1. Introduction
Metastases occur when cancer cells from the primary tumor spread to other organs and tissues, where they subsequently establish and colonize new sites. Certain types of cancer tend to spread to specific regions of the body, which may predominantly affect a specific organ, present organ-specific sequential colonization, or colonize different organs either simultaneously or sequentially. Bones are the third most common site for metastasis in the human body, immediately after the lungs and liver, with the spine being the most frequently affected area, followed by the pelvis, proximal femur, and humerus [
1,
2]. In cases of lesions in the proximal femur, 50% occur in the femoral neck, 30% in the subtrochanteric region, and 20% in the intertrochanteric region [
3].
Bone metastases occur when tumor cells disrupt the normal bone remodeling process, affecting the balance between bone resorption and formation and consequently compromising the bone’s integrity [
4]. In the metastization process, cells from the primary tumor first undergo an epithelial–mesenchymal transition, leading to a loss of cell adhesion and an increase in migratory potential, which promotes the invasion of surrounding tissues and entry into blood vessels (intravasation) [
5]. The cancer cells are then carried through the bloodstream and, if they manage to survive in this environment, they exit the blood vessels and migrate to a pre-metastatic niche formed in the bone marrow specifically prepared for their colonization [
6]. Once established, this niche evolves into a metastatic site where cancer cells interact with bone cells, stimulating osteoclast or osteoblast activity. If these cells stimulate osteoclast activity, excessive bone resorption occurs, resulting in osteolytic metastasis characterized by the destruction of bone tissue. On the other hand, when osteoblast activity is stimulated, it causes uncontrolled bone formation, resulting in osteoblastic metastasis [
6,
7].
The weakening of bones due to the interference of tumor cells in the remodeling process can lead to skeletal-related events, such as bone pain, pathological fractures, hypercalcemia, and compression of the spinal cord and nerve roots, resulting in neuropathic pain [
8,
9]. These events are associated with a loss of mobility, decreased autonomy, increased morbidity, an overall decline in a patient’s quality of life, and mortality [
10,
11].
The therapy applied to patients with bone metastases depends on several factors, including the primary tumor and histology, patient age and general status, response to previous treatments, the number and type of metastases, the location of the lesion, the presence of a pathological or imminent fracture, and patient expected survival [
7,
10]. Most patients exhibit a high number of metastases, leading to an unfavorable prognosis and a significantly reduced life expectancy. Thus, the treatment of multiple bone metastases is palliative, aiming for local control, pain relief, function preservation, and improved overall quality of life and, along with the oncological systemic treatment, expecting to slow the progression of the disease to extend patients’ survival [
7,
12]. Over half of pathological fractures occur in the proximal femur.
Surgical treatment focuses on pain relief and increasing load-bearing capacity. Conventional treatments like radiotherapy are not an option for proximal femur imminent or pathological fractures, leading to growing use of associated minimally invasive options, such as percutaneous cementoplasty and internal fixation with intramedullary nails [
13]. These strategies aim for local control and have been shown to significantly reduce pain and improve function, thereby enhancing patients’ quality of life [
14]. If patients are expected to live longer, tumoral endoprosthesis should be considered instead of internal fixation [
15,
16]. For intra-articular lesions at the proximal femur (femoral neck), palliative treatment requires a cemented hip replacement (arthroplasty).
Percutaneous cementoplasty involves the injection of bone cement, polymethylmethacrylate (PMMA), into an osteolytic lesion to fill and consolidate the bone weakened by the presence of metastases, relieve pain, and minimize local metastatic activity [
17]. Once injected into the lesion, PMMA rapidly polymerizes, producing heat that can induce thermal necrosis at the cement–tumor–bone interface. Excessive heat can lead to thermal necrosis of “healthy” bone tissue cells. Non-oncological studies suggest that to prevent this, the temperature should not exceed 47 °C for more than 1 min [
7,
18]. In addition to the thermal necrosis effect at the cement–tumor–bone interface, the generated heat also causes volumetric changes in the cement, which can induce thermal stresses. For lytic and mixed-type bone metastases, tumor necrosis will allow for local tumor control and inhibit local tumor growth. Besides that, as metastatic disease is systemic, affecting the local bone environment with cementoplasty in these situations as a reinforcement is not to be avoided as in degenerative non-tumoral cases.
In long bones, such as the femur, osteolytic and mixed-type lesions are associated with a high risk of pathological fractures, and cementoplasty should not be used alone, as PMMA is considered resistant to compressive forces but not to torsion forces [
3,
19,
20,
21]. As a result, this technique is combined with internal fixation using long intramedullary nails, and the main goal is to provide structural stabilization and local tumor necrosis to avoid progression, prevent imminent fractures, and stabilize pathological fractures, and to allow patients to be active, preserving quality of life. Additionally, internal fixation with intramedullary nails alone does not provide effective tumor control. Furthermore, pathological fractures should not be expected to have the biological capacity to heal; as nails are only a load-sharing device, in the presence of local tumor progression, this will lead to biomechanical and oncological failure over time if the patient has a longer survival [
7,
22]. For these specific patients, it is extremely important to provide a surgical treatment that preserves patient survival, avoiding any complication or failure during their remaining lifetime [
7,
22]. For this reason, in this study, women over 70 years of age were selected with some clinical problems, such as osteoporosis or other medical conditions, which affect bone loss, determined as critical.
This manuscript focuses on the study of two therapies used in the palliative treatment of multiple bone metastases—cementoplasty alone and cementoplasty combined with internal fixation using intramedullary nails—through thermal and thermomechanical analyses. Metastatic disease and bone metastases are exponentially increasing due to the oncological developments and extended survival of patients. The main objectives of this study involve evaluating the effect of heat resulting from the polymerization process of different types of bone cement (Palacos R®, Heraeus Medical GmbH, Wehrheim, Germany and CMW 3®, DePuy CMW, Raynham, MA, USA) inject ted into metastatic lesions and evaluating the benefits of combining them with internal fixation using intramedullary nails made of titanium (Ti6Al4V) and carbon fiber reinforced with polyetheretherketone (CF-PEEK).
Previous works of the present authors have studied the thermal effects of cement polymerization in the palliative treatments mentioned above [
14]. In this paper, as the main contribution, we went further and studied the thermomechanical effects of cement polymerization, focusing on the resulting stress fields and their implications for bone integrity.
This study aims to virtually predict the thermal and thermomechanical effect of cementoplasty when combined with intramedullary nailing for extra-articular osteolytic bone metastases at the proximal femur, comparing different materials, to analyze the benefits of this minimally invasive treatment.
2. Materials and Methods
Complex real-life problems can often be represented by mathematical models that can be solved using numerical methods, like the finite element method. In this work, two-dimensional models mimicking femoral metastatic lesions were created by using ANSYS
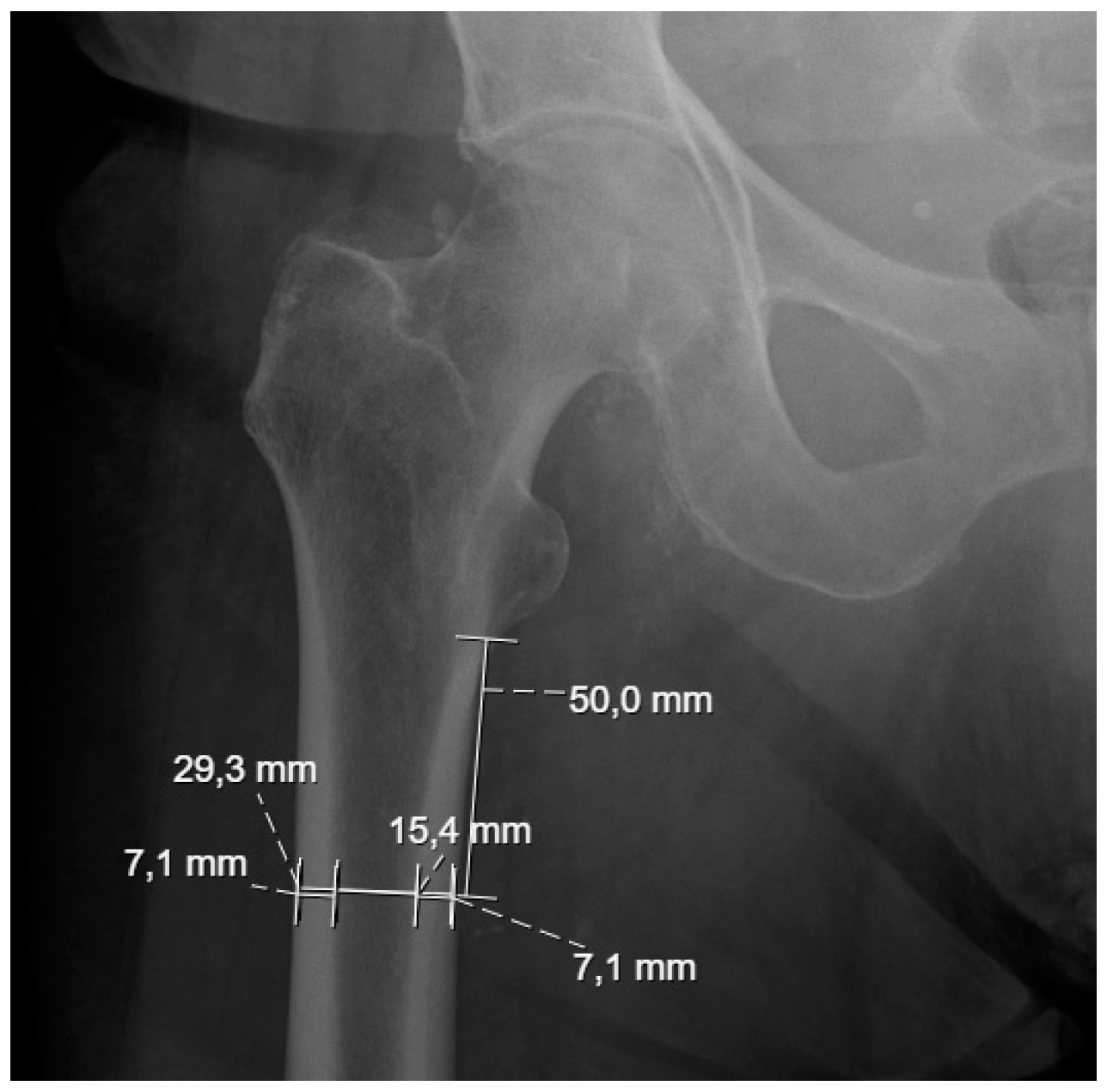
® software version 2024 R2 to evaluate, through thermal and thermomechanical analyses, the effects of different materials in the previously mentioned palliative treatments of bone metastases. The geometries were built using the average dimensions of the subtrochanteric region of 20 non-tumoral patients from the Centro Hospitalar Universitário de Santo António ULSSA (Porto), obtained from conventional pelvic radiographs, as shown in
Figure 1.
To build the numerical models, the patients were divided by gender, as shown in
Table 1. Each gender was segmented by age group, splitting those under and over 70 years old, which allowed for the creation of four distinct geometries from the average measurements resulting from the external bone diameter (DE) and internal bone diameter (DI). This ensured a more comprehensive representation of the physical and anatomical characteristics associated with gender and age. The data in
Table 1 were obtained from conventional pelvic radiographs of 20 non-tumor patients (10 female and 10 male) at the Centro Hospitalar do Porto. At 50 mm from the lesser trochanter, measurements were taken of the internal and external cortical thickness, the diameter of the cancellous bone (corresponding to 15.4 mm in
Figure 1), and the total femur diameter (29.3 mm in
Figure 1). Each measured value presented in
Table 1 was obtained from different measurements along the cancellous and cortical bone of each subject and represents the mean.
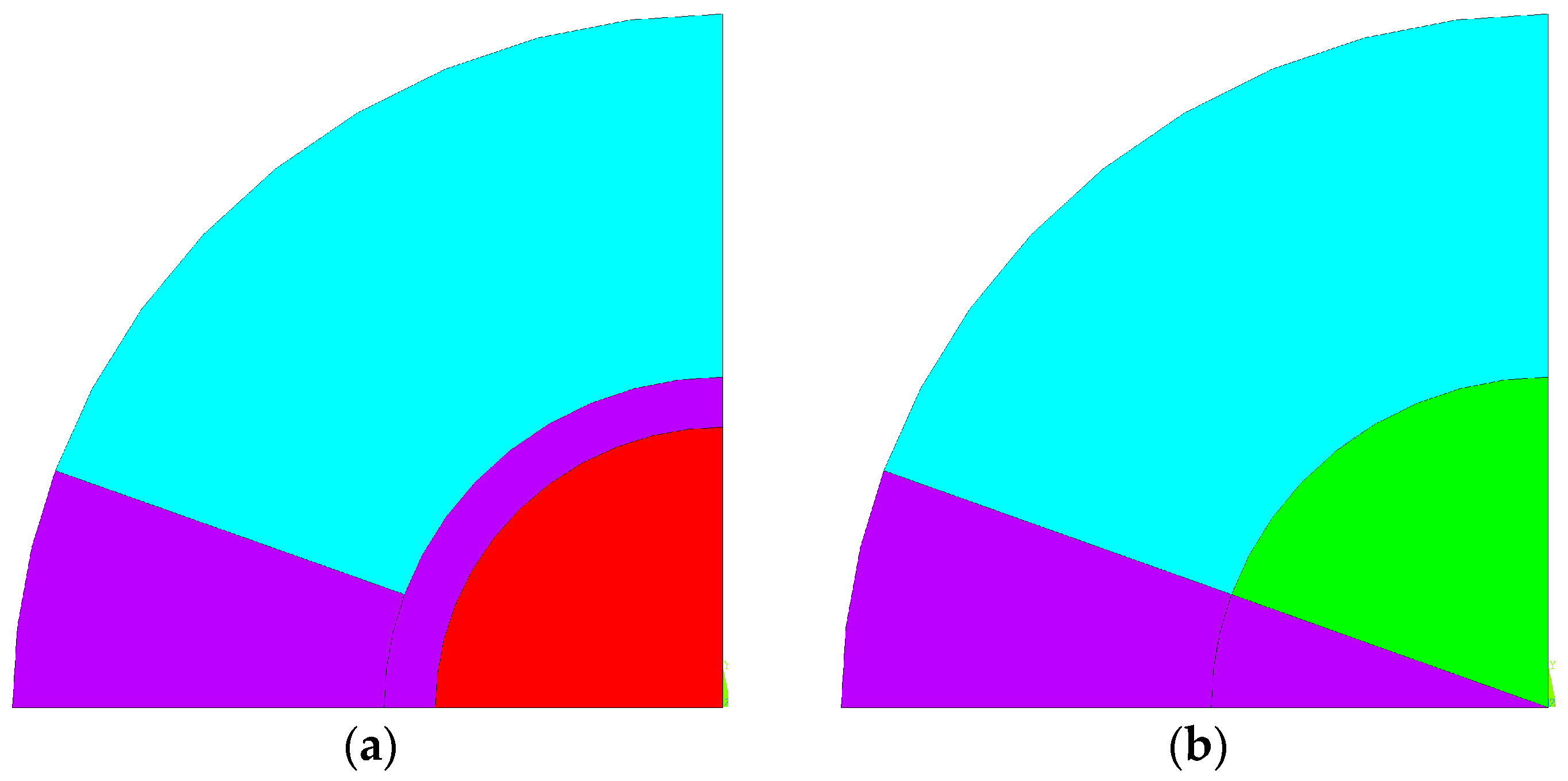
Using the previous dimensions, bone cement was introduced into the bone geometry to fill a metastatic lytic lesion, with an injection angle of 40°. Additionally, another model was developed that incorporated an intramedullary nail with a diameter of 11 mm to compare the effects of cementoplasty alone with those of cementoplasty in combination with internal fixation. These models, resembling a cylinder, allow any cross-sectional plane perpendicular to the cylinder’s axis to produce identical geometric slices along the region where bone cement was injected. This enables the problem to be treated as a plane strain state, where deformations occur only in the xy-plane, and deformation in the third direction is disregarded. To reduce the computational complexity of the solution, simplify visualization, and improve result interpretation, the circular slice was reduced to a single quadrant with symmetrical conditions, resulting in the geometries shown in
Figure 2.
Figure 2a,b represent the virtual numerical models with and without the intramedullary nail, respectively. Cortical tissue is represented in cyan, while cancellous tissue is shown in green. The purple zone indicates the area where bone cement was injected, and the red corresponds to the intramedullary nail. When cementoplasty is combined with internal fixation, the intramedullary nail is inserted into the cancellous bone, and the cement fills the remaining area. For each of the basic geometries related to the average dimensions, six thermal analyses and six thermomechanical analyses were carried out, using different materials. To evaluate the thermal behavior of the system during the PMMA polymerization process, a 2D thermal element (PLANE 77) with 8 nodes was used, where each node has a single degree of freedom, the temperature. All materials were considered isotropic in terms of their thermal properties, which are assumed to remain constant throughout the process. The thermal properties of each material are presented in
Table 2 and were sourced from the literature [
7,
23,
24,
25,
26,
27,
28,
29,
30]. Nevertheless, it is important to highlight that the material properties of degraded or osteolytic bone may differ from healthy bone tissue, which is presented in this table.
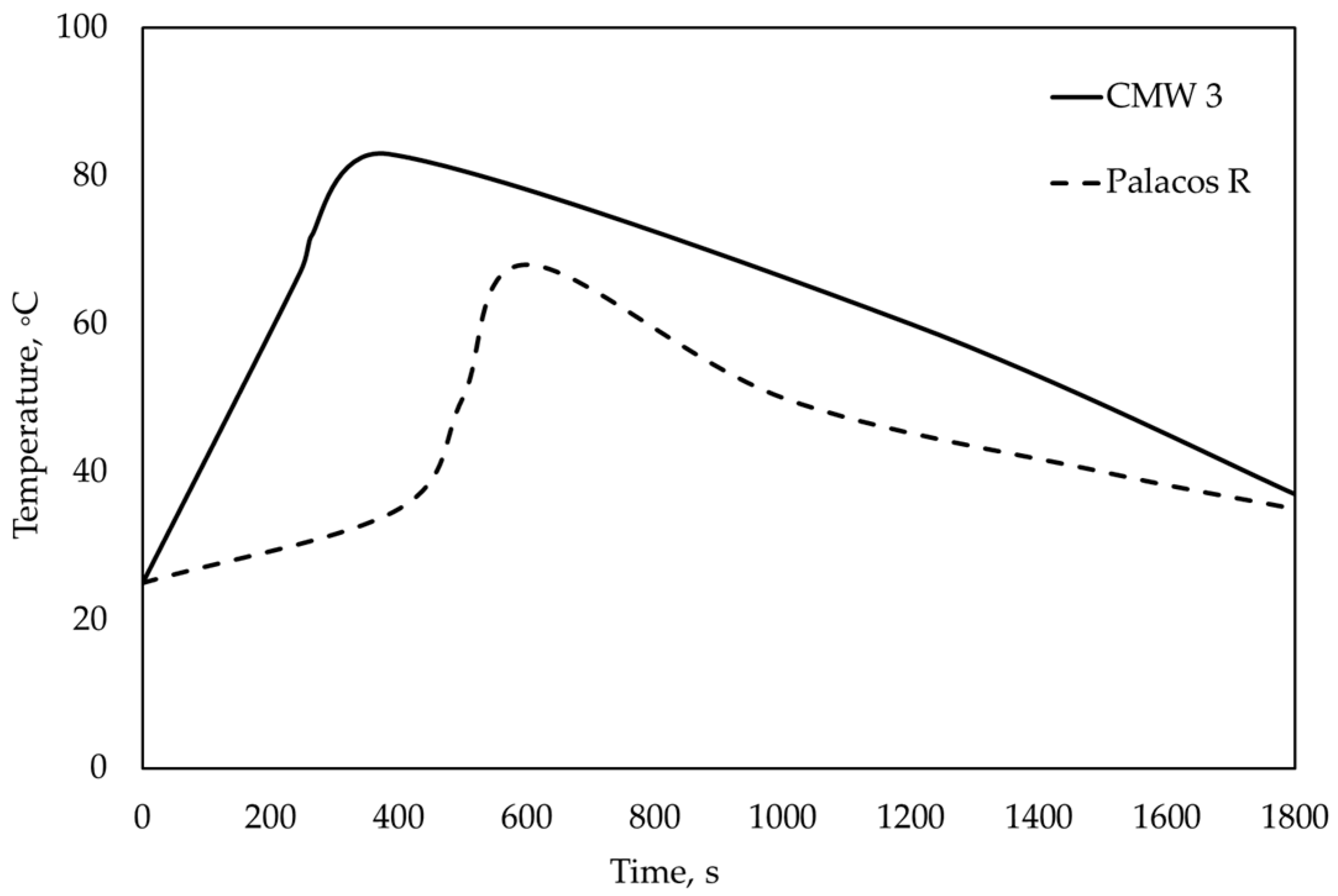
In this analysis, the initial temperature was 37 °C, corresponding to the normal human body temperature. The imposed boundary condition was the polymerization curve of bone cement, illustrated in
Figure 3, making it a thermal boundary with a prescribed temperature. These curves were constructed based on temperature data recorded during the polymerization process of Palacos R
® and CMW 3
® sourced from the literature [
7].
Table 3 represents 24 models which are used in the thermal and thermomechanical analyses, resulting in a total of 48 virtual models. Thermal and thermomechanical analyses will determine the effects produced by the polymerization of two types of PMMA bone cement with different viscosities: Palacos R
® and CMW 3
®. Virtual simulations were conducted with different materials for models incorporating an intramedullary nail, either Ti6Al4V or CF-PEEK.
Using the acquired temperature field, a thermomechanical analysis was performed to determine the resulting stress field. For this analysis, PLANE77 was replaced by an equivalent structural element, PLANE183, with 8 nodes, which has 2 degrees of freedom at each node: translations in the x and y directions. The boundary conditions consist of restrictions on displacement in the x and y directions. The mechanical properties required for this analysis are shown in
Table 4 and are available in the literature [
7,
23,
24,
25,
26,
27,
28,
29,
30].
To create the mesh, it was necessary to identify an element size that would provide stable numerical results when using an element edge with a dimension of 0.25 mm, as shown in
Figure 4.
3. Results and Discussion of Thermal and Thermomechanical Analyses
For each of the basic geometries under study, six thermal analyses and six thermomechanical analyses were carried out to determine the effect of bone cement polymerization. Still, for each type of cement, simulations were carried out in models with Ti and CF-PEEK intramedullary nails and without nails. Based on the results obtained, four types of evaluations were carried out and are presented as follows: 1—the influence of the geometry used; 2—the comparison between the two types of bone cement; 3—the influence of different intramedullary nails in the same type of bone cement; 4—the analysis of the effect of stress shielding produced by the different materials at the border with the cortical bone.
3.1. An Assessment of the Influence of the Geometry Used
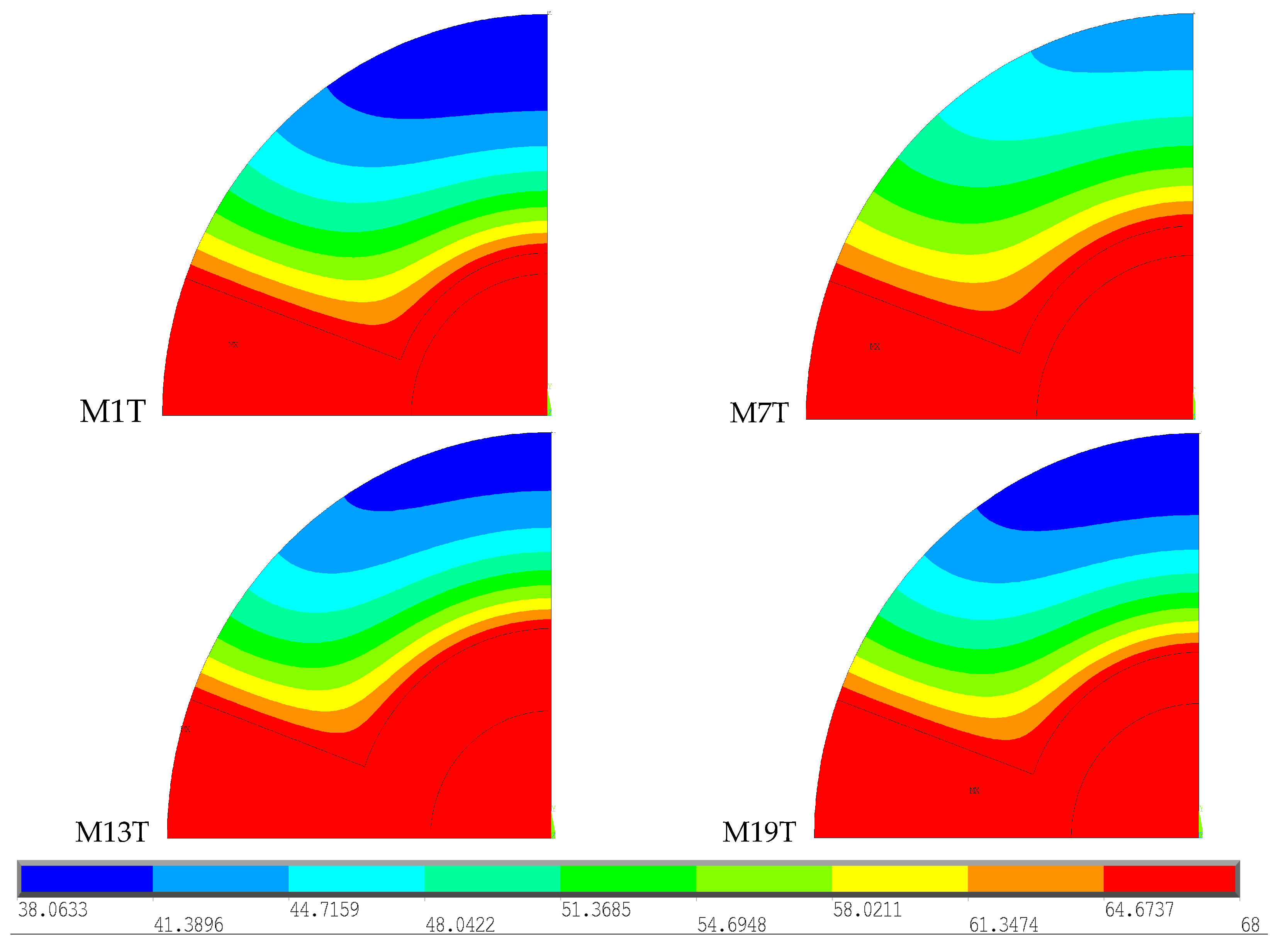
The first evaluation aimed to verify whether the temperature field varies with the gender and age of the patients, particularly female patients older than 70 years old who are expected to have a higher risk of osteopenia and osteoporosis. The 4 basic geometries were compared with the same type of bone cement (Palacos R
®) and with an intramedullary nail (Ti6A14V), represented in
Figure 5.
To facilitate analysis and interpretation of the results, the temperature scale was limited to the range 38–68 °C, the minimum value recorded among the 4 selected models and the polymerization temperature of the Palacos R®, respectively.
The virtual models demonstrate the polymerization effect of Palacos R
® cement after 600 s, when it reaches its maximum temperature. By observing the figures, it is possible to conclude that, in all of them, the temperature distribution in the region of the intramedullary nail and cement is identical, corresponding to the maximum temperature reached. In the cortical bone, the model that presents the greatest extent of higher temperatures, specifically above 47 °C, is M7T, therefore resulting in a greater area of thermal necrosis compared to the other models. M1T exhibits the lowest temperatures at a greater distance from the cement, while the M13T and M19T models demonstrate very similar temperature distributions throughout the cortical bone. M7T is the geometric model with an intramedullary nail made of Ti6A14V (
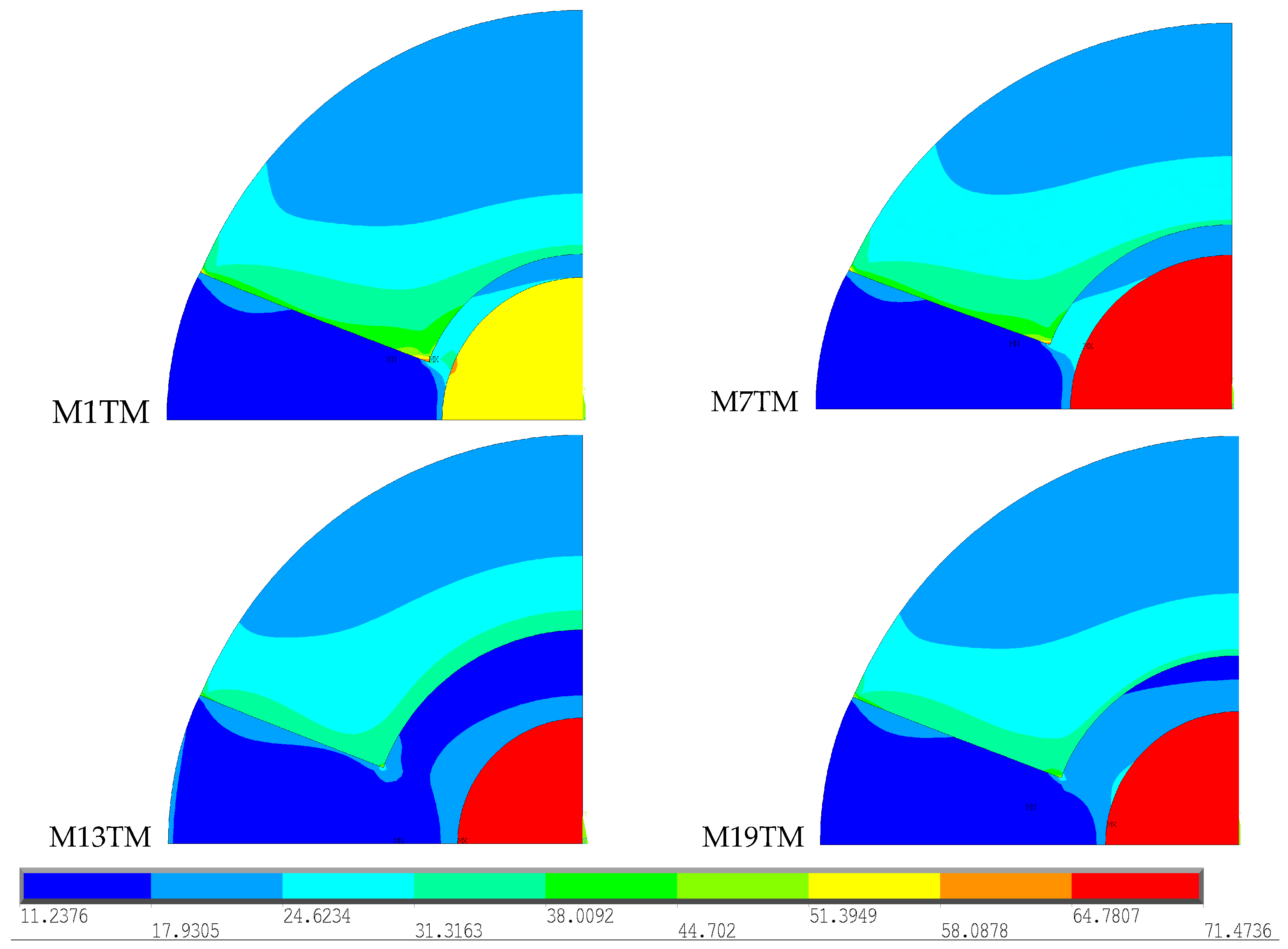
Table 3) with high thermal conductivity and the ability to conduct more heat inside the bone. With the calculation of temperature distribution, comparisons were made to verify whether the obtained thermal stress fields vary depending on the type of geometry used. Four geometries were considered with the same type of bone cement (Palacos R
®) and the same intramedullary nail material (Ti6Al4V), represented in
Figure 6.
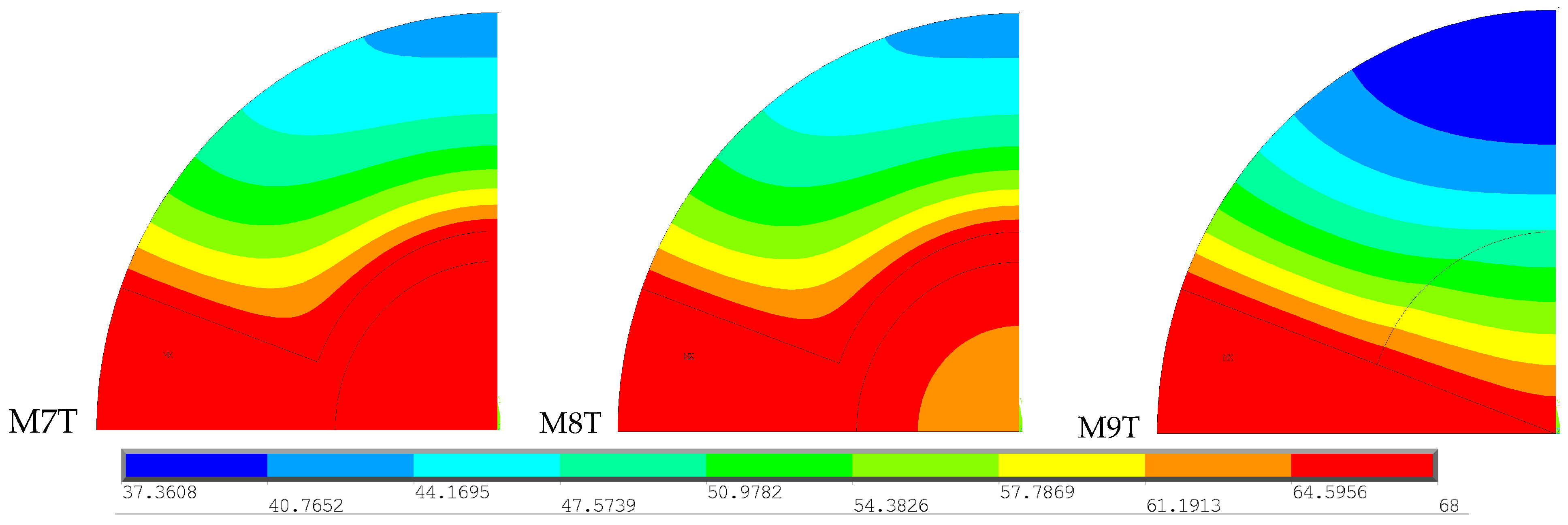
To facilitate the interpretation of the results, the stress range was limited to 11.24 to 71.47 MPa, the minimum and maximum stress recorded across the four models, respectively. These images show that M7TM has the largest area of higher stress values in the cortical bone, which can be attributed to the higher temperature values recorded at a greater distance, as verified in the thermal analysis carried out previously. The remaining models exhibit a very similar stress distribution along the cortical bone. As for the stem region, the M1T model presents significantly lower stresses than the other models, but higher stresses in the cement area.
3.2. Comparison Between Two Types of Bone Cements
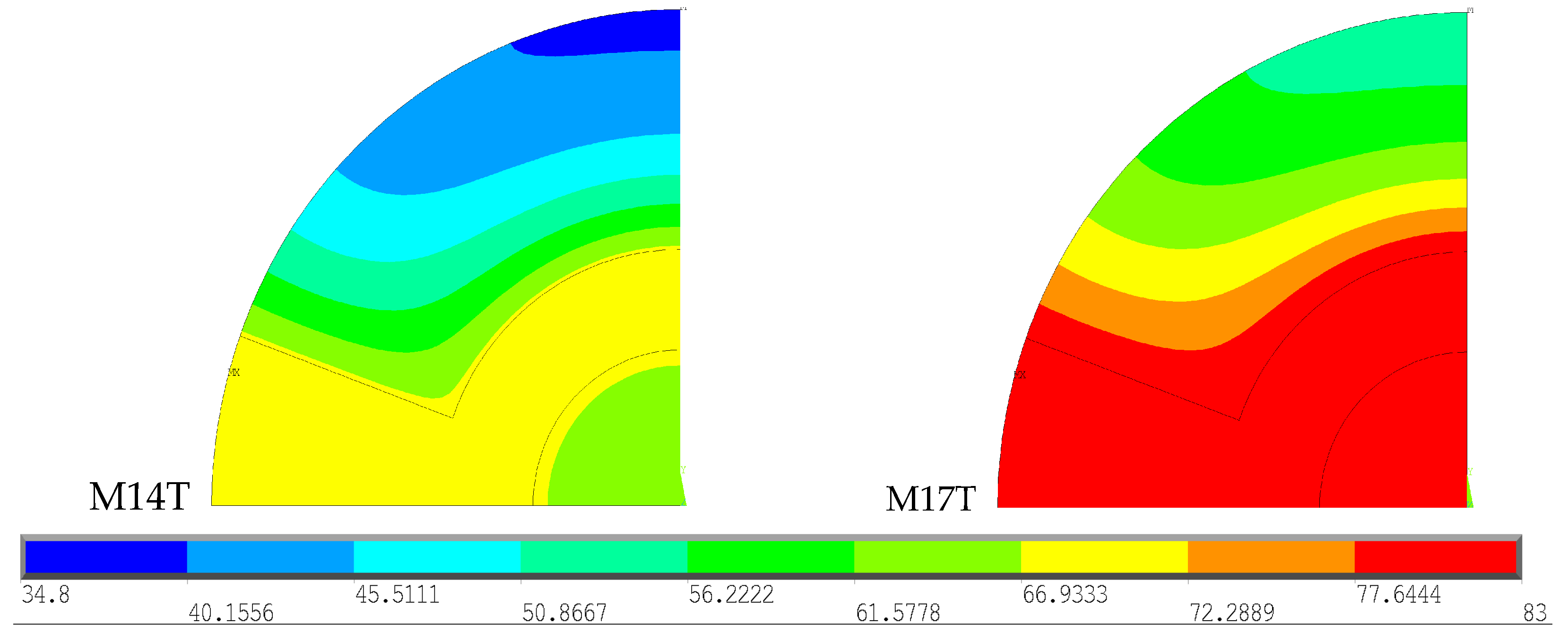
In this analysis, the results obtained for the geometries corresponding to the male gender, under 70 years, with an intramedullary nail made of the same material (CF-PEEK) were compared, varying only the type of bone cement. These results are represented in
Figure 7. To facilitate analysis and interpretation of the results, the temperature scale was limited to the range 34.8–83 °C, the value recorded among the models and the polymerization temperature of the types of cement.
The M14T model demonstrates the effect of Palacos R® polymerization after 600 s, while M17T represents the effect of CMW 3® polymerization after 384 s, corresponding to the point at which each cement reaches its peak temperature. CMW 3® has a higher polymerization temperature than Palacos R®, resulting in the production of a greater amount of heat. The intramedullary nail absorbs part of this heat; therefore, the greater the amount of heat generated, the greater the amount absorbed by the nail, which justifies the higher temperature values of the nail when using CMW 3® cement compared to Palacos R®. Regarding the results obtained in the cortical bone, the use of CMW 3® results in temperatures exceeding 47 °C throughout the cortical bone, causing thermal necrosis throughout its extension. Palacos R®, in contrast, produces temperatures below 47 °C, resulting in a smaller area of thermal necrosis.
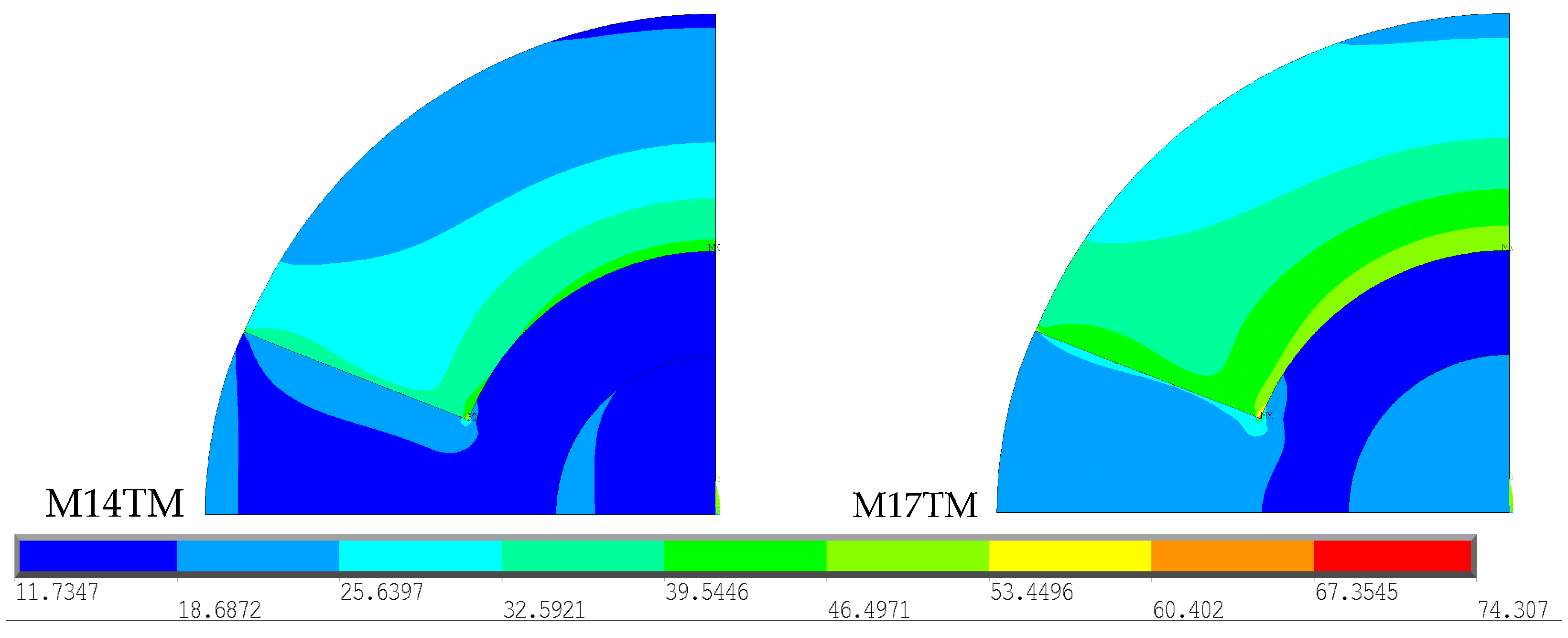
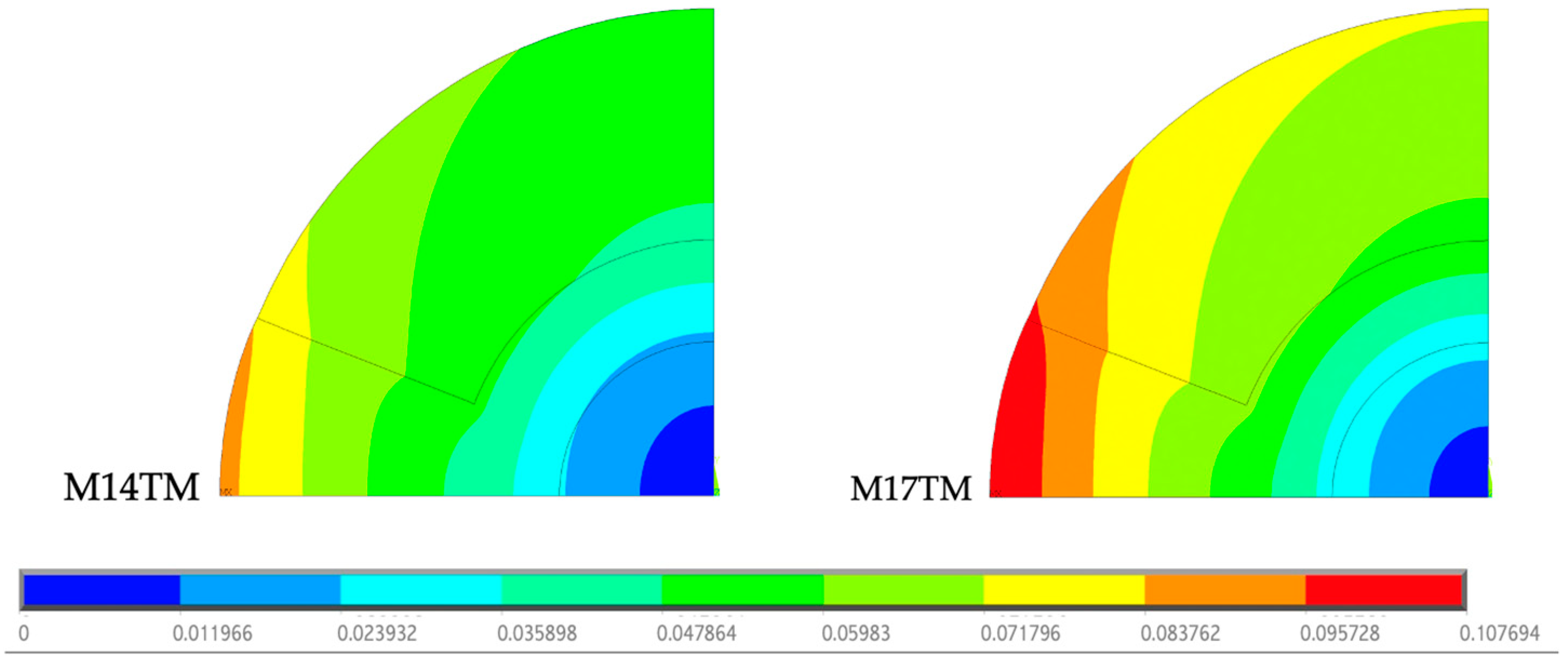
To determine whether the type of cement used causes changes in the thermal stress field obtained, a comparison was made between the two geometries described above, as shown in
Figure 8. For better interpretation and comparison of the results obtained for each type of cement, the stress scale was restricted to the range from 11.73 to 74.31 MPa, the limit values between the models. The model related to the polymerization of CMW 3
® (M17TM) reveals a wider distribution of high stress values when compared to Palacos R
® (M14TM). The polymerization temperature of CMW 3
® is considerably higher than that of Palacos R
®, generating a greater amount of heat, which is subsequently absorbed by the cortical bone, as previously proven.
For each thermal analysis carried out, the respective displacement fields were obtained. The scale presented varies between 0 and 0.1077 mm, representing the minimum and maximum values recorded in the geometries, as shown in
Figure 9.
The displacements observed in M17TM are greater than those in M14TM due to the more significant temperature variations. The greater the displacements, the greater the thermal stress values at a greater distance. In both models, the maximum stress is recorded at the border between the cement and the cortical bone, being superior in M17TM. However, this value is well below the yield stress of the cortical bone, suggesting that there is no risk of yielding, due to thermal loads, for any cement.
3.3. An Assessment of the Influence of Intramedullary Nails with the Same Type of Bone Cement
Other studies were carried out to understand the effects resulting from the presence of an intramedullary nail, as well as the type of material used, on temperature distribution.
Figure 10 presents the results obtained for the geometries corresponding to the female gender, aged 70 years or over, with the same type of bone cement (Palacos R
®), with and without an intramedullary nail. To enable a better analysis of the results, the temperature scale was restricted to the range from 37 to 68 °C, which corresponds to the lowest temperature recorded among the three models and the polymerization temperature of the cement used (Palacos R
®).
These models demonstrate the effect of Palacos R® polymerization after 600 s, when it reaches its peak temperature, revealing a wider distribution of higher temperatures in the cortical bone for the models with a stem (M7T and M8T) compared to the model without a stem (M9T). In models with an intramedullary nail, the injected PMMA is distributed around the stem, contributing to a greater area of cortical bone in contact with the cement. This produces higher temperatures over a greater distance and, consequently, a greater degree of thermal necrosis.
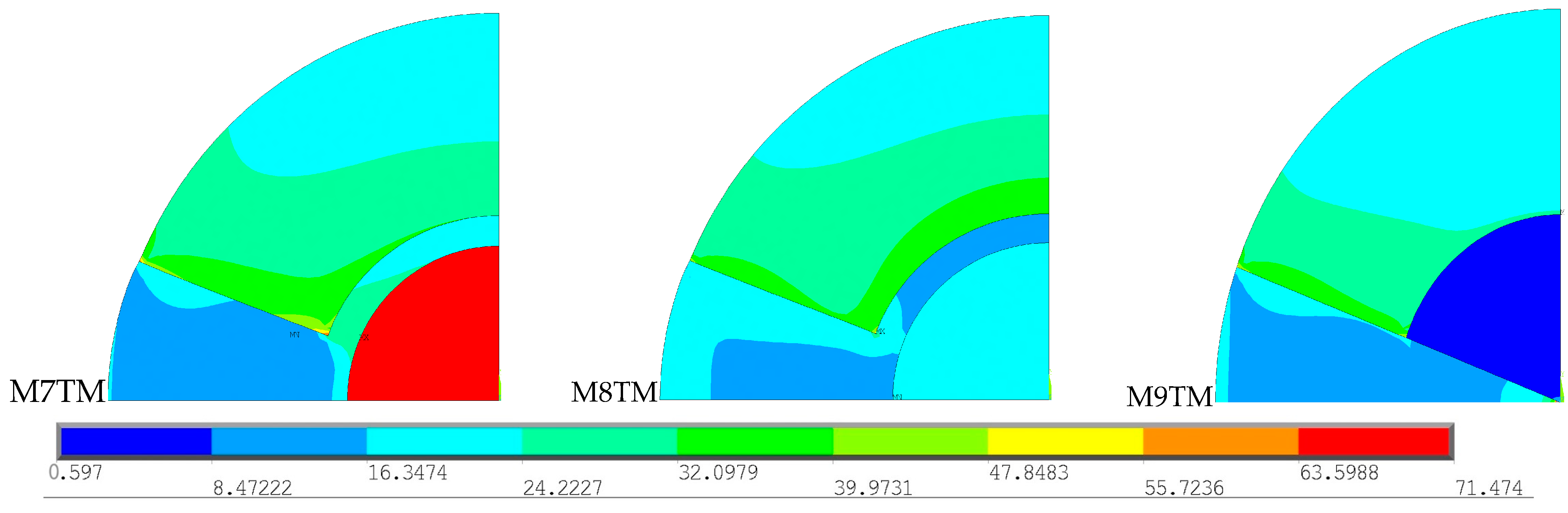
To understand the effects resulting from the presence of an intramedullary nail and the type of material in the thermal stress fields, the three previously analyzed geometries were compared. These results are presented in
Figure 11.
To facilitate the interpretation of the results, the stress scale varied in the range from 0.60 to 71.47 MPa, corresponding to the minimum and maximum stress recorded across the three models. Within the simulations with the same type of cement, there is a wider distribution of higher stress values for the models with intramedullary nails (M7TM and M8TM) compared to the model without intramedullary nails (M9TM). Regarding the stem material, the stress distribution in the cortical bone region is quite similar between the two models, since the amount of heat absorbed by the cortical bone is similar in both models, as previously demonstrated.
On the contrary, the stress values in the nail area exhibit significant differences between the two models. Due to its much lower thermal conductivity compared to Ti6Al4V, CF-PEEK absorbs less heat, resulting in lower stress values in this region. Additionally, the higher stiffness of Ti compared to CF-PEEK further contributes to the distinct stress patterns observed in each intramedullary nail. The stress values recorded in the Ti6Al4V intramedullary nail are significantly higher than in the CF-PEEK intramedullary nail, as the greater the elastic modulus of a material, the greater the stress generated. The lower stress values observed in the model without an intramedullary nail can be justified using the same criteria. Cancellous bone has a very low modulus of elasticity and therefore has stress values lower than those observed in models with a stem. Furthermore, the absence of an intramedullary nail results in a smaller cement area, which contributes to a smaller amount of heat generated and, consequently, a smaller extension of higher stress values.
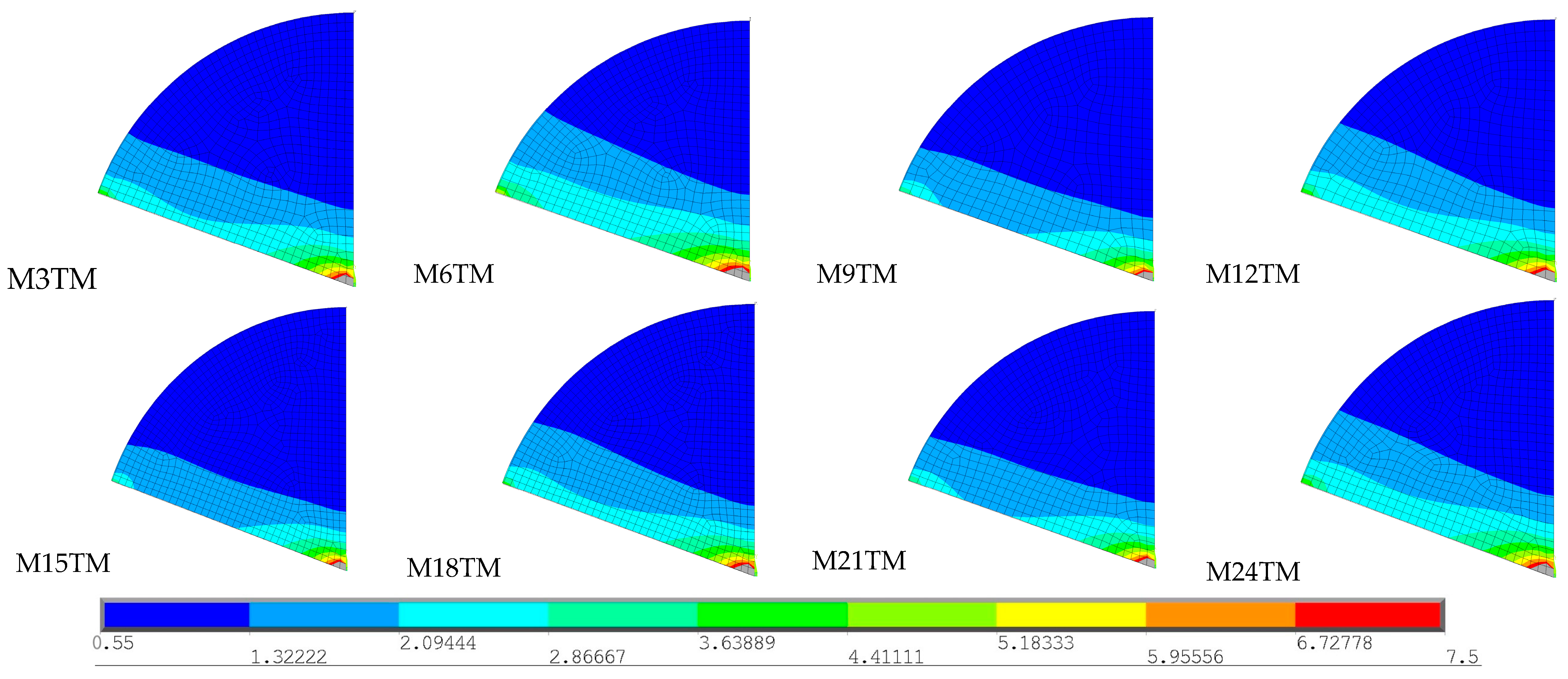
The stress values recorded in most materials are considerably below their yield stresses, which indicates that the thermal loads imposed are not sufficient to induce plastic deformation. The same does not happen with cancellous bone, which has a yield stress of just 7.5 MPa, significantly lower than that of other materials. By observing the images above, it is not possible to determine whether the recorded stresses exceed the yield stress of cancellous bone; therefore, additional analyses were carried out, as represented in
Figure 12.
The images illustrate all simulations conducted without an intramedullary nail for all basic geometries and with both types of bone cement. To determine whether the stress generated by the heat released during the cement polymerization process exceeds the yield stress of cancellous bone, a scale was chosen to vary in the range from 0.55 to 7.5 MPa, which corresponds to the minimum stress recorded in all models and the yield stress of cancellous bone, respectively. In all presented models, this stress was exceeded in the region shown in gray, indicating that the cancellous bone undergoes plastic deformation in this region. The gray area is larger in models with CMW 3® (M6TM, M12TM, M18TM, and M24TM), compared to those with Palacos R® (M1TM, M9TM, M15TM, and M21TM), as greater temperature variation results in greater displacements in cancellous bone and, consequently, higher thermal stress values. These results demonstrate the importance of applying internal fixation in conjunction with percutaneous cementoplasty for preventing pathological fractures.
3.4. Analysis of the Effect of Stress Shielding at the Border with the Cortical Bone
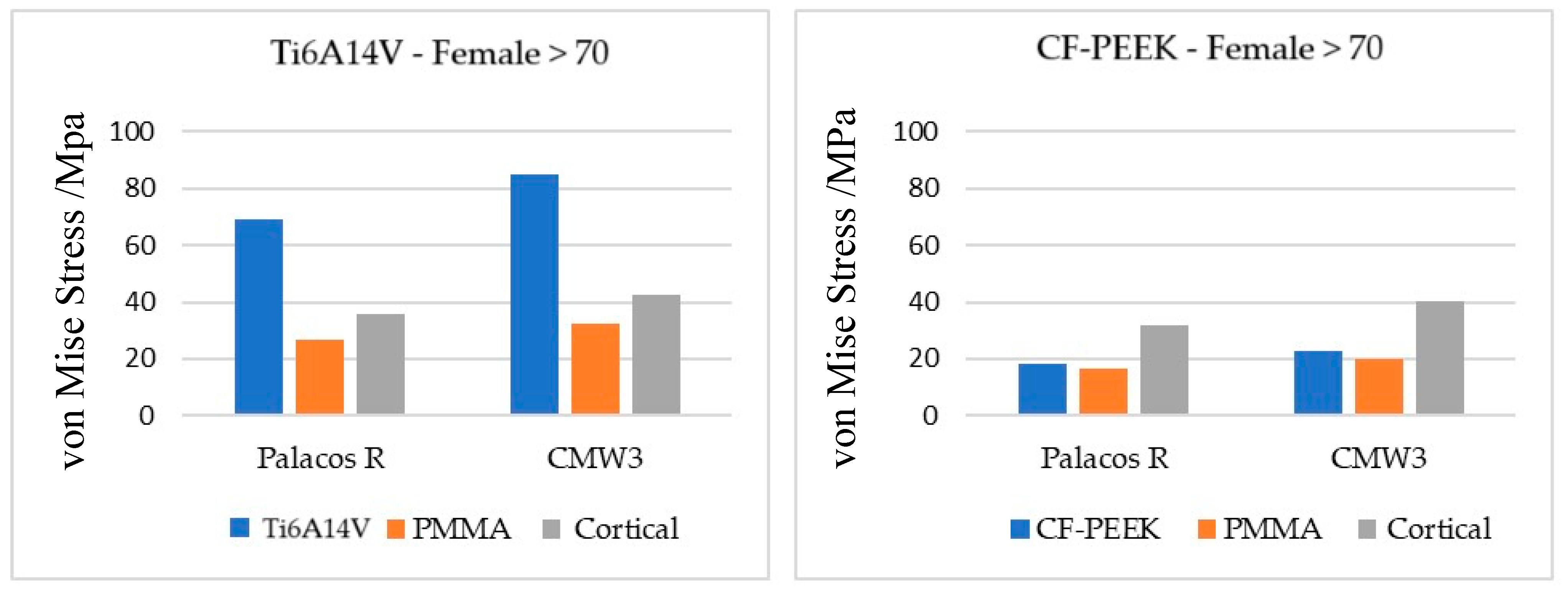
While internal fixation reduces the risk of pathological fractures, it can lead to complications when used alone. One notable issue is the stress shielding effect, caused by the difference in stiffness between the bone and the stem material used, which when significant does not provide effective tumor control or functional improvement. To minimize the stress shielding effect, materials with an elastic modulus closer to that of bone are used, such as intramedullary nails made of CF-PEEK and PMMA, in combination with percutaneous cementoplasty. To understand whether the stress shielding effect observed in this study is sufficiently significant for this type of problem to occur, an analysis of the stress distribution at the material boundaries was carried out for all models with intramedullary nails. The results obtained are divided by gender and age, type of cement, and stem material. The boundary stress distribution follows a similar trend for the same type of bone cement and for the same type of stem material, with only two cases analyzed in more detail.
Figure 13 shows the graphs referring to females aged over 70 years considering each type of cement and stem material.
In all models, the material that demonstrates the lowest stress values is PMMA, as it has the lowest modulus of elasticity. On the other hand, the one with the highest modulus of elasticity is Ti6Al4V, which presents considerably higher stresses than the other materials. The stress values recorded in all materials are higher when using CMW 3® compared to Palacos R®, because more significant temperature changes result in greater displacements and, consequently, greater thermal stresses, as previously proven.
In models with a Ti6A14V stem, for both types of bone cement, the greatest difference in stress is recorded between the stem and PMMA due to the significant disparity in the stiffness of both. In the absence of PMMA, the intramedullary nail would be in direct contact with the cortical bone, leading to a stress difference like the one observed between Ti6Al4V and PMMA. Since PMMA has an elastic modulus closer to that of cortical bone, the difference in stress recorded in the two materials is much smaller, contributing to a more natural stress distribution and thus reducing the effect of stress shielding at the cortical bone interface.
On the other hand, in models with a CF-PEEK intramedullary nail, the greatest disparity in stress values occurs between PMMA and cortical bone. Although the elastic moduli of PMMA, CF-PEEK, and cortical bone are relatively close, the stiffness difference between PMMA and CF-PEEK is less pronounced compared to the difference between PMMA and cortical bone. Therefore, in this case, PMMA does not have the role of stress shielding attenuator. However, the stress difference between PMMA and cortical bone is not sufficient to cause a significant stress shielding effect, which reveals that the impact on the bone remodeling process and the consequent loss of bone mass could be minimal.
4. Conclusions
To carry out thermal and thermomechanical analyses, virtual simulation models were developed using ANSYS® software. These models mimic the therapies used in the palliative treatment of femoral metastatic lesions, making it necessary to define the thermal and mechanical properties of the different materials as well as apply boundary conditions to simulate their behavior and subsequently analyze the effects produced.
The results reinforced the advantages of combining cementoplasty with internal fixation, as opposed to using cementoplasty alone, for the palliative treatment of bone metastases. When applied alone, cementoplasty limits the spread of bone cement to a smaller area, which generates less heat and, consequently, less extension of thermal necrosis in the cortical bone. This makes it difficult to control the local growth of the metastatic lesion, leading to little functional improvement and pain relief.
Furthermore, the absence of an intramedullary nail can cause excessive deformation in some regions of cancellous bone, increasing the risk of pathological fractures, especially for long load-bearing bones such as femurs. Additionally, the use of bone cement reduces the stress shielding effect at the cortical bone interface when Ti6A14V nails are used, minimizing changes in the bone remodeling process and, consequently, the loss of bone mass.
For the combination of cementoplasty and internal fixation to effectively enhance quality of life for patients with bone metastases, it is crucial to locally control tumor growth. A comparison between the two types of intramedullary nail materials, Ti6Al4V and CF-PEEK, revealed similar effects along the cortical bone, exhibiting the same extent of thermal necrosis. Nevertheless, thermal conductivity is responsible for conducting heat inside the model, which is noted in the results with Ti6Al4V. Also, this material, which is denser, tends to be more thermal conductive, transferring heat more quickly.
Regarding bone cement type, this analysis showed that CMW 3® induces a larger area of thermal necrosis due to its higher polymerization temperature and higher specific heat, which requires more energy to increase the temperature.
By producing a larger area of thermal necrosis, CMW 3® provides better control of tumor progression. However, the thermal necrosis produced by CMW 3® extends throughout the cortical bone, which indicates that the volume of cement used is excessive, resulting from a very high injection angle. It is extremely important to go further and optimize bone metastasis treatment for these patients.
As future work, it is suggested to continue this study, namely by obtaining more medical images and analyzing the same variables, with a focus on reducing the bone cement injection angle. The objective is to identify patterns or trends to aid in timely clinical decision-making. Additionally, it would be valuable to extend this virtual study to 3D models incorporating the full anatomical geometry obtained by tomographic images.