aneurysm is typically asymptomatic and not immediately life threatening, it poses a potential risk of rupture, which can lead to severe health complications or mortality. Therefore, it is crucial to detect and treat aneurysms during the unruptured phase. Moreover, a comprehensive understanding of the flow dynamics within the aneurysm and its parent artery is essential for accurate diagnosis and the prevention of aneurysm recurrence. While prior reviews have focused on computational fluid dynamics (CFD) studies on brain aneurysms, particularly patient-specific models from studies conducted over a decade ago, a more recent review is necessary. Additionally, reviewing various studies on the fluid dynamic behavior of treated aneurysms is crucial. Thus, the advancements in both experimental and computational studies on brain aneurysms must be explored to better understand their underlying fluid flow mechanisms and to develop robust treatment strategies. This review aims to summarize the different types of brain aneurysms, the screening and treatment processes, the key hemodynamic factors, and the fluid dynamic characteristics observed in aneurysms before and after treatment.
1. Introduction
Brain aneurysms, also known as cerebral aneurysms, are a cerebrovascular disease that forms a thin or weak spot in an artery in the brain, leading to bulging or ballooning of a blood vessel [1,2]. It happens due to disease, injury, or abnormality during birth [2]. Inflammation is the main reason behind brain aneurysms that alter the function of vascular smooth muscle cells (VSMCs), by causing endothelial injury. VSMC disfunction causes internal elastic lamina degradation, resulting in the disruption of collagen synthesis and extracellular matrix remodeling. These conditions enhance the activity of protein-breaking enzymes or proteases, accelerating the breakdown and weakening of the arterial wall, ultimately causing aneurysm formation, growth, and rupture [3].
Brain aneurysms typically occur in individuals aged between 35 and 60 [4]. Globally, approximately 5% of the adult population is affected by this cerebrovascular disease [5]. It was found by a clinical study that almost 50% of brain aneurysms are identified after they have ruptured [6]. In the US, around six million people have this condition. However, most brain aneurysms remain unruptured [4]. In general, most of these unruptured aneurysms tend to stabilize over time and do not lead to clinical consequences [7]. It was found that spontaneous thrombosis occurs in approximately 9–13% of cerebral aneurysms, wherein, for giant aneurysms, the likelihood of thrombosis increases with the aneurysm size, with reported rates ranging between 52 and 83%. Although partial healing or thrombus formation may occur in giant aneurysms, complete healing, as well as the disappearance of the aneurysm is rare [8]. While natural healing of an aneurysm can occur, unruptured aneurysms still pose a potential risk of rupture or blood leakage into the surrounding tissue, which can cause paralysis, permanent nerve damage, brain injury, and death [2,9]. Thus, treating an unruptured aneurysm in some cases is crucial to prevent future rupture. The treatment of unruptured aneurysms depends on the size and location of the lesion [5]. Moreover, treating an unruptured aneurysm is highly challenging due to the potential risk of rupture during surgery [10]. Various procedures have been utilized for aneurysm treatment, such as using flow diversion [11,12,13], bypass surgery [14,15], coil embolization [16,17], and surgical clipping [16,18]. These procedures involve a potential risk of morbidity [19]. For instance, surgical clipping of an unruptured aneurysm results in a mortality of 0.5–2.6% and a morbidity of 4.1–10.9% [20]. Poor outcomes are associated with the location of the aneurysm in the posterior circulation and the size of the aneurysm, particularly giant aneurysms [21]. A study performed by Nariai et al. that analyzed the outcomes of endovascular treatment of unruptured anterior communicating artery (AcomA) aneurysms found that the permanent morbidity rate was fairly minor (1%). However, the overall prevalence of procedure-related complications was ~19%, with thromboembolic complications, hemorrhagic complications, and intraprocedural aneurysm bleeding rates of 12.5%, 6.3%, and 3.1%, respectively. The study also found that an aneurysm size greater than 10 mm, a neck width greater than 4 mm, and the use of a stent, were each individually linked to stroke [22]. Irregularly shaped unruptured aneurysms may require earlier interventions, although their size did not reach the typical threshold for treatment [23]. Taken together, it is imperative to assess the risk of aneurysm rupture and the postoperative complications associated with surgery, which may result in permanent nerve damage or even mortality. Several factors must be considered when assessing the possible risk of aneurysm rupture or surgical complications, including the aneurysm’s size, shape, and location, the patient’s age and sex, as well as any underlying medical conditions (e.g., kidney disease) [24].
The flow dynamics in brain aneurysms before and after treatment, as well as the impact of hemodynamic properties on aneurysm growth and rupture, are topics that are already well-studied. Consequently, numerous reviews have already been performed on these topics. For instance, reviews have been conducted on various techniques and methods for assessing aneurysm growth and the risk of rupture, such as various imaging types, machine learning, deep learning, and computational approaches [25,26,27,28,29]. Other reviews highlight various treatment methods and their impact on hemodynamics, the healing of brain aneurysms, and the likelihood of retreatment [30,31,32,33,34]. Reviews on the role of hemodynamic properties on the aneurysm size, shape, growth, and rupture at different locations on the artery walls have also been conducted by different authors [35,36,37,38]. A review focusing on the computational fluid dynamics (CFD) of aneurysm growth and rupture, as well as the impact of hemodynamic factors on aneurysm growth and rupture, was conducted around a decade ago [28]. Additionally, no existing review has incorporated experimental outcomes on the fluid flow dynamics in aneurysms and their parent artery.
Significant advancements in this field since these reviews were conducted necessitate an updated review to reflect recent progress and developments in this area. This review summarizes the various types of aneurysms, hemodynamic factors, diagnostic processes, and fluid flow characteristics of brain aneurysms before and after treatment, using the available experimental and computational literature.
2. Types of Brain Aneurysms
Brain aneurysms are classified according to their relationship with nearby blood vessels within either the anterior or posterior circulation [39]. Additionally, other features, such as the etiology, size, shape, location, and branching, are vital for aneurysm classification [39]. The most common types of aneurysms are saccular and fusiform aneurysms [2,39]. Other types of aneurysms include mycotic, pseudo (dissecting), and blister aneurysms [40,41]. Figure 1 shows the different types of aneurysms, with the subsequent sections describing each type in detail.
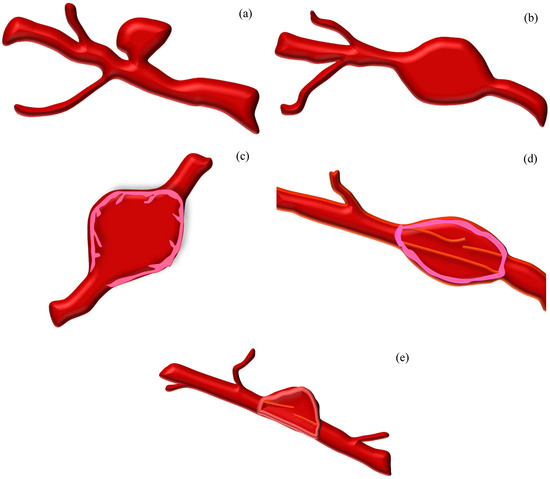
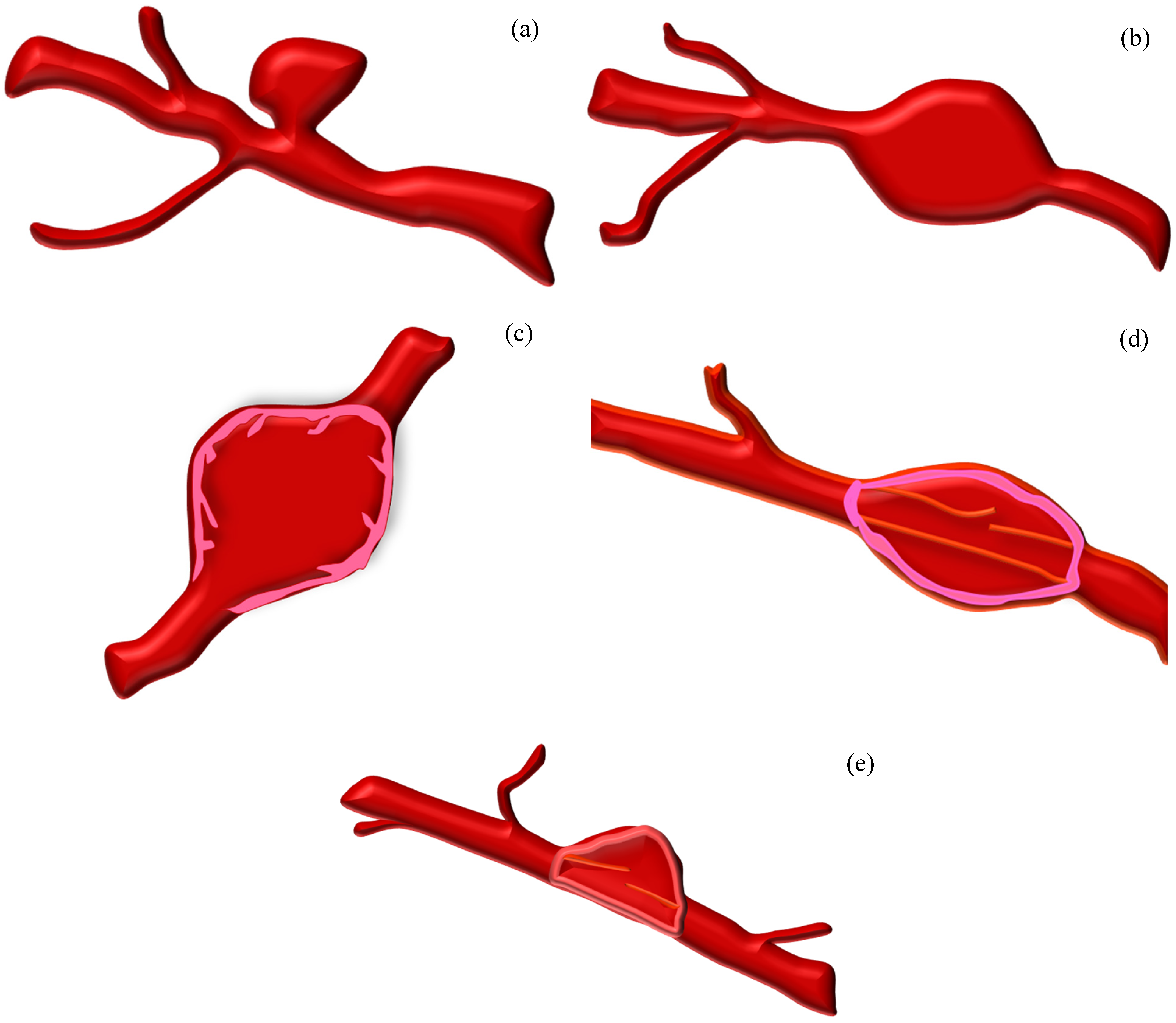
Figure 1.
Various types of aneurysms: (a) saccular, (b) fusiform, (c) mycotic, (d) pseudo (dissecting), and (e) blister aneurysm.
2.1. Saccular Aneurysm (SA)
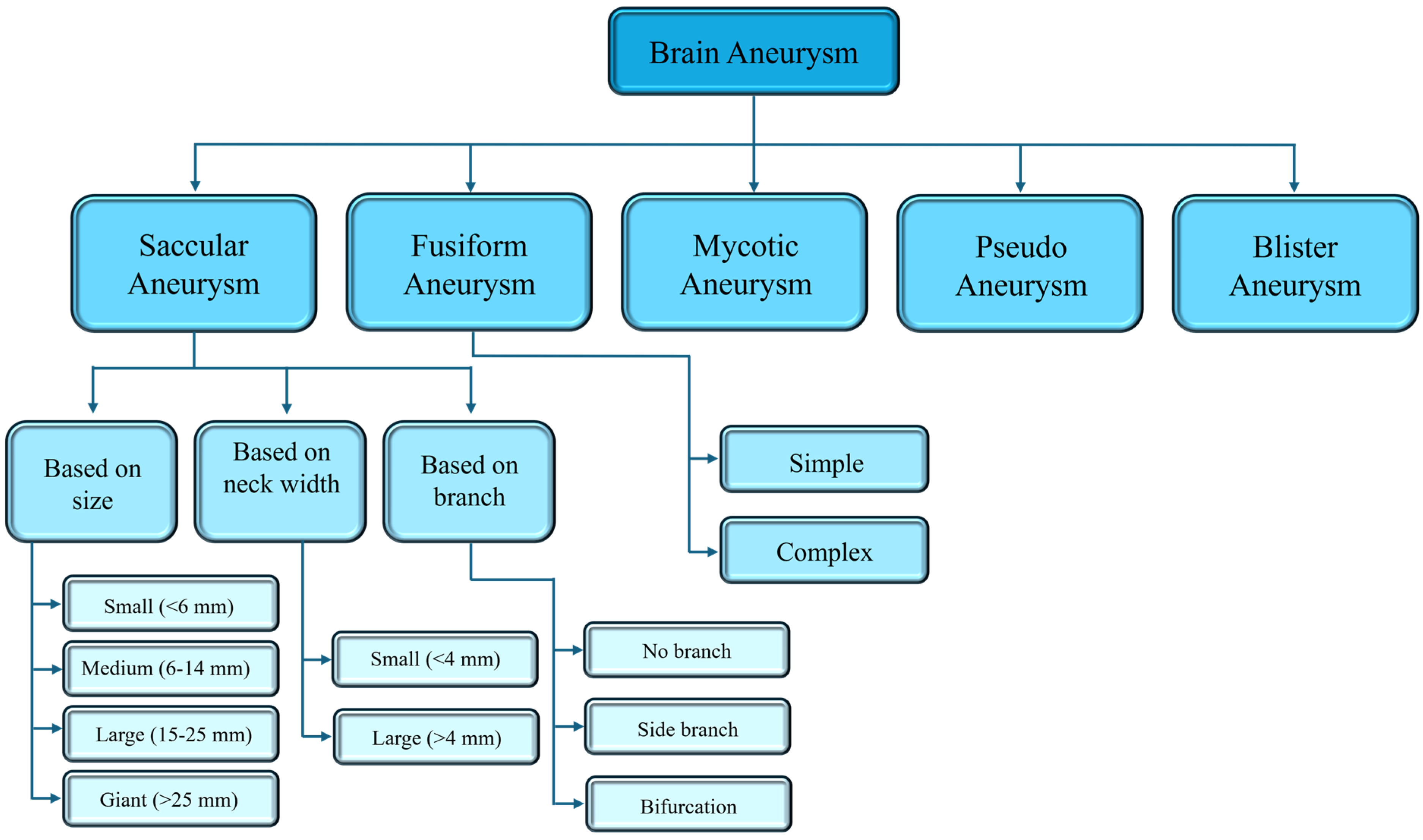
A saccular aneurysm (SA) is a berry or pouch-like pattern and develops as a thin-walled sac that protrudes from the arteries of the circle of Willis or its major branches [40,42]. It is a pathological dilation of intracranial arteries, due to a decreasing resistivity to withstand hemodynamic pressure and distension [43]. Approximately 90% of cerebral aneurysms are saccular, occurring in approximately 46–70% of the entire pediatric population [40,44]. While most SAs are stable, a few can rupture, because of changes in their structure, such as decellularization, matrix degeneration, or the formation of a disorganized blood clot [43]. Due to the aberrant blood flow associated with the pouch-like shape, cytotoxic and proinflammatory substances accumulate in the aneurysm wall, leading to decellularization and degeneration of the SA wall, which in turn promotes rupture [43]. SAs can be categorized based on their location, aneurysm size, and neck width, as shown in Figure 2.
2.2. Fusiform Aneurysm (FA)
A fusiform aneurysm (FA) is a severe form of artery widening, which can lead to serious complications, such rupture and thrombi formation [40,45]. Although rupture is uncommon, it can cause ischemia [40]. There are two main causes that may lead to this type of aneurysm: dissection and atherosclerosis. Other possible causes are the metabolic disorder of collagen and elastin, infections, neoplastic invasion of the arterial wall, and iatrogenesis [46]. Only 5% of aneurysms are of the fusiform type, and they typically occur in older patients [39,40]. A FA can be classified based on whether a side branch occurrence is absent (simple) or present (complex), with the complex type being less common compared to the simple type (Figure 2). The blood flow and vessel wall characteristics of these two types of FAs are different due to the presence or absence of branches, as well as the locations of the branches [39].
2.3. Mycotic Aneurysm (MA)
A mycotic or infected aneurysm (MA) involves the dilation of the artery wall caused by an infection, typically due to bacterial pathogens [47]. It usually develops due to a preexisting infection in the artery wall, rather than direct trauma [47]. MAs are rare, accounting for almost 0.6% of aneurysms in western countries [48]. However, they have a higher risk of rupture compared to uninfected aneurysms, which leads to high morbidity and mortality [48,49]. The treatments for MAs remain unclear because of the non-specific nature of the clinical presentations and the lack of clear diagnostic criteria [49].
2.4. Pseudo Aneurysm (PA)
A pseudo aneurysm (PA), also known as a dissecting aneurysm, is a rare, but well-known, condition that can occur at any arterial site, where the blood vessel expands, particularly at the outer wall, due to arterial puncture [50]. This causes localized turbulent blood flow, leading to the formation of a hematoma with a neck, which does not close naturally after reaching a specific size [50]. It usually occurs due to trauma, mycotic infection, the tearing of blood vessels, and congenital collagen deficiency [51]. This type of aneurysm has a higher risk of rupture, due to the inadequate support of the artery wall and, therefore, requires immediate treatment [52].
2.5. Blister Aneurysm (BA)
A blister aneurysm (BA) is a small dome-shaped bulge in the parent vessel, whose wall is fragile, consisting of a thin layer of adventitia and a poorly defined broad-based neck [53]. It usually forms on the internal carotid artery. The treatment of BAs through surgery is typically difficult, with a risk of a rupture of up to 50% [54]. The flow diversion technique to treat BAs has garnered attention due to its high success rate in treating the target affected wall, without manipulating the fragile fibrous wall [54].
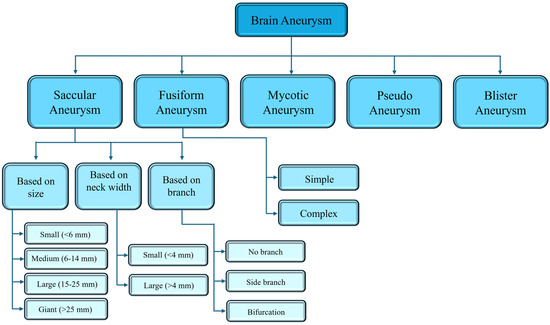
Figure 2.
Classification of brain aneurysms. The information is taken from the following references [39,40,53,55].
Figure 2.
Classification of brain aneurysms. The information is taken from the following references [39,40,53,55].
3. Diagnostic Methods for Brain Aneurysms
Brain aneurysms can be treated in many ways, but the first step is to identify the brain aneurysms and their condition (whether unruptured or ruptured). The diagnostic methods and steps are detailed below.
3.1. Screening of Brain Aneurysms
Various screening processes are used to detect brain aneurysms depending on the patient’s condition, as well as the state and severity of the aneurysm. Such methods include a computed tomography (CT) scan, computed tomographic angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), transcranial doppler ultrasonography (TCD), digital subtraction angiography (DSA), and a spinal tap [2,56,57,58]. Each of these methods is described in detail below, with Table 1 summarizing the roles, advantages, disadvantages, and limitations of each process:
- A CT scan is a specialized X-ray that provides a rapid diagnosis of aneurysm rupture by producing 2-D images [59]. It helps identify any bleeding around the brain, as well as the location of brain aneurysms [2,59]. While CT scans are rapid and painless, they do not always identify brain hemorrhage, nor do they provide information about the size and pattern of the brain aneurysm [59];
- CTA is a more advanced screening process, which combines the regular CT scan with the injection of a contrast dye into the vein or artery. As the dye travels through the vein, an image is captured by the CT scan to observe the blood flow in the region, providing a valuable insight into brain hemorrhage and aneurysm rupture [2,60];
- MRI is another fast and non-invasive screening process to identify brain aneurysms [2]. The image quality and visualization of an aneurysm wall depends on the magnetic field strength. For instance, 3T MRI (3 Tesla MRI) provides a 57% lower image resolution and a 15% thicker appearance of the aneurysm wall compared to 7T MRI. High spatial resolution MRI images can identify various biological processes occurring in the aneurysm wall and can help develop post-processing protocols [61];
- MRA is also an advanced, non-invasive screening process, which does not involve radiation exposure [62]. In addition to the detection of aneurysms, it can also provide information about the size, shape, and hemodynamic flow characteristics of aneurysms [62]. Some MRA processes require the injection of a contrast dye; however, this dye is lower in quantity and less toxic compared to the CTA contrast dye [2,63];
- DSA is a gold standard digital angiography technique for detecting aneurysms as small as 0.5 mm [64] and is more effective at identifying false positive aneurysms compared to other non-invasive techniques [65]. However, it is an invasive procedure requiring sedation and may cause neurological complications [66];
- TCD is a non-invasive, inexpensive screening process that is used to detect vasospasms (VSPs) associated with aneurysm rupture or subarachnoid hemorrhage. The detection of VSPs is an indication of severe aneurysm rupture or bleeding between the brain and its covering. It can also help monitor hemodynamic changes in the intracerebral vasculature due to an aneurysm rupture [67];
- A spinal tap or lumbar puncture is an invasive method used to detect ruptured aneurysms, which may not be detected by a CT scan [68]. If a subarachnoid hemorrhage occurs, red blood cells will be present in the cerebrospinal fluid (CSF). Performing a spinal tap to test the CSF allows for the identification of such a subarachnoid hemorrhage associated with aneurysm rupture [68,69].

Table 1.
Summary of roles, advantages, disadvantages, and limitations of various screening processes.
Table 1.
Summary of roles, advantages, disadvantages, and limitations of various screening processes.
| Screening Method | What It Identifies | Advantages | Disadvantages and Limitations |
|---|---|---|---|
| CT scan |
|
|
|
| MRI |
|
|
|
| CTA |
|
|
|
| MRA |
|
|
|
| TCD |
|
|
|
| DSA |
|
|
|
| Spinal tap or LP |
|
|
|
3.2. Treatment Methods
The factors that determine the appropriate method for the treatment of aneurysms and to prevent their rupture include the shape, size, and location of the aneurysm. Each technique includes further refinements aimed at improving treatment outcomes and reducing the need for retreatment. These treatment methods are briefly summarized as follows.
3.2.1. Surgical Techniques
Direct surgery for the treatment of an intracranial aneurysm (IA) was first established in the 1930s, when Sir Norman Dott successfully treated a ruptured aneurysm by opening up the brain or cranial cavity to access the aneurysm and reinforcing it using a muscle graft taken from the patient’s thigh [81]. Other surgical treatments were developed over the following decades. For example, aneurysm clipping, first applied by Walter Dandy in 1937 [81], involves the process of opening the skull, locating the aneurysm, and then ligating the aneurysm neck using single or multiple clips to prevent blood flow into the aneurysm. The success of the clipping process is dependent on effectively exposing the skull in order to visualize the aneurysm neck clearly [82]. Additionally, understanding the anatomy is crucial for selecting the optimal surgical approach, which could potentially impact the intraoperative exposure of the aneurysm and nearby blood vessels and post-surgical outcomes [83]. For instance, microsurgical clipping is the preferred method to treat aneurysms in the middle cerebral artery (MCA), due to ease of surgical access, and studies have shown promising outcomes for both ruptured and unruptured aneurysms [84,85]. This method is also preferrable for treating aneurysms with complex shapes, wherein endovascular techniques are not effective [86]. For instance, microsurgical repair is a viable option for treating vertebrobasilar dissecting (VBD) aneurysms, and the success rate is generally satisfactory, reaching 95% [87]. Sriamornrattanakul et al. [88] reviewed recent studies on the anterior temporal approach for clipping supraclinoid internal carotid artery (ICA) aneurysms with posterior projection to achieve better visualization of the aneurysm neck and related branches. They found that this new approach is safe and effectively clips the aneurysm, with a high success rate. The same technique used in clipping upper basilar artery aneurysms also showed promising success rates [89]. Other surgical clipping approaches have been employed depending on the type, size, shape, and location of the aneurysms. These approaches include the transorbital approach for MCA and ICA aneurysms [90,91]; the entire orifice blocking-assisted microsurgical approach for intracranial giant wide-neck paraclinoid aneurysms [92]; the keyhole approach for ICA aneurysms [93]; one-stage multiple craniotomies (OSMCs) for multiple cerebral aneurysms [94]; cotton-assisted clipping for very small intracranial aneurysms (IAs) [95]; and the mass reduction clipping technique for giant complex MCA aneurysms [96]. Although these studies have produced promising results, further research is required to evaluate the long-term durability of these treatments to reduce the need for repeated surgery and assess the potential for aneurysm growth.
3.2.2. Embolization Technique
The embolization technique, also known as the simple coil embolization process, is a minimally invasive procedure, whereby a platinum coil is inserted through a microcatheter to fill the aneurysm [97]. This promotes blood clotting, effectively sealing the aneurysm from active blood circulation to prevent rupture [82,97]. It is safer and significantly more effective than surgical clipping, with complication rates almost half that of surgical clipping [97,98]. Common complications associated with coil embolization are thromboembolic events, aneurysmal perforation by the microcatheter or coil, parent artery obstruction, coil misposition and migration, and coil blocking [97,99]. Modifications to this simple embolization process aim to alleviate these complications and address the challenges associated with treating aneurysms of different sizes and shapes, for example, the double catheter technique, balloon-assisted coiling, stent-assisted coiling, and utilizing an intermediate catheter [82]. Each of these modifications is described in detail as follows:
- The double catheter technique, suitable for treating wide-neck aneurysms, is a much easier process compared to stent- and ballon-assisted coiling. In regard to this procedure, two microcatheters of different shapes are inserted into different portions of the aneurysm to obtain a stable frame. This technique can also be used for aneurysms with a daughter sac, branch-incorporated aneurysms, and elongated aneurysms, wherein the coil insertion process for each condition is different [100]. Although the double catheter technique has a high recurrence rate, this technique is much safer and easier for the treatment of recurrent aneurysms compared to using a flow-diverting stent. Due to the irregular shape of the recanalized cavity inside preexisting coils, it is challenging to form a stable frame at the initial stage of coiling. Thus, it is recommended to use the double catheter technique, wherein a small-diameter coil, based on the maximum length of the recanalized aneurysm, is selected for the first coil, and the diameter of the second coil is kept nearly the same as the first coil. This approach helps create a safe and stable frame within the recanalized cavity [101];
- The balloon-assisted coiling process is also used for complex wide-necked aneurysms, where a balloon is inflated across the aneurysm neck to create enough space for the coil to be inserted [102,103]. The balloon is removed upon insertion of the coil into the aneurysm. Both single-lumen and double-lumen balloons may be deployed, with the double-lumen balloon enabling the option of placing a stent, along with a coil, into the aneurysm. Both the single- and double-lumen balloon procedures have similar rates of potential complications, with most studies reporting low mortality and morbidity risks, except for the study by Sluzewski et al. (14.1%) [104,105,106,107]. However, in general, this process carries a higher risk of ischemic and hemorrhagic complications compared to stent-assisted coiling for the acute management of ruptured aneurysms [108];
- Stent-assisted coiling is suitable for wider neck, fusiform, and dissecting aneurysms, as well as aneurysms with irregular or complex geometry. The stent is placed at the neck, which allows the coil to be fully inserted into the aneurysm without protruding into the main blood vessel. Additionally, the stent diverts blood flow towards the aneurysm and facilitates intra-aneurysm stasis and thrombosis [109]. Stent overlapping (multiple stents are placed in a way that results in their ends overlapping each other) can also be applied to prevent coil protrusion, stent malposition, and in-stent blood clotting [110]. Studies have shown that stent-assisted coiling exhibits higher occlusion rates compared to simple coiling without a stent [111] and is much safer and effective, particularly for unruptured aneurysms. While complications are more likely when used to treat ruptured aneurysms [112], stent-assisted coiling is still favorable compared to simple or microsurgical clipping [113];
- The placement of an intermediate catheter in the blood vessel for coil embolization is a recent advancement in aneurysm treatment. This catheter is designed to provide a buttress at tortuous blood vessels by combining a flexible distal tip with a supportive proximal shaft to aid in the insertion of the coil into the aneurysm, thereby improving maneuverability and stability. This enhanced design improved occlusion rates, as well as the coil packing density inside the aneurysm [114]. This modification is particularly suitable for unruptured aneurysms; for ruptured aneurysms, there are possible risks of intraprocedural rupture (IPR) during coil embolization [114,115].
3.2.3. Flow Diversion Technique
Flow diversion is a technique wherein blood flow is redirected away from the aneurysm with the aid of a stent. The stent facilitates blood occlusion by stagnating the blood flow within the aneurysm. Consequently, an endothelium can form across the neck, leading to the reconstruction of the parent vessel and the healing of the aneurysm [116]. Five different types of flow diversion stents are widely used: (a) a pipeline embolization device (PED), (b) a silk flow diverter (SFD), (c) a flow redirection endoluminal device (FRED), (d) a p64 flow modulation device (pFMD), and (e) a surpass flow diverter (SUFD) [117]. The PED exhibited better outcomes with less complications compared to coil embolization for large unruptured intracranial saccular aneurysms [118]. Although this method is widely used, some complications were observed, associated with the alteration of the mechanical properties and flow reduction effects [119]. Therefore, the optimization of flow diversion stent parameters is essential to enhance the efficacy of flow diversion and reduce such complications. A study reported a high success rate (96.3%) with a low incidence of complications (3.7%) for a stent with an average diameter of 4.3 mm and a length of 21.6 mm. This study utilized three different types of stents: PEDs, SFDs, and FREDs [120].
3.2.4. Other Techniques
Other methods have been utilized for aneurysm treatment that have shown various success rates and morbidity, such as salvation techniques, intrasaccular flow disruption, the use of liquid embolic agents, target therapy, Woven EndoBridge (WEB) therapy, shape memory polymer foam (SMPF) treatment, and foreign body granuloma (FBG) therapy [82,121,122]. However, these treatment methods are less commonly used for aneurysm treatment in real life.
4. Hemodynamic Properties and Their Impact
Hemodynamic properties are primarily responsible for the initiation, growth, and rupture of aneurysms. Different hemodynamic properties, such as wall shear stress (WSS), normal stress (NS), tangential stress (TS), the oscillatory shear index (OSI), and the oscillatory velocity index (OVI), help regulate blood flow through the vessel [36,123]. However, these hemodynamic or biomechanical properties are not only factors in aneurysm initiation and growth, but their interaction with biological properties is also important [124]. Blood is supplied to the brain through internal carotid arteries (ICAs) and basilar arteries (BAs), wherein these arteries are connected to each other by the circle of Willis (CoW). The CoW is a ring-like structure that acts as a backup system to circulate blood in the brain when any artery becomes blocked or absent. Any abnormalities in the circle of Willis may alter the hemodynamic properties and lead to the formation and potential rupture of an aneurysm [125]. Shine et al. [126] found in their computational study that the wall weakening of the CoW and its adjoining arteries alters the core velocity of the blood flow, WSS, and von Mises stress (VMS), which in turn increases the risk of aneurysm initiation and rupture, particularly in the anterior communicating artery (ACoA) and the posterior communicating artery (PCoA). Mohan et al. [127] identified that alterations in the arterial wall due to inflammation associated with external factors (smoke, arterial hypertension, etc.) lead to aneurysm initiation and growth. Since the physical state of the artery wall changes the hemodynamic properties, a detailed understanding of these properties is necessary. The sections below provide a summary of the most critical and common parameters that represent the hemodynamic properties.
4.1. Wall Shear Stress (WSS)
Wall shear stress (WSS) is one of the most impactful hemodynamic parameters responsible for brain aneurysms, defined as the dynamic force along the blood vessel surface, induced by the movement of viscous fluid through the vessel [3,36]. A time-averaged WSS can be estimated using Equation (1), where WSSi is the instantaneous wall shear stress vector and T is the duration of a cardiac cycle [36].
Both high and low WSS have adverse effects on aneurysm wall enhancement, depending on the aneurysm size [128]. In small aneurysms, the region of high WSS accelerates artery wall enhancement (AWE) due to inflammation. This inflammation occurs because high WSS causes the overproduction of nitric oxide, a decrease in arterial tone, and apoptosis of smooth muscle cells in vessel walls. Similarly, the region of low WSS in larger aneurysms also has adverse effects on AWE, due to an inflammatory response in the endothelium. Thus, both low and high WSS may lead to unruptured intracranial aneurysm (IA) initiation and growth [128]. Aneurysms with low WSS have a higher chance of rupturing compared to those with high WSS. This is because low WSS around the aneurysm area stimulates the proliferation of endothelial cells, and it also initiates programmable cell death by activating apoptotic pathways. The death of endothelial cells activates proinflammatory and procoagulant mediators, leading to the increased production of vasoconstrictive substances, while simultaneously reducing the production of vasodilators and antioxidative agents. Together, these changes weaken the vessel wall structure. Consequently, the weakened vessel wall is unable to tolerate the physiological hemodynamic stresses, leading to aneurysm formation and eventual rupture. In contrast, high WSS caused by the elevated blood flow, particularly in the bifurcation region, leads to endothelial dysfunction and damage over time [129]. Tang et al. [128] and Meng et al. [130] reported that both low and high WSS facilitate the growth and rupture of aneurysms, while Gao et al. [131] found that a larger aneurysm area with lower WSS promotes the risk of rupture. This adverse effect of WSS is primarily influenced by the aneurysm’s geometry and the blood flow dynamics within the aneurysm and its parent artery. In addition to the magnitude of the WSS (low or high), the WSS pattern also has significant effects on aneurysm rupture. For instance, higher and more heterogenous WSS, typically observed in aneurysms located in the bifurcation regions of the basilar, anterior, and posterior communicating artery, promotes bleb (blister-like protrusion) formation. During bleb formation, high intramural stresses develop at the bleb neck, ultimately causing aneurysm rupture. In contrast, lower and more homogenous WSS in sidewall aneurysms of the MCA and ICA facilitates wall thickening and may result in the rupture of aneurysms that are relatively larger in size without forming bleb [132].
4.2. Normal and Tensile (Circumferential) Stresses
Within a blood vessel, normal stresses act orthogonally in regard to a vessel wall (e.g., due to hydrostatic pressure), while circumferential stress is a force in the circumferential direction, acting against the blood vessel wall [36]. Both stresses cause the deformation of the aneurysm wall, as well as speed up the development of saccular aneurysms [133,134]. For instance, a traumatic brain injury (TBI) exerts a sudden load on the vessel wall, which causes mechanical failure or deformation, due to the stresses developing within the wall. A prior study found that TBI increases the deformation of the cerebral aneurysm wall by 0.072 mm [133]. The blood flow through the vessel also has an impact on the aneurysm wall. Jiang et al. [135] found that during peak systole, an increase in the blood hematocrit (HCT) level leads to elevated pressure on the aneurysm wall. This elevated pressure enhances the normal and tensile stress, causing wall deformation and potentially leading to rupture. Singh et al. [134] found that an increase in the aneurysm diameter and a decrease in the wall thickness also induce substantial strains on the aneurysm wall, which, in turn, can lead to rupture. Taken together, these studies demonstrate the importance of identifying the circumferential and normal stresses at play and how they affect brain aneurysms.
4.3. Oscillatory Shear Index (OSI)
The oscillatory shear index (OSI) is a non-dimensional parameter that characterizes oscillations during pulsatile flow. The OSI is calculated using Equation (2) [136] and ranges from 0 (steady flow) to 0.5 (intense oscillation) [36]. A higher OSI indicates a disturbed and non-uniform flow pattern (turbulent flow), which, along with irregular WSS, can damage the inner aneurysm wall and lead to aneurysm growth and rupture [137,138]. Although low WSS causes aneurysm rupture as discussed above, the presence of a low OSI alongside it can lead to reduced mechanical stress, thereby lowering the rupture risk [138]. In contrast, a low WSS combined with a high OSI usually indicate the location where an aneurysm is likely to rupture and suggest that the area is not exposed to the impact of direct blood flow [36]. The OSI intensity depends on the geometry of the aneurysm; a higher aspect ratio results in a higher OSI value, which leads to inflammation and remodeling of the aneurysm wall [137]. Note that although the OSI determines aneurysm growth and the risk of rupture, it does not differentiate between normal and hypertension conditions [137].
Here, the is the instantaneous wall shear stress, represents the sum of all the instantaneous wall shear stress vectors during a single cardiac cycle, and represents the sum of the magnitude of all the instantaneous wall shear stress vectors during a single cardiac cycle.
4.4. Aneurysm Formation Indicator (AFI)
The aneurysm formation indicator (AFI) is another hemodynamic index used to detect stagnation zones, by calculating the direction of the WSS vector blood flow in regard to the vessel wall [36]. Additionally, the AFI can measure fluctuations in the WSS vector at the location of the aneurysm formation [139]. Studies have shown that the AFI has a similar distribution pattern on the vascular wall as the OSI and minimal correlation with the area of intracranial aneurysm formation [140].
4.5. Oscillatory Velocity Index (OVI)
The oscillatory velocity index (OVI) quantifies the flow pattern, which is important for diagnosing aneurysm growth and the risk of rupture [141]. It is measured based on the local velocity magnitude and fluctuations in the flow patterns during a cardiac cycle [36]. A prior study reported that a high OVI is typically observed in ruptured aneurysms due to its complex and unstable flow patterns, along with the presence of multiple vortices at the rupture site [141,142,143].
4.6. Residence Time (RT)
The residence time (RT) is another important hydrodynamic parameter, defined as the average time that blood spends within the aneurysm. It plays a significant role in aneurysm rupture and the development of thrombi within the aneurysm [144]. The relative residence time (RRT) describes the blood flow distribution near the intra-aneurysmal vortex. It is used to estimate the RT of the blood cell flow circulation near the aneurysm wall [36]. An increased RT reflects impaired mass transfer between the blood flow and the vessel wall, which locally impacts the biological processes involving the artery wall [145]. However, the role of the RT in identifying aneurysm rupture and blood clotting (thrombus) is still unclear. For instance, Xu et al. [146] found that the relative RT (RRT) for ruptured and unruptured aneurysms is 0.34 ± 0.4 and 0.3 ± 0.26, respectively, indicating that the residence time for ruptured and unruptured aneurysms are not statistically different. Rayz et al. [145] found a correlation between the RT and WSS to predict the thrombus at the aneurysm site, wherein aneurysm wall regions with a high RT and a low WSS predict thrombus formation more effectively than either the RT or WSS alone. Hence, identifying strong correlations between the residence time and other hydrodynamic parameters may improve the predictive capabilities in regard to aneurysm rupture or thrombus formation.
Understanding the significance of hemodynamics through clinically relevant applications is important as it provides insights about the physical conditions of aneurysms and helps identify possible treatment strategies. For instance, microsurgical clipping is an effective method to identify rupture points. Li et al. [147] investigated the hemodynamics around a rupture point, wherein the rupture point was detected at the time of clipping, followed by hemodynamic analyses (e.g., WSS, OSI, etc.) of the rupture point and the entire aneurysm sac. It was found that the time-averaged WSS is significantly higher at the aneurysm sac compared to the rupture point, while the OSI is higher at the rupture point. Additionally, in about two-thirds of clinical cases, there was no blood flow impact observed at the aneurysm’s rupture point. Among the cases with daughter blebs, the rupture point was confirmed to be at the blebs in six cases. Liu et al. [148] investigated the risk factors related to intraoperative aneurysm rupture (IOR), by extracting both the morphological and hemodynamic features. They found that the average and maximum normalized WSS, as well as the aspect ratio, were lower for the IAs that ruptured during the pre-dissection stage compared to those that ruptured during the dissection stage. Suzuki et al. [149] conducted a CFD study based on the clinical outcomes of intraprocedural rupture (IPR) during coil embolization (CE) of an IA, examining the impact of hemodynamics on the rupture risk. They found that IPR occurred mostly in the ICA compared to the MCA and the anterior cerebral artery (ACA), and CFD analysis found that the rupture point coincided with a flow impingement zone, with maximum pressure. The time-averaged WSS in this location was also lower than the rest of the aneurysm dome. The unstable hemodynamics at this location were associated with a high risk of rupture. As such, to prevent rupture, a microcatheter was inserted into the inflow zone and directed towards the rupture point. Understanding patient-specific hemodynamic properties is also essential to ensure proper treatment, as the size, shape, and conditions of aneurysms vary from patient to patient. Davidson et al. [150] investigated the flow characteristics and hemodynamics of cerebral arteries before and after neurosurgical clipping. They selected two patient-specific cerebral artery geometries from an online dataset, one of which had an aneurysm located in the MCA bifurcation region. The CFD analysis showed that clipping altered the flow patterns and the WSS in the MCA, which confirmed the appropriateness of the treatment before proceeding. Taken together, these studies demonstrate the utility of clinical data to better understand the hemodynamics around an aneurysm, which could lead to the development of more effective diagnostic and treatment methods.
5. Fluid Flow Characteristics in the Aneurysm
Analyzing aneurysm fluid flow patterns before and after treatment helps assess the aneurysm’s condition and helps to guide the selection of the necessary precautions to reduce the risk of complications and death. Such fluid flow characteristics have been investigated using both experimental and computational approaches. However, computational approaches have been more prevalent compared to experimental approaches for the following reasons:
- (1)
- They can capture complex geometries with relative ease [28];
- (2)
- Such simulations can be used to investigate complex flow patterns and the corresponding hemodynamic properties [151];
- (3)
- They do not have the same limitations related to patient specific data as faced by experimental approaches [28,152];
- (4)
- Computational approaches allow for better control of the parameters and ease of executing multiple simulations [153,154];
- (5)
- They are more time and cost effective [153].
Computational studies on brain aneurysms are usually carried out in three different ways: (1) using computational fluid dynamics (CFD) models, (2) using fluid–structure interaction (FSI) models, and (3) using static structural analysis (SSA) models. Each model involves different aspects, based on the specific objectives of the study. In general, models that incorporate the vessel structure and properties (e.g., FSI and SSA), which includes the mechanical properties of the parent artery and aneurysm wall, are important because these properties influence aneurysm formation and rupture [155]. Excessive hemodynamic stress on the artery wall leads to the disruption of the internal elastic lamina, the loss of medial smooth muscle cells (SMCs), decreased SMC proliferation, and the loss of fibronectin, ultimately contributing to aneurysm initiation [19].
CFD models are used to simulate fluid domains only, wherein the vessel and aneurysm walls are assumed to be rigid. In contrast, FSI models account for flexible vessels and aneurysm walls, coupling them with the fluid domain [156]. This semi-realistic model enhances accuracy in regard to predicting fluid flow properties, by developing the interaction between the blood flow and the vessels’ structure [157]. Typically, the artery wall is modeled as either a linear or hyper-elastic model. While the linear elastic model is more stable and simpler compared to the hyper-elastic model, it does not accurately predict the nonlinear mechanical behavior of the parent artery [158]. In another study, Torii et al. found that the maximum displacement obtained from the hyper-elastic model was 36% smaller than that obtained from the linear elastic model [159]. Other hyper-elastic models used include the Mooney–Rivlin model, the Ogden model, the Yeoh model, the neo-Hookean model, the Exp–Ln model, and the Gent model [157,158,160]. A study found that the Exp–Ln model predicts the mechanical behavior of soft tissue more precisely than other hyper-elastic models [160]. SSA models primarily focus on the structural properties of blood vessels and aneurysm walls without accounting for fluid dynamic properties. Such models are less widely used compared to CFD and FSI models [156]. While computational approaches are more prevalent due to the reasons explained above, the use of experimental or clinical data are important for model validation. The following sections present a comprehensive review of various computational and experimental studies on the hemodynamics of aneurysms before and after treatment.
5.1. Hemodynamics Around Aneurysms Before Treatment
5.1.1. Aneurysm Initiation and Growth
The blood flow through the artery and its impact on altering the hemodynamic properties significantly influence the formation, growth, and rupture of aneurysms. As discussed above, in addition to inflammation, the aneurysm size, shape, location, and hemodynamic properties are also important variables. Various computational studies have been performed to investigate the effects of these variables on aneurysm formation. Lauric et al. [161] developed a CFD model to investigate the curvature effect at the ICA siphon on aneurysm formation. They found that sharp bends or curvature in a blood vessel cause fluctuations and high levels of WSS and WSS gradients at the inner wall. These high stresses are followed by a region in which the blood flow slows down and even recirculates, contributing to the weakening of blood vessel walls and, ultimately, initiating aneurysm formation. Lampropoulos et al. [162] also used CFD for multiphase blood flow analysis and found that local curvature in blood vessels has a significant effect on aneurysm formation. An increase in blood vessel curvature causes high stress and flow stagnation in localized regions, enhancing the risk of aneurysm formation. Csippa et al. [163] observed from their CFD analysis that blood vessel topology may impact the development of a strong secondary flow pattern at the site of aneurysm initiation. This magnitude of high velocity close to the wall and a high flow angle are the main reasons for the high WSS and OSI at the wall, which contribute to aneurysm initiation. Gao et al. [164] found through their CFD simulations that the initiation of an anterior communicating artery aneurysm mostly occurs in the daughter vessel, which has a smaller diameter, as well as a smaller angular distance from the parent blood vessel. They also reported that aneurysm formation is a self-adaptive process of the blood vessel wall to alleviate the impact of hemodynamic factors at the site of aneurysm initiation. Yu et al. [165] performed an experimental study to investigate the effect of the flow structure on the vortex strength, impinging location, and WSS. Their results indicate that a high Reynolds number (Re = 270) and Womersley number (α = 5) contribute to the movement and prolonged presence of a vortex structure within the aneurysm, leading to higher incidences of flow impingement on the aneurysm wall. Furthermore, a low bottleneck factor (BF = 1) allows more incoming blood flow to enter and circulate within the aneurysm sac. These factors, when taken together, facilitate aneurysm formation, growth, and rupture.
Experimental approaches to investigating the fluid flow characteristics in aneurysms before treatment are less prevalent. Chi et al. [166] experimentally investigated the effect of the aspect ratio (AR) of imaged-based aneurysms on wall deformation under various pulsatile flows, wherein wall deformation oscillations were detected using laser displacement sensors. They found that the wall deformation oscillations increase with an increasing pulsatile inflow frequency, which also causes the AR to increase. Additionally, the maximum wall deformation at the aneurysm dome decreases during the systolic phase. Souza et al. [167] used both particle-tracking velocimetry (PTV) and CFD to investigate the flow structure in aneurysms and found that recirculation, vorticity, and jet impingement and flow disturbances in localized regions occur due to an increase in the flow rate, providing insight into the state of aneurysms (e.g., initial state or fully grown aneurysm).
5.1.2. Aneurysm Rupture
Several studies have also been conducted both experimentally and computationally to identify possible factors for aneurysm rupture. For instance, Lai et al. [168] performed a study to identify the impact of the aspect ratio (aneurysm height: neck width) on blood flow dynamics, with experiments conducted to validate the CFD model. It was found that a blood stream jet entering the aneurysmal sac becomes high and narrower with an increase in the aspect ratio, potentially directly impinging on the weaker wall. Additionally, the distal portion of the neck exhibits variable shear stresses. The strong and narrow jet impingement, along with variable shear stress, enhances the chance of an aneurysm rupture. Shamloo et al. [169] developed a two-way FSI model to investigate the effect of aneurysm wall thickness on aneurysm rupture. They noticed that thicker walled aneurysms exhibit lower deformations and Von Mises stresses compared to aneurysms with thinner walls, hence lowering the risk of rupture. Philip et al. [170] found through their FSI model, that the shape of an aneurysm impacts the flow stability within the aneurysm. Pear- and beehive-shaped aneurysms exhibit high flow instability, while spherical-shaped aneurysms showed more stable flow; hence, the former two shapes have a higher risk of rupture. While aneurysm rupture depends primarily on the aneurysm’s size and shape, the flow structure also plays a critical role not just in its formation and growth, but also in regard to the likelihood of rupture. For instance, Huang et al. [171] developed a model to investigate the impact of flow fluctuations on aneurysm rupture. Generally, the fluid flow begins to fluctuate and becomes unstable immediately after peak systole, followed by a gradual decay, and, finally, returns to the laminar pulsatile phase during diastole. Thus, fluid flow instabilities start after the peak systole phase. This group found that pulsatile flow around the aneurysm neck enhances flow instability, which leads to rupture. Cebral et al. [172], based on their computational model, found that an abnormally high WSS causes aneurysm rupture at thinner and stiffer walls. Using another CFD model to investigate the effect of the flow conditions on wall remodeling and inflammation, they also found that a high WSS, high vorticity, high shear rate, and high viscous dissipation enhance inflammation, leading to aneurysm rupture [173]. Yu et al. [174] conducted an experimental study, wherein the inflow conditions in an aneurysm were investigated using particle image velocimetry (PIV). The study found that a high Reynolds number (Re = 150 and 250) and Womersley number (α = 5) enhance the vortex strength and generate secondary vortices. A high Re also alters the location of jet impingement. Taken together, these factors increase the risk of aneurysm rupture.
In addition, Table 2 provides a summary of the most recent articles (Year: 2020–2025) highlighting the impact of various factors associated with the alteration of the fluid flow behavior in brain aneurysms.

Table 2.
A compressive summary of most recent articles highlighting the flow conditions of brain or cerebral aneurysms.
5.2. Hemodynamics Around Aneurysms After Treatment
Flow diagnostics using computational approaches after aneurysm treatment are crucial for evaluating the effectiveness of the devices used, the healing process, the flow conditions in the treated regions, and the possibility of recurrence [195,196]. For instance, Cebral et al. [197] performed a CFD analysis to investigate the hemodynamics of treated ruptured and unruptured giant aneurysms. They found that for a successfully treated aneurysm, the flow diversion device reduces the flow velocity and WSS in the aneurysm, which validates the flow diversion device in regard to its ability to effectively divert the flow from the aneurysm sac into the parent artery. In contrast, for post-treatment aneurysms, the use of flow diversion devices caused the development of high pressures in the aneurysm. Otani et al. [17] conducted a blood flow analysis of coil-embolized aneurysms using CFD approaches and found that the blood flow velocity decreases with an increase in the packing density, although the effect of coil configuration was insignificant. However, the strength of the local shear flow varies with the coil configuration. The total shear rate decreases with an increase in the packing density, which is an important factor for blood clot formation inside the aneurysm, for successful aneurysm occlusion. The effectiveness of coil embolization also varies based on the patient’s gender. Sheidani at al. [198] performed a CFD study to examine the impact of hemodynamics on the coil porosity in both male and female patients. They found that a decrease in coil porosity from 0.89 to 0.79 reduces the maximum OSI value by 75% in males and 45% in females. Zhang et al. [199] compared the efficacy of using large-sized coils with the conventional coil for embolization. The diameter of the large-sized coil is 1.4–1.8 times higher than the diameter of the conventional coil. Their results show that the large-sized coil achieved a similar initial occlusion rate, while significantly reducing the percent packing volume (PPV), the coil number, the length of the total coil compared to the conventional coil, and the recurrence rate. Jeong et al. [200] investigated the effect of the coil shape and orientation on hemodynamics and found that the orthogonal orientation of a vortex coil and the parallel orientation of a cage-shaped coil exhibited a higher inflow velocity, WSS at the dome, and vorticity, which, in turn, is unfavorable for thrombus formation. Moreover, a thicker coil also negatively affects the hemodynamics. However, blocking the distal mid-transverse plane of the aneurysm significantly reduces the blood flow inside the aneurysm, ultimately creating favorable conditions for thrombus formation. Thus, the effectiveness of various aneurysm treatment methods primarily depends on the size, shape, and orientation of implanted devices, along with patient-specific conditions.
In addition, Table 3 provides a summary of the most recent articles (Year: 2020–2025) that highlight the hemodynamics of treated aneurysms.

Table 3.
Comprehensive summary of recent articles highlighting the hemodynamics and flow conditions of treated aneurysms.
The use of the computational process is still controversial, due to concerns regarding the validity of the governing models and the conflicting outcomes from several studies [28]. Thus, combining experimental approaches to complement and validate computational simulation predictions is a worthwhile endeavor to try to capture the relevant physiological characteristics accurately.
6. Conclusions
The identification and treatment of brain aneurysms are of great importance, regardless of their state (ruptured and unruptured). While unruptured aneurysms are not immediately life threatening, treatment is necessary because they carry the potential risk of rupture, due to the change in hemodynamic factors within the aneurysm and parent artery, leading to morbidity and mortality risks. This review highlights various types of brain aneurysms and their hemodynamic factors, the diagnostic methods used, and the fluid flow characteristics before and after treatment. Based on this review, the key observations are summarized as follows:
- While hemodynamic factors are crucial for aneurysm initiation, growth, and rupture, changes in the relevant biological properties accelerate the hemodynamic alterations both in the parent arteries and aneurysms. Additionally, the aneurysm’s geometry and location influence whether the hemodynamic factors become critical and whether they may lead to the rupture of the aneurysm. Thus, various screening and treatment processes are widely used to identify and treat both unruptured and ruptured aneurysms;
- An understanding of the fluid flow characteristics in the parent artery and adjacent aneurysms is important because this information helps to determine the state of the aneurysm in order to reduce the possible risk of complications and death. While computational methods are widely used compared to experimental approaches, because of their ease in capturing complex geometry, the ease of controlling the variables in hemodynamic investigations, the ability to execute multiple simulations across various parameters, and time efficiency, the accuracy of computational study is still questionable. Thus, validating computational simulations using experimental data is critical to help improve the model’s accuracy associated with its assumptions and governing equations;
- The majority of recent studies within the last 5 years (2020–2025) were computational simulations, with limited experimental studies, particularly using patient-specific models. This represents an important gap for future research, potentially contributing significantly to the advancement of knowledge in this field;
- Prior experimental and computational studies mostly focus on the aneurysm size, shape, and orientation, as well as the blood flow structure and stability in various arteries with aneurysms. These studies primarily highlight how hemodynamic changes due to changes in these properties ultimately lead to the formation, growth, and rupture of aneurysms. Further investigations are necessary to identify any correlation or interaction effects between these variables, which may change the interpretations of these findings;
- Preventing aneurysm recurrence after treatment is still challenging. The current research primarily focuses on hemodynamic changes before and after treatment, particularly on flow diversion and coil embolization techniques. Most of these studies rely on computational predictions, but the accuracy of the outcomes remains debated, due to the geometric complexity and model simplifications made for easier analysis, particularly in regard to stent or coil inclusions. Thus, combined experimental and computational approaches would provide a way for model validation to take place and enhance data reliability. Furthermore, extensive experimental investigations are necessary to evaluate the efficacy and reliability of embolization and flow diversion techniques in real-world conditions;
- It is crucial to explore new treatment techniques, instead of relying solely on coil embolization and flow diversion methods.
Author Contributions
P.R.C. worked on the outline, conducted the literature search, and wrote the initial draft. The review topic was developed jointly by all the authors. Both V.K.L. and R.Z. provided critical revisions and supervision and contributed to the writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the MnDRIVE Data Science Initiative (DSI) Seed Grant (2024–2025), University of Minnesota.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marbacher, S.; Strange, F.; Frösén, J.; Fandino, J. Preclinical extracranial aneurysm models for the study and treatment of brain aneurysms: A systematic review. J. Cereb. Blood Flow Metab. 2020, 40, 922–938. [Google Scholar] [CrossRef]
- Novitzke, J. The basics of brain aneurysms: A guide for patients. J. Vasc. Interv. Neurol. 2008, 1, 89–90. [Google Scholar] [PubMed]
- Texakalidis, P.; Sweid, A.; Mouchtouris, N.; Peterson, E.C.; Sioka, C.; Rangel-Castilla, L.; Reavey-Cantwell, J.; Jabbour, P. Aneurysm Formation, Growth, and Rupture: The Biology and Physics of Cerebral Aneurysms. World Neurosurg. 2019, 130, 277–284. [Google Scholar] [CrossRef]
- Toth, G.; Cerejo, R. Intracranial aneurysms: Review of current science and management. Vasc. Med. 2018, 23, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.K.; Nasztanovics, F.; Bojtár, I. Laboratory tests for strength paramaters of brain aneurysms. Acta Bioeng. Biomech. 2007, 9, 3. [Google Scholar]
- Brown, R.D.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Khoa, T.V.; Thuong, T.M.; Quang, P.X.; Thang, T.Q.; Linh, N.N.; Ngan, P.T.T.; Van, P.D.; Duong, P.N.; Duc, N.M. Spontaneous thrombosis of a large unruptured intracranial aneurysm causing ischemic stroke due to occlusion of the parent artery: A case report and literature review. Radiol. Case Rep. 2024, 19, 3405–3410. [Google Scholar] [CrossRef]
- Luo, C.B.; Chen, Y.L.; Hsu, S.W.; Alvarez, H.; Rodesch, G.; Lasjaunias, P. Spontaneous healing and complete disappearance of a giant basilar tip aneurysm in a child. Interv. Neuroradiol. 2001, 7, 141–145. [Google Scholar] [CrossRef]
- Crusius, C.; De Aguiar, P.; Crusius, M. Mixed aneurysm: A new proposed nomenclature for a rare condition. Surg. Neurol. Int. 2017, 8, 29. [Google Scholar] [CrossRef]
- Ajiboye, N.; Chalouhi, N.; Starke, R.M.; Zanaty, M.; Bell, R. Unruptured Cerebral Aneurysms: Evaluation and Management. Sci. World J. 2015, 2015, 954954. [Google Scholar] [CrossRef]
- Roy, J.M.; El Naamani, K.; Momin, A.A.; Ghanem, M.; Lan, M.; Ahmed, M.T.; Winiker, S.; Teichner, E.M.; Musmar, B.; Tjoumakaris, S.I.; et al. Telescoping Flow Diverters for the Treatment of Brain Aneurysms: Indications and Outcome. World Neurosurg. 2024, 191, e473–e479. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi-Farsani, H.; Scougal, E.; Herrmann, M.; Wei, W.; Frakes, D.; Chong, B. Numerical study of hemodynamics in brain aneurysms treated with flow diverter stents using porous medium theory. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.; Lozupone, E.; Milonia, L.; Pero, G.; Cervo, A.; Macera, A.; Quilici, L.; Visconti, E.; Valvassori, L.; Cenzato, M.; et al. Flow diverter devices in the treatment of complex middle cerebral artery aneurysms when surgical and endovascular treatments are challenging. J. Stroke Cerebrovasc. Dis. 2022, 31, 106760. [Google Scholar] [CrossRef]
- Kushi, Y.; Imamura, H.; Itazu, T.; Ozaki, S.; Niwa, A.; Shimonaga, K.; Ikedo, T.; Hamano, E.; Yamada, K.; Ohta, T.; et al. One-Stage Combined Open and Endovascular Treatment Using a Hybrid Operating Room is Safe and Effective for Distal Middle Cerebral Artery Aneurysms. World Neurosurg. 2024, 187, e731–e739. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Zeeshan, Q.; Ghodke, B.V.; Sekhar, L.N. Brain Bypass Surgery for Complex Middle Cerebral Artery Aneurysms: Evolving Techniques, Results, and Lessons Learned. World Neurosurg. 2019, 130, e272–e293. [Google Scholar] [CrossRef]
- Ishikawa, M.; Takahashi, S.; Hirai, S.; Sato, Y.; Shigeta, K.; Yoshimura, M.; Yamamura, T.; Taira, N.; Ishiwada, T.; Karakama, J.; et al. Efficacy of endovascular treatment for distal anterior cerebral artery aneurysms: A multicenter observational study. J. Stroke Cerebrovasc. Dis. 2024, 33, 107941. [Google Scholar] [CrossRef]
- Otani, T.; Ii, S.; Shigematsu, T.; Fujinaka, T.; Hirata, M.; Ozaki, T.; Wada, S. Computational study for the effects of coil configuration on blood flow characteristics in coil-embolized cerebral aneurysm. Med. Biol. Eng. Comput. 2017, 55, 697–710. [Google Scholar] [CrossRef]
- Lai, L.; Murtaza Mohsin, N.; Al-Farttoosi, H.; Raki, C.; Dhaliwal, T. Development of a predictive grading system for postoperative ischemia following middle cerebral artery aneurysm clipping. J. Clin. Neurosci. 2024, 130, 110914. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Hoh, B.L.; Hasan, D. Review of cerebral aneurysm formation, growth, and rupture. Stroke 2013, 44, 3613–3622. [Google Scholar] [CrossRef]
- Drexler, R.; Sauvigny, T.; Pantel, T.F.; Ricklefs, F.L.; Catapano, J.S.; Wanebo, J.E.; Lawton, M.T.; Sanchin, A.; Hecht, N.; Vajkoczy, P.; et al. Global Outcomes for Microsurgical Clipping of Unruptured Intracranial Aneurysms: A Benchmark Analysis of 2245 Cases. Neurosurgery 2024, 94, 369–378. [Google Scholar] [CrossRef]
- Raaymakers, T.W.M.; Rinkel, G.J.E.; Limburg, M.; Algra, A. Mortality and morbidity of surgery for unruptured intracranial aneurysms: A meta-analysis. Stroke 1998, 29, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Nariai, Y.; Takigawa, T.; Kawamura, Y.; Suzuki, R.; Hyodo, A.; Suzuki, K. Treatment results and long-term outcomes of endovascular treatment of 96 unruptured anterior communicating artery aneurysms: A large single-center study. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2021, 26, 101285. [Google Scholar] [CrossRef]
- Lampropoulos, D.S.; Hadjinicolaou, M. Investigating Hemodynamics in Intracranial Aneurysms with Irregular Morphologies: A Multiphase CFD Approach. Mathematics 2025, 13, 505. [Google Scholar] [CrossRef]
- Thompson, B.G.; Brown, R.D.; Amin-Hanjani, S.; Broderick, J.P.; Cockroft, K.M.; Connolly, E.S.; Duckwiler, G.R.; Harris, C.C.; Howard, V.J.; Johnston, S.C.; et al. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2368–2400. [Google Scholar] [CrossRef]
- Rubin, A.; Waszczuk, Ł.; Trybek, G.; Kapetanakis, S.; Bladowska, J. Application of susceptibility weighted imaging (SWI) in diagnostic imaging of brain pathologies—A practical approach. Clin. Neurol. Neurosurg. 2022, 221, 107368. [Google Scholar] [CrossRef]
- Xue, J.; Zheng, H.; Lai, R.; Zhou, Z.; Zhou, J.; Chen, L.; Wang, M. Comprehensive Management of Intracranial Aneurysms Using Artificial Intelligence: An Overview. World Neurosurg. 2025, 193, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Nafees Ahmed, S.; Prakasam, P. A systematic review on intracranial aneurysm and hemorrhage detection using machine learning and deep learning techniques. Prog. Biophys. Mol. Biol. 2023, 183, 1–16. [Google Scholar] [CrossRef]
- Sforza, D.M.; Putman, C.M.; Cebral, J.R. Computational fluid dynamics in brain aneurysms. Int. J. Numer. Method. Biomed. Eng. 2012, 28, 801–808. [Google Scholar] [CrossRef]
- Chandra Sahu, J.; Chakrabarti, S. A Review on Basic Understanding of Aneurysm and Possible Techniques in Assessing the Risk of Its Rupture. Int. J. Ser. Eng. Sci. Arch. Mech. Mach. Eng. 2017, 3, 1–21. [Google Scholar]
- Thompson, J.W.; Elwardany, O.; McCarthy, D.J.; Sheinberg, D.L.; Alvarez, C.M.; Nada, A.; Snelling, B.M.; Chen, S.H.; Sur, S.; Starke, R.M. In vivo cerebral aneurysm models. Neurosurg. Focus 2019, 47, E20. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Valizadeh, P.; Balabandian, M. Stent-assistant versus non-stent-assistant coiling for ruptured and unruptured intracranial aneurysms: A meta-analysis and systematic review. World Neurosurg. X 2024, 21, 100243. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Becerra, J.E.; Simoni, G.; Mendieta, C.D.; Acha Sánchez, J.L.; Palavani, L.B.; Wouters, K.; Punukollu, A.; Mangas, G.; Bertani, R.; Lopez-Gonzalez, M.A. Awake microsurgical management of brain aneurysms: A comprehensive systematic review and meta-analysis on rationale, safety and clinical outcomes. Neurochirurgie 2024, 70, 101600. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.A.; Naseri Alavi, S.A.; Mirjnani, M.S.; Aliasgary, A.; Delbari, P.; Ahmadvand, M.H.; Hatami, S.; Hasan, Z.; Dmytriw, A.A.; Kobets, A.J. Role of Statins in the Clinical and Radiologic Outcomes of Patients with Unruptured Intracranial Aneurysm Undergoing Microsurgery or Endovascular Treatment: A Systematic Review and Meta-Analysis. World Neurosurg. 2024, 194, 123497. [Google Scholar] [CrossRef]
- Kavi Fatania, D.T.P. Comprehensive review of the recent advances in devices for endovascular treatment of complex brain aneurysms. Br. J. Radiol. 2022, 95, 20210538. [Google Scholar] [CrossRef]
- Wang, Y.; Leng, X.; Zhou, X.; Li, W.; Siddiqui, A.H.; Xiang, J. Hemodynamics in a Middle Cerebral Artery Aneurysm Before Its Growth and Fatal Rupture: Case Study and Review of the Literature. World Neurosurg. 2018, 119, e395–e402. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.A.A.; Shuib, A.S.; Mohyi, M.H.H. A review of hemodynamic parameters in cerebral aneurysm. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2020, 22, 100716. [Google Scholar] [CrossRef]
- Shen, Y.; Molenberg, R.; Bokkers, R.P.H.; Wei, Y.; Uyttenboogaart, M.; van Dijk, J.M.C. The Role of Hemodynamics through the Circle of Willis in the Development of Intracranial Aneurysm: A Systematic Review of Numerical Models. J. Pers. Med. 2022, 12, 1008. [Google Scholar] [CrossRef] [PubMed]
- Maramkandam, E.B.; Kannan, A.; Valeti, C.; Manjunath, N.; Panneerselvam, N.K.; Alagan, A.K.; Panchal, P.M.; Kannath, S.K.; Darshan, H.R.; Nekkanti, R.K.; et al. Review of CFD Based Simulations to Study the Hemodynamics of Cerebral Aneurysms. J. Indian Inst. Sci. 2024, 104, 77–110. [Google Scholar] [CrossRef]
- Pritz, M.B. Cerebral aneurysm classification based on angioarchitecture. J. Stroke Cerebrovasc. Dis. 2011, 20, 162–167. [Google Scholar] [CrossRef]
- Gasparotti, R.; Liserre, R. Intracranial aneurysms. Eur. Radiol. 2005, 15, 441–447. [Google Scholar] [CrossRef]
- Liu, P.; Liu, L.; Zhang, C.; Lin, S.; Wang, T.; Xie, X.; Zhou, L.; Wang, C. Treatment of Blood Blister Aneurysms of the Internal Carotid Artery With Pipeline-Assisted Coil Embolization: A Single-Center Experience. Front. Neurol. 2022, 13, 882108. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey, L.; Origitano, T.C.; Biller, J. Subarachnoid Hemorrhage in Young Adults; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Frösen, J.; Tulamo, R.; Paetau, A.; Laaksamo, E.; Korja, M.; Laakso, A.; Niemelä, M.; Hernesniemi, J. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012, 123, 773–786. [Google Scholar] [CrossRef]
- Gemmete, J.J.; Toma, A.K.; Neurosurg, F.; Davagnanam, I.; Bch, M.B.; Robertson, F.; Brew, S. Pediatric Cerebral Aneurysms. Neuroimaging Clin. 2013, 23, 771–779. [Google Scholar] [CrossRef]
- David, C.; Bonovich, M.D. Stroke and Cerebral Aneurysms; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Barletta, E.A.; Ricci, R.L.; Silva, R.D.G.; Gaspar, R.H.M.L.; Araújo, J.F.M.; Neves, M.W.F.; De Aquino, J.L.B.; Belsuzarri, T.A.B. Fusiform aneurysms: A review from its pathogenesis to treatment options. Surg. Neurol. Int. 2018, 9, 189. [Google Scholar] [CrossRef]
- Gu, J.; Patel, S.; Kumaravel, M. Imaging of Musculoskeletal Infections Related to Recreational Drug Use. In Emergency Imaging of At-Risk Patients; Elsevier: Amsterdam, The Netherlands, 2023; pp. 166–185. [Google Scholar]
- Ravindra, A.; Naguthevar, S.; Kumar, D.; Rajagopal, R.; Khera, P.S.; Tak, V.; Ramankutty, N.T.; Meena, D.S.; Midha, N.; Bohra, G.K.; et al. Mycotic aneurysms: Uncommon pathogens and treatment conundrums. Access Microbiol. 2024, 6, 1–7. [Google Scholar] [CrossRef]
- Islam, S.; Harnarayan, P.; Naraynsingh, V. Mycotic Arterial Aneurysm. In Issues and Developments in Medicine and Medical Research; BP International Publisher: West Benga, Indian, 2022; Volume 10, pp. 95–112. [Google Scholar] [CrossRef]
- Rivera, P.A.; Dattilo, J.B. Pseudoaneurysm; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2024. [Google Scholar] [PubMed]
- Nomura, M.; Mori, K.; Tamase, A.; Kamide, T.; Seki, S.; Iida, Y.; Nakano, T.; Kawabata, Y.; Kitabatake, T.; Nakajima, T.; et al. Pseudoaneurysm formation due to rupture of intracranial aneurysms: Case series and literature review. Neuroradiol. J. 2017, 30, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Meola, M.; Marciello, A.; Di Salle, G.; Petrucci, I. Ultrasound evaluation of access complications: Thrombosis, aneurysms, pseudoaneurysms and infections. J. Vasc. Access 2021, 22 (Suppl. 1), 71–83. [Google Scholar] [CrossRef] [PubMed]
- Meling, T.R. What are the treatment options for blister-like aneurysms? Neurosurg. Rev. 2017, 40, 587–593. [Google Scholar] [CrossRef]
- Kan, P.; Sweid, A.; Srivatsan, A.; Jabbour, P. Expanding Indications for Flow Diverters: Ruptured Aneurysms, Blister Aneurysms, and Dissecting Aneurysms. Neurosurgery 2020, 86, S96–S103. [Google Scholar] [CrossRef]
- Henkes, H.; Fischer, S.; Mariushi, W.; Weber, W.; Liebig, T.; Miloslavski, E.; Brew, S.; Kühne, D. Angiographic and clinical results in 316 coil-treated basilar artery bifurcation aneurysms. J. Neurosurg. 2005, 103, 990–999. [Google Scholar] [CrossRef]
- Rinkel, G.J.E.; Ruigrok, Y.M. Preventive screening for intracranial aneurysms. Int. J. Stroke 2022, 17, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Kwoon, J.V.; Lavine, S.D. Intracranial aneurysms: Current evidence and clinical practice. Am. Fam. Physician 2002, 66, 601–608. [Google Scholar] [PubMed]
- Li, H.; Wang, W. Evaluation of the Effectiveness of Lumbar Punctures in Aneurysmal Subarachnoid Hemorrhage Patient with External Ventricular Drainage. World Neurosurg. 2021, 151, e1–e9. [Google Scholar] [CrossRef]
- Hsiang, J.N.K.; Liang, E.Y.; Lam, J.M.K.; Zhu, X.L.; Poon, W.S. The role of computed tomographic angiography in the diagnosis of intracranial aneurysms and emergent aneurysm clipping. Neurosurgery 1996, 38, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, V.; Shaqdan, K.; Hedgire, S.; Ghoshhajra, B. Vascular computed tomography angiography technique and indications. Cardiovasc. Diagn. Ther. 2019, 9, 14–27. [Google Scholar] [CrossRef]
- Samaniego, E.A. Brain Aneurysm Biology: What Can We Learn From Imaging? Stroke Vasc. Interv. Neurol. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Kapsalaki, E.Z.; Rountas, C.D.; Fountas, K.N. The role of 3 Tesla MRA in the detection of intracranial aneurysms. Int. J. Vasc. Med. 2012, 2012, 792834. [Google Scholar] [CrossRef]
- Green, D.; Parker, D. CTA and MRA: Visualization without catheterization. Semin. Ultrasound CT MRI 2003, 24, 185–191. [Google Scholar] [CrossRef]
- Van Rooij, W.J.; Sprengers, M.E.; De Gast, A.N.; Peluso, J.P.P.; Sluzewski, M. 3D rotational angiography: The new gold standard in the detection of additional intracranial aneurysms. Am. J. Neuroradiol. 2008, 29, 976–979. [Google Scholar] [CrossRef]
- Rustemi, O.; Alaraj, A.; Shakur, S.F.; Orning, J.L.; Du, X.; Aletich, V.A.; Amin-Hanjani, S.; Charbel, F.T. Detection of unruptured intracranial aneurysms on noninvasive imaging. Is there still a role for digital subtraction angiography? Surg. Neurol. Int. 2015, 6, 170029. [Google Scholar] [CrossRef]
- Uricchio, M.; Gupta, S.; Jakowenko, N.; Levito, M.; Vu, N.; Doucette, J.; Liew, A.; Papatheodorou, S.; Khawaja, A.M.; Aglio, L.S.; et al. Computed Tomography Angiography Versus Digital Subtraction Angiography for Postclipping Aneurysm Obliteration Detection: A Meta-Analysis. Stroke 2019, 50, 381–388. [Google Scholar] [CrossRef]
- Kalanuria, A.; Nyquist, P.A.; Armonda, R.A.; Razumovsky, A. Use of Transcranial Doppler (TCD) Ultrasound in the Neurocritical Care Unit. Neurosurg. Clin. N. Am. 2013, 24, 441–456. [Google Scholar] [CrossRef]
- Inder, T.E.; Perlman, J.M.; Volpe, J.J. Intracranial Hemorrhage. Volpe’s Neurol. Newborn 2018, 30, 593–622.e7. [Google Scholar] [CrossRef]
- Jane, L.A.; Wray, A.A. Lumbar Puncture. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557553/ (accessed on 5 March 2025).
- Vymazal, J.; Rulseh, A.M.; Keller, J.; Janouskova, L. Comparison of CT and MR imaging in ischemic stroke. Insights Imaging 2012, 3, 619–627. [Google Scholar] [CrossRef]
- van Beek, E.J.R.; Kuhl, C.; Anzai, Y.; Desmond, P.; Ehman, R.L.; Gong, Q.; Gold, G.; Gulani, V.; Hall-Craggs, M.; Leiner, T.; et al. Value of MRI in medicine: More than just another test? J. Magn. Reson. Imaging 2019, 49, e14–e25. [Google Scholar] [CrossRef] [PubMed]
- Katti, G.; Ara, S.A.; Shireen, A. Magnetic Resonance Imaging (MRI)—A Review. Int. J. Dent. Clin. 2011, 3, 65–70. [Google Scholar] [CrossRef]
- Turan, N.; Heider, R.A.; Roy, A.K.; Miller, B.A.; Mullins, M.E.; Barrow, D.L.; Grossberg, J.; Pradilla, G. Current Perspectives in Imaging Modalities for the Assessment of Unruptured Intracranial Aneurysms: A Comparative Analysis and Review. World Neurosurg. 2018, 113, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, N.N.; Mngomezulu, V.M.; Buthelezi, T.E. Accuracy of CT angiography for detecting ruptured intracranial aneurysms. S. Afr. J. Radiol. 2023, 27, 1–6. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; White, P.M. The detection and management of unruptured intracranial aneurysms. Brain 2000, 123, 205–221. [Google Scholar] [CrossRef]
- Ali, M.F. Transcranial Doppler ultrasonography (uses, limitations, and potentials): A review article. Egypt. J. Neurosurg. 2021, 36, 20. [Google Scholar] [CrossRef]
- Bittar, J.; Hannawi, Y. Transcranial Doppler in Subarachnoid Hemorrhage. In Neurovascular Sonography; Springer: Cham, Switherland, 2022; pp. 81–98. [Google Scholar] [CrossRef]
- Sumner, D.S.; Porter, D.J.; Moore, D.J.; Winders, R.E. Digital subtraction angiography: Intravenous and intra-arterial techniques. J. Vasc. Surg. 1985, 2, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Migdal, V.L.; Wu, W.K.; Long, D.; McNaughton, C.D.; Ward, M.J.; Self, W.H. Risk-Benefit Analysis of Lumbar Puncture to Evaluate for Nontraumatic Subarachnoid Hemorrhage in Adult ED Patients Victoria. Am J Emerg Med. 2016, 33, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Horstman, P.; Linn, F.H.H.; Voorbij, H.A.M.; Rinkel, G.J.E. Chance of aneurysm in patients suspected of SAH who have a “negative” CT scan but a “positive” lumbar puncture. J. Neurol. 2012, 259, 649–652. [Google Scholar] [CrossRef]
- Lai, L.T.; O’Neill, A.H. History, Evolution, and Continuing Innovations of Intracranial Aneurysm Surgery. World Neurosurg. 2017, 102, 673–681. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, H.; Summers, R.; Yang, M.; Cousins, B.G.; Tsui, J. Current Treatment Strategies for Intracranial Aneurysms: An Overview. Angiology 2018, 69, 17–30. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Zhu, X.; Chen, Y.; Zhang, C.; Shi, W.; Chen, Q.; Wang, Y. Anterior Communicating Artery Aneurysms: Anatomical Considerations and Microsurgical Strategies. Front. Neurol. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Van Dijk, J.M.C.; Groen, R.J.M.; Ter Laan, M.; Jeltema, J.R.; Mooij, J.J.A.; Metzemaekers, J.D.M. Surgical clipping as the preferred treatment for aneurysms of the middle cerebral artery. Acta Neurochir. 2011, 153, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Metayer, T.; Leclerc, A.; Borha, A.; Derrey, S.; Langlois, O.; Barbier, C.; Aldea, S.; le Guerinel, C.; Piotin, M.; Vivien, D.; et al. Microsurgical Clipping of Middle Cerebral Artery Aneurysms: Complications and Risk Factors for Complications. World Neurosurg. 2022, 168, e87–e96. [Google Scholar] [CrossRef]
- Rutledge, C.; Baranoski, J.F.; Catapano, J.S.; Lawton, M.T.; Spetzler, R.F. Microsurgical Treatment of Cerebral Aneurysms. World Neurosurg. 2022, 159, 250–258. [Google Scholar] [CrossRef]
- Frisoli, F.A.; Srinivasan, V.M.; Catapano, J.S.; Rudy, R.F.; Nguyen, C.L.; Jonzzon, S.; Korson, C.; Karahalios, K.; Lawton, M.T. Vertebrobasilar dissecting aneurysms: Microsurgical management in 42 patients. J. Neurosurg. 2022, 137, 393–401. [Google Scholar] [CrossRef]
- Sriamornrattanakul, K.; Wongsuriyanan, S. Anterior Temporal Approach for Clipping Posterior-Projecting Supraclinoid Carotid Artery Aneurysms: A More Lateral Corridor to Better Visualize the Aneurysm Neck and Related Branches. World Neurosurg. 2021, 149, e549–e562. [Google Scholar] [CrossRef] [PubMed]
- Sriamornrattanakul, K.; Wongsuriyanan, S.; Akharathammachote, N. Anterior Temporal Approach for Clipping of Upper Basilar Artery Aneurysms: Surgical Techniques and Treatment Outcomes. World Neurosurg. 2019, 131, e530–e542. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.; Saez-Alegre, M.; Perillo, T.; Peto, I.; Najera, E.; Williams, J.; Breton, J.; Felbaum, D.R.; Jean, W.C. Transorbital approach clipping of middle cerebral artery aneurysm: A virtual reality morphometric anatomic study. World Neurosurg. 2024, 191, e429–e437. [Google Scholar] [CrossRef] [PubMed]
- Mosteiro, A.; Manfrellotti, R.; Torné, R.; Gagliano, D.; Codes, M.; Perera, D.; Di Somma, A.; Prats-Galino, A.; Enseñat, J. The Transorbital Approach to the Internal Carotid and Middle Cerebral Arteries. A Dissection Study Toward Targeted Access Aneurysm Clipping. World Neurosurg. 2024, 194, 123486. [Google Scholar] [CrossRef]
- Chen, R.; Guo, R.; Wen, D.; You, C.; Ma, L. Entire Orifice Blocking-Assisted Microsurgical Treatment: Clipping of Intracranial Giant Wide-Neck Paraclinoid Aneurysms. World Neurosurg. 2018, 114, e861–e868. [Google Scholar] [CrossRef]
- Toyooka, T.; Wada, K.; Otani, N.; Tomiyama, A.; Takeuchi, S.; Tomura, S.; Nishida, S.; Ueno, H.; Nakao, Y.; Yamamoto, T.; et al. Potential Risks and Limited Indications of the Supraorbital Keyhole Approach for Clipping Internal Carotid Artery Aneurysms. World Neurosurg. X 2019, 2, 100025. [Google Scholar] [CrossRef]
- Seo, D.; Jo, H.; Jeong, H.G.; Kim, Y.D.; Lee, S.U.; Ban, S.P.; Kim, T.; Kwon, O.K.; Oh, C.W.; Bang, J.S. Simultaneous Craniotomies for Multiple Intracranial Aneurysm Clippings—One-Stage Surgery with Multiple Craniotomies. World Neurosurg. 2022, 158, e689–e696. [Google Scholar] [CrossRef]
- Liu, J.; Gao, G.; Zhang, S.; Huang, Y.; Wu, J.; Hu, X.; Lu, J.; Zhang, Q.; Zhou, L.; Huang, Y. Cotton-Assisted Surgical Clipping of Very Small Aneurysms: A Two-Center Study. World Neurosurg. 2019, 127, e242–e250. [Google Scholar] [CrossRef]
- Takeda, R.; Kurita, H. “Mass Reduction” Clipping Technique for Large and Complex Intracranial Middle Cerebral Artery Aneurysm. World Neurosurg. 2019, 125, 150–155. [Google Scholar] [CrossRef]
- Medical Advisory Secretariat. Coil embolization for intracranial aneurysms: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2006, 6, 1–114. [Google Scholar]
- Byoun, H.S.; Lim, J.W.; Han, M.H.; Jeong, E.O.; Koh, H.S.; Kwon, H.J. Coil embolization of the middle cerebral artery bifurcation aneurysms: Feasibility and durability. J. Clin. Neurosci. 2024, 126, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Ihn, Y.K.; Shin, S.H.; Baik, S.K.; Choi, I.S. Complications of endovascular treatment for intracranial aneurysms: Management and prevention. Interv. Neuroradiol. 2018, 24, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Liu, J.; Qin, F.; Hu, L.; Li, Z. Safety and effectiveness of double microcatheter technique in the treatment of ruptured aneurysms of anterior cerebral circulation. Front. Neurol. 2022, 13, 1015304. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Koizumi, S.; Shojima, M.; Ishikawa, O.; Kiyofuji, S.; Umekawa, M.; Saito, N. Double-catheter technique for the embolization of recurrent cerebral aneurysms: A single-center experience. Surg. Neurol. Int. 2023, 14, 273. [Google Scholar] [CrossRef]
- Wallace, A.N.; Samaniego, E.; Kayan, Y.; Derdeyn, C.P.; Delgado Almandoz, J.E.; Dandapat, S.; Fease, J.L.; Thomas, M.; Milner, A.M.; Scholz, J.M.; et al. Balloon-assisted coiling of cerebral aneurysms with the dual-lumen Scepter XC balloon catheter: Experience at two high-volume centers. Interv. Neuroradiol. 2019, 25, 414–418. [Google Scholar] [CrossRef]
- Santillan, A.; Gobin, Y.P.; Mazura, J.C.; Meausoone, V.; Leng, L.Z.; Greenberg, E.; Riina, H.A.; Patsalides, A. Balloon-assisted coil embolization of intracranial aneurysms is not associated with increased periprocedural complications. J. Neurointerv. Surg. 2013, 5, iii56–iii61. [Google Scholar] [CrossRef]
- Vignesh, S.; Prasad, S.N.; Singh, V.; Phadke, R.V.; Balaguruswamy, M.M.; Udiya, A.; Shetty, G.S.; Dhull, V. Balloon-Assisted Coiling of Intracranial Aneurysms: Technical Details and Evaluation of Local Complications. Neurol. India 2022, 70, 643–651. [Google Scholar] [CrossRef]
- Sluzewski, M.; van Rooij, W.J.; Beute, G.N.; Nijssen, P.C. Balloon-assisted coil embolization of intracranial aneurysms: Incidence, complications, and angiography results. J. Neurosurg. 2006, 105, 396–399. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Quadri, S.; Sodhi, A.; Cortez, V.; Taqi, M. E-064 Safety and Efficacy of Balloon-Assisted Coiling of Intracranial Aneurysms: A Single-Center Study. J. Neurointerv. Surg. 2014, 6, A68–A69. [Google Scholar] [CrossRef]
- Pop, R.; Harsan, O.; Martin, I.; Mihoc, D.; Richter, J.S.; Manisor, M.; Simu, M.; Chibbaro, S.; Cebula, H.; Proust, F.; et al. Balloon-assisted coiling of intracranial aneurysms using the Eclipse 2L double lumen balloon. Interv. Neuroradiol. 2020, 26, 291–299. [Google Scholar] [CrossRef]
- Vivanco-Suarez, J.; Wallace, A.N.; Dandapat, S.; Lopez, G.V.; Mendez-Ruiz, A.; Kayan, Y.; Copelan, A.Z.; Dajles, A.; Zevallos, C.B.; Quispe-Orozco, D.; et al. Stent-Assisted Coiling Versus Balloon-Assisted Coiling for the Treatment of Ruptured Wide-Necked Aneurysms: A 2-Center Experience. Stroke Vasc. Interv. Neurol. 2023, 3, 1–10. [Google Scholar] [CrossRef]
- Irie, K.; Negoro, M.; Hayakawa, M.; Eayashi, J.; Kanno, T. Stent assisted coil embolization: The treatment of wide-necked, dissecting, and fusiform aneurysms. Interv. Neuroradiol. 2003, 9, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.S.; Kim, E.J.; Sung, S.M.; Hwangbo, L.; Lee, T.H.; Ko, J.K. Additional rescue stenting with Neuroform Atlas stents during stent-assisted coiling of saccular aneurysms. Clin. Neurol. Neurosurg. 2023, 230, 107777. [Google Scholar] [CrossRef]
- Jahshan, S.; Abla, A.A.; Natarajan, S.K.; Drummond, P.S.; Kan, P.; Karmon, Y.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Results of stent-assisted vs non-stent-assisted endovascular therapies in 489 cerebral aneurysms: Single-center experience. Neurosurgery 2013, 72, 232–239. [Google Scholar] [CrossRef]
- Chalouhi, N.; Jabbour, P.; Singhal, S.; Drueding, R.; Starke, R.M.; Dalyai, R.T.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Rosenwasser, R.; et al. Stent-assisted coiling of intracranial aneurysms: Predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013, 44, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.C.H.; Ng, Y.T.; Yu, S.C.H.; Zhuang, J.T.F.; Wong, G.K.C. Mid-term outcomes in stent-assisted coil embolization for ruptured cerebral aneurysms in the acute period: A single institution retrospective review. Brain Hemorrhages 2024, 5, 267–273. [Google Scholar] [CrossRef]
- Fuga, M.; Ishibashi, T.; Aoki, K.; Tachi, R.; Irie, K.; Kato, N.; Kan, I.; Hataoka, S.; Nagayama, G.; Sano, T.; et al. Intermediate catheter use is associated with complete occlusion and dense packing in coil embolization of unruptured cerebral aneurysms: A propensity score matched study. J. Neurointerv. Surg. 2024, 17, 174–180. [Google Scholar] [CrossRef]
- Fuga, M.; Ishibashi, T.; Aoki, K.; Kato, N.; Kan, I.; Hataoka, S.; Nagayama, G.; Sano, T.; Tanaka, T.; Murayama, Y. Intermediate catheter use is associated with intraprocedural rupture during coil embolization of ruptured intracranial aneurysms: A retrospective propensity score-matched study. Front. Neurol. 2024, 15, 1401378. [Google Scholar] [CrossRef]
- Walcott, B.P.; Koch, M.J.; Stapleton, C.J.; Patel, A.B. Blood Flow Diversion as a Primary Treatment Method for Ruptured Brain Aneurysms—Concerns, Controversy, and Future Directions. Neurocrit. Care 2017, 26, 465–473. [Google Scholar] [CrossRef]
- Briganti, F.; Leone, G.; Marseglia, M.; Mariniello, G.; Caranci, F.; Brunetti, A.; Maiuri, F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol. J. 2015, 28, 365–375. [Google Scholar] [CrossRef]
- Chalouhi, N.; Tjoumakaris, S.; Starke, R.M.; Gonzalez, L.F.; Randazzo, C.; Hasan, D.; McMahon, J.F.; Singhal, S.; Moukarzel, L.A.; Dumont, A.S.; et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013, 44, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takao, H.; Fujimura, S.; Dahmani, C.; Ishibashi, T.; Mamori, H.; Fukushima, N.; Yamamoto, M.; Murayama, Y. Selection of helical braided flow diverter stents based on hemodynamic performance and mechanical properties. J. Neurointerv. Surg. 2017, 9, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Binh, N.T.; Luu, V.D.; Thong, P.M.; Cuong, N.N.; Anh, N.Q.; Tuan, T.A.; Linh, L.T.; Thien, N.T.; Uddin, M.J.; Dinh, T.C.; et al. Flow diverter stent for treatment of cerebral aneurysms: A report of 130 patients with 134 aneurysms. Heliyon 2020, 6, e03356. [Google Scholar] [CrossRef] [PubMed]
- Essibayi, M.A.; Jabal, M.S.; Musmar, B.; Adeeb, N.; Salim, H.; Aslan, A.; Cancelliere, N.M.; McLellan, R.M.; Algin, O.; Ghozy, S.; et al. Off-Label use of Woven EndoBridge device for intracranial brain aneurysm treatment: Modeling of occlusion outcome. J. Stroke Cerebrovasc. Dis. 2024, 33, 107897. [Google Scholar] [CrossRef]
- María Ontañón Garcés, J.; Saralegui Prieto, I.; Zaldumbide-Duenas, L.; Fernandez-Ruanova, B.; Cabrera Zubizarreta, A.; Vicente Olabarria, I. Foreign body granuloma as a complication of endovascular treatment of brain aneurysm. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2021, 24, 101068. [Google Scholar] [CrossRef]
- Singla, R.; Gupta, S.; Chanda, A. A Computational Fluid Dynamics-Based Model for Assessing Rupture Risk in Cerebral Arteries with Varying Aneurysm Sizes. Math. Comput. Appl. 2023, 28, 90. [Google Scholar] [CrossRef]
- Sforza, D.M.; Putman, C.M.; Cebral, J.R. Hemodynamics of cerebral aneurysms. Annu. Rev. Fluid Mech. 2009, 41, 91–107. [Google Scholar] [CrossRef]
- Diagbouga, M.R.; Morel, S.; Bijlenga, P.; Kwak, B.R. Role of hemodynamics in initiation/growth of intracranial aneurysms. Eur. J. Clin. Invest. 2018, 48, e12992. [Google Scholar] [CrossRef]
- Shine, S.R.; Shantanu, S.; Harshavardhan, E.; Sudhir, B.J. Fluid–Structure Interaction Model for Assessing Aneurysm Initiation at the Circle of Willis. J. Eng. Sci. Med. Diagn. Ther. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Mohan, D.; Munteanu, V.; Coman, T.; Ciurea, A.V. Genetic factors involves in intracranial aneurysms--actualities. J. Med. Life 2015, 8, 336–341. [Google Scholar]
- Tang, Y.; Wei, H.; Zhang, Z.; Fu, M.; Feng, J.; Li, Z.; Liu, X.; Wu, Y.; Zhang, J.; You, W.; et al. Transition of intracranial aneurysmal wall enhancement from high to low wall shear stress mediation with size increase: A hemodynamic study based on 7T magnetic resonance imaging. Heliyon 2024, 10, e30006. [Google Scholar] [CrossRef] [PubMed]
- Shantha Kumar, V.; Shantha Kumar, V. High Wall Shear Incites Cerebral Aneurysm Formation and Low Wall Shear Stress Propagates Cerebral Aneurysm Growth. J. Neurol. Res. 2023, 13, 1–11. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or Low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Ding, H.; Ren, Y.; Bai, D.; Wu, Z. Study of Typical Ruptured and Unruptured Intracranial Aneurysms Based on Fluid–Structure Interaction. World Neurosurg. 2023, 175, e115–e128. [Google Scholar] [CrossRef]
- Salimi Ashkezari, S.F.; Mut, F.; Robertson, A.M.; Cebral, J.R. Differences Between Ruptured Aneurysms with and Without Blebs: Mechanistic Implications. Cardiovasc. Eng. Technol. 2023, 14, 92–103. [Google Scholar] [CrossRef]
- Razaghi, R.; Biglari, H.; Karimi, A. Risk of rupture of the cerebral aneurysm in relation to traumatic brain injury using a patient-specific fluid-structure interaction model. Comput. Methods Programs Biomed. 2019, 176, 9–16. [Google Scholar] [CrossRef]
- Singh, G.; Yadav, P.N.; Gupta, S.; Chanda, A. Modeling of aneurysm progression in anterior cerebral arteries to estimate rupture risk: A computational study. Biomed. Eng. Adv. 2023, 6, 100106. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, Z.; Gerdroodbary, M.B.; Sabernaeemi, A.; Salavatidezfouli, S. The influence of sac centreline on saccular aneurysm rupture: Computational study. Sci. Rep. 2023, 13, 11288. [Google Scholar] [CrossRef]
- Lee, U.Y.; Kwak, H.S. Analysis of morphological-hemodynamic risk factors for aneurysm rupture including a newly introduced total volume ratio. J. Pers. Med. 2021, 11, 744. [Google Scholar] [CrossRef]
- Muhib, F.; Islam, M.D.; Arafat, M.T. A study on the computational hemodynamic and mechanical parameters for understanding intracranial aneurysms of patients with hypertension and atrial fibrillation. Inform. Med. Unlocked 2022, 32, 101031. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, Z.; Guo, Z. Computational evaluating on the role of sac centerline length on hemorrhage risk of anterior cerebral artery with saccular aneurysm. Chin. J. Phys. 2024, 92, 975–986. [Google Scholar] [CrossRef]
- Mantha, A.; Karmonik, C.; Benndorf, G.; Strother, C.; Metcalfe, R. Hemodynamics in a cerebral artery before and after the formation of an aneurysm. Am. J. Neuroradiol. 2006, 27, 1113–1118. [Google Scholar]
- Cmiel-Smorzyk, K.; Ladziński, P.; Kaspera, W. Biology, Physics and Genetics of Intracranial Aneurysm Formation: A Review. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, S.; Ishida, F.; Kishimoto, T.; Tsuji, M.; Tanaka, K.; Shimosaka, S.; Toyoda, M.; Kashiwagi, N.; Sano, T.; Suzuki, H. Quantification of hemodynamic irregularity using oscillatory velocity index in the associations with the rupture status of cerebral aneurysms. J. Neurointerv. Surg. 2019, 11, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Ishida, F.; Tsuji, M.; Furukawa, K.; Shimosaka, S.; Suzuki, H. Hemodynamic Differences Between Ruptured and Unruptured Cerebral Aneurysms Simultaneously Existing in the Same Location: 2 Case Reports and Proposal of a Novel Parameter Oscillatory Velocity Index. World Neurosurg. 2017, 98, 868.e5–868.e10. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of Hemodynamic Characteristics and Cerebral Aneurysm Rupture. AJNR Am. J. Neuroradiol. 2011, 32, 264. [Google Scholar] [CrossRef]
- Li, Y.; Amili, O.; Moen, S.; Van de Moortele, P.F.; Grande, A.; Jagadeesan, B.; Coletti, F. Flow residence time in intracranial aneurysms evaluated by in vitro 4D flow MRI. J. Biomech. 2022, 141, 111211. [Google Scholar] [CrossRef]
- Rayz, V.L.; Boussel, L.; Ge, L.; Leach, J.R.; Martin, A.J.; Lawton, M.T.; McCulloch, C.; Saloner, D. Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Ann. Biomed. Eng. 2010, 38, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, H.; Chen, Y.; Dai, Y.; Lin, B.; Liang, F.; Wan, J.; Yang, Y.; Zhao, B. Morphological and Hemodynamic Factors Associated with Ruptured Middle Cerebral Artery Mirror Aneurysms: A Retrospective Study. World Neurosurg. 2020, 137, e138–e143. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Liu, J.; Zhao, C.; Yang, X. Hemodynamics in Ruptured Intracranial Aneurysms with Known Rupture Points. World Neurosurg. 2018, 118, e721–e726. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, P.; Wu, J.; Gao, B.; Wang, S. The Morphological and Hemodynamic Characteristics of the Intraoperative Ruptured Aneurysm. Front. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hasegawa, H.; Shibuya, K.; Fujiwara, H.; Oishi, M. Clinical and Hemodynamic Features of Aneurysm Rupture in Coil Embolization of Intracranial Aneurysms. Diagnostics 2024, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.; Scardino, B.; Gamage, P.T.; Taebi, A. Variations of Middle Cerebral Artery Hemodynamics Due to Aneurysm Clipping Surgery. J. Eng. Sci. Med. Diagn. Ther. 2023, 7, 11008. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, S.; Kheiri, A.A. Influence of physiological conditions on hemodynamics of internal carotid artery aneurysms. Sci. Rep. 2024, 14, 23106. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, T.; Panaitescu, C.; Li, R.; Wu, K.; Zhou, Y.; Pokrajac, D.; Dini, D.; Zhan, W. Exploring tissue permeability of brain tumours in different grades: Insights from pore-scale fluid dynamics analysis. Acta Biomater. 2024, 190, 398–409. [Google Scholar] [CrossRef]
- Deuter, D.; Haj, A.; Brawanski, A.; Krenkel, L.; Schmidt, N.O.; Doenitz, C. Fast simulation of hemodynamics in intracranial aneurysms for clinical use. Acta Neurochir. 2025, 167, 56. [Google Scholar] [CrossRef] [PubMed]
- Paritala, P.K.; Anbananthan, H.; Hautaniemi, J.; Smith, M.; George, A.; Allenby, M.; Mendieta, J.B.; Wang, J.; Maclachlan, L.; Liang, E.S.; et al. Reproducibility of the computational fluid dynamic analysis of a cerebral aneurysm monitored over a decade. Sci. Rep. 2023, 13, 219. [Google Scholar] [CrossRef]
- Campo-Deano, L.; Oliveira, M.S.N.; Pinho, F.T. A review of computational hemodynamics in middle cerebral aneurysms and rheological models for blood flow. Appl. Mech. Rev. 2015, 67, 030801. [Google Scholar] [CrossRef]
- Sun, H.T.; Sze, K.Y.; Chow, K.W.; On Tsang, A.C. A comparative study on computational fluid dynamic, fluid-structure interaction and static structural analyses of cerebral aneurysm. Eng. Appl. Comput. Fluid Mech. 2022, 16, 262–278. [Google Scholar] [CrossRef]
- Rostam-Alilou, A.A.; Jarrah, H.R.; Zolfagharian, A.; Bodaghi, M. Fluid–structure interaction (FSI) simulation for studying the impact of atherosclerosis on hemodynamics, arterial tissue remodeling, and initiation risk of intracranial aneurysms. Biomech. Model. Mechanobiol. 2022, 21, 1393–1406. [Google Scholar] [CrossRef]
- M. Fauzi, M.F.; Johari, N.H.; M. Mokhtarudin, M.J. Brief Review of Recent Study on Fluid–Structure Interaction Modeling of Blood Flow in Peripheral Arterial Disease. In Proceedings of the 2nd Human Engineering Symposium; Hassan, M.H.A., Omar, M.N., Johari, N.H., Zhong, Y., Eds.; Springer Nature: Singapore, 2024; pp. 185–197. [Google Scholar]
- Torii, R.; Oshima, M.; Kobayashi, T.; Takagi, K.; Tezduyar, T.E. Fluid-structure interaction modeling of a patient-specific cerebral aneurysm: Influence of structural modeling. Comput. Mech. 2008, 43, 151–159. [Google Scholar] [CrossRef]
- Dwivedi, K.K.; Lakhani, P.; Kumar, S.; Kumar, N. A hyperelastic model to capture the mechanical behaviour and histological aspects of the soft tissues. J. Mech. Behav. Biomed. Mater. 2022, 126, 105013. [Google Scholar] [CrossRef]
- Lauric, A.; Hippelheuser, J.; Safain, M.G.; Malek, A.M. Curvature effect on hemodynamic conditions at the inner bend of the carotid siphon and its relation to aneurysm formation. J. Biomech. 2014, 47, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, D.S.; Hadjinicolaou, M. Investigating the impact of vessel geometry on cerebral aneurysm formation using multi-phase blood flow models. Comput. Math. Appl. 2024, 176, 257–269. [Google Scholar] [CrossRef]
- Csippa, B.; Závodszky, G.; Paál, G.; Szikora, I. A new hypothesis on the role of vessel topology in cerebral aneurysm initiation. Comput. Biol. Med. 2018, 103, 244–251. [Google Scholar] [CrossRef]
- Gao, B.L.; Hao, W.L.; Ren, C.F.; Li, C.H.; Wang, J.W.; Liu, J.F. Greater hemodynamic stresses initiated the anterior communicating artery aneurysm on the vascular bifurcation apex. J. Clin. Neurosci. 2022, 96, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Durgesh, V. Experimental study of flow structure impact on the fluid parameters in saccular aneurysm models. Exp. Therm. Fluid Sci. 2022, 138, 110675. [Google Scholar] [CrossRef]
- Chi, Q.; Li, X.; Chang, S.; Mu, L.; He, Y.; Gao, G. In-vitro experimental study on the fluid-structure interaction in an image-based flexible model with a lateral cerebral aneurysm. Med. Nov. Technol. Devices 2019, 3, 100019. [Google Scholar] [CrossRef]
- Souza, A.; Lopes, D.; Souza, S.; Ribeiro, J.; Lima, R.A.; Ferrera, C. Experimental and numerical analyses of the hemodynamics impact on real Experimental and numerical analyses of the hemodynamics impact on real intracranial aneurysms: A particle tracking velocimetry approach. Results Eng. 2024, 24, 103566. [Google Scholar] [CrossRef]
- Lai, S.S.M.; Tang, A.Y.S.; Tsang, A.C.O.; Leung, G.K.K.; Yu, A.C.H.; Chow, K.W. A joint computational-experimental study of intracranial aneurysms: Importance of the aspect ratio. J. Hydrodyn. 2016, 28, 462–472. [Google Scholar] [CrossRef]
- Shamloo, A.; Nejad, M.A.; Saeedi, M. Fluid–structure interaction simulation of a cerebral aneurysm: Effects of endovascular coiling treatment and aneurysm wall thickening. J. Mech. Behav. Biomed. Mater. 2017, 74, 72–83. [Google Scholar] [CrossRef]
- Philip, N.T.; Bolem, S.; Sudhir, B.J.; Patnaik, B.S. V Hemodynamics and bio-mechanics of morphologically distinct saccular intracranial aneurysms at bifurcations: Idealised vs Patient-specific geometries. Comput. Methods Programs Biomed. 2022, 227, 107237. [Google Scholar] [CrossRef]
- Huang, F.; Janiga, G.; Berg, P.; Hosseini, S.A. On flow fluctuations in ruptured and unruptured intracranial aneurysms: Resolved numerical study. Sci. Rep. 2024, 14, 19658. [Google Scholar] [CrossRef]
- Cebral, J.R.; Vazquez, M.; Sforza, D.M.; Houzeaux, G.; Tateshima, S.; Scrivano, E.; Bleise, C.; Lylyk, P.; Putman, C.M. Analysis of hemodynamics and wall mechanics at sites of cerebral aneurysm rupture. J. Neurointerv. Surg. 2015, 7, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Cebral, J.; Ollikainen, E.; Chung, B.J.; Mut, F.; Sippola, V.; Jahromi, B.R.; Tulamo, R.; Hernesniemi, J.; Niemelä, M.; Robertson, A.; et al. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. Am. J. Neuroradiol. 2017, 38, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Durgesh, V. Experimental study of large-scale flow structures in an aneurysm. In Proceedings of the ASME 2018 5th Joint US-European Fluids Engineering Division Summer Meeting, Montreal, QC, Canada, 15–20 July 2018; pp. 1–10. [Google Scholar] [CrossRef]
- Alagan, A.K.; Valeti, C.; Bolem, S.; Karve, O.S.; Arvind, K.R.; Rajalakshmi, P.; Sabareeswaran, A.; Gopal, S.; Matham, G.; Darshan, H.R.; et al. Histopathology-based near-realistic arterial wall reconstruction of a patient-specific cerebral aneurysm for fluid-structure interaction studies. Comput. Biol. Med. 2025, 185, 109579. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, S.; Tanaka, K.; Takao, H.; Okudaira, T.; Koseki, H.; Hasebe, A.; Suzuki, T.; Uchiyama, Y.; Ishibashi, T.; Otani, K.; et al. Computational fluid dynamic analysis of the initiation of cerebral aneurysms. J. Neurosurg. 2022, 137, 335–343. [Google Scholar] [CrossRef]
- Cherkaoui, I.; Bettaibi, S.; Barkaoui, A.; Kuznik, F. Numerical study of pulsatile thermal magnetohydrodynamic blood flow in an artery with aneurysm using lattice Boltzmann method (LBM). Commun. Nonlinear Sci. Numer. Simul. 2023, 123, 107281. [Google Scholar] [CrossRef]
- Moghadasi, K.; Ghayesh, M.H.; Li, J.; Hu, E.; Amabili, M.; Żur, K.K.; Fitridge, R. Nonlinear biomechanical behaviour of extracranial carotid artery aneurysms in the framework of Windkessel effect via FSI technique. J. Mech. Behav. Biomed. Mater. 2024, 160, 106760. [Google Scholar] [CrossRef]
- Sharzehee, M.; Khalafvand, S.S.; Han, H.C. Fluid-structure interaction modeling of aneurysmal arteries under steady-state and pulsatile blood flow: A stability analysis. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Barzegar Gerdroodbary, M.; Abazari, A.M.; Moradi, R. Computational study of blood flow characteristics on formation of the aneurysm in internal carotid artery. Eur. Phys. J. Plus 2021, 136, 541. [Google Scholar] [CrossRef]
- Fattahi, M.; Abdollahi, S.A.; Alibak, A.H.; Hosseini, S.; Dang, P. Usage of computational method for hemodynamic analysis of intracranial aneurysm rupture risk in different geometrical aspects. Sci. Rep. 2023, 13, 20749. [Google Scholar] [CrossRef]
- Cho, K.C.; Yang, H.; Kim, J.J.; Oh, J.H.; Kim, Y.B. Prediction of rupture risk in cerebral aneurysms by comparing clinical cases with fluid–structure interaction analyses. Sci. Rep. 2020, 10, 18237. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.L.; Santos, G.B.; Militzer, J.; Baccin, C.E.; Tatit, R.T.; Gasche, J.L. A longitudinal study of a lateral intracranial aneurysm: Identifying the hemodynamic parameters behind its inception and growth using computational fluid dynamics. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 138. [Google Scholar] [CrossRef]
- Zhu, Y.; Zou, R.; Sun, X.; Lei, X.; Xiang, J.; Guo, Z.; Su, H. Assessing the risk of intracranial aneurysm rupture using computational fluid dynamics: A pilot study. Front. Neurol. 2023, 14, 1277278. [Google Scholar] [CrossRef]
- Rahma, A.G.; Abdelhamid, T. Hemodynamic and fluid flow analysis of a cerebral aneurysm: A CFD simulation. SN Appl. Sci. 2023, 5, 62. [Google Scholar] [CrossRef]
- Závodszky, G.; Gyürki, D.; Károlyi, G.; Szikora, I.; Paál, G. Fractals and Chaos in the Hemodynamics of Intracranial Aneurysms. Adv. Neurobiol. 2024, 36, 397–412. [Google Scholar] [CrossRef]
- Salimi Ashkezari, S.F.; Mut, F.; Chung, B.J.; Robertson, A.M.; Frösen, J.; Cebral, J.R. Analysis of hemodynamic changes from aneurysm inception to large sizes. Int. J. Numer. Method. Biomed. Eng. 2021, 37, e3415. [Google Scholar] [CrossRef]
- Usmani, A.Y.; Muralidhar, K. Unsteady hemodynamics in intracranial aneurysms with varying dome orientations. J. Fluids Eng. Trans. ASME 2021, 143, 061206. [Google Scholar] [CrossRef]
- Li, Y.; Yoneyama, Y.; Isoda, H.; Terada, M.; Kosugi, T.; Kosugi, T.; Zhang, M.; Ohta, M. Haemodynamics in a patient-specific intracranial aneurysm according to experimental and numerical approaches: A comparison of PIV, CFD and PC-MRI. Technol. Health Care 2021, 29, 253–267. [Google Scholar] [CrossRef]
- Wu, X.; Gürzing, S.; Schinkel, C.; Toussaint, M.; Perinajová, R.; van Ooij, P.; Kenjereš, S. Hemodynamic Study of a Patient-Specific Intracranial Aneurysm: Comparative Assessment of Tomographic PIV, Stereoscopic PIV, In Vivo MRI and Computational Fluid Dynamics. Cardiovasc. Eng. Technol. 2022, 13, 428–442. [Google Scholar] [CrossRef]
- Tupin, S.; Saqr, K.M.; Ohta, M. Effects of wall compliance on multiharmonic pulsatile flow in idealized cerebral aneurysm models: Comparative PIV experiments. Exp. Fluids 2020, 61, 164. [Google Scholar] [CrossRef]
- Ikeya, N.; Yamazaki, T.; Tanaka, G.; Ohta, M.; Yamaguchi, R. Experimental evaluation of wall shear stress in an elastic cerebral aneurysm model. J. Biorheol. 2022, 36, 58–67. [Google Scholar] [CrossRef]
- Shen, F.; Lu, X.; Pang, Y.; Liu, Z. Experimental study on transient flow patterns in simplified saccular intracranial aneurysm models using particle image velocimetry. Acta Mech. Sin. 2022, 38, 322162. [Google Scholar] [CrossRef]
- Yazdi, S.G.; Mercier, D.; Bernard, R.; Tynan, A.; Ricci, D.R. Particle image velocimetry measurements of the flow-diverting effects of a new generation of the eclips implant for the treatment of intracranial bifurcation aneurysms. Appl. Sci. 2020, 10, 8639. [Google Scholar] [CrossRef]
- Murayama, Y.; Fujimura, S.; Suzuki, T.; Takao, H. Computational fluid dynamics as a risk assessment tool for aneurysm rupture. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef]
- Hadad, S.; Mut, F.; Kadirvel, R.; Ding, Y.H.; Kallmes, D.; Cebral, J.R. Evaluation of outcome prediction of flow diversion for intracranial aneurysms. Am. J. Neuroradiol. 2021, 42, 1973–1978. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Raschi, M.; Scrivano, E.; Ceratto, R.; Lylyk, P.; Putman, C.M. Putman Aneurysm rupture following treatment with flow-diverting stents: Computational hemodynamics analysis of treatment. Am. J. Neuroradiol. 2011, 32, 27–33. [Google Scholar] [CrossRef]
- Sheidani, A.; Barzegar Gerdroodbary, M.; Poozesh, A.; Sabernaeemi, A.; Salavatidezfouli, S.; Hajisharifi, A. Influence of the coiling porosity on the risk reduction of the cerebral aneurysm rupture: Computational study. Sci. Rep. 2022, 12, 19082. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.L.; Gao, B.L.; Xue, J.Y.; Li, T.X.; Zhao, T.Y.; Cai, D.Y.; He, Y.K. Use of a First Large-Sized Coil Versus Conventional Coils for Embolization of Cerebral Aneurysms: Effects on Packing Density, Coil Length, and Durable Occlusion. World Neurosurg. 2019, 127, e685–e691. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Han, M.H.; Rhee, K. Effects of framing coil shape, orientation, and thickness on intra-aneurysmal flow. Med. Biol. Eng. Comput. 2013, 51, 981–990. [Google Scholar] [CrossRef]
- Yao, Z.; Wen, H. Impact of aneurysm sac size on the effectiveness of endovascular coiling in patient-specific middle cerebral artery aneurysms: A computational study. Sci. Rep. 2025, 15, 8825. [Google Scholar] [CrossRef]
- Sadeh, A.; Kazemi, A.; Bahramkhoo, M.; Barzegar Gerdroodbary, M. Computational study of blood flow inside MCA aneurysm with/without endovascular coiling. Sci. Rep. 2023, 13, 4560. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, A.; Fallah, K.; Rostamiyan, Y.; Alinejad, J. Computational study of the blood hemodynamic inside the cerebral double dome aneurysm filling with endovascular coiling. Sci. Rep. 2023, 13, 2909. [Google Scholar] [CrossRef]
- Yang, R.; Yang, L.; Ghane, G. Computational and statistical analyses of blood hemodynamic inside cerebral aneurysms for treatment evaluation of endovascular coiling. Sci. Rep. 2023, 13, 20461. [Google Scholar] [CrossRef] [PubMed]
- Boniforti, M.A.; Magini, R.; Orosco Salinas, T. Hemodynamic Investigation of the Flow Diverter Treatment of Intracranial Aneurysm. Fluids 2023, 8, 189. [Google Scholar] [CrossRef]
- Kim, S.; Yang, H.; Hong, I.; Oh, J.H.; Kim, Y.B. Computational Study of Hemodynamic Changes Induced by Overlapping and Compacting of Stents and Flow Diverter in Cerebral Aneurysms. Front. Neurol. 2021, 12, 705841. [Google Scholar] [CrossRef]
- Pei, J.; Xu, X.; Zhao, L.C. Hemodynamic evaluation of endovascular techniques of stenting and coiling for the treatment of internal carotid artery aneurysm: A computational study. Sci. Rep. 2024, 14, 29117. [Google Scholar] [CrossRef]
- Horn, J.D.; Maitland, D.J.; Hartman, J.; Ortega, J.M. Computational study of clot formation in aneurysms treated with shape memory polymer foam. Med. Eng. Phys. 2020, 75, 65–71. [Google Scholar] [CrossRef]
- Chen, L.; Leng, X.; Zheng, C.; Shan, Y.; Wang, M.; Bao, X.; Wu, J.; Zou, R.; Liu, X.; Xu, S.; et al. Computational fluid dynamics (CFD) analysis in a ruptured vertebral artery dissecting aneurysm implanted by Pipeline when recurrent after LVIS-assisted coiling treatment: Case report and review of the literatures. Interv. Neuroradiol. 2023, 29, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.; Scardino, B.; Hollingsworth, L.; Thibbotuwawa Gamage, P.; Taebi, A. A Comparative Study of Middle Cerebral Artery Hemodynamics Pre- and Post-Clipping of Cerebral Aneurysm. In Proceedings of the ASME 2023 International Mechanical Engineering Congress and Exposition, New Orleans, LA, USA, 29 October–2 November 2023. [Google Scholar] [CrossRef]
- Sanches, A.F.; Shit, S.; Özpeynirci, Y.; Liebig, T. CFD to Quantify Idealized Intra-Aneurysmal Blood Flow in Response to Regular and Flow Diverter Stent Treatment. Fluids 2022, 7, 254. [Google Scholar] [CrossRef]
- Shi, J.; Wu, P.; Zhou, Y.; Chen, Z. An experimental study on effects of interventional stent treatment on hemodynamics in elastic aneurysms. Phys. Fluids 2023, 35, 051906. [Google Scholar] [CrossRef]
- Roloff, C.; Berg, P. Effect of flow diverter stent malposition on intracranial aneurysm hemodynamics—An experimental framework using stereoscopic particle image velocimetry. PLoS ONE 2022, 17, e0264688. [Google Scholar] [CrossRef]
- Chodzyǹski, K.J.; Uzureau, P.; Nuyens, V.; Rousseau, A.; Coussement, G.; Zouaoui Boudjeltia, K. The impact of arterial flow complexity on flow diverter outcomes in aneurysms. Sci. Rep. 2020, 10, 10337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).