The Effects of Midfoot/Hindfoot Fusions on the Behaviour of Peroneus Longus Tendon in Adult-Acquired Flatfoot Deformity: A Biomechanical and Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Meshing
2.2. Tissue Modeling and Boundary Conditions
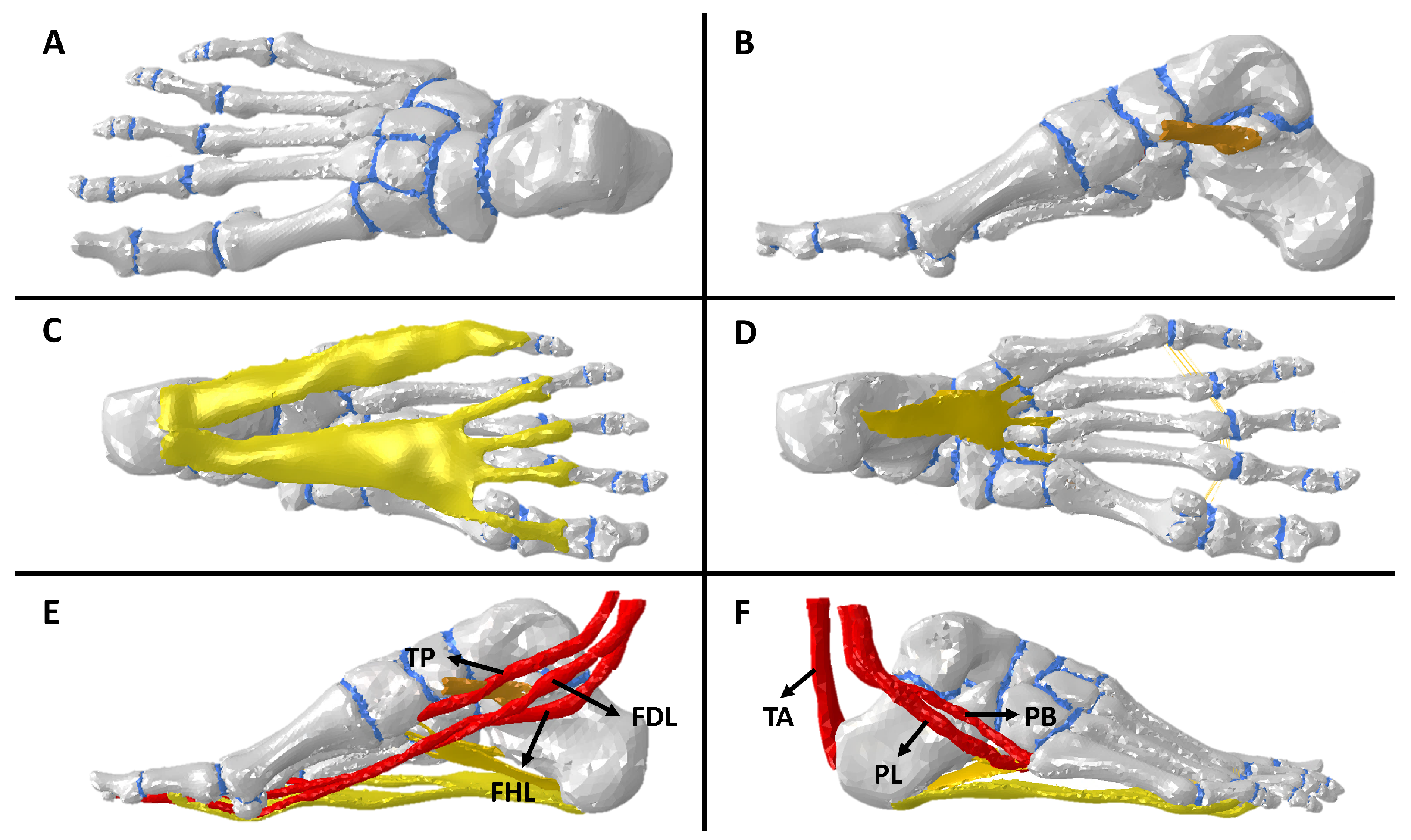
2.2.1. Tissue Modeling
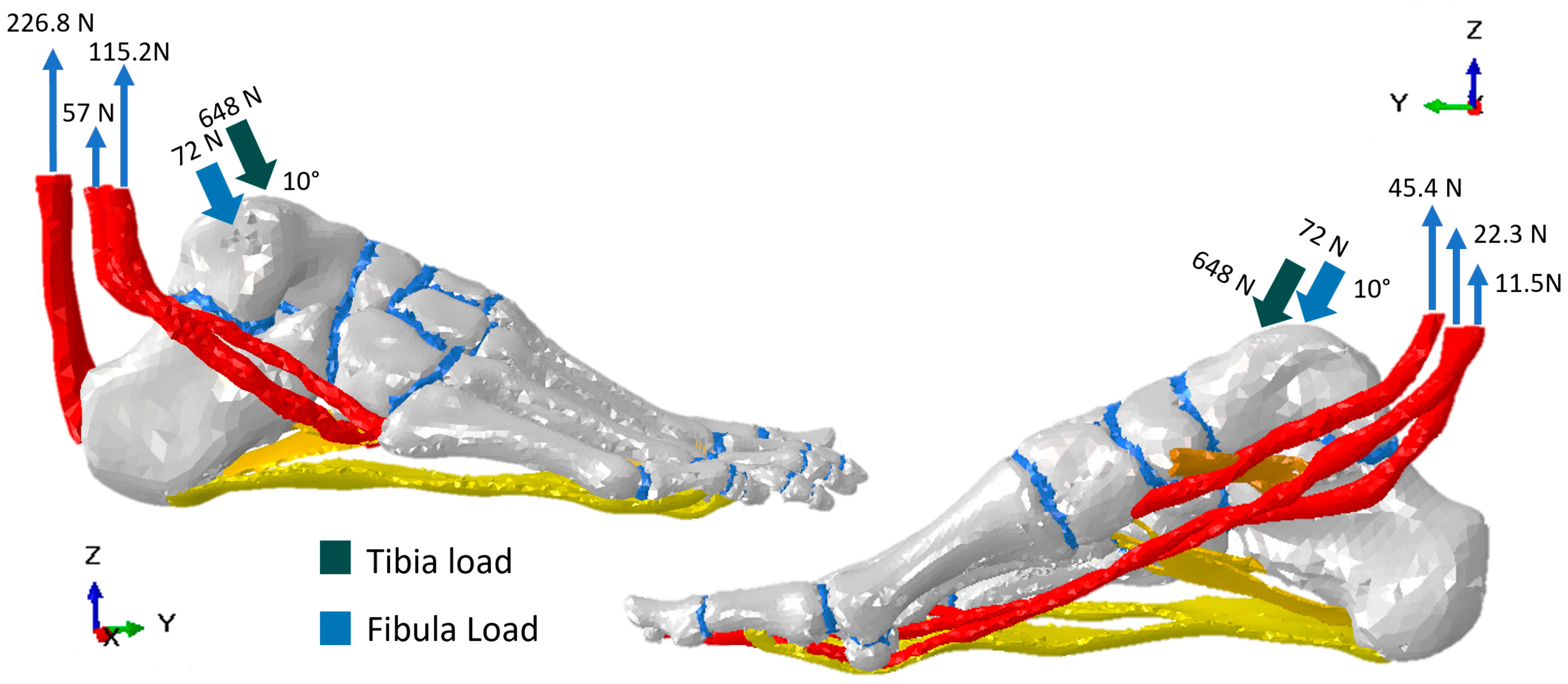
2.2.2. Boundary Conditions
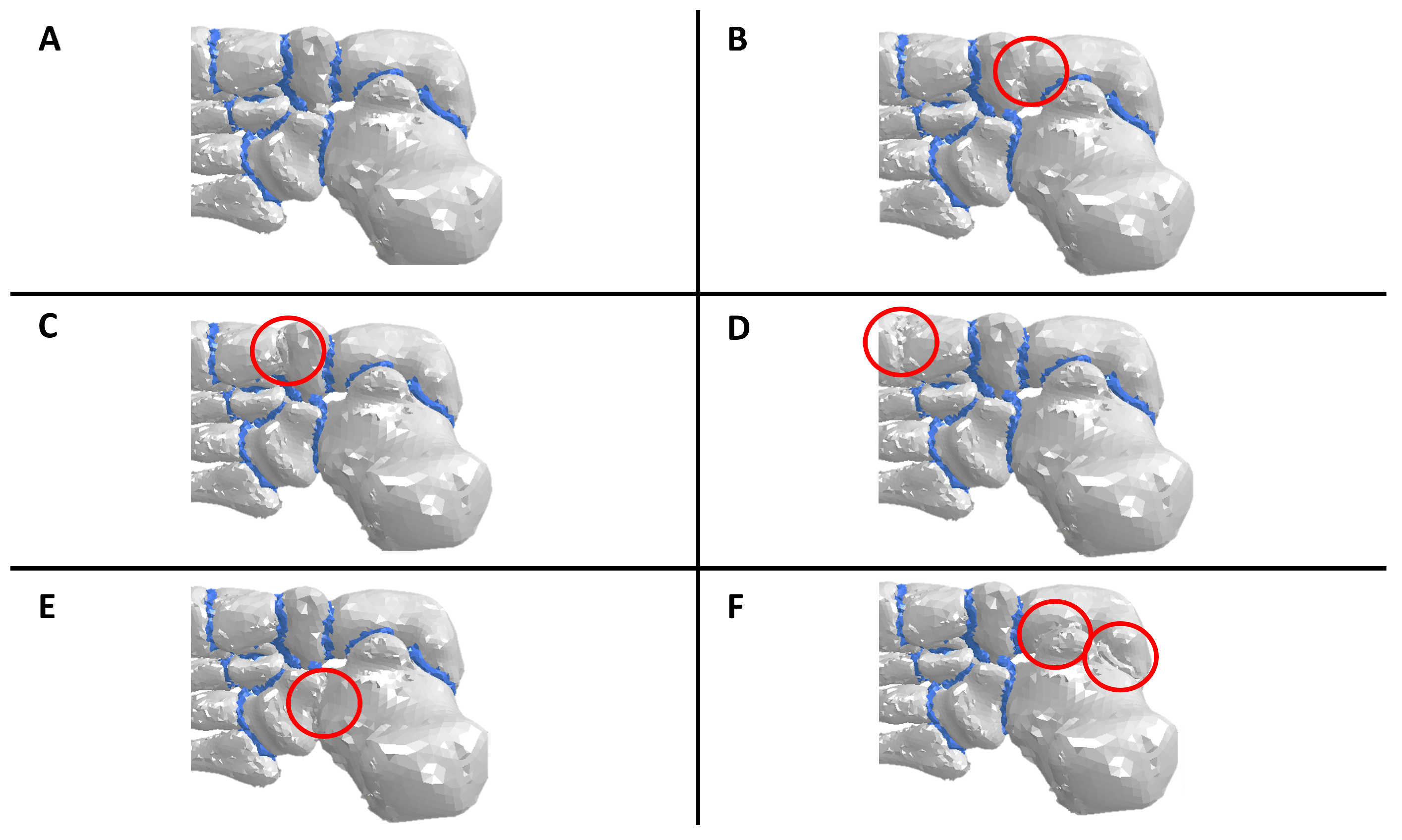
2.3. Flatfoot, Arthrodesis, and Fusion Representations in the Computational Model
2.4. Model Analysis and Evaluation Criteria
2.5. Foot Model Validation
3. Results
3.1. About Model Validation
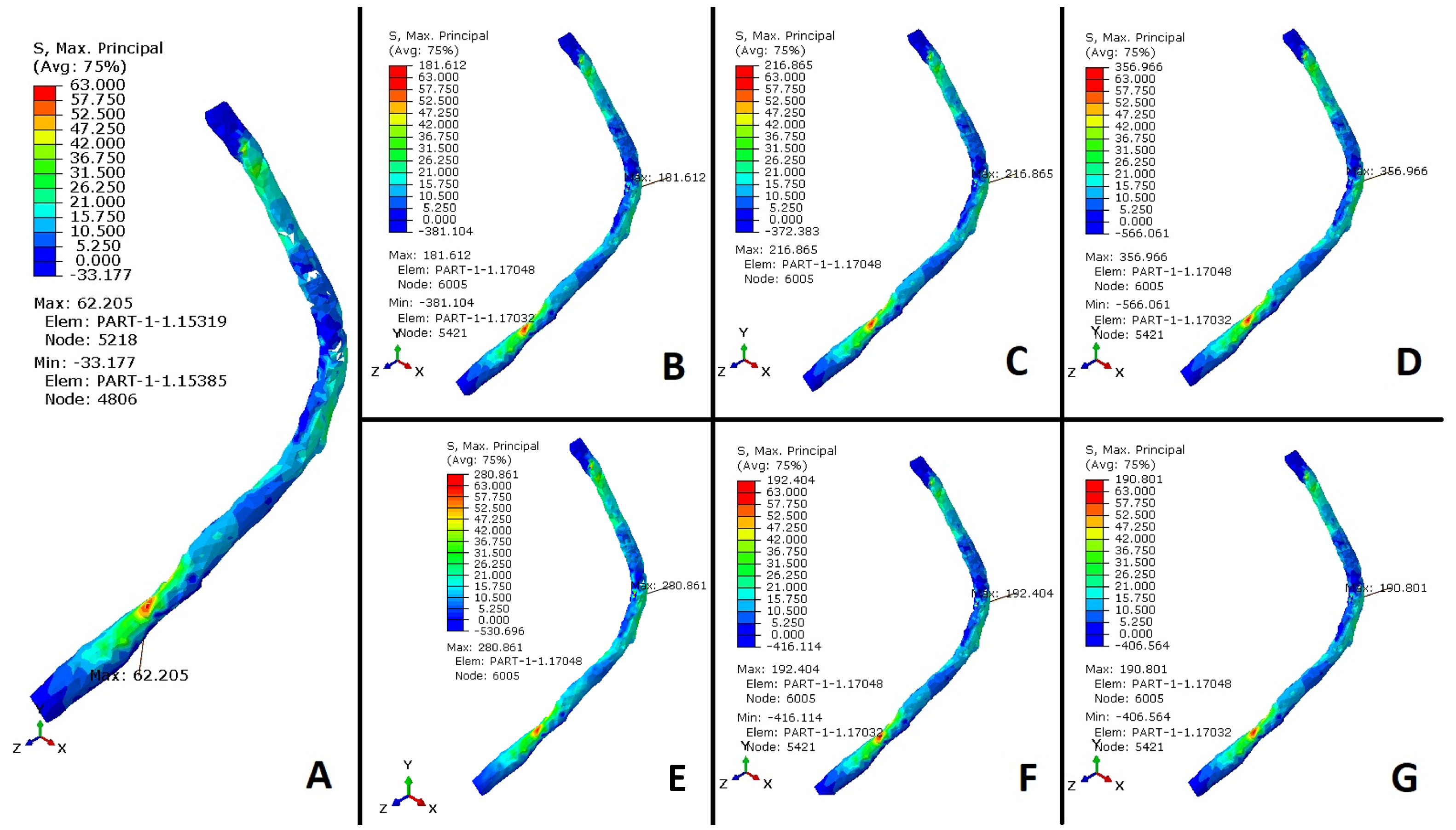
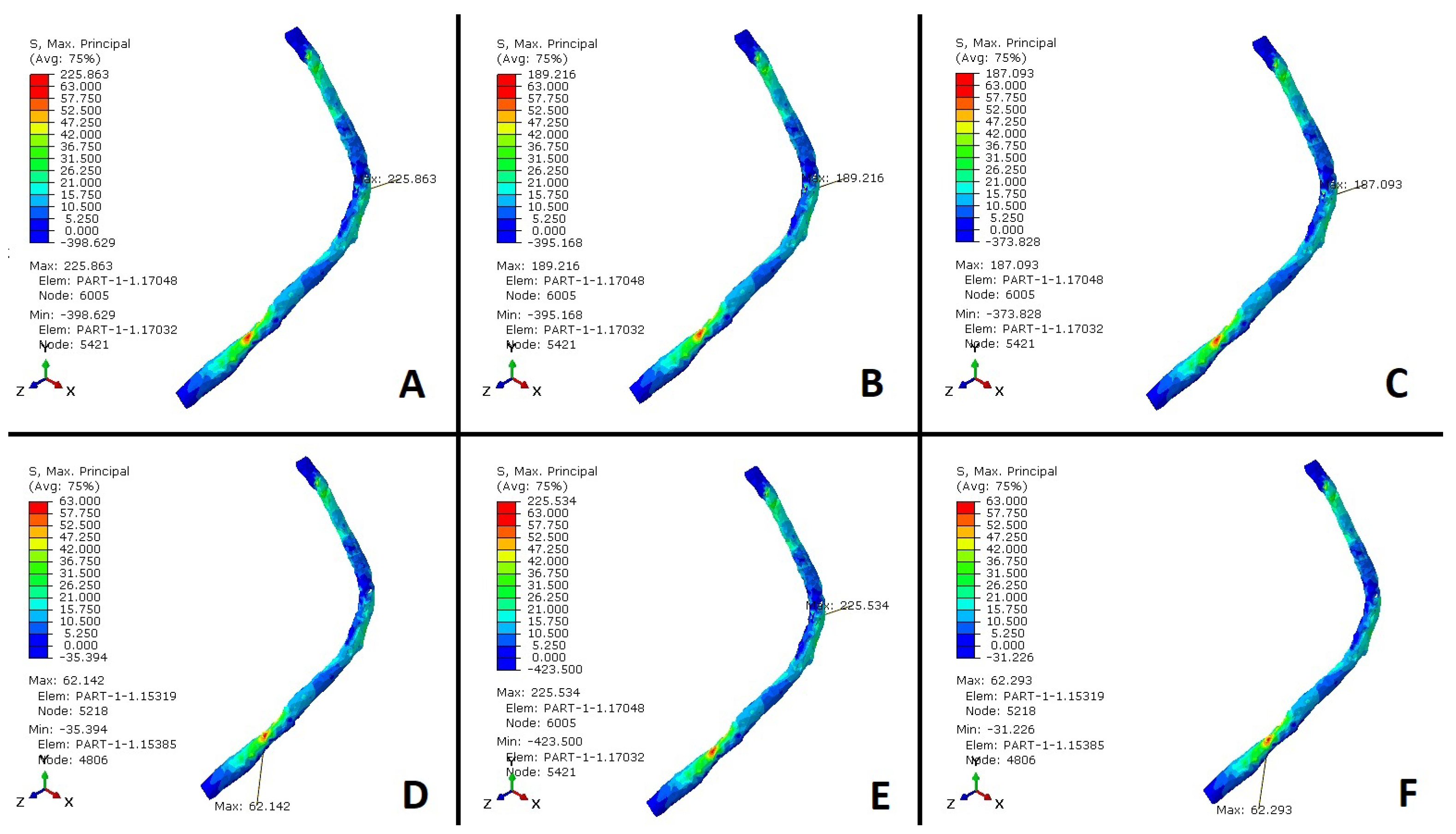
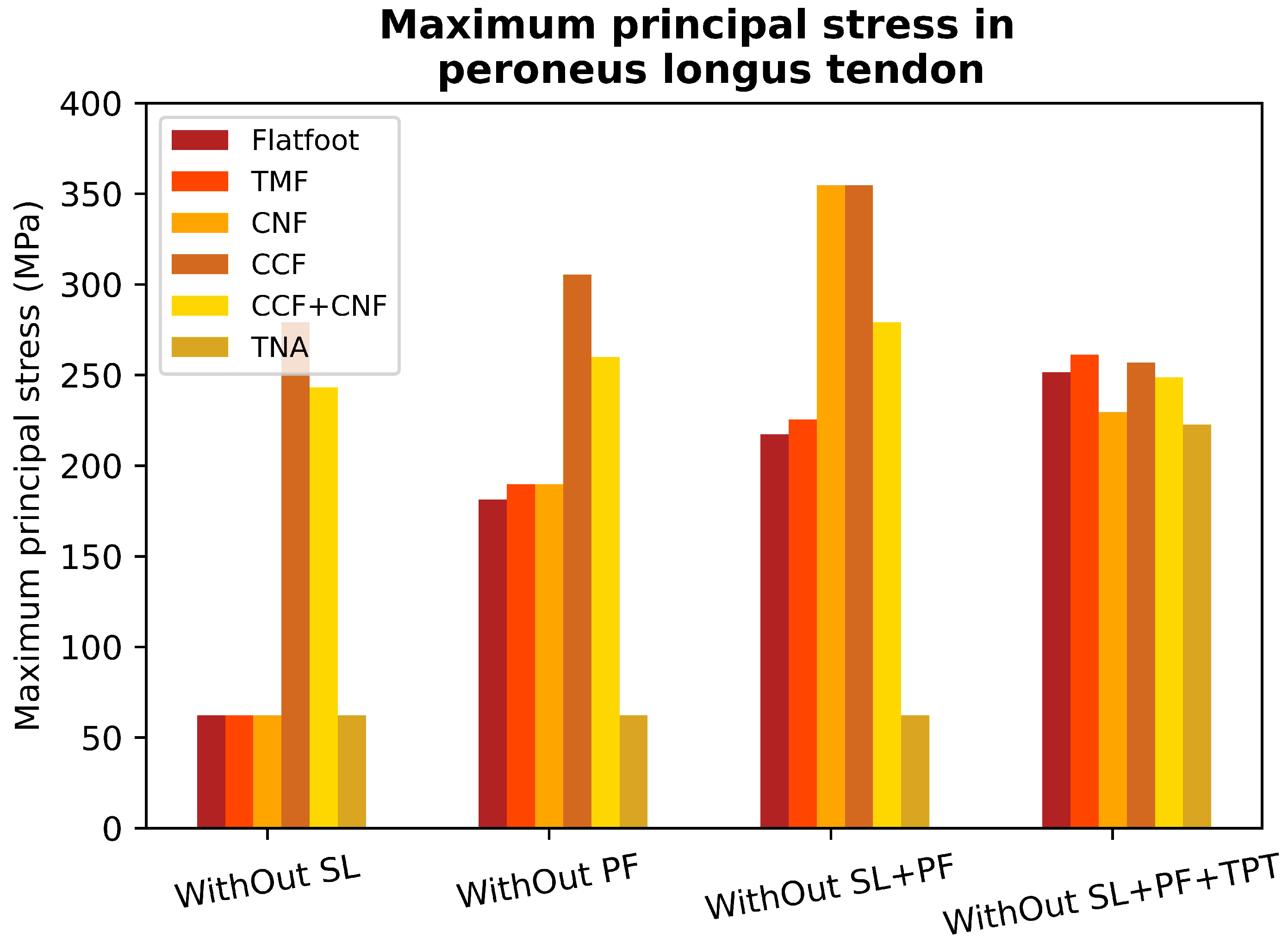
3.2. Peroneus Longus Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAFD | Adult-acquired flatfoot deformity; |
| PF | Plantar fascia; |
| SL | Spring ligament; |
| ATFL | Cavus and anterior talofibular ligament; |
| LPL | Long plantar ligament; |
| SPL | Short plantar ligament; |
| AT | Achilles tendon; |
| PBT | Peroneus brevis tendon; |
| TPT | Tibialis posterior tendon; |
| PLT | Peroneal longus tendon; |
| FDL | Flexor digitorum longus; |
| FHL | Flexor hallucis longus; |
| STA | Subtalar arthrodesis; |
| TNA | Talonavicular arthrodesis; |
| TMF | First tarsometatarsal fusion; |
| CCF | Calcaneocuboid fusion; |
| CNF | Cuneonavicular fusion; |
| FE | Finite element; |
| FL | Foot lengthening; |
| AST | Astragalus; |
| NAV | Navicular; |
| CUN | Cuneiform; |
| MTH1 | First metatarsal head. |
References
- Johnson, K.A.; Strom, D.E. Tibialis posteroir tendon dysfunction. Clin. Orthop. Relat. Res. 1989, 239, 196–206. [Google Scholar] [CrossRef]
- Jennings, M.M.; Christenson, J. The The effects of sectioning the spring ligament on rearfoot stability and posterior tibial tendon efficiency. J. Foot Ankle Surg. 2008, 47, 219–224. [Google Scholar] [CrossRef]
- Cifuentes-De la Portilla, C.; Larrainzar-Garijo, R.; Bayod, J. Analysis of biomechanical stresses caused by hindfoot joint arthrodesis in the treatment of adult acquired flatfoot deformity: A finite element study. Foot Ankle Surg. 2020, 26, 412–420. [Google Scholar] [CrossRef]
- Murley, S.G.; Menz, B.H.C.; Landorf, K.B. Foot posture influences the electromyographic activity of selected lower limb muscles during gait. J. Foot Ankle Res. 2009, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-De la Portilla, C.; Pasapula, C.; Gutiérrez-Narvarte, B.; Larrainzar-Garijo, R.; Bayod, J. Peroneus Longus overload caused by soft tissue deficiencies associated with early adult acquired flatfoot: A finite element analysis. Clin. Biomech. 2021, 86, 105383. [Google Scholar] [CrossRef]
- Burkhart, T.A.; Andrews, D.M.; Dunning, C.E. Finite element modeling mesh quality, energy balance and validation methods: A review with recommendations associated with the modeling of bone tissue. J. Biomech. 2013, 46, 1477–1488. [Google Scholar] [CrossRef]
- Schneider, T.; Hu, Y.; Gao, X.; Dumas, J.; Zorin, D.; Panozzo, D. A large-scale comparison of tetrahedral and hexahedral elements for solving elliptic pdes with the finite element method. ACM Trans. Graph. (TOG) 2022, 41, 1–14. [Google Scholar] [CrossRef]
- García-Aznar, J.M.; Bayod, J.; Rosas, A.; Larrainzar, R.; García-Bógalo, R.; Doblaré, M.; Llanos, L.F. Load transfer mechanism for different metatarsal geometries: A finite element study. J. Biomech. Eng. 2009, 131, 021011. [Google Scholar] [CrossRef]
- Tao, K.; Ji, W.T.; Wang, D.M.; Wang, C.T.; Wang, X. Relative contributions of plantar fascia and ligaments on the arch static stability: A finite element study. Biomed. Eng. Tech. 2010, 55, 265–271. [Google Scholar] [CrossRef]
- Wright, D.G.; Rennels, D.C. A study of the elastic properties of plantar Fascia. J. Bone Jt. Surg. 1964, 46, 482–492. [Google Scholar] [CrossRef]
- Forriol Campos, F. El cartílago articular: Aspectos mecánicos y su repercusión en la reparación tisular. Rev. Ortop. Traumatol. 2002, 380–390. [Google Scholar]
- Mansour, J.M. Biomechanics of cartilage. In Kinesiology: The Mechanics and Pathomechanics of Human Movement; Oxford University Press: Oxford, UK, 2003; pp. 66–79. [Google Scholar]
- Wu, L. Nonlinear finite element analysis for musculoskeletal biomechanics of medial and lateral plantar longitudinal arch of Virtual Chinese Human after plantar ligamentous structure failures. Clin. Biomech. 2007, 22, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Morales Orcajo, E.; Barbosa de las Casas, E.; Bayod López, J. Computational Foot Modeling for Clinical Assessment. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, España, 2015. [Google Scholar]
- Arangio, G.A.; Salathe, E.P. A biomechanical analysis of posterior tibial tendon dysfunction, medial displacement calcaneal osteotomy and flexor digitorum longus transfer in adult acquired flat foot. Clin. Biomech. 2009, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Louwerens, J.W.K.; Linge, B.V.; de Klerk, L.W.; Mulder, P.G.; Snijders, C.J. Peroneus longus and tibialis anterior muscle activity in the stance phase: A quantified electromyographic study of 10 controls and 25 patients with chronic ankle instability. Acta Orthop. Scand. 1995, 66, 517–523. [Google Scholar] [CrossRef]
- Hyer, C.F.; Dawson, J.M.; Philbin, T.M.; Berlet, G.C.; Lee, T.H. The peroneal tubercle: Description, classification, and relevance to peroneus longus tendon pathology. Foot Ankle Int. 2005, 26, 947–950. [Google Scholar] [CrossRef]
- Johnson, C.H.; Christensen, J.C. Biomechanics of the first ray part I. The effects of peroneus longus function: A three-dimensional kinematic study on a cadaver model. J. Foot Ankle Surg. 1999, 38, 313–321. [Google Scholar] [CrossRef]
- Sumal, A.S.; Jarvis, G.E.; Norrish, A.R.; Brassett, C.; Whitaker, R.H. The role of the angle of the fibularis longus tendon in foot arch support. Clin. Anat. 2021, 34, 651–658. [Google Scholar] [CrossRef]
- Pecheva, M.; Devany, A.; Nourallah, B.; Cutts, S.; Pasapula, C. Long-term follow-up of patients undergoing tibialis posterior transfer: Is acquired pes planus a complication? Foot 2018, 34, 83–89. [Google Scholar] [CrossRef]
- Yeap, J.S.; Singh, D.; Birch, R. Tibialis posterior tendon dysfunction: A primary of secondary problem? Foot Ankle Int. 2001, 22, 51–55. [Google Scholar] [CrossRef]
- Pasapula, C.; Ali, A.; Kiliyanpilakkil, B.; Hardcastle, A.; Koundu, M.; Ghaooni, A.; Kabwama, S.; Cutts, S. High Incidence of spring ligament laxity in ankle fractures with complete deltoid ruptures and secondary first ray instabilty. Foot 2021, 46, 101720. [Google Scholar] [CrossRef]
- Chu, I.T.; Myerson, M.S.; Nyska, M.; Parks, B.G. Experimental flatfoot model: The contribution of dynamic loading. Foot Ankle Int. 2001, 22, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.A. Subtalar joint axis location and rotational equilibrium theory of foot function. J. Am. Podiatr. Med. Assoc. 2001, 91, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Ringleb, S.I.; Kavros, S.J.; Kotajarvi, B.R.; Hansen, D.K.; Kitaoka, H.B.; Kaufman, K.R. Changes in gait associated with acute stage II posterior tibial tendon dysfunction. Gait Posture 2007, 25, 555–564. [Google Scholar] [CrossRef]
- Crary, J.L.; Hollis, J.M.; Manoli, A. The effect of plantar fascia release on strain in the spring and long plantar ligaments. Foot Ankle Int. 2003, 24, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, Y.; Qiang, M.; Hao, Y. Effects of five hindfoot arthrodeses on foot and ankle motion: Measurements in cadaver specimens. Sci. Rep. 2016, 6, 35493. [Google Scholar] [CrossRef]
- Huang, C.K.; Kitaoka, H.B.; An, K.N.; Chao, E.Y. Biomechanical evaluation of longitudinal arch stability. Foot Ankle 1993, 14, 353–357. [Google Scholar] [CrossRef]

| Tissue | Mesh Size (mm) | Element Type |
|---|---|---|
| Cortical bone | 5 | Tetrahedral (C3D4) |
| Trabecular bone | 4 | Tetrahedral (C3D4) |
| Plantar fascia | 2 | Tetrahedral (C3D4) |
| Spring ligament | 2 | Tetrahedral (C3D4) |
| Tendons | 3 | Tetrahedral (C3D4) |
| Short plantar ligament | 2 | Tetrahedral (C3D4) |
| Long plantar ligament | 2 | Tetrahedral (C3D4) |
| Cartilages | 1–2 | Tetrahedral (C3D4) |
| Talocalcaneal and metatarsal ligament | Bar element (1D) |
| Quality Metric | Assessment Criteria | Accurate Elements | Inaccurate Elements |
|---|---|---|---|
| Element jacobian | >0.2 | 99.2% | 0.8% |
| Aspect ratio | >0.3 | 95.5% | 4.5% |
| Min Angles | >30° | 97.6% | 2.4% |
| Max Angles | >120° | 98.7% | 1.3% |
| Reference Point | Model Prediction (mm) | Patient Average (mm) | Patient Std. Deviation |
|---|---|---|---|
| T | −0.292 | −0.291 | 0.03 |
| NAV | −0.33 | −0.278 | 0.056 |
| CUN | −0.324 | −0.205 | 0.122 |
| MTH1 | −0.056 | −0.064 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanguma-Muñoz, N.; Quevedo, B.D.S.; Pasapula, C.; Austin, I.; Larrainzar-Garijo, R.; Bayod, J.; Cifuentes-De la Portilla, C. The Effects of Midfoot/Hindfoot Fusions on the Behaviour of Peroneus Longus Tendon in Adult-Acquired Flatfoot Deformity: A Biomechanical and Finite Element Analysis. Biomechanics 2024, 4, 494-506. https://doi.org/10.3390/biomechanics4030035
Yanguma-Muñoz N, Quevedo BDS, Pasapula C, Austin I, Larrainzar-Garijo R, Bayod J, Cifuentes-De la Portilla C. The Effects of Midfoot/Hindfoot Fusions on the Behaviour of Peroneus Longus Tendon in Adult-Acquired Flatfoot Deformity: A Biomechanical and Finite Element Analysis. Biomechanics. 2024; 4(3):494-506. https://doi.org/10.3390/biomechanics4030035
Chicago/Turabian StyleYanguma-Muñoz, Nicolás, Brayan David Solorzano Quevedo, Chandra Pasapula, Isabel Austin, Ricardo Larrainzar-Garijo, Javier Bayod, and Christian Cifuentes-De la Portilla. 2024. "The Effects of Midfoot/Hindfoot Fusions on the Behaviour of Peroneus Longus Tendon in Adult-Acquired Flatfoot Deformity: A Biomechanical and Finite Element Analysis" Biomechanics 4, no. 3: 494-506. https://doi.org/10.3390/biomechanics4030035
APA StyleYanguma-Muñoz, N., Quevedo, B. D. S., Pasapula, C., Austin, I., Larrainzar-Garijo, R., Bayod, J., & Cifuentes-De la Portilla, C. (2024). The Effects of Midfoot/Hindfoot Fusions on the Behaviour of Peroneus Longus Tendon in Adult-Acquired Flatfoot Deformity: A Biomechanical and Finite Element Analysis. Biomechanics, 4(3), 494-506. https://doi.org/10.3390/biomechanics4030035







