Synthesis, Investigation, Biological Evaluation, and Application of Coordination Compounds with Schiff Base—A Review
Abstract
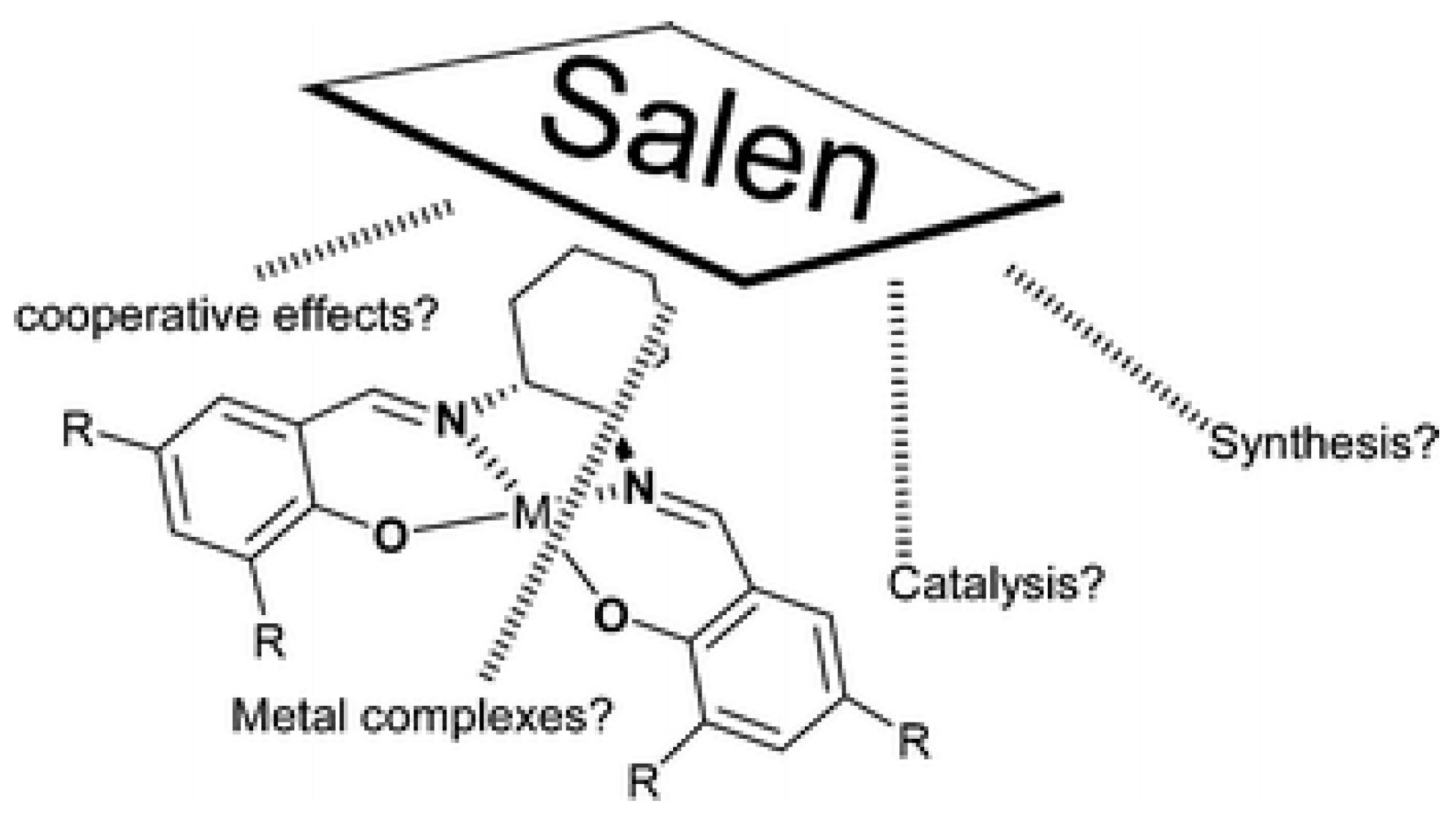
1. Introduction
2. Methods for Characterization of Coordination Compounds
3. Some Aspects of the Biological Significance of Coordination Compounds with Schiff Base
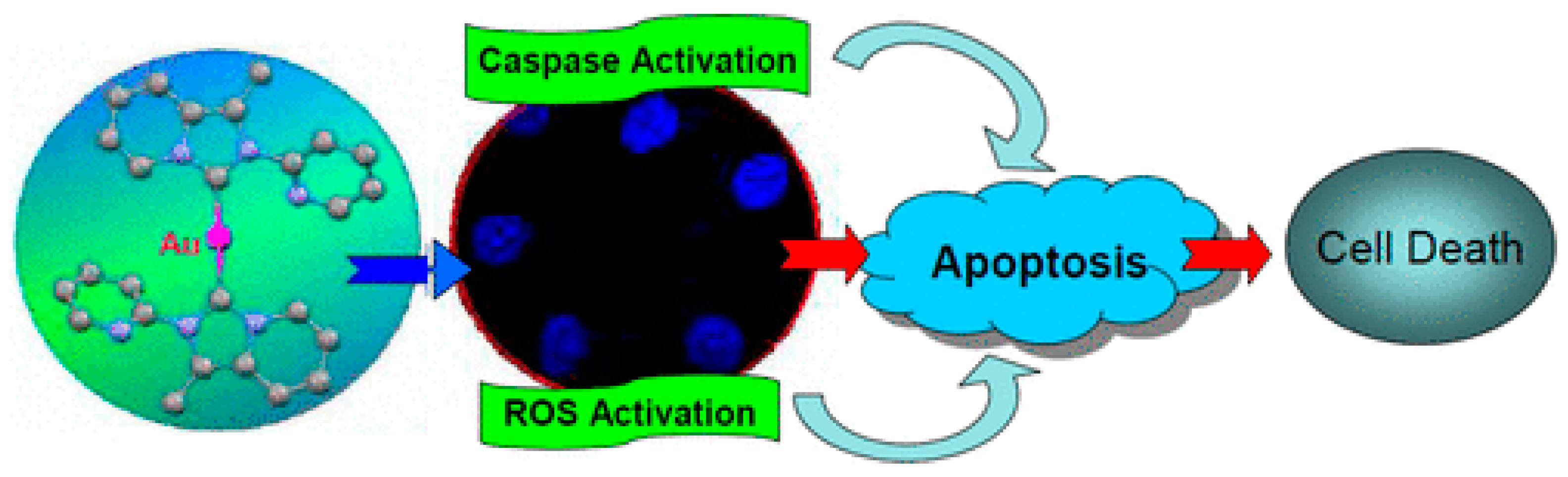
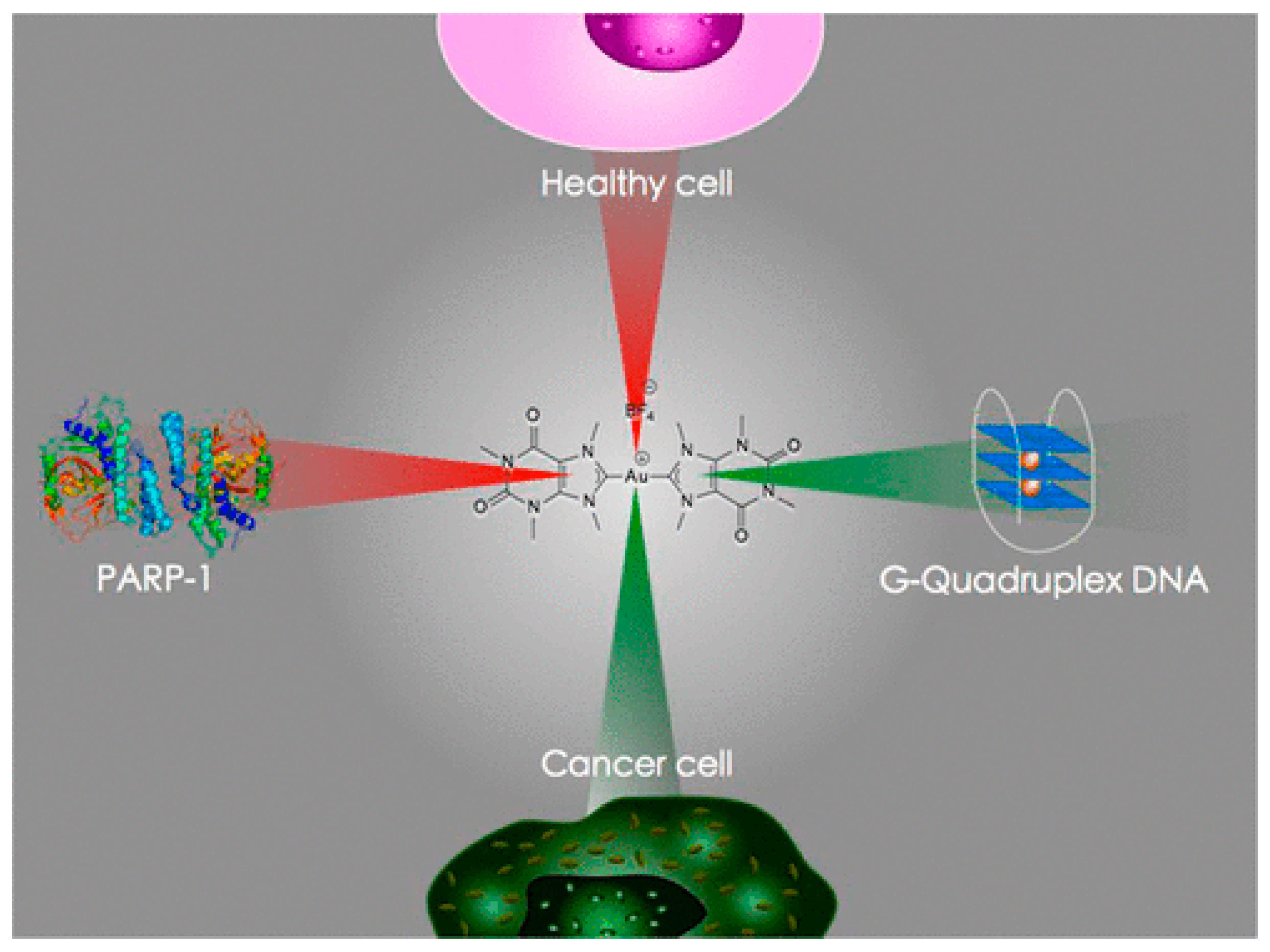
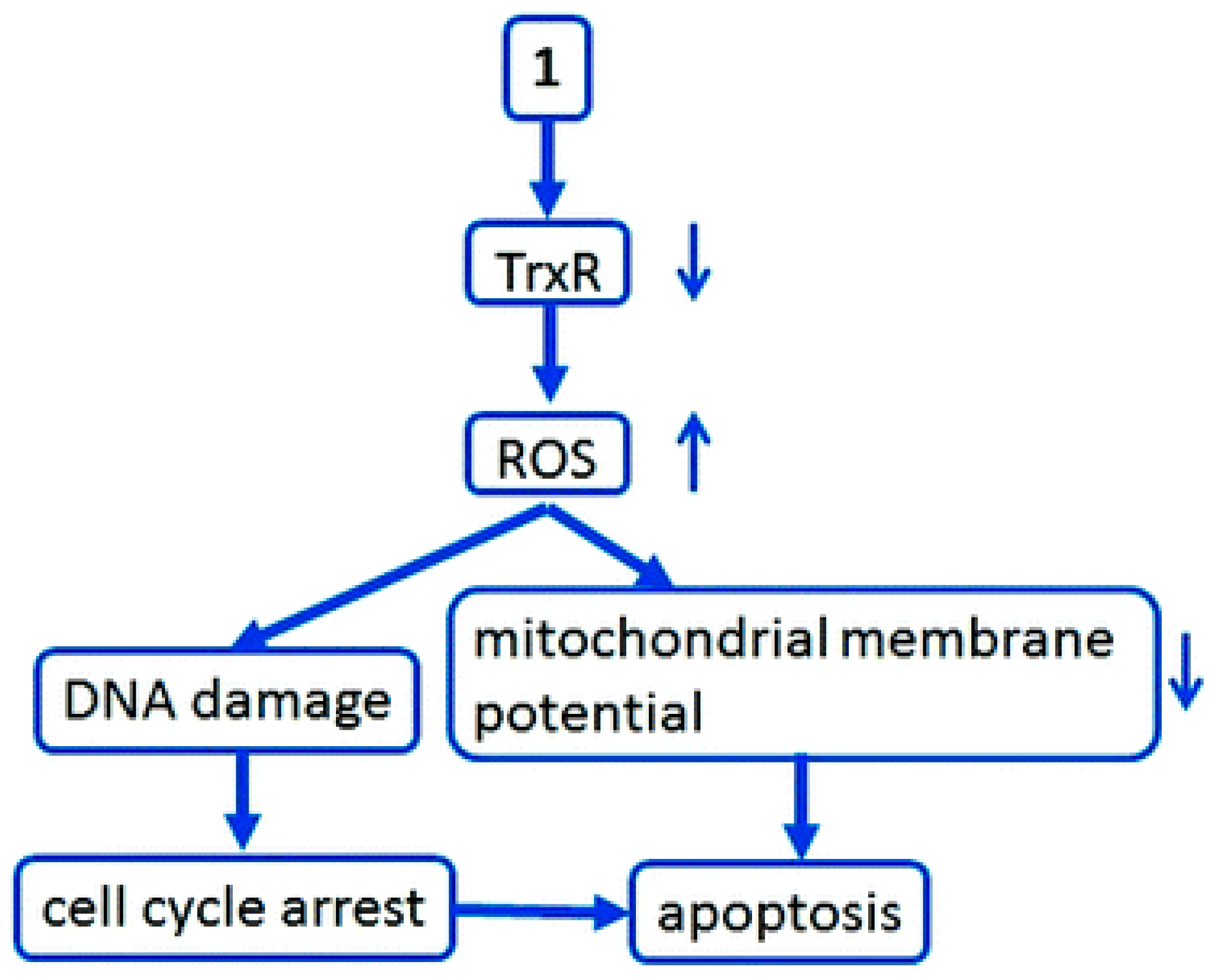
3.1. Anticancer Properties
3.1.1. Therapeutic Potential in Cancer Treatment
- − Exhibit redox activity;
- − Form complexes targeting specific biomolecules;
- − Disrupt cellular mechanisms of proliferation.
3.1.2. Safety Issues with Metal Complexes
- 1.
- Toxicity Challenges: Despite their effectiveness, metal-based cancer drugs like cisplatin are associated with severe side effects, including nephrotoxicity, neurotoxicity, and ototoxicity [113]. These challenges have spurred the development of derivatives such as carboplatin, which, while promising, still face regulatory hurdles due to adverse effects.
- 2.
- Examples of Failed Derivatives: Several platinum-based drugs (e.g., JM-11, ormaplatin, zeniplatin, and spiroplatin) failed to gain market approval due to severe or unpredictable toxicities [114].
- 3.
- 4.
- Strategies to Mitigate Toxicity: Structural modifications of metal complexes aim to improve their selectivity for cancer cells and reduce adverse effects on healthy tissues.
3.1.3. Nanoparticles in Cancer Therapy
- 1.
- Advantages of Nanotechnology: Nanoparticles (NPs) offer targeted drug delivery, improving therapeutic index and reducing off-target effects [117]. They enhance bioavailability, solubility, and stability while facilitating sustained release and selective targeting of cancer cells.
- 2.
- Metal-Based Nanoparticles: Metal-based NPs (e.g., nickel, gold, silver, iron oxide, gadolinium) provide significant advantages in drug delivery and diagnosis due to their large surface area, which can carry higher drug loads.
- 3.
- Tumor-Specific Targeting: NPs can be functionalized with peptides, proteins, nucleic acids, or small molecules to target tumor-specific receptors or biomarkers, ensuring precise delivery [118]. This reduces toxicity in non-cancerous tissues.
- 4.
- Imaging and Therapeutic Applications: NP-based platforms are used for advanced optical imaging and therapeutic delivery. Their multifunctional nature enables combined diagnostic and therapeutic applications, paving the way for synergistic effects when combined with multidrug regimens.
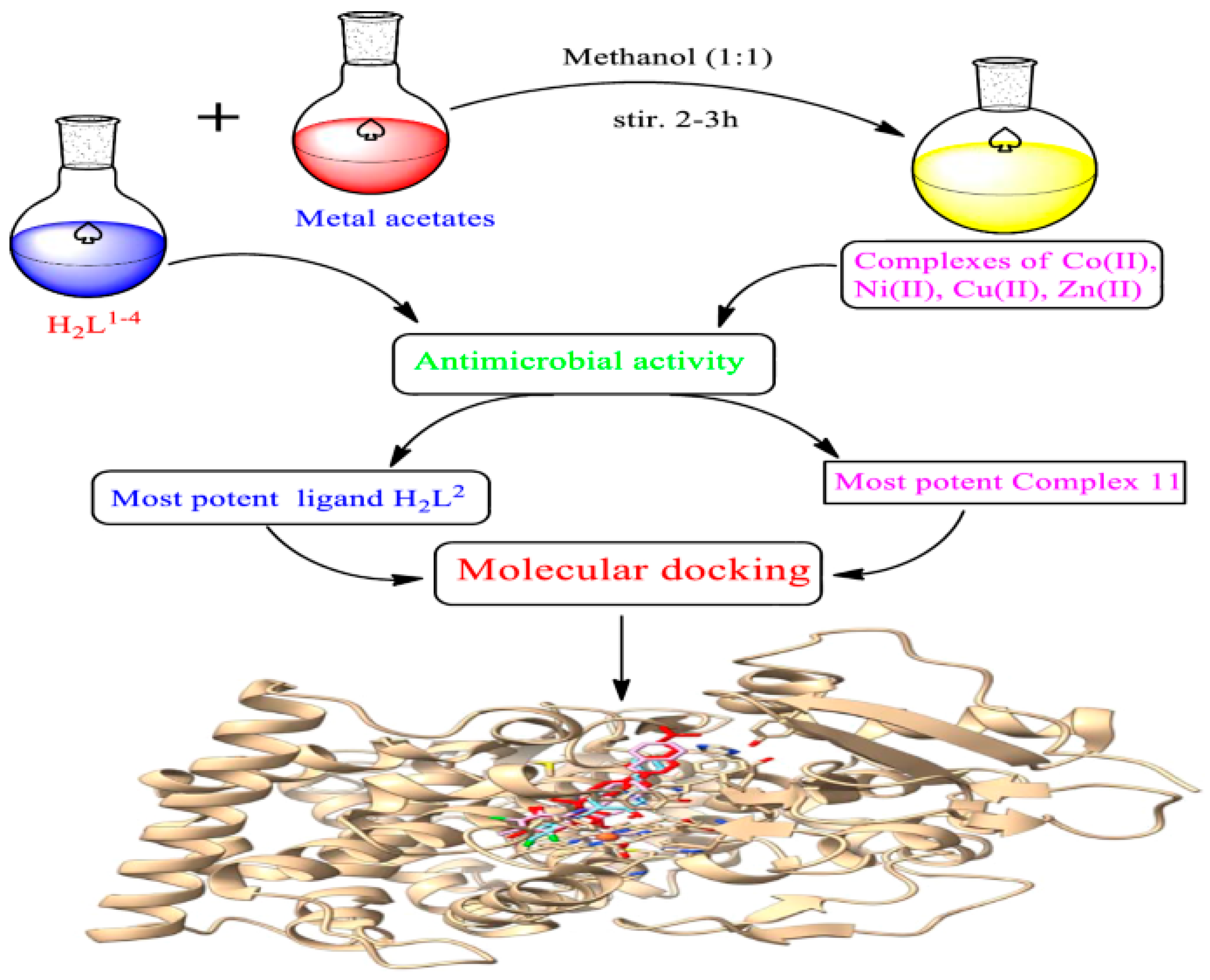
3.2. Antimicrobial Activity (Antibacterial and Antifungal)
3.3. Antioxidant Activity
3.4. Enzyme-Inhibitory Activities
4. Schiff Base Complexes as Catalysts
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| NMR | Nuclear magnetic resonance |
| FAB-MS | Fast atom bombardment mass spectrometry |
| MALDI | Matrix-assisted laser desorption/ionization |
| XRD | X-ray diffraction |
| UV-Vis | Ultraviolet-visible spectroscopy |
| IR | Infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| TrxR | Thioredoxin reductase |
| ROS | Reactive oxygen species |
| DNA | Deoxyribonucleic acid |
| CR | Congo red |
| EA | Ethacrynic acid |
| MMP-3 | Matrix metalloproteinase-3 |
References
- Ahmedova, A.; Marinova, P.; Paradowska, K.; Stoyanov, N.; Wawer, I.; Mitewa, M. Spectroscopic aspects of the coordination modes of 2,4-dithiohydantoins: Experimental and theoretical study on copper and nickel complexes of cyclohexanespiro-5-(2,4-dithiohydantoin). Inorg. Chim. Acta 2010, 363, 3919–3925. [Google Scholar] [CrossRef]
- Ahmedova, A.; Marinova, P.; Paradowska, K.; Marinov, M.; Wawer, I.; Mitewa, M. Structure of 2,4-dithiohydantoin complexes with copper and nickel—Solid-state NMR as verification method. Polyhedron 2010, 29, 1639–1645. [Google Scholar] [CrossRef]
- Ahmedova, A.; Pavlović, G.; Marinov, M.; Marinova, P.; Momekov, G.; Paradowska, K.; Yordanova, S.; Stoyanov, S.; Vassilev, N.; Stoyanov, N. Synthesis and anticancer activity of Pt(II) complexes of spiro-5-substituted 2,4-dithiohydantoins. Inorganica Chim. Acta 2021, 528, 120605. [Google Scholar] [CrossRef]
- Ahmedova, A.; Marinova, P.; Paradowska, K.; Marinov, M.; Mitewa, M. Synthesis and characterization of Copper(II) and Ni(II) complexes of (9′-fluorene)-spiro-5-dithiohidantoin. J. Mol. Struct. 2008, 892, 13–19. [Google Scholar] [CrossRef]
- Ahmedova, A.; Paradowska, K.; Wawer, I. 1H, 13C MAS NMR and DFT GIAO study of quercetin and its complex with Al(III) in solid state. J. Inorg. Biochem. 2012, 110, 27–35. [Google Scholar] [CrossRef]
- Marinova, P.; Hristov, M.; Tsoneva, S.; Burdzhiev, N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization, and Antibacterial Studies of New Cu(II) and Pd(II) Complexes with 6-Methyl-2-Thiouracil and 6-Propyl-2-Thiouracil. Appl. Sci. 2023, 13, 13150. [Google Scholar] [CrossRef]
- Marinova, P.; Stoitsov, D.; Burdzhiev, N.; Tsoneva, S.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Investigation of the Complexation Activity of 2,4-Dithiouracil with Au(III) and Cu(II) and Biological Activity of the Newly Formed Complexes. Appl. Sci. 2024, 14, 6601. [Google Scholar] [CrossRef]
- Marinova, P.; Burdzhiev, N.; Blazheva, D.; Slavchev, A. Synthesis and Antibacterial Studies of a New Au(III) Complex with 6-Methyl-2-Thioxo-2,3-Dihydropyrimidin-4(1H)-One. Molbank 2024, 2024, M1827. [Google Scholar] [CrossRef]
- Ahmedova, A.; Marinova, P.; Paradowska, K.; Tyuliev, G.; Marinov, M.; Stoyanov, N. Spectroscopic study on the solid state structure of Pt(II) complexes of cycloalkanespiro-5-(2,4-dithiohydantoins). Bulg. Chem. Commun. 2024, 56, 89–95. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Soliman, S.M.; Al-Wahaib, D.; Barakat, A.; Ali, A.E.; Elbadawy, H.A. Synthesis of a New Dinuclear Ag(I) Complex with Asymmetric Azine Type Ligand: X-ray Structure and Biological Studies. Inorganics 2022, 10, 209. [Google Scholar] [CrossRef]
- Zimina, A.M.; Somov, N.V.; Malysheva, Y.B.; Knyazeva, N.A.; Piskunov, A.V.; Grishin, I.D. 12-Vertex clo-so-3,1,2-Ruthenadicarba-dodecaboranes with Chelate POP-Ligands: Synthesis, X-ray Study and Electrochemical Properties. Inorganics 2022, 10, 206. [Google Scholar] [CrossRef]
- Al-Shboul, T.M.A.; El-khateeb, M.; Obeidat, Z.H.; Ababneh, T.S.; Al-Tarawneh, S.S.; Al Zoubi, M.S.; Alshaer, W.; Abu Seni, A.; Qasem, T.; Moriyama, H.; et al. Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes. Inorganics 2022, 10, 112. [Google Scholar] [CrossRef]
- Ahmedova, A.; Atanasov, V.; Marinova, P.; Stoyanov, N.; Mitewa, M. Synthesis, characterization and spectroscopic properties of some 2-substituted 1,3-indandiones and their metal complexes. Cent. Eur. J. Chem. 2009, 7, 429–438. [Google Scholar] [CrossRef]
- Ahmedova, A.; Marinova, P.; Ciattini, S.; Stoyanov, N.; Springborg, M.; Mitewa, M. A combined experimental and theoretical approach for structural study on a new cinnamoyl derivative of 2-acetyl-1,3-indandione and its metal(II) complexes. Struct. Chem. 2009, 20, 101–111. [Google Scholar] [CrossRef]
- Ahmedova, A.; Marinova, P.; Pavlović, G.; Guncheva, M.; Stoyanov, N.; Mitewa, M. Structure and properties of a series of 2-cinnamoyl-1,3-indandiones and their metal complexes. J. Iran. Chem. Soc. 2012, 9, 297–306. [Google Scholar] [CrossRef]
- Nikolova, I.; Marinov, M.; Marinova, P.; Dimitrov, A.; Stoyanov, N. Cu(II) complexes of 4- and 5- nitro-substituted heteroaryl cinnamoyl derivatives and determining their anticoagulant activity. Ukr. Food J. 2016, 5, 326–349. [Google Scholar] [CrossRef]
- Marinova, P.E.; Nikolova, I.D.; Marinov, M.N.; Tsoneva, S.H.; Dimitrov, A.N.; Stoyanov, N.M. Ni(II) complexes of 4- and 5-nitro-substituted heteroaryl cinnamoyl derivatives. Bulg. Chem. Commun. 2017, 49, 183–187. [Google Scholar]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Blazheva, D.; Slavchev, A.; Sbirkova-Dimitrova, H.; Sarafian, V.; Stoyanov, N. Crystal Structure of 5′-oxospiro-(fluorene-9, 4′-imidazolidine)-2′-thione and biological activities of its derivatives. Russ. J. Gen. Chem. 2021, 91, 939–946. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Blazheva, D.; Slavchev, A.; Georgiev, D.; Nikolova, I.; Sbirkova-Dimitrova, H.; Sarafian, V.; et al. Copper(II) complex of bis(1′,3′-hydroxymethyl)-spiro-(fluorine-9,4′-Imidazolidine)-2′,5′-dione, cytotoxicity and antibacterial activity of its derivative and crystal structure of free ligand. Russ. J. Inorg. Chem. 2021, 66, 1925–1935. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Georgiev, D.; Becheva, M.; Penchev, P.; Stoyanov, N. Synthesis and antimicrobial study of new Pt(IV) and Ru(III) complexes of fluorenylspirohydantoins. Rev. Roum. Chim. 2019, 64, 595–601. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Penchev, P.; Sarafian, V.; Stoyanov, N. Synthesis and in vitro activity of platinum (II) complexes of two fluorenylspirohydantoins against a human tumor cell line. Biotechnol. Biotechnol. Equip. 2014, 28, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Marinova, P.; Marinov, M.; Stoyanov, N. New metal complexes of cyclopentanespiro-5-hydantoine. Trakia J. Sci. 30 Years High. Med. Educ. Stara Zagor. 2012, 10 (Suppl. S1), 84–87. [Google Scholar]
- Marinova, P.E.; Marinov, M.N.; Kazakova, M.H.; Feodorova, Y.N.; Sarafian, V.S.; Stoyanov, N.M. Synthesis and bioactivity of new platinum and ruthenium complexes of 4-bromo-spiro-(fluorene-9,4′-imidazolidine)-2′,5′-dithione. Bulg. Chem. Commun. 2015, 47, 75–79. [Google Scholar]
- Ahmedova, A.; Marinova, P.; Marinov, M.; Stoyanov, N. An integrated experimental and quantum chemical study on the complexation properties of (9′-fluorene)-spiro-5-hydantoin and its thio-analogues. J. Mol. Struct. 2016, 1108, 602–610. [Google Scholar] [CrossRef]
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev, A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies. Russ. J. Gen. Chem. 2022, 92, 1578–1584. [Google Scholar] [CrossRef]
- Marinova, P.; Nikolova, S.; Tsoneva, S. Synthesis of N-(1-(2-acetyl-4,5-dimethoxyphenyl)propan-2-yl)benzamide and its copper(II) complex. Russ. J. Gen. Chem. 2023, 93, 161–165. [Google Scholar] [CrossRef]
- Uzal-Varela, R.; Lucio-Martínez, F.; Nucera, A.; Botta, M.; Esteban-Gómez, D.; Valencia, L.; Rodríguez-Rodríguez, A.; Platas-Iglesias, C. A systematic investigation of the NMR relaxation properties of Fe(III)-EDTA derivatives and their potential as MRI contrast agents. Inorg. Chem. Front. 2023, 10, 1633–1649. [Google Scholar] [CrossRef]
- Üngör, Ö.; Sanchez, S.; Ozvat, T.M.; Zadrozny, J.M. Asymmetry-enhanced 59Co NMR thermometry in Co(III) complexes. Inorg. Chem. Front. 2023, 10, 7064–7072. [Google Scholar] [CrossRef]
- Trindade, I.B.; Coelho, a.; Cantini, F.; Piccioli, M.; Louro, I.O. NMR of paramagnetic metalloproteins in solution: Ubi venire, quo vadis? J. Inorg. Biochem. 2022, 234, 111871. [Google Scholar] [CrossRef]
- Raducka, A.; Świątkowski, M.; Korona-Głowniak, I.; Kaproń, B.; Plech, T.; Szczesio, M.; Gobis, K.; Szynkowska-Jóźwik, M.I.; Czylkowska, A. Zinc Coordination Compounds with Benzimidazole Derivatives: Synthesis, Structure, Antimicrobial Activity and Potential Anticancer Application. Int. J. Mol. Sci. 2022, 23, 6595. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Muenzner, J.K.; el Maaty, M.A.A.; Karge, B.; Schobert, R.; Wölfl, S.; Ott, I. A Multi-target caffeine derived rhodium(I) N-heterocyclic carbene complex: Evaluation of the mechanism of action. Dalton Trans. 2016, 45, 13161. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.K.; Nandy, A.; Bertolasi, V.; Bielawski, C.W.; Das Saha, K.; Dinda, J. Novel gold(I)−and gold(III)−N-heterocyclic carbene complexes: Synthesis and evaluation of their anticancer properties. Organometallics 2014, 33, 2544–2548. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Marinelli, M.; Marzano, C.; Yousufuddin, M.; Dias, H.V.R.; Santini, C. Synthesis and biological activity of ester- and amide-functionalized imidazolium salts and related water-soluble coinage metal N-heterocyclic carbene complexes. Inorg. Chem. 2012, 51, 9873–9882. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2010, 109, 4921–4960. [Google Scholar] [CrossRef]
- Yan, Y.K.; Melchart, M.; Habtemariam, A.; Sadler, P.J. Organometallic chemistry, biology and medicine: Ruthenium arene anticancer complexes. Chem. Commun. 2005, 14, 4764–4776. [Google Scholar] [CrossRef]
- Salga, M.S.; Ali, H.M.; Abdulla, M.A.; Abdelwahab, S.I. Acute oral toxicity evaluations of some zinc(II) complexes derived from 1-(2-Salicylaldiminoethyl)piperazine schiff bases in rats. Int. J. Mol. Sci. 2012, 13, 1393–1404. [Google Scholar] [CrossRef]
- Sekine, Y.; Nihei, M.; Kumai, R.; Nakao, H.; Murakami, Y.; Oshio, H. Investigation of the light-induced electron-transfer-coupled spin transition in a cyanide-bridged [Co2Fe2] complex by X-ray diffraction and absorption measurements. Inorg. Chem. Front. 2014, 1, 540–543. [Google Scholar] [CrossRef]
- Matteppanavar, S.; Rayaprol, S.; Singh, K.; Raghavendra Reddy, V.; Angadi, B. Evidence for magneto-electric and spin–lattice coupling in PbFe0.5Nb0.5O3 through structural and magneto-electric studies. J. Mater. Sci. 2015, 50, 4980–4993. [Google Scholar] [CrossRef]
- Mustafin, E.S.; Mataev, M.M.; Kasenov, R.Z.; Pudov, A.M.; Kaykenov, D.A.; Bogzhanova, Z.K. X-ray diffraction study of the YbMII3Fe5O12 (MII = Mg, Ca, Sr) ferrites. Inorg. Mater. 2014, 50, 622–624. [Google Scholar] [CrossRef]
- Cheong, S.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Valencia, S.; Konstantinovic, Z.; Schmitz, D.; Gaupp, A. X-ray magnetic circular dichroism study of cobalt-doped ZnO. Phys. Rev. B 2011, 84, 024413. [Google Scholar] [CrossRef]
- Harder, R.; Robinson, I.K. Three-dimensional mapping of strain in nanomaterials using X-ray diffraction microscopy. New J. Phys. 2010, 12, 035019. [Google Scholar] [CrossRef]
- Newton, M.C.; Leake, S.J.; Harder, R.; Robinson, I.K. Three-dimensional imaging of strain in ZnO nanorods using coherent X-ray diffraction. Nat. Mater. 2010, 9, 120–125. [Google Scholar] [CrossRef]
- Cha, W.; Ulvestad, A.; Kim, J.W. Coherent diffraction imaging of crystal strains and phase transitions in complex oxide nanocrystals. Nat. Commun. 2018, 9, 3422. [Google Scholar] [CrossRef]
- Diao, J.; Ulvestad, A. In situ study of ferroelastic domain wall dynamics in BaTiO₃ nanoparticles by X-ray diffraction microscopy. Phys. Rev. Mater. 2020, 4, 053601. [Google Scholar] [CrossRef]
- Kim, J.; Robinson, I.K. Real-time observation of defect dynamics during oxidation in Pt nanoparticles using coherent X-ray diffraction imaging. Nano Lett. 2015, 15, 5044–5051. [Google Scholar] [CrossRef]
- Pfeifer, M.A.; Williams, G.J.; Vartanyants, I.A.; Harder, R.; Robinson, I.K. Three-dimensional mapping of a deformation field inside a nanocrystal using coherent X-ray diffraction. Nature 2006, 442, 63–66. [Google Scholar] [CrossRef]
- Song, C.; Nam, D. Quantitative imaging of single, unstained viruses with coherent X-rays. Phys. Rev. Lett. 2014, 101, 158101. [Google Scholar] [CrossRef]
- Shrestha, P. UV-Vis Spectroscopy: Principle, Parts, Uses, Limitations. 2023. Available online: https://microbenotes.com/uv-vis-spectroscopy/ (accessed on 7 April 2025).
- Khalid, K.; Ishak, R.; Chowdhury, Z.Z. Chapter 15—UV–Vis spectroscopy in non-destructive testing. In Non-Destructive Material Characterization Methods; Elsevier: Amsterdam, The Netherlands, 2024; pp. 391–416. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; 3rd printing; W. H. Freeman: New York, NY, USA, 2007. [Google Scholar]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef]
- Namioka, T. Diffraction Gratings. In Vacuum Ultraviolet Spectroscopy; Experimental Methods in Physical Sciences; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1, pp. 347–377. [Google Scholar] [CrossRef]
- Abramowitz, M.; Michael, W. Davidson. Photomultiplier Tubes. Molecular Expressions. Available online: https://micro.magnet.fsu.edu/primer/digitalimaging/concepts/photomultipliers.html (accessed on 25 April 2021).
- Picollo, M.; Aceto, M.; Vitorino, T. UV-Vis spectroscopy. Phys. Sci. Rev. 2019, 4, 20180008. [Google Scholar] [CrossRef]
- What Is a Photodiode? Working, Characteristics, Applications. Published Online 30 October 2018. Available online: https://www.electronicshub.org/photodiode-working-characteristics-applications/ (accessed on 29 April 2021).
- Amelio, G. Charge-Coupled Devices. Sci. Am. 1974, 230, 22–31. Available online: http://www.jstor.org/stable/24950003 (accessed on 7 April 2025).
- Hackteria. DIY NanoDrop. Available online: https://hackteria.org/wiki/File:NanoDropConceptSpectrometer2.png (accessed on 15 June 2021).
- Sharpe, M.R. Stray light in UV-VIS spectrophotometers. Anal. Chem. 1984, 56, 339A–356A. [Google Scholar] [CrossRef]
- Liu, P.-F.; Avramova, L.V.; Park, C. Revisiting absorbance at 230 nm as a protein unfolding probe. Anal. Biochem. 2009, 389, 165–170. [Google Scholar] [CrossRef]
- Kalb, V.; Bernlohr, R. A New Spectrophotometric Assay for Protein in Cell Extracts. Anal. Biochem. 1977, 82, 362–371. [Google Scholar] [CrossRef]
- Bosch Ojeda, C.; Sanchez Rojas, F. Recent applications in derivative ultraviolet/visible absorption spectrophotometry: 2009–2011. Microchem. J. 2013, 106, 1–16. [Google Scholar] [CrossRef]
- Domingo, C.; Saurina, J. An overview of the analytical characterization of nanostructured drug delivery systems: Towards green and sustainable pharmaceuticals: A review. Anal. Chim. Acta 2012, 744, 8–22. [Google Scholar] [CrossRef]
- Gaikwad, J.; Sharma, S.; Hatware, K.V. Review on Characteristics and Analytical Methods of Tazarotene: An Update. Crit. Rev. Anal. Chem. 2020, 50, 90–96. [Google Scholar] [CrossRef]
- Gendrin, C.; Roggo, Y.; Collet, C. Pharmaceutical applications of vibrational chemical imaging and chemometrics: A review. J. Pharm. Biomed. Anal. 2008, 48, 533–553. [Google Scholar] [CrossRef]
- Lourenço, N.D.; Lopes, J.A.; Almeida, C.F.; Sarraguça, M.C.; Pinheiro, H.M. Bioreactor monitoring with spectroscopy and chemometrics: A review. Anal. Bioanal. Chem. 2012, 404, 1211–1237. [Google Scholar] [CrossRef]
- Sánchez Rojas, F.; Bosch Ojeda, C. Recent development in derivative ultraviolet/visible absorption spectrophotometry: 2004–2008. Anal. Chim. Acta 2009, 635, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016, 6, 38828. [Google Scholar] [CrossRef] [PubMed]
- Tadesse Wondimkun, Z. The Determination of Caffeine Level of Wolaita Zone, Ethiopia Coffee Using UV-Visible Spectrophotometer. Am. J. Appl. Chem. 2016, 4, 59. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Zhan, J.; Huang, W. Review of recent UV–Vis and infrared spectroscopy researches on wine detection and discrimination. Appl. Spectrosc. Rev. 2018, 53, 65–86. [Google Scholar] [CrossRef]
- Leong, Y.S.; Ker, P.J.K.; Jamaludin, M.Z.; Nomanbhay, S.M.; Ismail, A.; Abdullah, F.; Looe, H.M.; Lo, C.K. UV-Vis Spectroscopy: A New Approach for Assessing the Color Index of Transformer Insulating Oil. Sensors 2018, 18, 2175. [Google Scholar] [CrossRef]
- Brown, J.Q.; Vishwanath, K.; Palmer, G.M.; Ramanujam, N. Advances in quantitative UV–visible spectroscopy for clinical and pre-clinical application in cancer. Curr. Opin. Biotechnol. 2009, 20, 119–131. [Google Scholar] [CrossRef]
- Pinheiro, H.M.; Touraud, E.; Thomas, O. Aromatic amines from azo dye reduction: Status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dye. Pigment. 2004, 61, 121–139. [Google Scholar] [CrossRef]
- Kristo, E.; Hazizaj, A.; Corredig, M. Structural Changes Imposed on Whey Proteins by UV Irradiation in a Continuous UV Light Reactor. J. Agric. Food Chem. 2012, 60, 6204–6209. [Google Scholar] [CrossRef]
- Lange, R.; Balny, C. UV-visible derivative spectroscopy under high pressure. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 2002, 1595, 80–93. [Google Scholar] [CrossRef]
- Tom, J.; Jakubec, P.J.; Andreas, H.A. Mechanisms of Enhanced Hemoglobin Electroactivity on Carbon Electrodes upon Exposure to a Water-Miscible Primary Alcohol. Anal. Chem. 2018, 90, 5764–5772. [Google Scholar] [CrossRef]
- Patel, M.U.M.; Demir-Cakan, R.; Morcrette, M.; Tarascon, J.-M.; Gaberscek, M.; Dominko, R. Li-S Battery Analyzed by UV/Vis in Operando Mode. ChemSusChem 2013, 6, 1177–1181. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Naseem, K.; Ali, F.; Batool, M.; Xiao, J.; Irfan, A. Applications of UV/Vis Spectroscopy in Characterization and Catalytic Activity of Noble Metal Nanoparticles Fabricated in Responsive Polymer Microgels: A Review. Crit. Rev. Anal. Chem. 2018, 48, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Ghasemi, F.; Ghalkhani, M.; Ashkarran, A.A.; Akbari, S.M.; Pakpour, S.; Hormozi-Nezhad, M.R.; Jamshidi, Z.; Mirsadeghi, S.; Dinarvand, R.; et al. Determination of nanoparticles using UV-Vis spectra. Nanoscale 2015, 7, 5134–5139. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- El-Azazy, M.; Al-Saad, K.; El-Shafie, A.S. (Eds.) Infrared Spectroscopy: Perspectives and Applications; Books on Demand: London, UK, 2023; ISBN 1803562811/9781803562810. [Google Scholar]
- James, M. Thompson, Infrared Spectroscopy, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Barbara, H. Stuart, Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 9780470854273. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Al-Saad, K. Infrared Spectroscopy—Principles, Advances, and Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Robert, T. Conley, Infrared Spectroscopy; Allyn and Bacon: Boston, MA, USA, 1972. [Google Scholar]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Iglesias-Reguant, A.; Reis, H.; Medveď, M.; Luis, J.M.; Zaleśny, R. A New Computational Tool for Interpreting the Infrared Spectra of Molecular Complexes. Phys. Chem. Chem. Phys. 2023, 25, 11658–11664. [Google Scholar] [CrossRef]
- Golea, C.M.; Codină, G.G.; Oroian, M. Prediction of Wheat Flours Composition Using Fourier Transform Infrared Spectrometry (FT-IR). Food Control 2023, 143, 109318. [Google Scholar] [CrossRef]
- Yaman, H.; Aykas, D.P.; Rodriguez-Saona, L.E. Monitoring Turkish White Cheese Ripening by Portable FT-IR Spectroscopy. Front. Nutr. 2023, 10, 1107491. [Google Scholar] [CrossRef]
- An, Y.; Sedinkin, S.L.; Venditti, V. Solution NMR methods for structural and thermodynamic investigation of nanoparticle adsorption equilibria. Nanoscale Adv. 2022, 4, 2583–2607. [Google Scholar] [CrossRef]
- Mohan, M.; Andersen, A.B.A.; Mareš, J.; Jensen, N.D.; Nielsen, U.G.; Vaara, J. Unravelling the effect of paramagnetic Ni2+ on the 13C NMR shift tensor for carbonate in Mg2−xNix Al layered double hydroxides by quantum-chemical computations. Phys. Chem. Chem. Phys. 2023, 25, 24081–24096. [Google Scholar] [CrossRef]
- Ju, M.; Kim, J.; Shin, J. EPR spectroscopy: A versatile tool for exploring transition metal complexes in organometallic and bioinorganic chemistry. Bull. Korean Chem. Soc. 2024, 45, 835–862. [Google Scholar] [CrossRef]
- Devreux, M.; Henoumont, C.; Dioury, F.; Boutry, S.; Vacher, O.; Elst, L.V.; Port, M.; Muller, R.N.; Sandre, O.; Laurent, S. Mn2+ Complexes with Pyclen-Based Derivatives as Contrast Agents for Magnetic Resonance Imaging: Synthesis and Relaxometry Characterization. Inorg. Chem. 2021, 60, 3604–3619. [Google Scholar] [CrossRef]
- Shin, H.K.; Lee, S.J.; Kim, T.J.; Lee, K.G.; Kang, D.H.; Son, J.T. Analyzing 31P NMR shifts in molybdenum phosphide nanoclusters. J. Mol. Struct. 2023, 1289, 135896. [Google Scholar] [CrossRef]
- Parigi, G.; Ravera, E.; Luchinat, C. Paramagnetic effects in NMR for protein structures and ensembles: Studies of metalloproteins. Curr. Opin. Struc. Biol. 2022, 74, 102386. [Google Scholar] [CrossRef] [PubMed]
- Charette, B.J.; Griffin, P.J.; Zimmermana, C.M.; Olshansky, L. Conformationally dynamic copper coordination complexes. Dalton Trans. 2022, 51, 6212–6219. [Google Scholar] [CrossRef]
- Brett, G.L.; Armstrong, R.D.; Thomas, S.P.; McQueen, C.J. Exploring transition metal complex environments via 1H and 31P NMR spectroscopy. Chem. Eur. J. 2023. [Google Scholar] [CrossRef]
- Middleton, D.A.; Griffin, J.; Esmann, M.; Fedosova, N.U. Solid-state NMR chemical shift analysis for determining the conformation of ATP bound to Na,K-ATPase in its native membrane. RSC Adv. 2023, 13, 34836–34846. [Google Scholar] [CrossRef]
- Smith, M.E. Recent progress in solid-state NMR of spin-½ low-γ nuclei applied to inorganic materials. Phys. Chem. Chem. Phys. 2023, 25, 26–47. [Google Scholar] [CrossRef]
- Desyatkin, V.G.; Martin, W.B.; Aliev, A.E.; Chapman, N.E.; Fonseca, A.F.; Galvão, D.S.; Miller, E.R.; Stone, K.H.; Wang, Z.; Zakhidov, D.; et al. Advanced Solid-State NMR Studies of Transition Metal Complexes. J. Am. Chem. Soc. 2022, 144, 17999–18008. [Google Scholar] [CrossRef]
- Shin, J.; Lim, M.H.; Han, J. NMR spectroscopic investigations of transition metal complexes in organometallic and bioinorganic chemistry. Bull. Korean Chem. Soc. 2024, 45, 593–613. [Google Scholar] [CrossRef]
- Novotný, J.; Jeremias, L.; Nimax, P.; Komorovsky, S.; Heinmaa, I.; Marek, R. Crystal and Substituent Effects on Paramagnetic NMR Shifts in Transition-Metal Complexes. Inorg. Chem. 2021, 60, 9368–9377. [Google Scholar] [CrossRef]
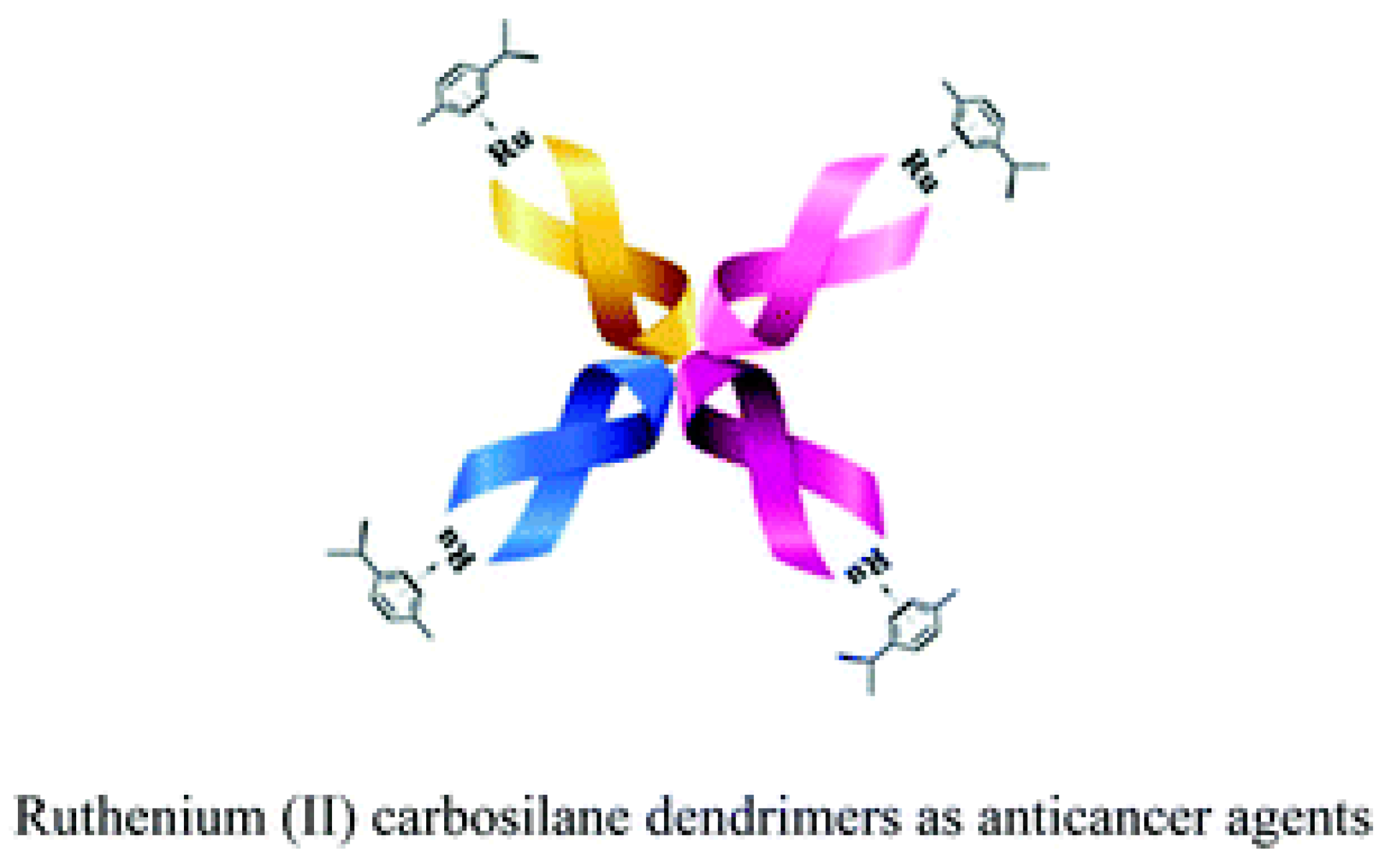
- Maroto-Díaz, M.; Elie, B.T.; Gómez-Sal, P.; Pérez-Serrano, J.; Gómez, R.; Contel, M.; de la Mata, F.J. Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium (II) complexes. Dalton Trans. 2016, 45, 7049–7066. [Google Scholar] [CrossRef]
- El-Tabl, A.S.; El-Waheed, M.M.A.; Wahba, M.A.; El-Halim, N.A.; El-Fadl, A. Synthesis, characterization, and anticancer activity of new metal complexes derived from 2-Hydroxy-3-(hydroxyimino)-4-oxopentan-2-ylidene benzohydrazide. Bioinorg. Chem. Appl. 2015, 1, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Parkinson, J.A.; Parsons, S.; Coxall, R.A.; Gould, R.O.; Sadler, P.J. Organometallic ruthenium (II) diamine anticancer complexes: Arene-nucleobase stacking and stereospecific hydrogen-bonding in guanine adducts. J. Am. Chem. Soc. 2002, 124, 3064. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Melchart, M.; Habtemariam, A.; Parsons, S.; Sadler, P.J. Use of Chelating Ligands to Tune the Reactive Site of Half-Sandwich Ruthenium (II)–Arene Anticancer Complexes. Chem. Eur. J. 2004, 10, 5173. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, A.; Bargan, A. Advanced and Biomedical Applications of Schiff-Base Ligands and Their Metal Complexes: A Review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Jorge, J.; Santos, K.F.D.P.; Timóteo, F.; Vasconcelos, R.R.P.; Cáceres, O.I.A.; Granja, I.J.A.; de Souza, D.M.; Frizon, T.E.A.; Botteselle, G.D.V.; Braga, A.L.; et al. Recent Advances on the Antimicrobial Activities of Schiff Bases and their Metal Complexes: An Updated Overview. Curr. Med. Chem. 2024, 31, 2330–2344. [Google Scholar] [CrossRef]
- Bertrand, B.; Stefan, L.; Pirrotta, M.; Monchaud, D.; Bodio, E.; Richard, P.; Le Gendre, P.; Warmerdam, E.; de Jager, M.H.; Groothuis, G.M.; et al. Caffeine-based gold(I) N—Heterocyclic carbenes as possible anticancer agents: Synthesis and biological properties. Inorg. Chem. 2014, 53, 2296–2303. [Google Scholar] [CrossRef]
- Nandaniya, B.K.; Das, S.P.; Chhuchhar, D.R.; Pithiya, M.G.; Khatariya, M.D.; Jani, D.; Dangar, K.B. Schiff base metal complexes: Advances in synthesis, characterization, and exploration of their biological potential. Int. J. Chem. Stud. 2024, 12, 131–134. [Google Scholar]
- Farrell, N. Transition metal complexes as drugs and chemotherapeutic agents. Met. Complexes Drugs Chemother. Agents 1989, 11, 809–840. [Google Scholar]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2012, 39, 8113–8127. [Google Scholar] [CrossRef]
- Uversky, V.N.; Kretsinger, R.H.; Permyakov, E.A. Encyclopedia of Metalloproteins; Springer: New York, NY, USA, 2013; Volume 1, pp. 1–89. [Google Scholar]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and its complexes in medicine: A biochemical approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.R.; Vivas-Mejia, P.E. Nanoparticles as drug delivery systems in cancer medicine: Emphasis on RNAi-containing nanoliposomes. Pharmaceuticals 2013, 6, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The nanomedicine revolution: Part 2: Current and future clinical applications. Pharm. Ther. 2012, 37, 582–591. [Google Scholar] [PubMed] [PubMed Central]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.A.; Ardakani, A.A.; Sedighi-Khavidak, S.; Munawarf, K.S.; Ashfaq, M.; Tahir, M.N. Synthesis, spectral characterization, crystal structures, biological activities, theoretical calculations and substitution effect of salicylidene ligand on the nature of mono and dinuclear Zn(II) Schiff base complexes. Polyhedron 2022, 213, 115636. [Google Scholar] [CrossRef]
- Nath, B.D.; Islam, M.; Karim, R.; Rahman, S.; Shaikh, A.A.; Georghiou, P.E.; Menelaou, M. Recent Progress in Metal-Incorporated Acyclic Schiff-Base Derivatives: Biological Aspects. Chem. Sel. 2022, 7, e20210429. [Google Scholar] [CrossRef]
- Abid, K.K.; Al-Bayati, R.H.; Faeq, A.A. Transition metal complexes of new N-amino quinolone derivative; synthesis, characterization, thermal study and antimicrobial properties. J. Am. Chem. Soc. 2016, 6, 29–35. [Google Scholar]
- Abu-Dief, M.; Nassr, L.A.E. Tailoring, physicochemical characterization, antibacterial and DNA binding mode studies of Cu(II) Schiff bases amino acid bioactive agents incorporating 5-bromo- 2-hydroxybenzaldehyde. J. Iran. Chem. Soc. 2015, 12, 943–955. Available online: https://link.springer.com/article/10.1007/s13738-014-0557-9 (accessed on 7 April 2025). [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Hashem, N.A.; Seleem, A.A. Recent advances in synthesis, characterization and biological activity of nano sized Schiff base amino acid M(II) complexes. Int. J. Nanomater. Chem. 2015, 1, 79–95. [Google Scholar] [CrossRef]
- Yousif, E.; Majeed, A.; Al-Sammarrae, K.; Salih, N.; Salimon, J.; Abdullah, B. Metal complexes of Schiff base: Preparation, characterization and antibacterial activity. Arab. J. Chem. 2017, 10, 1639–1644. [Google Scholar] [CrossRef]
- Horozic, E.; Suljagic, J.; Suljkanovic, M. Synthesis, characterization, antioxidant and antimicrobial activity of Copper (II) complex with Schiff base derived from 2,2-dihydroxyindane-1, 3-dione and Tryptophan. Am. J. Org. Chem. 2019, 9, 9–13. [Google Scholar] [CrossRef]
- Abu-Yamin, A.A.; Abduh, M.S.; Saghir, S.A.M.; Al-Gabri, N. Synthesis, Characterization and Biological Activities of New Schiff Base Compound and Its Lanthanide Complexes. Pharmaceuticals 2022, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Arjmand, F.; Zhang, Q.; Ahmad, M.; Khan, A.; Toupet, L. Molecular docking, DFT and antimicrobial studies of Cu(II) complex as topoisomerase I inhibitor. J. Biomol. Struct. Dyn. 2020, 39, 2092–2105. [Google Scholar] [CrossRef] [PubMed]
- Lobana, T.S.; Kaushal, M.; Bala, R.; Nim, L.; Paul, K.; Arora, D.S.; Bhatia, A.; Arora, S.; Jasinski, J.P. Di-2-pyridylketone-N(1)-substituted thiosemicarbazone derivatives of copper(II): Biosafe antimicrobial potential and high anticancer activity against immortalized L6 rat skeletal muscle cells. J. Inorg. Biochem. 2020, 212, 111205. [Google Scholar] [CrossRef]
- Halawa, A.H.; El-Gilil, S.M.A.; Bedair, A.H.; Shaaban, M.; Frese, M.; Sewald, N.; Eliwa, E.M.; El-Agrody, A.M. Synthesis, biological activity and molecular modeling study of new Schiff bases incorporated with indole moiety. Z. Naturforsch. 2017, 72, 467–475. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, V.K.; Kumar, G. Synthesis, Antimicrobial Evaluation of Substituted Indole and Nitrobenzenamine based Cr(III), Mn(III) and Fe(III) Metal Complexes. Drug Res. 2021, 71, 455–461. [Google Scholar] [CrossRef]
- Al Zamil, N.O. Synthesis, DFT calculation, DNA-binding, antimicrobial, cytotoxic and molecular docking studies on new complexes VO(II), Fe(III), Co(II), Ni(II) and Cu(II) of pyridine Schiff base ligand. Mater. Res. Express 2020, 7, 065401. [Google Scholar] [CrossRef]
- Nayak, S.G.; Poojary, B. Synthesis of novel Schiff bases containing arylpyrimidines as promising antibacterial agents. Heliyon 2019, 5, e02318. [Google Scholar] [CrossRef]
- Benabid, W.; Ouari, K.; Bendia, S.; Bourzami, R.; Ali, M.A. Crystal structure, spectroscopic studies, DFT calculations, cyclic voltammetry and biological activity of a copper (II) Schiff base complex. J. Mol. Struct. 2020, 1203, 127313. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Hashemi, M.; Hassan, M.; Kobarfard, F.; Mohebbi, S. Synthesis and antibacterial activity of Schiff bases of 5-substituted isatins. Chin. Chem. Lett. 2016, 27, 221–225. [Google Scholar] [CrossRef]
- El-Faham, A.; Hozzein, W.N.; Wadaan, M.A.M.; Khattab, S.N.; Ghabbour, H.A.; Fun, H.-K.; Siddiqui, M.R. Microwave Synthesis, Characterization, and Antimicrobial Activity of Some Novel Isatin Derivatives. J. Chem. 2015, 2015, 716987. [Google Scholar] [CrossRef]
- Dikio, C.W.; Okoli, B.J.; Mtunzi, F.M. Synthesis of new anti-bacterial agents: Hydrazide Schiff bases of vanadium acetylacetonate complexes. Cogent Chem. 2017, 3, 1336864. [Google Scholar] [CrossRef]
- Al-Hiyari, B.A.; Shakya, A.K.; Naik, R.R.; Bardaweel, S. Microwave-Assisted Synthesis of Schiff Bases of Isoniazid and Evaluation of Their Anti-Proliferative and Antibacterial Activities. Molbank 2021, 2021, M1189. [Google Scholar] [CrossRef]
- Fonkui, T.Y.; Ikhile, M.I.; Njobeh, P.B.; Ndinteh, D.T. Benzimidazole Schif base derivatives: Synthesis, characterization and antimicrobial activity. BMC Chem. 2019, 13, 127. [Google Scholar] [CrossRef]
- Alterhoni, E.; Tavman, A.; Hacioglu, M.; Sahin, O.; Tan, A.S.B. Synthesis, structural characterization and antimicrobial activity of Schiff bases and benzimidazole derivatives and their complexes with CoCl2, PdCl2, CuCl2 and ZnCl2. J. Mol. Struct. 2021, 1229, 129498. [Google Scholar] [CrossRef]
- Kais, R.; Adnan, S. Synthesis, Identification and Studying Biological Activity of Some Heterocyclic Derivatives from 3,5-Dinitrosalicylic Acid. IOP Conf. Ser. J. Phys. Conf. Ser. 2019, 1234, 01209. [Google Scholar] [CrossRef]
- Govindarao, K.; Srinivasan, N.; Suresh, R. Synthesis, Characterization and Antimicrobial Evaluation of Novel Schiff Bases of Aryl Amines Based 2-Azetidinones and 4-Thiazolidinones. Res. J. Pharm. Technol. 2020, 13, 168–172. [Google Scholar] [CrossRef]
- Mohanty, P.; Behera, S.; Behura, R.; Shubhadarshinee, L.; Mohapatra, P.; Barick, A.K.; Jali, B.R. Antibacterial Activity of Thiazole and its Derivatives: A Review. Biointerface Res. Appl. Chem. 2022, 12, 2171–2195. [Google Scholar] [CrossRef]
- Zhu, J.; Teng, G.; Li, D.; Hou, R.; Xia, Y. Synthesis and antibacterial activity of novel Schiff bases of thiosemicarbazone derivatives with adamantane moiety. Med. Chem. Res. 2021, 30, 1534–1540. [Google Scholar] [CrossRef]
- Yakan, H. Preparation, structure elucidation, and antioxidant activity of new bis(thiosemicarbazone) derivatives. Turk. J. Chem. 2020, 44, 1085–1099. [Google Scholar] [CrossRef]
- Vimala Joice, M.; Metilda, P. Synthesis, characterization and biological applications of curcumin-lysine based Schiff base and its metal complexes. J. Coord. Chem. 2021, 74, 2395–2406. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. A review on biological activities of Schiff base, hydrazone, and oxime derivatives of curcumin. RSC Adv. 2020, 10, 30186–30202. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A. Cell sensitivity assays: Clonogenic assay. Methods Mol. Med. 2004, 88, 159–164. Available online: https://link.springer.com/protocol/10.1385/1-59259-687-8:17 (accessed on 6 April 2025).
- Sundriyal, S.; Sharma, R.K.; Jain, R. Current advances in antifungal targets and drug development. Curr. Med. Chem. 2006, 13, 1321–1335. [Google Scholar] [CrossRef]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. Human Fungal Pathogen Identification; Springer: New York, NY, USA, 2017; Volume 1508. [Google Scholar]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole Antifungals: 35 Years of Invasive Fungal Infection Management. Expert Rev. Anti-Infect. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef]
- Ejidike, I. Cu(II) Complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino]ethyl}ethanimidoyl]benzene-1,3-diol Schiff base: Synthesis, spectroscopic, in-vitro antioxidant, antifungal and antibacterial studies. Molecules 2018, 23, 1581. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Tu, J.; Ji, C.; Han, G.; Sheng, C. Discovery of Simplified Sampangine Derivatives with Potent Antifungal Activities against Cryptococcal Meningitis. ACS Infect. Dis. 2019, 5, 1376–1384. [Google Scholar] [CrossRef]
- Shafiei, M.; Toreyhi, H.; Firoozpour, L.; Akbarzadeh, T.; Amini, M.; Hosseinzadeh, E.; Hashemzadeh, M.; Peyton, L.; Lotfali, E.; Foroumad, A. Design, Synthesis, and In Vitro and In Vivo Evaluation of Novel Fluconazole-Based Compounds with Promising Antifungal Activities. ACS Omega 2021, 6, 24981–25001. [Google Scholar] [CrossRef]
- Su, H.; Han, L.; Huang, X. Potential Targets for the Development of New Antifungal Drugs. J. Antibiot. 2018, 71, 978–991. [Google Scholar] [CrossRef]
- Montoya, M.C.; Didone, L.; Heier, R.F.; Meyers, M.J.; Krysan, D.J. Antifungal Phenothiazines: Optimization, Characterization of Mechanism, and Modulation of Neuroreceptor Activity. ACS Infect. Dis. 2018, 4, 499–507. [Google Scholar] [CrossRef]
- Malik, M.A.; Lone, S.A.; Gull, P.; Dar, O.A.; Wani, M.Y.; Ahmad, A.; Hashmi, A.A. Efficacy of Novel Schiff base Derivatives as Antifungal Compounds in Combination with Approved Drugs Against Candida Albicans. Med. Chem. 2019, 15, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring. Polymers 2019, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.P.; Wang, Z.F.; Huang, X.L.; Tan, M.X.; Shi, B.B.; Liang, H. High In Vitro and In Vivo Tumor-Selective Novel Ruthenium(II) Complexes with 3-(20-Benzimidazolyl)-7-fluoro-coumarin. ACS Med. Chem. Lett. 2019, 10, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Pahontu, E.; Julea, F.; Rosu, T.; Purcarea, V.; Chumakov, Y.; Petrenco, P.; Gulea, A. Antibacterial, antifungal and in vitro antileukaemia activity of metal complexes with thiosemicarbazones. J. Cell. Mol. Med. 2015, 19, 865–878. [Google Scholar] [CrossRef]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-based Drugs According to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
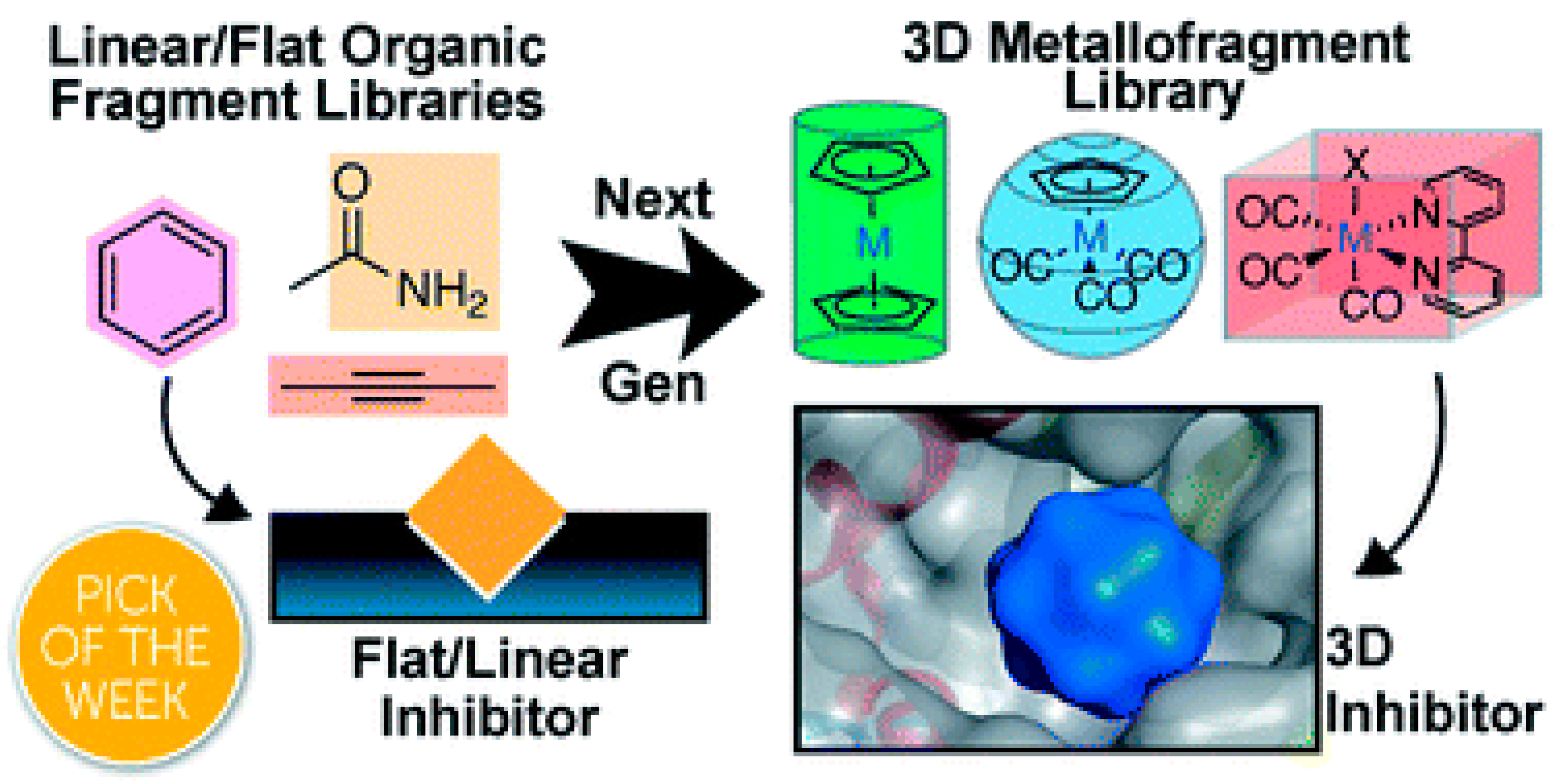
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding medicinal chemistry into 3D space: Metallofragments as 3D scaffolds for fragment-based drug discovery. Chem. Sci. 2020, 11, 1216–1225. [Google Scholar] [CrossRef]
- Frei, A.; King, A.P.; Lowe, G.J.; Cain, A.K.; Short, F.L.; Dinh, H.; Elliott, A.G.; Zuegg, J.; Wilson, J.J.; Blaskovich, M.A.T. Nontoxic Cobalt(III) Schiff Base Complexes with Broad-Spectrum Antifungal Activity. Chem. Eur. J. 2021, 27, 2021–2029. [Google Scholar] [CrossRef]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef]
- Devi, J.; Kumar, S.; Kumar, B.; Asija, S.; Kumar, A. Synthesis, structural analysis, in vitro antioxidant, antimicrobial activity and molecular docking studies of transition metal complexes derived from Schiff base ligands of 4-(benzyloxy)-2-hydroxybenzaldehyde. Res. Chem. Intermed. 2022, 48, 1541–1576. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni Suef. Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef]
- Borase, J.N.; Mahale, R.G.; Rajput, S.S.; Shirsath, D.S. Design, synthesis and biological evaluation of heterocyclic methyl substituted pyridine Schiff base transition metal complexes. SN Appl. Sci. 2021, 3, 197. [Google Scholar] [CrossRef]
- Inan, A.; Ikiz, M.; Tayhan, S.E.; Bilgin, S.; Genç, N.; Sayın, K.; Ceyhan, G.; Kose, M.; Dag, A.; Ispir, E. Antiproliferative, antioxidant, computational and electrochemical studies of new azo-containing Schiff base ruthenium (II) complexes. New J. Chem. 2018, 42, 2952–2963. [Google Scholar] [CrossRef]
- Uddin, M.N.; Ahmed, S.S.; Alam, S.M.R. REVIEW: Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Che, C.-M.; Siu, F.-M. Metal complexes in medicine with a focus on enzyme inhibition. Curr. Opin. Chem. Biol. 2010, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kizilkaya, H.; Dag, B.; Aral, T.; Genc, N.; Erenler, R. Synthesis, characterization, and antioxidant activity of heterocyclic Schiff bases. J. Chin. Chem. Soc. 2020, 67, 1696–1701. [Google Scholar] [CrossRef]
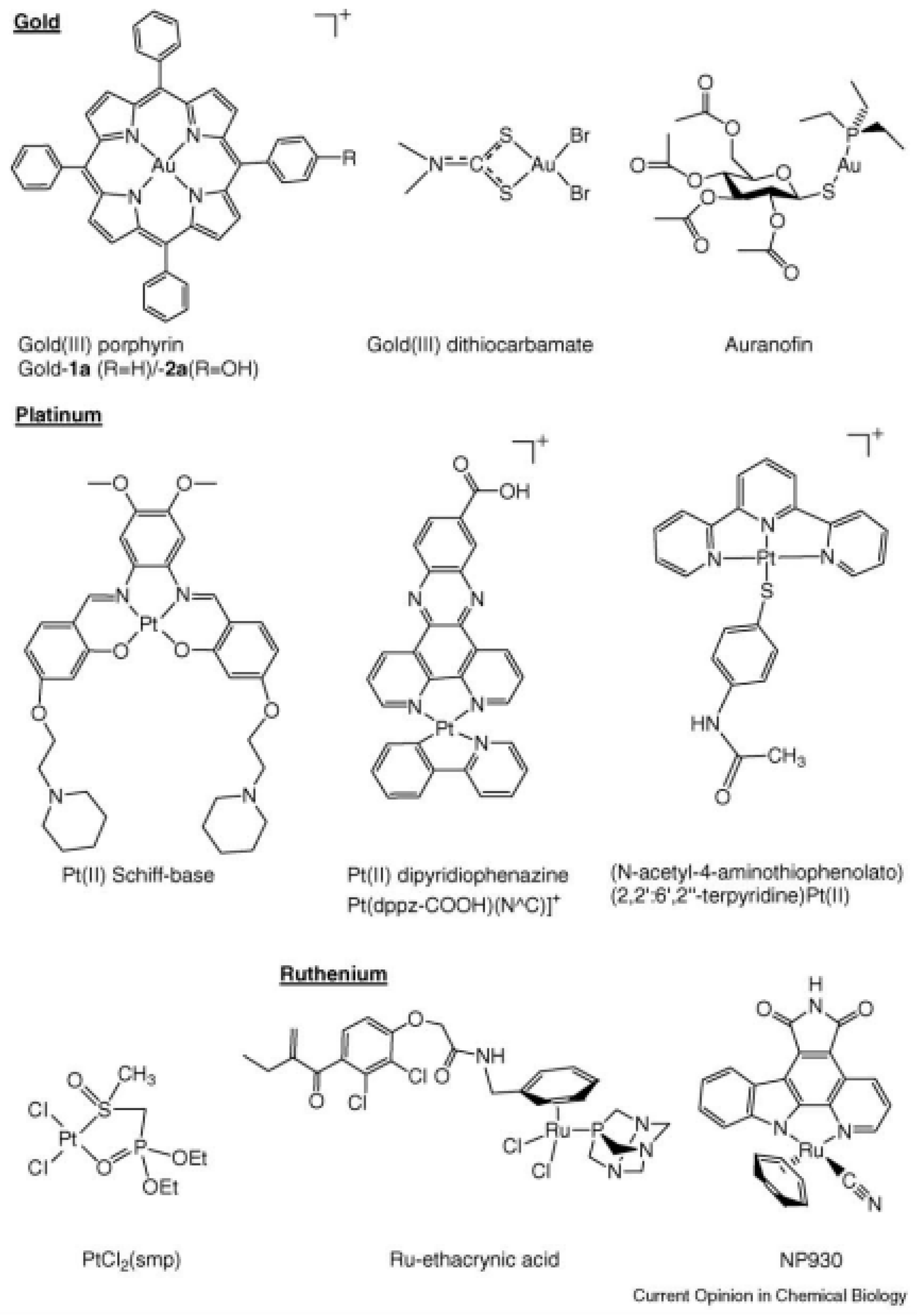
- Angelucci, F.; Sayed, A.A.; Williams, D.L.; Boumis, G.; Brunori, M.; Dimastrogiovanni, D.; Miele, A.E.; Pauly, F.; Bellelli, A. Inhibition of Schistosoma mansoni thioredoxin glutathione reductase by auranofin: Structural and kinetic aspects. J. Biol. Chem. 2009, 284, 28977–28985. [Google Scholar] [CrossRef]
- Mirabelli, C.K.; Johnson, R.K.; Sung, C.M.; Faucette, L.; Muirhead, K.; Crooke, S.T. Evaluation of the in vivo antitumor activity and in vitro cytotoxic properties of auranofin, a coordinated gold compound, in murine tumor models. Cancer Res. 1985, 45, 32–39. [Google Scholar]
- Ott, I.; Qian, X.; Xu, Y.; Vlecken, D.H.; Marques, I.J.; Kubutat, D.; Will, J.; Sheldrick, W.S.; Jesse, P.; Prokop, A.; et al. A gold(I) phosphine complex containing a naphthalimide ligand functions as a TrxR inhibiting antiproliferative agent and angiogenesis inhibitor. J. Med. Chem. 2009, 52, 763–770. [Google Scholar] [CrossRef]
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, K.R.; Fregona, D.; Dou, Q.P. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar] [CrossRef]
- Becker, K.; Herold-Mende, C.; Park, J.J.; Lowe, G.; Schirmer, R.H. Human thioredoxin reductase is efficiently inhibited by (2,2′:6′,2″-terpyridine)platinum(II) complexes. Possible implications for a novel antitumor strategy. J. Med. Chem. 2001, 44, 2784–2792. [Google Scholar] [CrossRef]
- Lo, Y.C.; Ko, T.P.; Su, W.C.; Su, T.L.; Wang, A.H.J. Terpyridine-platinum(II) complexes are effective inhibitors of mammalian topoisomerases and human thioredoxin reductase 1. J. Inorg. Biochem. 2009, 103, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
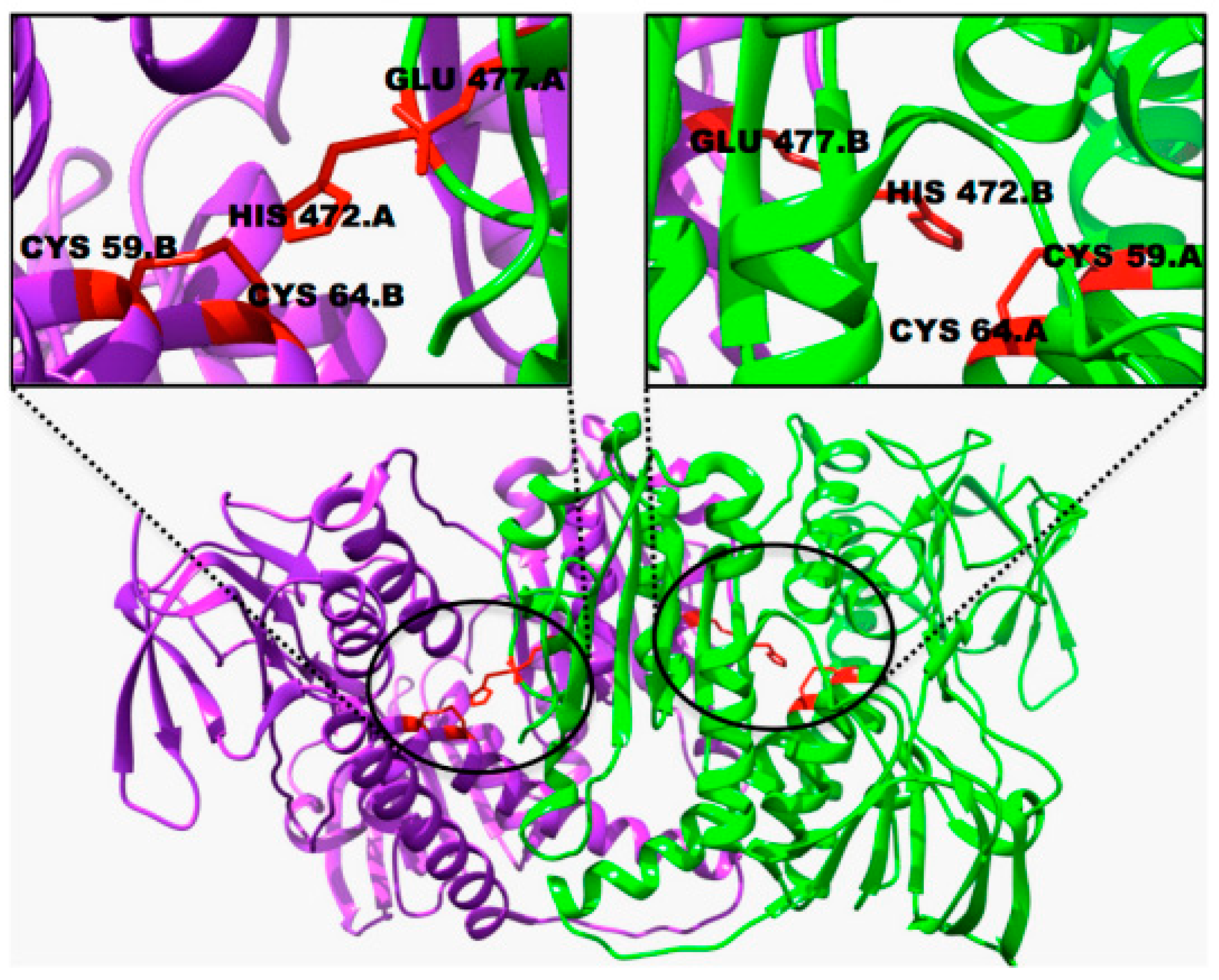
- Arnesano, F.; Boccarelli, A.; Cornacchia, D.; Nushi, F.; Sasanelli, R.; Coluccia, M.; Natile, G. Mechanistic insight into the inhibition of matrix metalloproteinases by platinum substrates. J. Med. Chem. 2009, 52, 7847–7855. [Google Scholar] [CrossRef] [PubMed]
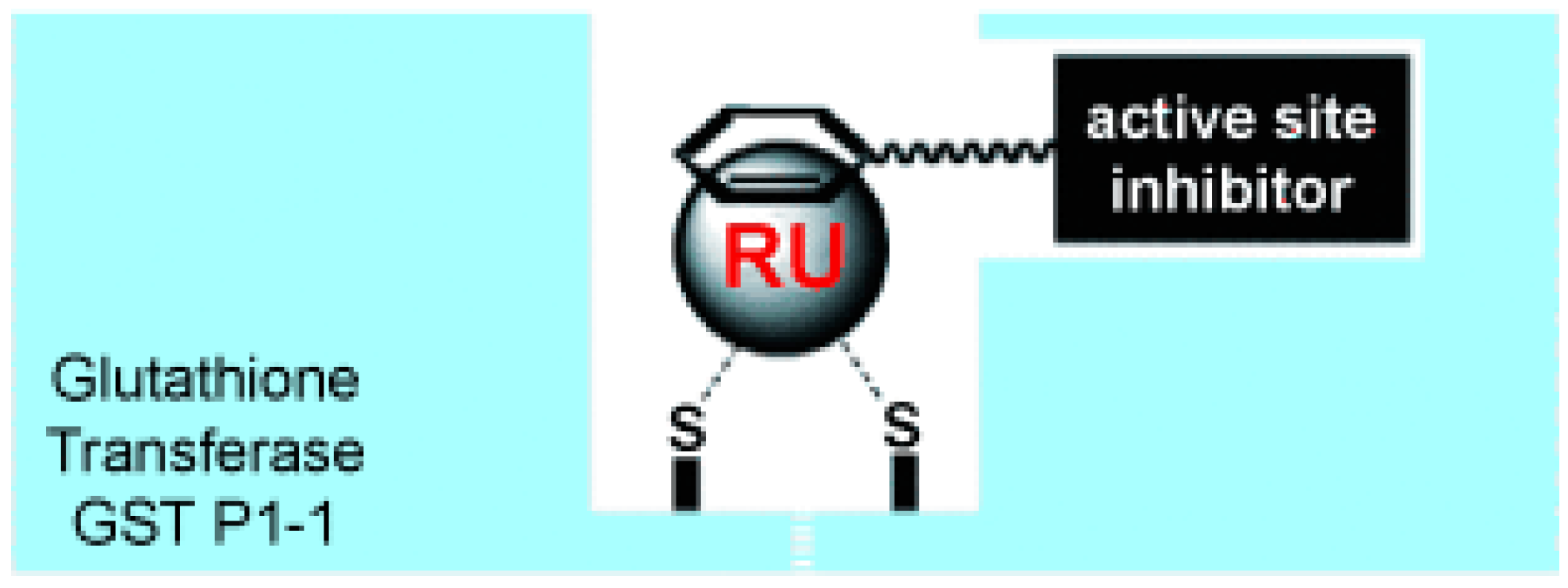
- Ang, W.H.; Parker, L.J.; De Luca, A.; Juillerat-Jeanneret, L.; Morton, C.J.; Lo Bello, M.; Parker, M.W.; Dyson, P.J. Rational design of an organometallic glutathione transferase inhibitor. Angew. Chem. Int. Ed. Engl. 2009, 48, 3854–3857. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef]
- Montana, A.M.; Batalla, C. The rational design of anticancer platinum complexes: The importance of the structure–activity relationship. Curr. Med. Chem. 2009, 16, 2235–2260. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Kettunen, M. Platinum group antitumor chemistry: Design and development of new anticancer drugs complementary to cisplatin. Curr. Med. Chem. 2006, 13, 1337–1357. [Google Scholar] [CrossRef]
- Caires, A.C.F. Recent Advances Involving Palladium (II) Complexes for the Cancer Therapy. Anticancer Agents Med. Chem. 2007, 7, 484–491. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, H. Metal Complexes with Aromatic N-Containing Ligands as Potential Agents in Cancer Treatment. Curr. Med. Chem. Anticancer Agents 2005, 5, 137–147. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Ranninger, C.N. Contribution to the SAR field of metallated and coordination complexes: Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord. Chem. Rev. 2004, 248, 119–133. [Google Scholar] [CrossRef]
- Reedijk, J. Metal-Ligand Exchange Kinetics in Platinum and Ruthenium Complexes. Platin. Met. Rev. 2008, 52, 2–11. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.G.; Keppler, B.K. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 2, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Dougan, S.J.; Sadler, P.J. The Design of Organometallic Ruthenium Arene Anticancer Agents. Chimia 2007, 61, 704–715. [Google Scholar] [CrossRef]
- Bratsos, I.; Jedner, S.; Gianferrara, T.; Alessio, E. Ruthenium Anticancer Compounds: Challenges and Expectations. Chimia 2007, 61, 692–697. [Google Scholar] [CrossRef]
- Wai-Yin Sun, R.; Ma, D.; Wong, E.; Che, C. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans. 2007, 43, 4884–4892. [Google Scholar] [CrossRef]
- Melchart, M.; Sadler, P.J. Bioorganomet; Jaouen, G., Ed.; Wiley-VCH: Weinheim, Germany, 2006; Volume 1, pp. 39–64. [Google Scholar]
- Ott, I.; Gust, R. Non Platinum Metal Complexes as Anti-cancer Drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Bergamo, A.; Sava, G. Ruthenium complexes can target determinants of tumour malignancy. Dalton Trans. 2007, 13, 1267–1272. [Google Scholar] [CrossRef]
- Brabec, V.; Novakova, O. DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Drug Resist. Updates 2006, 9, 111–122. [Google Scholar] [CrossRef]
- Kostova, I. Ruthenium Complexes as Anticancer Agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef]
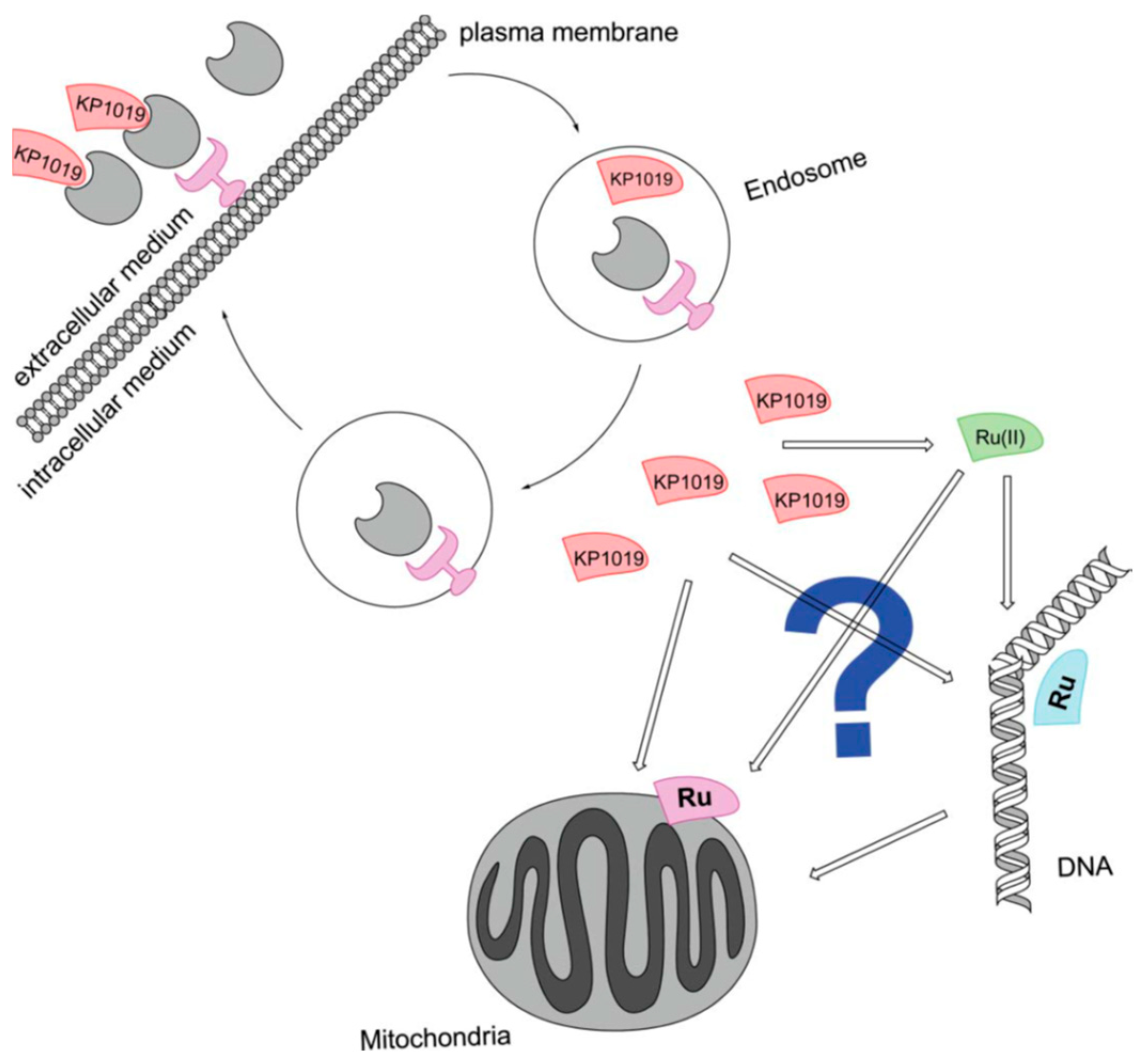
- Hartinger, C.G.; Zorbas-Seifried, S.; Jakupec, M.A.; Kynast, B.; Zorbas, H.; Keppler, B.K. From bench to bedside--preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). J. Inorg. Biochem. 2006, 100, 891–904. [Google Scholar] [CrossRef]
- Gonzalez-Vilchez, F.; Vilaplana, R. Metal Compounds in Cancer Chemotherapy. In Metal Compounds in Cancer Chemotherapy; Pérez, J.M., Fuertes, M.A., Alonso, C., Eds.; Research Signpost: Kerala, India, 2005; pp. 321–354. [Google Scholar]
- Wang, X.; Guo, Z. Towards the rational design of platinum(II) and gold(III) complexes as antitumour agents. Dalton Trans. 2008, 12, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marcon, G. Gold complexes as antitumor agents. Met. Ions Biol. Syst. 2004, 42, 385–424. [Google Scholar] [PubMed]
- Marcon, G.; Carotti, S.; Coronnello, M.; Messori, L.; Mini, E.; Orioli, P.; Mazzei, T.; Cinellu, M.A.; Minghetti, G. Gold(III) complexes with bipyridyl ligands: Solution chemistry, cytotoxicity, and DNA binding properties. J. Med. Chem. 2002, 45, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Gabbiani, C. Recent Trends in Antitumor Gold(III) Complexes: Innovative cytotoxic metallodrugs for cancer treatment. In Metal Compounds in Cancer Chemotherapy; Pérez, J.M., Fuertes, M.A., Alonso, C., Eds.; Research Signpost: Kerala, India, 2005; pp. 355–375. [Google Scholar]
- Messori, L.; Orioli, P.; Gabbiani, C. Antitumor gold(III) complexes. Farmatsiya 2005, 52, 64–67. Available online: https://flore.unifi.it/handle/2158/332076 (accessed on 6 April 2025).
- Scharwitz, M.A.; Ott, I.; Geldmacher, Y.; Gust, R.; Sheldrick, W.S. Cytotoxic half-sandwich rhodium(III) complexes: Polypyridyl ligand influence on their DNA binding properties and cellular uptake. J. Organomet. Chem. 2008, 693, 2299–2309. [Google Scholar] [CrossRef]
- Rahman, M.M.; Yasuda, H.; Katsura, S.; Mizuno, A. Inhibition of endonuclease cleavage and DNA replication of E. coli plasmid by the antitumor rhodium(II) complex. Arch. Biochem. Biophys. 2007, 464, 28–35. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Ko, Y.G. Schiff base complexes and their versatile applications as catalysts in oxidation of organic compounds: Part I. Appl. Organomet. Chem. 2016, 31, e3574. [Google Scholar] [CrossRef]
- Balas, M.; KBidi, L.; Launay, F.; Villanneau, R. Chromium-Salophen as a Soluble or Silica-Supported Co-Catalyst for the Fixation of CO2 Onto Styrene Oxide at Low Temperatures. Front. Chem. 2021, 9, 765108. [Google Scholar] [CrossRef]
- North, M.; Quek, S.C.Z.; Pridmore, N.E.; Whitwood, A.C.; Wu, X. Aluminum(salen) Complexes as catalysts for the Kinetic Resolution of Terminal Epoxides via CO2 Coupling. ACS Catal. 2015, 5, 3398–3402. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) Complex Catalyzed Cyclic Carbonate Synthesis at Ambient Temperature and Pressure. ACS Catal. 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- Uğur, T.; Tuna, M. Investigation of The Effects of Diaminopyridine and o-Vanillin Derivative Schiff Base Complexes of Mn(II), Mn(III), Co(II) and Zn(II) Metals on The Oxidative Bleaching Performance of H2O2. Sak. Univ. J. Sci. 2021, 25, 984–994. [Google Scholar] [CrossRef]
- Bulduruna, K.; Özdemir, M. Ruthenium(II) complexes with pyridine-based Schiff base ligands: Synthesis, structural characterization and catalytic hydrogenation of ketones. J. Mol. Struct. 2020, 1202, 127266. [Google Scholar] [CrossRef]
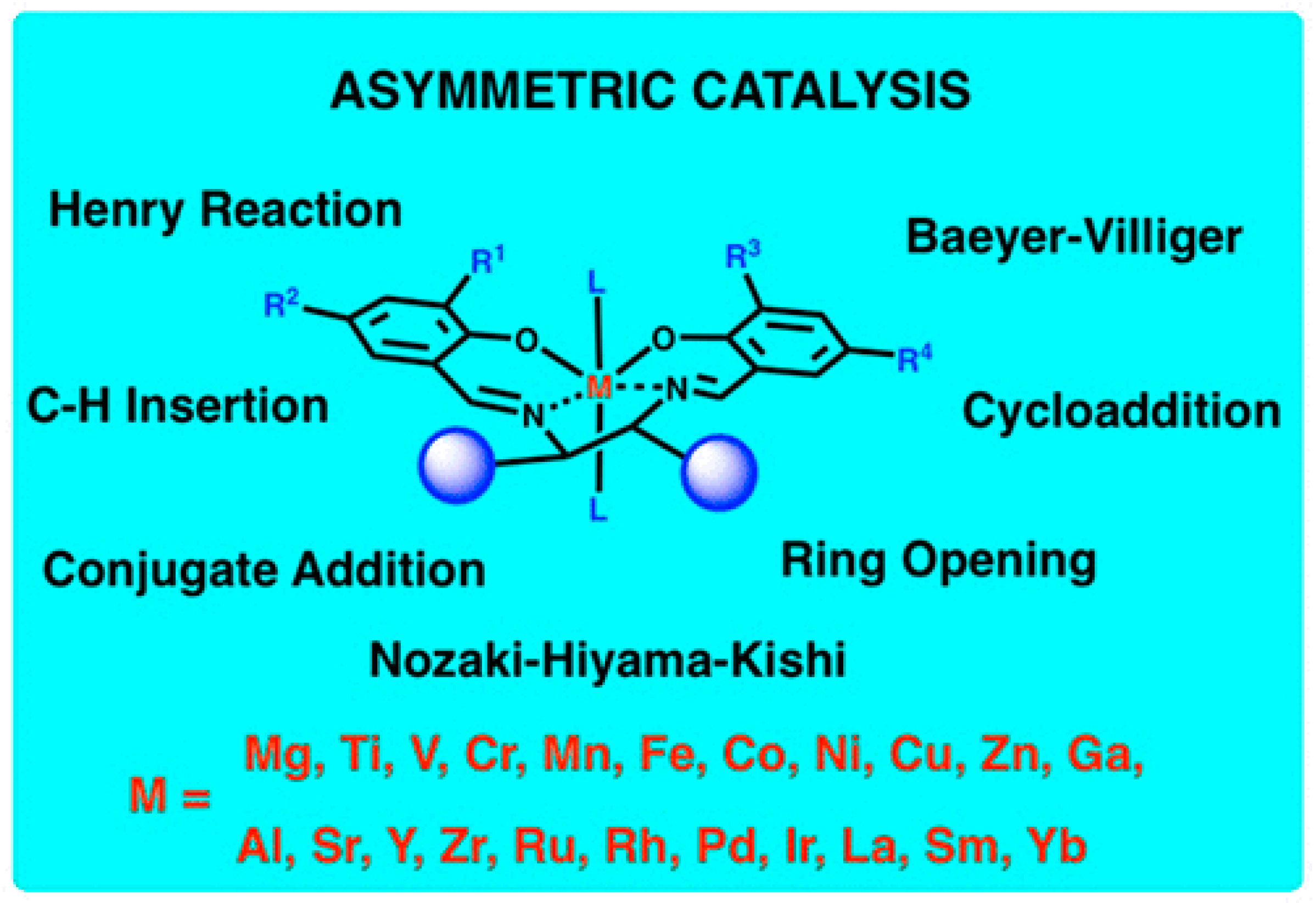
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, P.G. Metal-salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.F.; Iacob, M.; Silion, M.; Avadanei, M.; Bargan, A. Siloxane-based metal–organic frameworks with re markable catalytic activity in mild environmental photodegradation of azo dyes. Appl. Catal. B Environ. 2017, 205, 78–92. [Google Scholar] [CrossRef]
- Maharana, T.; Nath, N.; Pradhan, H.C.; Mantri, S.; Routaray, A.; Sutar, A.K. Polymer-supported first-row transition metal schiff base complexes: Efficient catalysts for epoxidation of alkenes. React. Funct. Polym. 2022, 171, 105142. [Google Scholar] [CrossRef]
- Salman, A.T.; Ismail, A.H.; Rheima, A.M.; Abd, A.N.; Habubi, N.F.; Abbas, Z.S. Nano-Synthesis, characterization and spectroscopic Studies of chromium (III) complex derived from new quinoline-2-one for solar cell fabrication. J. Phys. Conf. Ser. 2021, 1853, 012021. [Google Scholar] [CrossRef]

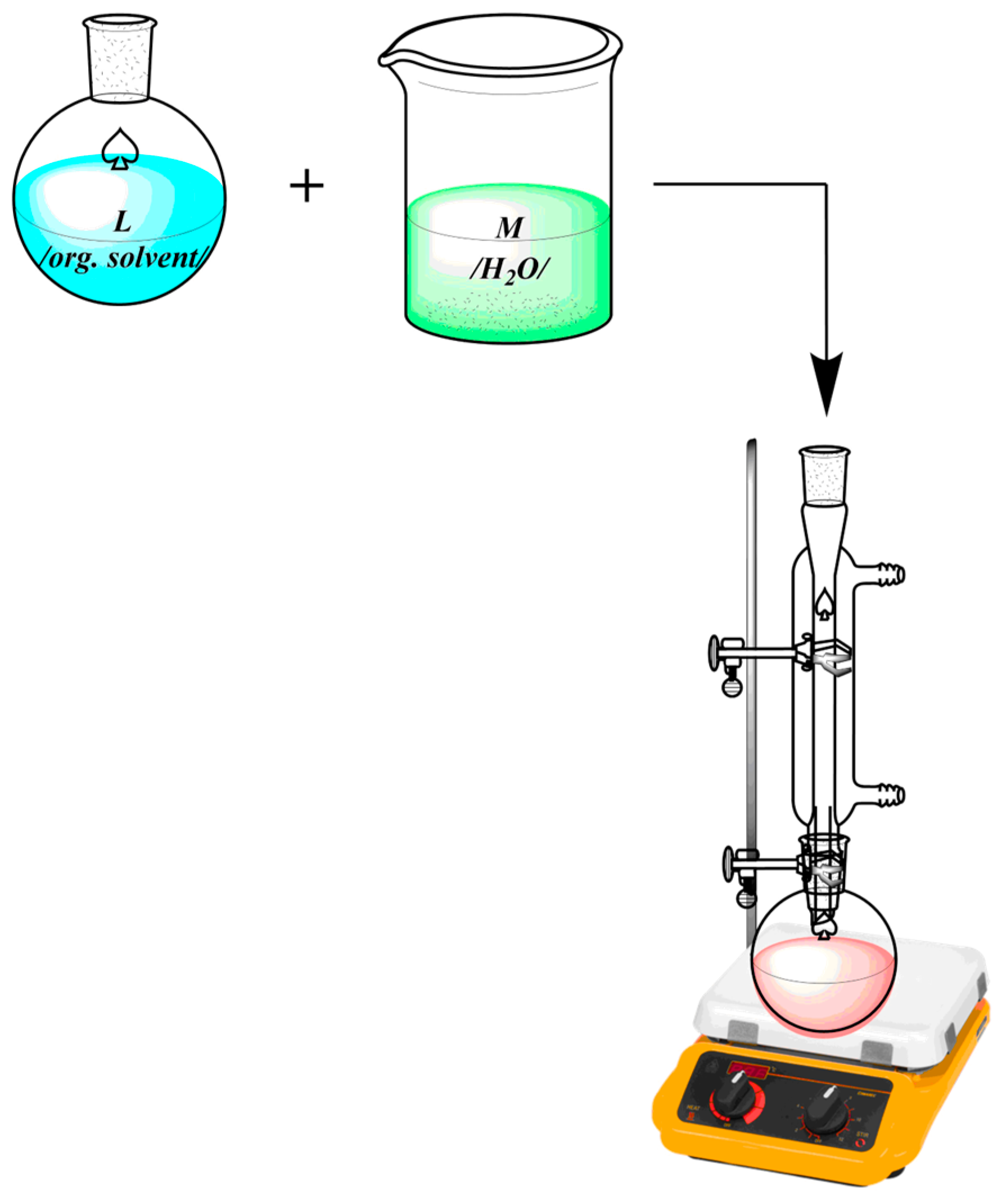
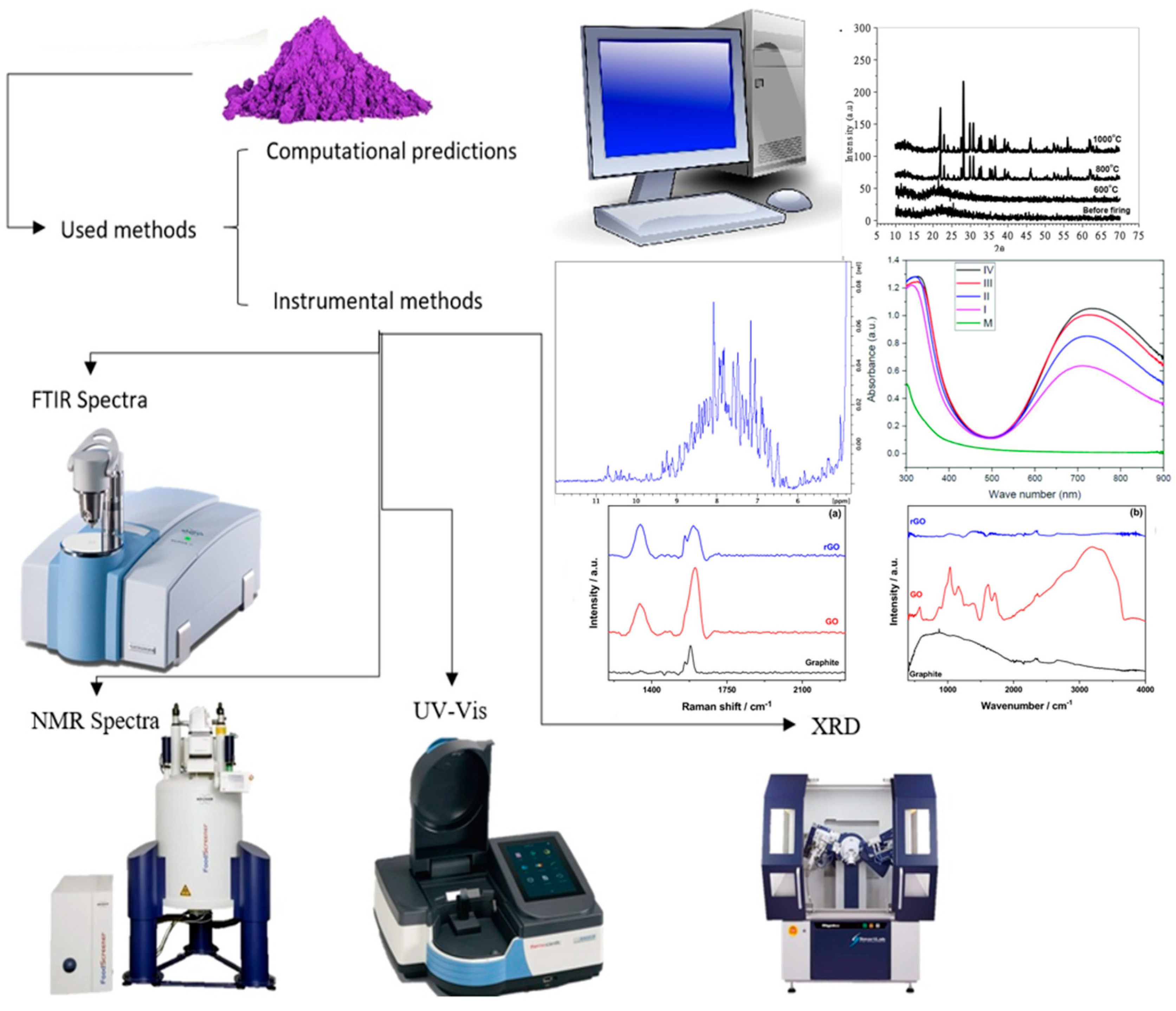
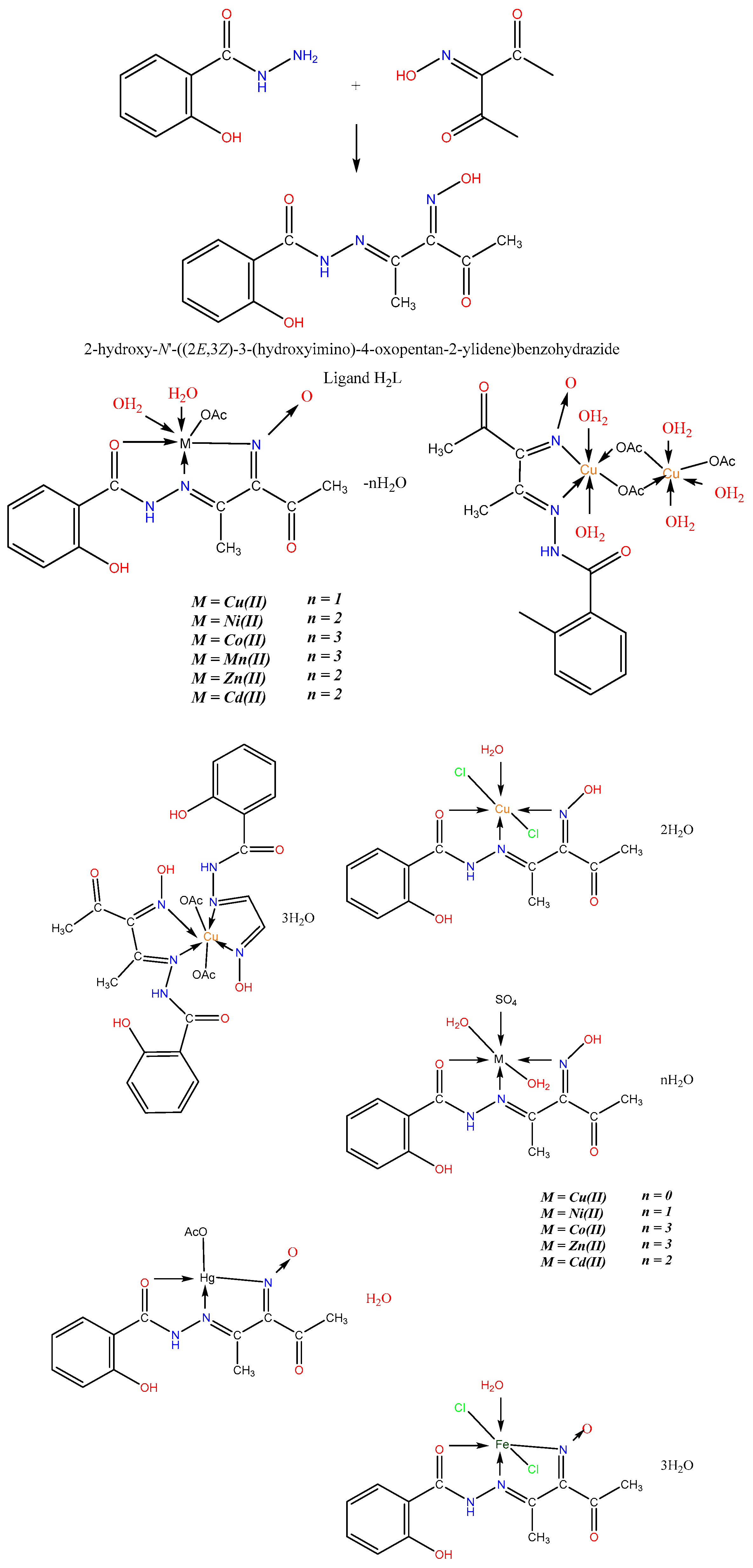
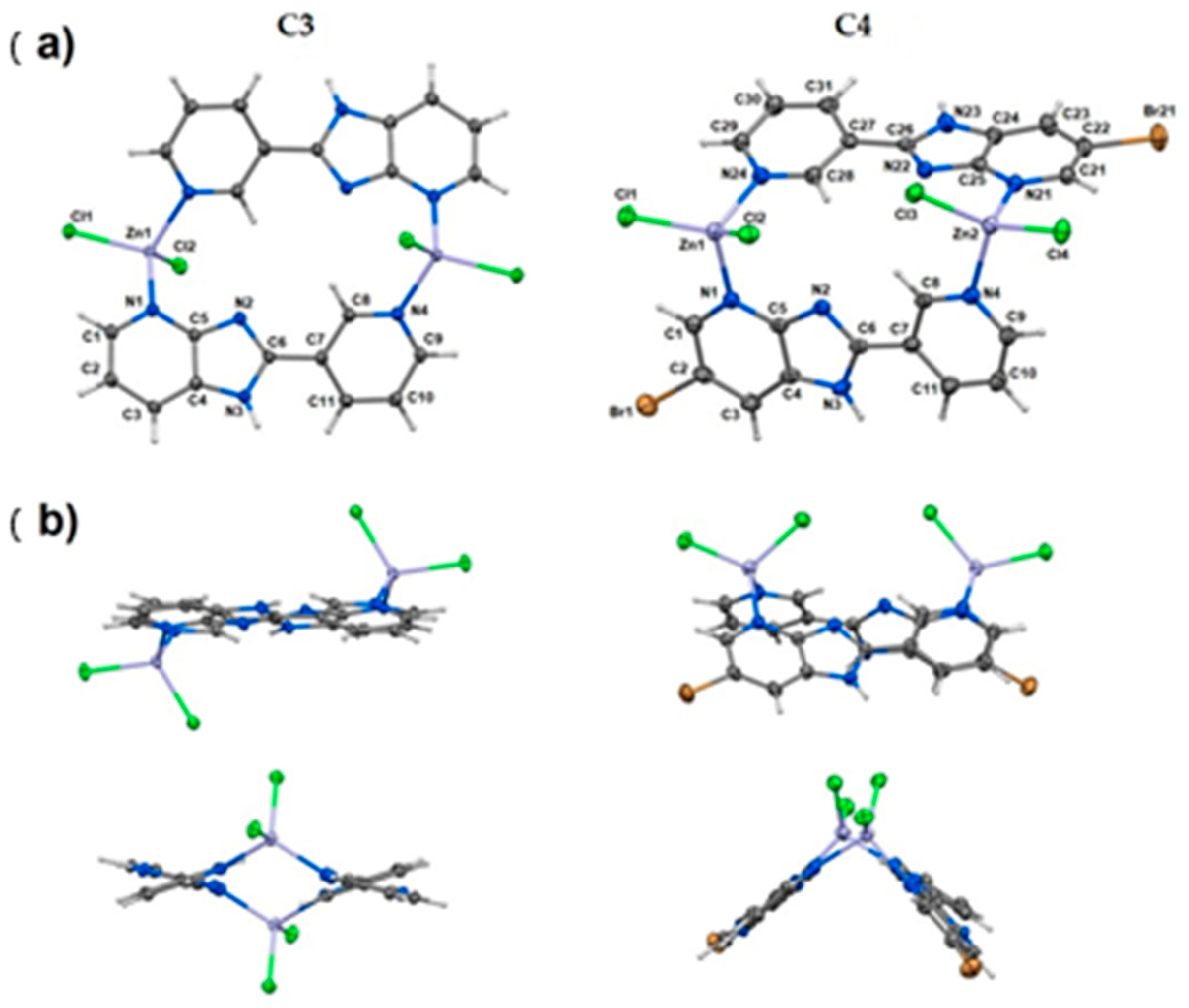
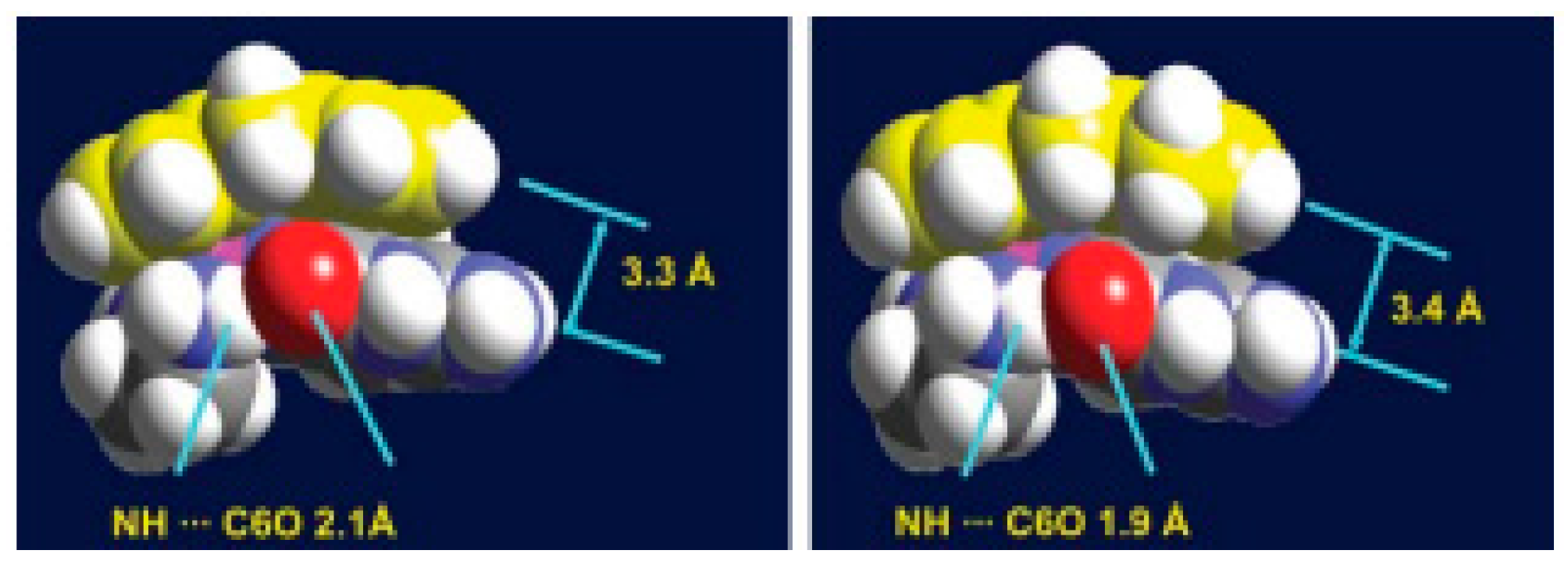
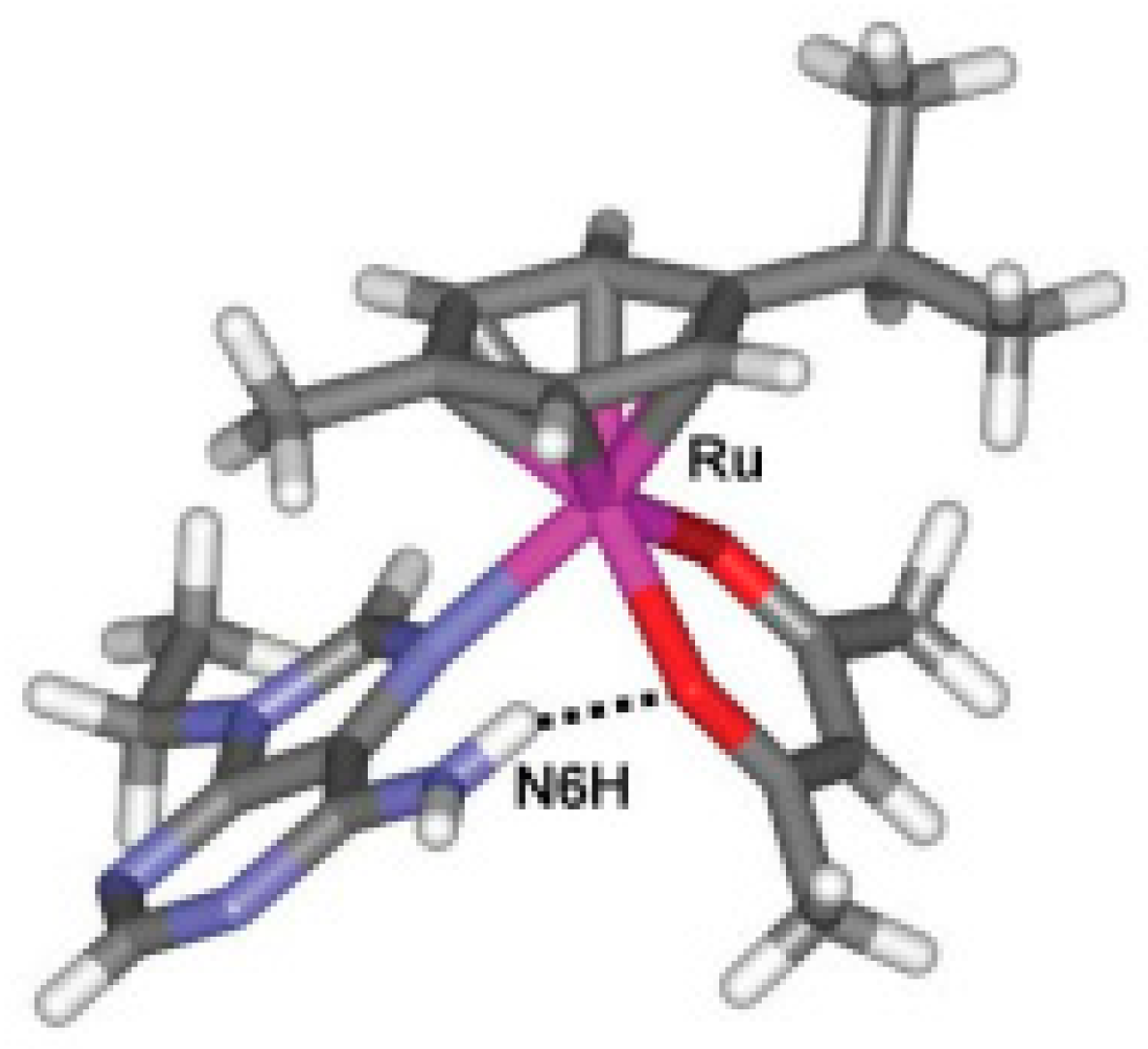

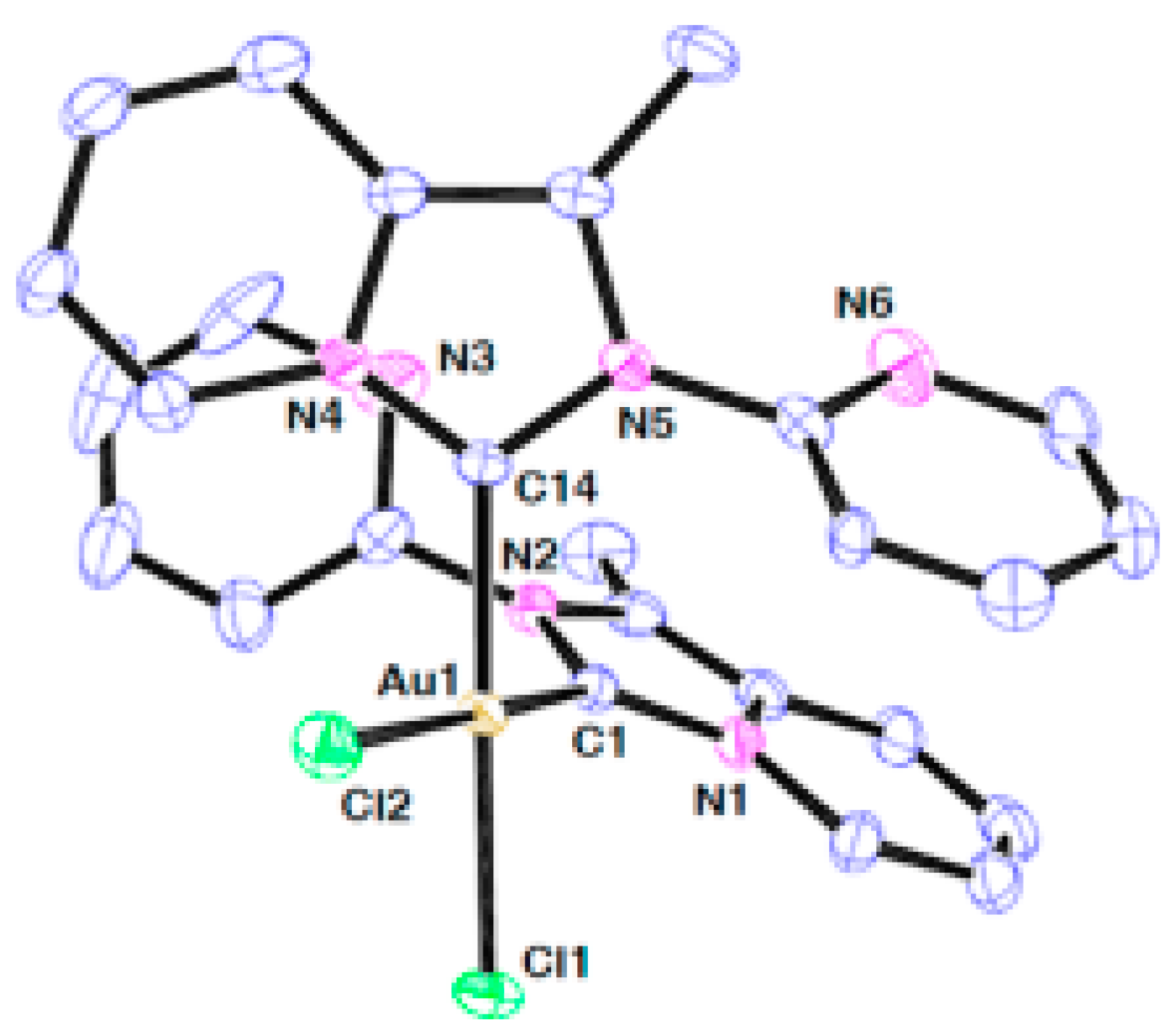





| Technique | Donor Atom | Metal | Structure | References |
|---|---|---|---|---|
| 13C CPMAS NMR, IR and FAB-MS and theoretical DFT studies | N3^S4-bridging coordination | Cu(I) and Ni(II) | dimeric structures | [1] |
| 13C CPMAS NMR and theoretical DFT studies | N3^S2-bridging coordination for cyclohexanespiro-5-(2,4-dithiohydantoin with Cu(I); monodentate coordination (N3- and S2-) of two non-equivalent ligand molecules for cyclooctanespiro-5-(2,4-dithiohydantoin) with Cu(I); N3^S4- bridging way for Ni(II) | Cu(I) and Ni(II) | dimeric structure for Cu(I) with cycloheptanespiro-5-(2,4-dithiohydantoin); square planar for Ni(II) with cycloheptanespiro-5-(2,4-dithiohydantoin) and cyclooctanespiro-5-(2,4-dithiohydantoin) | [2] |
| IR and 13C CPMAS NMR and theoretical DFT studies | N and S | Pt(II) | square planar | [3] |
| 13C-NMR-CP-MAS, EPR, IR and quantum-chemical (DFT/B3LYP-6-31G (d,p)) methods | N for Cu(II) and N3 and S2 for Ni(II) | Cu(II) and Ni(II) | distorted tetrahedral for Cu(II) and square planar for Ni(II) | [4] |
| 13C CPMAS NMR and theoretical DFT studies, X-ray | O, Cl | Al(III) | six-membered chelate rings | [5] |
| melting point analysis, MP-AES for Cu and Pd, UV-Vis, IR, ATR, 1H NMR, 13C NMR and Raman, Solid-state NMR spectroscopy | O, S for 6-methyl-2-thiouracil and S for 6-propyl-2-thiouracil with Cu(II); N, S, O with Pd(II) | Cu(II) and Pd(II) | tetrahedral for Cu(II) with 6-methyl-2-thiouracil and octahedral for 6-propyl-2-thiouracil; chelate for Pd(II) with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil | [6] |
| MP-AES for Cu and Au, ICP-OES for S, ATR, solution and solid-state NMR, and Raman spectroscopy | N,S for Au(III) and O,S for Cu(II) | Au(III) and Cu(II) | chelate structure | [7] |
| UV-Vis, IR, ATR, 1H NMR, HSQC, and Raman, solid-state NMR spectroscopy | O, S | Au(III) | tetrahedral | [8] |
| IR, FAB-MS, XPS, solid-state NMR spectroscopy and theoretical DFT studies | N, S | Pt(II) | dimer, chelate structure | [9] |
| X-ray | O, N | Ag(I) | dinuclear complex, chelate structure | [10] |
| X-ray, ESR, MALDI mass-spectrometry, NMR spectroscopy | P, O, P | Ru(II) and Ru(III) | chelate structure | [11] |
| X-ray and 1H-, 13C-NMR, IR and UV-Vis spectroscopy and elemental analysis and theoretical DFT studies | O, N | Cu(II), Fe(II) and Zn(II) | chelate structure | [12] |
| elemental analysis, FAAS, FT-IR, MS, TG methods and X-ray for C3 and C4 | N, Cl | Zn(II) | tetrahedral geometry, dinuclear coordination compounds | [30] |
| Elemental analysis, NMR and ESI-MS | C, Cl | Rh(I) and Ru(II) | Tetrahedral or square planar | [31] |
| X-ray and 1H-, 13C-NMR, IR and UV-Vis spectroscopy and elemental analysis | C, Cl | Au(III) | square planar | [32] |
| NMR and mass spectroscopy, X-ray | C, Cl | Au(I) and Ag(I) | Linear | [33] |
| Ligand | Pa | Pi | Cell-Line Name | Tissue | Tumor Type |
|---|---|---|---|---|---|
| * L3 | 0.587 | 0.029 | Oligodendroglioma | Brain | Glioma |
| L3 | 0.538 | 0.010 | Colon adenocarcinoma | Colon | Adenocarcinoma |
| L3 | 0.490 | 0.022 | Non-small-cell lung carcinoma | Lung | Carcinoma |
| L3 | 0.475 | 0.009 | Pancreatic carcinoma | Pancreas | Carcinoma |
| L3 | 0.439 | 0.043 | Pancreatic carcinoma | Pancreas | Carcinoma |
| * L4 | 0.559 | 0.006 | Pancreatic carcinoma | Pancreas | Carcinoma |
| L4 | 0.554 | 0.009 | Colon adenocarcinoma | Colon | Adenocarcinoma |
| L4 | 0.415 | 0.038 | Cervical adenocarcinoma | Cervix | Adenocarcinoma |
| L4 | 0.426 | 0.099 | Oligodendroglioma | Brain | Glioma |
| Complex | T98G | SK-N-AS | A549 | CCD-1059-Sk |
|---|---|---|---|---|
| * L1 | 41.25 ± 2.30 | >100 | >100 | >100 |
| C1 | 32.22 ± 0.92 | 35.59 ± 1.03 | 33.51 ± 1.29 | 18.42 ± 0.37 |
| * L2 | 34.98 ± 1.44 | 81.35 ± 3.31 | 43.08 ± 2.17 | >100 |
| C2 | 24.29 ± 0.11 | 33.72 ± 0.39 | 34.44 ± 0.75 | 27.27 ± 1.05 |
| L3 | >100 | >100 | >100 | >100 |
| C3 | 46.54 ± 1.86 | 41.60 ± 1.93 | 41.34 ± 2.17 | 30.84 ± 1.11 |
| L4 | >100 | >100 | >100 | >100 |
| C4 | 30.05 ± 1.81 | 36.17 ± 0.44 | 35.01 ± 0.86 | 33.62 ± 0.85 |
| Etoposide | >100 | 67.83 ± 2.03 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinova, P.E.; Tamahkyarova, K.D. Synthesis, Investigation, Biological Evaluation, and Application of Coordination Compounds with Schiff Base—A Review. Compounds 2025, 5, 14. https://doi.org/10.3390/compounds5020014
Marinova PE, Tamahkyarova KD. Synthesis, Investigation, Biological Evaluation, and Application of Coordination Compounds with Schiff Base—A Review. Compounds. 2025; 5(2):14. https://doi.org/10.3390/compounds5020014
Chicago/Turabian StyleMarinova, Petya Emilova, and Kristina Dimova Tamahkyarova. 2025. "Synthesis, Investigation, Biological Evaluation, and Application of Coordination Compounds with Schiff Base—A Review" Compounds 5, no. 2: 14. https://doi.org/10.3390/compounds5020014
APA StyleMarinova, P. E., & Tamahkyarova, K. D. (2025). Synthesis, Investigation, Biological Evaluation, and Application of Coordination Compounds with Schiff Base—A Review. Compounds, 5(2), 14. https://doi.org/10.3390/compounds5020014







