Abstract
The purpose of this study was to analyze the effects of locking plate fixation used for bridging of tibial segmental ostectomy and of cast immobilization on gait biomechanics in goats. We hypothesized that stable fixation of a segmental bone defect, using a locking plate construct, would result in minimal changes in biomechanical variables of gait in goats, but full-limb immobilization would result in lasting alterations in the immobilized limb’s gait kinetics. A pressure-sensing walkway was used to measure biomechanical characteristics for stride, gait, and walking vertical force. Thirteen, non-lame adult Boer-cross goats were trained to walk over a pressure-sensing walkway prior to instrumentation. Segmental ostectomy was performed on the right hind tibia of each goat and the defect was stabilized using bridging plate fixation with a locking compression plate. Per the protocol of an ongoing orthopedic study, the same goats underwent right hindlimb cast immobilization between one and four months postoperatively. Data was collected preoperatively and then over twelve months postoperatively in goats with unrestricted mobility. Statistical analysis revealed no significant alterations in hindlimb kinematics or maximum force when comparing the period after surgery with that after cast immobilization; significant decreases in forelimb stride length and velocity were noted postoperatively but normalized prior to cast placement, suggesting the overall functional stability of fixation. Cast immobilization had a profound and sustained effect on gait with significant alterations in both forelimb kinetics and hindlimb kinetics and kinematics for the remainder of the trial period; increased hindlimb asymmetry characterized by greater weight distribution and impulse to the left hindlimb was observed, suggesting the potential for long-term and/or permanent detrimental effects of prolonged limb immobilization.
1. Introduction
Small ruminants such as sheep and goats remain an important species model for translational bone healing research, effectively modeling human osteoporosis, osteoarthritis, bony trauma, and fracture physiology [1,2,3,4]. The caprine tibial segmental defect model of bone healing is well described and offers the opportunity to model multiple in vivo healing prognostic factors, such as defect size, anatomic location, and soft tissue coverage [2,5,6]. Benefits of the caprine model include clinically relevant body weight and long bone dimensions to human subjects, allowing use of human implants, bone tissue macro- and microstructural similarities to human bone, similar metabolic and bone remodeling rates to those of human bone, subject trainability, and affordable housing and upkeep in normal group social structure [1,5,7,8]. The caprine model of segmental ostectomy also allows for translational capability in biomaterials and tissue engineering research when combined with plate fixation [9]. Plate osteosynthesis with rigid fixation (i.e., dynamic compression plate, limited contact dynamic compression plate, and locking plate) represents the standard treatment for metaphyseal fractures, and it provides long-term stabilization while leaving an open space for biomaterial/construct implantation [5,7,10]. However, at the early stages of fixation, this technique diverts load eccentrically from the tibial shaft to the plate, and the balance of fixation rigidity and mechanical stimulation across the fracture/ostectomy site remains a focus of biomedical research [10,11,12]. The dynamic compression plate (DCP) offers adequate biomechanical stiffness of fixation, but it is associated with potential complications from excessive periosteal contact and pressure, including potential avascular necrosis and/or impaired healing [7,13,14]. The limited contact DCP (LC-DCP) reduces the area of periosteal contact and inherent contact-related risks, but it still relies on the plate–bone interface for fixation stability [13]. Conversely, locking plate fixation relies on the screw plate interface for fixation stability and can maintain sufficient strength in cases with weak metaphyseal bone (i.e., osteoporosis, osteomalacia, or severe fracture comminution) that would warrant tissue engineering or biomaterial-based intervention [12,15]. This construct carries similar mechanical stability to external fixation but reduces risks associated with long-term external fixation, including infection or patient-associated trauma to the fixation [6,10,11,12]. Studies have confirmed similar rates and quality of fracture healing between DCP and locking plate fixation, despite a significant increase in fixation rigidity with the locking plate [13,16]. However, few studies describe biomechanical analysis of gait following locking plate fixation of segmental ostectomy, assessing the subject’s perceived stability of fixation and subsequent weight distribution [17,18]. Given the translational importance of the caprine and ovine tibial segmental ostectomy models in biomaterials research, further characterization of the subject’s biomechanical response to surgery is necessary.
Cast immobilization is a common clinical practice in both human and veterinary medicine, allowing for necessary immobilization and stability for bone, ligament, tendon, and other soft tissue healing [19,20]. However, prolonged cast immobilization is associated with common, severe complications, including muscle atrophy, intramuscular fibrosis, joint contracture, central and peripheral neural impairment, venous thromboembolism, tendon atrophy, cartilage degeneration, hyperalgesia, and osteoporosis [19,20,21,22,23,24,25]. Despite ample research into the biomechanical changes in gait induced during joint immobilization and into the physiologic effects of cast placement on bone, muscle, tendon, and nervous tissue, few studies document long-term alterations in gait following long-term immobilization [23,26,27,28,29]. Caplan et al. described significantly reduced plantar-flexor strength and overall balance impairment after only seven days of ankle immobilization in a prospective human trial [26]. In an equine prospective trial testing the effect of distal limb cast immobilization for 56 days, Stewart et al. observed a 24-fold increase in the likelihood of lameness after cast placement, and complications such as lameness, deep digital flexor tendonitis, decreased metacarpophalangeal joint range of motion, and increased sensitivity to flexion that did not resolve during a 12-week rehabilitation period after cast removal [19]. We aimed to further add to the body of knowledge regarding the effects of extended full limb cast immobilization using serial biomechanical assessment of gait in goat models used in orthopedic research.
Biomechanical assessment of gait via plantar-pressure-sensing technology provides an objective tool for use in orthopedic research to monitor response to treatment, lameness, and animal welfare [30]. Historically, both subjective and objective measurements of gait have been employed to detect pain in goats. Subjective modalities include visual lameness scores, behavior scoring, and numeric rating scales, but these methods are variable and prone to biases, including observer effect and categorical bias (in which an increase in lameness score does not correspond to an equivalent degree of behavioral change) [31,32,33]. Objective modalities include biomechanical assessment of gait, infrared thermography of an area of interest, pedometer and accelerometer tracking, and biochemical analysis such as plasma cortisol [30,31,32,34]. The number of studies utilizing two-dimensional and three-dimensional biomechanical investigation of gait in veterinary research are rapidly increasing, and implementation of biomechanical gait analysis adds weight to both veterinary and translational interpretation of results [35,36]. In particular, biomechanical analysis of gait via pressure-sensing systems is a portable, time-efficient and affordable option for quantitative and serial evaluation of orthopedic pain, and, as opposed to ground reaction force measurement with force plates, it allows the observer to evaluate multiple steps within and among strides in the same pass [30,37,38]. Multi-step analysis enables determination of paired-limb symmetry in quadrupedal research species, and calculated asymmetry indices, described as a percentage of paired-limb symmetry, allow for subject-to-subject comparison without confounding factors of heterogenous body size, conformation, body mass, and gait velocity [38,39,40,41]. The current study utilizes long-term monitoring of plantar pressure to evaluate goats’ biomechanical responses to surgical stabilization of tibial segmental ostectomy and to extended full limb cast immobilization. We hypothesized that stable fixation of a segmental bone defect, using a locking plate construct, would result in minimal changes in biomechanical variables of gait, but full-limb immobilization would result in alterations in gait kinetics of the affected limb.
2. Materials and Methods
2.1. Goats
The goats involved in this study were part of an orthopedic research project assessing bone healing over 12 months. Thirty-two female, Boer-cross adult goats were purchased from a licensed, commercial vendor. Criteria for inclusion in this study were goats who completed the 12-month duration orthopedic research study of segmental defects that were non-load-sharing and who had complete sets of biomechanical data at the desired time points. All study procedures were approved by the University of Tennessee Animal Care and Use Committee (protocol number 2741) and adhered to the National Institute of Health’s Guide for the Care and Use of Laboratory Animals [42]. Of the thirty-two goats enrolled in the ongoing orthopedic research project, thirteen female Boer-cross, adult goats weighing 52.8 ± 7.9 kg (range 29–67 kg) met the inclusion criteria for this study. Preoperatively, goats were judged to be free of lameness based on a visual lameness score of 0 out of 4. Hooves and feet were inspected and trimmed to ensure consistent, normal balance and conditioning. Preoperatively, goats were housed in small group pens in groups of four to six (≥17 ft2 per goat); postoperatively, goats were housed individually in adjacent pens (≥20 ft2 per goat). Flooring included a layer of wood shavings laid on top of rubber mats over concrete flooring in a conditioned housing facility for the duration of the study. The goats were fed a balanced ration of grass hay, supplemental grain mix, and alfalfa as needed based on body condition and weight change. Free-choice fresh water was provided via automatic waterers in group housing and in water buckets in individual pens. Goats were weighed at study entry, weekly for the first thirty days postoperatively, and monthly for the remainder of the study.
2.2. Surgery
A mid-diaphyseal segmental ostectomy was performed on the right hind tibia of each goat, and it was stabilized using a custom-designed low contact round double threaded 8-hole, 4.5 mm thick locking plate (Veterinary Orthopedic Implants, St. Augustine, FL, USA) with a solid central portion between the screw holes [5]. The plate was stabilized with eight 4.0 mm diameter locking-head self-tapping screws (Veterinary Orthopedic Implants, St. Augustine, FL, USA), four in the proximal segment and four in the distal segment.
Surgical procedures were as follows. Goats received perioperative antibiotics (ceftiofur sodium 2.2 mg/kg IV, q12 h, Zoetis®, Parsippany, NJ., USA, and oxytetracycline 20 mg/kg IV, single dose, Zoetis®, Parsippany, NJ., USA), non-steroidal anti-inflammatory medication (flunixin meglumine 1.1 mg/kg IV, q12 h, Merck®, Keniworth, NJ, USA), and opioid analgesic (fentanyl transdermal patch 75 mcg/h, placed 12 h preoperatively, Mallinckrodt, Surrey, UK). After placement of a jugular intravenous catheter (18GA × 2 in polyurethane catheter, Terumo Medical Corporation, Somerset, NJ, USA), goats were sedated with xylazine (0.05 mg/kg IV, MWI Animal Health, Boise, ID, USA) and induced into general anesthesia using a mixture of ketamine hydrochloride (5 mg/kg IV, MWI Animal Health, Boise, ID, USA) and midazolam (0.25 mg/kg IV, West-Ward Pharmaceuticals, Eatontown, NJ, USA) titrated to effect. The goats were intubated, and general anesthesia was maintained using isoflurane vaporized into oxygen (1–2 L/min, MWI Animal Health, Boise, ID, USA) and administered via endotracheal intubation. Goats were placed in dorsal recumbency and the right hindlimb was suspended in an extended position, clipped, cleaned, and aseptically prepped. A roughly 20 cm incision was made along the medial surface of the tibia, extending from immediately proximal to the medial malleolus to immediately distal to the medial condyle of the tibia. The periosteum was stripped from the bone surface using periosteal elevators (Veterinary Orthopedic Implants, St. Augustine, FL, USA). The locking plate was applied to the craniomedial surface of the tibia and stabilized with screws. Screws were placed using standard techniques, in brief: a 3.2 mm diameter locking-head drill sleeve (Veterinary Orthopedic Implants, St. Augustine, FL, USA) was secured in the intended hole of the locking plate, a guide hole was drilled using a battery-powered orthopedic drill (Model Number: ND-1001, Anhui, China) and 3.2 mm diameter drill bit (Veterinary Orthopedic Implants, St. Augustine, FL, USA) with continuous lavage with sterile saline for cooling and debris clearance, depth and appropriate screw length were determined using a depth gauge (Veterinary Orthopedic Implants, St. Augustine, FL, USA), and each self-tapping screw was manually driven into the bone using a hand-held screwdriver (Veterinary Orthopedic Implants, St. Augustine, FL, USA). After initial placement of screws in the 1st, 4th, 5th, and 8th position with unicortical engagement, the plate was distracted away from the bone to allow for ostectomy using an oscillating bone saw (DEJUN, Shenzhen, China) to create a 2.0 cm segmental defect in the mid-diaphysis. The plate was realigned in contact with the bone and all screws were hand-tightened until tight engagement between the screw and plate threads. Soft tissues were closed in a routine fashion and a full limb bandage with medial and lateral plastic splints (Premier1Supplies, Washington, IA, USA) that spanned the limb from foot to stifle was applied for recovery. Postoperatively, the goats were continued on an antibiotic (ceftiofur sodium 2.2 mg/kg IV, q12h, Zoetis®, Parsippany, NJ., USA) and nonsteroidal anti-inflammatory medication (flunixin meglumine 1.1 mg/kg IV, q12h, Merck®, Keniworth, NJ, USA) for three days. Supplemental analgesia was provided via a transdermal opioid (fentanyl patch 75 mcg/h, q72h, Mallinckrodt, Surrey, UK) and/or a non-steroidal anti-inflammatory medication (meloxicam 0.5–1 mg/kg PO, q24h, MWI Animal Health, Boise, ID, USA) at the discretion of the attending veterinarian. Immediately after surgery, goats were maintained in full limb bandages with medial and lateral plastic splints (Premier1Supplies, Washington, IA, USA) that spanned the limb from foot to stifle for the first month. Bandage changes occurred every two days for the first two weeks, then twice weekly for the remainder of the month.
2.3. Cast Immobilization
Based on the ongoing research protocol, goats included in this study had full limb casts placed on the treated limbs for a period of three months, beginning one month after surgery. Casts were changed monthly. Briefly, each goat was sedated using midazolam (0.25 mg/kg IV, West-Ward Pharmaceuticals, Eatontown, NJ, USA) and xylazine (0.01–0.02 mg/kg IV, MWI Animal Health, Boise, ID, USA) and placed in left lateral recumbency. A fiberglass cast was placed on the right hindlimb encasing the hoof and extending proximally to the level of the femorotibial joint. Goats were maintained in individual housing (≥20 ft2 per goat) throughout the immobilization period. Following cast removal, the goats were transitioned to a full limb bandage with medial and lateral plastic splints that spanned the limb from foot to stifle. Bandages were changed twice weekly for a minimum of two weeks and then the goats were returned to unrestricted activity. Biomechanical data collection was reinitiated when the goat had returned to unrestricted mobility.
2.4. Biomechanical Data Collection
Gait parameters were objectively assessed using an automated, real-time pressure sensing system (Walkway Pressure Mapping System, Tekscan Inc., South Boston, MA, USA) with a sensor matrix of 87.1 cm by 36.9 cm and a sensor density of 1.4 sensors/cm2. The mat was calibrated and equilibrated according to the manufacturer’s instructions. Recordings were manually triggered and ended by the investigator based on the goat’s approach and exit from the mat. Maximum recorded frames were set at 1000 frames with a recording rate of 15 frames per second. Goats were trained to walk on a halter through an alleyway that housed the mat into a small pen (~20 sq. ft.). The mat was placed at the midpoint of the alley and covered with a soft, rubber overlay for device protection and to avoid slipping during ambulation. One side of the alleyway was formed using a single plexiglass barrier to allow for perpendicular video recording of each pass (Figure 1). Prior to training, goats were weighed on a digital scale and the weight was recorded in the computer system software for future data. Prior to surgery, the goats were trained to walk at a uniform pace across the mat from a starting handler to a second handler holding a halter with long lead. The lead was held without tension applied to the halter during the pass to allow for free movement at a normal pace. The investigator was positioned at the midpoint of the mat and each pass was recorded using a digital video camera (Microsoft LifeCam Cinema, Microsoft Corporation, Redmond, WA, USA) with the same frame rate of 15 frames per second and positioned at the same location as the investigator to allow for accurate determination of extremity strike and overall gait.
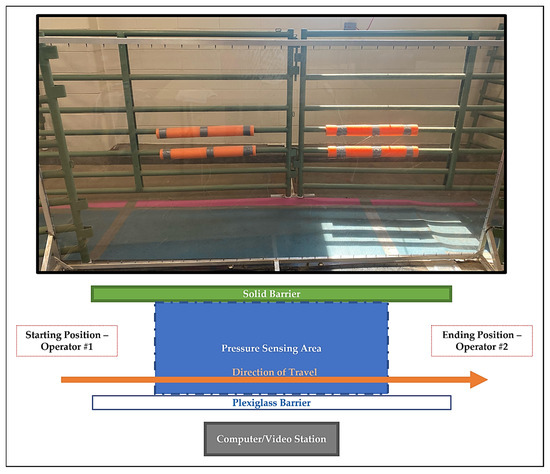
Figure 1.
Photograph and associated diagram of data collection area. Goats were allowed to walk at a self-selected pace in the indicated direction of travel from a starting handler to a second handler holding a halter with long lead. The lead was held without tension applied to the halter during the pass to allow for free movement at a normal pace. Enclosure surrounding ending position not pictured above.
Biomechanical assessment of gait was collected from the three best-fit recordings in one direction, on each of the following timepoints: preoperative (Day −1) and Days 1, 7, 30, 180, 240, 300, and 360 postoperative. The Day 1, Day 7, and Day 30 timepoints were obtained after surgery and without external coaptation. The remaining postoperative timepoints occurred after goats had reached unrestricted mobility following cast immobilization of the treated hindlimb. During each data collection session, each goat was walked across the mat until at least three valid walking passes were obtained. A valid walking pass was defined as traversal of the mat at a progressive and calm walking gait with all four limbs having at least one contact with the pressure sensing surface and without stopping, excessive lateral or medial deviation, distraction, or tension applied on the halter. The data from the first three valid passes on each day were recorded and averaged for each goat and this averaged data was used for further analysis. On Days 1, 7, and 30 postoperatively, each goat’s splint bandage was removed prior to pressure mat analysis and replaced immediately after each day’s data collection was completed. At the completion of each session, pressure-sensing data was exported from the Tekscan software to Microsoft Excel (Microsoft, Redmond, WA, USA) for backup and storage within an external hard drive (Seagate Technology, Cupertino, CA, USA).
Kinematic variables of interest included the number of stances, gait time-front (s), gait distance-front (cm), gait velocity-front (cm/s), cycles per minute, stance time (s), swing time (s), stride time (s), stride length (cm), and stride velocity (cm/s). Kinetic variables of interest included maximum vertical force (kg), maximum vertical force normalized to body weight (%BW), vertical impulse (kg*s), vertical impulse normalized to body weight (%BW*s), and maximum peak pressure (kPa). These variables were defined as previously reported [30]. Maximum force (kg) and maximum force normalized to body weight (%BW) were collected during the stance phase of each extremity, and when multiple stances were present within the same pass, the values were averaged for further data analysis. Impulse (kg*s) and impulse normalized to body weight (%BW*s) utilized the average impulse for a given extremity.
2.5. Asymmetry Indices
To further characterize the effects of surgery and cast immobilization on gait, asymmetry indices (ASI) were employed for both the forelimbs and hindlimbs as previously described [40,41]. Briefly, data was transformed using the following equation:
where RL is the right limb and LL is the paired left limb. Asymmetry indices were calculated for the variables of limb stance time, stride length, stride velocity, normalized maximum vertical force, normalized maximum impulse, and maximum peak pressure. Variable-specific ASI values, expressed as percent asymmetry, with a value of 0 reflecting perfect symmetry between paired limbs, were generated for each timepoint for each goat and were utilized for statistical analysis.
2.6. Statistical Analysis
Descriptive statistics for each kinematic and kinetic variable of interest were generated, including the mean, standard deviation, range, minimum, and maximum values. The effects of time and limb on kinematic and kinetic variables were analyzed using mixed model analysis for randomized block design, respectively, with the individual animal as the block effect. Ranked transformation was applied when diagnostic analysis using Shapiro–Wilk test and Levene’s test on residuals exhibited violation of normality and equal variance assumption. Post hoc multiple comparisons were performed with Tukey’s adjustment.
Asymmetry indices were calculated for limb stance time, stride length, stride velocity, normalized maximum vertical force, normalized maximum impulse, and maximum peak pressure as described above for all timepoints completed by each goat. Descriptive statistics were generated including mean, standard deviation, and range. ASI were analyzed using repeated-measures analysis of variance and post hoc comparisons were performed with Tukey’s adjustment. All biomechanical and asymmetry analyses were conducted in SAS 9.4 TS1M7 (SAS Institute Inc., Cary, NC, USA), and statistical significance was identified at the level of 0.05.
3. Results
3.1. Goats
All goats completed the study to the 12-month endpoint. Due to a software error, data loss was sustained for five goats, affecting the Day 1, Day 7, and Day 30 timepoints. The adjusted sample sizes are reported in Table 1, Table 2 and Table 3. Briefly, data from 12 of 13 goats was included in the Day 1 and Day 7 subsets, and data from 10 of 13 goats was included in the Day 30 subset.

Table 1.
Forelimb and hindlimb kinematics following right hindlimb tibial segmental defect locking plate stabilization and following right hindlimb cast immobilization. All values are presented as mean ± standard deviation.

Table 2.
Forelimb and hindlimb kinetics following right hindlimb tibial segmental defect and locking plate stabilization and right hindlimb cast immobilization. All values are presented as mean ± standard deviation.

Table 3.
Forelimb and hindlimb kinetics following right hindlimb tibial segmental defect and locking plate stabilization and right hindlimb cast immobilization. All values are presented as mean ± standard deviation.
3.2. Post-Surgical Biomechanics (Days 1–30)
Limb-specific kinematics are presented in Table 1 and the variables of stance time, stride length, and stride velocity are illustrated in Figure 2 (forelimbs) and Figure 3 (hindlimbs). During the initial postoperative period, prior to limb immobilization, significant shortening of stride length was noted in the right forelimb (p = 0.010) and left forelimb (p = 0.026) on Day 1 compared to preoperative baseline, but stride length normalized by the Day 7 and Day 30 timepoints. No significant changes in hindlimb kinematics were appreciated during the first thirty days postoperative.
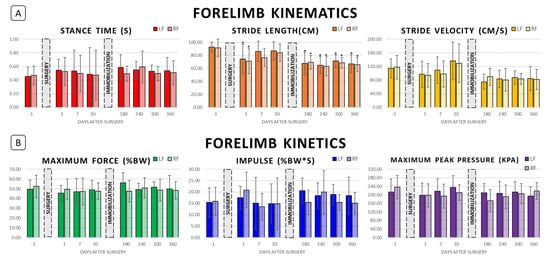
Figure 2.
Forelimb biomechanics following right hindlimb tibial segmental defect locking plate stabilization and following right hindlimb cast immobilization. (A) Forelimb kinematics preoperatively (Day −1) and postoperatively at Days 1, 7, 30, 180, 240, 300, and 360. (B) Forelimb kinetics at the same timepoints. “*” represents value significantly different from the respective preoperative baseline in pairwise comparison; p < 0.05.
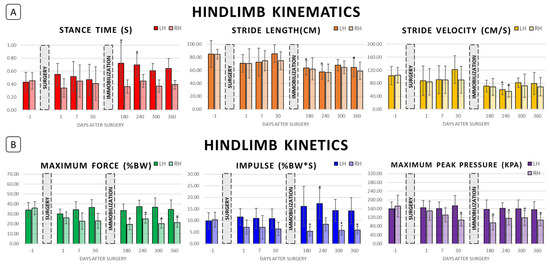
Figure 3.
Hindlimb biomechanics following right hindlimb tibial segmental defect locking plate stabilization and following right hindlimb cast immobilization. (A) Hindlimb kinematics preoperatively (Day −1) and postoperatively at Days 1, 7, 30, 180, 240, 300, and 360. (B) Hindlimb kinetics at the same timepoints. “*” represents value significantly different from the respective preoperative baseline in pairwise comparison; p < 0.05.
Limb-specific kinetics are presented in Table 2 and the variables of weight-normalized maximum vertical force, weight-normalized impulse, and maximum peak pressure are illustrated in Figure 2 (forelimbs) and Figure 3 (hindlimbs). No significant alterations in forelimb kinetics were appreciated in the first thirty days postoperative. Right hindlimb impulse (%BW*s) and maximum peak pressure decreased postoperatively compared to preoperative baseline, reaching significance on Day 30 (p = 0.041 and p = 0.001, respectively), but no significant change in left hindlimb kinetics was noted.
3.3. Post-Immobilization Biomechanics (Days 180–360)
Post-immobilization limb-specific kinematics are included in Table 1 and Figure 2 and Figure 3. Significant shortening of forelimb stride length compared with preoperative baseline was found at all post-immobilization timepoints (p < 0.003). In addition, average right forelimb stride lengths on Days 240 and 360 were significantly shorter than that on Day 30 (p = 0.006 and p = 0.025, respectively). Similarly, average left forelimb stride lengths on Days 180, 240, and 360 were significantly shorter as compared with that on Days 7 (p = 0.020, p = 0.003, and p = 0.010, respectively) and 30 (p = 0.042, p = 0.008, and p = 0.025, respectively). Significant decreases in forelimb stride velocity compared with Day 30 were found for the right forelimb on Days 240, 300, and 360 (p = 0.024, p = 0.049, and p = 0.029, respectively) and for the left forelimb on Days 180, 240, 300, and 360 (p = 0.003, p = 0.014, p = 0.037, and p = 0.018, respectively). Throughout the entire postoperative period, no significant alterations in forelimb stance time, swing time, or stride time were noted.
Hindlimb stride length was significantly shortened compared to preoperative baseline at all post-immobilization timepoints with the exception of Day 300 for the left hindlimb (p < 0.015 for RH Days 180, 240, 300, and 360 and LH Days 180, 240, and 360). In addition, left hindlimb average stride lengths on Days 180, 240, and 360 were significantly shorter than that of Day 30 (p = 0.018, p < 0.001, and p = 0.020, respectively). Left hindlimb stance time was significantly less on Days 180 and 240 compared to preoperative baseline (p = 0.010 and p = 0.029). Both left and right hindlimb stride velocity was significantly less on Day 240 compared to their respective preoperative baselines (p = 0.020 and p = 0.003, respectively). In addition, left hindlimb average stride velocities on Days 180, 300, and 360 were significantly less than that of Day 30 (p = 0.007, p < 0.001, and p = 0.034, respectively). Finally, left hindlimb stance times on Days 180 and 240 were significantly greater than preoperative baseline (p = 0.010 and p = 0.029, respectively). Although reciprocal lessening of right hindlimb stance time can be appreciated in Table 1, these differences were not statistically significant. Throughout the entire postoperative period, no significant differences in left hindlimb stride time and in right hindlimb stance, swing, and stride times were noted.
Post-immobilization limb-specific kinetics are included in Table 2 and Figure 2 and Figure 3. No significant alterations in forelimb kinetics were noted post-immobilization. For the left hindlimb, normalized impulse (%BW*s) on Day 240 was significantly greater than preoperative baseline (p = 0.019), but no significant changes in maximum vertical force (%BW) or maximum peak pressure (kPa) were appreciated. Significant alterations of right hindlimb kinetics were noted for all variables throughout the post-immobilization period. Right hindlimb maximum vertical force (%BW) and maximum peak pressure values were significantly less than preoperative baseline on Days 180, 240, 300, and 360 (p < 0.005). Right hindlimb impulse (%BW*s) was significantly less than preoperative baseline on Days 180, 300, and 360 (p < 0.005).
3.4. Asymmetry Indices
Asymmetry index values, expressed as % asymmetry for stance time, stride length, stride velocity, maximum vertical force, impulse, and maximum peak pressure, are presented in Table 3 and illustrated in Figure 4. No significant changes in forelimb asymmetry indices were found. No significant differences in stride length, stride velocity, and maximum peak pressure asymmetry indices were found. Hindlimb stance time ASI was significantly greater than baseline on Days 1, 180, 240, 300, and 360 (p = 0.006, p < 0.001, p = 0.026, p = 0.004, and p = 0.006, respectively). Hindlimb maximum force ASI was significantly greater than preoperative baseline on Days 180 and 300 (p = 0.028 and p = 0.022, respectively), and hindlimb impulse ASI was significantly greater than baseline on Days 180, 240, 300, and 360 (p < 0.001, p = 0.013, p < 0.001, and p = 0.002, respectively).
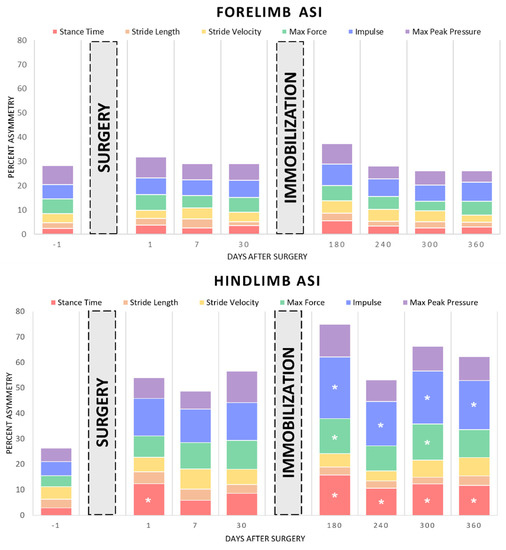
Figure 4.
Asymmetry indices for the variables of stance time, stride length, stride velocity, maximum weight-normalized vertical force, impulse, and maximum peak pressure following right hindlimb tibial segmental defect locking plate stabilization and following right hindlimb cast immobilization. “*” represents value significantly different from the respective preoperative baseline in pairwise comparison; p < 0.05.
4. Discussion
This study is the first of its kind to characterize the effects of locking plate stabilization of tibial segmental ostectomy and extended cast immobilization on the biomechanical characteristics of gait in goats. The results of this study showed that goats recovered substantially from creation of the surgical model in the first 30 days after surgery; however, prolonged immobilization of the limb resulted in profound alterations in gait kinetics of the affected limb. Notable findings can be categorized temporally into two postoperative periods: pre-immobilization and post-immobilization. Pre-immobilization biomechanical analysis supported our hypothesis and documented the short-term functional stability of locking plate fixation of bone defects. However, marked alterations in gait kinetics and kinematics were noted post-immobilization, suggesting the potential for long-term and/or permanent detrimental effects of prolonged limb immobilization. Unfortunately, the study design of the ongoing orthopedic research project limited our ability to define or control the factors associated with alterations in gait. However, the results reported here could be used to design more specific studies in which risk factors can be isolated.
An important finding in this study is that the transient significant alterations in gait kinematics associated with the locking plate model used in goats normalized by 30 days postoperative. Initially, forelimb stride length significantly decreased bilaterally despite increased stance time. Interestingly, concurrent significant changes in hindlimb kinematics were not present on Day 1, but a significant increase in hindlimb stance time asymmetry was detected. Taken together, these kinematic alterations describe voluntary gait alteration to compensate for a surgically induced lameness. Similar shortening of forelimb stride length and increase in forelimb stance time in response to hindlimb lameness were observed in an ovine segmental ostectomy model stabilized with external fixators [17,18]. These kinematic alterations create an offloading effect, allowing for more weight to be distributed across the three unoperated limbs. Although postoperative reductions in right hindlimb maximum vertical force, impulse, and maximum peak pressure were mirrored by increases on the forelimbs and left hindlimb, these compensatory kinetic changes did not reach significance. Kinematic alterations in gait normalized by Day 30 postoperative, supporting the overall functional stability of ostectomy fixation using the locking plate.
Kinetic depreciations in right hindlimb impulse and maximum peak pressure were noted in the pre-immobilization period. This represented an offloading effect similar to that described in an ovine model of tibial segmental ostectomy stabilized by a rigid external fixator, with maximal offloading (defined as local minima in maximum vertical force on the operated limb) occurring at two weeks postoperative and with normalization by nine weeks postoperative [17,18]. However, as interfragmentary stability was experimentally decreased, the offloading effect became more pronounced and rapid, reaching maximal offloading between 2–7 days postoperative [18]. A notable limitation of the current study is the short pre-immobilization period of 30 days compared to six months post-immobilization. Due to the nature of the ongoing study, long term effects of surgical fixation without cast immobilization could not be assessed, but the timing of maximal offloading may give insight into the functional rigidity of fixation. Unlike that observed by Schell et al., significant decreases in right hindlimb maximum vertical force were not observed in the first thirty days postoperative, and the observed offloading pattern more closely resembles that of the stable, rigid external fixation documented by Seebeck et al. [17,18] In contrast to both ovine studies, no significant changes in left hindlimb (unoperated paired limb) kinetics or in hindlimb kinetic asymmetry were observed in the current trial’s pre-immobilization period. Therefore, the normalization of forelimb kinematics, the lack of significant alterations to hindlimb symmetry, and the gradual and relatively minor changes to hindlimb kinetics support the use of locking plate osteosynthesis as a functionally stable fixation for tibial segmental ostectomy in goats.
Long-term full limb cast immobilization in goats had marked and prolonged effects on gait kinematics and kinetics following cast removal. Both forelimbs exhibited significantly shortened stride lengths and slower stride velocities throughout the post-immobilization period, without apparent normalization. To the authors’ knowledge, previous biomechanical analysis of the long-term effects of hindlimb cast immobilization in small ruminants has not been documented. These seemingly permanent alterations in forelimb kinematics may reflect the true treatment effect of hindlimb immobilization, representing an adaptation during cast immobilization, or they may be the result of training effect, with the goats’ gait variability and overall stride frequency altered as the number of biomechanical data collection sessions increased. Follow-up studies analyzing either the long-term biomechanical effects of cast immobilization in small ruminants or the trends in normal goat biomechanics over a year of data collection are necessary. However, in this study, a return to preoperative baseline forelimb kinematics post-immobilization was not achieved, and these findings in conjunction with the hindlimb kinetic alterations discussed below suggest potentially permanent detrimental effects of prolonged limb immobilization on gait in goats.
Cast immobilization had significant, lasting effects on hindlimb kinetics and kinematics. Similar to the forelimbs, both hindlimbs exhibited significantly shorter stride lengths compared to preoperative baseline, but unlike in the forelimbs, hindlimb stride velocity was not as consistently affected. Notably, right hindlimb stride velocity did not differ significantly from preoperative baseline during the post-immobilization period. A consistent, concurrent decrease in velocity on all four limbs would explain the significant reductions in stride lengths, but the documented changes in forelimb and left hindlimb kinematics, without concurrent alteration in right hindlimb velocity, suggests a true treatment effect of cast immobilization. Marked decreases in right hindlimb maximum vertical force, impulse, and maximum peak pressure throughout the post-immobilization period support this treatment effect and emphasize the clinical impact that cast immobilization can have on a limb. Previous studies have extensively documented the physiologic changes that cast immobilization can elicit, including muscle atrophy, neuromuscular impairment, cartilage degeneration, tendon atrophy, and joint stiffness [20,21,22,23]. These physiologic changes manifest as functional impairment, as measured by impulse, weight distribution, and muscular strength (isometric force); in a study assessing the effect of one week of hindlimb immobilization in rats, significant reductions in the immobilized limb’s strength, load before irreversible deformation (ex vivo), and stiffness were documented following remobilization [20]. In a meta-analysis comparing results of internal fixation and cast immobilization for scaphoid fractures (a non-loadbearing carpal bone) in humans, no difference in overall healing or reported pain was noted between modalities, but functional parameters such as grip strength were impaired in the cast-immobilized group [43].
The current study provides further evidence of functional impairment following immobilization through biomechanical analysis of gait. The immobilized right hindlimb exhibited significant kinematic alterations throughout the post-immobilization period, including reduced stride length and increased asymmetry favoring the left hindlimb in stance time. Marked, significant increases in hindlimb impulse ASIs, reaching over 24% asymmetry favoring the left hindlimb, were noted at all post-immobilization timepoints, and concurrent significant depreciations in right hindlimb impulse, reaching almost a 50% decrease in impulse compared to preoperative baseline, were appreciated. Weight bearing was shifted away from the right hindlimb, as shown by significant decreases in maximum vertical force and maximum peak pressure as well as significant increases in hindlimb maximum force ASI. One limitation of this study is that further characterization of the muscular, neural, and soft tissue changes in both the immobilized and contralateral limb were outside of the scope of the ongoing orthopedic research project. We can hypothesize that the biomechanical changes documented in both hindlimbs were secondary to the physiologic complications described above, but quantification and characterization of atrophy or other degenerative process was not performed. However, the most consistently and significantly affected kinetic variable in this study was impulse; directly related to muscular force and the time over which the motion was completed, a significant drop in impulse without concurrent drop in stride velocity or stride time, as seen in the right hindlimb, indicates a decrease in muscle force (torque) generated for the given motion, most commonly secondary to a decrease in functional muscular mass or activation [44]. Together, these biomechanical changes illustrate the lasting functional impairment of the right hindlimb following long term immobilization, without apparent normalization at six months post-immobilization (Day 360). Questions remain as to the capability for gait normalization after extended immobilization and future research is required to characterize the long-term potential compensatory changes in quadrupedal gait after hindlimb immobilization. Further, studies aimed to quantitate the temporal effect of limb immobilization may aid researchers and clinicians in post-operative rehabilitative plans to minimize the potential for detrimental effects on animals and patients.
One limitation of the present study is the limited number of subjects included in data analysis. Thirteen goats out of a total population of thirty-two goats met the inclusion criteria for this study. These subjects were selected from an ongoing orthopedic research project exploring bone healing in goats, and they met inclusion criteria ensuring uniformity of gender, surgical fixation, postoperative care, and cast immobilization protocol. Pre-determination of sample size of the study population was not performed because we intended to enroll all qualified goats. On post hoc sample size analysis with type II error set at 0.2, the minimum sample size needed for statistical analysis was eleven goats. Therefore, total population statistical analysis met the appropriate power for interpretation, but further stratification based on demographic factors such as weight or age was not feasible given the current dataset.
5. Conclusions
This study documents the effects that locking plate stabilization of a tibial segmental defect and cast immobilization have on goat gait biomechanics. Postoperative data during the pre-immobilization period documented the functional stability of locking plate fixation of segmental bone defects. Due to the nature of the ongoing study, long-term effects of surgical fixation without cast immobilization could not be assessed, but the normalization of forelimb kinematics and lack of significant alterations to hindlimb symmetry and kinetics during the first month after surgery are encouraging. However, marked alterations in gait kinetics and kinematics were noted following cast stabilization, suggesting the potential for long-term and/or permanent detrimental effects of prolonged limb immobilization. In particular, the persistence of gait alteration for six months following coaptation and recovery highlights the profound effects that joint immobilization can have on force distribution and ambulation. Though both procedures affected gait, prolonged cast immobilization had significant and lasting effects which should be considered in the design of future orthopedic research using goat models.
Author Contributions
Conceptualization, K.M.B. and D.E.A.; methodology, K.M.B., E.G.C., L.D.T., H.S.A.III, P.-Y.M. and D.E.A.; data curation, K.M.B.; writing—original draft preparation, K.M.B.; writing—review and editing, K.M.B., D.E.A., P.-Y.M. and H.S.A.III; visualization, K.M.B.; project administration, D.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the USAMRAA, grant number JWM180123.
Institutional Review Board Statement
The animal study protocol was approved by the University of Tennessee Institutional Animal Care and Use Committee (Protocol #2741-0220).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge and thank Xiaocun Sun of the Office of Information and Technology at the University of Tennessee for her assistance in statistical analysis. We would also like to thank Alex Anderson, Tammy Howard, and all the staff of the UTK Johnson Research and Teaching Unit for their assistance in goat care and husbandry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dias, I.R.; Camassa, J.A.; Bordelo, J.A.; Babo, P.S.; Viegas, C.A.; Dourado, N.; Reis, R.L.; Gomes, M.E. Preclinical and Translational Studies in Small Ruminants (Sheep and Goat) as Models for Osteoporosis Research. Curr. Osteoporos. Rep. 2018, 16, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Oliver, R.A.; Pelletier, M.H.; Walsh, W.R. Ovine model for critical-size tibial segmental defects. Comp. Med. 2014, 64, 377–385. [Google Scholar] [PubMed]
- Atarod, M.; Frank, C.B.; Shrive, N.G. Kinematic and Kinetic Interactions During Normal and ACL-Deficient Gait: A Longitudinal In Vivo Study. Ann. Biomed. Eng. 2014, 42, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Diogo, C.C.; Camassa, J.A.; Fonseca, B.; Maltez da Costa, L.; Pereira, J.E.; Filipe, V.; Couto, P.A.; Raimondo, S.; Armada-da-Silva, P.A.; Maurício, A.C.; et al. A Comparison of Two-Dimensional and Three-Dimensional Techniques for Kinematic Analysis of the Sagittal Motion of Sheep Hindlimbs During Walking on a Treadmill. Front. Vet. Sci. 2021, 8, 545708. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, R.M.; Rifkin, R.E.; Croy, E.G.; Steiner, R.C.; Seddighi, R.; Mulon, P.-Y.; Adair, H.S.; Anderson, D.E. Temporal Changes in Reverse Torque of Locking-Head Screws Used in the Locking Plate in Segmental Tibial Defect in Goat Model. Front. Surg. 2021, 8, 637268. [Google Scholar] [CrossRef] [PubMed]
- McKinley, T.O.; Natoli, R.M.; Fischer, J.P.; Rytlewski, J.D.; Scofield, D.C.; Usmani, R.; Kuzma, A.; Griffin, K.S.; Jewell, E.; Childress, P.; et al. Internal Fixation Construct and Defect Size Affect Healing of a Translational Porcine Diaphyseal Tibial Segmental Bone Defect. Mil. Med. 2021, 186, e1115–e1123. [Google Scholar] [CrossRef]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; Van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef]
- Zeiter, S.; Koschitzki, K.; Alini, M.; Jakob, F.; Rudert, M.; Herrmann, M. Evaluation of Preclinical Models for the Testing of Bone Tissue-Engineered Constructs. Tissue Eng. Part C Methods 2020, 26, 107–117. [Google Scholar] [CrossRef]
- Xu, G.-H.; Liu, B.; Zhang, Q.; Wang, J.; Chen, W.; Liu, Y.-J.; Peng, A.Q.; Zhang, Y.-Z. Biomechanical comparison of gourd-shaped LCP versus LCP for fixation of comminuted tibial shaft fracture. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 250–257. [Google Scholar] [CrossRef]
- Stoffel, K.; Dieter, U.; Stachowiak, G.; Gächter, A.; Kuster, M.S. Biomechanical testing of the LCP—How can stability in locked internal fixators be controlled? Injury 2003, 34 (Suppl. 2), B11–B19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.A.; Aithal, H.P.; Amarpal; Kinjavdekar, P.; Gope, P.C.; Madhu, D.N. Biomechanical properties of a novel locking compression plate to stabilize oblique tibial osteotomies in buffaloes. Vet. Surg. 2021, 50, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Xu, H.; Ding, H.; Qin, H.; An, Z. Comparison of the effect on bone healing process of different implants used in minimally invasive plate osteosynthesis: Limited contact dynamic compression plate versus locking compression plate. Sci. Rep. 2016, 6, 37902. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Medeiros Savi, F.; Berner, A.; Fountain, S.; Saifzadeh, S.; Steck, R.; Epari, D.R.; Woodruff, M.A.; Knackstedt, M.; Schuetz, M.A.; et al. Scaffold-guided bone regeneration in large volume tibial segmental defects. Bone 2021, 153, 116163. [Google Scholar] [CrossRef]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra93. [Google Scholar] [CrossRef]
- Marcondes, G.D.M.; Paretsis, N.F.; Souza, A.F.D.; Ruivo, M.R.B.A.; Rego, M.A.F.; Nóbrega, F.S.; Cortopassi, S.R.G.; De Zoppa, A.L.D.V. Locking compression plate fixation of critical-sized bone defects in sheep. Development of a model for veterinary bone tissue engineering. Acta Cirúrgica Bras. 2021, 36, e360601. [Google Scholar] [CrossRef]
- Seebeck, P.; Thompson, M.S.; Parwani, A.; Taylor, W.R.; Schell, H.; Duda, G.N. Gait evaluation: A tool to monitor bone healing? Clin. Biomech. 2005, 20, 883–891. [Google Scholar] [CrossRef]
- Schell, H.; Thompson, M.S.; Bail, H.J.; Hoffmann, J.-E.; Schill, A.; Duda, G.N.; Lienau, J. Mechanical induction of critically delayed bone healing in sheep: Radiological and biomechanical results. J. Biomech. 2008, 41, 3066–3072. [Google Scholar] [CrossRef]
- Stewart, H.L.; Werpy, N.M.; McIlwraith, C.W.; Kawcak, C.E. Physiologic effects of long-term immobilization of the equine distal limb. Vet. Surg. 2020, 49, 840–851. [Google Scholar] [CrossRef]
- Oliveira Milani, J.G.P.; Matheus, J.P.C.; Gomide, L.B.; Volpon, J.B.; Shimano, A.C. Biomechanical Effects of Immobilization and Rehabilitation on the Skeletal Muscle of Trained and Sedentary Rats. Ann. Biomed. Eng. 2008, 36, 1641–1648. [Google Scholar] [CrossRef]
- Kaneguchi, A.; Ozawa, J.; Minamimoto, K.; Yamaoka, K. Morphological and biomechanical adaptations of skeletal muscle in the recovery phase after immobilization in a rat. Clin. Biomech. 2020, 75, 104992. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Taylor, J.L.; Hoffman, R.L.; Dearth, D.J.; Thomas, J.S. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve 2010, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kannus, R.; Jòzsa, L.; Renström, R.; Järvtoen, M.; Kvist, M.; Lento, M.; Oja, P.; Vuorl, I. The effects of training, immobilization and remobilization on musculoskeletal tissue. Scand. J. Med. Sci. Sport. 1992, 2, 100–118. [Google Scholar] [CrossRef]
- Aufwerber, S.; Heijne, A.; Edman, G.; Grävare Silbernagel, K.; Ackermann, P.W. Early mobilization does not reduce the risk of deep venous thrombosis after Achilles tendon rupture: A randomized controlled trial. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Oga, S.; Goto, K.; Sakamoto, J.; Sasaki, R.; Honda, Y.; Kataoka, H.; Okita, M. Voluntary Forelimbs Exercise Reduces Immobilization-Induced Mechanical Hyperalgesia in the Rat Hind Paw. Pain Res. Manag. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caplan, N.; Forbes, A.; Radha, S.; Stewart, S.; Ewen, A.; Gibson, A.S.C.; Kader, D. Effects of 1 Week of Unilateral Ankle Immobilization on Plantar-Flexor Strength, Balance, and Walking Speed: A Pilot Study in Asymptomatic Volunteers. J. Sport Rehabil. 2015, 24, 156–162. [Google Scholar] [CrossRef]
- Nahm, N.; Bey, M.J.; Liu, S.; Guthrie, S.T. Ankle Motion and Offloading in Short Leg Cast and Low and High Fracture Boots. Foot Ankle Int. 2019, 40, 1416–1423. [Google Scholar] [CrossRef]
- Zhang, S.; Clowers, K.G.; Powell, D. Ground reaction force and 3D biomechanical characteristics of walking in short-leg walkers. Gait Posture 2006, 24, 487–492. [Google Scholar] [CrossRef]
- Kadel, N.J.; Segal, A.; Orendurff, M.; Shofer, J.; Sangeorzan, B. The Efficacy of Two Methods of Ankle Immobilization in Reducing Gastrocnemius, Soleus, and Peroneal Muscle Activity during Stance Phase of Gait. Foot Ankle Int. 2004, 25, 406–409. [Google Scholar] [CrossRef]
- Rifkin, R.E.; Grzeskowiak, R.M.; Mulon, P.-Y.; Adair, H.S.; Biris, A.S.; Dhar, M.; Anderson, D.E. Use of a pressure-sensing walkway system for biometric assessment of gait characteristics in goats. PLoS ONE 2019, 14, e0223771. [Google Scholar] [CrossRef]
- Reppert, E.J.; Kleinhenz, M.D.; Viscardi, A.; Montgomery, S.R.; Crane, A.R.; Coetzee, J.F. Development and evaluation of two different lameness models in meat goats, a pilot study. Transl. Anim. Sci. 2020, 4, txaa193. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Oliveira, M.D.; Nunes, T.; Stilwell, G. Making the case for developing alternative lameness scoring systems for dairy goats. Appl. Anim. Behav. Sci. 2015, 171, 94–100. [Google Scholar] [CrossRef]
- Battini, M.; Renna, M.; Giammarino, M.; Battaglini, L.; Mattiello, S. Feasibility and Reliability of the AWIN Welfare Assessment Protocol for Dairy Goats in Semi-extensive Farming Conditions. Front. Vet. Sci. 2021, 8, 731927. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Mosher, R.A.; Anderson, D.E.; Robert, B.; Kohake, L.E.; Gehring, R.; White, B.J.; Kukanich, B.; Wang, C. Impact of oral meloxicam administered alone or in combination with gabapentin on experimentally induced lameness in beef calves1. J. Anim. Sci. 2014, 92, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Netukova, S.; Duspivova, T.; Tesar, J.; Bejtic, M.; Baxa, M.; Ellederova, Z.; Szabo, Z.; Krupicka, R. Instrumented pig gait analysis: State-of-the-art. J. Vet. Behav. 2021, 45, 51–59. [Google Scholar] [CrossRef]
- Egenvall, A.; Marr, C.M.; Byström, A. Study design synopsis: How to conduct, prepare, analyse and report equine biomechanical studies. Equine Vet. J. 2021, 53, 645–648. [Google Scholar] [CrossRef]
- Connor, P.; Ross, A. Biometric recognition by gait: A survey of modalities and features. Comput. Vis. Image Underst. 2018, 167, 1–27. [Google Scholar] [CrossRef]
- Meijer, E.; Bertholle, C.P.; Oosterlinck, M.; Van Der Staay, F.; Back, W.; Van Nes, A. Pressure mat analysis of the longitudinal development of pig locomotion in growing pigs after weaning. BMC Vet. Res. 2014, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Meijer, E.; Oosterlinck, M.; Van Nes, A.; Back, W.; Van Der Staay, F.J. Pressure mat analysis of naturally occurring lameness in young pigs after weaning. BMC Vet. Res. 2014, 10, 193. [Google Scholar] [CrossRef]
- Fanchon, L.; Grandjean, D. Accuracy of asymmetry indices of ground reaction forces for diagnosis of hind limb lameness in dogs. Am. J. Vet. Res. 2007, 68, 1089–1094. [Google Scholar] [CrossRef]
- Kano, W.T.; Rahal, S.C.; Agostinho, F.S.; Mesquita, L.R.; Santos, R.R.; Monteiro, F.O.B.; Castilho, M.S.; Melchert, A. Kinetic and temporospatial gait parameters in a heterogeneous group of dogs. BMC Vet. Res. 2016, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. The National Academies Collection: Reports Funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, DC, USA; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- Johnson, N.A.; Fairhurst, C.; Brealey, S.D.; Cook, E.; Stirling, E.; Costa, M.; Divall, P.; Hodgson, S.; Rangan, A.; Dias, J.J. One-year outcome of surgery compared with immobilization in a cast for adults with an undisplaced or minimally displaced scaphoid fracture: A meta-analysis of randomized controlled trials. Bone Jt. J. 2022, 104, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.K.; Falvo, M.J.; Chiu, L.Z.F. Force-velocity, impulse-momentum relationships: Implications for efficacy of purposefully slow resistance training. J. Sports Sci. Med. 2008, 7, 299–304. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).