Technologies and Sensor Design for the Measurement of Ground Reaction Forces in Mice: A Review
Abstract
1. Introduction
2. Methods
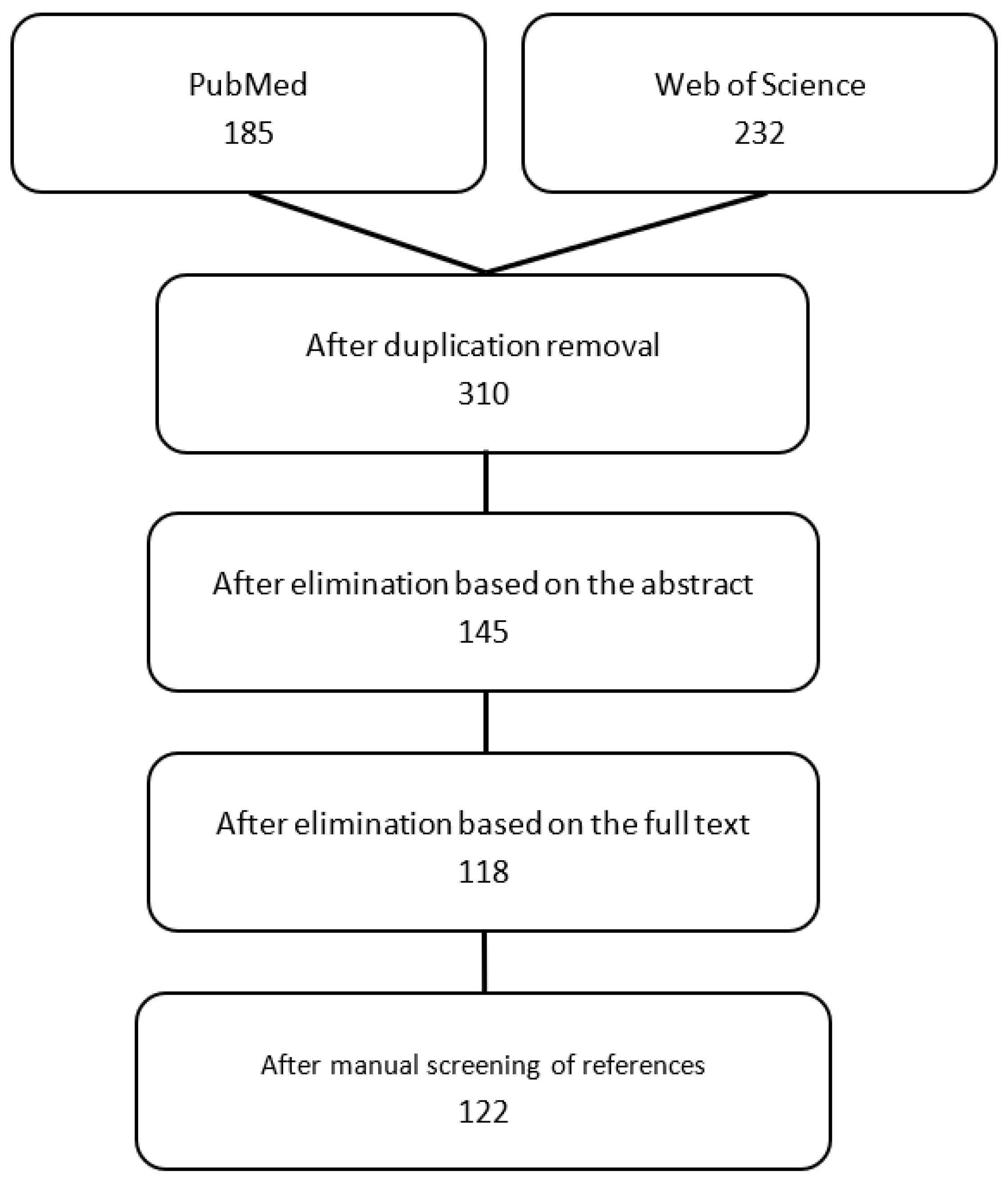
2.1. Literature Selection
2.2. Criteria for Platform Classification
- -
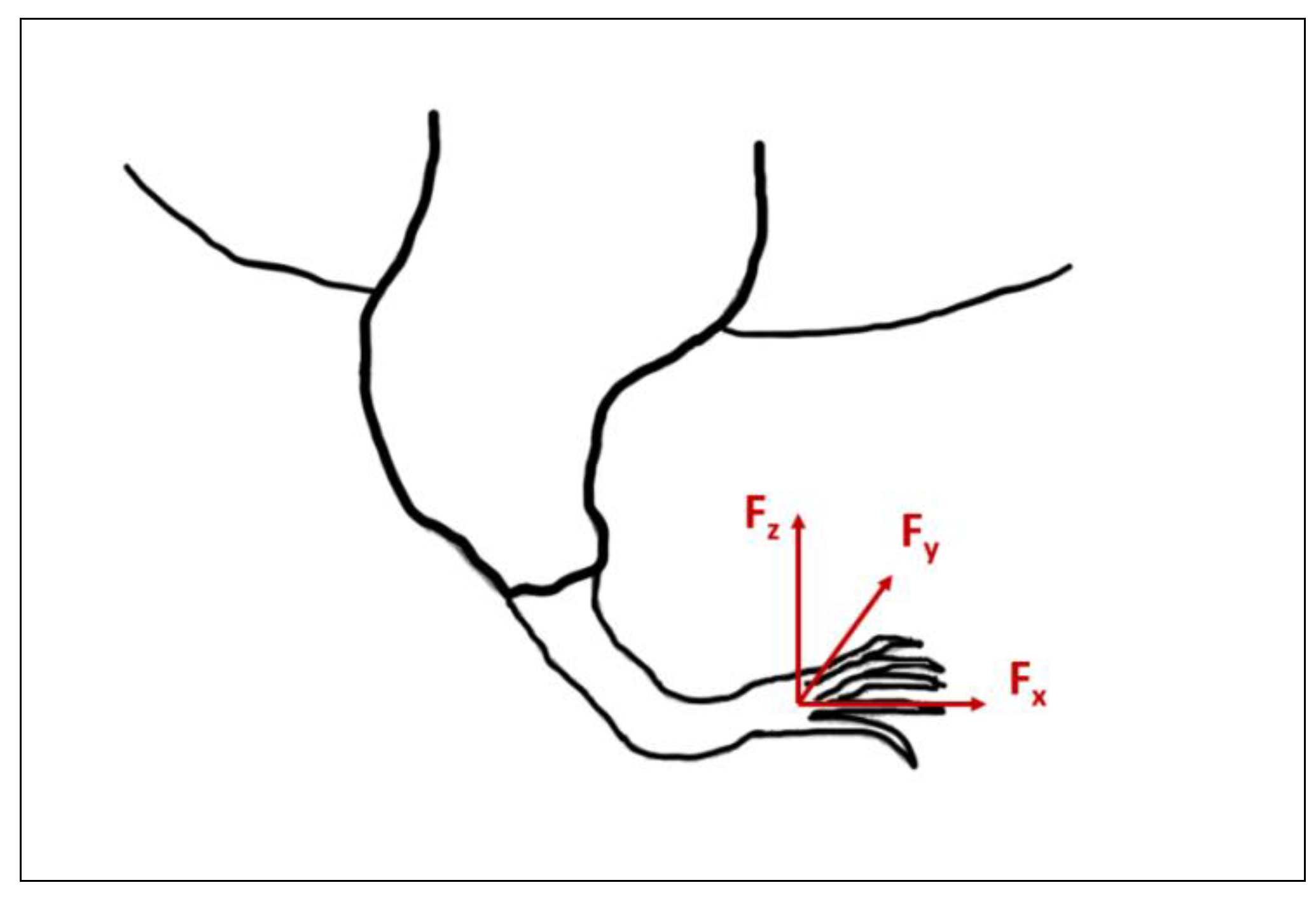
- Independent measurement of 3 orthogonal components of the GRF.
- -
- Determination of the centre of pressure (CoP).
- -
- Low ‘crosstalk’ between the components: where an upper limit of 3% crosstalk was considered acceptable [26].
- -
- Sensing technique (load cells, strain gauges, gelatin slabs, water sensors…) and how easily the technology can be adapted for use in the assessment of mice GRFs.
- -
- Sufficient sensitivity and resolution: the expected forces for a 20 g mouse are 0.01 N to 0.12 N for the vertical direction, 0.004 N to 0.036 N for the fore-aft direction, and 0.002 N to 0.02 N for the mediolateral direction [27]. Accordingly, the force plate needs to have a minimum resolution of 10 mN in the vertical direction and 2 mN for the horizontal directions, while for sensitivity, 10 V/N should be sufficient.
- -
- Linear response: According to the expected forces, the linear response should cover a range of about 0.008 N to 0.15 N for vertical forces and a range of 0.001 to 0.05 N for horizontal forces.
- -
- Uniform response over the plate surface: Previous research suggests a maximum variation of 3% for mice gait [26], even though the origin of this number was not further discussed.
- -
- High natural frequency: If the mouse gait contains any frequencies at the natural frequency of the plate, this will lead to resonance, which causes high noise or could even damage the mechanical structure. Thus, it is important to ensure that the plate’s natural frequency is well above any frequency components of the mouse movement under assessment. During mice gait, frequencies up to 30 Hz [28] can occur, and therefore, the natural frequency of the force plate should be at least 100 Hz.
- -
- Force plate size: A mouse hind paw about 17.5 mm long and about 5.8 mm wide [23], while the stride length is around 60 mm, which is defined as the distance from the centre of the front paw to the centre of the ipsilateral hind paw. So, in order to measure the forces per paw, the top plate on which the animal will step cannot be smaller than 18 mm to fit a whole paw and not bigger than 20 mm to avoid overlapping paws for full-width plates (three paws per stride length). If the separate left and right plates are used in the runway, the plates can be 30 mm long (two paws per stride length).
3. Results and Discussion
3.1. Running Wheels
| GRF Components | Sensing Techniques | Sensitivity/Resolution | Crosstalk | Linearity of the Response | Natural Frequency | Variation across the Platform | Shape and Size | Application | Complexity Level of Adaptation | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Fx and Fz | Resistance strain gauges | Vertical: 1.5%; Fore-aft: 38% | Tested between 10 mN and 402 mN | Strain magnitude varied linearly (R2 > 0.99) | A standard stainless-steel mouse running wheel | Mice | Cannot be adapted | [34] | ||
| Fx, Fy and Fz | Hall-effect sensors | 94.3 ± 12.1 Hz | Running Wheel | Mice | Hard to adapt | [35] |
3.2. Flat Force Plates
3.2.1. Devices That Can Measure Only the Vertical Component
3.2.2. Devices That Can Measure Two Components of the GRF
| GRF Components | Sensing Techniques | Sensitivity/Resolution | Crosstalk | Linearity of the Response | Natural Frequency | Variation Across the Platform | Shape and Size | Application | Complexity Level of Adaptation | References and Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Fz and Fx | Semiconductor strain gauges | Resolution 0.5 mN | <2% | In the range 0.001–0.1 N | 400–650 Hz | Less than 7% | 107 mm × 60 mm | Cockroach | Can be adapted | [39,50,51] |
| MEMS Piezoresistive strain gauges | Resolution 1 μN | 560 Hz | 2 mm × 0.98 mm | Ants | Hard to adapt | [44] | ||||
| Resistance strain gauges | <2% | 180 Hz (the large plates) 300 Hz (the lighter plates) | 2% | Runway each plate 250 mm × 250 mm | Kangaroo rats | Hard to adapt | [41,42,43] | |||
| Fx and Fy | IR-emitting diode and a phototransistor | Resolution 1 mN | Linear in the range of ±100 µm | 100 Hz | 150 mm in diameter | Mice | Hard to adapt | [21,28,46,52] | ||
| Fz and (Fx or Fy) | Resistance strain gauges | 5% | 11% | Area 220 mm2 | Crayfish | Hard to adapt | [45] |
3.2.3. Devices That Can Measure Three Components of the GRF
- Load-cell based devices
- Resistance and semiconductor strain gauge based devices
| GRF Components | Sensing Techniques | Sensitivity/Resolution | Crosstalk | Linearity of the Response | Natural Frequency | Variation Across the Platform | Shape and Size | Application | Complexity Level of Adaptation | References and Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Fv, Fx, and Fy | Resistance strain gauges | 8.5 V/N in the Z direction and 5.0 V/N in the Y and X directions | Between vertical and lateral 2% | In the range of 0.1 to 4 N | 105 mm × 105 mm | Rats | Can be adapted | [26,27,55,71,72,73,74,75,76,77,78,79,80,81] Was also used in runways | ||
| Resistance strain gauges | Resolution 500 mN | <5% | In the range of 0.1 to 4 N | 240 Hz | <7% | 100 mm × 80 mm | Chicks | Can be adapted | [26,40,56] | |
| Resistance strain gauges | 16% between horizontal directions | ≥128 Hz | 74.6 mm × 155 mm | Vampire bats | Can be adapted | [58,82] | ||||
| Resistance strain gauges | Resolution 2 mN in the X and Y directions and 3 mN in the Z direction | <5% | 277 Hz | 30 mm × 30 mm | Lizard Frog | Can be adapted | [60,61,62,63,64,83,84,85] | |||
| Semiconductor strain gauges | <5% | In the range of 0.0001–0.1 N | 400 Hz | 110 mm × 60 mm | Gecko | Can be adapted | [57] | |||
| Semiconductor strain gauges | Resolution Fx = 5.4 μN, Fy = 2.9 μN and Fv = 10.8 μN | 4%–6%. | 201 Hz | 4 mm × 4 mm | Ants | Can be adapted | [30,65,86,87] | |||
| Semiconductor stain gauges | Sensitivity 12.60 V/N | 3% | 527 Hz | 15 mm × 7.5 mm | Locust | Can be adapted | [59,88] | |||
| Resistance strain gauges | Resolution 15 mN | 200 mm × 600 mm | Lizard | Hard to adapt | [68,69] | |||||
| Resistance strain-gauges | Resolution 0.026 N, in the Z direction, 0.062 N in the X direction, and 0.095 N in the Y direction | 3.2% of vertical in the X direction and 4.4% of the vertical force in the Y direction | Lizard | Hard to adapt | [70] Supplementary information of [70] | |||||
| Resolution 0.05 N in the Z direction, 0.03 N in the X direction, and 0.02 N in the Y direction | Vertical force was 3.2% in the X direction and 4.4% in the Y direction | Bird | Hard to adapt | |||||||
| Semiconductor strain gauges | Resolution 0.05 mN | 5 mm × 5 mm | Stick insect | Hard to adapt | [66,67,89,90] | |||||
| Semiconductor strain gauges for X and Y directions + Water pressure sensors for the Z-direction | In the range 0–3 mN for the X and Y directions and in the range 0–5 mN for the Z direction | Stick insect and cockroaches | Hard to adapt | [91] | ||||||
| MEMS piezoresistive strain gauges | Sensitivity of 55 V/N in the vertical direction and 12 V/N in horizontal directions | Linear in the in range of 1–100 mN | 900 Hz | 5.3 mm square plate | Cockroach | Hard to adapt | [92] |
| GRF Components | Sensing Techniques | Sensitivity/Resolution | Crosstalk | Linearity of the Response | Natural Frequency | Variation Across the Platform | Shape and Size | Application | Complexity Level of Adaptation | References and Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Fz | Uniaxial load cell | Resolution 0.02 N | Range of measurement 0–2.5 N | Force plate 300 mm × 300 mm | Mice | Cannot be adapted | [93,94,95,96] | |||
| Uniaxial load cell | Sensitivity: 112, 410 mV/kN | Force plate 460 mm × 510 mm | Rats | Cannot be adapted | [97] | |||||
| uniaxial load cells | Force plate 177.8 mm × 177.8 mm | Mice Rats | Cannot be adapted | [98,99] | ||||||
| Uniaxial lBased i1oad cell | Linear between 0–1.94 N | Runway of 1000 mm–1200 mm length and adjustable width | Cannot be adapted | [100,101,102,103,104] | ||||||
| Strain gauges load cell | Runway of 420 mm with four force plate of 38 mm × 30 mm each | Mice | Cannot be adapted | [105] | ||||||
| load cells 5-OMEGA, model LCL-227G | Elevated force plates 40 mm × 40 mm each | Rats | Cannot be adapted | [106] | ||||||
| Resistance strain gauges | <2% | linearity 99% | Glass plate under the mouse cage | Mice | Cannot be adapted | [107] | ||||
| Fz and Fx | Semiconductor strain gauges | 80 Hz | 5 mm × 5 mm | Ants | Cannot be adapted | [49] | ||||
| Gelatin slab (Photoelastic material) between polarizing filter and a light source) | 102 mm × 305 mm 286 mm × 286 mm 245 mm × 245 mm | Cockroaches | Cannot be adapted | [47,48] | ||||||
| Uniaxial force sensors | Beetles | Insufficient information | [108] | |||||||
| Strain gauges | Caterpillars | Insufficient information | [109,110] | |||||||
| Strain gauges | Rats | Insufficient information | [111] | |||||||
| Fv, Fx and Fz | Load cell (Kistler platform 9286A) | Resolution Fz < 250 mN | <0.05% between vertical and lateral | Linearity of 0.5% for a range of −2.5 kN to 2.5 kN for lateral direction and a range of 0 to 10 kN for the vertical direction | 200 Hz | 400 mm × 600 mm | Rats | Cannot be adapted | [112] data sheet of Kistler 9286A | |
| Load cell | Sensitivity 2 mV/V | Runway with four force plate | Rats | Cannot be adapted | [113] | |||||
| Load cell (kistler, type 9251A) | Resolution 0.01 N | 1% between vertical and lateral | Range of measurement 0–2500 N | Runway 1200 mm × 88 mm | Rats | Cannot be adapted | [114,115] Datasheet | |||
| Load cells | Runway of 760 mm × 80 mm | Rats | Cannot be adapted | [11,25,116,117] | ||||||
| Load cell FT3/10 ATI | Resolution 0.01 N | Runway of four separated force plates | Rats | Cannot be adapted | [118,119,120,121] | |||||
| Load cell FSG15N1A Honeywell | Sensitivity 0.24 V/N | The measuring range of 0–1500 g | Sensor array in a runway of 125 mm × 75 mm | Rats | Cannot be adapted | [122] Datasheet | ||||
| Load cell Nano43, ATI Industrial Automation | Resolution 1/512 N | Rats | Cannot be adapted | [123] Sensor Specifications | ||||||
| Load cell (nano 17, ATI) | Resolution 1/160 N | 70 mm × 150 mm | Rats | Cannot be adapted | [124] Sensor Specification | |||||
| Load cell (ATI nano17) | Resolution 300 mN | 200 Hz | 80 mm × 9 mm | Small Birds/Lizard/Frogs | Cannot be adapted | [125,126,127,128,129,130] | ||||
| Load cell AMTI MC3A-100 | Sensitivity Fv = 1.35 µV/(V × N), Fx = Fy 5.4 µV/(V × N) | <2% | 300 Hz | 150 mm × 150 mm | Frog | Cannot be adapted | [54,131] | |||
| Load cell kistler force plates | threshold Fz <250 mN | <2% | In the range of 2.5 to 2.5 kN in X and Y and 0 to 10 kN in Z | 200 Hz | 400 mm × 600 mm | Birds | Cannot be adapted | [132] + data sheet of Kistler 9286A | ||
| Load cell kistler force plates | Resolution ±0.01 N | 200 Hz | 200 mm × 100 mm | Birds | Cannot be adapted | [53] | ||||
| Load cell Bertec force plate | Sensitivities 5 mN for horizontal and 10 mN for vertical force components | The measurement range −10 to 10 kN | 800 Hz | 400 mm × 600 mm 150 mm × 150 mm | Birds | Cannot be adapted | [133,134,135] [53,136] | |||
| Hall Effect (HE6X6 by AMTI) | Resolution 2.5 mN | 1% in the X and Y and 2% in the Z direction | 38 Hz | 152 mm × 152 mm 105 mm × 110 mm | Rats Mice | Cannot be adapted | [24,137,138,139,140,141,142,143,144,145,146,147] | |||
| Load cell | 600 mm × 600 mm | Marmosets | Insufficient information | [148] | ||||||
| Strain gauges | 600 mm × 200 mm | Lizards | Insufficient information | [69] | ||||||
| Strain gauges | cylindrical sensitive region of 38 mm | Opossums | Insufficient information | [149,150] | ||||||
| Not mentioned | Resolution 1 mN | Locust | Insufficient information | [151] |
4. Synopsis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef]
- Cronin, J.B.; Bressel, E.; Fkinn, L. Augmented Feedback Reduces Ground Reaction Forces in the Landing Phase of the Volleyball Spike Jump. J. Sport Rehabil. 2008, 17, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Bissas, A. Ground reaction forces of Olympic and World Championship race walkers. Eur. J. Sport Sci. 2014, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Wang, R.-L.; Liou, D.-J.; Shaw, J.-S. Gait Disorders in Parkinson’s Disease: Assessment and Management. Int. J. Gerontol. 2013, 7, 189–193. [Google Scholar] [CrossRef]
- Muniz, A.M.S.; Liu, H.; Lyons, K.E.; Pahwa, R.; Liu, W.; Nadal, J. Quantitative Evaluation of the Effects of Subthalamic Stimulation on Gait in Parkinson’s Disease Patients Using Principal Component Analysis. Int. J. Neurosci. 2010, 120, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mora-Macías, J.; Reina-Romo, E.; Morgaz, J.; Dominguez, J. In Vivo Gait Analysis During Bone Transport. Ann. Biomed. Eng. 2015, 43, 2090–2100. [Google Scholar] [CrossRef]
- Gaudreault, N.; Gravel, D.; Nadeau, S.; Houde, S.; Gagnon, D. Gait patterns comparison of children with Duchenne muscular dystrophy to those of control subjects considering the effect of gait velocity. Gait Posture 2010, 32, 342–347. [Google Scholar] [CrossRef]
- Kloefkorn, H.E.; Jacobs, B.Y.; Loye, A.M.; Allen, K.D. Spatiotemporal gait compensations following medial collateral ligament and medial meniscus injury in the rat: Correlating gait patterns to joint damage. Arthritis Res. 2015, 17, 287. [Google Scholar] [CrossRef]
- Yusof, M.I.; Shaharudin, S.; Sivalingarajah, P. Does Vertical Ground Reaction Force of the Hip, Knee, and Ankle Joints Change in Patients with Adolescent Idiopathic Scoliosis after Spinal Fusion? Asian Spine J. 2018, 12, 349–355. [Google Scholar] [CrossRef]
- Nejad, Z.I.; Khalili, K.; Nasab, S.H.H.; Schütz, P.; Damm, P.; Trepczynski, A.; Taylor, W.R.; Smith, C.R. The Capacity of Generic Musculoskeletal Simulations to Predict Knee Joint Loading Using the CAMS-Knee Datasets. Ann. Biomed. Eng. 2020, 48, 1430–1440. [Google Scholar] [CrossRef]
- Tang, W.; Lovering, R.M.; Roche, J.A.; Bloch, R.J.; Neerchal, N.K.; Tasch, U. Gait analysis of locomotory impairment in rats before and after neuromuscular injury. J. Neurosci. Methods 2009, 181, 249–256. [Google Scholar] [CrossRef][Green Version]
- Brand, R.V.D.; Heutschi, J.; Barraud, Q.; DiGiovanna, J.; Bartholdi, K.; Huerlimann, M.; Friedli, L.; Vollenweider, I.; Moraud, E.M.; Duis, S.; et al. Restoring Voluntary Control of Locomotion after Paralyzing Spinal Cord Injury. Science 2012, 336, 1182–1185. [Google Scholar] [CrossRef]
- Scheuren, A.C.; Vallaster, P.; Kuhn, G.A.; Paul, G.R.; Malhotra, A.; Kameo, Y.; Müller, R. Mechano-Regulation of Trabecular Bone Adaptation Is Controlled by the Local in vivo Environment and Loga-rithmically Dependent on Loading Frequency. Front. Bioeng. Biotechnol. 2020, 8, 566346. [Google Scholar] [CrossRef]
- Paul, G.R.; Malhotra, A.; Müller, R. Mechanical Stimuli in the Local In Vivo Environment in Bone: Computational Approaches Linking Organ-Scale Loads to Cellular Signals. Curr. Osteoporos. Rep. 2018, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Fokkenrood, S.A.; Menger, J.T.; Van Der Kooij, H.; Veltink, P.H. Evaluation of instrumented shoes for ambulatory assessment of ground reaction forces. Gait Posture 2007, 26, 39–47. [Google Scholar] [CrossRef]
- Liu, T.; Inoue, Y.; Shibata, K.; Shiojima, K. Three-dimensional lower limb kinematic and kinetic analysis based on a wireless sensor system. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 842–847. [Google Scholar]
- Shahabpoor, E.; Pavic, A. Measurement of Walking Ground Reactions in Real-Life Environments: A Systematic Review of Tech-niques and Technologies. Sensors 2017, 17, 2085. [Google Scholar] [CrossRef]
- Robin, D.; Chateau, H.; Falala, S.; Valette, J.; Pourcelot, P.; Ravary, B.; Denoix, J.-M.; Crevier-Denoix, N. Ground reaction forces in the horse at the walk, trot and gallop measured with an instrumented shoe. Comput. Methods Biomech. Biomed. Eng. 2008, 11, 195–196. [Google Scholar] [CrossRef]
- Jay, G.D.; Elsaid, K.A.; Kelly, K.A.; Anderson, S.C.; Zhang, L.; Teeple, E.; Waller, K.A.; Fleming, B.C. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2011, 64, 1162–1171. [Google Scholar] [CrossRef]
- Hsu, J.E.; Reuther, K.E.; Sarver, J.J.; Lee, C.S.; Thomas, S.J.; Glaser, D.L.; Soslowsky, L.J. Restoration of anterior-posterior rotator cuff force balance improves shoulder function in a rat model of chronic massive tears. J. Orthop. Res. 2011, 29, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Campolo, D.; Cavallo, G.; Keller, F.; Accoto, D.; Dario, P.; Guglielmelli, E. Design and development of a miniaturized 2-axis force sensor for tremor analysis during locomotion in small-sized animal models. IEEE 2005, 5, 5054–5057. [Google Scholar]
- Bogert, A.J.V.D. Analysis and simulation of mechanical loads on the human musculoskeletal system: A methodological overview. Exerc. Sport Sci. Rev. 1994, 22, 23–51. [Google Scholar] [CrossRef]
- Wong, J.; Bennett, W.; Ferguson, M.W.; McGrouther, D.A. Microscopic and histological examination of the mouse hindpaw digit and flexor tendon arrangement with 3D recon-struction. J. Anat. 2006, 209, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Zumwalt, A.C.; Hamrick, M.; Schmitt, D. Force plate for measuring the ground reaction forces in small animal locomotion. J. Biomech. 2006, 39, 2877–2881. [Google Scholar] [CrossRef]
- Tasch, U.; Moubarak, P.; Tang, W.; Zhu, L.; Lovering, R.M.; Roche, J.; Bloch, R.J. An Instrument That Simultaneously Measures Spatiotemporal Gait Parameters and Ground Reaction Forces of Loco-moting Rats. In Proceedings of the 9th Biennial Conference on Engineering Systems Design and Analysis, Haifa, Israel, 7–9 July 2008; Volume 2, pp. 45–49. [Google Scholar]
- Biewener, A.; Full, R.J. Force platform and kinematic analysis. Biomech. Struct. Syst. A Pract. Approach 1992, 45–73. [Google Scholar]
- Muir, G.D.; Whishaw, I.Q. ; Whishaw, I.Q. Ground reaction forces in locomoting hemi-parkinsonian rats: A definitive test for impairments and com-pensations. Exp. Brain Res. 1999, 126, 307–314. [Google Scholar] [CrossRef]
- Cavallo, G.; Campolo, D.; Keller, F.; Guglielmelli, E. A Modular Platform for In-plane Ground Reaction Forces Detection in a Mouse Model: Design, Development and Verification. Adv. Robot. 2008, 22, 141–157. [Google Scholar] [CrossRef]
- Costello, K.E.; Guilak, F.; Setton, L.A.; Griffin, T.M. Locomotor activity and gait in aged mice deficient for type IX collagen. J. Appl. Physiol. 2010, 109, 211–218. [Google Scholar] [CrossRef][Green Version]
- Reinhardt, L.; Blickhan, R. Ultra-miniature force plate for measuring triaxial forces in the micronewton range. J. Exp. Biol. 2014, 217, 704–710. [Google Scholar] [CrossRef]
- Cobos, E.J.; Ghasemlou, N.; Araldi, D.; Segal, D.; Duong, K.; Woolf, C.J. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 2012, 153, 876–884. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Fediuc, S.; Campbell, J.E.; Riddell, M.C. Metabolic effects of voluntary wheel running in young and old Syrian golden hamsters. Physiol. Behav. 2006, 87, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.H.; Robbers, Y. Wheel running in the wild. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140210. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.C.; Edke, M.; Griffin, T.M. A novel mouse running wheel that senses individual limb forces: Biomechanical validation and in vivo testing. J. Appl. Physiol. 2012, 113, 627–635. [Google Scholar] [CrossRef]
- Smith, B.J.H.; Cullingford, L.; Usherwood, J.R. Identification of mouse gaits using a novel force-sensing exercise wheel. J. Appl. Physiol. 2015, 119, 704–718. [Google Scholar] [CrossRef]
- Poulet, B. Non-invasive Loading Model of Murine Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Charles, J.P.; Cappellari, O.; Spence, A.J.; Wells, D.J.; Hutchinson, J.R. Muscle moment arms and sensitivity analysis of a mouse hindlimb musculoskeletal model. J. Anat. 2016, 229, 514–535. [Google Scholar] [CrossRef]
- Charles, J.P.; Cappellari, O.; Spence, A.J.; Hutchinson, J.R.; Wells, D.J. Musculoskeletal Geometry, Muscle Architecture and Functional Specialisations of the Mouse Hindlimb. PLoS ONE 2016, 11, e0147669. [Google Scholar] [CrossRef] [PubMed]
- Full, R.J.; Tu, M.S. Mechanics of six-legged runners. J. Exp. Biol. 1990, 148, 129–146. [Google Scholar]
- Muir, G.D.; Katz, S.L.; Gosline, J.M.; Steeves, J.D. Asymmetric bipedal locomotion--an adaptive response to incomplete spinal injury in the chick. Exp. Brain Res. 1998, 122, 275–282. [Google Scholar] [CrossRef]
- Heglund, N.C. A Simple Design for a Force-Plate to Measure Ground Reaction Forces. J. Exp. Biol. 1981, 93, 333–338. [Google Scholar]
- Biewener, A.A.; Blickhan, R.; Perry, A.K.; Heglund, N.C.; Taylor, C.R. Muscle forces during locomotion in kangaroo rats: Force platform and tendon buckle measurements compared. J. Exp. Biol. 1988, 137, 191–205. [Google Scholar]
- Biewener, A.A.; Blickhan, R. Kangaroo rat locomotion: Design for elastic energy storage or acceleration? J. Exp. Biol. 1988, 140, 243–255. [Google Scholar]
- Takahashi, H.; Thanh-Vinh, N.; Jung, U.G.; Matsumoto, K.; Shimoyama, I. MEMS two-axis force plate array used to measure the ground reaction forces during the running motion of an ant. J. Micromech. Microeng. 2014, 24, 65014. [Google Scholar] [CrossRef]
- Klarner, D.; Barnes, W.J.P. The Cuticular Stress Detector (Csd2) of the Crayfish Activity during Walking and Influences on Leg Coordination. J. Exp. Biol. 1986, 122, 161–175. [Google Scholar]
- Campolo, D.; Cavallo, G.; Keller, F.; Accoto, D.; Dario, P.; Guglielmelli, E. A mechatronic system for in-plane ground-reaction-force measurement for tremor analysis in animal models. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, AB, Canada, 2–6 August 2005; Volume 1–4, pp. 2505–2510. [Google Scholar]
- Full, R.; Yamauchi, A.; Jindrich, D. Maximum single leg force production: Cockroaches righting on photoelastic gelatin. J. Exp. Biol. 1995, 198, 2441–2452. [Google Scholar] [PubMed]
- Jindrich, D.L.; Full, R.J. Many-legged maneuverability: Dynamics of turning in hexapods. J. Exp. Biol. 1999, 202, 1603–1623. [Google Scholar] [PubMed]
- Endlein, T.; Federle, W. On Heels and Toes: How Ants Climb with Adhesive Pads and Tarsal Friction Hair Arrays. PLoS ONE 2015, 10, e0141269. [Google Scholar] [CrossRef] [PubMed]
- Full, R.J.; Tu, M.S. Mechanics of a rapid running insect: Two-, four- and six-legged locomotion. J. Exp. Biol. 1991, 156, 215–231. [Google Scholar]
- Full, R.J.; Blickhan, R.; Ting, L.H. Leg design in hexapedal runners. J. Exp. Biol. 1991, 158, 369–390. [Google Scholar]
- Cavallo, G.; Campolo, D.; Guglielmelli, E.; Vollaro, S.; Keller, F. Mechatronics and Phenomics: A case-study on tremor detection during locomotion in small-sized animals. In Proceedings of the the First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, BioRob, Pisa, Italy, 20–22 February 2006; Volume 1–3, p. 376. [Google Scholar]
- Provini, P.; Tobalske, B.W.; Crandell, K.E.; Abourachid, A. Transition from leg to wing forces during take-off in birds. J. Exp. Biol. 2012, 215, 4115–4124. [Google Scholar] [CrossRef]
- Nauwelaerts, S.; Aerts, P. Take-off and landing forces in jumping frogs. J. Exp. Biol. 2006, 209, 66–77. [Google Scholar] [CrossRef]
- Muir, G.D.; Whishaw, I.Q. Complete locomotor recovery following corticospinal tract lesions: Measurement of ground reaction forces during overground locomotion in rats. Behav. Brain Res. 1999, 103, 45–53. [Google Scholar] [CrossRef]
- Muir, G.D.; Gosline, J.M.; Steeves, J.D. Ontogeny of bipedal locomotion: Walking and running in the chick. J. Physiol. 1996, 493, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Peattie, A.M.; Autumn, K.; Full, R.J. Differential leg function in a sprawled-posture quadrupedal trotter. J. Exp. Biol. 2006, 209, 249–259. [Google Scholar] [CrossRef]
- Riskin, D.K.; Bertram, J.E.A.; Hermanson, J.W. Testing the hindlimb-strength hypothesis: Non-aerial locomotion by Chiroptera is not constrained by the dimensions of the femur or tibia. J. Exp. Biol. 2005, 208, 1309–1319. [Google Scholar] [CrossRef]
- Katz, S.L. and J.M. Gosline, Ontogenic Scaling of Jump Performance in the African Desert Locust (Schistocerca-Gregaria). J. Exp. Biol. 1993, 177, 81–111. [Google Scholar]
- Dai, Z.; Wang, Z.; Ji, A. Dynamics of gecko locomotion: A force-measuring array to measure 3D reaction forces. J. Exp. Biol. 2011, 214, 703–708. [Google Scholar] [CrossRef]
- Endlein, T.; Ji, A.; Yuan, S.; Hill, I.; Wang, H.; Barnes, W.J.P.; Dai, Z.; Sitti, M. The use of clamping grips and friction pads by tree frogs for climbing curved surfaces. Proc. R. Soc. B Boil. Sci. 2017, 284, 20162867. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Yuan, S.; Endlein, T.; Hill, I.D.C.; Wang, W.; Wang, H.; Jiang, N.; Zhao, Z.; Barnes, W.J.P.; Dai, Z.; et al. A force-measuring and behaviour-recording system consisting of 24 individual 3D force plates for the study of single limb forces in climbing animals on a quasi-cylindrical tower. Bioinspiration Biomim. 2019, 14, 046004. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, A.; Endlein, T.; Samuel, D.; Yao, N.; Wang, Z.; Dai, Z. The role of fore- and hindlimbs during jumping in the Dybowski’s frog (Rana dybowskii). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 324–333. [Google Scholar] [CrossRef]
- Wang, Z.; Gorb, S.N.; Dai, Z. Control strategies of gecko’s toe in response to reduced gravity. Appl. Sci. 2020, 10, 2257. [Google Scholar] [CrossRef]
- Reinhardt, L.; Blickhan, R. Level locomotion in wood ants: Evidence for grounded running. J. Exp. Biol. 2014, 217, 2358–2370. [Google Scholar] [CrossRef]
- Dallmann, C.J.; Hoinville, T.; Dürr, V.; Schmitz, J. A load-based mechanism for inter-leg coordination in insects. Proc. R. Soc. B Boil. Sci. 2017, 284, 20171755. [Google Scholar] [CrossRef]
- Dallmann, C.J.; Dürr, V.; Schmitz, J. Motor control of an insect leg during level and incline walking. J. Exp. Biol. 2019, 222, jeb188748. [Google Scholar] [CrossRef]
- McElroy, E.J.; Wilson, R.; Biknevicius, A.R.; Reilly, S.M. A comparative study of single-leg ground reaction forces in running lizards. J. Exp. Biol. 2013, 217, 735–742. [Google Scholar] [CrossRef]
- McElroy, E.J.; Reilly, S.M. The relationship between limb morphology, kinematics, and force during running: The evolution of loco-motor dynamics in lizards. Biol. J. Linn. Soc. 2009, 97, 634–651. [Google Scholar] [CrossRef]
- Clemente, C.J.; Bishop, P.J.; Newman, N.; Hocknull, S.A. Steady bipedal locomotion with a forward situated whole-body centre of mass: The potential importance of tempo-rally asymmetric ground reaction forces. J. Zool. 2018, 304, 193–201. [Google Scholar] [CrossRef]
- Kanagal, S.G.; Muir, G.D. Bilateral dorsal funicular lesions alter sensorimotor behaviour in rats. Exp. Neurol. 2007, 205, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.W.; Lanovaz, J.L.; Muir, G.D. The biomechanics of locomotor compensation after peripheral nerve lesion in the rat. Behav. Brain Res. 2012, 229, 391–400. [Google Scholar] [CrossRef]
- Webb, A.A.; Muir, G.D. Compensatory Locomotor Adjustments of Rats with Cervical or Thoracic Spinal Cord Hemisections. J. Neurotrauma 2002, 19, 239–256. [Google Scholar] [CrossRef]
- Webb, A.A.; Muir, G.D. Course of motor recovery following ventrolateral spinal cord injury in the rat. Behav. Brain Res. 2004, 155, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kanagal, S.; Muir, G. The differential effects of cervical and thoracic dorsal funiculus lesions in rats. Behav. Brain Res. 2008, 187, 379–386. [Google Scholar] [CrossRef]
- Muir, G.D.; Webb, A.A.; Kanagal, S.; Taylor, L. Dorsolateral cervical spinal injury differentially affects forelimb and hindlimb action in rats. Eur. J. Neurosci. 2007, 25, 1501–1510. [Google Scholar] [CrossRef]
- Webb, A.A.; Gowribai, K.; Muir, G.D. Fischer (F-344) rats have different morphology, sensorimotor and locomotor abilities com-pared to Lewis, Long-Evans, Sprague-Dawley and Wistar rats. Behav. Brain Res. 2003, 144, 143–156. [Google Scholar] [CrossRef]
- Wehner, T.; Wolfram, U.; Henzler, T.; Niemeyer, F.; Claes, L.; Simon, U. Internal forces and moments in the femur of the rat during gait. J. Biomech. 2010, 43, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Muir, G.D.; Whishaw, I.Q. Red nucleus lesions impair overground locomotion in rats: A kinetic analysis. Eur. J. Neurosci. 2000, 12, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Kanagal, S.G.; Muir, G.D. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp. Neurol. 2009, 216, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.A.; Muir, G.D. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur. J. Neurosci. 2003, 18, 412–422. [Google Scholar] [CrossRef]
- Riskin, D.K.; Parsons, S.; Schutt, W.A.; Carter, G.G.; Hermanson, J.W. Terrestrial locomotion of the New Zealand short-tailed bat Mystacina tuberculata and the common vampire bat Des-modus rotundus. J. Exp. Biol. 2006, 209, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Endlein, T.; Ji, A.; Samuel, D.; Yao, N.; Wang, Z.; Barnes, W.J.P.; Federle, W.; Kappl, M.; Dai, Z. Sticking like sticky tape: Tree frogs use friction forces to enhance attachment on overhanging surfaces. J. R. Soc. Interface 2013, 10, 20120838. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z.; Ji, A.; Ren, L.; Xing, Q.; Dai, L. Biomechanics of gecko locomotion: The patterns of reaction forces on inverted, vertical and horizontal substrates. Bioinspir. Biomim. 2015, 10, 16019. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z.; Li, W.; Ji, A.; Wang, W. How do the substrate reaction forces acting on a gecko’s limbs respond to inclines? Naturwissenschaften 2015, 102, 7–1259. [Google Scholar] [CrossRef]
- Reinhardt, L.; Weihmann, T.; Blickhan, R. Dynamics and kinematics of ant locomotion: Do wood ants climb on level surfaces? J. Exp. Biol. 2009, 212, 2426–2435. [Google Scholar] [CrossRef] [PubMed]
- Wöhrl, T.; Reinhardt, L.; Blickhan, R. Propulsion in hexapod locomotion: How do desert ants traverse slopes? J. Exp. Biol. 2017, 220, 1618–1625. [Google Scholar] [CrossRef]
- Katz, S.L.; Gosline, J.M. Scaling Modulus as a Degree-of-Freedom in the Design of Locust Legs. J. Exp. Biol. 1994, 187, 207–223. [Google Scholar] [PubMed]
- Dallmann, C.J.; Dürr, V.; Schmitz, J. Joint torques in a freely walking insect reveal distinct functions of leg joints in propulsion and posture control. Proc. R. Soc. B Boil. Sci. 2016, 283, 20151708. [Google Scholar] [CrossRef]
- Lévy, J.; Cruse, H. Controlling a system with redundant degrees of freedom. I. Torque distribution in still standing stick insects. J. Comp. Physiol. A 2008, 194, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Bartling, C.; Schmitz, J. Reaction to disturbances of a walking leg during stance. J. Exp. Biol. 2000, 203, 1211–1223. [Google Scholar]
- Bartsch, M.S.; Federle, W.; Full, R.J.; Kenny, T.W. A Multiaxis Force Sensor for the Study of Insect Biomechanics. J. Microelectromech. Syst. 2007, 16, 709–718. [Google Scholar] [CrossRef]
- Fowler, S.; Birkestrand, B.; Chen, R.; Moss, S.; Vorontsova, E.; Wang, G.; Zarcone, T. A force-plate actometer for quantitating rodent behaviors: Illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J. Neurosci. Methods 2001, 107, 107–124. [Google Scholar] [CrossRef]
- Fowler, S.C.; Miller, B.R.; Gaither, T.W.; Johnson, M.A.; Rebec, G.V. Force-plate quantification of progressive behavioral deficits in the R6/2 mouse model of Huntington’s disease. Behav. Brain Res. 2009, 202, 130–137. [Google Scholar] [CrossRef]
- Fowler, S.C.; Muma, N.A. Use of a force-sensing automated open field apparatus in a longitudinal study of multiple behavioral defi-cits in CAG140 Huntington’s disease model mice. Behav. Brain Res. 2015, 294, 7–16. [Google Scholar] [CrossRef][Green Version]
- Dai, Y.; Dudek, N.L.; Li, Q.; Fowler, S.C.; Muma, N.A. Striatal Expression of a Calmodulin Fragment Improved Motor Function, Weight Loss, and Neuropathology in the R6/2 Mouse Model of Huntington’s Disease. J. Neurosci. 2009, 29, 11550–11559. [Google Scholar] [CrossRef]
- Handley, D.; Ross, J.; Carr, G. A force plate system for measuring low-magnitude reaction forces in small laboratory animals. Physiol. Behav. 1998, 64, 661–669. [Google Scholar] [CrossRef]
- Welch, J.M.; Wade, J.A.; Hillberry, B.M.; Weaver, C.M. Force platform for rats measures fore and hind forces concurrently. J. Biomech. 2009, 42, 2734–2738. [Google Scholar] [CrossRef]
- Welch, J.M.; Weaver, C.M.; Turner, C.H. Adaptations to free-fall impact are different in the shafts and bone ends of rat forelimbs. J. Appl. Physiol. 2004, 97, 1859–1865. [Google Scholar] [CrossRef]
- Clarke, K.; Still, J. Development and consistency of gait in the mouse. Physiol. Behav. 2001, 73, 159–164. [Google Scholar] [CrossRef]
- Clarke, K.A.; Still, J. Gait Analysis in the Mouse. Physiol. Behav. 1999, 66, 723–729. [Google Scholar] [CrossRef]
- Clarke, K.A.; Heitmeyer, S.A.; Smith, A.G.; Taiwo, Y.O. Gait analysis in a rat model of osteoarthrosis. Physiol. Behav. 1997, 62, 951–954. [Google Scholar] [CrossRef]
- Clarke, K. Differential fore- and hindpaw force transmission in the walking rat. Physiol. Behav. 1995, 58, 415–419. [Google Scholar] [CrossRef]
- Clarke, K.A.; Smart, L.; Still, J. Ground reaction force and spatiotemporal measurements of the gait of the mouse. Behav. Res. Methodsinstrumentscomput. 2001, 33, 422–426. [Google Scholar] [CrossRef][Green Version]
- Manske, S.L.; Boyd, S.K.; Zernicke, R.F. Vertical ground reaction forces diminish in mice after botulinum toxin injection. J. Biomech. 2011, 44, 637–643. [Google Scholar] [CrossRef]
- Miklyaeva, E.I.; Woodward, N.C.; Nikiforov, E.G.; Tompkins, G.J.; Klassen, F.; Ioffe, M.E.; Whishaw, I.Q. The ground reaction forces of postural adjustments during skilled reaching in unilateral dopamine-depleted hem-iparkinson rats. Behav. Brain Res. 1997, 88, 143–152. [Google Scholar] [CrossRef]
- Rantalainen, T.; Silvennoinen, M.; Kainulainen, H.; Sievänen, H. Vertical ground reaction force measurements and video measurements provide comparable estimates of distance moved by mice during artificial light and dark periods. J. Neurosci. Methods 2011, 197, 104–108. [Google Scholar] [CrossRef]
- Busshardt, P.; Gorb, S.N. Ground reaction forces in vertically ascending beetles and corresponding activity of the claw retractor muscle on smooth and rough substrates. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2014, 200, 385–398. [Google Scholar] [CrossRef]
- Lin, H.T.; Trimmer, B.A. The substrate as a skeleton: Ground reaction forces from a soft-bodied legged animal. J. Exp. Biol. 2010, 213, 1133–1142. [Google Scholar] [CrossRef]
- Vaughan, S.C.; Lin, H.-T.; Trimmer, B.A. Caterpillar Climbing: Robust, Tension-Based Omni-Directional Locomotion. J. Insect Sci. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Hochman, S.; Gozal, E.A.; Hayes, H.B.; Anderson, J.T.; DeWeerth, S.P.; Chang, Y.H. Enabling techniques for in vitro studies on mammalian spinal locomotor mechanisms. Front Biosci. 2012, 17, 2158–2180. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-I.; Sone, T.; Ohnaru, K.; Tanaka, K.; Yamaguchi, H.; Fukunaga, M. Effects of Different Types of Jump Impact on Trabecular Bone Mass and Microarchitecture in Growing Rats. PLoS ONE 2014, 9, e107953. [Google Scholar] [CrossRef]
- Wu, P.T.; Hsu, C.H.; Su, F.C.; Jou, I.M.; Chen, S.Y.; Wu, C.L.; Kuo, L.C. Dynamic weight bearing analysis is effective for evaluation of tendinopathy using a customized corridor with mul-ti-directional force sensors in a rat model. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Howard, C.S.; Blakeney, D.C.; Medige, J.; Moy, O.J.; Peimer, C.A. Functional assessment in the rat by ground reaction forces. J. Biomech. 2000, 33, 751–757. [Google Scholar] [CrossRef]
- Diogo, C.C.; Da Costa, L.M.; Pereira, J.E.; Filipe, V.; Couto, P.A.; Geuna, S.; Armada-Da-Silva, P.A.; Maurício, A.C.; Varejão, A.S. Kinematic and kinetic gait analysis to evaluate functional recovery in thoracic spinal cord injured rats. Neurosci. Biobehav. Rev. 2019, 98, 18–28. [Google Scholar] [CrossRef]
- Tang, W.; McDowell, K.; Limsam, M.; Neerchal, N.K.; Yarowsky, P.; Tasch, U. Locomotion analysis of Sprague–Dawley rats before and after injecting 6-OHDA. Behav. Brain Res. 2010, 210, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tasch, U.; Neerchal, N.K.; Zhu, L.; Yarowsky, P. Measuring early pre-symptomatic changes in locomotion of SOD1-G93A rats—A rodent model of amyotrophic lateral sclerosis. J. Neurosci. Methods 2009, 176, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Giszter, S.F.; Davies, M.R.; Graziani, V. Motor Strategies Used by Rats Spinalized at Birth to Maintain Stance in Response to Imposed Perturbations. J. Neurophysiol. 2007, 97, 2663–2675. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giszter, S.F.; Davies, M.R.; Graziani, V. Coordination strategies for limb forces during weight-bearing locomotion in normal rats, and in rats spinalized as neonates. Exp. Brain Res. 2008, 190, 53–69. [Google Scholar] [CrossRef][Green Version]
- Nessler, J.A.; Moustafa-Bayoumi, M.; Soto, D.; Duhon, J.; Schmitt, R. Assessment of Hindlimb Locomotor Strength in Spinal Cord Transected Rats through Animal-Robot Contact Force. J. Biomech. Eng. 2011, 133, 121007. [Google Scholar] [CrossRef] [PubMed]
- Pardes, A.; Freedman, B.; Soslowsky, L. Ground reaction forces are more sensitive gait measures than temporal parameters in rodents following rotator cuff injury. J. Biomech. 2016, 49, 376–381. [Google Scholar] [CrossRef]
- Silva, N.; Sousa, J.J.; Peres, E.; Sousa, A.; Ruiz-Armenteros, A.M.; Varejão, A.; Morais, R. A cost-effective instrumented walkway for measuring ground reaction forces in rats to assess gait pattern. Measurement 2017, 103, 241–249. [Google Scholar] [CrossRef]
- Song, H.; Polk, J.D.; Kersh, M.E. Rat bone properties and their relationship to gait during growth. J. Exp. Biol. 2019, 222, jeb203554. [Google Scholar] [CrossRef] [PubMed]
- Sarver, J.J.; Dishowitz, M.I.; Kim, S.-Y.; Soslowsky, L.J. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J. Biomech. 2010, 43, 778–782. [Google Scholar] [CrossRef][Green Version]
- Andrada, E.; Nyakatura, J.A.; Bergmann, F.; Blickhan, R. Adjustments of global and local hindlimb properties during terrestrial locomotion of the common quail (Coturnix coturnix). J. Exp. Biol. 2013, 216, 3906–3916. [Google Scholar] [CrossRef]
- Andrada, E.; Rode, C.; Blickhan, R. Grounded running in quails: Simulations indicate benefits of observed fixed aperture angle between legs before touch-down. J. Theor. Biol. 2013, 335, 97–107. [Google Scholar] [CrossRef]
- Andrada, E.; Rode, C.; Sutedja, Y.; Nyakatura, J.A.; Blickhan, R. Trunk orientation causes asymmetries in leg function in small bird terrestrial locomotion. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141405. [Google Scholar] [CrossRef]
- Jagnandan, K.; Russell, A.P.; Higham, T.E. Tail autotomy and subsequent regeneration alter the mechanics of locomotion in lizards. J. Exp. Biol. 2014, 217, 3891–3897. [Google Scholar] [CrossRef]
- Rode, C.; Sutedja, Y.; Kilbourne, B.M.; Blickhan, R.; Andrada, E. Minimizing the cost of locomotion with inclined trunk predicts crouched leg kinematics of small birds at realistic levels of elastic recoil. J. Exp. Biol. 2016, 219, 485–490. [Google Scholar] [CrossRef]
- Porro, L.B.; Collings, A.J.; Eberhard, E.A.; Chadwick, K.P.; Richards, C.T. Inverse dynamic modelling of jumping in the red-legged running frog, Kassina maculata. J. Exp. Biol. 2017, 220, 1882–1893. [Google Scholar] [CrossRef]
- Reilly, S.M.; Montuelle, S.J.; Schmidt, A.; Krause, C.; Naylor, E.; Essner, R.L. Functional evolution of jumping in frogs: Interspecific differences in take-off and landing. J. Morphol. 2016, 277, 379–393. [Google Scholar] [CrossRef]
- Main, R.P.; Biewener, A.A. Skeletal strain patterns and growth in the emu hindlimb during ontogeny. J. Exp. Biol. 2007, 210, 2676–2690. [Google Scholar] [CrossRef]
- Hancock, J.A.; Stevens, N.J.; Biknevicius, A.R. Elegant-crested Tinamous Eudromia elegans do not synchronize head and leg movements during head-bobbing. Ibis 2014, 156, 198–208. [Google Scholar] [CrossRef]
- Body Motion Analysis. In Sensors in Medicine and Health Care; Wiley-VCH: Weinheim, Germany; pp. 243–281.
- Hancock, J.A.; Stevens, N.J.; Biknevicius, A.R. Whole-body mechanics and kinematics of terrestrial locomotion in the Ele-gant-crested Tinamou Eudromia elegans. Ibis 2007, 149, 605–614. [Google Scholar] [CrossRef]
- Provini, P.; Tobalske, B.W.; Crandell, K.E.; Abourachid, A. Transition from wing to leg forces during landing in birds. J. Exp. Biol. 2014, 217, 2659–2666. [Google Scholar] [CrossRef]
- Johnson, W.L.; Jindrich, D.L.; Roy, R.R.; Edgerton, V.R. Quantitative metrics of spinal cord injury recovery in the rat using motion capture, electromyography and ground reaction force measurement. J. Neurosci. Methods 2012, 206, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.W.; Webb, A.A.; Dhaliwal, S.; Syed, S.; Walsh, S.K.; Midha, R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp. Neurol. 2011, 229, 460–470. [Google Scholar] [CrossRef]
- Bain, S.D.; Huber, P.; Ausk, B.J.; Kwon, R.Y.; Gardiner, E.M.; Srinivasan, S.; Gross, T.S. Neuromuscular dysfunction, independent of gait dysfunction, modulates trabecular bone homeostasis in mice. J. Musculoskelet. Neuronal Interact. 2019, 19, 79–93. [Google Scholar] [PubMed]
- Rapp, A.E.; Kroner, J.; Baur, S.; Schmid, F.; Walmsley, A.; Mottl, H.; Ignatius, A. Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. J. Orthop. Res. 2015, 33, 1235–1241. [Google Scholar] [CrossRef]
- Röntgen, V.; Blakytny, R.; Matthys, R.; Landauer, M.; Wehner, T.; Göckelmann, M.; Jermendy, P.; Amling, M.; Schinke, T.; Claes, L.; et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. 2010, 28, 1456–1462. [Google Scholar] [CrossRef]
- Roemhildt, M.L.; Gardner-Morse, M.; Rowell, C.; Beynnon, B.D.; Badger, G.J. Gait alterations in rats following attachment of a device and application of altered knee loading. J. Biomech. 2010, 43, 3227–3231. [Google Scholar] [CrossRef]
- Schmitt, D.; Zumwalt, A.C.; Hamrick, M.W. The relationship between bone mechanical properties and ground reaction forces in normal and hypermuscular mice. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 339–351. [Google Scholar] [CrossRef]
- Alant, J.D.D.V.; Kemp, S.W.P.; Khu, K.J.O.L.; Kumar, R.; Webb, A.A.; Midha, R. Traumatic Neuroma in Continuity Injury Model in Rodents. J. Neurotrauma 2012, 29, 1691–1703. [Google Scholar] [CrossRef]
- Allen, K.D.; Mata, B.A.; Gabr, M.A.; Huebner, J.L.; Adams, S.B.; Kraus, V.B.; Schmitt, D.O.; Setton, L.A. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res. 2012, 14, R78. [Google Scholar] [CrossRef]
- Webb, A.A.; Kerr, B.; Neville, T.; Ngan, S.; Assem, H. Kinematics and Ground Reaction Force Determination: A Demonstration Quantifying Locomotor Abilities of Young Adult, Middle-aged, and Geriatric Rats. J. Vis. Exp. 2011, 2011, e2138. [Google Scholar] [CrossRef]
- Kemp, S.W.P.; Alant, J.; Walsh, S.K.; Webb, A.A.; Midha, R. Behavioural and anatomical analysis of selective tibial nerve branch transfer to the deep peroneal nerve in the rat. Eur. J. Neurosci. 2010, 31, 1074–1090. [Google Scholar] [CrossRef]
- Shimada, H.; Kanai, R.; Kondo, T.; Yoshino-Saito, K.; Uchida, A.; Nakamura, M.; Ogihara, N. Three-dimensional kinematic and kinetic analysis of quadrupedal walking in the common marmoset (Callithrix jac-chus). Neurosci. Res. 2017, 125, 11–20. [Google Scholar] [CrossRef]
- Lammers, A.R. Mechanics of generating friction during locomotion on rough and smooth arboreal trackways. J. Exp. Biol. 2009, 212, 1163–1169. [Google Scholar] [CrossRef][Green Version]
- Lammers, A.R.; Gauntner, T. Mechanics of torque generation during quadrupedal arboreal locomotion. J. Biomech. 2008, 41, 2388–2395. [Google Scholar] [CrossRef]
- Wan, C.; Cao, R.; Hao, Z. The Effect of Ground Type on the Jump Performance of Adults of the Locust Locusta migratoria manilen-sis: A Preliminary Study. Insects 2020, 11, 259. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limam, T.; Vogl, F.; Taylor, W.R. Technologies and Sensor Design for the Measurement of Ground Reaction Forces in Mice: A Review. Biomechanics 2021, 1, 53-72. https://doi.org/10.3390/biomechanics1010005
Limam T, Vogl F, Taylor WR. Technologies and Sensor Design for the Measurement of Ground Reaction Forces in Mice: A Review. Biomechanics. 2021; 1(1):53-72. https://doi.org/10.3390/biomechanics1010005
Chicago/Turabian StyleLimam, Tayssir, Florian Vogl, and William R. Taylor. 2021. "Technologies and Sensor Design for the Measurement of Ground Reaction Forces in Mice: A Review" Biomechanics 1, no. 1: 53-72. https://doi.org/10.3390/biomechanics1010005
APA StyleLimam, T., Vogl, F., & Taylor, W. R. (2021). Technologies and Sensor Design for the Measurement of Ground Reaction Forces in Mice: A Review. Biomechanics, 1(1), 53-72. https://doi.org/10.3390/biomechanics1010005







