A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation
Abstract
1. Introduction
- (1)
- Changes in the spatial structures and temporal profiles for different conditions.
- (2)
- No change in spatial structures but changes in the temporal profiles for different conditions.
- (3)
- Changes in the number of spatial and their temporal profiles for different conditions.
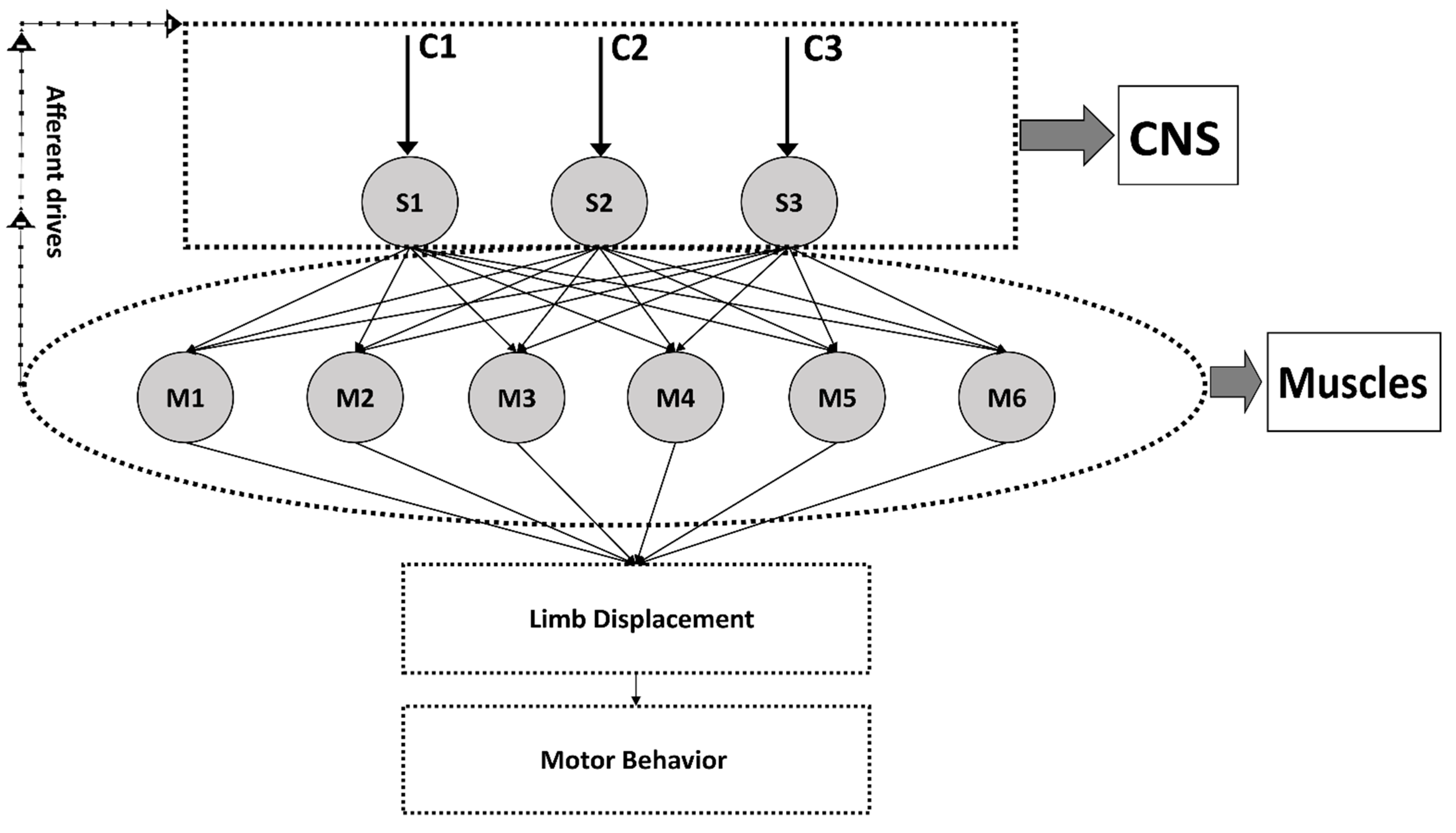
2. Altered Synergies and Altered Recruitment Profiles
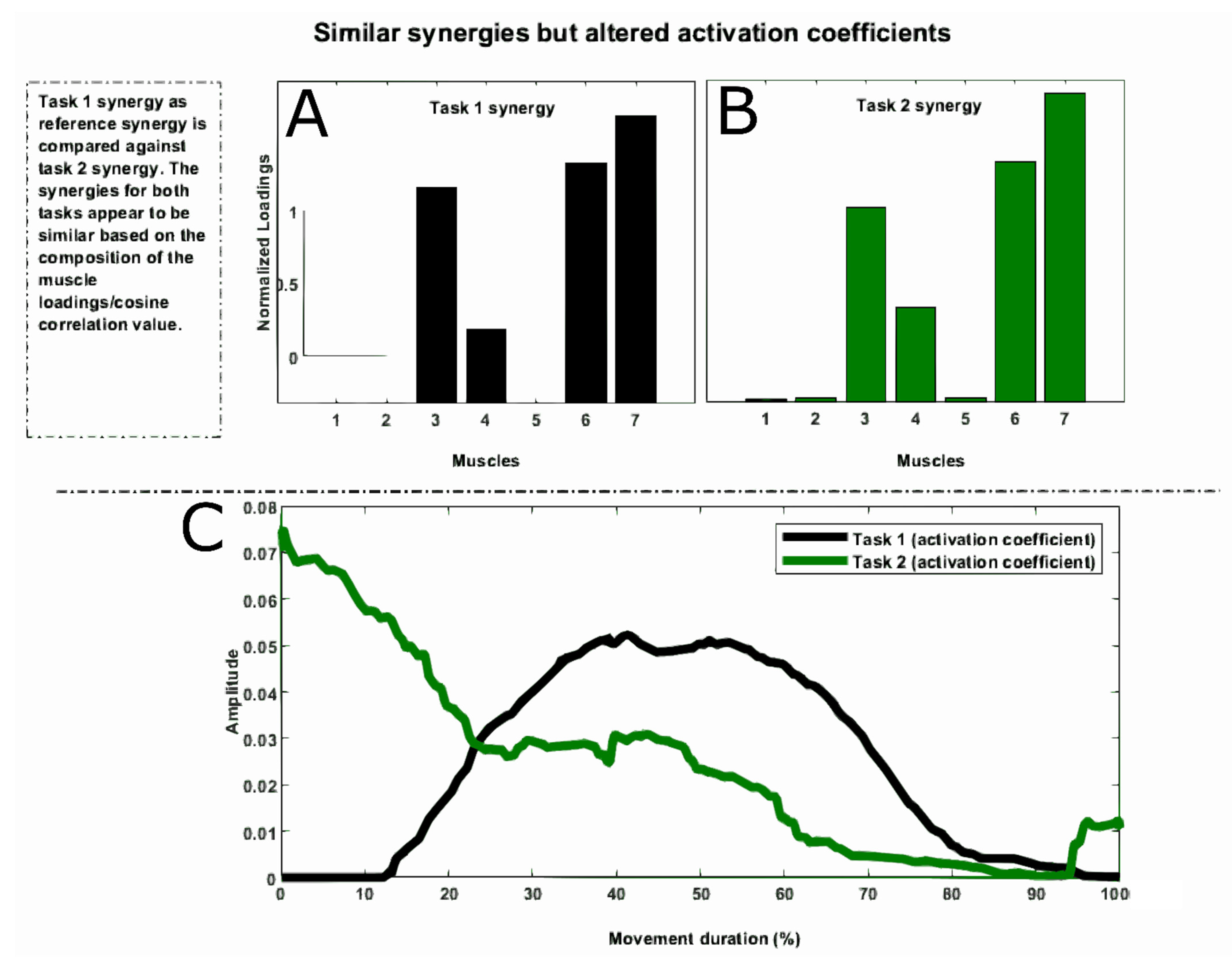
3. Preserved Synergies and Altered Recruitment Profiles
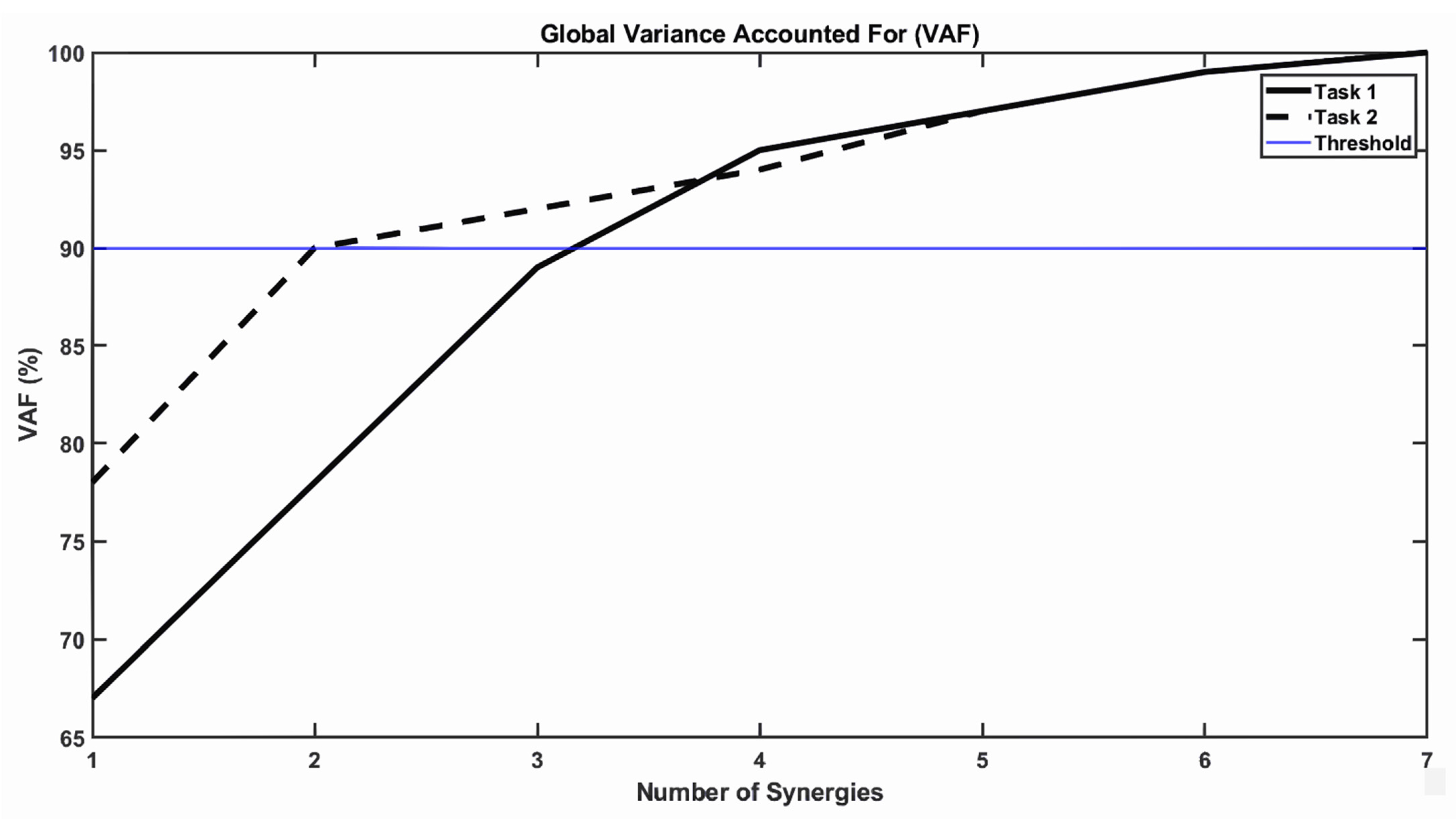
4. Changes in the Number of Muscle Synergies (Modular Organization)
5. Alternate Theories
- The uncontrolled manifold hypothesis (UCM). As observed by Bernstein, the degrees of freedom involved in a postural or movement task exceeds the number of control variables necessary to describe the task [34]. The UCM hypothesis states that the CNS focuses on controlling the task relevant DOF and leaves others uncontrolled. The concept provides a basis for distinguishing between different degrees of freedom in terms of their control-theoretical stability [35]. In other words, UCM indicates that the control of essential performance variables among duplications of a task is achieved by taking advantage of the available motor abundance rather than opting for a distinctive solution to the organization problem. According to the UCM theory, collaborations among the outputs of the motor components are arranged such that their variance is essentially limited to a subspace (a UCM) in the state space of those components that corresponds to the desired value of a specific performance variable [36].
- The equilibrium point hypothesis (EPH). The EPH explains how motor neuron thresholds have been controlled out of the value of lambda (λ), which correlates to an organization for a muscle, joint, or group of joints [37]. Researchers consider the combination of posture and movement control into a single mechanism as a very important characteristic of the equilibrium point hypothesis [37]. Some researchers defined the equilibrium point hypothesis (EPH) as method that the central nervous system utilizes to control the movement of extremities via a simple shift in equilibrium place, and the central nervous system doesn’t need to compensate for task dynamics [38].
- The internal model theory is one of numerous theories that consider the strategy of the central nervous (CNS) to perform human movements in which the CNS processes neural interpretations of the environment around the human and utilizes these to anticipate and regulate any actions [39,40]. Some researchers state that humans can learn and adapt when the dynamics of extremities change [41].
6. Application of Muscle Synergies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of Muscle Synergies in the Construction of a Natural Motor Behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- D’Avella, A.; Bizzi, E. Shared and Specific Muscle Synergies in Natural Motor Behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef]
- Alessandro, C.; Rellinger, B.A.; Barroso, F.O.; Tresch, M.C. Adaptation after Vastus Lateralis Denervation in Rats Demonstrates Neural Regulation of Joint Stresses and Strains. eLife 2018, 7, e38215. [Google Scholar] [CrossRef]
- Barroso, F.O.; Alessandro, C.; Tresch, M.C. Adaptation of Muscle Activation after Patellar Loading Demonstrates Neural Control of Joint Variables. Sci. Rep. 2019, 9, 20370. [Google Scholar] [CrossRef]
- Ting, L.H.; Macpherson, J.M. A Limited Set of Muscle Synergies for Force Control During a Postural Task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef]
- Cheung, V.C.K. Central and Sensory Contributions to the Activation and Organization of Muscle Synergies during Natural Motor Behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef] [PubMed]
- Tresch, M.C.; Jarc, A. The Case for and against Muscle Synergies. Curr. Opin. Neurobiol. 2009, 19, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, E.; Cheung, V.C.K. The Neural Origin of Muscle Synergies. Front. Comput. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- Kutch, J.J.; Valero-Cuevas, F.J. Challenges and New Approaches to Proving the Existence of Muscle Synergies of Neural Origin. PLoS Comput. Biol. 2012, 8, e1002434. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Clark, D.J.; Kautz, S.A. Modular Control of Human Walking: A Simulation Study. J. Biomech. 2009, 42, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Logan, D.; Giszter, S.F. Motor Primitives Are Determined in Early Development and Are Then Robustly Conserved into Adulthood. Proc. Natl. Acad. Sci. USA 2019, 116, 12025–12034. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.E.; White, G.; Delis, I.; Iqbal, K. Alteration of Muscle Synergy Structure While Walking under Increased Postural Constraints. Cogn. Comput. Syst. 2020, 2, 50–56. [Google Scholar] [CrossRef]
- Singh, R.E.; Iqbal, K.; White, G.; Hutchinson, T.E. A Systematic Review on Muscle Synergies: From Building Blocks of Motor Behavior to a Neurorehabilitation Tool. Appl. Bionics Biomech. 2018, 2018, 3615368. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Sun, Y.; Xie, B.; Huang, Z.; Wu, J.; Hou, J.; Liu, Y.; Huang, Z.; Zhang, Z. Alterations of Muscle Synergies During Voluntary Arm Reaching Movement in Subacute Stroke Survivors at Different Levels of Impairment. Front. Comput. Neurosci. 2018, 12, 69. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging Human Locomotion: Stability and Modular Organisation in Unsteady Conditions. Sci. Rep. 2018, 8, 2740. [Google Scholar] [CrossRef]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in Upper Limb Muscle Synergy Structure in Chronic Stroke Survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- Fox, E.J.; Tester, N.J.; Kautz, S.A.; Howland, D.R.; Clark, D.J.; Garvan, C.; Behrman, A.L. Modular Control of Varied Locomotor Tasks in Children with Incomplete Spinal Cord Injuries. J. Neurophysiol. 2013, 110, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; Rozumalski, A.; Schwartz, M.H. Muscle Synergies and Complexity of Neuromuscular Control during Gait in Cerebral Palsy. Dev. Med. Child Neurol. 2015, 57, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Torres-Oviedo, G.; Ting, L.H. Subject-Specific Muscle Synergies in Human Balance Control Are Consistent Across Different Biomechanical Contexts. J. Neurophysiol. 2010, 103, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Ting, L.H. Common Muscle Synergies for Balance and Walking. Front. Comput. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef]
- Martino, G.; Ivanenko, Y.P.; d’Avella, A.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Cappellini, G.; Casali, C.; Lacquaniti, F. Neuromuscular Adjustments of Gait Associated with Unstable Conditions. J. Neurophysiol. 2015, 114, 2867–2882. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.; Torricelli, D.; Moreno, J.C.; Taylor, J.; Gomez-Soriano, J.; Esteban, E.B.; Santos, C.; Pons, J.L. Similarity of Muscle Synergies in Human Walking and Cycling: Preliminary Results. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6933–6936. [Google Scholar] [CrossRef]
- Abd, A.T.; Singh, R.E.; Iqbal, K.; White, G. Investigation of Power Specific Motor Primitives in an Upper Limb Rotational Motion. J. Mot. Behav. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five Basic Muscle Activation Patterns Account for Muscle Activity during Human Locomotion: Basic Muscle Activation Patterns. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef]
- Dominici, N.; Ivanenko, Y.P.; Cappellini, G.; d’Avella, A.; Mondi, V.; Cicchese, M.; Fabiano, A.; Silei, T.; Di Paolo, A.; Giannini, C.; et al. Locomotor Primitives in Newborn Babies and Their Development. Science 2011, 334, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Rimini, D.; Agostini, V.; Knaflitz, M. Intra-Subject Consistency during Locomotion: Similarity in Shared and Subject-Specific Muscle Synergies. Front. Hum. Neurosci. 2017, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Abd, A.T.; Singh, R.E.; Iqbal, K.; White, G. Muscle Synergies Are Robust across Participants in Upper Limb Rotational Motion. In Proceedings of the 2020 7th International Conference on Electrical and Electronics Engineering (ICEEE), Antalya, Turkey, 14–16 April 2020; pp. 310–314. [Google Scholar] [CrossRef]
- Safavynia, S.; Torres-Oviedo, G.; Ting, L. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.N.G.; Ballekere, A.N.; Fregly, B.J.; Roh, J. Are Muscle Synergies Useful for Stroke Rehabilitation? Curr. Opin. Biomed. Eng. 2021, 19, 100315. [Google Scholar] [CrossRef]
- Matsunaga, N.; Kaneoka, K. Comparison of Modular Control during Smash Shot between Advanced and Beginner Badminton Players. Appl. Bionics Biomech. 2018, 2018, 6592357. [Google Scholar] [CrossRef]
- Bowden, M.G.; Clark, D.J.; Kautz, S.A. Evaluation of Abnormal Synergy Patterns Poststroke: Relationship of the Fugl-Meyer Assessment to Hemiparetic Locomotion. Neurorehabil. Neural Repair 2010, 24, 328–337. [Google Scholar] [CrossRef]
- Tan, C.K.; Kadone, H.; Watanabe, H.; Marushima, A.; Hada, Y.; Yamazaki, M.; Sankai, Y.; Matsumura, A.; Suzuki, K. Differences in Muscle Synergy Symmetry Between Subacute Post-stroke Patients With Bioelectrically-Controlled Exoskeleton Gait Training and Conventional Gait Training. Front. Bioeng. Biotechnol. 2020, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- Bernshtein, N.A. The Coordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- Scholz, J.P.; Schöner, G. The Uncontrolled Manifold Concept: Identifying Control Variables for a Functional Task. Exp. Brain Res. 1999, 126, 289–306. [Google Scholar] [CrossRef]
- Schoner, G. Recent Developments and Problems in Human Movement Science and Their Conceptual Implications. Ecol. Psychol. 1995, 7, 291–314. [Google Scholar] [CrossRef]
- Sainburg, R.L. Should the Equilibrium Point Hypothesis (EPH) Be Considered a Scientific Theory? Motor Control 2015, 19, 142–148. [Google Scholar] [CrossRef][Green Version]
- Hinder, M.R.; Milner, T.E. The Case for an Internal Dynamics Model versus Equilibrium Point Control in Human Movement. J. Physiol. 2003, 549, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Control of Mental Activities by Internal Models in the Cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef]
- Kawato, M. Internal Models for Motor Control and Trajectory Planning. Curr. Opin. Neurobiol. 1999, 9, 718–727. [Google Scholar] [CrossRef]
- Gillespie, R.B.; Ghasemi, A.H.; Freudenberg, J. Human Motor Control and the Internal Model Principle. IFAC-PapersOnLine 2016, 49, 114–119. [Google Scholar] [CrossRef]
- Ting, L.H.; Chiel, H.J.; Trumbower, R.D.; Allen, J.L.; McKay, J.L.; Hackney, M.E.; Kesar, T.M. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron 2015, 86, 38–54. [Google Scholar] [CrossRef]
- Mussa–Ivaldi, F.A.; Bizzi, E. Motor Learning through the Combination of Primitives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1755–1769. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd, A.T.; Singh, R.E.; Iqbal, K.; White, G. A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation. Biomechanics 2021, 1, 253-263. https://doi.org/10.3390/biomechanics1020021
Abd AT, Singh RE, Iqbal K, White G. A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation. Biomechanics. 2021; 1(2):253-263. https://doi.org/10.3390/biomechanics1020021
Chicago/Turabian StyleAbd, Ashar Turky, Rajat Emanuel Singh, Kamran Iqbal, and Gannon White. 2021. "A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation" Biomechanics 1, no. 2: 253-263. https://doi.org/10.3390/biomechanics1020021
APA StyleAbd, A. T., Singh, R. E., Iqbal, K., & White, G. (2021). A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation. Biomechanics, 1(2), 253-263. https://doi.org/10.3390/biomechanics1020021






