The Effects of Altered Blood Flow, Force, Wrist Posture, Finger Movement Speed, and Population on Motion and Blood Flow in the Carpal Tunnel: A Mega-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomechanical Influences on Tendon and SSCT Motion
2.2. Median Nerve Blood Flow Changes
2.3. Effect of Induced Blood Flow Alteration
2.4. Statistics
3. Results
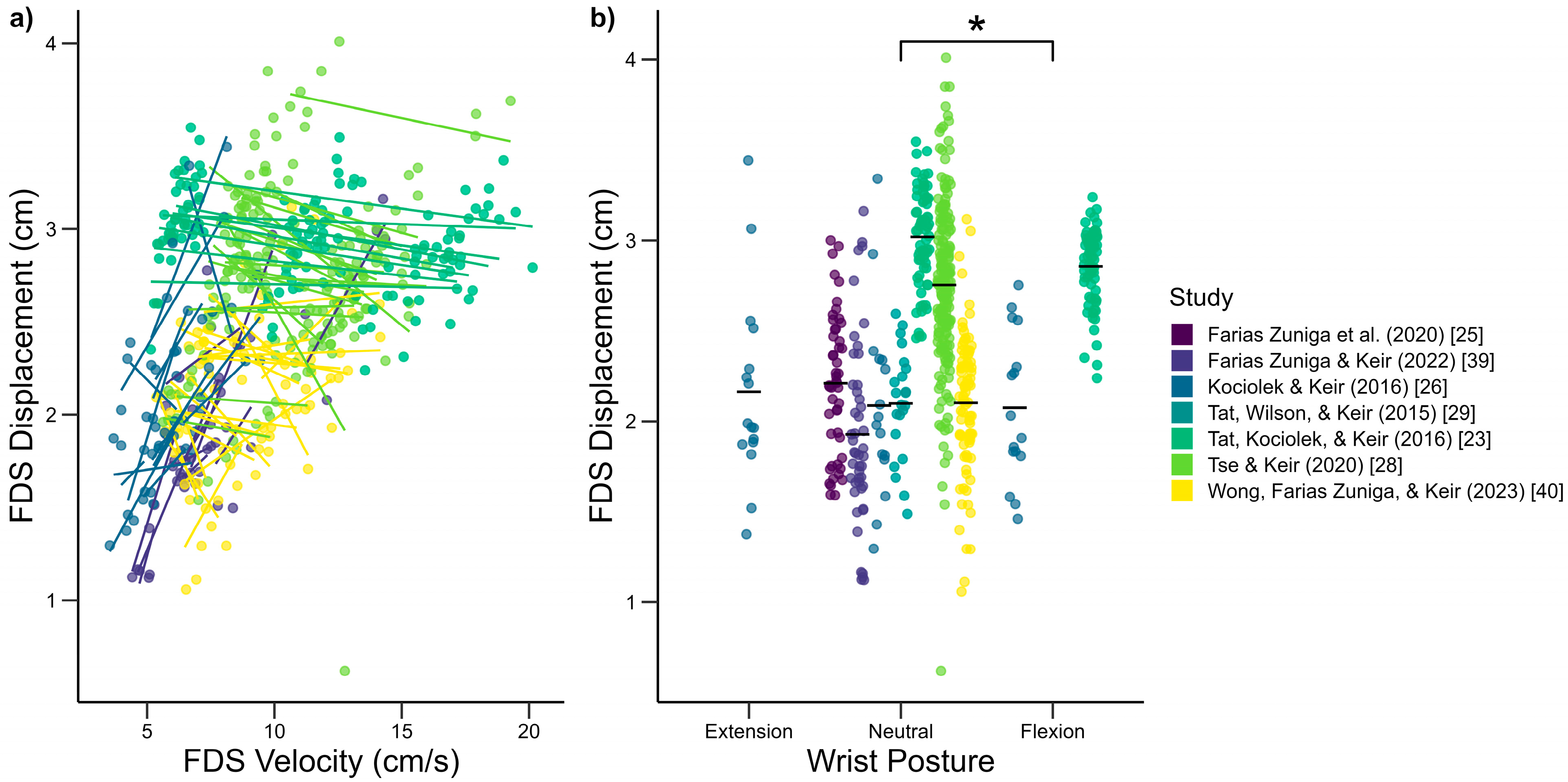
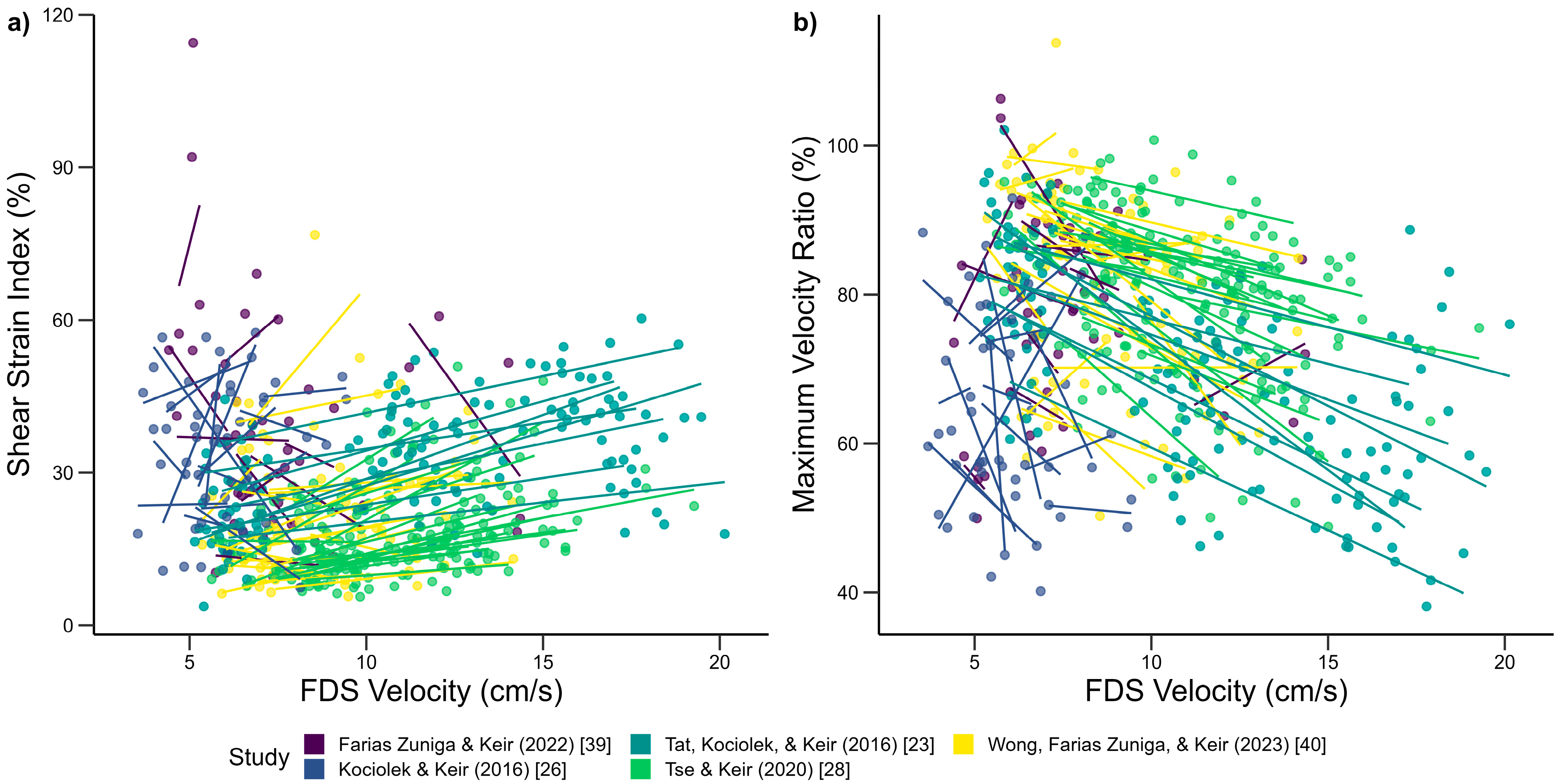
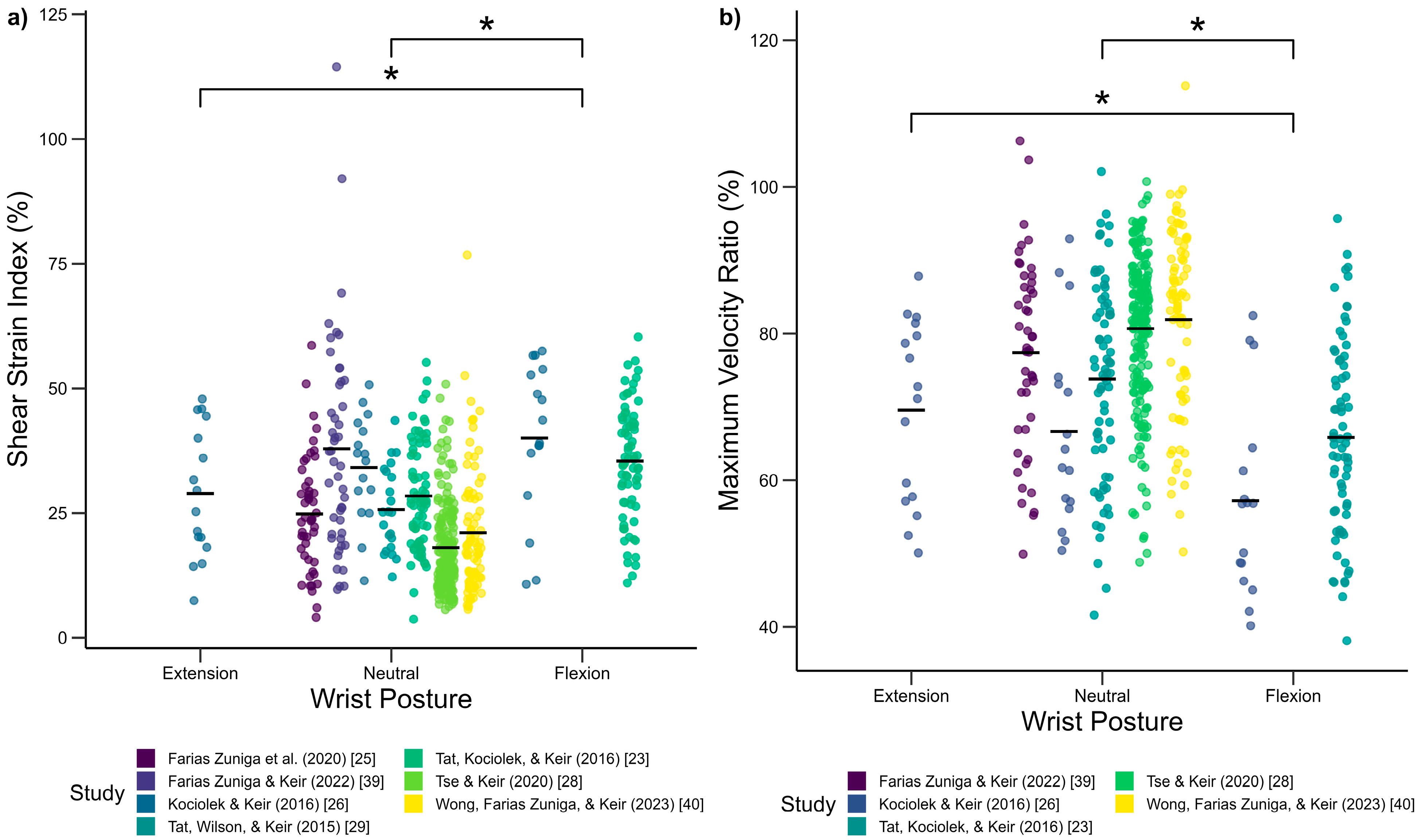
3.1. Biomechanical Influences on Tendon and SSCT Motion
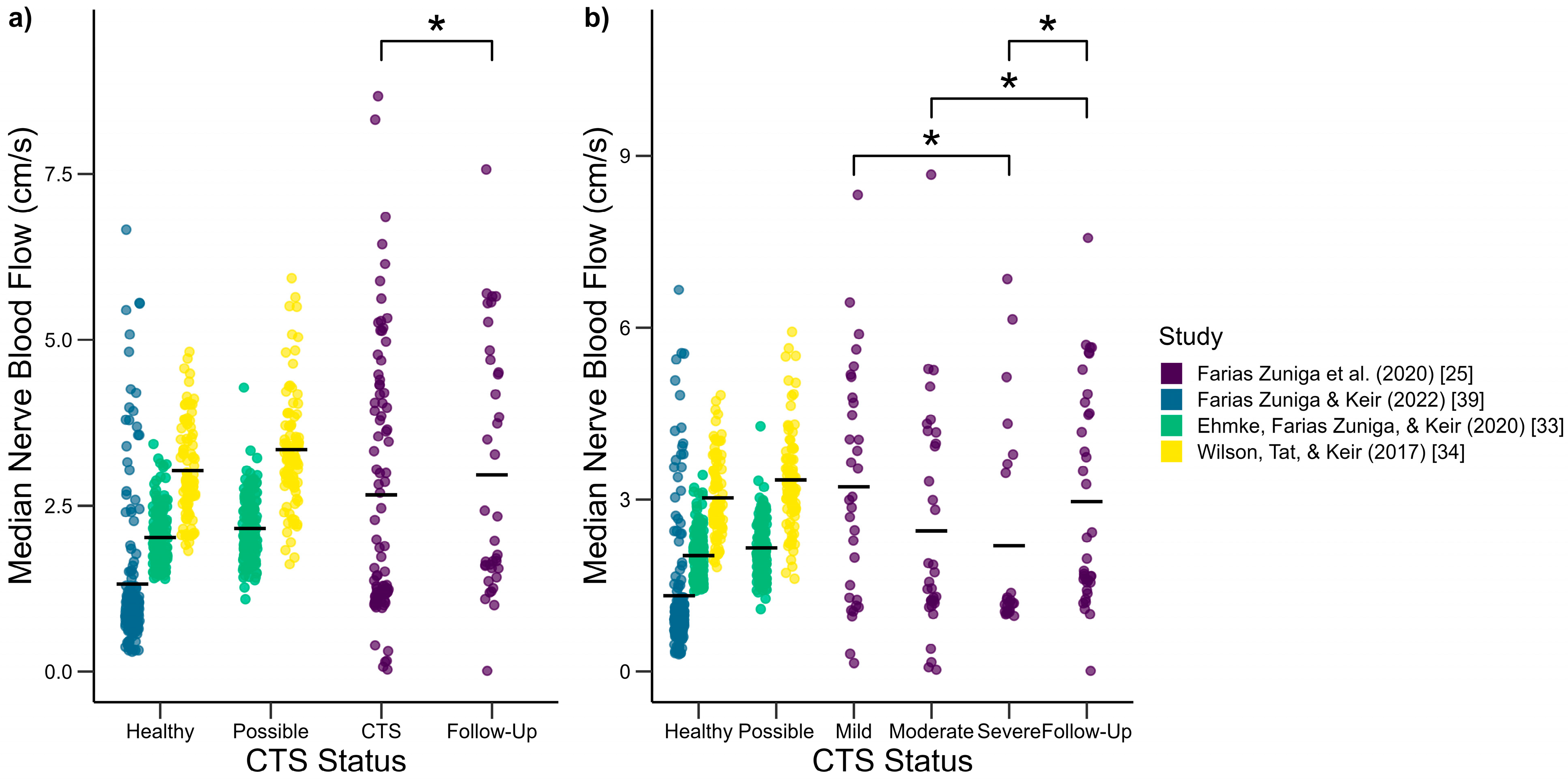
3.2. Median Nerve Blood Flow Changes
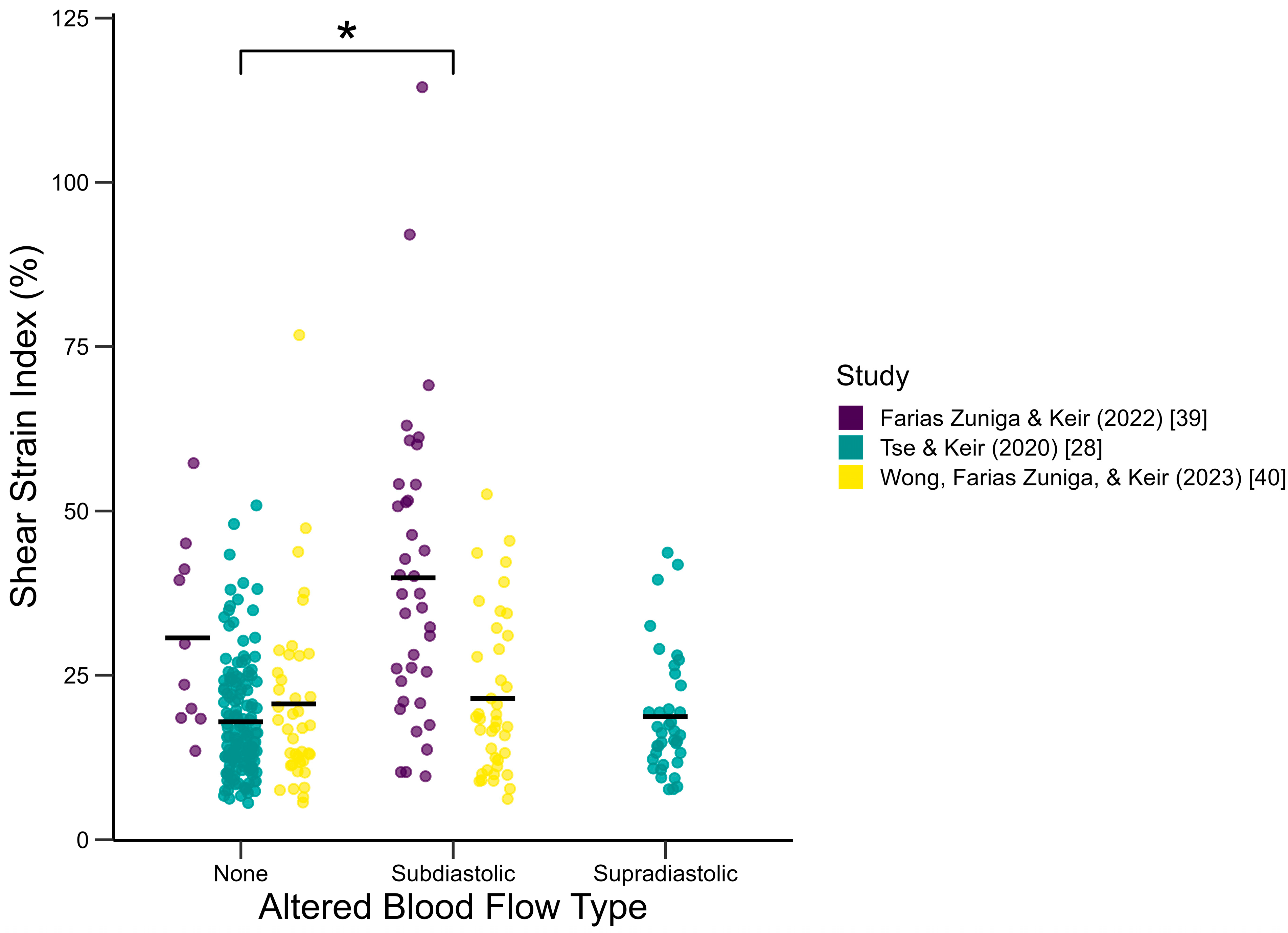
3.3. Effect of Induced Blood Flow Alteration
4. Discussion
4.1. Biomechanical Influences on Tendon and SSCT Motion
4.2. Median Nerve Blood Flow Changes
4.3. Effect of Induced Blood Flow Alteration
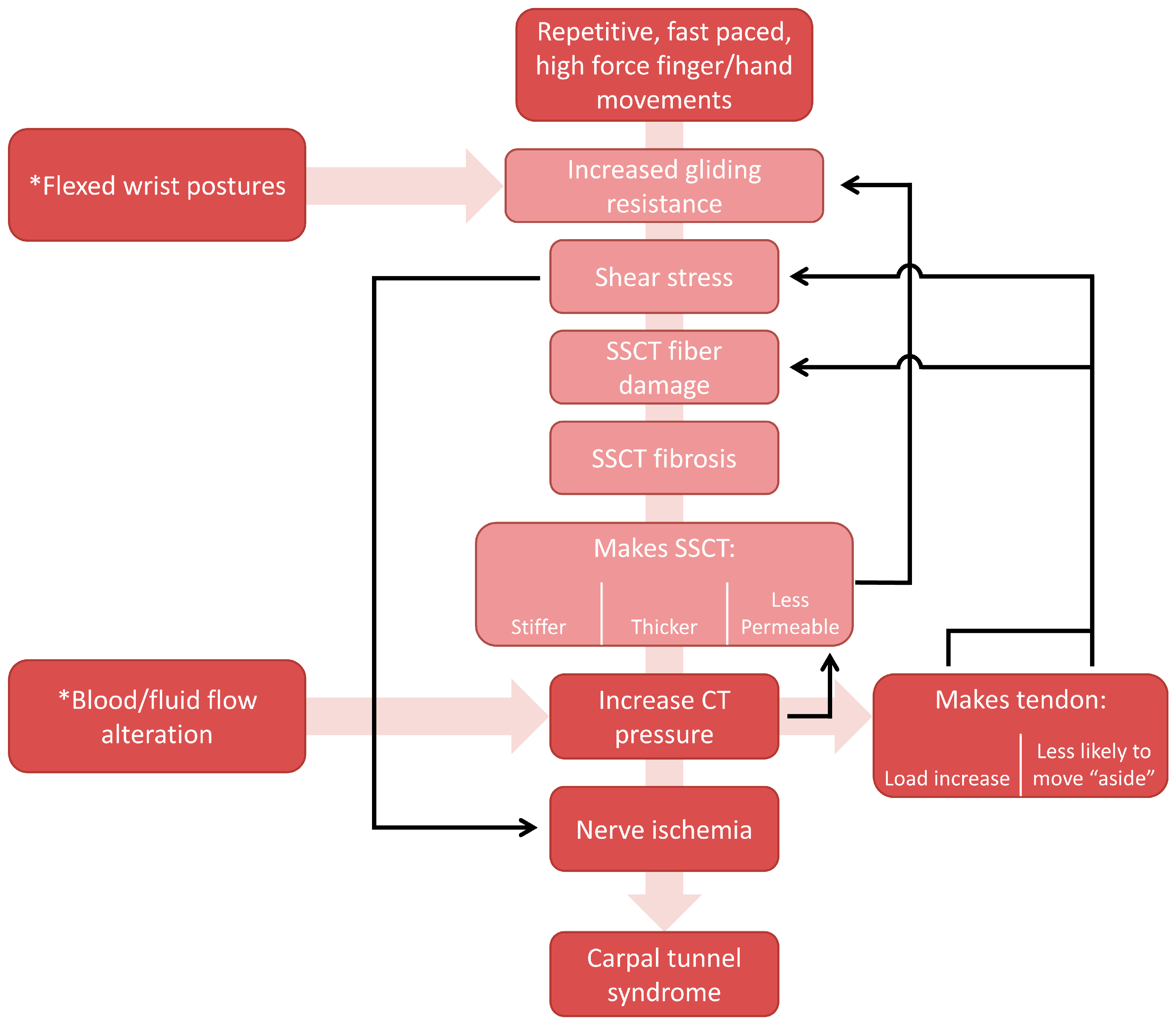
4.4. Updated Mechanism of Injury
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of Carpal Tunnel Syndrome in a General Population. J. Am. Med. Assoc. 1999, 282, 153–158. [Google Scholar] [CrossRef]
- Dale, A.M.; Harris-Adamson, C.; Rempel, D.; Gerr, F.; Hegmann, K.; Silverstein, B.; Burt, S.; Garg, A.; Kapellusch, J.; Merlino, L.; et al. Prevalence and Incidence of Carpal Tunnel Syndrome in US Working Populations: Pooled Analysis of Six Prospective Studies. Scand. J. Work Environ. Health 2013, 39, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Shiri, R.; Falah-Hassani, K. Computer Use and Carpal Tunnel Syndrome: A Meta-Analysis. J. Neurol. Sci. 2014, 349, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Thomsen, J.F.; Overgaard, E.; Lassen, C.F.; Brandt, L.P.A.; Vilstrup, I.; Kryger, A.I.; Mikkelsen, S. Computer Use and Carpal Tunnel Syndrome: A 1-Year Follow-Up Study. JAMA 2003, 289, 2963. [Google Scholar] [CrossRef]
- Silverstein, B.A.; Fine, L.J.; Armstrong, T.J. Occupational Factors and Carpal Tunnel Syndrome. Am. J. Ind. Med. 1987, 11, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Harris-Adamson, C.; Eisen, E.A.; Kapellusch, J.; Garg, A.; Hegmann, K.T.; Thiese, M.S.; Marie Dale, A.; Evanoff, B.; Burt, S.; Bao, S.; et al. Biomechanical Risk Factors for Carpal Tunnel Syndrome: A Pooled Study of 2474 Workers. Occup. Environ. Med. 2015, 72, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Musolin, K.M.; Ramsey, J.G. Carpal Tunnel Syndrome Prevalence: An Evaluation of Workers at a Raw Poultry Processing Plant. Int. J. Occup. Environ. Health 2017, 23, 282–290. [Google Scholar] [CrossRef]
- Keir, P.J.; Farias Zuniga, A.; Mulla, D.M.; Somasundram, K.G. Relationships and Mechanisms Between Occupational Risk Factors and Distal Upper Extremity Disorders. Hum. Factors 2021, 63, 5–31. [Google Scholar] [CrossRef]
- Ettema, A.M.; Amadio, P.C.; Zhao, C.; Wold, L.E.; An, K.-N. A Histological and Immunohistochemical Study of the Subsynovial Connective Tissue in Idiopathic Carpal Tunnel Syndrome. J. Bone Jt. Surg. Am. 2004, 86, 1458–1466. [Google Scholar] [CrossRef]
- Ettema, A.M.; Amadio, P.C.; Zhao, C.; Wold, L.E.; O’Byrne, M.M.; Moran, S.L.; An, K.-N. Changes in the Functional Structure of the Tenosynovium in Idiopathic Carpal Tunnel Syndrome: A Scanning Electron Microscope Study. Plast. Reconstr. Surg. 2006, 118, 1413–1422. [Google Scholar] [CrossRef]
- Freeland, A.E.; Tucci, M.A.; Barbieri, R.A.; Angel, M.F.; Nick, T.G. Biochemical Evaluation of Serum and Flexor Tenosynovium in Carpal Tunnel Syndrome. Microsurgery 2002, 22, 378–385. [Google Scholar] [CrossRef]
- Jinrok, O.; Zhao, C.; Amadio, P.C.; An, K.-N.; Zobitz, M.E.; Wold, L.E. Vascular Pathologic Changes in the Flexor Tenosynovium (Subsynovial Connective Tissue) in Idiopathic Carpal Tunnel Syndrome. J. Orthop. Res. 2004, 22, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Neal, N.C.; McManners, J.; Stirling, G.A. Pathology of the Flexor Tendon Sheath in the Spontaneous Carpal Tunnel Syndrome. J. Hand Surg. 1987, 12, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Phalen, G.S. The Carpal-Tunnel Syndrome. Seventeen Years’ Experience in Diagnosis and Treatment of Six Hundred Fifty-Four Hands. J. Bone Jt. Surg. 1966, 48, 211–228. [Google Scholar] [CrossRef]
- Guimberteau, J.C.; Delage, J.P.; McGrouther, D.A.; Wong, J.K.F. The Microvacuolar System: How Connective Tissue Sliding Works. J. Hand Surg. Eur. Vol. 2010, 35, 614–622. [Google Scholar] [CrossRef]
- van Doesburg, M.H.M.; Mink van der Molen, A.; Henderson, J.; Cha, S.S.; An, K.N.; Amadio, P.C. Sonographic Measurements of Subsynovial Connective Tissue Thickness in Patients with Carpal Tunnel Syndrome. J. Ultrasound Med. 2012, 31, 31–36. [Google Scholar] [CrossRef]
- Werthel, J.-D.; Zhao, C.; An, K.-N.; Amadio, P. Carpal Tunnel Syndrome Pathophysiology: Role of Subsynovial Connective Tissue. J. Wrist Surg. 2014, 03, 220–226. [Google Scholar] [CrossRef]
- Kerr, C.D.; Sybert, D.R.; Albarracin, N.S. An Analysis of the Flexor Synovium in Idiopathic Carpal Tunnel Syndrome: Report of 625 Cases. J. Hand Surg. 1992, 17, 1028–1030. [Google Scholar] [CrossRef]
- Robben, E.; Dever, J.; De Groef, A.; Degreef, I.; Peers, K. Subsynovial Connective Tissue Thickness in Carpal Tunnel Syndrome: A Systematic Review. Clin. Biomech. 2020, 75, 105002. [Google Scholar] [CrossRef]
- Schrier, V.J.M.M.; Evers, S.; Geske, J.R.; Kremers, W.K.; Villarraga, H.R.; Selles, R.W.; Hovius, S.E.R.; Gelfman, R.; Amadio, P.C. Relative Motion of the Connective Tissue in Carpal Tunnel Syndrome: The Relation with Disease Severity and Clinical Outcome. Ultrasound Med. Biol. 2020, 46, 2236–2244. [Google Scholar] [CrossRef]
- Kociolek, A.M.; Tat, J.; Keir, P.J. Biomechanical Risk Factors and Flexor Tendon Frictional Work in the Cadaveric Carpal Tunnel. J. Biomech. 2015, 48, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Belohlavek, M.; Zhao, C.; Osamura, N.; Zobitz, M.E.; An, K.N.; Amadio, P.C. Detection of Differential Gliding Characteristics of the Flexor Digitorum Superficialis Tendon and Subsynovial Connective Tissue Using Color Doppler Sonographic Imaging. J. Ultrasound Med. 2007, 26, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tat, J.; Kociolek, A.M.; Keir, P.J. Relative Displacement of the Tendon and Subsynovial Connective Tissue Using Ultrasound Captures Different Phenomena than Mechanical Tendon Shear. J. Biomech. 2016, 49, 3682–3687. [Google Scholar] [CrossRef]
- Tat, J.; Kociolek, A.M.; Keir, P.J. Validation of Color Doppler Sonography for Evaluating Relative Displacement Between the Flexor Tendon and Subsynovial Connective Tissue. J. Ultrasound Med. 2015, 34, 679–687. [Google Scholar] [CrossRef]
- Farias Zuniga, A.; Ghavanini, A.A.; Israelian, G.; Keir, P.J. Blood Flow Velocity but Not Tendon Mechanics Relates to Nerve Function in Carpal Tunnel Syndrome Patients. J. Neurol. Sci. 2020, 411, 116694. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, A.M.; Keir, P.J. Relative Motion between the Flexor Digitorum Superficialis Tendon and Paratenon in Zone V Increases with Wrist Flexion Angle. J. Orthop. Res. 2016, 34, 1248–1255. [Google Scholar] [CrossRef]
- Tat, J.; Kociolek, A.M.; Keir, P.J. Repetitive Differential Finger Motion Increases Shear Strain between the Flexor Tendon and Subsynovial Connective Tissue. J. Orthop. Res. 2013, 31, 1533–1539. [Google Scholar] [CrossRef]
- Tse, C.T.F.; Keir, P.J. External Compression and Partial Ischemia Decrease Human Finger Flexor Tendon and Subsynovial Connective Tissue Relative Motion. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 1038–1044. [Google Scholar] [CrossRef]
- Tat, J.; Wilson, K.E.; Keir, P.J. Pathological Changes in the Subsynovial Connective Tissue Increase with Self-Reported Carpal Tunnel Syndrome Symptoms. Clin. Biomech. 2015, 30, 360–365. [Google Scholar] [CrossRef]
- Joy, V.; Therimadasamy, A.K.; Chan, Y.C.; Wilder-Smith, E.P. Combined Doppler and B-Mode Sonography in Carpal Tunnel Syndrome. J. Neurol. Sci. 2011, 308, 16–20. [Google Scholar] [CrossRef]
- Motomiya, M.; Funakoshi, T.; Ishizaka, K.; Nishida, M.; Matsui, Y.; Iwasaki, N. Blood Flow Changes in Subsynovial Connective Tissue on Contrast-Enhanced Ultrasonography in Patients with Carpal Tunnel Syndrome before and after Surgical Decompression. J. Ultrasound Med. 2018, 37, 1597–1604. [Google Scholar] [CrossRef]
- Evans, K.D.; Roll, S.C.; Volz, K.R.; Freimer, M. Relationship Between Intraneural Vascular Flow Measured with Sonography and Carpal Tunnel Syndrome Diagnosis Based on Electrodiagnostic Testing. J. Ultrasound Med. 2012, 31, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Ehmke, S.; Farias Zuniga, A.; Keir, P.J. Effect of Force, Posture, and Repetitive Wrist Motion on Intraneural Blood Flow in the Median Nerve. J. Ultrasound Med. 2020, 40, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.E.; Tat, J.; Keir, P.J. Effects of Wrist Posture and Fingertip Force on Median Nerve Blood Flow Velocity. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karahan, A.Y.; Arslan, S.; Ordahan, B.; Bakdik, S.; Ekiz, T. Superb Microvascular Imaging of the Median Nerve in Carpal Tunnel Syndrome: An Electrodiagnostic and Ultrasonographic Study. J. Ultrasound Med. 2018, 37, 2855–2861. [Google Scholar] [CrossRef]
- Seiler, J.G.; Milek, M.A.; Carpenter, G.K.; Swiontkowski, M.F. Intraoperative Assessment of Median Nerve Blood Flow during Carpal Tunnel Release with Laser Doppler Flowmetry. J. Hand Surg. 1989, 14, 986–991. [Google Scholar] [CrossRef]
- Sunderland, S. The Nerve Lesion in the Carpal Tunnel Syndrome. J. Neurol. Neurosurg. Psychiatry 1976, 39, 615–626. [Google Scholar] [CrossRef]
- Guimberteau, J.C. New Ideas in Hand Flexor Tendon Surgery; Sauramps Medical: Maisons-Alfort, France, 2001; ISBN 2-84023-268-5. [Google Scholar]
- Farias Zuniga, A.; Keir, P.J. Thirty Minutes of Sub-Diastolic Blood Flow Occlusion Alters Carpal Tunnel Tissue Function and Mechanics. Ultrasound Med. Biol. 2022, 48, 1110–1121. [Google Scholar] [CrossRef]
- Wong, A.Y.W.; Farias Zuniga, A.; Keir, P.J. Carpal Tunnel Tendon and Sub-Synovial Connective Tissue Mechanics Are Affected by Reduced Venous Return. Clin. Biomech. 2023, 107, 106039. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R 2 from Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2013, 2, 133–142. [Google Scholar] [CrossRef]
- Yoshii, Y.; Zhao, C.; Henderson, J.; Zhao, K.D.; An, K.N.; Amadio, P.C. Velocity-Dependent Changes in the Relative Motion of the Subsynovial Connective Tissue in the Human Carpal Tunnel. J. Orthop. Res. 2011, 29, 62–66. [Google Scholar] [CrossRef]
- Yoshii, Y.; Zhao, C.; Henderson, J.; Zhao, K.D.; Zobitz, M.E.; An, K.-N.; Amadio, P.C. Effects of Carpal Tunnel Release on the Relative Motion of Tendon, Nerve, and Subsynovial Connective Tissue in a Human Cadaver Model. Clin. Biomech. 2008, 23, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Zhao, C.; Zhao, K.D.; Zobitz, M.E.; An, K.N.; Amadio, P.C. The Effect of Wrist Position on the Relative Motion of Tendon, Nerve, and Subsynovial Connective Tissue within the Carpal Tunnel in a Human Cadaver Model. J. Orthop. Res. 2008, 26, 1153–1158. [Google Scholar] [CrossRef]
- Gabra, J.N.; Gordon, J.L.; Marquardt, T.L.; Li, Z.M. In Vivo Tissue Interaction between the Transverse Carpal Ligament and Finger Flexor Tendons. Med. Eng. Phys. 2016, 38, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- An, K.-N. Tendon Excursion and Gliding: Clinical Impacts from Humble Concepts. J. Biomech. 2007, 40, 713–718. [Google Scholar] [CrossRef]
- Korstanje, J.-W.H.; Scheltens-De Boer, M.; Blok, J.H.; Amadio, P.C.; Hovius, S.E.; Stam, H.J.; Selles, R.W. Ultrasonographic Assessment of Longitudinal Median Nerve and Hand Flexor Tendon Dynamics in Carpal Tunnel Syndrome. Muscle Nerve 2012, 45, 721–729. [Google Scholar] [CrossRef]
- van Beek, N.; Gijsbertse, K.; Selles, R.W.; de Korte, C.L.; Veeger, D.J.; Stegeman, D.F.; Maas, H. Tendon Displacements during Voluntary and Involuntary Finger Movements. J. Biomech. 2018, 67, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Filius, A.; Thoreson, A.R.; Ozasa, Y.; An, K.N.; Zhao, C.; Amadio, P.C. Delineation of the Mechanisms of Tendon Gliding Resistance within the Carpal Tunnel. Clin. Biomech. 2017, 41, 48–53. [Google Scholar] [CrossRef]
- Filius, A.; Thoreson, A.R.; Wang, Y.; Passe, S.M.; Zhao, C.; An, K.-N.; Amadio, P.C. The Effect of Tendon Excursion Velocity on Longitudinal Median Nerve Displacement: Differences Between Carpal Tunnel Syndrome Patients and Controls. J. Orthop. Res. 2015, 33, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Villarraga, H.R.; Henderson, J.; Zhao, C.; An, K.-N.; Amadio, P.C. Speckle Tracking Ultrasound for Assessment of the Relative Motion of Flexor Tendon and Subsynovial Connective Tissue in the Human Carpal Tunnel. Ultrasound Med. Biol. 2009, 35, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Zhao, C.; Henderson, J.; Zhao, K.D.; An, K.-N.; Amadio, P.C. Shear Strain and Motion of the Subsynovial Connective Tissue and Median Nerve During Single Digit Motion. J. Hand Surg. Am. 2009, 34, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ettema, A.M.; An, K.-N.; Zhao, C.; O’Byrne, M.M.; Amadio, P.C. Flexor Tendon and Synovial Gliding During Simultaneous and Single Digit Flexion in Idiopathic Carpal Tunnel Syndrome. J. Biomech. 2008, 41, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ugbolue, U.C.; Hsu, W.-H.; Goitz, R.J.; Li, Z.-M. Tendon and Nerve Displacement at the Wrist during Finger Movements. Clin. Biomech. 2005, 20, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rempel, D.; Keir, P.J.; Smutz, W.P.; Hargens, A. Effects of Static Fingertip Loading on Carpal Tunnel Pressure. J. Bone Jt. Surg. 1997, 15, 422–426. [Google Scholar] [CrossRef]
- Gupta, R.; Gray, M.; Chao, T.; Bear, D.; Modafferi, E.; Mozaffar, T. Schwann Cells Upregulate Vascular Endothelial Growth Factor Secondary to Chronic Nerve Compression Injury. Muscle Nerve 2005, 31, 452–460. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghasemi-Rad, M.; Mladkova-Suchy, N.; Ansari, S. Correlation between the Severity of Carpal Tunnel Syndrome and Color Doppler Sonography Findings. Am. J. Roentgenol. 2012, 198, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, G.A.K.B.; Meys, V.E.W.H.; Beekman, R. Doppler Sonography for the Diagnosis of Carpal Tunnel Syndrome: A Critical Review. Muscle Nerve 2014, 50, 159–163. [Google Scholar] [CrossRef]
- Lundborg, G.; Gelberman, R.H.; Minteer-Convery, M.; Lee, Y.F.; Hargens, A.R. Median Nerve Compression in the Carpal Tunnel—Functional Response to Experimentally Induced Controlled Pressure. J. Hand Surg. 1982, 7, 252–259. [Google Scholar] [CrossRef]
- Diao, E.; Shao, F.; Liebenberg, E.; Rempel, D.; Lotz, J.C. Carpal Tunnel Pressure Alters Median Nerve Function in a Dose-Dependent Manner: A Rabbit Model for Carpal Tunnel Syndrome. J. Orthop. Res. 2005, 23, 218–223. [Google Scholar] [CrossRef]
- Festen-Schrier, V.J.M.M.; Amadio, P.C. The Biomechanics of Subsynovial Connective Tissue in Health and Its Role in Carpal Tunnel Syndrome. J. Electromyogr. Kinesiol. 2018, 38, 232–239. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, A.Y.W.; Kociolek, A.M.; Keir, P.J. The Effects of Altered Blood Flow, Force, Wrist Posture, Finger Movement Speed, and Population on Motion and Blood Flow in the Carpal Tunnel: A Mega-Analysis. Biomechanics 2025, 5, 15. https://doi.org/10.3390/biomechanics5010015
Wong AYW, Kociolek AM, Keir PJ. The Effects of Altered Blood Flow, Force, Wrist Posture, Finger Movement Speed, and Population on Motion and Blood Flow in the Carpal Tunnel: A Mega-Analysis. Biomechanics. 2025; 5(1):15. https://doi.org/10.3390/biomechanics5010015
Chicago/Turabian StyleWong, Andrew Y. W., Aaron M. Kociolek, and Peter J. Keir. 2025. "The Effects of Altered Blood Flow, Force, Wrist Posture, Finger Movement Speed, and Population on Motion and Blood Flow in the Carpal Tunnel: A Mega-Analysis" Biomechanics 5, no. 1: 15. https://doi.org/10.3390/biomechanics5010015
APA StyleWong, A. Y. W., Kociolek, A. M., & Keir, P. J. (2025). The Effects of Altered Blood Flow, Force, Wrist Posture, Finger Movement Speed, and Population on Motion and Blood Flow in the Carpal Tunnel: A Mega-Analysis. Biomechanics, 5(1), 15. https://doi.org/10.3390/biomechanics5010015





