Progress in Cellulose-Based Polymer Ionic Conductors: From Performance Optimization to Strain-Sensing Applications
Abstract
1. Introduction
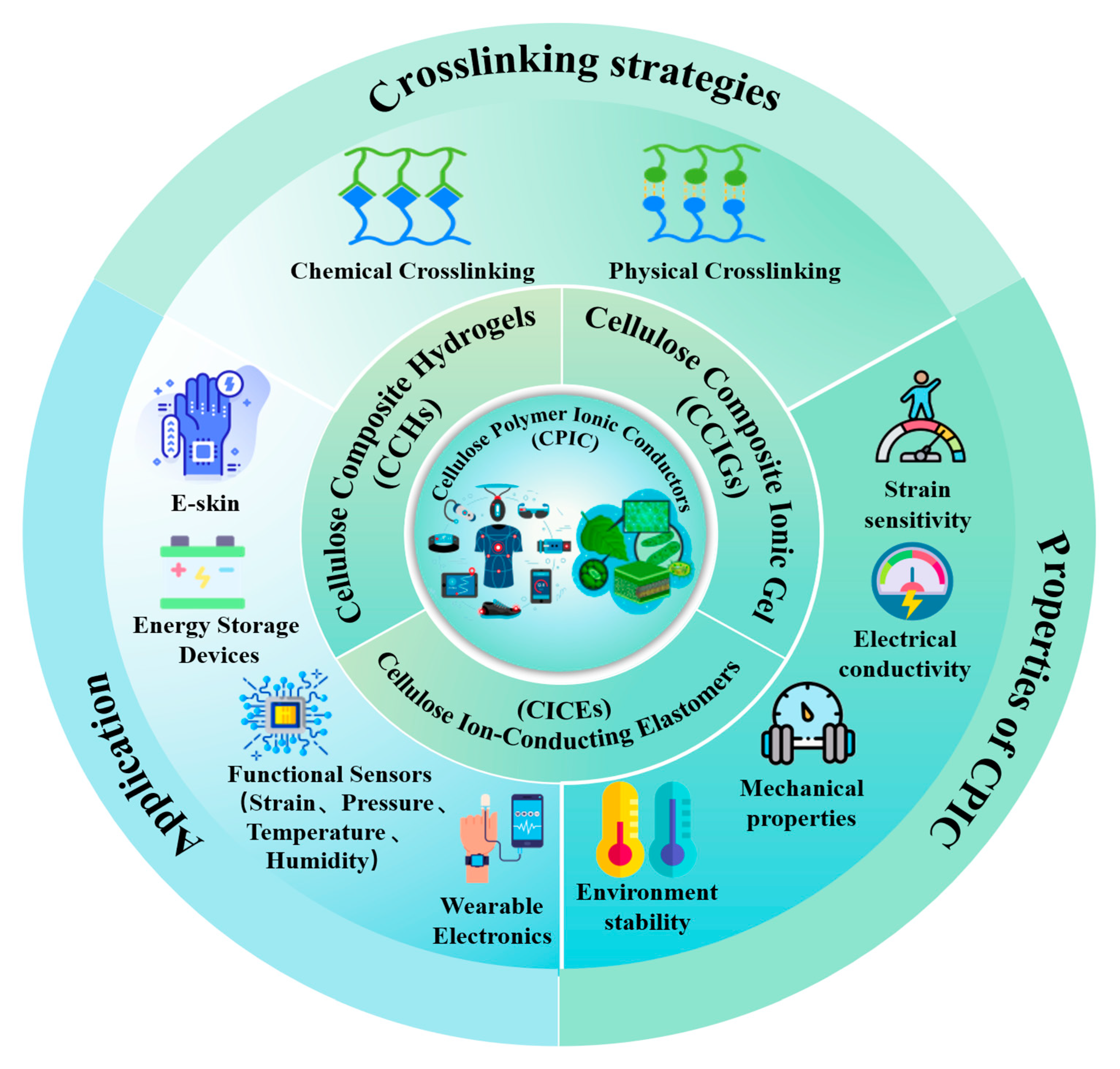
2. Classification of CPIC
2.1. Cellulose Composite Hydrogels
2.2. Cellulose Composite Ionic Gel
2.3. Cellulose Ion-Conducting Elastomer
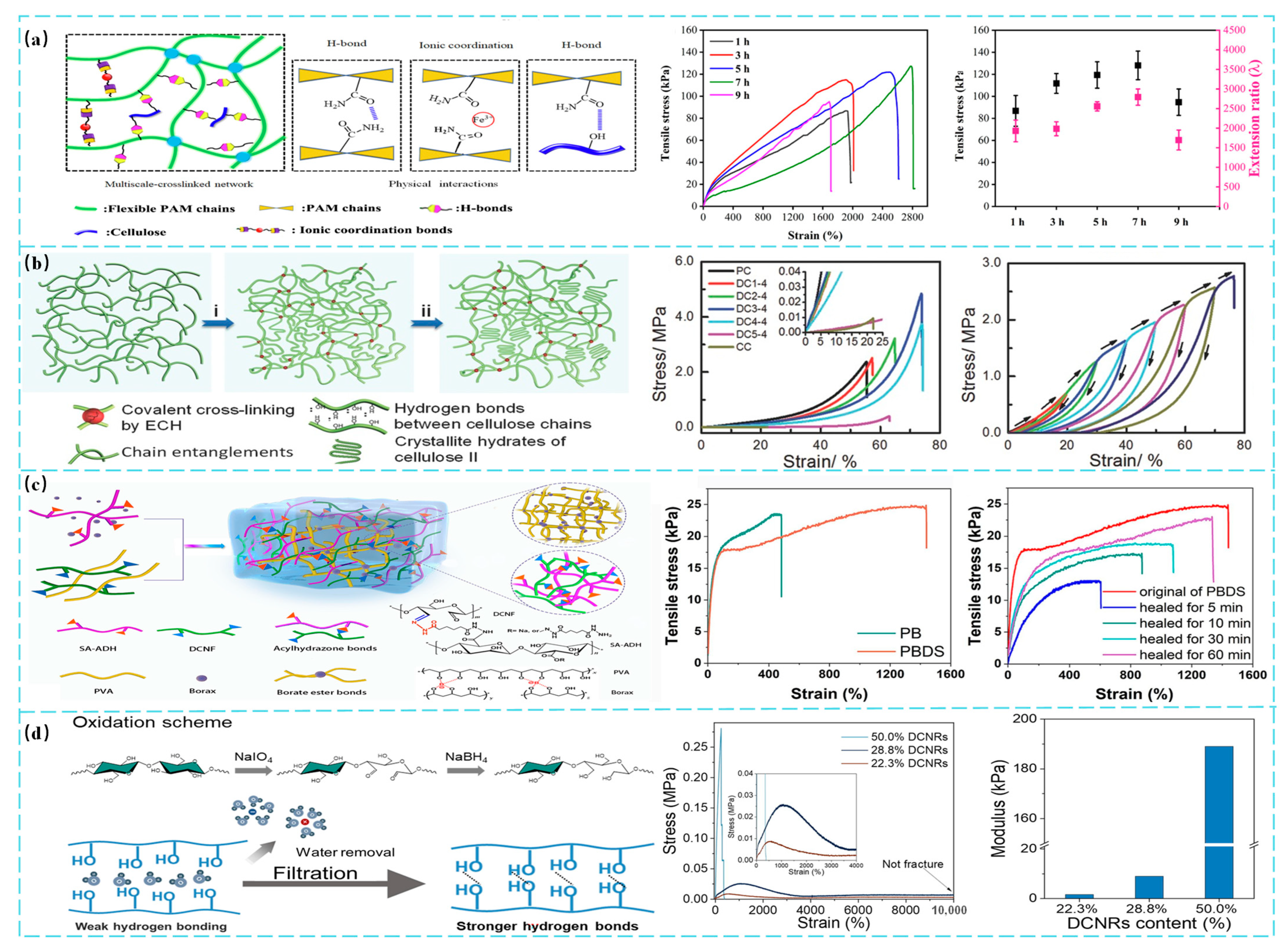
3. Optimization of Mechanical Properties
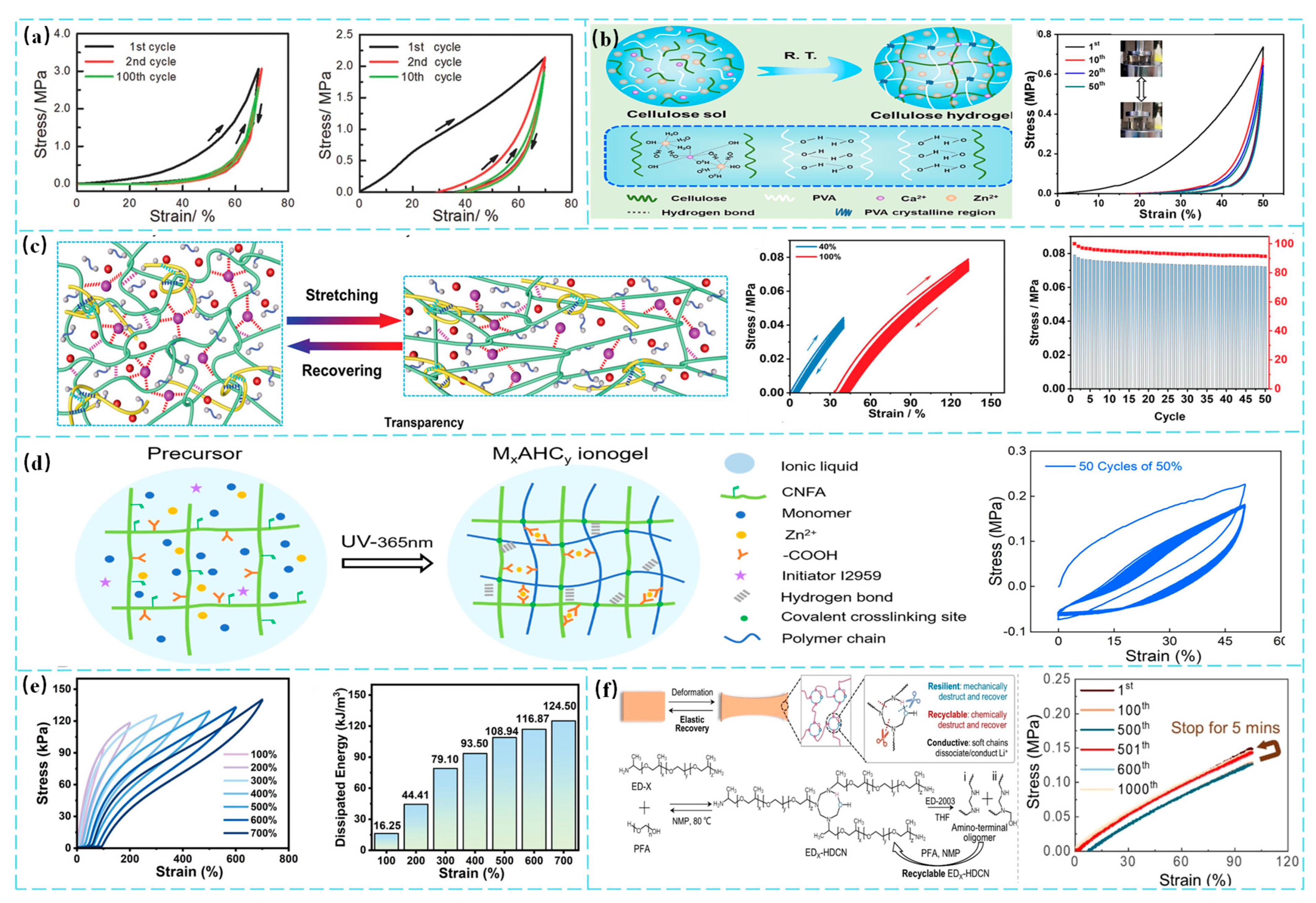
3.1. Enhancement of Mechanical Strength

3.2. Enhancement of Fatigue Resistance
4. Optimization of Electrical Conductivity
5. Optimization of Environmental Stability
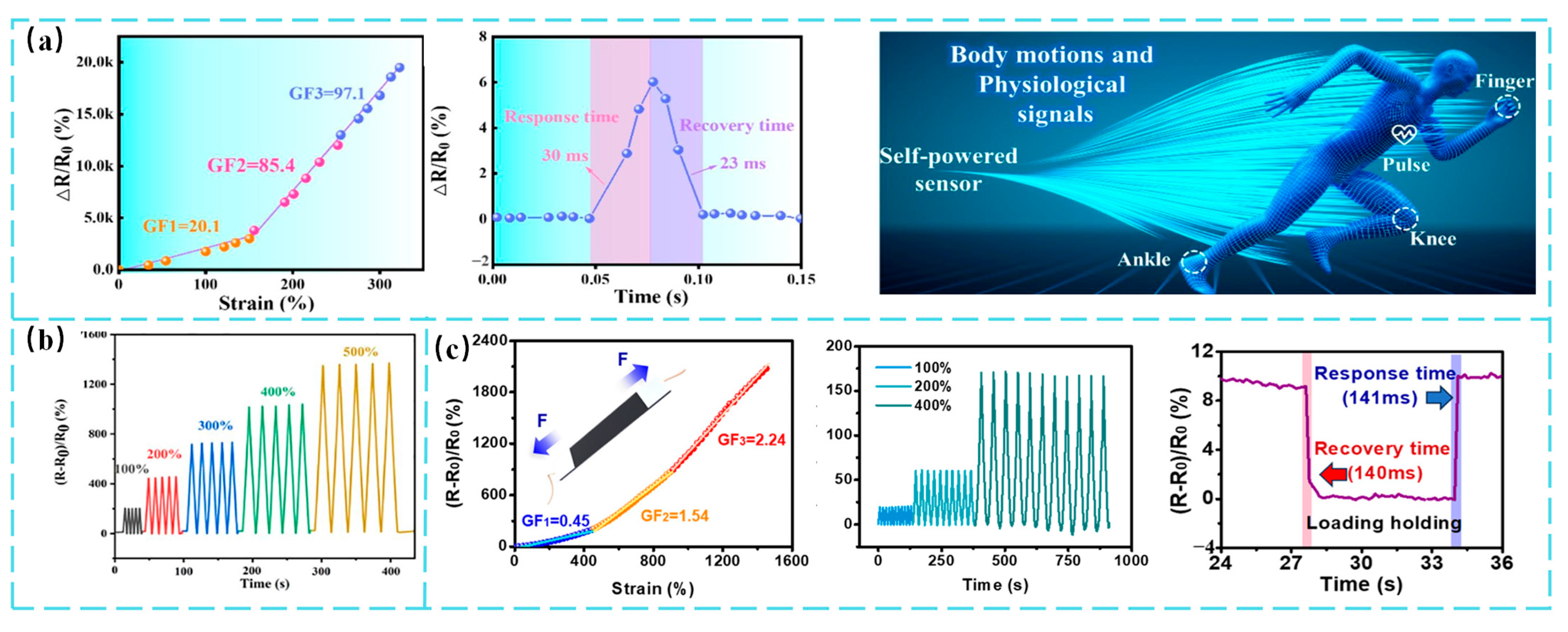
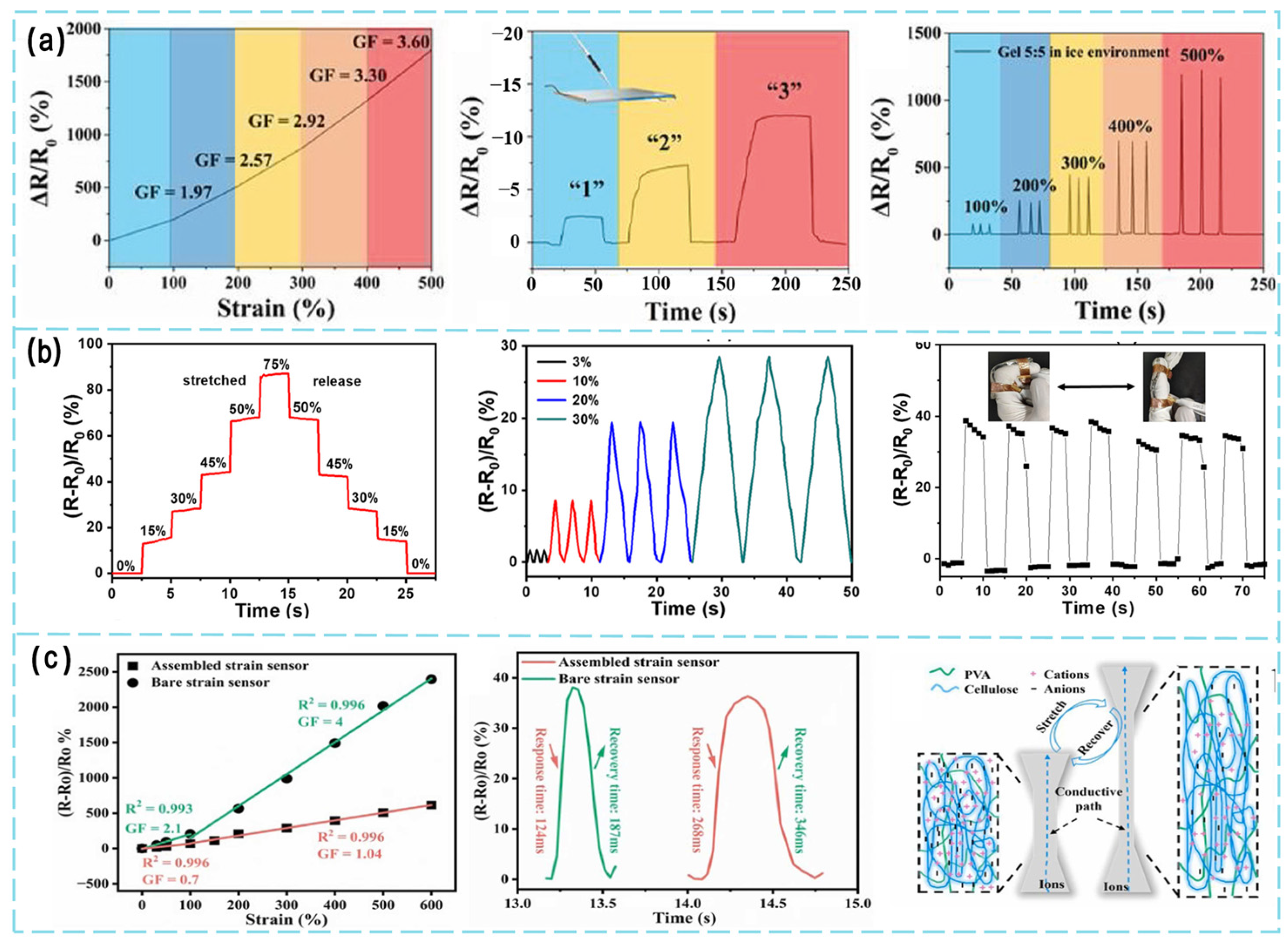
6. Optimization of Strain-Sensing Performance
| CPIC | Material | GF | Response Time(s) | Strain Range | Cyclic Stability | Reference |
|---|---|---|---|---|---|---|
| CCH | CMC + LiCl + AAM | 3.15 | 360 | 1200% | 500 cycles at a compressive strain of 300% | [111] |
| QACNF + MXene | 2.24 | 140 | 1465% | 100 cycles at a compressive strain of 60% | [112] | |
| PVA + PPY + CNF + TA | 1.92 | 400 | 414% | 2000 cycles at a compressive strain of 30% | [113] | |
| cotton linter cellulose + ZnCl2/CaCl2 + PVA | 1.14 | 200 | 50 cycles at a compressive strain of 50% | [72] | ||
| MoS2 + CMOF | 20.1 | 23 | 320% | 10,000 cycles at a compressive strain of 320% | [110] | |
| CNF + AAM + Mxenes + LiCl + Chitosan | 4.17 | 130 | 800% | [117] | ||
| CNC + PANI + PVA | 2.56 | 7 | 1085% | Over 1000 cycles of wrist bending | [118] | |
| CMC + AM + FeCI3 | 1.4 | 980 | 1086% | [119] | ||
| CNF + PAM + PBA-IL | 3.41 | 160 | 1810% | 500 cycles at a compressive strain of 100% | [120] | |
| CMC + FeCI3 + PAAm | 1.99 | 260 | 690% | 200 cycles at a tensile strain of 100% | [121] | |
| Methylcellulose + PAMC + TA@CNCs + LiCl | 1.63 | 227 | 600.00% | 300 cycles at a tensile strain of 100% | [122] | |
| BC + LiCl + DMAc + Mxene | 1.51 | 130 | 120% | 250 cycles at a tensile strain of 10% | [123] | |
| HECM + PAA + PPy | 1.67 | 180.6 | 254% | 1000 cycles at a tensile strain of 40% | [124] | |
| CNCs + AMM + BA + NaCl | 7.4 | 140 | 750% | Continuous cyclic stretching-release for 700 s under a tensile strain of 300% | [125] | |
| CNC + PAM + rGO | 3.4 | 125 | 400 | [126] | ||
| CCIG | CMC + P(AA-MEA) + AlCl3 | 1.47 | 400 | 500% | 300 cycles at a tensile strain of 400% | [114] |
| Cellulose + PVA + ZnCl2 + CaCl2 | 2.1 | 187 | 600% | 10 cycles at a tensile strain of 100% | [115] | |
| Cellulose + [Bmim]Cl + P(AM-DMAA-MAA) | 3.95 | 140 | 350% | 100 cycles at a tensile strain of 40% | [127] | |
| CNF + PAZ + FeCl3 + Gly | 1.52 | 427 | 900% | 500 cycles at a tensile strain of 30% | [128] | |
| CNF + PAZ + +LiCl + ZILs | 1.98 | 142 | 1400% | 100 cycles at a tensile strain of 100% | [129] | |
| BC + PAM + [EMIM]Cl | 4.28 | 177.7 | 1000% | 500 cycles at a tensile strain of 100% | [130] | |
| HPC + LA + AA + [C2VIm]Br | 2.29 | 4222% | 1000 cycles at a tensile strain of 50% | [31] | ||
| HPC + PAA + [EMIM][DEP] | 2.65 | 846 | 800% | 300 cycles at a tensile strain of 40% | [131] | |
| PA + DES + [EMIM]BF4 | 1.15 | 2500% | 45 cycles at a tensile strain of 100% | [132] | ||
| CICEs | CNC + PAA + TA + ChCl | 1.59 | 2400% | 75 | 100 cycles at a tensile strain of 100% | [74] |
| HPC + ZnCl2 + EG + AA | 197.70% | 42.8 | 700 cycles at a tensile strain of 40% | [6] | ||
| Cellulose + MCC-M-A + PDES | 1.06 | 395% | 114 | 500 cycles at a tensile strain of 15% | [116] | |
| EC-g-P(LMA-co-FMA) + ESOM + CNT | 1.744 | 47.20% | 277 | 200 cycles at a tensile strain of 25% | [133] | |
| SCNC + CNT + PDMS | 10.77 | 100% | 1000 cycles at a tensile strain of 50% | [134] | ||
| C-CNC@PANI + ENR | 1.45 | 300% | 162.3 | 5 cycles at a tensile strain of 300% | [135] | |
| MCNF + CNT + SR | 20.8 | 300% | 510 cycles at a tensile strain of 100% | [136] | ||
| CMC + S-PAM + LiCl + S-PDMS | 3.8 | 70% | 100 cycles at a tensile strain of 70% | [137] |
7. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; He, S.; Liu, A.; Zhang, J.; Liu, J.; He, S.; Shao, W. Preparation and characterization of self-healable and wearable hydrogels with ultrasensitive sensing performances. Compos. Part B Eng. 2022, 239, 109982. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Yu, Y.; Wang, C.-H. Strategies for Designing Stretchable Strain Sensors and Conductors. Adv. Mater. Technol. 2020, 5, 1900908. [Google Scholar] [CrossRef]
- Riess, I. Polymeric mixed ionic electronic conductors. Solid State Ion. 2000, 136, 1119–1130. [Google Scholar] [CrossRef]
- Takada, A.; Kadokawa, J.-i. Preparation of cellulosic soft and composite materials using ionic liquid media and ion gels. Cellulose 2022, 29, 2745–2754. [Google Scholar] [CrossRef]
- Li, X. Preparation of Lignocellulosic Hydrogel Electrolyte and Its Application in Supercapacitors. Master’s Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar]
- Lu, C.; Wang, X.; Shen, Y.; Xu, S.; Huang, C.; Wang, C.; Xie, H.; Wang, J.; Yong, Q.; Chu, F. Skin-like transparent, high resilience, low hysteresis, fatigue-resistant cellulose-based eutectogel for self-powered E-skin and human–machine interaction. Adv. Funct. Mater. 2024, 34, 2311502. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, H.; Zhou, Y.; Zhou, L.; Fu, J. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater. Horiz. 2020, 7, 1872–1882. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, L.; Lizundia, E.; Zhu, Y.; Chen, C.; Jiang, F. Cellulose-based ionic conductor: An emerging material toward sustainable devices. Chem. Rev. 2023, 123, 9204–9264. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Yin, R. Fiber/Yarn and Textile-Based Piezoresistive Pressure Sensors. Adv. Fiber Mater. 2025, 7, 34–71. [Google Scholar] [CrossRef]
- Xi, J.; Yang, H.; Li, X.; Wei, R.; Zhang, T.; Dong, L.; Yang, Z.; Yuan, Z.; Sun, J.; Hua, Q. Recent advances in tactile sensory systems: Mechanisms, fabrication, and applications. Nanomaterials 2024, 14, 465. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Recent progress in processing cellulose using ionic liquids as solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
- Ning, C.; Zhang, X.; Jiang, C.; Liu, W.; Sun, H.; Hou, Q. Study on Preparation and Properties of Nanocellulose-Polymerizable Deep Eutectic Solvents Ionic Conductive Elastomer. China Pulp Pap. 2023, 42, 43–49. [Google Scholar]
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef]
- Chen, Z.; Du, J.; Wang, Y.; Li, Z.; Song, W.; Wei, R.; Chen, D.; Hua, Q.; Fu, X.; Huang, S. Fully Integrated Multifunctional Flexible Ultrasonic–Electric-Coupled Patches for Advancing Wound Care Paradigms. Adv. Funct. Mater. 2025, 35, 2425025. [Google Scholar] [CrossRef]
- Cui, Z.; Hua, Q.; Shi, Y.; Wei, R.; Dong, Z.; Dai, X.; Huang, T.; Shen, G.; Wang, Z.L.; Hu, W. Skin-integrated haptic interface system based on a stretchable pressure sensor array for wireless tactile visualization applications. Nano Energy 2025, 139, 110911. [Google Scholar] [CrossRef]
- Shen, J.; Fu, S. Research progress of cellulose-based hydrogels. Chem. Ind. Eng. Prog. 2022, 41, 3022–3037. [Google Scholar]
- Fu, Q.; Liu, Y.; Mo, J.; Lu, Y.; Cai, C.; Zhao, Z.; Wang, S.; Nie, S. Improved capture and removal efficiency of gaseous acetaldehyde by a self-powered photocatalytic system with an external electric field. ACS Nano 2021, 15, 10577–10586. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-stimuli-responsive and cellulose conductive ionic hydrogel for smart wearable devices and thermal actuators. ACS Appl. Mater. Interfaces 2020, 13, 1353–1366. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, B.; Ma, M.; Wu, B.; Shi, Y.; Wang, X. Multiporous microstructure for enhancing the water absorption and swelling rate in poly (sodium acrylic acid) superabsorbent hydrogels based on a novel physical and chemical composite foaming system. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, M.; Qin, C.; Qian, X.; Zhang, L.; Zhou, J.; Lu, A. Transparent, conductive cellulose hydrogel for flexible sensor and triboelectric nanogenerator at subzero temperature. Carbohydr. Polym. 2021, 265, 118078. [Google Scholar] [CrossRef]
- Rathika, R.; Suthanthiraraj, S.A. Influence of 1-ethyl-3-methylimidazolium bis (trifluoromethyl sulfonyl) imide plasticization on zinc-ion conducting PEO/PVdF blend gel polymer electrolyte. J. Mater. Sci. Mater. Electron. 2018, 29, 19632–19643. [Google Scholar] [CrossRef]
- Cho, K.G.; Kim, H.S.; Jang, S.S.; Kyung, H.; Kang, M.S.; Lee, K.H.; Yoo, W.C. Optimizing electrochemically active surfaces of carbonaceous electrodes for ionogel based supercapacitors. Adv. Funct. Mater. 2020, 30, 2002053. [Google Scholar] [CrossRef]
- Ju, M.; Wu, B.; Sun, S.; Wu, P. Redox-active iron-citrate complex regulated robust coating-free hydrogel microfiber net with high environmental tolerance and sensitivity. Adv. Funct. Mater. 2020, 30, 1910387. [Google Scholar] [CrossRef]
- Yan, C.-C.; Li, W.; Liu, Z.; Zheng, S.; Hu, Y.; Zhou, Y.; Guo, J.; Ou, X.; Li, Q.; Yu, J.; et al. Ionogels: Preparation, Properties and Applications. Adv. Funct. Mater. 2024, 34, 2314408. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E.; Marcinkowska, A.; Zgrzeba, A. Ionogels–materials containing immobilized ionic liquids. Polimery 2017, 62, 344–352. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Yang, J.; Tao, T.; Chen, L.; Ni, Y.; Li, J. Preparation and properties of cellulose ionic gel. Acta Mater. Compos. Sin. 2021, 38, 4247–4254. [Google Scholar]
- Kong, L.; Lu, R.; Wang, Y.; Ran, Y.; Jv, J.; Sui, W.; Peng, Y. Transparent bamboo as a replacement for glass: Effects of lignin decolorisation methods on weatherability. Int. J. Biol. Macromol. 2024, 277, 134470. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, B.; Ji, J.; Cheng, F.; Cai, C.; Fu, Y. Unmatched resilience and fatigue resistance in a novel cellulose-derived ionic gel with hierarchical superstructured synergy for advanced human-computer interaction. Chem. Eng. J. 2024, 497, 154672. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Zhou, X.; Gui, S.; Wei, L.; Yang, H.; Li, H.; Guo, X. Ionic conduction in polymer-based solid electrolytes. Adv. Sci. 2023, 10, 2201718. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Wang, D.; Xie, H.; Liu, Q.; Mu, K.; Song, Z.; Xu, W.; Tian, L.; Zhu, C.; Xu, J. Low-Cost, High-Strength Cellulose-based Quasi-Solid Polymer Electrolyte for Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302767. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, M.; Lin, Z.; Shen, Z. Recent advances in cellulose-based polymer electrolytes. Cellulose 2022, 29, 8997–9034. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, Z.; Xu, J.; Sun, Z.; Wu, X.; Han, J.; Wang, Y.-H.; Wang, M.-S. Zwitterionic Cellulose-Based Polymer Electrolyte Enabled by Aqueous Solution Casting for High-Performance Solid-State Batteries. Angew. Chem. Int. Ed. 2024, 63, e202400477. [Google Scholar] [CrossRef]
- Gao, C. Construction of Composite Polymer Electrolytes Based onCellulose Derivatives and Performance in Lithium Batteries. Ph.D. Thesis, Shaanxi University of Science and Technology, Xi’an, China, 2023. [Google Scholar]
- Jinisha, B.; Anilkumar, K.; Manoj, M.; Pradeep, V.; Jayalekshmi, S. Development of a novel type of solid polymer electrolyte for solid state lithium battery applications based on lithium enriched poly (ethylene oxide)(PEO)/poly (vinyl pyrrolidone)(PVP) blend polymer. Electrochim. Acta 2017, 235, 210–222. [Google Scholar]
- Mindemark, J.; Törmä, E.; Sun, B.; Brandell, D. Copolymers of trimethylene carbonate and ε-caprolactone as electrolytes for lithium-ion batteries. Polymer 2015, 63, 91–98. [Google Scholar] [CrossRef]
- Liu, F.; Bin, F.; Xue, J.; Wang, L.; Yang, Y.; Huo, H.; Zhou, J.; Li, L. Polymer electrolyte membrane with high ionic conductivity and enhanced interfacial stability for lithium metal battery. ACS Appl. Mater. Interfaces 2020, 12, 22710–22720. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Ji, K.; Jiang, B.; Cui, X.; Sha, W.; Wang, B.; Dai, X.; Hua, Q.; Wan, L. Flexible, stretchable, and transparent InGaN/GaN multiple quantum wells/polyacrylamide hydrogel-based light emitting diodes. Nano Res. 2022, 15, 5492–5499. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-dependent stress relaxing semi-interpenetrating networks of hydroxyethyl cellulose in gelatin-PEG hydrogel with good mechanical stability and reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-strength and high-toughness double-cross-linked cellulose hydrogels: A new strategy using sequential chemical and physical cross-linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Rana, H.H.; Park, J.H.; Gund, G.S.; Park, H.S. Highly conducting, extremely durable, phosphorylated cellulose-based ionogels for renewable flexible supercapacitors. Energy Storage Mater. 2020, 25, 70–75. [Google Scholar] [CrossRef]
- Kale, S.B.; Nirmale, T.C.; Khupse, N.D.; Kale, B.B.; Kulkarni, M.V.; Pavitran, S.; Gosavi, S.W. Cellulose-derived flame-retardant solid polymer electrolyte for lithium-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 1559–1567. [Google Scholar] [CrossRef]
- Villar-Chavero, M.M.; Dominguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Chitosan-reinforced cellulosic bionogels: Viscoelastic and antibacterial properties. Carbohydr. Polym. 2020, 229, 115569. [Google Scholar] [CrossRef]
- Kunchornsup, W.; Sirivat, A. Electromechanical properties study of 1-butyl-3-methylimidazolium chloride/cellulosic gel blended with polydiphenylamine. Sens. Actuators A Phys. 2014, 220, 249–261. [Google Scholar] [CrossRef]
- Lee, H.; Erwin, A.; Buxton, M.L.; Kim, M.; Stryutsky, A.V.; Shevchenko, V.V.; Sokolov, A.P.; Tsukruk, V.V. Shape persistent, highly conductive ionogels from ionic liquids reinforced with cellulose nanocrystal network. Adv. Funct. Mater. 2021, 31, 2103083. [Google Scholar] [CrossRef]
- Song, H.; Luo, Z.; Zhao, H.; Luo, S.; Wu, X.; Gao, J.; Wang, Z. High tensile strength and high ionic conductivity bionanocomposite ionogels prepared by gelation of cellulose/ionic liquid solutions with nano-silica. RSC Adv. 2013, 3, 11665–11675. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.-Z.; Zhao, R.-Q.; Rao, W.; Liu, J. Liquid metal composites. Matter 2020, 2, 1446–1480. [Google Scholar] [CrossRef]
- Liu, X.; Taiwo, O.O.; Yin, C.; Ouyang, M.; Chowdhury, R.; Wang, B.; Wang, H.; Wu, B.; Brandon, N.P.; Wang, Q. Aligned ionogel electrolytes for high-temperature supercapacitors. Adv. Sci. 2019, 6, 1801337. [Google Scholar] [CrossRef]
- Kunchornsup, W.; Sirivat, A. Effects of crosslinking ratio and aging time on properties of physical and chemical cellulose gels via 1-butyl-3-methylimidazolium chloride solvent. J. Sol-Gel Sci. Technol. 2010, 56, 19–26. [Google Scholar] [CrossRef]
- Zhang, L.M.; He, Y.; Cheng, S.; Sheng, H.; Dai, K.; Zheng, W.J.; Wang, M.X.; Chen, Z.S.; Chen, Y.M.; Suo, Z. Self-healing, adhesive, and highly stretchable ionogel as a strain sensor for extremely large deformation. Small 2019, 15, 1804651. [Google Scholar] [CrossRef]
- Huang, F.; Wei, W.; Fan, Q.; Li, L.; Zhao, M.; Zhou, Z. Super-stretchable and adhesive cellulose Nanofiber-reinforced conductive nanocomposite hydrogel for wearable Motion-monitoring sensor. J. Colloid Interface Sci. 2022, 615, 215–226. [Google Scholar] [CrossRef]
- Shu, L.; Wang, Z.; Zhang, X.-F.; Yao, J. Highly conductive and anti-freezing cellulose hydrogel for flexible sensors. Int. J. Biol. Macromol. 2023, 230, 123425. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, Z.; Wang, Z.; Guo, Z.; Jiang, J.; Xie, Y. Highly stretchable, fast self-healing nanocellulose hydrogel combining borate ester bonds and acylhydrazone bonds. Int. J. Biol. Macromol. 2023, 245, 125471. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Ye, Y.; Oguzlu, H.; Zhu, Y.; Zhu, J.; Le, K.; Yang, P.; Jiang, F. All-cellulose hydrogel with ultrahigh stretchability exceeding 40000%. Mater. Today 2024, 74, 67–76. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Dickey, M.D. Tough ionogels: Synthesis, toughening mechanisms, and mechanical properties—A perspective. JACS Au 2022, 2, 2645–2657. [Google Scholar] [CrossRef]
- Long, Q.; Jiang, G.; Zhou, J.; Zhao, D.; Yu, H. A cellulose ionogel with rubber-like stretchability for low-grade heat harvesting. Research 2024, 7, 0533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Bai, R.; Zhao, Y.; Shi, S.; Jiang, G.; Zhao, D. Mechanically robust, highly conductive, wide-voltage cellulose ionogels enabled by molecular network reconstruction. Adv. Funct. Mater. 2025, 35, 2503512. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, K.; Feng, Z.; Hou, Y.; Li, H.; Zhao, J.; Tian, Y.; Song, H. High performance liquid crystalline bionanocomposite ionogels prepared by in situ crosslinking of cellulose/halloysite nanotubes/ionic liquid dispersions and its application in supercapacitors. Appl. Surf. Sci. 2018, 455, 599–607. [Google Scholar] [CrossRef]
- Chen, W.; Bu, Y.; Li, D.; Liu, C.; Chen, G.; Wan, X.; Li, N. High-strength, tough, and self-healing hydrogel based on carboxymethyl cellulose. Cellulose 2020, 27, 853–865. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Destro, M.; Gerbaldi, C. Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochim. Acta 2015, 174, 185–190. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, S.; Li, Y.; Xin, S.; Manthiram, A.; Goodenough, J.B. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 2016, 138, 9385–9388. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Huang, C.; Luo, L.; Deng, Z.; Guo, W.; Xu, J.; Meng, Z. A novel cellulose membrane from cattail fibers as separator for Li-ion batteries. Cellulose 2021, 28, 9309–9321. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Cai, C.; Wei, Z.; Fu, Y. 3D-cellulose acetate-derived hierarchical network with controllable nanopores for superior Li+ transference number, mechanical strength and dendrites hindrance. Carbohydr. Polym. 2021, 274, 118620. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Ma, R.; Yu, J.; Zhang, X.; Shi, C.; Ma, L.; Li, T.; Huang, Y.; Hu, Y. A novel one-pot strategy to construct 3D-printable cellulose nanofiber/poly (deep eutectic solvent) conductive elastomers. Chem. Eng. J. 2023, 454, 140022. [Google Scholar] [CrossRef]
- Wang, X.; Wei, R.; Chen, Z.; Pang, H.; Li, H.; Yang, Y.; Hua, Q.; Shen, G. Bioinspired Intelligent Soft Robotics: From Multidisciplinary Integration to Next-Generation Intelligence. Adv. Sci. 2025, 12, e06296. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Li, H.; Sun, J.; Deng, Y.; Jiao, F.; Han, Y.; Zhang, K.; Meng, J.; Li, X.; Wang, L. All-Optical Synapses Based on a Mechanoluminescent Material. Adv. Mater. 2025, 37, 2503376. [Google Scholar] [CrossRef]
- Wei, R.; Li, H.; Chen, Z.; Hua, Q.; Shen, G.; Jiang, K. Revolutionizing wearable technology: Advanced fabrication techniques for body-conformable electronics. npj Flex. Electron. 2024, 8, 83. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.-F.; Wu, Y.; Wang, Z.; Yao, J. Facile fabrication of strong and conductive cellulose hydrogels with wide temperature tolerance for flexible sensors. Int. J. Biol. Macromol. 2023, 240, 124438. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Qie, X.; Feng, H.; Min, Z.; Zhang, J.; Ren, S.; Ren, J. Tough and highly stretchable multifunctional ionogels based on phase-separated structure and nanocellulose macromolecular covalent cross-linker. Cell Rep. Phys. Sci. 2024, 5, 102073. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Q.; Wang, Y.; Zhao, H.; Hao, S.; Ma, C.; Xu, F.; Yang, J. Tough liquid-free ionic conductive elastomers with robust adhesion and self-healing properties for ionotronic devices. Adv. Funct. Mater. 2024, 34, 2307400. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, P.; Yang, J.; Meng, J.; Wu, M.; Pu, X. A Dual-Bond Crosslinking Strategy Enabling Resilient and Recyclable Electrolyte Elastomers for Solid-State Lithium Metal Batteries. Angew. Chem. Int. Ed. 2024, 63, e202404769. [Google Scholar] [CrossRef]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel-Based Flexible Electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic salts induce thermally reversible and anti-freezing cellulose hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Wang, C.; Zhang, F.; Liang, W.; Kuga, S.; Dong, Z.; Zhao, Y.; Huang, Y.; Wu, M. Flexible double-cross-linked cellulose-based hydrogel and aerogel membrane for supercapacitor separator. J. Mater. Chem. A 2018, 6, 24468–24478. [Google Scholar] [CrossRef]
- Yan, X.; Lin, X.; Liu, H.; Lu, J.; Wang, H.; Huang, X.; Liu, H.; Xu, X. Tough and temperature-tolerance cellulose/polyacrylic acid/bentonite hydrogel with high ionic conductivity enables self-powered triboelectric wearable electronic devices. Carbohydr. Polym. 2024, 344, 122552. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Lu, A. Transparent, antifreezing, ionic conductive cellulose hydrogel with stable sensitivity at subzero temperature. ACS Appl. Mater. Interfaces 2019, 11, 41710–41716. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Wei, X.; Zhang, J.; Wang, D.; Lu, H.; Jia, P. Ultrastretchable, tough, antifreezing, and conductive cellulose hydrogel for wearable strain sensor. ACS Appl. Mater. Interfaces 2020, 12, 53247–53256. [Google Scholar] [CrossRef]
- Cao, K.; Zhu, Y.; Zheng, Z.; Cheng, W.; Zi, Y.; Zeng, S.; Zhao, D.; Yu, H. Bio-inspired multiscale design for strong and tough biological ionogels. Adv. Sci. 2023, 10, 2207233. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Xu, G.; Wang, Q.; Liu, S.; Li, J.; Chen, C.; Yu, H.; Hu, L. A dynamic gel with reversible and tunable topological networks and performances. Matter 2020, 2, 390–403. [Google Scholar] [CrossRef]
- Yim, C.-H.; Tam, J.; Soboleski, H.; Abu-Lebdeh, Y. On the correlation between free volume, phase diagram and ionic conductivity of aqueous and non-aqueous lithium battery electrolyte solutions over a wide concentration range. J. Electrochem. Soc. 2017, 164, A1002. [Google Scholar] [CrossRef]
- Han, J.; Mariani, A.; Passerini, S.; Varzi, A. A perspective on the role of anions in highly concentrated aqueous electrolytes. Energy Environ. Sci. 2023, 16, 1480–1501. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Hu, P.; Liu, Z.; Qin, B.; Zhang, B.; Wang, Q.; Ding, G.; Zhang, C.; Zhou, X. Taichi-inspired rigid-flexible coupling cellulose-supported solid polymer electrolyte for high-performance lithium batteries. Sci. Rep. 2014, 4, 6272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, R.; Jia, D.; Cui, Y.; Liu, Q.; Liu, S.; Wu, D. Ultrathin yet robust single lithium-ion conducting quasi-solid-state polymer-brush electrolytes enable ultralong-life and dendrite-free lithium-metal batteries. Adv. Mater. 2021, 33, 2100943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, J.; Bautista, F.; Lyons, L.; Shariatzadeh, N.; Sherlock, D.; Amine, K.; West, R. Ion conductive characteristics of cross-linked network polysiloxane-based solid polymer electrolytes. Solid State Ion. 2004, 170, 233–238. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.H.; Schiff, T.; Eyler, A.; Li, B. A Particle-Controlled, High-Performance, Gum-Like Electrolyte for Safe and Flexible Energy Storage Devices. Adv. Energy Mater. 2015, 5, 1400463. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Feng, W.; Zhen, Y.; Zhao, P.; Cai, Z.; Li, L. Effects of the shapes of BaTiO3 nanofillers on PEO-based electrolytes for all-solid-state lithium-ion batteries. Ionics 2019, 25, 1471–1480. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Gosselink, D.; Chen, P. Synthesis of poly (ethylene-oxide)/nanoclay solid polymer electrolyte for all solid-state lithium/sulfur battery. Ionics 2015, 21, 381–385. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.; Lee, J.-I.; Lee, C. Ionic conductivity and electrochemical properties of cross-linked solid polymer electrolyte using star-shaped siloxane acrylate. J. Power Sources 2007, 165, 92–96. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Li, X.; Yan, M.; Wang, Y.; Ma, J.; Wang, Z. Compressible, anti-freezing, and ionic conductive cellulose/polyacrylic acid composite hydrogel prepared via AlCl3/ZnCl2 aqueous system as solvent and catalyst. Int. J. Biol. Macromol. 2023, 253, 126550. [Google Scholar] [CrossRef]
- Li, N.; Qiu, Y.; Ma, J.; Qiu, L.; Sun, W.; Li, J.; Han, Z.; Chen, W.; Ji, X. Mechanically robust and multifunctional ionogels based on cellulose and polymerizable 1-butyl-3-methylimidazolium chloride/acrylamide deep eutectic solvent. ACS Appl. Polym. Mater. 2023, 5, 9974–9986. [Google Scholar] [CrossRef]
- Wu, D.; Wang, M.; Yu, W.; Wang, G.-G.; Zhang, J. A robust, biodegradable and recyclable all-cellulose ionogel from low-value wood. Chem. Eng. J. 2024, 486, 150121. [Google Scholar] [CrossRef]
- Li, J.; Hu, Z.; Zhang, S.; Zhang, H.; Guo, S.; Zhong, G.; Qiao, Y.; Peng, Z.; Li, Y.; Chen, S. Molecular engineering of renewable cellulose biopolymers for solid-state battery electrolytes. Nat. Sustain. 2024, 7, 1481–1491. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, J.; Guo, B.; Li, Z.; Wang, W.; Li, W. Cellulose-based eutectogel electrolyte with high ionic conductivity for solid-state lithium-ion batteries. Chem. Eng. J. 2024, 491, 151783. [Google Scholar] [CrossRef]
- Wang, R.; Dong, W.; Song, Z.; Tan, J.; Liu, Q.; Mu, K.; Xu, W.; Huang, H.; Zhang, Z.; Yin, G. Ion-Conducting Molecular-Grafted Sustainable Cellulose Quasi-Solid Composite Electrolyte for High Stability Solid-State Lithium-Metal Batteries. Adv. Funct. Mater. 2024, 34, 2402461. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Lu, A. Highly stretchable, transparent cellulose/PVA composite hydrogel for multiple sensing and triboelectric nanogenerators. J. Mater. Chem. A 2020, 8, 13935–13941. [Google Scholar] [CrossRef]
- Wu, R.; Wang, Y.; Liu, Y.; Yuan, B. Functionalizing chitosan-based film with highly sensitive fire response and commendable flame retardancy for intelligent fire-alarm system. Compos. Part A Appl. Sci. Manuf. 2024, 178, 107999. [Google Scholar] [CrossRef]
- Zhao, D.; Pang, B.; Zhu, Y.; Cheng, W.; Cao, K.; Ye, D.; Si, C.; Xu, G.; Chen, C.; Yu, H. A stiffness-switchable, biomimetic smart material enabled by supramolecular reconfiguration. Adv. Mater. 2022, 34, 2107857. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Zhang, J.; Hao, S.; Duan, X.; Song, H.; Zhang, J. Novel chemically cross-linked chitosan-cellulose based ionogel with self-healability, high ionic conductivity, and high thermo-mechanical stability. Cellulose 2020, 27, 5121–5133. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Zhang, Y.; Long, Q.; Jiang, G.; Zeng, S.; Zhou, J.; Zhao, D. Stimulation-Reinforced Cellulose–Protein Ionogels with Superior Mechanical Strength and Temperature Resistance. Adv. Funct. Mater. 2024, 34, 2408160. [Google Scholar] [CrossRef]
- Kotobuki, M.; Suzuki, Y.; Munakata, H.; Kanamura, K.; Sato, Y.; Yamamoto, K.; Yoshida, T. Electrochemical property of honeycomb type all-solid-state Li battery at high temperature. Electrochemistry 2011, 79, 464–466. [Google Scholar] [CrossRef]
- Samad, Y.A.; Asghar, A.; Lalia, B.S.; Hashaikeh, R. Networked cellulose entrapped and reinforced PEO-based solid polymer electrolyte for moderate temperature applications. J. Appl. Polym. Sci. 2013, 129, 2998–3006. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Zhou, X.; Hu, B.; Wang, Y.; Li, Y.; Jing, X. Research Progress in Conducting Polymer Hydrogels and Their StrainSensing Properties. Mater. Rep. 2024, 38, 22120184-11. [Google Scholar]
- Wang, B.; Shi, Y.; Li, H.; Hua, Q.; Ji, K.; Dong, Z.; Cui, Z.; Huang, T.; Chen, Z.; Wei, R. Body-Integrated Ultrasensitive All-Textile Pressure Sensors for Skin-Inspired Artificial Sensory Systems. Small Sci. 2024, 4, 2400026. [Google Scholar] [CrossRef]
- Wang, S.; Yang, T.; Zhang, D.; Hua, Q.; Zhao, Y. Unveiling gating behavior in piezoionic effect: Toward neuromimetic tactile sensing. Adv. Mater. 2024, 36, 2405391. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Li, C.; Tao, Y.; Hu, J.; Lu, J.; Du, J.; Wang, H. Synergistic defect and heterojunction engineering of carbonized MOF@ MoS2 for self-powered sensing micro-system with photothermal therapy. Chem. Eng. J. 2024, 495, 153367. [Google Scholar] [CrossRef]
- Zhu, T.; Cheng, Y.; Cao, C.; Mao, J.; Li, L.; Huang, J.; Gao, S.; Dong, X.; Chen, Z.; Lai, Y. A semi-interpenetrating network ionic hydrogel for strain sensing with high sensitivity, large strain range, and stable cycle performance. Chem. Eng. J. 2020, 385, 123912. [Google Scholar] [CrossRef]
- Ni, Q.-Y.; He, X.-F.; Zhou, J.-L.; Yang, Y.-Q.; Zeng, Z.-F.; Mao, P.-F.; Luo, Y.-H.; Xu, J.-M.; Jiang, B.; Wu, Q. Mechanical tough and stretchable quaternized cellulose nanofibrils/MXene conductive hydrogel for flexible strain sensor with multi-scale monitoring. J. Mater. Sci. Technol. 2024, 191, 181–191. [Google Scholar] [CrossRef]
- Lin, F.; Yang, W.; Lu, B.; Xu, Y.; Chen, J.; Zheng, X.; Liu, S.; Lin, C.; Zeng, H.; Huang, B. Muscle-Inspired Robust Anisotropic Cellulose Conductive Hydrogel for Multidirectional Strain Sensors and Implantable Bioelectronics. Adv. Funct. Mater. 2025, 35, 2416419. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Chen, Y.; Rehman, H.U.; Guo, Y.; Li, H.; Liu, H. Ionic conductive organohydrogels with dynamic pattern behavior and multi-environmental stability. Adv. Funct. Mater. 2021, 31, 2101464. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ren, P.; Yu, S.; Cui, P.; Nielsen, C.B.; Abrahams, I.; Briscoe, J.; Lu, Y. Versatile and recyclable double-network PVA/cellulose hydrogels for strain sensors and triboelectric nanogenerators under harsh conditions. Nano Energy 2024, 125, 109599. [Google Scholar] [CrossRef]
- Luo, T.; Guo, X.; Qi, J.; Yu, J.; Lu, C.; Wang, C.; Chu, F.; Wang, J. Fabrication of liquid-free ionic conductive elastomer (ICE) from cellulose-rosin derived poly (esterimide) towards temperature-tolerant and solvent-resistant UV shadowless adhesive and sensor. Int. J. Biol. Macromol. 2024, 278, 134921. [Google Scholar] [CrossRef]
- Geng, L.; Liu, W.; Fan, B.; Wu, J.; Shi, S.; Huang, A.; Hu, J.; Peng, X. Anisotropic double-network hydrogels integrated superior performance of strength, toughness and conductivity for flexible multi-functional sensors. Chem. Eng. J. 2023, 462, 142226. [Google Scholar] [CrossRef]
- Song, M.; Yu, H.; Zhu, J.; Ouyang, Z.; Abdalkarim, S.Y.H.; Tam, K.C.; Li, Y. Constructing stimuli-free self-healing, robust and ultrasensitive biocompatible hydrogel sensors with conductive cellulose nanocrystals. Chem. Eng. J. 2020, 398, 125547. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, X.; Gao, G.; Duan, L. High strength, anti-freezing and strain sensing carboxymethyl cellulose-based organohydrogel. Carbohydr. Polym. 2019, 223, 115051. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super stretchable, self-healing, adhesive ionic conductive hydrogels based on tailor-made ionic liquid for high-performance strain sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F. Dual physically cross-linked carboxymethyl cellulose-based hydrogel with high stretchability and toughness as sensitive strain sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Xu, L.; Yan, M.; Bi, H.; Wang, Q. Transparent multifunctional cellulose-based conductive hydrogel for wearable strain sensors and arrays. Carbohydr. Polym. 2024, 329, 121784. [Google Scholar] [CrossRef]
- Gai, Y.; Yang, L.; Shen, W.; Tan, F.; Yu, Q.; Zhang, L.; Sun, D. A flexible piezoresistive strain sensor based on MXene/bacterial cellulose hydrogel with high mechanical strength for real-time monitoring of human motions. J. Mater. Chem. C 2024, 12, 1763–1772. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Hua, W.; Wang, S.; Wang, S.; Yu, J.; Wang, J.; Yong, Q.; Chu, F.; Lu, C. Self-strengthening and conductive cellulose composite hydrogel for high sensitivity strain sensor and flexible triboelectric nanogenerator. Int. J. Biol. Macromol. 2023, 248, 125900. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Shah, L.A.; Khan, M.T. Cellulose nanocrystals boosted hydrophobically associated self-healable conductive hydrogels for the application of strain sensors and electronic devices. Int. J. Biol. Macromol. 2024, 260, 129376. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, W.; Ding, S.; Liu, E.; Liu, D. Preparation and properties of polyacrylamide/cellulose nanocrystal/reduced graphene oxide interpenetrating network composite hydrogels. New J. Chem. 2023, 47, 14273–14281. [Google Scholar] [CrossRef]
- Xu, A.; Xia, Q.; Ju, Y.; Wang, Y.; Xiao, Z.; Wang, H.; Xie, Y. Cellulose enhanced highly sensitive and durable dual-network ionogel sensor for human motion monitoring. Chem. Eng. J. 2024, 499, 156608. [Google Scholar] [CrossRef]
- Fu, D.; Xing, L.; Xie, Y.; Li, P.; Yang, F.; Sui, X.; Liu, J.; Chi, J.; Huang, B.; Shen, J. Hybrid crosslinking cellulose nanofibers-reinforced zwitterionic poly (ionic liquid) organohydrogel with high-stretchable, anti-freezing, anti-drying as strain sensor application. Carbohydr. Polym. 2025, 353, 123253. [Google Scholar] [CrossRef]
- Fu, D.; Xing, L.; Xie, Y.; Shen, J. High-mechanical properties, anti-freezing, and self-regeneration zwitterionic poly (ionic liquid) hydrogel reinforced by cellulose nanofibers for strain sensor applications. Int. J. Biol. Macromol. 2025, 320, 145999. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Ding, Q.; Chen, L.; Yang, S.; Lou, J.; Liu, Z.; Li, X.; Jiang, Y.; Wang, X.; Han, W. Hyper strength, high sensitivity integrated wearable signal sensor based on non-covalent interaction of an ionic liquid and bacterial cellulose for human behavior monitoring. Mater. Horiz. 2024, 11, 2420–2427. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Liu, Y.; Luo, X.; He, Y.; Niu, Y.; Xu, Q. Transparent, stretchable, self-healing, and self-adhesive ionogels for flexible multifunctional sensors and encryption systems. Chem. Eng. J. 2024, 484, 149632. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Shu, H.; Sun, A.; Zhao, T.; Chen, Y.; Chen, Y. High-Performance Deep Eutectic/Ionic Liquid Gels for Zinc-Ion Battery and Flexible Sensor Applications in Extreme Environments. Adv. Funct. Mater. 2025, e14358. [Google Scholar] [CrossRef]
- Lu, C.; Guo, X.; Wang, C.; Wang, J.; Chu, F. Integration of metal-free ATRP and Diels-Alder reaction toward sustainable and recyclable cellulose-based thermoset elastomers. Carbohydr. Polym. 2020, 242, 116404. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Lu, Y.; Wang, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Fu, Q.; Han, J. Inherently conductive poly (dimethylsiloxane) elastomers synergistically mediated by nanocellulose/carbon nanotube nanohybrids toward highly sensitive, stretchable, and durable strain sensors. ACS Appl. Mater. Interfaces 2021, 13, 59142–59153. [Google Scholar] [CrossRef]
- Xu, S.; Jia, Q.; Zhang, K.; Lu, C.; Wang, C.; Wang, J.; Yong, Q.; Chu, F. Recyclable and mechanically tough nanocellulose reinforced natural rubber composite conductive elastomers for flexible multifunctional sensor. Int. J. Biol. Macromol. 2024, 268, 131946. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yue, Y.; Lu, Y.; Xiang, K.; Wang, J.; Lu, W.; Tian, H.; Jia, L.; Wu, G.; Xiao, J. Three-dimensional printed cellulose nanofibers/carbon nanotubes/silicone rubber flexible strain sensor for wearable body monitoring. J. Mater. Chem. C 2024, 12, 5972–5984. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Jiang, S.; He, S.; Wu, Q.; Xiao, H.; Han, J. Environment-tolerant ionic hydrogel–elastomer hybrids with robust interfaces, high transparence, and biocompatibility for a mechanical–thermal multimode sensor. InfoMat 2023, 5, e12409. [Google Scholar] [CrossRef]


| CPIC | Material | Salt Medium | Conductivity (mS/cm) | Reference |
|---|---|---|---|---|
| CCH | Cellulose | ZnCl2/CaCl2 | 749 | [77] |
| Cellulose + ECH | ZnCl2/CaCl2 | 548 | [56] | |
| Cellulose + PAM | AlCl3/ZnCl2 | 270 | [94] | |
| Cellulose + PAA + BT | ZnCl2 | 88.9 | [79] | |
| Cellulose + BT | LiCl | 89.9 | [80] | |
| Cellulose + BzMe3NOH | NOH | 237 | [81] | |
| Cellulose + PDA + PAM | FeCI3 | 240 | [78] | |
| HPMC + AN + AM | ZnCI2 | 154 | [82] | |
| CCIG | Cellulose + AM | [Bmim]Cl | 244 | [95] |
| HPC + LA + AA | [C2VIm]Br | 3.29 | [31] | |
| Cellulose | [Emim]OAc | 59.5 ± 4.7 | [96] | |
| Cellulose | [Bmim]Cl | 40 | [84] | |
| Cellulose + silk fibers | [Bmim]Cl | 49.6 | [83] | |
| Cyanate Cellulose | [Bmim]Cl | 21.35 | [60] | |
| CICEs | CLA + LATP | 1.1 | [35] | |
| CP | [Amim]Cl | 1.09 | [97] | |
| Cellulose + TEMPO | 2.83 | [66] | ||
| HEC + NMA | LiTFSI | 1.61 | [98] | |
| CLA + LATP | LiTFSI | 1.25 | [99] |
| CPIC | Optimization Method | Feasible Temp. (°C) | Performance | Reference |
|---|---|---|---|---|
| CCH | ZnCl2/CaCl2 + Glycerol | −70–50 | Max. Conductivity: 74.9 mS/cm | [77] |
| PVA + BzMe3NOH | −27.8–62.1 | Transparency: >90%; Stable mechanical properties; Conductivity: 18 mS/cm at 38 °C | [81] | |
| ZnCl2 + PAA + BT | −60–60 | Conductivity: 30.3 mS/cm at −60 °C; 120.2 mS/cm at 60 °C | [79] | |
| ZnCl2 + PAA | −70–25 | Conductivity: 13.9 mS/cm at 25 °C; 6.2 mS/cm at −70 °C | [77] | |
| CCIG | PAAm | −50 | Impact strength: 96.35 kJ·m2/(g·cm3) | [96] |
| Chitosan + [EMIM]OAc | −50–120 | Conductivity: 10−1 to 102 mS/cm | [103] | |
| Soy Protein + [Bmim]Cl | −20–85 | Tensile strength: >30 MPa at 25 °C; >10 MPa at −20 °C; >15 MPa at 85 °C | [104] | |
| [EMIM]OAc | −25–50 | Conductivity: 11.7 ± 1.1 mS/cm at −25 °C; 6.4 ± 0.7 mS/cm at −50 °C | [96] | |
| CICEs | HEC + DES | 60–280 | Conductivity: 1.61 mS/cm at 25 °C | [98] |
| PEO + NC | 30–80 | Conductivity: 5 × 10−3 mS/cm at 30 °C; 0.9 mS/cm at 80 °C | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R.; Wang, Y.; Pang, H.; Zhang, P.; Hua, Q. Progress in Cellulose-Based Polymer Ionic Conductors: From Performance Optimization to Strain-Sensing Applications. Nanoenergy Adv. 2025, 5, 12. https://doi.org/10.3390/nanoenergyadv5040012
Lu R, Wang Y, Pang H, Zhang P, Hua Q. Progress in Cellulose-Based Polymer Ionic Conductors: From Performance Optimization to Strain-Sensing Applications. Nanoenergy Advances. 2025; 5(4):12. https://doi.org/10.3390/nanoenergyadv5040012
Chicago/Turabian StyleLu, Rouyi, Yinuo Wang, Hao Pang, Panpan Zhang, and Qilin Hua. 2025. "Progress in Cellulose-Based Polymer Ionic Conductors: From Performance Optimization to Strain-Sensing Applications" Nanoenergy Advances 5, no. 4: 12. https://doi.org/10.3390/nanoenergyadv5040012
APA StyleLu, R., Wang, Y., Pang, H., Zhang, P., & Hua, Q. (2025). Progress in Cellulose-Based Polymer Ionic Conductors: From Performance Optimization to Strain-Sensing Applications. Nanoenergy Advances, 5(4), 12. https://doi.org/10.3390/nanoenergyadv5040012







