Recent Advances in Green-Solvent-Processable Organic Photovoltaics
Abstract
:1. Introduction
- i.
- Halogenated solvents, such as chlorobenzene (CB), 1,2-dichlorobenzene (o-DCB), and chloroform (CF);
- ii.
- Non-halogenated and aromatic solvents, such as toluene, o-xylene, ethylbenzene, 1,2,4-trimethylbenzene (1,2,4-TMB), and p-cymene;
- iii.
- Non-halogenated and non-aromatic solvents, such as tetrahydrofuran (THF), 2-methyl-THF, and cyclopentyl methyl ether (CPME);
- iv.
- Food additives such as 2-methylanisole (2-MA) and (R)-(+)-limonene;
- v.
- Alcohols and water such as ethanol and 2-propanol.
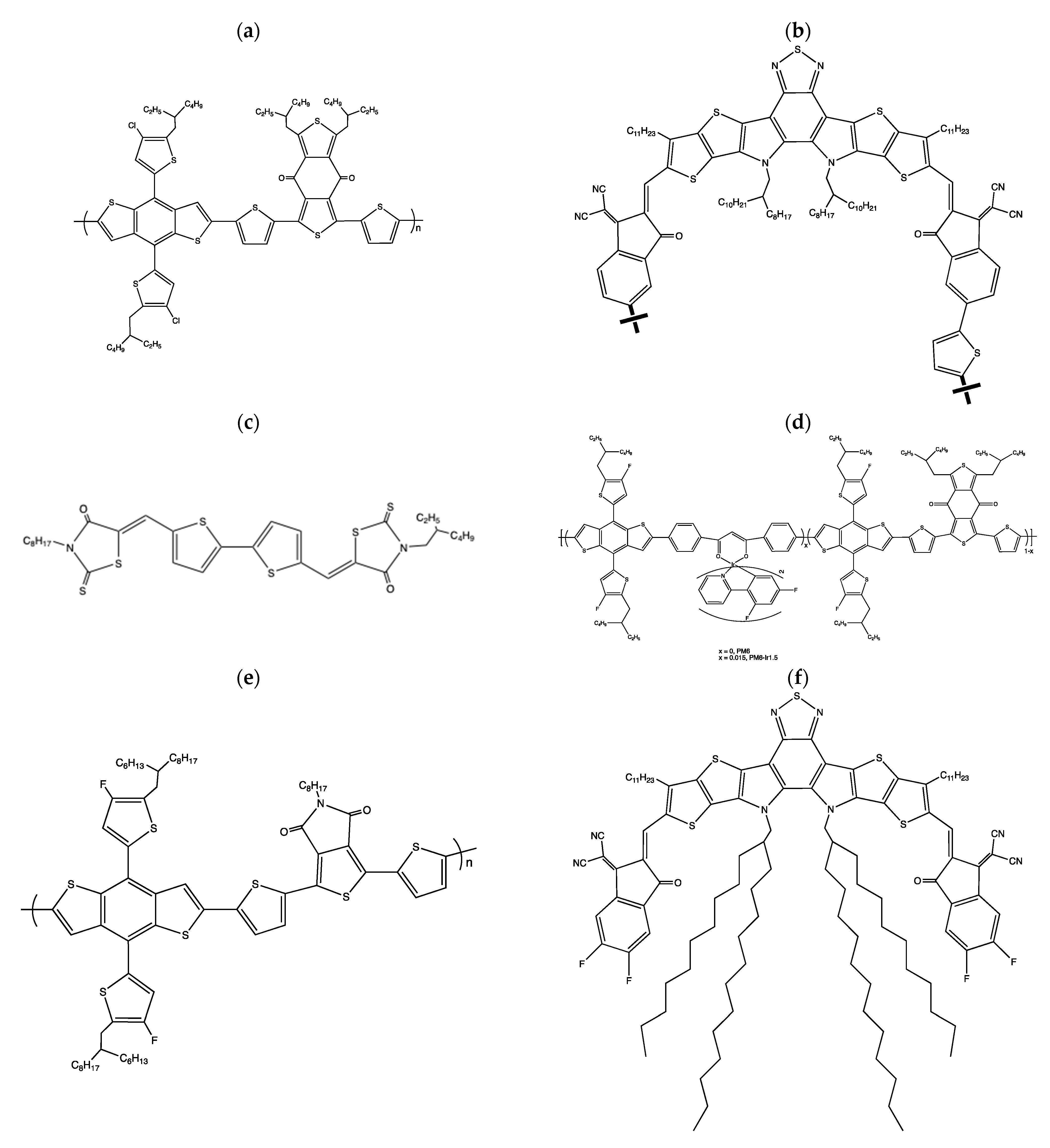
2. Molecular Engineering
3. Solvent Selection
4. Nanoparticle Ink Technology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inganäs, O. Organic Photovoltaics over Three Decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar]
- Cui, C.; Li, Y. High-performance conjugated polymer donor materials for polymer solar cells with narrow-bandgap nonfullerene acceptors. Energy Environ. Sci. 2019, 12, 3225–3246. [Google Scholar] [CrossRef]
- An, C.; Zheng, Z.; Hou, J. Recent progress in wide bandgap conjugated polymer donors for high-performance nonfullerene organic photovoltaics. Chem. Commun. 2020, 56, 4750–4760. [Google Scholar] [CrossRef] [PubMed]
- Armin, A.; Li, W.; Sandberg, O.J.; Xiao, Z.; Ding, L.; Nelson, J.; Neher, D.; Vandewal, K.; Shoaee, S.; Wang, T.; et al. A History and Perspective of Non-Fullerene Electron Acceptors for Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003570. [Google Scholar] [CrossRef]
- Lu, B.; Wang, J.; Zhang, Z.; Yuan, X.; Ding, Y.; Wang, Y.; Yao, Y. Recent progress of Y-series electron acceptors for organic solar cells. Nano Sel. 2021, 2, 2029–2039. [Google Scholar] [CrossRef]
- Li, S.; Li, C.-Z.; Shi, M.; Chen, H. New Phase for Organic Solar Cell Research: Emergence of Y-Series Electron Acceptors and Their Perspectives. ACS Energy Lett. 2020, 5, 1554–1567. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart-NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 15 October 2021).
- Bernardo, G.; Lopes, T.; Lidzey, D.G.; Mendes, A. Progress in Upscaling Organic Photovoltaic Devices. Adv. Energy Mater. 2021, 11, 2100342. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, B.; Gong, Y.; Deng, J.; Tan, Z. Green-solvent-processable strategies for achieving large-scale manufacture of organic photovoltaics. J. Mater. Chem. A 2019, 7, 22826–22847. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Zhang, H.; Hou, J. Green-solvent-processable organic solar cells. Mater. Today 2016, 19, 533–543. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.A.; Ryu, S.U.; Chung, D.; Park, T.; Son, S.Y. Green-solvent-processable organic semiconductors and future directions for advanced organic electronics. J. Mater. Chem. A 2020, 8, 21455–21473. [Google Scholar] [CrossRef]
- Campana, F.; Kim, C.; Marrocchi, A.; Vaccaro, L. Green solvent-processed organic electronic devices. J. Mater. Chem. C 2020, 8, 15027–15047. [Google Scholar] [CrossRef]
- McDowell, C.; Bazan, G.C. Organic solar cells processed from green solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 49–54. [Google Scholar] [CrossRef]
- Hong, L.; Yao, H.; Cui, Y.; Ge, Z.; Hou, J. Recent advances in high-efficiency organic solar cells fabricated by eco-compatible solvents at relatively large-area scale. APL Mater. 2020, 8, 120901. [Google Scholar] [CrossRef]
- Liao, H.-H.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- McDowell, C.; Abdelsamie, M.; Toney, M.F.; Bazan, G.C. Solvent Additives: Key Morphology-Directing Agents for Solution-Processed Organic Solar Cells. Adv. Mater. 2018, 30, e1707114. [Google Scholar] [CrossRef]
- Bernardo, G.; Gaspar, H.; Perez, G.; Shackleford, A.S.; Parnell, A.J.; Bleuel, M.; Mendes, A.; King, S.M.; Parnell, S.R. Impact of 1,8-diiodooctane on the morphology of organic photovoltaic (OPV) devices—A Small Angle Neutron Scattering (SANS) study. Polym. Test. 2019, 82, 106305. [Google Scholar] [CrossRef]
- Zhang, Y.; Parnell, A.J.; Pontecchiani, F.; Cooper, J.F.K.; Thompson, R.; Jones, R.; King, S.M.; Lidzey, D.G.; Bernardo, G. Understanding and controlling morphology evolution via DIO plasticization in PffBT4T-2OD/PC71BM devices. Sci. Rep. 2017, 7, 44269. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, G.; Washington, A.L.; Zhang, Y.; King, S.M.; Toolan, D.T.W.; Weir, M.P.; Dunbar, A.D.F.; Howse, J.R.; Dattani, R.; Fairclough, J.P.A.; et al. Does 1,8-diiodooctane affect the aggregation state of PC71BM in solution? R. Soc. Open Sci. 2018, 5, 180937. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, J.H. Solubility of Non-Electrolytes; Reinhold Publising Corporation: New York, NY, USA, 1935. [Google Scholar] [CrossRef]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient; Danish Technical: Copenhagen, Denmark, 1967. [Google Scholar]
- Walker, B.; Tamayo, A.; Duong, D.T.; Dang, X.-D.; Kim, C.; Granstrom, J.; Nguyen, T.-Q. A Systematic Approach to Solvent Selection Based on Cohesive Energy Densities in a Molecular Bulk Heterojunction System. Adv. Energy Mater. 2011, 1, 221–229. [Google Scholar] [CrossRef]
- Machui, F.; Langner, S.; Zhu, X.; Abbott, S.; Brabec, C.J. Determination of the P3HT:PCBM solubility parameters via a binary solvent gradient method: Impact of solubility on the photovoltaic performance. Sol. Energy Mater. Sol. Cells 2012, 100, 138–146. [Google Scholar] [CrossRef]
- Burgués-Ceballos, I.; Machui, F.; Min, J.; Ameri, T.; Voigt, M.M.; Luponosov, Y.N.; Ponomarenko, S.A.; Lacharmoise, P.D.; Campoy-Quiles, M.; Brabec, C.J. Solubility Based Identification of Green Solvents for Small Molecule Organic Solar Cells. Adv. Funct. Mater. 2014, 24, 1449–1457. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters—A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Landfester, K. The Generation of Nanoparticles in Miniemulsions. Adv. Mater. 2001, 13, 765–768. [Google Scholar] [CrossRef]
- Landfester, K.; Montenegro, R.; Scherf, U.; Güntner, R.; Asawapirom, U.; Patil, S.; Neher, D.; Kietzke, T. Semiconducting Polymer Nanospheres in Aqueous Dispersion Prepared by a Miniemulsion Process. Adv. Mater. 2002, 14, 651–655. [Google Scholar] [CrossRef]
- Darwis, D.; Elkington, D.; Sesa, E.; Cooling, N.; Bryant, G.; Zhou, X.; Belcher, W.; Dastoor, P. Surfactant Free P3HT/PCBM Nanoparticles for Organic Photovoltaics (OPV). AIP Conf. Proc. 2011, 1415, 120–123. [Google Scholar]
- Fan, Q.; Zhu, Q.; Xu, Z.; Su, W.; Chen, J.; Wu, J.; Guo, X.; Ma, W.; Zhang, M.; Li, Y. Chlorine substituted 2D-conjugated polymer for high-performance polymer solar cells with 13.1% efficiency via toluene processing. Nano Energy 2018, 48, 413–420. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, L.; Rech, J.J.; Hu, J.; Zhang, Q.; You, W. Green-Solvent-Processed Conjugated Polymers for Organic Solar Cells: The Impact of Oligoethylene Glycol Side Chains. ACS Appl. Polym. Mater. 2019, 1, 804–814. [Google Scholar] [CrossRef]
- Jin, L.; Ma, R.; Liu, H.; Xu, W.; Luo, Z.; Liu, T.; Su, W.; Li, Y.; Lu, R.; Lu, X.; et al. Boosting Highly Efficient Hydrocarbon Solvent-Processed All-Polymer-Based Organic Solar Cells by Modulating Thin-Film Morphology. ACS Appl. Mater. Interfaces 2021, 13, 34301–34307. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, S.; Rasool, S.; Song, C.E.; Kim, D.; Lee, S.K.; Shin, W.S.; Lim, E. Non-halogenated solvent-processed ternary-blend solar cells via alkyl-side-chain engineering of a non-fullerene acceptor and their application in large-area devices. J. Mater. Chem. A 2020, 8, 10318–10330. [Google Scholar] [CrossRef]
- Sun, R.; Wang, T.; Luo, Z.; Hu, Z.; Huang, F.; Yang, C.; Min, J. Achieving Eco-Compatible Organic Solar Cells with Efficiency > 16.5% Based on an Iridium Complex-Incorporated Polymer Donor. Sol. RRL 2020, 4, 2000156. [Google Scholar] [CrossRef]
- Du, X.; Liu, B.; Li, L.; Kong, X.; Zheng, C.; Lin, H.; Tong, Q.; Tao, S.; Zhang, X. Excimer emission induced intra-system self-absorption enhancement—A novel strategy to realize high efficiency and excellent stability ternary organic solar cells processed in green solvents. J. Mater. Chem. A 2018, 6, 23840–23855. [Google Scholar] [CrossRef]
- Rasool, S.; Van Vu, D.; Song, C.E.; Lee, H.K.; Lee, S.K.; Lee, J.; Moon, S.; Shin, W.S. Room Temperature Processed Highly Efficient Large-Area Polymer Solar Cells Achieved with Molecular Engineering of Copolymers. Adv. Energy Mater. 2019, 9, 1900168. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Chen, Y.; Lee, C.-C.; Wang, G.; Teng, N.-W.; Lee, C.-H.; Li, W.-L.; Chen, Y.-K.; Li, C.-H.; Ho, H.-L.; et al. Processing Strategies for an Organic Photovoltaic Module with over 10% Efficiency. Joule 2020, 4, 189–206. [Google Scholar] [CrossRef]
- Xia, P.; Wu, M.; Zhang, S.; Hu, J.; Chen, L.; Bu, T.; Yi, J.; Wu, D.; Xia, J. High performance PDI based ternary organic solar cells fabricated with non-halogenated solvent. Org. Electron. 2019, 73, 205–211. [Google Scholar] [CrossRef]
- Dong, S.; Jia, T.; Zhang, K.; Jing, J.; Huang, F. Single-Component Non-halogen Solvent-Processed High-Performance Organic Solar Cell Module with Efficiency over 14%. Joule 2020, 4, 2004–2016. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, R.; Chen, X.; Zeng, G.; Kobera, L.; Abbrent, S.; Zhang, B.; Chen, W.; Xu, G.; Oh, J.; et al. A guest-assisted molecular-organization approach for >17% efficiency organic solar cells using environmentally friendly solvents. Nat. Energy 2021, 6, 1045–1053. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, G.; Yu, L.; Li, R.; Peng, Q. P3HT-Based Polymer Solar Cells with 8.25% Efficiency Enabled by a Matched Molecular Acceptor and Smart Green-Solvent Processing Technology. Adv. Mater. 2019, 31, 1906045. [Google Scholar]
- Qin, Y.; Ye, L.; Zhang, S.; Zhu, J.; Yang, B.; Ade, H.; Hou, J. A polymer design strategy toward green solvent processed efficient non-fullerene polymer solar cells. J. Mater. Chem. A 2018, 6, 4324–4330. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Xu, Y.; Xian, K.; Gao, B.; Qin, J.; Zhang, J.; Wei, Z.; et al. Achieving Over 15% Efficiency in Organic Photovoltaic Cells via Copolymer Design. Adv. Mater. 2019, 31, e1808356. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Sun, C.; Angunawela, I.; Qiu, B.; Du, J.; Qin, S.; Meng, L.; Zhang, Z.; Ade, H.; et al. Green solvent-processed organic solar cells based on a low cost polymer donor and a small molecule acceptor. J. Mater. Chem. C 2020, 8, 7718–7724. [Google Scholar] [CrossRef]
- Hong, L.; Yao, H.; Wu, Z.; Cui, Y.; Zhang, T.; Xu, Y.; Yu, R.; Liao, Q.; Gao, B.; Xian, K.; et al. Eco-Compatible Solvent-Processed Organic Photovoltaic Cells with Over 16% Efficiency. Adv. Mater. 2019, 31, 1903441. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhu, P.; Xin, J.; Li, N.; Ying, L.; Zhong, W.; Li, Z.; Ma, W.; Huang, F.; Cao, Y. High-Performance Thick-Film All-Polymer Solar Cells Created Via Ternary Blending of a Novel Wide-Bandgap Electron-Donating Copolymer. Adv. Energy Mater. 2018, 8, 1703085. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Xu, X.; Liu, F.; Li, Y.; Peng, Q. Green solvent-processed efficient non-fullerene organic solar cells enabled by low-bandgap copolymer donors with EDOT side chains. J. Mater. Chem. A 2019, 7, 716–726. [Google Scholar] [CrossRef]
- Dayneko, S.V.; Hendsbee, A.D.; Welch, G.C. Combining Facile Synthetic Methods with Greener Processing for Efficient Polymer-Perylene Diimide Based Organic Solar Cells. Small Methods 2018, 2, 1800081. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Q.; Chen, L.; Xiao, Y. Amino-acid ester derived perylene diimides electron acceptor materials: An efficient strategy for green-solvent-processed organic solar cells. Dye. Pigment. 2019, 164, 384–389. [Google Scholar] [CrossRef]
- Son, S.Y.; Kim, J.W.; Lee, J.; Kim, G.-W.; Hong, J.; Kim, J.Y.; Park, T. A donor–acceptor semiconducting polymer with a random configuration for efficient, green-solvent-processable flexible solar cells. J. Mater. Chem. A 2018, 6, 24580–24587. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.; Lee, C.; Kim, Y.; Kim, C.; Nguyen, T.L.; Gautam, B.; Gundogdu, K.; Woo, H.Y.; Kim, B.J. Aqueous Soluble Fullerene Acceptors for Efficient Eco-Friendly Polymer Solar Cells Processed from Benign Ethanol/Water Mixtures. Chem. Mater. 2018, 30, 5663–5672. [Google Scholar] [CrossRef]
- Lee, C.; Lee, H.R.; Choi, J.; Kim, Y.; Nguyen, T.L.; Lee, W.; Gautam, B.; Liu, X.; Zhang, K.; Huang, F.; et al. Efficient and Air-Stable Aqueous-Processed Organic Solar Cells and Transistors: Impact of Water Addition on Processability and Thin-Film Morphologies of Electroactive Materials. Adv. Energy Mater. 2018, 8, 1802674. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Wu, Z.; Lee, C.; Oh, S.J.; Luan, N.T.; Lee, J.; Jeong, D.; Zhang, K.; Huang, F.; et al. Aqueous-Soluble Naphthalene Diimide-Based Polymer Acceptors for Efficient and Air-Stable All-Polymer Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 45038–45047. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xiong, Y.; Zhang, Q.; Li, S.; Wang, C.; Jiang, Z.; Hou, J.; You, W.; Ade, H. Surpassing 10% Efficiency Benchmark for Nonfullerene Organic Solar Cells by Scalable Coating in Air from Single Nonhalogenated Solvent. Adv. Mater. 2018, 30, 1705485. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Liao, C.; Chang, C.; Huang, K.; Chen, J.; Chen, C.; Meng, H.-F.; Zan, H.-W.; Horng, S.-F.; Lin, Y.-C.; et al. Large-area blade-coated organic solar cells processed from halogen-free solvent. Org. Electron. 2019, 75, 105376. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Chen, Z.; Zhang, Q.; Fei, Z.; Henry, R.; Heeney, M.; O’Connor, B.T.; You, W.; Ade, H. Sequential Deposition of Organic Films with Eco-Compatible Solvents Improves Performance and Enables Over 12%-Efficiency Nonfullerene Solar Cells. Adv. Mater. 2019, 31, e1808153. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, L.; Yan, H.; Li, R.; Peng, Q. Highly efficient non-fullerene organic solar cells enabled by a delayed processing method using a non-halogenated solvent. Energy Environ. Sci. 2020, 13, 4381–4388. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, S.; Zhang, Y.; Li, S.; Liu, X.; He, C.; Zheng, Z.; Hou, J. Environmentally Friendly Solvent-Processed Organic Solar Cells that are Highly Efficient and Adaptable for the Blade-Coating Method. Adv. Mater. 2018, 30, 1704837. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, G.; Wang, Y.; Xiao, T.; Adil, M.A.; Lu, G.; Zhang, J.; Wei, Z. A Sequential Slot-Die Coated Ternary System Enables Efficient Flexible Organic Solar Cells. Sol. RRL 2019, 3, 1800333. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, K.; Xie, B.; Xiao, J.; Yip, H.-L.; Yan, H.; Huang, F.; Cao, Y. High-Performance Large-Area Organic Solar Cells Enabled by Sequential Bilayer Processing via Nonhalogenated Solvents. Adv. Energy Mater. 2019, 9, 1802832. [Google Scholar] [CrossRef]
- Dayneko, S.V.; Pahlevani, M.; Welch, G.C. Indoor Photovoltaics: Photoactive Material Selection, Greener Ink Formulations, and Slot-Die Coated Active Layers. ACS Appl. Mater. Interfaces 2019, 11, 46017–46025. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-M.; Liao, C.-Y.; Lee, C.-C.; Lin, S.-Y.; Teng, N.-W.; Tan, P.H.-S. All solution and ambient processable organic photovoltaic modules fabricated by slot-die coating and achieved a certified 7.56% power conversion efficiency. Sol. Energy Mater. Sol. Cells 2019, 202, 110064. [Google Scholar] [CrossRef]
- Strohm, S.; Machui, F.; Langner, S.; Kubis, P.; Gasparini, N.; Salvador, M.; McCulloch, I.; Egelhaaf, H.-J.; Brabec, C.J. P3HT: Non-fullerene acceptor based large area, semi-transparent PV modules with power conversion efficiencies of 5%, processed by industrially scalable methods. Energy Environ. Sci. 2018, 11, 2225–2234. [Google Scholar] [CrossRef]
- Yang, P.; Zhai, T.; Yu, B.; Du, G.; Mi, B.; Zhao, X.; Deng, W. Toward all aerosol printing of high-efficiency organic solar cells using environmentally friendly solvents in ambient air. J. Mater. Chem. A 2021, 9, 17198–17210. [Google Scholar] [CrossRef]
- Perkhun, P.; Köntges, W.; Pourcin, F.; Esteoulle, D.; Barulina, E.; Yoshimoto, N.; Pierron, P.; Margeat, O.; Videlot-Ackermann, C.; Bharwal, A.K.; et al. High-Efficiency Digital Inkjet-Printed Non-Fullerene Polymer Blends Using Non-Halogenated Solvents. Adv. Energy Sustain. Res. 2021, 2, 2000086. [Google Scholar] [CrossRef]
- Bouzid, H.; Prosa, M.; Bolognesi, M.; Chehata, N.; Gedefaw, D.; Albonetti, C.; Andersson, M.R.; Muccini, M.; Bouazizi, A.; Seri, M. Impact of environmentally friendly processing solvents on the properties of blade-coated polymer solar cells. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 487–494. [Google Scholar] [CrossRef]
- Fan, B.; Zhong, W.; Ying, L.; Zhang, D.; Li, M.; Lin, Y.; Xia, R.; Liu, F.; Yip, H.-L.; Li, N.; et al. Surpassing the 10% efficiency milestone for 1-cm2 all-polymer solar cells. Nat. Commun. 2019, 10, 4100. [Google Scholar] [CrossRef]
- Li, Z.; Ying, L.; Zhu, P.; Zhong, W.; Li, N.; Liu, F.; Huang, F.; Cao, Y. A generic green solvent concept boosting the power conversion efficiency of all-polymer solar cells to 11%. Energy Environ. Sci. 2019, 12, 157–163. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Z.; Armin, A.; Dong, S.; Xia, R.; Yip, H.-L.; Shoaee, S.; Huang, F.; Cao, Y. Efficient Large Area Organic Solar Cells Processed by Blade-Coating With Single-Component Green Solvent. Sol. RRL 2018, 2, 1700169. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.H.; Byranvand, M.M.; Choi, K.; Kim, H.I.; Park, S.A.; Kim, J.Y.; Park, T. Green-solvent processable semiconducting polymers applicable in additive-free perovskite and polymer solar cells: Molecular weights, photovoltaic performance, and thermal stability. J. Mater. Chem. A 2018, 6, 5538–5543. [Google Scholar] [CrossRef]
- An, K.; Zhong, W.; Ying, L. Enhanced performance of P3HT-based non-fullerene polymer solar cells by optimizing film morphology using non-halogenated solvent. Org. Electron. 2020, 82, 105701. [Google Scholar] [CrossRef]
- Xie, C.; Classen, A.; Späth, A.; Tang, X.; Min, J.; Meyer, M.; Zhang, C.; Li, N.; Osvet, A.; Fink, R.H.; et al. Overcoming Microstructural Limitations in Water Processed Organic Solar Cells by Engineering Customized Nanoparticulate Inks. Adv. Energy Mater. 2018, 8, 1702857. [Google Scholar] [CrossRef]
- Xie, C.; Heumüller, T.; Gruber, W.; Tang, X.; Classen, A.; Schuldes, I.; Bidwell, M.; Späth, A.; Fink, R.H.; Unruh, T.; et al. Overcoming efficiency and stability limits in water-processing nanoparticular organic photovoltaics by minimizing microstructure defects. Nat. Commun. 2018, 9, 5335. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Liu, T.; Ma, R.; Xiao, Y.; Zhan, L.; Zhang, G.; Sun, H.; Ni, F.; Chai, G.; Wang, J.; et al. Precisely Controlling the Position of Bromine on the End Group Enables Well-Regular Polymer Acceptors for All-Polymer Solar Cells with Efficiencies over 15%. Adv. Mater. 2020, 32, e2005942. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, R.; Zhong, C.; Liu, T.; Zhang, G.; Zou, Y.; Jiao, X.; Min, J.; Yang, C. Altering alkyl-chains branching positions for boosting the performance of small-molecule acceptors for highly efficient nonfullerene organic solar cells. Sci. China Chem. 2020, 63, 361–369. [Google Scholar] [CrossRef]
- Xiao, J.; Jia, X.; Duan, C.; Huang, F.; Yip, H.; Cao, Y. Surpassing 13% Efficiency for Polythiophene Organic Solar Cells Processed from Nonhalogenated Solvent. Adv. Mater. 2021, 33, 2008158. [Google Scholar] [CrossRef]
- Wu, M.; Yi, J.-P.; Chen, L.; He, G.; Chen, F.; Sfeir, M.Y.; Xia, J. Novel Star-Shaped Helical Perylene Diimide Electron Acceptors for Efficient Additive-Free Nonfullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 27894–27901. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Bin, H.; Zhang, J.; Xue, L.; Qiu, B.; Wei, Z.; Zhang, Z.-G.; Li, Y. A low cost and high performance polymer donor material for polymer solar cells. Nat. Commun. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, H.; Angunawela, I.; Zhou, J.; Du, J.; Liebman-Pelaez, A.; Zhu, C.; Zhang, Z.; Meng, L.; Xie, Z.; et al. Effects of Short-Axis Alkoxy Substituents on Molecular Self-Assembly and Photovoltaic Performance of Indacenodithiophene-Based Acceptors. Adv. Funct. Mater. 2020, 30, 1906855. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer−Fullerene Solar Cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Lee, C.; Kim, H.; Kim, Y.; Lee, W.; Oh, J.H.; Kim, B.J.; Woo, H.Y. Ethanol-Processable, Highly Crystalline Conjugated Polymers for Eco-Friendly Fabrication of Organic Transistors and Solar Cells. Macromolecules 2017, 50, 4415–4424. [Google Scholar] [CrossRef]
- Du, Z.; Mainville, M.; Vollbrecht, J.; Dixon, A.L.; Schopp, N.; Schrock, M.; Peng, Z.; Huang, J.; Chae, S.; Ade, H.; et al. Insights into Bulk-Heterojunction Organic Solar Cells Processed from Green Solvent. Sol. RRL 2021, 5, 2100213. [Google Scholar] [CrossRef]
- La Notte, L.; Cataldi, P.; Ceseracciu, L.; Bayer, I.S.; Athanassiou, A.; Marras, S.; Villari, E.; Brunetti, F.; Reale, A. Fully-sprayed flexible polymer solar cells with a cellulose-graphene electrode. Mater. Today Energy 2018, 7, 105–112. [Google Scholar] [CrossRef]
- Aïch, B.R.; Lu, J.; Moisa, S.; Movileanu, R.; Estwick, E.; Tao, Y. Ink formulation for organic photovoltaic active layers using non-halogenated main solvent for blade coating process. Synth. Met. 2020, 269, 116513. [Google Scholar] [CrossRef]
- van der Poll, T.S.; Love, J.A.; Nguyen, T.-Q.; Bazan, G.C. Non-Basic High-Performance Molecules for Solution-Processed Organic Solar Cells. Adv. Mater. 2012, 24, 3646–3649. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, H.; Ko, S.-J.; Uddin, M.A.; Walker, B.; Yum, S.; Jeong, J.-E.; Yun, M.H.; Shin, T.J.; Hwang, S.; et al. Semi-crystalline photovoltaic polymers with efficiency exceeding 9% in a ∼300 nm thick conventional single-cell device. Energy Environ. Sci. 2014, 7, 3040–3051. [Google Scholar] [CrossRef] [Green Version]
- Prosa, M.; Tessarolo, M.; Bolognesi, M.; Margeat, O.; Gedefaw, D.; Gaceur, M.; Videlot-Ackermann, C.; Andersson, M.; Muccini, M.; Seri, M.; et al. Enhanced Ultraviolet Stability of Air-Processed Polymer Solar Cells by Al Doping of the ZnO Interlayer. ACS Appl. Mater. Interfaces 2016, 8, 1635–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, T.-Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J.-R.; Wakim, S.; Zhou, J.; Leclerc, M.; Li, Z.; Ding, J.; et al. Bulk Heterojunction Solar Cells Using Thieno[3,4-c]pyrrole-4,6-dione and Dithieno[3,2-b:2′,3′-d]silole Copolymer with a Power Conversion Efficiency of 7.3%. J. Am. Chem. Soc. 2011, 133, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Ying, L.; Zhu, P.; Pan, F.; Liu, F.; Chen, J.; Huang, F.; Cao, Y. All-Polymer Solar Cells Based on a Conjugated Polymer Containing Siloxane-Functionalized Side Chains with Efficiency over 10%. Adv. Mater. 2017, 29, 1703906. [Google Scholar] [CrossRef] [PubMed]
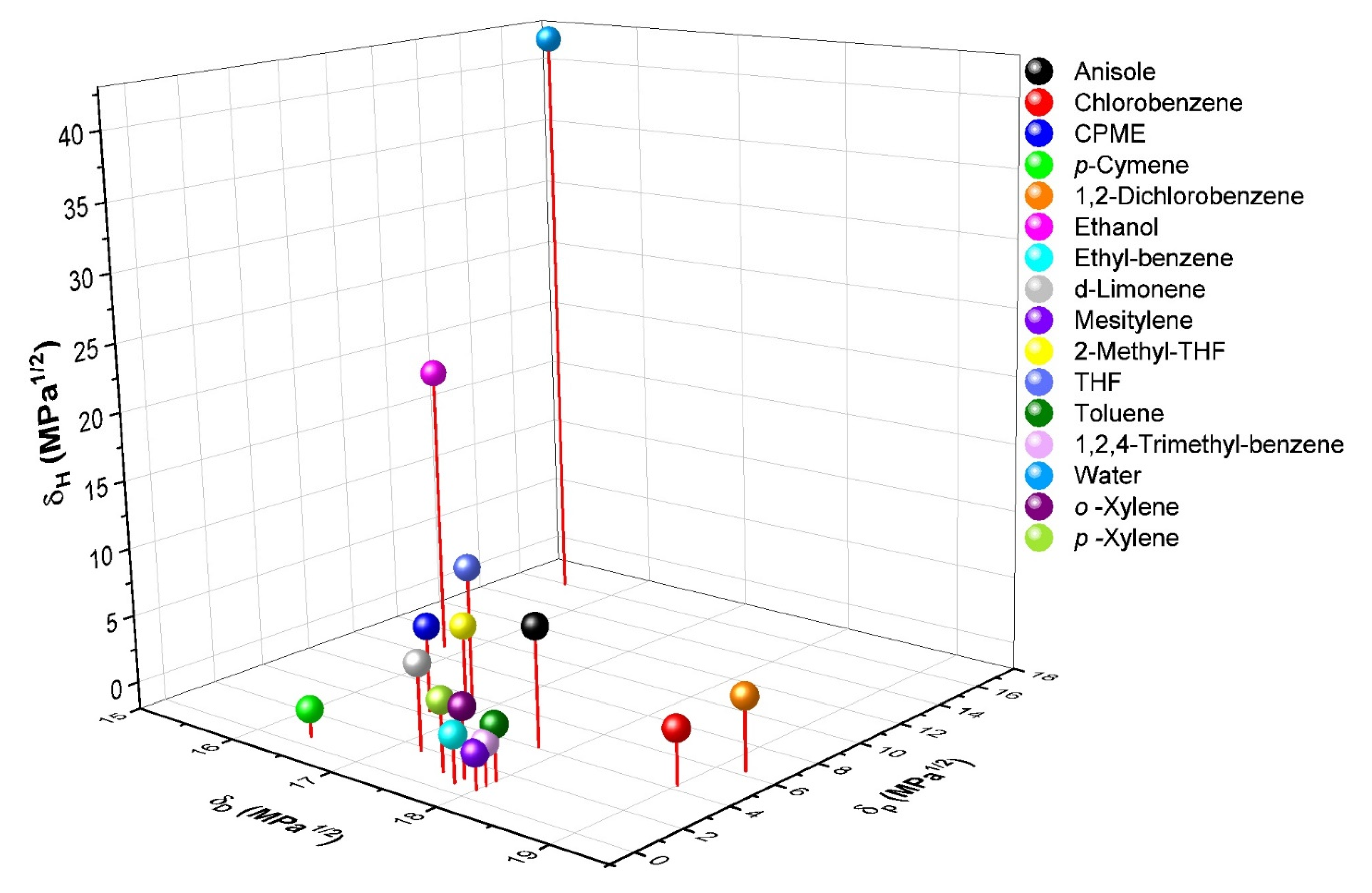
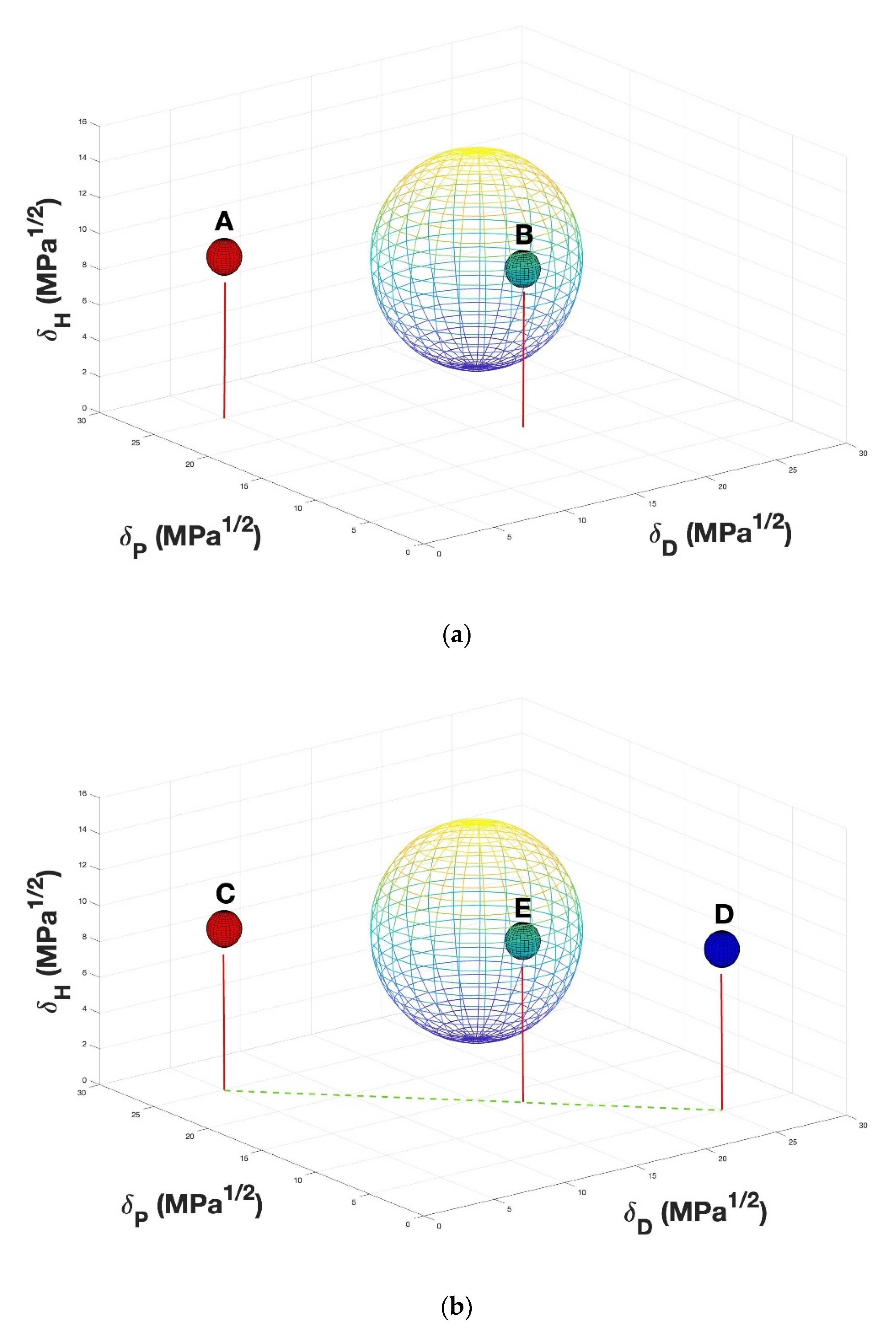
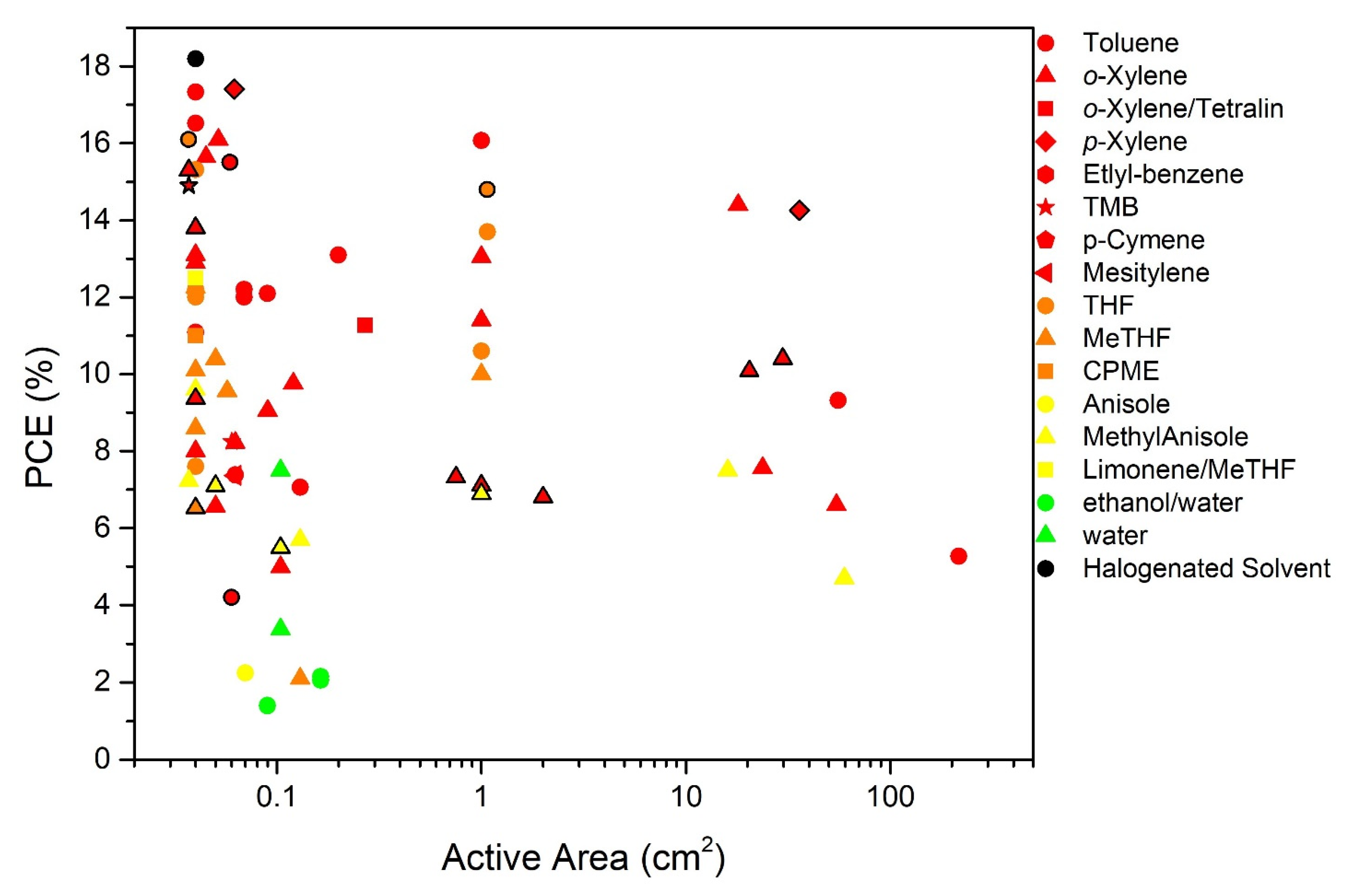
| Solvent | CAS Number | δD (a) | δP (a) | δH (a) | Molar Volume |
|---|---|---|---|---|---|
| Anisole | 100-66-3 | 17.8 | 4.1 | 6.7 | 119.1 |
| Chlorobenzene | 108-90-7 | 19.0 | 4.3 | 2.0 | 102.1 |
| Cyclopentyl Methyl Ether (CPME) | 5614-37-9 | 16.7 | 4.3 | 4.3 | |
| p-Cymene | 99-87-6 | 16.4 | 0.6 | 0.0 | |
| 1,2-Dichlorobenzene | 95-50-1 | 19.2 | 6.3 | 3.3 | 112.8 |
| Ethanol | 64-17-5 | 15.8 | 8.8 | 19.4 | 58.5 |
| Ethyl-benzene | 100-41-4 | 17.8 | 0.6 | 1.4 | 123.1 |
| d-Limonene | 5989-27-5 | 17.2 | 1.8 | 4.3 | |
| Mesitylene | 108-67-8 | 18.0 | 0.6 | 0.6 | 139.8 |
| 2-Methyl-THF | 96-47-9 | 16.9 | 5.0 | 4.3 | |
| THF | 109-99-9 | 16.8 | 5.7 | 8.0 | 81.7 |
| Toluene | 108-88-3 | 18.0 | 1.4 | 2.0 | 106.8 |
| 1,2,4-Trimethyl-benzene | 95-63-6 | 18.0 | 1.0 | 1.0 | 137.3 |
| Water | 7732-18-5 | 15.5 | 16.0 | 42.3 | 18.0 |
| o-Xylene | 95-47-6 | 17.8 | 1.0 | 3.1 | 121.2 |
| p-Xylene | 106-42-3 | 17.6 | 1.0 | 3.1 | 123.3 |
| Device Structure | Photoactive Layer (PAL) (wt:wt) | PAL Deposition Technique (a) | PAL Solvent (b) | Additive (% v/v) (b) | PAL Thick (nm) | Active Area (cm2) | PCE (%) Average/Maximum AM 1.5G | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ITO/NP-ZnO/PAL/MoO3/Al | PM7:IT-4F | SC | Toluene | - | 120 | 0.2 | 12.6/13.1 | 2018 | [30] |
| PBDB-T:IT-4F | 5.5/5.8 | ||||||||
| ITO/ZnO/PAL/MoO3/Al | P2:IT-M | SC | Toluene | - | 80–90 | 0.13 | 7.06 | 2019 | [31] |
| P3:IT-M | 3.57 | ||||||||
| P6:IT-M | 1.23 | ||||||||
| P3:IT-M | 2-MeTHF | 2.10 | |||||||
| P6:IT-M | 1.02 | ||||||||
| ITO/PEDOT:PSS/PAL/PNDIT-F3N/Ag | PM6:PY-IT (1:1) | SC | Toluene | 1-CN (1%) | 100 | 0.059 | 15.28/15.51 | 2021 | [32] |
| CF | 14.74/15.00 | ||||||||
| ITO/ZnO/PEIE/PAL/MoOx/Ag. | PTB7-Th:EH-IDTBR:T2-ORH (1:1:1) | SC | CF | DPE (1%) | 100 | 0.09 | 11.25/11.47 | 2020 | [33] |
| Toluene | DPE (1%) | 1.38/2.35 | |||||||
| PTB7-Th:EH-IDTBR:T2-OEHRH (1:1:1) | CF | DPE (1%) | 11.20/11.44 | ||||||
| Toluene | - | 7.89/8.16 | |||||||
| DPE (1%) | 11.89/12.10 | ||||||||
| D-bar-C | Toluene | DPE (1%) | 55.5 | 9.32 | |||||
| ITO/PEDOT:PSS/PAL/PNDIT-F3N-Br/Ag | PM6-Ir1.5:Y6-2C (1:1.2) | SC | THF | - | ~120 | 0.04 | 15.19/15.31 | 2020 | [34] |
| Toluene | 16.46/16.52 | ||||||||
| 1.00 | 15.92/16.07 | ||||||||
| ITO/ZnO/PAL/MoO3/Ag | PTB7-Th:PTN:PC71BM (85:15:130) | SC | ETB | DIO (3%) | 120 | 0.02 | 11.13/11.44 | 2018 | [35] |
| PTB7-Th:PPN:PC71BM (85:15:130) | 9.43/9.77 | ||||||||
| ITO/ZnO-NPs/PAL/MoOx/Ag | PNTz4T:PC71BM | SC | o-xylene | DPE (3%) | ~240 | 0.12 | 9.58/9.76 | 2019 | [36] |
| PNTz4T-3MTC:PC71BM | 9.30/9.59 | ||||||||
| PNTz4T-5MTC:PC71BM | ~250 | 9.45/9.66 | |||||||
| PNTz4T-7MTC:PC71BM | 7.27/7.47 | ||||||||
| PNTz4T-10MTC:PC71BM | 6.92/7.12 | ||||||||
| PNTz4T-5MTC:PC71BM | D-bar-C | 240 | 54.45 | 6.46/6.61 | |||||
| PNTz4T:PC71BM | 100 | 3.91/4.29 | |||||||
| ITO/ZnO/PAL/MoO3/Ag | TPD-1:IT-4F (1:1) | SC | o-xylene | DIO (0.5%) | 100–110 | 0.04 | 11.2/11.7 | 2020 | [37] |
| TPD-2:IT-4F (1:1) | 11.5/11.8 | ||||||||
| TPD-3:IT-4F (1:1) | 11.9/12.1 | ||||||||
| TPD-3F:IT-4F (1:1) | 13.6/13.8 | ||||||||
| TPD-3F:IT-4F (1:1) | CB | 13.4/13.5 | |||||||
| TPD-3F:IT-4F (1:1) | o-xylene | 20.4 | 10.08 | ||||||
| 29.75 | 10.13 | ||||||||
| BC | 10.40 | ||||||||
| ITO/ZnO/PAL/MoO3/Ag | PTB7-Th:TPDI2 | SC | o-xylene | ---- | 65 | 0.0625 | 6.83/6.95 | 2019 | [38] |
| PTB7-Th:TPDI2:ITIC (1:1:0.4) | 69 | 8.11/8.22 | |||||||
| Mesitylene | ---/7.37 | ||||||||
| Toluene | ---/7.38 | ||||||||
| CB | ---/7.28 | ||||||||
| ITO/PEDOT:PSS/PM6:NFA/PFNBr/Ag | PM6:Y6 (1:1.2) | SC | CF | - | ~110 | 0.0516 | 14.4/14.9 | 2020 | [39] |
| CF | CN (0.5%) | 15.3/15.7 | |||||||
| xylene | - | 10.5/10.8 | |||||||
| PM6:DTY6 (1:1.2) | CF | - | 16.1/16.3 | ||||||
| xylene | - | 15.9/16.1 | |||||||
| ITO/PEDOT:PSS/PAL/PNDIT-F3NBr/Ag | PM6:DTY6 | BC | o-xylene | - | 18 | 14.4 | |||
| ITO/AZO/PAL/MoO3/Al | PM6:Y6 | SC | p-xylene | CN (1.2%) | ~105 | 0.062 | 11.06/11.25 | 2021 | [40] |
| PM6:Y6:20%BTO | 16.46/16.59 | ||||||||
| PM6:Y6:20%BTO:PC71BM | 17.30/17.41 | ||||||||
| PM6:Y6 | BC | 36 | 7.31 | ||||||
| PM6:Y6:20%BTO:PC71BM | 14.26 | ||||||||
| ITO/PEDOT:PSS/PAL/OTF/Al | P3HT: TrBTIC | SC | 1,2,4-TMB (0 min aging) | - | ~110 | 0.06 | 6.31/6.62 | 2019 | [41] |
| 1,2,4-TMB (40 min aging) | 7.96/8.25 | ||||||||
| ITO/ PEDOT:PSS/PAL/PFN-Br/Al | PBDB-T:IT-M | SC | THF | - | ~100 | Small area (d) | 6.20/6.41 | 2018 | [42] |
| CB | 11.64/11.74 | ||||||||
| PBDB-T-BO:IT-M | THF | 10.64/10.78 | |||||||
| CB | 7.85/7.87 | ||||||||
| PBDB-BzT:IT-M | THF | 11.68/12.10 | |||||||
| CB | 9.04/9.18 | ||||||||
| ITO/ZnO/PAL/MoO3/Al | PBDB-TF-T1:IT-4F (1:1) | SC | CB | DIO (0.5%) | 100 | 0.0375 | 14.8/15.1 | 2019 | [43] |
| THF | NMP (0.5%) | 13.8/14.2 | |||||||
| 1.07 | 13.3/13.7 | ||||||||
| BC | 140 | 12.3/13.1 | |||||||
| ITO/PEDOT:PSS/PAL/PDINO/Al | PTQ10:HO-IDIC-2F (BHJ) (1:1) | SC | THF | - | Small area (d) | 11.84/12.20 | 2020 | [44] | |
| CF | 11.94/12.43 | ||||||||
| PTQ10/HO-IDIC-2F (LbL) | THF | 12.03 | |||||||
| BC | THF | 11.85 | |||||||
| ITO/PEDOT:PSS/PAL/PFN-Br/Al | PBDB-TF-T1:BTP-4F-12 (1:1.2) | SC | CB | DIO (0.5%) | ~100 | 0.037 | 15.0/15.5 | 2019 | [45] |
| o-DCB | 12.2/12.6 | ||||||||
| CF | 15.1/15.5 | ||||||||
| o-xylene | 15.1/15.3 | ||||||||
| 1,2,4-TMB | 14.6/14.9 | ||||||||
| THF | 15.7/16.1 | ||||||||
| SC | THF | 1.07 | 14.8 | ||||||
| BC | 14.4 | ||||||||
| ITO/PEDOT:PSS/PAL/PFN-Br/Ag | PTzBI-Si:PBTA-Si:N2200 (1:1:1) | SC | MeTHF | - | 150 | 0.057 | 9.41/9.56 | 2018 | [46] |
| ITO/ZnO/PAL/MoO3/Al | PTB-EDOT:ITIC-Th (1:1.5) | SC | CB | 1-CN (2%) | 100 | 0.04 | 7.01/7.31 | 2019 | [47] |
| MeTHF | - | 8.30/8.59 | |||||||
| PTB-EDOTS:ITIC-Th (1:1.5) | CB | 1-CN (2%) | 8.98/9.28 | ||||||
| MeTHF | - | 9.90/10.18 | |||||||
| PTB-EDOTS:J71:ITIC-Th (1-x, x, 1.5) (x = 10 wt%) | 10.70/11.04 | ||||||||
| PTB-EDOTS:J71:ITIC-Th (1-x, x, 1.5) (x = 20 wt%) | 11.94/12.26 | ||||||||
| PTB-EDOTS:J71:ITIC-Th (1-x, x, 1.5) (x = 30 wt%) | 11.57/11.79 | ||||||||
| ITO/ZnO/PAL/MoO3/Ag | PTB7-Th:4C1 (3:7) | SC | 2Me-THF | - | 0.04 | 4.27/4.81 | 2018 | [48] | |
| PTB7-Th:4C2 (3:7) | 4.64/4.81 | ||||||||
| PTB7-Th:4C3 (3:7) | 2.99/3.69 | ||||||||
| PTB7-Th:4C4 (3:7) | 4.77/5.21 | ||||||||
| PTB7-Th:4C5 (3:7) | 4.62/4.89 | ||||||||
| PTB7-Th:4C6 (3:7) | 5.16/5.55 | ||||||||
| PTB7-Th:4C8 (3:7) | 2.80/3.37 | ||||||||
| PTB7-Th:4C8b (3:7) | 5.33/5.47 | ||||||||
| PTB7-Th:4C8b (3:7) | DIO (0.25%) | 6.39/6.52 | |||||||
| ITO/ZnO/PAL/MoO3/Al | PBDTTT-C:oo-2PDIate (1:1) | SC | Anisole | - | 80 | 0.07 | ---/2.24 | 2019 | [49] |
| CB | ---/2.52 | ||||||||
| ITO/ZnO/PAL/MoO3/Ag. | PffBT-RT4:PC71BM | SC | DCB | - | 400–450 | 0.037 | 8.53/8.84 | 2018 | [50] |
| 2-MA | 7.10/7.23 | ||||||||
| ITO/ZnO/PAL/MoO3/Ag | PPDT2FBT-A: PCBO12 | SC | ethanol/water mixture 88:12 (v/v) | - | 70–90 | 0.09 | 1.20/1.40 | 2018 | [51] |
| PDT2FBT-A: PCBO15 | 0.96/1.11 | ||||||||
| PPDT2FBT-A: PCBO27 | 0.35/0.37 | ||||||||
| ITO/PEDOT:PSS (+0.1 vol% GOPS)/PAL/PNDIT-F3N/Ag | PPDT2FBT-A: PC61BO12 (1:2.5) | SC | ethanol/water mixture 90:10 (v/v) | - | 60–70 | 0.164 | 0.62/0.83 | 2018 | [52] |
| ethanol/water mixture 85:15 (v/v) | 1.86/2.05 | ||||||||
| ethanol/water mixture 75:25 (v/v) | 1.36/1.74 | ||||||||
| ethanol/water mixture 65:35 (v/v) | 1.16/1.39 | ||||||||
| ITO/PEDOT:PSS/PAL/ PNDIT-F3N/Ag | PPDT2FBT-A: P(NDIDEG-T) (3:1) | SC | Ethanol/water 85:15 (v/v) | - | 70–90 | 0.164 | 1.89/2.15 | 2019 | [53] |
| PPDT2FBT-A: P(NDITEG-T) (3:1) | 1.22/1.43 | ||||||||
| PPDT2FBT-A: P(NDITEG-T2) (3:1) | 1.38/1.56 | ||||||||
| ITO/PEDOT:PSS/PAL/PFN-Br/Al | FTAZ:IT-M (1:1) | SC | CB | - | ~105 | 0.069 | 10.5/11.1 | 2018 | [54] |
| Toluene | 0.069 | 12.0/12.2 | |||||||
| BC | 0.56 | ---/9.8 | |||||||
| ITO/PEDOT:PSS/PAL/LiF/Al | PTB7-Th:PC71BM | BC | CB | DIO (3%) | - | 0.04 | 9.45/9.75 | 2019 | [55] |
| ITO/PEDOT:PSS/PAL/LiF/Al | Toluene | NMP (3%) | 9.26/9.39 | ||||||
| ITO/PEDOT:PSS/PAL/ZrAcac/Al | Toluene | NMP (3%) | 10.91/11.09 | ||||||
| ITO/PEDOT:PSS/PAL/LiF/Al | CB | DIO (3%) | 216 | 5.16/5.20 | |||||
| ITO/PEDOT:PSS/PAL/LiF/Al | Toluene | NMP (3%) | 5.00/5.03 | ||||||
| ITO/PEDOT:PSS/PAL/ZrAcac/Al | Toluene | NMP (3%) | 5.12/5.27 | ||||||
| ITO/PEDOT:PSS/PAL/PFN-Br/Al | FTAZ/IT-M (bi-layer) | SSC | (R)-(+)-limonene/2-Me-THF (c) | - | ~105 | 0.069 | 12.2/12.5 | 2019 | [56] |
| OTAZ/IT-M (bi-layer) | 4.7/4.8 | ||||||||
| F-OTAZ/IT-M (bi-layer) | 5.8/6.1 | ||||||||
| FTAZ: IT-M (BHJ) | SC | Toluene | 11.7/12.0 | ||||||
| OTAZ: IT-M (BHJ) | 3.8/4.1 | ||||||||
| F-OTAZ: IT-M (BHJ) | 5.2/5.7 | ||||||||
| ITO/PEDOT:PSS/PAL/PFN-Br/Al | PM6:BTP-BO-4Cl (1:1.2) | SC | Toluene (0 min del. time) | BV (0.4%) | ~110 | 0.04 | 15.28/15.69 | 2020 | [57] |
| Toluene (40 min del. time) | 16.92/17.33 | ||||||||
| Toluene (100 min del. time) | 12.10/12.59 | ||||||||
| ITO/PEDOT:PSS/PAL/PFN-Br/Al | PBTA-TF:IT-M | SC | o-xylene | 1-PN | 0.04 | 12.7/13.1 | 2018 | [58] | |
| THF | IPA | 11.7/12.0 | |||||||
| BC | THF | IPA | ~100 | 1.0 | 10.6 | ||||
| ITO/ZnO/PAL/MoOx/Ag | [PTB7-Th] + (p-DTS(FBTTH2)2: PC71BM) | SDC | o-xylene | DIO (3%) | 200 | 0.04 | 9.36 | 2019 | [59] |
| 0.75 | 7.32 | ||||||||
| 1 | 7.11 | ||||||||
| 2 | 6.80 | ||||||||
| ITO/PEDOT/PAL/PNDIT-F3N/Ag | PffBT4T-2OD/PC71BM (bilayer) | SC | CB/DCB | - | 90/30 | 0.04 | 9.1/9.4 | 2019 | [60] |
| PffBT4T-2OD:PC71BM (BHJ) | 120 | 9.0/9.3 | |||||||
| PM6/IT-4F (bilayer) | SC | xylene | 0.04 | 12.5/12.9 | |||||
| PM6:IT-4F (BHJ) | 11.4/12.0 | ||||||||
| PffBT4T-2OD/PC71BM (bilayer) | 8.7/8.9 | ||||||||
| PffBT4T-2OD:PC71BM (BHJ) | 3.9/4.1 | ||||||||
| PM6/IT-4F (bilayer) | BC | xylene | 1 | 11.0/11.4 | |||||
| PM6:IT-4F (BHJ) | 10.1/10.6 | ||||||||
| PffBT4T-2OD/PC71BM (bilayer) | 7.8/8.0 | ||||||||
| PffBT4T-2OD:PC71BM (BHJ) | 2.8/3.0 | ||||||||
| ITO/ZnO/PEIE/PAL/MoOx/Ag | PPDT2FBT:PC61BM | SC | o-DCB | DPE | 6.8 | 2019 | [61] | ||
| PPDT2FBT:ITIC-M | 5.6 | ||||||||
| PPDT2FBT:PC61BM | SC | o-xylene | DPE (3%) | 0.09 | 6.12/8.03 | ||||
| SDC | 6.86/8.48 | ||||||||
| PPDT2FBT:ITIC-M | SC | DPE (1%) | 7.08/7.60 | ||||||
| SDC | 5.87/6.70 | ||||||||
| PPDT2FBT:ITIC-F | SC | DPE (1%) | 7.72/9.05 | ||||||
| SDC | 7.00/8.35 | ||||||||
| PPDT2FBT:tPDI2N-EH | SC | DPE (0.75%) | 5.62/6.48 | ||||||
| SDC | 3.16/4.66 | ||||||||
| ITO/PEI/PAL/PEDOT:PSS/Ag | PV2000:PCBM | SC | o-xylene | ---- | 0.04 | 8.00 | 2019 | [62] | |
| SDC | ---- | 200-250 | 23.7 | 7.56 | |||||
| ITO/ZnO/PAL/PEDOT:PSS/AgNW | P3HT:IDTBR | BC | o-xylene | ---- | 0.104 | 3.71 | 2018 | [63] | |
| 1-MN | 4.99 | ||||||||
| THN | 4.99 | ||||||||
| p-cymene | ---- | 4.17 | |||||||
| BrA | 5.30 | ||||||||
| 2-MA | ---- | 5.41 | |||||||
| BrA | 5.49 | ||||||||
| BC | CB | BrA | 200 | 59.52 | 4.8/5.0 | ||||
| SDC | 4.3/4.4 | ||||||||
| BC | 2-MA | - | 4.5/4.7 | ||||||
| ITO/PEDOT:PSS/PAL/PDINO/Ag | PTQ10:Y6-BO (1:1.2) | AC | o-xylene | - | ~100 | 0.045 | 15.40/15.65 | 2021 | [64] |
| 1.0 | 12.51/13.05 | ||||||||
| ITO/ZnO/PAL/MoOx/Ag | PM6:ITIC-4F | SC | o-xylene:Tetralin | - | 100 | 0.27 | 10.76/11.27 | 2021 | [65] |
| AC | 120 | 9.10/9.93 | |||||||
| ITO/PEDOT:PSS/PAL/LiF/Al. | PFQ2T-BDT:PC61BM (1:1) | BC | o-DCB | - | 0.06 | 3.68 | 2019 | [66] | |
| o-DCB | DIO (2.5%) | 3.75 | |||||||
| TMB | - | 3.53 | |||||||
| TMB | DIO (2.5%) | 4.20 | |||||||
| ITO/PEDOT:PSS/PAL/PFNDI-Br/Ag | PBTA-Si:PTzBI-Si:N2200 | SC | MeTHF | - | 130 | 0.05 | 9.1 | 2019 | [67] |
| - | 320 | 8.7 | |||||||
| DBE | 130 | 9.3 | |||||||
| DBE | 380 | 10.4 | |||||||
| DBE | 130 | 1.0 | 9.8 | ||||||
| DBE | 350 | 10.0 | |||||||
| ITO/PEDOT:PSS/PAL/PFNDI-Br/Ag. | PTzBI-Si:N2200 | SC | 2-MeTHF | DBE (0.5%) | 130 | 0.04 | 9.9/10.1 | 2019 | [68] |
| THF | 7.4/7.6 | ||||||||
| CPME | 10.8/11.0 | ||||||||
| CB | - | 1.2 | |||||||
| DCB | 1.1 | ||||||||
| CF | 5.1 | ||||||||
| ITO/PEDOT/PAL/PFNBr/Ag | PTB7-Th:PC71BM | SC | 2-MA | - | 220 | 0.04 | 9.2/9.6 | 2018 | [69] |
| ITO/ZnO/PAL/MoO3/Ag | BC | ~230 | 16.0 | 7.1/7.5 | |||||
| ITO/PEDOT:PSS/PAL/Al. | asy-BTBDTs:PC71BM (1:1) | SC | 2-MA | - | 0.13 | 5.7 | 2018 | [70] | |
| ITO/PEDOT:PSS/PAL/PFN-Br/Ag | P3HT:O-IDTBR (1:1) | SC | o-xylene | - | 0.05 | 5.42/5.61 | 2020 | [71] | |
| 2-MA | - | 6.31/6.56 | |||||||
| 1-MN | 6.95/7.10 | ||||||||
| 1.0 | 6.67/6.89 | ||||||||
| ITO/ZnO/PAL/MoOx/Ag | pDPP5T2 + PC71BM bpNPs | SC | Water | - | 0.104 | 2.32 | 2018 | [72] | |
| pDPP5T2:PC71BM bNPs | 3.38 | ||||||||
| ITO/ZnO/PAL/MoOx/Ag | P3HT:o-IDTBR NPs | SC | THF | - | 210 | 0.104 | 3.11/3.45 | 2018 | [73] |
| H2O with SDS | 210 | 2.53/2.73 | |||||||
| H2O with F127 | 230 | 4.95/5.23 | |||||||
| PCE10:o-IDTBR NPs | H2O with SDS | 90 | 3.61/4.12 | ||||||
| H2O with F127 | 100 | 4.94/5.19 | |||||||
| PBQ-QF:o-IDTBR NPs | H2O with SDS | 90 | 3.45/4.02 | ||||||
| H2O with F127 | 90 | 5.96/6.52 | |||||||
| PBQ-QF:ITIC | H2O with SDS | 110 | 3.98/4.42 | ||||||
| H2O with F127 | 110 | 6.97/7.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, H.; Bernardo, G.; Mendes, A. Recent Advances in Green-Solvent-Processable Organic Photovoltaics. Nanoenergy Adv. 2022, 2, 1-28. https://doi.org/10.3390/nanoenergyadv2010001
Gaspar H, Bernardo G, Mendes A. Recent Advances in Green-Solvent-Processable Organic Photovoltaics. Nanoenergy Advances. 2022; 2(1):1-28. https://doi.org/10.3390/nanoenergyadv2010001
Chicago/Turabian StyleGaspar, Hugo, Gabriel Bernardo, and Adélio Mendes. 2022. "Recent Advances in Green-Solvent-Processable Organic Photovoltaics" Nanoenergy Advances 2, no. 1: 1-28. https://doi.org/10.3390/nanoenergyadv2010001
APA StyleGaspar, H., Bernardo, G., & Mendes, A. (2022). Recent Advances in Green-Solvent-Processable Organic Photovoltaics. Nanoenergy Advances, 2(1), 1-28. https://doi.org/10.3390/nanoenergyadv2010001








